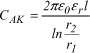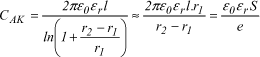Chapter 18
Electrolytic Capacitors 1
18.1. Introduction
Considering their ability to store energy in the form of electrostatic charges, capacitors are indispensable passive components in electronic and electronic power circuits. The physical and geometrical properties of the dielectric which constitutes them have a major impact on their characteristics.
Electrolytic capacitors are amongst the most commonly used capacitors because they associate a strong capacity with unit volume, an operating voltage which can reach several hundred volts and an attractive price. Their main fault is their relatively high rate of failure compared with the other constituents of electronic circuits. The presentation of different constituents, their foils and the technology used for the manufacture of these capacitors allows us to understand and deduce their main characteristics. Owing to the elaboration of equivalent electrical diagrams related to the different elements making up the capacitor, the behavior of this latter and the influences of electrical parameters, temperature or ageing can be analyzed.
This chapter starts with a presentation of parameters influencing the characteristics of capacitors, which permits an inventory to be made of their different families. The most used electrolytic capacitors are those with liquid electrolyte aluminum or solid electrolyte tantalum. The principle, the constituents and the foils of these two components are detailed. The models and the characteristics for the capacitors are then presented and the variations of parameters analyzed as a function of voltage and temperature for both types of electrolytic capacitors presented. The failures of these components are then listed and explained. To conclude, a glimpse of the current and future evolution of electrolytic capacitors is given.
18.2. Generalities
18.2.1. Characteristic parameters
18.2.1.1. Presentation
A capacitor is a device permitting energy to be stored in the form of electrostatic charges. It is composed of two conductive electrodes (the anode and the cathode) separated by an insulating material (the dielectric).
When a potential difference U is applied between both electrodes, an electric charge Q proportional to the applied voltage U and the capacity CAK is accumulated in the capacitor.
18.2.1.2. Energy and capacity
For a capacitor under a voltage U of capacitance CAK, the stored energy W is given by the following relationship [18.1]:
In the case of an ideal plane capacitor (see Figure 18.1), the value of the capacitance CAK is proportional to the surface S of the electrodes and inversely proportional to the thickness of the dielectric e (see equation [18.2]):
where:
– ε0 is the absolute vacuum permittivity (ε0 = 8,854.10−12 F/m);
– εr is the relative permittivity or dielectric constant of the dielectric material;
– S is the surface of the electrodes (m2);
– e is the thickness of the dielectric (m).
Considering equations [18.1] and [18.2], the stored energy W in the capacitors is therefore a function of four parameters: U, εr, S and e. To increase the stored energy, we must therefore increase the voltage U, the relative permittivity εr, the surface S and decrease the thickness e.
18.2.1.3. Dielectric constant and rigidity
The parameters U, εr and e are interdependent of one another. Indeed, one of the essential characteristics of the dielectric is its performance in voltage as a function of its thickness.
Table 18.1 gives the value of the dielectric constant εr and the dielectric rigidity ev of different materials used for the manufacture of capacitors [PER 03], [BES 90], [LAG 96]. These materials define the type of capacitor. For certain materials, the dielectric rigidity is not linear depending on their thickness [MEN 97]. The technological stresses due to materials and to the manufacture of capacitors therefore impose restrictions on the dielectric thickness and its voltage performance.
The use of a dielectric given under an appropriate voltage allows a strong capacitance. The associated dielectric constant is therefore a key factor of this capacitance. Let us note that ceramic capacitors possess a very strong relative permittivity value but, unfortunately, the minimal thickness of the dielectric is relatively large (see below).
Table 18.1. Dielectric constant and rigidity of the main materials used for the manufacture of capacitors

This leads us to tackle the second dimension parameter of capacitance, i.e. the dielectric thickness.
18.2.1.4. Thickness of the dielectric
The minimal dielectric thickness emin is a function of dielectric rigidity ev and the rated voltage Un of the component according to the following relationship:
Table 18.2 gives the minimal thickness of the dielectric emin ([BES 90], [LAG 96]) for the different families of capacitors and the order of magnitude of the rated voltage range. For electrolytic capacitors, this thickness, which constitutes that of the oxide formed by the electrolyze process, is a function of the applied voltage during this process.
The thickness of the dielectric is a determining element for the value of the capacitance. Indeed, we see in Table 18.2 that electrolytic capacitors, which can have a minimal thickness of dielectric emin up to 1,000 times less than the other components, possess a very strong capacitance in comparison with these latter.
