Chapter 15
Physico-Chemical Characterization Techniques of Dielectrics 1
15.1. Introduction
The physico-chemical characterization techniques of gaseous, liquid and solid dielectrics have been used simultaneously to better understand their nature, improve their characteristics, and follow their behavior as a function of the stresses they are subjected to in the role of electrical insulating materials.
It is evident that these techniques have evolved in time, their resolution power has increased, the acquisition of data modernized and their range widened. They allow, in particular, the analysis of materials for their selection and development, to interpret their evolution and degradation, including analysis of samples extracted from operating equipment (when possible), or from damaged material to try to explain the causes.
We can therefore, for example, use the same physico-chemical characterization techniques for operating transformers or power capacitors as for a buried cable which has been damaged. For this reason, only one description will be made of all the domains where they are used, and only the information they provide will be commented on.
15.2. Domains of application
15.2.1. Transformers and power capacitors
Electrical discharges of higher or weaker intensity generating variable current densities, as well as the local rising of temperature, can lead to the degradation of the oil and/or the paper in this type of equipment. Degradation of cellulose can be caused by hydrolysis, oxidation and thermal effect. The latter leads to a formation of water, monoxide and carbon dioxide. The products which appear are essentially in gaseous form. A small quantity of products (a few ppm) is enough for analysis by gaseous chromatography (GC) i.e. chromatography in the gaseous phase. This routine analysis has been practiced for over 40 years. The furanic by-products are analyzed by high performance liquid chromatography (HPLC) [DUV 77], [LAM 81], [DUV 82]. A good description of all the analyzes realized on transformers has been made by Vergne [VER 92] and Dhiba [DHI 95].
15.2.1.1. Principle of chromatographic analysis and results
The products to be analyzed in gaseous chromatography pass through an inert material, during a stationary phase, such as alumina or activated carbon, on which the selection is made from retention time. These materials with a porous character fill a stainless metal tube of diameter varying from 2 to 4 mm; described as a column, this tube (from 1 to 6 m long) presents itself in the form of a coil. The products injected in the column are conveyed by an inert gas such as helium, hydrogen, nitrogen or argon. The samples are sent into an injection chamber and led by the vector gas which puts them in contact with the inert phase. At the exit of the column we proceed, in general, to electrical detection by a heated filament mounted on a resistance bridge. The electrical equilibrium of this latter varies because of the cooling created by the organic component; a comparison is made with the resistance of the filament around which the effluent circulates alone. It is the area of current peaks which gives the concentration of the products, this concentration being a linear function of the areas of recorded peaks. Other types of detectors are used: flame ionization, argon ionization, electron capture. A chromatograph can be connected to a mass spectrometer or an infrared spectrophotometer.
In practice, we take, for example with the aid of a syringe, an oil sample at the bottom or, in certain cases, from the upper part of the transformer. This sample is sent to a chemical laboratory for separation of gaseous products, which are analyzed by chromatography. The techniques of sampling are variable depending on the country. The analyzed gases are: H2, CH4, C2H2, C2H6, CO and CO2, whose concentrations depend on the type of defect. For example, acetylene (CH ≡ CH) principally comes from an arc which produces temperatures of several thousand degrees, ethylene (CH2 = CH2) comes from hot spots where temperatures vary between 150°C and 1,000°C. The partial discharges, corresponding to a cool plasma, will particularly generate hydrogen (H2) [DUV 84], [DUV 89], [DUV 02].
Let us note that this technique can be applied to the study of the degradation of solid insulating materials used for other types of insulation. This is the case of polyethylene, for example, to which we want to know the method of degradation during exceptional thermal stress (> 250°C). Protection against the effects of dangerous toxic gases is needed, since mixtures of oxygen and low weight molecular products such as aldehydes, ketones and acids are often found. By associating the chromatograph with a mass spectrometer, 44 components have thus been identified, fatty acids representing the principal organic products with formic acid [HOF 81].
15.2.1.2. High performance liquid chromatography (HPLC)
This technique works on the same principles as chromatography on a column where the phenomena of sharing, absorption, ion exchange or exclusion are produced. This depends on the stationary phase, constituting the column, which can be polar (amine, nitrile) or apolar (alkyl, phenyl). The polar character of the stationary phase is aimed at a weakly mobile polar phase like a hydrocarbon.
In their furan analysis, certain researchers use steel capillary columns from 10 to 30 cm long, with an inner diameter ranging from 3 to 5 mm, filled with solid particles. For the furan analysis, these latter are composed of silicium to which carbon chains (octadecyl) are chemically bonded. The sample is injected in the column through which an organic solvent is pumped. Depending on their chemical nature, the components interact differently with the solid support. At the output, different detectors, electrochemical or thermal conductivity, can be associated. For example, a UV detector operating between 200 and 400 nm was used by Unsworth [UNS 90]. The location of the recorded peaks characterizes the component and, as previously, the peak area gives its concentration.
15.2.1.3. Gel Permeation Chromatography (GPC)
Sometimes called exclusion or molecular sieving chromatography, this method leads to the separation of molecules as a function of their size. It is the grains of porous gel filling the columns which play the role of molecular sieve. The smallest molecules remain included in the gel and are eluted after a delay. The diameters of pores lower than 50 nm can be used for molecules present in oils. The technique turns out to be very practical for the analysis of additives incorporated in transformer oils; we note, for example, the clear separation revealed on a chromatogram between a UV stabilizer and an antioxidant [BUR 88].
