Chapter 22
Recycling of Plastic Materials 1
22.1. Introduction
It is difficult to imagine our life today without plastic materials. The wide variety of plastic materials with their very large range of properties (light, heavy, flexible, rigid, thermal insulators, electric insulators, electric conductors, good optical properties) and their relatively simple transformation at a profitable cost, are the main reasons for their increasing use for the production of products and consumer goods.
The technological developments of the last decades, especially from the 1950s– 1960s, have entailed a large evolution of the use and development of new plastics.
In numerous applications, plastics have replaced other traditional materials (metal, ceramics and wood) but their use has also extended owing to their application in new domains of science and technology: in microelectronics, biomedicine, telecommunications, etc.
Once their useful life is finished, plastic products and components must be disposed of. The issue of the final processing of plastic residues, as for a large part of the solid residues generated by our society, has not been completely solved. It seems logical to seek solutions other than their accumulation in rubbish tips, not only because of the impact on the environment but also because the materials in the residues could be reused; it is a waste of non-renewable resources.
22.2. Plastic materials
22.2.1. Introduction to plastic materials
Plastic materials are organic polymers characterized by their plasticity, or ability to be molded under the effect of pressure and temperature. This is why, despite their diversity and their particularities, they are known under the generic name of plastics, which stems from the Greek plastikos: to form or prepare to mold.
The most used synthetic polymers are obtained from a fraction of petroleum. We estimate that 6% of petroleum is transformed into plastic. Its chemical structure is characterized by the repetition of small molecular units called monomers.
Polymers are macromolecules of high molecular mass, synthesized through poly-condensation or poly-addition reactions, from monomers. They contain carbon and hydrogen atoms and other elements (oxygen, nitrogen, etc.), combined altogether by chemical covalent bonds.
Plastics can be listed in three large groups according to the structure of the macromolecules and the bonds between them (this has a high influence on their thermal and mechanical behavior):
– thermoplastics: consisting of macromolecules of linear or ramified chains but without cross-linking. By increasing the temperature, they melt and can be molded in a reversible way. This characteristic permits their recycling, since owing to the application of heat, they can be remelted and remolded;
– elastomers: with an elastic and gummy aspect, their molecules are distributed by forming a tridimensional lattice, with not many inter-twinings and a low degree of cross-linking, presenting a restricted mobility. The molecular bonds between the chains break at high temperatures and do not reform when the temperature decreases;
– thermosettings: thermosetting or thermohardenable resins react to give a tridimensional lattice of macromolecules, with a high degree of cross-linking. Under the effect of heat, they conserve their shape and are maintained rigid until they reach the temperature which destroys them. The intertwining is not reversible and, consequently, the thermally stable residues and their composites cannot be recycled as easily as thermoplastics;
– plastic, thermoplastic or thermally stable materials are usually presented with other substances: strengthenings, charges and additives. These products of organic or inorganic nature are mixed up with the polymeric matrix, resulting in a plastic which can be better worked on and/or have improved properties;
– charges are organic or inorganic filling materials, which can reach relatively high percentages, and make the material more economical. They can modify and improve a few of their rheological and mechanical properties as well as their physical aspect. Silicone, silicates, calcium carbonate, clay, talc, lime and even well-pulverized synthesis polymers can be used as charges;
– strengthenings are usually fibers which are added to the plastics by forming composed or composite materials. Fiberglass, carbon, graphite, aramide, and cellulose fiber can all be used, and thus increase the solidity, the resistance to impact, the rigidity, etc. Epoxy-type resins and polyesters are much used for these thermohardenable composites;
– the term “additive” covers a wide range of chemical products which are added to plastics: antioxidants, thermal stabilizers and stabilizers exposed to light, lubricants, plasticizers, coloring agents, etc.
22.2.2. Consumption of plastic materials
In 2007, the consumption of plastics in Europe exceeded 52.5 million tonnes.
The consumption distribution of thermoplastics by sector is represented in Figure 22.1.
Figure 22.1. Consumption of plastics by sector in Europe, 2007. Source: Plasticeurope Market Research Group (PEMRG)
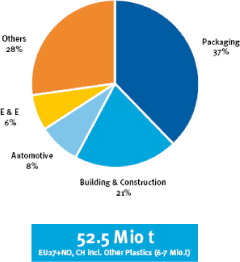
More than half of the total consumed plastics (Figure 22.2), correspond to four types of thermoplastics: polyethylene (PE), polypropylene (PP), polystyrene (PS) and vinyl polychloride (PVC) which are known as commercial plastics. Their good characteristics for use and their economical price allow their use for technical applications as well as for the manufacture of consumer products.
Figure 22.2. Consumption of plastics by materials in Europe, 2007. Source: Plasticeurope Market Research Group (PEMRG)

22.2.3. Plastics in electrical engineering
Polymeric materials in electrical engineering are fundamentally used for their dielectric and mechanical properties. They are used as thermoplastics and thermohardenables depending on their applications.
PE is used as an internal insulating material and as the covering for conductive cables for the transport of electric energy in high, average and low voltage lattices.
PP is used as an insulating material and as a dielectric in power capacitors, possibly impregnated by oil; it supports high voltages and is characterized by weak losses.
Epoxy resin composites with fiberglass and/or mineral charges are used in the cores of line insulators which are used in suspended air cables of the electric grid.
Figure 22.3. Plastics Materials demand by type, 2005–2007.
Source: Plasticeurope Market Research Group (PEMRG). Western Europe (WE) EU15 + CH + N (+ Malta + Cyprus)