Table 18.2. Minimal thickness of the main dielectric materials used for the manufacture of capacitors and associated voltage ranges

18.2.1.5. Surface of electrodes
The last important parameter permitting the value of the capacitance to be increased is the surface of the electrodes S. To obtain a large surface of electrodes for a given package volume, two processes are used:
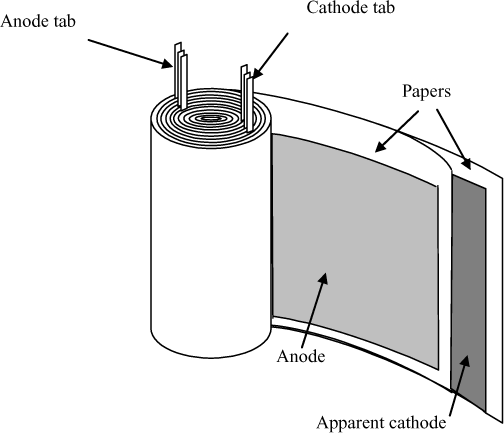
– the dielectric material (or electrolyte support for aluminum electrolytic capacitors (see section 18.4)) is coiled with the electrodes (see Figure 18.2). The package used is most often cylindrical or possibly parallelepipede-shaped if the coil is flattened;
– the dielectric material (or electrolyte support for aluminum electrolytic capacitors (see section 18.4)) is piled up with electrodes on different layers for the insertion in a generally parallelepipedic package (see Figure 18.3). According to the type and the technology of capacitors, there are several bonding possibilities of different layers of the same nature.
Let us note that the capacitance CAK of a cylindrical capacitor with only one layer having a length l, of electrodes with radius r1 and r2 (see Figure 18.4) is given by the following expression:
In general, the difference r2-r1, corresponding to the thickness e of the dielectric, is small compared to r1. The expression can therefore be written:
where e = r2-r1 and S = 2nr1.l corresponding to the surface of the electrodes.
The capacitance CAK of a cylindrical capacitor is therefore comparable to that of a flat capacitor.
For aluminum electrolytic capacitors (see section 18.4), the electrode surface on which the oxide is left by electrolysis is engraved, which allows it to have an effective surface larger than the apparent surface. The multiplicative factor between the apparent surface and the real surface S can vary from 20 to 100 according to the voltage range of capacitors. Let us note that the quality of the engraving has an important influence on the capacitance of the capacitor and it determines a large part of its tolerance range.
18.2.2. Conclusions on the different families of capacitors
The different parameters detailed above allow both main parameters to be taken into account for the choice of a type of capacitors, i.e. capacitance CAK and use of the voltage U of the component. Figure 18.5 represents the orders of magnitude of capacitance and voltage ranges for the different families of capacitors on the market [ELE 04], [SAR 98], [WIL 92].
The components presenting the strongest volume capacitance value are electrolytic capacitors. For example, the theoretical volume v of a capacitor 4,700 µF / 500 V as a function of the used technology is as follows [PER 03]:
– for an aluminum electrolytic capacitor: v ≈ 0.4 dm3;
– for a polypropylene film capacitor: v ≈ 70 dm3;
– for a mica capacitor: v ≈ 500 dm3.
18.3. Electrolytic capacitors
The next part of this chapter deals with electrolytic capacitors. Owing to their strong capacitance volume associated with a rated voltage which can reach several hundred volts, these components are widely used in the electronic and power electronic domains. The frequential and thermal electrical stresses present in electronic circuits have a direct influence on the behavior of capacitors. The main disadvantage of electrolytic capacitors is their relatively weak reliability.
There are two large families of electrolytic capacitors: liquid electrolyte or solid electrolyte.
Aluminum electrolytic capacitors dominate the market of capacitors with strong capacitance. They are therefore much used in power electronics. They represent over a third of the world market of capacitors [LAG 96], [NIS 96]; 99% of these are liquid electrolyte [NIS 96]. This is mainly due to the relatively high cost of solid electrolyte capacitors and the technological difficulty of uniformly fitting the electrodes of these components with the aid of a solid electrolyte.
In the domain of electronics, the most used solid electrolyte capacitors are tantalum electrolytic capacitors which, at a reasonable price, allow a high degree of miniaturization necessary in computer circuits, cameras, flat screens, telephones, etc. The capacitance obtained for a reduced-volume capacitor is important owing to, amongst other things, a dielectric constant practically three times higher than that of aluminum electrolytic capacitors. These components represent over 10% of the world market of capacitors [LAG 96], [NIS 96].
The vast majority of electrolytic capacitors present in power and electronic circuits are therefore liquid electrolyte aluminum capacitors and solid electrolyte tantalum capacitors. It is therefore these which will be presented below. Most analyzes, interpretations or conclusions will nevertheless be transposable to other electrolytic capacitors.
18.4. Aluminum liquid electrolytic capacitors
18.4.1. Principles and composition [PER 03], [ALV 95]
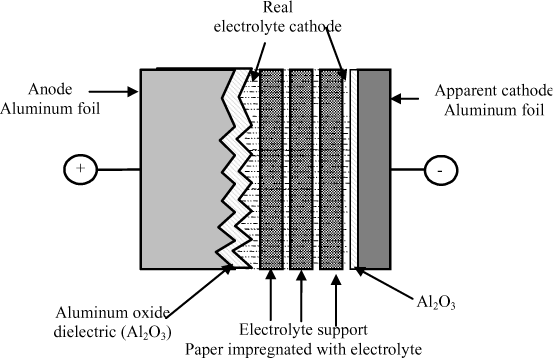
Aluminum electrolytic capacitors are composed of two aluminum foils, an electrolyte support (also called a separator) made up of paper foils impregnated by the electrolyte (generally boric acid dissolved in a solvent) and an aluminum oxide (AL2O3) layer constituting the dielectric and formed on the surface of the anode foil by electrolysis. The composition of such a capacitor is represented in Figure 18.6.