A recent report, processing the diagnostics on power equipment containing oil, traces a critical history of chromatographic analysis and proposes automatization as a means to avoid experimental errors [ARA 02].
15.2.2. Energy transport cables and dry capacitors
Low density polyethylene (LDPE), chemically reticulated polyethylene (XLPE), ethylene-propylene-hexadiene 1-4 terpolymer (EPR) and, for certain applications, chlorosulfonated polyethylene (HYPALON) [GUE 92], are generally used for the insulation of high voltage cables. For dry capacitors, we keep the use of terephthalate polyethylene (PET), polypropylene (PP) or polyethylene naphthalene (PEN) films.
These materials are most often examined by the same techniques which are used for their selection, or for the understanding of ageing or failure. The most current ones are microscopy, infrared spectrophotometry, calorimetric analysis, thermally stimulated currents, and electron paramagnetic resonance.
15.2.2.1. Microscopy
In optical microscopy, using the shortest possible waves, i.e. ultraviolet, the resolution limit is about 200 nm. Since the photon does not allow us to go further, we use another elementary particle, the electron, instead. The wavelength associated with an electron is indeed much lower than that of the ultraviolet photon and the final resolution is thus of the order of a nanometer. This wavelength is of the same order as that of atomic bonds. Thanks to electromagnetic lenses, the electron beam emitted by an electron gun is focused on the material crossing it with a bigger or smaller absorption. The image forms behind the target on a fluorescent screen.
Except for the fact that electron absorbers are heavy metals, the same revelation techniques as for microscopy in direct light can be used. There are two variants of electron microscopy: scanning electron microscopy and transmission microscopy.
Scanning electron microscopy (SEM) consists of exciting a conductive surface by an electron beam and recreating its topography by capturing the reemitted electrons. The difference in mechanical resistance between amorphous and crystalline zones of polyethylene, for example, makes a difference in relief appearing when test tubes were obtained by the sudden fracture of the polymer at a temperature lower than glass transition temperature. A gold metallic layer of about 30 nm is left on the insulating material by a cool plasma in order to avoid it from being charged during bombardment by the electron beam. We thus observe a dense spherolitic structure.
The observation of the same material by transmission (TEM) permits the contrast between the amorphous zone and the crystalline zone to appear. However, this latter technique is tricky in its development because the thickness of the sample must be reduced to about 0.1 µm and the contrast method is difficult to control. This method requires a coloring of the polymer first, by diffusing chlorosulfonic acid in the amorphous zones of polyethylene, then uranyl acetate which is fixed on these same zones, making them opaque to the electron beam. The contrast then appears between white crystalline lamella (C) and the dark amorphous zones (Figure 15.1).
Fluorescence microscopy, derived from light microscopy, is a technique well known in the field of biology. It allows fluorescent objects or those marked by fluorescent substances to be observed.
Figure 15.2. describes the principle of the microscope. The image formed by the luminous beam is collected after reflection; this is called the epi-fluorescence mode. The fluorescent marker can be rhodamine, for example, which, when excited by blue light, will emit in red. Owing to filters, the sample is lit in blue and the red light is captured. The selection of wavelengths improves the contrast by comparison to clear field light microscopy, owing to the elimination in the final image of incident light reflection. A variant of this type of microscopy consists of replacing the beam of light by a laser beam of wavelength adjusted to the fluorescent substance and focused on the sample; this is laser scanning confocal microscopy. The acquisition of the image is made by scanning of the beam according to the XY focus plane and by displacement of the object according to the Z axis, using precise motors. A computing system processes the images, which allows a tri-dimensional structure to be revealed, by using successive optical sections, superposing them and reconstituting a stereographic view.
One of the problems posed by this type of defect, water treeing, is its structure. Indeed, the characterization methods described above can lead to erroneous conclusions: semi-spherical cavities, micro-channels, etc. This latter gives different forms according to the section plane; this is due to the preparation technique for the observations by SEM (fracture) or TEM (100 nm thick sections). Also, the processing in order to improve the contrast creates artefacts [BAM 83,] [MEL 81]. We note that this purely experimental approach can support hypotheses made on the breakdown mechanism of the insulating material and perhaps, back up certain proposed models.
In practice, we most often remember that an energy transport cable can continue to ensure its function, despite the presence of defects of the type represented in Figure 15.4. Confocal microscopy has also been used to reveal microcavities which can exist in different thin (25 µm) films, amongst which certain were destined for the manufacture of capacitors: polypropylene, polyimide, polyvinylidene fluorure, etc. The obtained information concerns the surface of the film, but also a sub-layer which can reveal micro voids [SUT 92].
All these observation modes are particularly developed to compare PE and XLPE with samples where water treeing have progressed [MOR 92], [MOR 93].
The X-fluorescence induced by particles, i.e. the emission of X-rays induced by charged particles (PIXE), most often uses protons and allows the search for chemical species. By penetrating the material, a beam particle ejects an electron near the core of an atom which thus finds itself in an excited state. An electron in an outer orbit fills the gap and the atom then emits an X-ray expressing its energy excess, which is a proper value for each element. The device itself is composed of a 3 MeV particle accelerator, lenses which allow a focus of beams on a diameter < 1µm and an X-ray detector which realizes the counting and measurement of energy. In a few minutes, we can simultaneously take measurements from sodium (Na) to uranium (U), the weakest quantity being of the order of 1 part/106. For Z ranging from 20 to 30, the sensitivity is 10 ppm. The beryllium windows of the vacuum-packed detector limit its response to elements whose atomic number Z is greater than 11 (Na).
The technique has been applied to the research of impurities in cable insulation and, more particularly, to one of its defects: a bowtie-type of water treeing. The penetration depth in XLPE of electrons accelerated under 50 kV, for example, is 50 µm and 12 µm for protons accelerated under 1 MeV. The first measurements of the target surface being concerned with the emission of X-rays, only a few nanogram of polymer are analyzed, hence care must brought to the preparation of test tubes in order to avoid contamination. Taken on a 20 kV cable, this type of defect revealed an important quantity of chlorine comparatively to the rest of the material. The antioxidant is the source of sulfur that we also detect. The concentration of lead is decreasing, showing its conductor diffusion towards the outside, in this specific case. Despite the sensitivity of the method and the richness of the species found, it is difficult to reach a decision on the role of each one of them regarding the degradation process [HIN 88], [HOU 92]. From the sulfur measurement, the radial distribution of the concentration in the antioxidant was studied in XLPE insulated cables. As we have previously mentioned, the analysis was completed by using IR and UV spectrophotometries. We could thus notice a uniform antioxidant concentration in the core of the insulating material and decreasing towards the semi-conductor screen. Both types of antioxidants (Irganox and Santonox) have been tested here [MAN 83], [PAR 99].
Electronic paramagnetic resonance (EPR), in the area we are concerned with, is used for the detection of free radicals in old insulating materials. In the same way as free electrons in metals or semi-conductors, free radicals possess an unpaired electron. The magnetic moments of an atom or a molecule originate from the circulation of electrons around the cores, the orbital moments and the rotation of the electron on itself called the spin moment. When a material containing unpaired electrons is subjected to a magnetic field of frequency ν, an energy can be absorbed if a static magnetic field of value H is simultaneously applied to it. In practice, we make H increase in a continuous manner, such that the energy absorbed passes a maximum which corresponds to an energy level transition of the unpaired electron hν = gβH, which is called the resonance condition, H and β being constants and the factor g having a value close to 2. The resonance condition implies that, at a value of the applied magnetic field, only a frequency ν corresponds. Experimentally, the absorption lines present a certain width due to the interaction of spins between themselves and their environment. Amongst the paramagnetic bodies we shall evoke here, the free radicals are not generally at a natural state but are created by a certain number of stresses, such as interactions of the insulating material with electric discharges, irradiations, or chain ruptures consecutive to electrical or mechanical stresses.
When discharges are produced in cavities within solid insulation, their noxiousness leads to the breakdown of the material over a more or less long term; when they are used to modify the surface state of a film before metallizing it and making it a capacitor, the interaction can be considered positive. In both cases, we know that ions and electrons are elements of the plasma which constitute these discharges and whose efficiency is good to know. The experiments made in particular on PP films destined for the manufacture of capacitors have shown the role played by ions and electrons accelerated between 50 and 240 eV, as well as the high efficiency when these same species work in synergy. Owing to ESR, it was particularly shown that the observed radical was of a peroxide (COO) type, the signal presenting an increasing amplitude by passing from argon ions to oxygen ions [GOM 89]. The same technique was used for the study of PEN irradiated by γ-rays [ROG 71]. The existence of fracture kinetics of polymers subjected to a mechanical stress was also demonstrated by this type of analysis. We observed in particular that the formation speed of the free radicals increased in an exponential manner as a function of the applied stress [ZHU 66]. At last used for the first time in the study of treeing phenomenon in LDPE and XLPE, this type of analysis revealed different signals according to whether it was a tree or dielectric breakdown phenomenon; however, the cores could not be identified during these preliminary experiments [BAC 78].
15.3. The materials themselves
We have just examined a few examples of current applications of physico-chemical analyzes on the ageing of systems with, for some of them, the possibility of leading to an intervention before serious damage, whilst for others leading to better understanding the system’s ageing. The methods presented in the following sections are more particularly focused on the material before its use.
15.3.1. Infrared spectrophotometry
Infrared spectrophotometry is one of the most ancient methods at the disposal of researchers and engineers in the dielectric material field. Its use has surely been emphasized by the introduction of Fourier transform (IRTF) devices. The central organ of the Fourier transform device is a Michelson interferometer, a device destined for the analysis of multiple frequencies from a signal. Microscopes and accessories allow, in particular, surface analyzes of insulating materials which are presented in the form of films, similarly to those destined for the coils of dry capacitors. The transmission method calls on the absorption principle according to which the ratio between the transmitted light and the incident light follows a decreasing exponential law expressed by the Beer–Lambert law: I = I0 e−kx, where k is a function of the wavelength λ and the molecular concentration, x representing the matter crossed thickness. In the process of multiple reflections, the law remains applicable, but the coefficient k also contains parameters relative to the contact surface and the number of reflections. In the case where interest only lies on particular points of the surface, we can adjoin a beam condenser to the apparatus. When the absorbant centers characteristic of a degradation or a simple transformation are in weak concentration and situated near the surface, it is necessary to resort to two experimental processes, specular reflection and internal multiple reflections: in the first case, the incident beam traverses the sample (tacked on a mirror) twice, the reflecting sides of a prism at 120° ensuring the transfer of the beam; in the second case, the sample is placed on both sides of a crystal, fitted for its transmission to the infrared beam which will meet the material to be analyzed n times; the value of n only depends on the crystal thickness. These two techniques increase the encounter probability of absorbant centers in a significant manner. Thus, a 2 mm thick thallium iodo-bromure crystal (KRS5) allows about 20 reflections, with a penetration depth from 0.64 to 5.1 µm in the range from 4,000 to 400 cm−1. Let us note here that we express the spectral space analyzed in wave number (cm−1) which is the inverse of wavelength. If we take PE for example, particular attention is often paid to the formation of carbonyl (C = O) groups around 1,720 cm−1, and we then know that the penetration depth of the infrared beam for this wavelength is about 1.85 µm. An example is given in 15.5. Decomposition powders and products can be analyzed after mixture in the potassium bromide powder (KBr).