As we saw above, the thin oxide layer (a few nm to a few hundred nm) and the engraving of the aluminum foil constituting the anode means large capacitances can be achieved.
This type of capacitor is polarized and can only support a very weak inverse voltage (of a few volts). This performance with inverse voltage is due to a thin oxide layer which is naturally created on the aluminum cathode of the component (see Figure 18.6).
The dielectric, i.e. the aluminum oxide layer, is formed by electrolysis on the anode during the manufacturing process of the component. The electrolyte which impregnates the paper has two main roles:
– to ensure the best possible electrical conduction between the aluminum foil brought to the negative potential and the aluminum oxide (the real cathode is therefore made up of the impregnated paper);
– to regenerate the integrity of the aluminum oxide in case of defect.
These latter defects are related to the quality and the greater or lesser thickness of the aluminum oxide layer. In thinner zones which can appear, the leakage current IL can increase locally. The current thus created transforms the water of the electrolyte into oxygen and hydrogen. This electrochemical phenomenon permits an oxidation of the anode, which eliminates the possible weaknesses of the aluminum oxide layer. This phenomenon is called self-regeneration of the oxide.
18.4.2. Assembly and connections [PER 03]
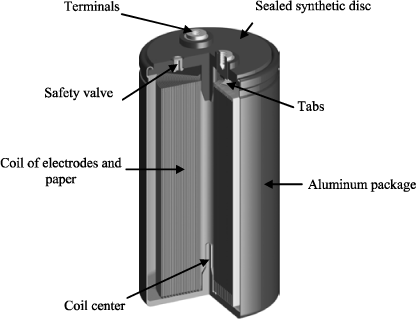
Tabs link the coil of the capacitor to the terminals on the component package. These tabs are set on the anode and on the cathode. Figure 18.7 represents the connections realized on the coil for a capacitor with radial outputs. There are several coil loops by tab. The path of the current entering the anode is therefore axial in the tabs and mainly orthoradial in the electrodes.
Knowledge of the direction and distribution of the currents in the different elements of the capacitor are essential to determine the magnetic field within and near the component, the associated parasitic inductance, the losses and the heating generated [JOU 96], [PER 05].
The coil is placed in a waterproof aluminum package. For example, Figure 18.8 represents the composition of a large electrolytic capacitor possessing a connection with screwed terminals. Depending on the package, a rupture point or a safety valve (see Figure 18.8) allows the evacuation of possible over pressure in case of evaporation of the electrolyte due to internal heating (see section 18.7.3.1).
Figure 18.8. Structure of an electrolytic capacitor with screwed terminals. (Image courtesy of Vishay Intertechnology, Inc.)
18.5. (Solid electrolyte) tantalum electrolytic capacitors
18.5.1. Principle, composition and glimpse of the manufacture [BES 90], [KEM 01], [LAG 96], [PRY 01]
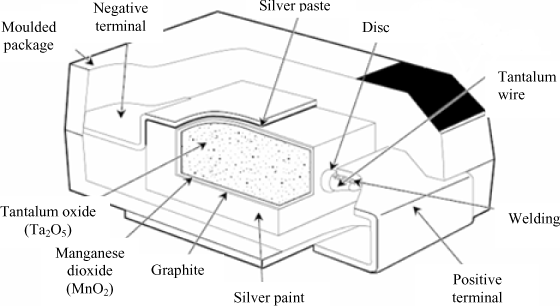
The basic structure of a tantalum electrolytic capacitor is represented in Figure 18.9. The role and the manufacture of the different elements are explained below.
Tantalum (Ta) is a rare metal. To obtain the anode, some tantalum powder mixed with a binding organic is pressed with a tantalum connection wire. In general, the conglomerated powder (pellet) has a cylindrical shape for traversing components (drop-shaped or cylindrical) and parallelepipedic for the SMC (Surface Mounted Components). A tantalum connection wire is placed in the pellet axis. The strengthening of the pellet is made by placing the device under vacuum at very high temperature (generally between 1,200°C and 1,800°C). This latter process is called fritting.
The formation of the dielectric (Ta2O5) is realized by immersing the pellet in an acid bath and by subjecting it to a continuous voltage (3 and 4 times higher than the rated voltage of the capacitor). This operation oxidizes the tantalum, and a thin layer of tantalum pentoxide (Ta2O5) (with thickness of about 1.4 to 2 nm/V) is formed at its surface. Owing to the use of the porous structure made up of tantalum powder, the surface affected by the oxidation can be up to 100 times greater than the apparent surface of the pellet. The thinness of the dielectric combined with this large surface and the relatively strong dielectric constant allows strong capacitance values to be obtained.
The cathode is made by dipping the pellet in a manganese nitrate solution which penetrates the porous structure and is left on the dielectric. By heating the whole, the nitrate is decomposed in manganese dioxide (MnO2). The manganese dioxide can be replaced by a polymer, which allows the equivalent series resistance of the capacitor to be improved [PRY 01]. It is quite tricky to link the manganese dioxide (MnO2) connection wire. For this purpose, the pellet is first dipped into a graphite solution, dried, and then a silver (Ag) layer is left on the whole. The connection wire of the cathode is welded on the silver layer or is placed in contact with the silver via the the component package.
As for the electrolyte of the aluminum electrolytic capacitor, the manganese dioxide (MnO2) of the tantalum capacitor has two main roles:
– to ensure the best possible electrical conduction between the negative terminal of the component and the tantalum oxide (ta2O5);
– to insulate the possible defects of the tantalum oxide (by a self-healing phenomenon).
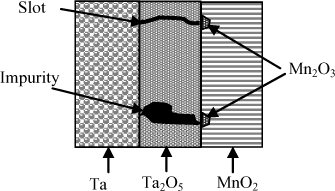
These latter defects are related to the quality and the presence of possible impurities in the tantalum oxide (Ta2O5) layer. If a defect zone is present, the leakage current IL increases locally in this latter and in the manganese dioxide adjacent to this zone. The current thus created locally heats the manganese dioxide (MnO2), which by liberating oxygen is decomposed into manganese sesquioxide (Mn2O3) of much higher resistivity. If this mechanism is successful, a zone of very high resistivity adjacent to the defect decreases the current and prevents a short circuit (see Figure 18.10) [PRY 01]. This phenomenon is called self-healing. It is only possible if the defect zone is small and if the energy dissipation is not too high. In the opposite case, local heating near the defect zone can lead to an extension of this zone and therefore make self-healing impossible.
18.5.2. Assembly and connections
The assembly described above is then coated with epoxy resin, moulded or placed in a plastic or metallic package. The traversing components can have different shapes (cylindrical, parallelepipedic, “drop”) whereas the SMC (Surface Mounted Components) are generally shaped like a cobblestone; for example, Figure 18.11 represents the structure of a SMC tantalum capacitor [KEM 06].
18.6. Models and characteristics
18.6.1. Representative electrical diagram
There are several diagrams representing the frequential behavior of capacitors. The most used model, combining simplicity and relatively good precision, is represented in Figure 18.12, in which:
– CAK represents the ideal capacitance between anode and cathode;
– Rp is parallel resistance, representing the losses in the dielectric and leakage between both electrodes (considering the defects of the dielectric). This element representing the insulating resistance of the capacitor induces a leakage current IL. The orders of magnitude of this current are variable according to the type of dielectric and the technology of the capacitor;
– Rl is the series resistance of the connections and the electrodes (including the paper impregnated with electrolyte for aluminum electrolytic capacitors);
– L is the equivalent series inductance of the connections and coils.
The capacitance CAK is a function of the relative permittivity (see equation [18.2]) which mainly depends on temperature, applied voltage and a lower frequency measurement [ROB 79].
The resistance Rp depends on the applied voltage and the temperature.
The resistance Rl is a function of the temperature and the frequency (this latter variation, visible in high frequency, is due to the skin effect and the non-homogenous distribution of currents in the electrodes [JOU 96]).
These different influences on the elements of the diagram will be detailed in later sections.
We can simplify this diagram according to the normalized representation of Figure 18.13. It is composed of elements function of the frequency which are:
– C, the capacitance;
– ESR, the Equivalent Series Resistance, representing all the losses in the capacitor;
– ESL, the Equivalent Series Inductance.
From the identity of impedances of the circuit represented in Figure 18.12 and that represented in Figure 18.13, we deduce the following relationships:
where ω is the electric pulsation.
This representation is important because it can be deduced quasi directly from the characterization of the capacitor during frequential measurements of the impedance Z of the capacitor. Indeed, the ESR resistance represents the real part of the impedance while the imaginary part is comparable to reactances 1/(C.ω) at low frequency and ESL.ω at high frequency. The resonance frequency fr is thus expressed:
The modulus variation of the impedance Z with the frequency for an aluminum electrolytic real capacitor 4,700 µF / 500 V is drawn in Figure 18.14 on a log-log scale. The normalized equivalent diagram represents its behavior precisely.
Figure 18.14. Modulus of the impedance Z as a function of the frequency for a capacitor 4,700 µF. Values deduced from the measurement: C = 4,620 µF, ESR = 9,2 mΩ ESL = 12 nH

18.6.2. Loss factors, loss angles
The elements Rp and CAK of the equivalent circuit in Figure 18.12 are directly related to the dielectric. By considering the voltage Up to the terminal of Rp, the losses in the dielectric are written:
These are null if the resistance Rp tends to infinity, which corresponds to a dephasing ![]() p of the current in the capacitor I on the voltage Up at -π/2. In the opposite case, the dephasing
p of the current in the capacitor I on the voltage Up at -π/2. In the opposite case, the dephasing ![]() p is greater than -π/2 and the loss angle of the dielectric δd is then defined as being the complement of the phase angle
p is greater than -π/2 and the loss angle of the dielectric δd is then defined as being the complement of the phase angle ![]() p with respect to -π/2. This angle is visualizable on the complex plane by representing the complex admittance Yp made up by Rp in parallel with CAK (see Figure 18.15).
p with respect to -π/2. This angle is visualizable on the complex plane by representing the complex admittance Yp made up by Rp in parallel with CAK (see Figure 18.15).
The loss (or dissipation) factor of the dielectric is defined by expressing the loss angle tangent of the dielectric, which amounts to expressing the ratio of the current in Rp with the current in CAK (see equation [18.11]).