We then proceed to observation by transmission of a chip obtained by compression of the mixture. Obviously, liquid and gas cells containing transparent windows in the same range of wavelengths are also used.
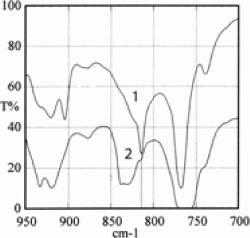
The comparison between spectra obtained by direct transmission and by the multiple reflection technique (Figure 15.6) has allowed a difference between the volume (1) and the surface (2) of a 12 µm PEN film destined for the manufacture of capacitors to be shown. The manufacturing method of the film, generating its stretching, gives rise to a more crystalline surface morphology. The analysis shows that the surface morphology favors motions in the naphthalene plane, while in volume, vibrations outside the plane prevail [KRA 96].
Even though manufacture technologies of energy transport cables have evolved since they first came out, the phenomenon of water treeing has always caught the attention of manufacturers, as well as researchers. After several years of service of 5 kV cables, XLPE samples containing antioxidants were taken and subjected to a certain number of analyzes, amongst which was the IRTF. The principal difficulty in this case is the extraction of the information of spectra to numerous differences when they are compared to the basic material. Thus, sulfate (1,130, 625 cm−1) and carboxylate (1,575 cm−1) anions and the classic oxidation attributed to carbonyl groups (evoked above) (~1,725 cm−1) appeared. The differences between spectra, which can be realized on the apparatus, by comparing the clean and contaminated parts of the polymer, show the more important oxidation of the degraded part (in the region exhibiting treeing). This analysis thus reveals the early ageing of the insulating material, in the regions in which the antioxidant vanishing was important and, simultaneously, the catalytic effect of contaminants regarding oxidation. We can then better understand the weakening of dielectric behavior [GAR 87]. In 1994, to celebrate the 25th anniversary of the discovery of the phenomenon, an examination of theories was published, in which we underline that most researches are based on the infrared technique (micro-IRTF). An analysis of the literature published until then underlines the differences between the results concerning cable samples aged in a laboratory and those aged under an electrical field stress. The normal chemical entities given as examples are: carboxylate ions (RCOO-), ketones (R2C = CO), and esters (RCOOR) which we detect in the region ranging from 1,700 to 1,730 cm−1 [XU 94]. The breakdown process of polyepoxide resins also seems preceded by a chemical modification of the material, revealed by IRTF, favoring the creation of electrical treeing [MIT 81]. In section 15.6.2, we emphasized the techniques permitting the control of liquid insulation; obviously IRTF contributes to the analysis of this type of insulation and what has been particularly shown through the detection of impurities is that we can identify the nature and give the proportion in the transformer oils for concentrations of the order of 30 ppm. The normal corresponding resolution of specters is 4 cm−1 [PER 98].
It is now common to use several techniques to study the same type of ageing of electrical insulating materials. The last two analysis methods which we just presented, ESR and IRTF, were used to study the chemical evolutions due to the impact on high density PE of ions accelerated between 50 and 150 kV; the goal being to find an analogy with the evolution of the insulating material of a cable under voltage [SCH 87].
15.3.2. Calorimetric analysis
The state change of a dielectric material leads to a variation of temperature, the heat being absorbed or emitted. If the comparison is made with an inert material, we can measure the necessary supply for the preservation of the equilibrium between these two systems. U being the energy of the system, this energy can evolve from a state A to a state B. The energy therefore depends on the state of the system, like pressure P and volume V. The function of state H, possessing the same properties is the enthalpy: H = U + PV. Differential thermal systems record the difference between the enthalpy changes of an analyzed material and an inert reference during simultaneous heating. When the sample and the reference are heated by the same heating source we record, as a function of time, the temperature difference between them owing to sensors placed near them both, or fixed to the bowls containing them. We then have a Differential Thermal Analysis (DTA). The most used technique is the Differencial Entropic Analysis (DEA) or Differential Scanning Calorimetry (DSC). The difference with the previous technique is the use of a heating element intrinsic to each part, sample and reference. The apparatus then consists of two loops, one controlling the temperature as a function of a predetermined growth speed, the other allows the input power to be adjusted as a function of the temperature difference which can appear between the reference and the sample and if, for example, a reaction is produced in this latter, whether it is exo- or endo-thermal. This process is sometimes called power compensation. It is important to mention that the head temperature measurement of the sample is maintained constant until the head temperature measurement of the reference is adjusted in a continuous manner. We record a signal proportional to the difference of heat supplied to the sample and the reference (dh/dt). This type of analysis was most often practiced for the study of a material before its use. It thus permitted the reticulation of PE to be studied, the phenomena resulting from thermal processing of this material destined for the manufacture of cables [CAI 72]. Associated with infrared spectrophotometry, it completes the analysis of oxidation phenomena of PE when it is in contact with metals with greater or lesser, according to whether we are dealing with Cu, Pb, Ag and Zn or Al and Au, etc. Oxidation is once again characterized by the optical density of the absorption at 1,720 cm−1 which corresponds to the vibrations of the carbonyl (C = O) groups. With metals such as copper and lead, whose chemical activity is important, the formed components diffuse easier in the polymer when the temperature of the material is high. We remember that these two metals can be an essential part of a cable, the first as a conductor, the second in the role of a screen [EGO 75]. DEA, associated with thermally stimulated depolarization currents, dielectrical spectroscopic analysis and dynamic mechanical analysis, allow a comparative study of an amorphous and a partially crystalline PEN. The interest of the study was on the effect of the degree of crystallinity on the electrical properties in particular, knowing that this dielectric is used for the manufacture of capacitors and that, comparatively to PET, its fusion temperature (268°C) is greater by about 10°C and its glass transition temperature Tg is also higher than 50°C [CAN 00]. A similar approach had been realized for the study of the formation of aggregates due to water and which we know can lead to the breakdown of cable insulations [JOH 80]. By assuming the nature of the physical properties of polyepoxides from biphenol-A or cyclo-aliphatics to be unstable at temperatures less than the glass transition temperature Tg, this technique used at the same time as dielectric measurements shows the interest of annealing [LI 94].