Thus, by considering equation [18.10], the losses in the dielectric Pd can be expressed as a function of this factor and the reactive power dissipated in the dielectric Qd:
The total losses in the capacitor P are the sum of the losses in the dielectric Pd and the losses by Joule effect due to the resistance Rl of the connections and the electrodes. By considering I, the current in the capacitor, the equivalent diagrams in Figure 18.12 and Figure 18.13, they are therefore written:
For a perfect capacitor, there are no losses P, the resistance ESR is null and the dephasing ![]() of the current in the capacitor I on the voltage at the terminals of the component is at -π/2. For a real capacitor, the dephasing
of the current in the capacitor I on the voltage at the terminals of the component is at -π/2. For a real capacitor, the dephasing ![]() is greater than -π/2 and the loss angle δ is then defined as being the complement of the phase angle
is greater than -π/2 and the loss angle δ is then defined as being the complement of the phase angle ![]() with respect to -π/2. By neglecting ESL, the loss angle δ is visualizable on the complex plane by representing the complex equivalent impedance Z of the circuit in Figure 18.13 (see Figure 18.16).
with respect to -π/2. By neglecting ESL, the loss angle δ is visualizable on the complex plane by representing the complex equivalent impedance Z of the circuit in Figure 18.13 (see Figure 18.16).
The loss (or dissipation) factor of the capacitor is defined by expressing the loss angle tangent:
It is this factor entitled DF (Dissipation Factor) which is given by the manufacturers of capacitors. It permits the total losses P in the component as a function of the total reactive power Q considering equations [18.13] and [18.14] to be determined:
The loss factor of the capacitor tan δ must not be confused with the loss factor of the dielectric tan δd. The procedure to obtain the relationship relating these two factors is given below.
By neglecting ESL, the expression for the equality of reactive powers of equivalent circuits in Figure 18.12 and Figure 18.13 gives a relationship between the current I and the voltage Up:
By expressing the total losses in the capacitor with the aid of equations [18.13] and [18.15], and by considering equations [18.10], [18.11] and [18.16], we find the relationship between both loss factors:
By only taking into account the circuit elements in Figure 18.12 and Equation [18.6], this equality can thus also be written:
There is equality between both factors only if the losses by Joule effect due to the resistance Rl are neglected.
18.6.3. Variation as a function of the voltage
The polarization voltage U (continuous voltage) applied to the terminals of the capacitor has a minor influence (1 or 2% at the maximum) on the value of the capacitance CAK and the series resistance of the connections and the electrodes Rl [KEM 06], [PER 03]. On the other hand, its influence is important on the leakage current and therefore on the resistance Rp which represents, among others, these leaks between both electrodes.
This voltage sensitivity is easily understood knowing that the stronger the electric field is, the more the electrolyte (the manganese dioxide for tantalum capacitors) is able to emit electrons which migrate towards the anode via the dielectric. The leakage current is therefore mainly a function of this electric field applied between the anode and the cathode, as well as the thickness and the quality of the oxide. It is therefore higher for capacitors with a strong capacitance value, because statisticly the larger the surface of the dielectric is, the higher the number of possible defects (impurities or irregularities in the layer). Let us note that the leakage current is also variable as a function of the polarization time and depends on the previous state for liquid electrolyte capacitors. Indeed, the aluminum oxide layer of a stored aluminum electrolytic capacitor is degraded. The leakage current when brought into service can be large. It then decreases in time owing to the self-healing phenomenon.
For an aluminum electrolytic capacitor, this leakage current IL can be written as a function of the polarization voltage U in the following form [PER 03], [RIF 95]:
where Un is the rated voltage of the capacitor, R is the resistance of the dielectric considered constant for weak voltages (less than Un/2), ILn is the leakage current at Un, and ξ a coefficient depending on the component.
Figure 18.17 shows the leakage current IL versus applied polarization voltage U, measured 5 minutes after the beginning of the charge for an aluminum electrolytic capacitor of 4,700 µF / 500 V [PER 03]. This curve was deduced from equation [18.19].
Figure 18.17. Leakage current at 5mn as a function of the polarization voltage for a capacitor 4,700 µF / 500 V at 25°C

The resistance Rp decreases exponentially with the voltage U because this latter can thus be expressed (knowing that the resistance Rl is negligible compared to Rp):
The variations with the voltage for tantalum capacitors are near those of aluminum electrolytic capacitors.
18.6.4. Variation as a function of the ambient temperature
18.6.4.1. Electrolyte liquid aluminum electrolytic capacitors
Aluminum electrolytic capacitors are very sensitive to the variations of temperatures because of the property changes of the electrolyte. For example, Figure 18.18 represents the modulus of the impedance Z as a function of the frequency for different ambient temperatures for a capacitor 4,700 µF/500 V. When the temperature increases, we note a strong decrease of the equivalent series resistance (ESR) (corresponding to the value of Z at the resonance frequency fr i.e. the minimum of Z as a function of f), a shift (decrease) of the resonance frequency due to an increase of the capacity C (see equation [18.9]).
Figure 18.18. Modulus of the impedance Z as a function of the frequency and defined by the ambient temperature for a capacitor 4,700 µF/500 V

These variations are above all due to the change in viscosity of the electrolyte when the temperature decreases, which decreases its conductivity. Further, the temperature variations can generate a dilation of electrodes which also modifies the capacitance value.
ESR decreases exponentially with temperature T according to the formula:
α, β and γ are coefficients which depend on the component. ESR decreases (a few tens per cent) between a temperature of 0°C and the maximal temperature acceptable by the component, but it can increase by a factor of more than 10 if the temperature decreases to negative values.
To a first approximation, the capacitance C increases linearly a few tens per cent with temperature.
The leakage current increases with the temperature T according to an exponential law in the form:
χ, λ, θ are coefficients which depend on the component. The increase between the negative minimal and the positive maximal temperatures is important since it can reach a factor near 100.
The elements of Figure 18.12 can be determined as a function of temperature [PER 03]. The inductance L can be considered constant. The variations of the other parameters are given below, knowing that the different coefficients (Greek letters) depend on the component:
ILn(t) is determined using equation [18.22].
The model in Figure 18.12 is valid as long as the temperature is positive. For negative temperatures, Figure 18.18 shows that the modulus curve of the impedance Z as a function of the frequency presents an inflection point. The basic model in Figure 18.12 is then no longer satisfactory and its order must be increased by adjunction of elements in parallel or in series. Possible models, representing correctly the functioning at a negative temperature, are given in [PER 06].
18.6.4.2. Solid electrolyte tantalum capacitors
For tantalum capacitors, variations with temperature are of a similar form to those of aluminum electrolytic capacitors. However, their amplitudes are low because the electrolyte is solid, so there is no modification related to the state change of the electrolyte. This aspect constitutes one of the advantages of solid electrolyte capacitors.
18.7. Failures of electrolytic capacitors
In comparison to other electronic components, electrolytic capacitors present relatively large failure rates [LAH 98], [USM 95] hence the importance of this section.
18.7.1. Modes and failure rates of components
The failure rate λ(t) is the conditional probability of failure by unit time. It gives a measurement of the risks for a device to break down during a time interval ]t, t+Δt] when Δt tends to zero, knowing that this device lasted until time t. The failure rate λ(t) is expressed in 10−6/hour or 10−9/hour (implied: failure/106 h or failure/109 h) or in FIT (Failure In Time with 1 FIT = 1 failure/109 h). Thus, if 1,000 capacitors are used for 1 million hours (114 years), 1 failure corresponds to 1 FIT. For electrolytic capacitors, according to the stress and the components, the failure rates can range from a few FIT to a few thousand (even a few tens of thousand) FIT (for reasonable ambient temperatures).
The failure rate λ(t) follows for many components the bathtub curve represented in Figure 18.19.
This curve is split in three parts:
– the early failure period (t ≤ ta), due to the youth defects of capacitors, where the failure rate λ(t) decreases;
– the intermediary period (t ![]() ]ta, tb[), where the failure rate is approximately constant, corresponding to the normal lifetime period;
]ta, tb[), where the failure rate is approximately constant, corresponding to the normal lifetime period;
– the wear period (t ≥ tb) where the failure rate increases. In this period, the failures take a systematic character. Generally, these are failures by degradation (drifts).
There are two main failure modes, by degradation and by catastrophy:
– failures by degradation are defined by a change of characteristics of the component which drifts out of its specific tolerances. The most current degradations are an increase of the equivalent series resistance (ESR) or of the loss factor tan δ, a decrease in the capacitance C, an increase in the leakage current IL;
– catalectic failures (sometimes called catastrophic by manufacturers) are sudden failures corresponding to the disappearance of the component function. They are most often characterized by a short circuit or an open circuit of the capacitor.
18.7.2. Influence of temperature
Temperature is a major stress with a direct influence on failure rate λ(t). The temperature of a component is a function of the ambient temperature and of the dissipation of power P in the component. The current I which traverses the capacitor and the equivalent series resistance ESR (see equation [18.13]) therefore causes an increase in the temperature of the component package and influences the lifetime. It is recognized that the breakdown process is equivalent to a chemical reaction. Now, the speed constant of chemical reaction kv depends on the absolute temperature T, according to the Arrhenius Law:
Ea is the activation energy of the reaction (which depends on the type of failure) expressed in electronvolts (eV) and k the Boltzmann constant (k = 8.62.10−5 eV/K).
The time of apparition of a failure is inversely proportional to the speed of apparition of this failure, so this time t1 can therefore be written for a temperature T1 considering equation [18.26]:
A is a coefficient which depends on the component. The time t1 until the failure can be accelerated (until time t2) by increasing the temperature (of value T1 to T2) by the multiplicative acceleration factor of the failure Acc. This factor can thus be written:
This formula is much used to extrapolate the duration of apparition of a failure at any temperature following an ageing accelerated under maximal temperature.
18.7.3. Failures of liquid electrolyte aluminum electrolytic capacitors
18.7.3.1. Ageing of liquid electrolyte aluminum capacitors
The most probable failure of liquid electrolyte capacitors is related to its ageing. Like all components based on liquid electrolyte, the aluminum electrolytic capacitors present a wear period (t ≥ tb in Figure 18.19), their failure then becoming inevitable.
This is the main disadvantage of this component. The life end of the capacitors is caused by an internal failure phenomenon, evaporation of the electrolyte which manifest itself by the following degradation of electric parameters: the capacitance C decreases (a few tens per cent) and the equivalent series resistance ESR increases (by more than 100%).