More recently, the study of total and partial isothermal crystallization by DEA permitted the existence of a negligible proportion of rigid amorphous phases in PEN to be quantified. The complement of analysis brought by IRTF led to a quantitative study from certain absorption bands (813 and 839 cm−1), showing that the crystallization process induced an increase of trans conformations. All the results show which semi-crystalline structure we should tend towards for good use of PEN as a capacitor dielectric [ZOU 02].
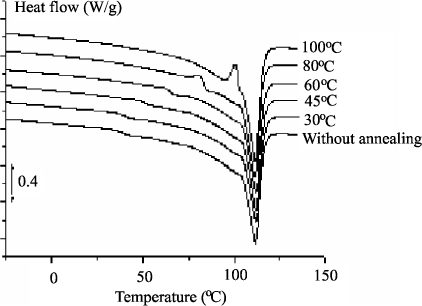
A better knowledge of the morphology of an organic insulating material in particular can help to understand the conduction and breakdown phenomena of this type of material. When LDPE is used for the manufacture of energy transport cables, we can take an interest in the gradual evolution of the material, from the basic resin, as a function of a temperature rise, then as a function of its transformation such as a reticulation from dicumyle peroxide and, finally, the adjunction of antioxidants already mentioned. Fusion specters obtained in DEA for samples annealed at different temperatures are represented in Figure 15.7. Three zones of temperature could then be defined: Tf < 45°C, 45°C < Tf < 100°C and Tf > 100°C. The same technique used to analyze the material with its antioxidant reveals an increase of the fusion peak which is interpreted as a reduction of the number of defects inside crystallites. A correlation was found with electrical behavior [BOU 01].
Figure 15.7. Influence of the annealing temperature on an LDPE and its antioxidant analyzed by DEA [BOU 01]
15.3.3. Thermostimulated currents
This technique, proposed 40 years ago [BUC 66] to characterize the punctual defects in alkali halogen crystals has since been widely applied to other materials, like those used in electrical insulating [LAV 93] shows the schematic representation of an experimental device which is composed of a measurement cell (1), placed in an enclosure regulated between the temperature of liquid nitrogen and 300°C. The cryostat (2) is constituted of a triple wall permitting it to be insulated by a secondary vacuum (3); the liquid nitrogen (4) is destined to cool the cell. The thermal exchange between the cell and the walls of the nitrogen reservoir is ensured by the gaseous helium under weak pressure (20 mbar) (5). A heating resistance (6) surrounding a removable stainless steel cylinder (7) is connected to a power supply controlled by a regulator. A probe placed in the vicinity of the sample measures its temperature (8). The electrodes (9), between which the sample is placed (10), are connected to a rotating relay allowing the application of a static electric field and the measurement of the depolarization current, as a function of its position. The detection of the depolarization current is recorded by an electrometer sensitive to 10−16 A.
At given polarization temperature Tp, the samples are subjected to a static electric field Fp for about 2 mn to allow the orientation of mobile dipoles at this temperature, according to the field Fp; the equilibrium polarization thus obtained, we proceed to a decrease of the temperature until T0 << Tp in order to fix the dipoles. After suppression of the field, we place the test tube in short circuit, which allows the flow of surface charges. A linear growth of the temperature at the speed of a few degrees/mn induces the gradual relaxation of dipolar entities. We then record a depolarization current. The recording of this current as a function of the temperature constitutes a global or complex spectrum of the dielectric relaxation modes. Each peak being associated with a relaxation mode, certain criteria ensure their dipolar nature: these are reproducibility, proportionality between their intensity and Fp, and invariance with different polarization temperatures. There is a relaxation time distribution which makes the exploitation of this type of spectra difficult; also another procedure is applied, that of fractional polarizations. Here, the temperature is decreased under field until a value Td = Tp − ΔT. After the suppression of the field, the temperature is maintained constant for a time td allowing the return to the equilibrium of entities whose relaxation time τ(Td) is smaller than td. The spectrum is then recorded as in the previous method. By displacing the polarization window [Tp, Td] along the axis of temperatures, we obtain a set of elementary spectra corresponding to the decomposition of a complex spectrum.