The decreasing rate in the capacitance C is lower than the increasing rate of ESR, so the loss factor tan δ defined by equation [18.14] increases.
The equivalent series resistance ESR is the parameter which is the most rapidly degraded with the component ageing. It therefore constitutes an indicator of ageing preferred for these components.
The equivalent series resistance mainly depends on the resistance of the electrolyte which impregnates the papers.
Since the electrolyte is evaporated, the equivalent surface of this latter decreases, leading to the increase of ESR. The increase in this resistance as a function of time depends on the type of electrolyte, the component package and, more particularly, its watertightness. These leaks are not always visible on the component. They are on the capacitor represented in Figure 18.20.
Several models describe its evolution as a function of time. A simple linear model of the evolution as a function of the ageing time of ESR in 1/ESR was developed by [RHO 84]. Other more precise models show an increase of the ESR according to the exponential law [PER 03].
The variable elements of Figure 18.12 as a function of time t1 for a temperature T1 are represented by the following equations [PER 03]:
Rl(0) and CAK(0) are the values of Rl and CAK for a healthy capacitor. The different coefficients aR, bR and aC are functions of the component. They depend on the geometry of this latter, of its technological characteristics and of the electrolyte used. Considering equation [18.28], we can deduce the evolution of the parameters of this model as a function of time for a temperature T2. The activation energy Ea of this equation was estimated at 0.405 eV by [RHO 84] for this failure mode.
Since the drifts of the different parameters are known and can be expressed as equations, predictive maintenance systems of electrolytic capacitors can be elaborated [LAH 98]. Some propose an individual supervision of the capacitors [BES 03], [VEN 02] by measuring their impedance near resonance frequency, the equivalent series resistance ESR (see Figure 18.14) can be determined whilst functioning and compared to that of a healthy component.
18.7.3.2. Other failures of liquid electrolyte aluminum capacitors
A large number of catalectic failures related to the manufacture defect of the capacitor or its use can come occur. Figure 18.21 summarizes the main modes, mechanisms and causes of failure [PER 03], [ABD 08].
18.7.4. Failures of solid electrolyte tantalum capacitors
Since the electrolyte of the tantalum capacitors considered is solid, there is no electrolyte leakage risk and therefore these components do not present any wear period (see Figure 18.19). Their failure rate therefore does not increase in time, which is one of their advantages.
The majority of defects of these components are related to their thinness and to the quality of the tantalum oxide layer (Ta2O5); 90% of the failures induce an increase of the leakage current IL or short circuits, which can lead to the flammability of the component.
The stresses revealing these failures are temperature, polarization voltage and current.
These failures are related to the absence of the self-healing phenomenon (see section 18.5.1). As for the component, this latter is only possible if:
– the manganese dioxide (MnO2) thickness is sufficient and regular;
– the tantalum (Ta) pellets do not present any impurities;
– silver or graphite do not migrate to the tantalum oxide (Ta2O5) dielectric film through a possible crack of the manganese dioxide (see Figure 18.9). In these penetration zones, the self-healing effect of the capacitor is held, leading to a deterioration of the dielectric film, then a breakdown of the dielectric [POZ 98].
Moreover, for the self-healing to take place, the energy dissipated in the capacitor needs to remain at a decent level. The charge/discharge resistance of the capacitor must be higher than a few Ω/V for a good self-healing process. By default, locally, a very high temperature can appear, which associated with the electric field can lead to a crystallization of the tantalum pentoxide (Ta2O5) and lead to an increase of the leakage current or to a short circuit.
These different defects are inherent to manufacturing problems, storage under high humidity or to a poor use of the component.
18.8. Conclusion and perspectives
Electronic devices must be more and more compact. Since the complexity of electronic circuits increases, the power demanded for their supplies is therefore higher. The current devices consequently require the use of not too large electrolytic capacitors capable of supplying a relatively high power. This is the case for the market of SMC-type electrolytic capacitors, which is today boosted by the increase of IT and popular electronics (flat screens, DVD players and recorders, etc.).
To answer these criteria, several routes are conceivable for tantalum electrolytic capacitors. Some of them are cited below:
– the component miniaturization first needs an increase of its volume capacity. The use of tantalum nanopowder allows this need to be answered. The use of new dielectrics to replace tantalum pentoxide (Ta2O5) is also considered. Niobium pentoxide (Nb2O5), for example, presents a larger dielectric constant than that of tantalum pentoxide (41 for Nb2O5 against 27 for Ta2O5);
– the use of polymers to replace manganese dioxide (MnO2) will allow components with weaker ESR to be made, therefore with less losses and a higher energy density;
– the manufacture of components including several tantalum pellets in parallel (multi-anodes) allows the ESR to be reduced, and capacitance C to be increased.
Liquid electrolyte aluminum capacitors are also subject to miniaturization. To reduce their ESR, weak resistivity electrolytes are studied. To improve their reliability, devices to monitor their healthy state are conceived. Finally, to combine the advantages of solid and liquid electrolyte capacitors, hybrid capacitors are investigated.