The electric stress can be replaced by a mechanical cutting one σ; we then realize the thermo-stimulated flow (TSF) which allows the molecular mobility to be studied. The mechanical stress is applied for a time tσ of the order of 2 mn at temperature Tσ, which allows the mobile entities of the polymer to get oriented. This configuration out of equilibrium is frozen by a rapid quench which prevents any molecular displacement. The stress is then removed and the return to equilibrium of the sample is generated by a rise of temperature at constant speed (~7 K/mn); the mobile entities can then reorient themselves. In the ageing domain of cable insulation affected by water treeing, this technique brought certain physical indications on the role of water and electrical field. Thus, the decrease of activation enthalpy appears as a consequence of water absorption which transforms the structure under the influence of an electrical field [MAR 92].
The few references which we have given here simultaneously take into account the most fundamental aspects of results for this type of analysis, and also the aspects purely applied in the world of insulation, knowing that this selection is not exhaustive and that further examples can be found in the references supplied.
The effect of the thermal expansion of elastomers on TSC was revealed on both sides of Tg in this type of polymer [VAN 87]. In spite of wide use in the insulation domain, the complexity of the poly-epoxy structure which we have already evoked makes it difficult to understand the conduction mechanism, in particular because of the ionic or electronic character that can be attributed to it as a function of the applied electrical field range. Photo-currents and TSC can come in helpful for understanding this [KAW 88]. The interest in simultaneously using stimulated thermoluminescence and TSC has been shown in several insulating materials such as XLPE, PET, and EPR [FLE 89]. More recently, by choosing three poly-epoxides whose architecture is well defined, the distinction was made between the processes of localized and non-localized relaxation, owing to the study of the influence of growth speed in temperature [HEN 00].
15.4. Conclusion
We have attempted to inspect a few analysis techniques practiced in the domain of electrical insulating materials. As we have underlined, the apparatus and the investigations have a history; with progress being due to developments in electronics and computer science. We have also pointed out that several techniques can be used simultaneously, sometimes in complement to electrical measurements, to try to gain a better understanding of the initial structure and behavior of materials subjected to device stresses. Published papers contain more and more results obtained by this type of approach, even if it is costly. Among other analyzes, we could have cited for example: UV spectrophotometry, ESCA, XPS, the interaction of weak energy electrons with the surface of solid dielectrics (LEET), and ESD, described in a synthesis article by Sanche [SAN 93].
We have insisted on using a more commonly practiced method because, to our mind, beyond the results it already brings, it could help towards the validation of certain models for which outlines have already been conceived and which rely on the existence of free volumes. This method belongs to the series of spectroscopic methods using γ-rays, PASCA (Positron Annihilation Spectroscopy for Chemical Analysis). The positron, known for about half a century, is the antimatter of the electron carrying a positive charge of the same order as the negative charge of the electron, and with properties identical to those of electron. The most common source is 22Na. This new probe (PASCA) is well described by Stevens [STE 90] and Jean [JEA 90] and a demonstration of its use was made, for example, in the ageing study of cables insulated by cross-linked polyethylene [BER 92].
15.5. Bibliography
[ARA 02] ARAKELIAN V.G., “Effective diagnostics for oil-filled equipment”, IEEE Electrical Insulation Magazine, vol. 18, no. 6, p. 26–38, 2002.
[BAC 78] BACQUET G., DIB J., WU C.Y., DENSLEY J.R., BOGGS S.A., “ESR study of free radicals in electrical trees in polyethylene”, IEEE Trans. Electr. Insul., vol. 13, no. 3, p. 157–163, 1978.
[BAM 83] BAMJI S.S., BULINSKI A., DENSLEY J., GARTON A., “Etching and the morphology of cross-linked PE cable insulation”, IEEE Trans. Electr. Insul., vol. 18, no. 1, p. 32–41, 1983.
[BER 92] BERNSTEIN B.S., STINIVAS N., “Accelerated aging of crosslinked polyethylene cable insulation; electric Positron Annihilation Spectroscopy study”, Conference Record of the 1992 IEEE Intern. Symp. Electr. Insul. Dielectr. Phenom., 92CH3150-0, p. 104–109, 1992.
[BOU 01] BOUDOU L., Influence des paramètres de mise en œuvre, sur la morphologie et la conductivité électrique d’un polyéthylène soumis destiné à la fabrication des câbles, Doctoral Thesis, University of Paul Sabatier, Toulouse, 2001.
[BUC 66] BUCCI C., FIESCHI R., GUILDI G., “Ionic thermocurrents in dielectrics”, Phys. Rev., vol. 148, no. 2, p. 816–823, 1966.
[BUR 88] BURTON M.J., CARBALLEIRA M., DUVAL M., FULLER C.W., GRAHAM J., DE PABLO A., SAMAT J., SPICAR E., “Applications de la chromatographie liquide à l’analyse des matériaux isolants électriques”, Proceedings of the International Council on Large Electric Systems Conference (CIGRE), 15–03, 1988.
[CAI 72] CAILLOT C., AUDOUX C., AUCLAIR H., “Application de l’analyse enthalpique differentielle à l’étude du polyéthylène haute pression pour câbles”, Rev. Gén. Ele., vol. 81, no. 11, p. 740–745, 1972.
[DHI 95] DHIBA D., Etude du vieillissement de l’isolation papier/huile dans les transformateurs de puissance. Influence des inhibiteurs d’oxydation, Doctoral Thesis, University of Paul Sabatier, Toulouse, 1995.
[DUV 77] DUVAL M., LAMARRE C., “The characterisation of electrical insulating oil by HPLC”, IEEE Trans. Electr. Insul, vol. 12, no. 5, p. 340–344, 1977.
[DUV 82] DUVAL M., GIGUÈRE Y., “Simultaneous determination of the antioxidant, the crosslinking-agent and decomposition products in polyethylene by reverse-phase HPLC”, J. Liquid Chromatography, vol. 5, no. 10, p. 1847–1857, 1982.
[DUV 84] DUVAL M., LAMARRE C., GIGUERE Y., “Reversed-phase high-performance liquid chromatographic analysis of polar oxidation products in transformer oils”, J. Chromatography, vol. 284, no. 1, p. 273–280, 1984.
[DUV 89] DUVAL M., “Dissolved gas analysis: it can save your transformer”, IEEE Electrical Insulation Magazine, vol. 5, no. 6, p. 22–23, 1989.
[DUV 02] DUVAL M., “A review of faults detectables by gas-in oil analysis in transformers”, IEEE Electrical Insulation Magazine, vol. 18, no. 3, p. 8–17, 2002.
[EGO 75] EGORENKOV D.G., LIN D.G., BELY V.A., “Effect of metals on melt oxidation of polyethylene”, J. Polym. Sc., vol. 13, no. 7, p. 1493–1498, 1975.
[FLE 89] FLEMING R.J., “Thermally-stimulated conductivity and luminescence in organic polymers”, IEEE Trans. Electr. Insul., vol. 24, no. 3, p. 523–531, 1989.
[GAR 87] GARTON M., BAMJI S., BULINSKI A., DENSLEY J., “Oxidation and water tree formation in service-aged cable insulation”, IEEE Trans. Electr. Insul., vol. 22, no. 4, p. 405–412, 1987.
[GOM 89] GOMÈS DE LIMA P., LOPEZ J., DESPAX B., MAYOUX C., “Sur l’efficacité des ions et des électrons dans un plasma froid venant en contact avec un polymère”, Rev. Phys. Appl., vol. 24, no. 3, p. 331–335, 1989.
[GUE 92] GUEGUEN V., Vieillissement d’élastomères utilisés comme isolants électriques en ambiance nucléaires, Thesis, Ecole Normale Supérieure d’Arts et Métiers, Paris, 1992.
[HEN 00] HENN F., GLUNTINI J.C., HALARI J.L., VANDERSCHUEREN J., MURACCIOLE J.M., “TSDC in epoxy networks of well-controlled architecture. Experimental evidence for local and non-local relaxation process”, IEEE Trans. Electr. Insul., vol. 7, no. 4, p. 551–555, 2000.
[HIN 88] HINRICHSEN P.F., HOUDAYER A., BELHADFA A., CRINE J.P., PELISSOU S., CHOLEWA M., “A localized trace element analysis of water trees in XLPE cable insulation by micro-PIXE and EDX”, IEEE Trans. Electr. Insul., vol. 23, no. 6, p. 971–978, 1988.
[HOU 92] HOUDAYER A.J., HINRICHSEN P.F., KAJRIS G., PARPAL J.L., FOURMIGUÉ J.M., CRINE J.P., “µ-PIXE analysis of impurity migration in polyethylene”, Proceedings of the 4th International Conference on Conduction and Breakdown in Solid Dielectrics, Catalog 92CH3094-6, p. 433–439, 1992.
[HOF 81] HOFF A, JACOBSSON S., “Thermo-oxidative degradation of low-density polyethylene close to industrial processing conditions”, J. Appl. Polymer Sci., vol. 26, no. 10, p. 3409–3423, 1981.
[JEA 90] JEAN Y.C., “Positron Annihilation Spectroscopy for Chemical Analysis: A new probe for microstructural analysis of polymers”, Microchem., vol. 42, no. 1, p. 72–102, 1990.
[JOH 80] JOHNSON G.E., BAIR H.E., MATSUOKA S., ANDERSON E.W., SCOTT J.E., “Water sorption and its effect on a polymer’s dielectric behaviour”, Water in Polymers, 27, p. 451–468, American Chemical Society, 1980.
[KAW 88] KAWAMOTO A., SUZUOKI. Y., IKERJIRI T., MIZUTANI T., IEDA M., “Photocurrents and thermally stimulated currents in epoxy resin. Effects of mechanical stress”, IEEE Trans. Electr. Insul., vol. 23, no. 2, p. 201–208, 1988.
[KRA 96] KRAUS E., Comportement électrique et physicochimique de films de poly (éthylène-naphtalène 2,6 dicarboxylate) soumis à des contraintes électriques et thermiques, Thesis, No. 2288, Toulouse, 1996.
[LAM 81] LAMARRE C., DUVAL M., GAUTHIER J., “Dosage par CLHP du DBPC dans les huiles de transformateurs neuves oxydées”, J. Chromatography., vol. 213, no. 3, p. 481–490, 1981.
[LAV 93] LAVERGNE C., LACABANNE C., “A review of thermo-stimulated current”, IEEE Electrical Insulation Magazine, vol. 9, no. 2, p. 5–21, 1993.
[LI 94] LI Y., UNSWORTH J., “Effect of physical aging on dielectric, thermal and mechanical properties of cast-epoxy insulators”, IEEE Trans. Dielec. Electr. Insul., vol. 1, no. 1, p. 9–17, 1994.