We cannot finish this chapter, and particularly this section, on the perspectives of electrolytic capacitors without tackling the new component in the domain of energy storage, i.e. the supercapacitor. This component is particular as its principle does not rely on the presence of a dielectric but on that of the double electric layer which is created at the interface between a solid electrode and a liquid electrolyte in the presence of an electric field. It is the thickness of the double electric layer of a few nanometers (related to the diameters of the solvent molecules) which defines the component capacitance. This thinness, associated with a very large surface of electrodes owing to its use of porous materials such as activated carbon, allows very high capacitances (of several thousand farads for the largest components) to be obtained. By principle, the voltage performance of this component is limited to a few volts, which corresponds to the electrolyte decomposition. The capacitance of this component is very rapidly degraded as a function of the frequency, which reserves it to the exclusive domain of energy storage. The supercapacitor can therefore only challenge electrolytic capacitors in this specific domain.
Electrolytic capacitors are therefore essential today in most applications such as for filtering, decoupling, etc.
18.9. Bibliography
[ABD 08] ABDENNADHER K., VENET P., ROJAT G., RETIF J.M., ROSSET C., “A real time predictive maintenance system of Aluminium Electrolytic Capacitors used in Uninterrupted Power Supplies”, IEEE Industry Applications Society Annual Meeting, Edmonton, Canada, 2008.
[ALV 95] ALVSTEN B., Electrolytic capacitors theory and application, Rifa Electrolytic Capacitors, Sweden, 1995.
[BES 90] BESSON R., Technologie des composants électroniques, vol. 1, Editions Radio, Paris, 1990.
[BES 03] BESSEYRE D., Développement d’un condensateur intelligent, Mémoire d’ingénieur CNAM, Lyon, 2003.
[ELE 04] ELECTRONIQUE INTERNATIONAL HEBDO, Les condensateurs évoluent à leur rythme, 2004.
[JOU 96] JOUBERT C., Etude des phénomènes électromagnétiques dans les condensateurs à films métallisés – Nouvelle génération de condensateurs, Doctoral Thesis, Ecole Centrale de Lyon, December 1996.
[KEM 01] General Notes of the KEMET capacitance company, “What is a capacitor?”, 2001.
[KEM 06] General Notes of the KEMET capacitance company, “Surface Mount Capacitors”, 2006.
[LAG 96] LAGRANGE A., “Condensateurs”, Techniques de l’Ingénieur, traité Electronique, E 2 060, Editions TI, 1996.
[LAH 98] LAHYANI A., VENET P., GRELLET G., VIVERGE P.-J. “Failure Prediction of Electrolytic Capacitors During Operation of a Switchmode Power Supply”, IEEE Transactions on Power Electronics, vol. 13, no. 6, november 1998.
[MEN 97] MENGUY C., “Mesure des caractéristiques des matériaux isolants solides”, Techniques de l’Ingénieur, traité Génie Electrique D 2 310, Editions TI, 1997.
[NIS 96] NISHINO A., “Capacitors: operating principles, current market and technical trends”, Journal of Power Sources, vol. 60, no. 2, June 1996.
[PER 03] PERISSE F., Etude et analyse des modes de défaillances des condensateurs électrolytiques à l’aluminum et des thyristors, appliquées au système de protection du LHC (Large Hadron Collider), Doctoral Thesis, Claude Bernard University, Lyon 1, July 2003.
[PER 05] PERRET R., Mise en œuvre des composants électroniques de puissance, Hermes, 2005.
[PER 06] PERISSE F., VENET P., RETIF J.M., ROJAT G., “Study of an Electrolytic Capacitor Model as a function of Temperature”, EPE Journal, vol. 16, no. 2, 2006.
[POZ 98] POZDEEV Y., “Reliability Comparison of Tantalum and Niobium Solid Electrolytic Capacitors”, Quality and Reliability Engineering International, vol. 14, no. 2, 1998.
[PRY 01] PRYMAK J., “Improvements with Polymer Cathodes in Aluminum and Tantalum Capacitors”, Applied Power Electronics Conference and Exposition, APEC 2001. Sixteenth Annual IEEE Volume 2, 2001.
[RHO 84] RHOADES G.E., SMITH A.W.H., “Expected Life of Capacitor with Non-solid Electrolyte”, 34th Component Conference Proc, 1984.
[RIF 95] RIFA, Electrolytic Capacitors Theory and Application, 1995.
[ROB 79] ROBERT P, Matériaux de l’électrotechnique, Traité d’électricité, St Saphorin, Editions Georgi, vol II, 1979.
[SAR 98] SARJEANT W.J., ZIRNHELD J., MACDOUGALL F.W., “Capacitors”, IEEE Transactions on Plasma Science, vol. 26, no. 5, October 1998.
[USM 95] U.S. MIL SPECS, Military Handbook Reliability Prediction of Electronic Equipment, MIL-HDBK-217F, notice 1: July 1992, notice 2: February 1995.
[VEN 02] VENET P., PERISSE F., EL-HUSSEINI M.H., ROJAT G., “Realization of a Smart Electrolytic Capacitor Circuit”, IEEE Industrial Applications Magazine, vol. 8, no. 1, January/February 2002.
[WIL 92] WILLIAMS B.W., Power Electronics: Devices, Drivers, Applications and Passive Components, 2nd Edition, Hong Kong, Editions ELBS, 1992.
1 Chapter written by Pascal VENET.