[MAN 83] MANGARAJ D., MONDRON P., EPSTEIN M.M., “Analysis of antioxidant in aged cables”, 1983 Annual Report of the Conference on Electrical Insulation and Dielectric Phenomena, catalog 83CH1902-6, p. 286–293, 1983.
[MAR 92] MARTINEZ J.J., BOULOUZ M., MAYOUX C., LACABANNE C., “Thermally stimulated creep for the study of ageing in insulating polymers under electric field”, Conference Record of the 1992 IEEE International Symposium on Electrical Insulation and Dielectric Phenomena, Catalog 92CH3150-0, p. 62–65, 1992.
[MAY 00] MAYOUX CH., Polymérisation radicalaire en suspension inverse d’un poly (acide acrylique) réticulé. Etude de la dynamique de chaîne, Thesis, No.3802, Toulouse 2000.
[MEL 81] MELTON C.W., MANGARAJ D., EPSTEIN M.M., “Morphology of thermoplastic and cross-linked polyethylene cable insulation”, Conference Record of the 1981 IEEE Conference on Electrical Insulation and Dielectric Phenomena, catalog 81CH1668-3, p. 299–305, 1981.
[MIT 81] MITSUI H., YOSHIMITSU T., MIZUTANI Y., UMEMOTO K., “Electrical failure properties of cast epoxy resins”, IEEE Trans. Electr. Insul., vol. 16, no. 6, p. 533–541, 1981.
[MOR 92] MOREAU E., Un phénomène de vieillissement du polyéthylène sous champ électrique: les arborescences d’eau, leur structure fine et leur caractéristique électrique, Thesis, No. 1294, Toulouse, 1992.
[MOR 93] MOREAU E., BOUDET A., MAYOUX C., LAURENT C., WRIGHT M., “Fine structure of defects in polyethylene used for power cable insulation observed by fluorescence microscopy”, J. Mater. Sc., vol. 28, no. 1, p. 161–169, 1993.
[PAR 99] PARPAL J.L., GUDDEMI C., HINRICHSEN P.F., “Antioxydant concentration distribution measurement in XLPE cable insulation by PIXE analysis, FTIR and UV spectroscopy”, Proceedings of the 5th International Conference on Insulated Power Cables, p. 324–328, 1999.
[PER 98] PERCHERANCIER J.P., VUARCHEIX P.J., “Fourier Transform Infrared (FT-IR) spectrometry to detect additives and contaminants in insulating oils”, IEEE Electrical Insulation Magazine, vol. 14, no. 3, p. 23–29, 1998.
[ROG 71] ROGOWSKI R.S., PEZDIRTZ G.F., “Electron spin resonance of γ-irradiated poly(ethylene-2,6-naphtalene dicarboxylate)”, J. Polym. Sc. A2, vol. 9, p. 2111–2117, 1971.
[SAN 93] SANCHE L., “Electronic aging. Electron interactions in thin-film dielectrics”, IEEE Trans. Electr. Insul., vol. 28, no. 5, p. 789–819, 1993.
[SCH 87] SCHAIBLE M., HAYDEN H., TANAKA, “Chemical changes created by high energy ions in polyethylene”, IEEE Trans. Electr. Insul., vol. 22, no. 6, p. 699–708, 1987.
[SCH 88] SCHUPPE W.H., SAURE M., ANDRESS H., MÖLLER K., MEURER D., KRAUSE K., “Méthodes analytiques et physiques sensibles pour diagnostiquer les modifications d’état dans des plaques ou des isolations de câbles”, Proceedings of the International Council on Large Electric Systems Conference (CIGRE), Papier 15–03, 1988.
[STE 90] STEVENS J.R., “Probe and label techniques”, Methods of experimental physics, vol. 16A, Ch. 5, Academic Press, p. 371–403, 1990.
[SUT 02] SUTAR J.L., LAGHARI J.R., CHENG P.C., “LSCM: A non-destructive diagnostic tool for examining the microstructure of polymer dielectric films”, IEEE Electrical Insulation Magazine, vol. 8, no. 4, p. 20–24, 1992.
[UNS 90] UNSWORTH J., MITCHELL F., “Degradation of electrical insulating paper monitored with high performance liquid chromatography”, IEEE Trans. Electr. Insul., vol. 25, no. 4, p. 737–746, 1990.
[VAN 87] VANDERSHUEREN J., LADANG M., NIEZETTE J., CORAPCI M. “The effect of thermal expansion on thermally stimulated currents in polymers; 1. Elastomeric materials changes”, IEEE Trans. Electr. Insul., vol. 22, no. 1, p. 19–22, 1987.
[VER 92] VERGNE J., Etude du vieillissement physico-chimique du complexe papier-huile dans les transformateurs électriques, Thesis no. 522, Toulouse, 1992.
[XU 94] XU J.J., BOGGS S.A., “The chemical nature of water treeing: Theories and evidence”, IEEE Electrical Insulation Magazine, vol.10, no. 5, p. 29–37, 1994.
[ZHU 66] ZHURKOV S.N., TOMASHEVSKI E.E., “An investigation of fracture process of polymers by the Electon Spin Resonance method”, Proc. Conf. Physical basis of yield and fracture, Oxford, p.200–208, 1966.
[ZOU 02] Zouzou N., Etude de la mobilité moléculaire du PEN destiné à la fabrication des condensateurs: influence de la microstructure, Thesis, University of Paul Sabatier, Toulouse, 2002.
1 Chapter written by Christine MAYOUX and Christian MAYOUX.