22.3. Plastic residues
22.3.1. Generation and recovery of plastic residues
From the estimations of the Association of Plastics Manufacturers in Europe (APME), the total amount of plastic residues after their consumption reached 20,608,000 tonnes in 2002, amongst which 38% of plastics were recovered as materials or energy. For the remaining 62%, their final destination was incineration or rubbish tips.
The highest amounts recovered, in urban residues, are due on the one hand to the high percentage of plastics in these residues (mainly as wrappings) and, on the other hand, to the strong development of selective recovery systems required to be applied by all countries of the EU, in order to reach the following recycling percentages: 15% of materials in 2001 and 22.5% of plastics in 2008.
However, the efficiency is lower than in other sectors (agriculture or distribution) where the percentages of recovery are higher. This is because plastics, after consumption in urban residues, are mixed up with other types of materials, organic waste, papers, metals, etc. which make their recycling a lot more complicated.
Plastics in agriculture have a high recovery rate, although there is no specific legislation in the EU. The success of voluntary initiatives in this sector is probably due to the homogeneity of plastics and to the relative ease of recovering them in large quantities.
Figure 22.4. Strong continued growth of recycling and energy recovery, 1995-2007. Source: Plasticeurope

Figure 22.5. Total plastic residues by sectors in Europe, 2002. Source: APME, Plastics in Europe 2002 &3

On the other hand, in the automobile, electric and/or electronic and building equipments sectors, the recovery results are still fairly weak. These residues are generally heterogenous and often mixed up with other non-plastic materials, which makes their separation or their selection complicated. Different alternatives to recover plastics in out of service vehicles and in electrical and electronic equipment are being evaluated to reach recycling objectives required by different corresponding directives.
Table 22.1. Amount of recovered plastic residues in 2002, by sector and by recovery mode. Source: APME, Plastics in Europe 2002 and 2003

22.3.2. Processings at the end of life
Once their useful life is finished, the final destination of plastic residues, like that of most solid residues produced by our society, is their accumulation in rubbish tips.
Outside of the use of the materials themselves, we induce a waste of non-renewable resources and, furthermore, another major issue is raised: that of the rubbish tips themselves.
Figure 22.6. Options for the recovery of plastic residues. Source: APME. http://www.plasticseurope.org

Plastic residues represent resources which can be recovered as materials or in the form of energy. The three alternatives for their recovery are:
1. mechanical recycling;
2. chemical recycling or feedstock;
3. energy recovery.
22.3.2.1. Mechanical recycling of thermoplastics
The mechanical recycling of thermoplastics is a process in several stages which requires a specific technology and equipment. Fundamentally, mechanical recycling consists of selecting and listing the plastic residues, grinding them, cleaning them, melting and extruding them in the shape of pellets or granules.
Subsequently, the recovered material can be transformed again to produce new pieces or products.
The quality of recycling depends to a great extent on the separation of different types of used polymers (particularly those which are not compatible), the degree of cleaning and the absence of impurities, such as metals, glass, paper, etc.
Ferrous contaminants are eliminated by using electromagnetic separators and/or metal detectors, and non-ferrous contaminants by using the Foucault current technique. Glass and paper are separated in the heavy or light fraction depending on their weights, by using water or air currents (via hydrocyclones, centrifuges, etc.).
Figure 22.7. Stages for the processing of mechanical recycling of plastics. Source: VIPLAT, recycling equipment supplier, www.viplat.com

To separate different types of plastics from one another, we use their density differences: 0.94–0.96 g/cm3 for high density PE, 0.89–0.91 g/cm3 for PP and 1.16– 1.38 g/cm3 for PVC. For this purpose, we apply flotation separation in water or in water mixed with other solvents or tensioactives.
From the differences in transmission/reflection of light, and for wavelengths characteristic of the absorption spectrum, optical detectors with different emission sources (polarized light, UV, IR, X-ray, etc.), combined with air ejectors, can separate colored plastics, different types of plastics and plastics which contain certain unwanted charges or additives.
22.3.2.2. Mechanical recycling of thermohardenables
The recycling of thermostables and their composites with charges and strengthening fibers is essentially a division process followed by a thinner grinding until a fibrous powder with an appropriate particle size is obtained, which varies between 100 mm and 50 micrometers.
All components of the original composite are found in the recycled material, which is a mixture of polymer, fibers and charges. This recovered material possesses shorter fibers but can partially replace the charges to be used in the production of new composites. In certain cases, when the recycled material contains a larger amount of fibers, it can be used to partially replace the strengthening fiber in the molding of new pieces. However, in all cases the mechanical properties are reduced when the amounts of recycled material are large.
They can also be used as strengthening material in thermoplastic composites or mixed with tar or thermoplastics. They can also replace wood fibers for the production of posts and pylons (wood-plastics) [PIC 05].
22.3.2.3. Chemical recycling, or feedstock
Chemical recycling, also called feedstock, is the decomposition of polymers contained in residues to obtain starting monomers or a mixture of basic chemical products: hydrocarbons with different molecular masses, synthetic gas, etc., which could be used as combustibles or as raw material for the chemical industry.
There are two types of processes: chemolysis or chemical depolymerization, and thermolysis.
22.3.2.3.1. Chemolysis or chemical depolymerization
This process is about adding reactive chemical products which, in the presence of catalysts and under certain pressure and temperature conditions, break the macromolecules down. The reaction products are monomers or oligomers, which can be used to synthesize the same starting polymer again, or even another type of polymer.
The process can be applied to condensation polymers with functional groups combined with weak bonds likely to be dissociated by the action of certain reactives. We distinguish different types of depolymerization reactions depending on the reactive used:
– glycolysis: with polyglycols, polymeric by-products of ethylene oxide such as glycol polyethylene, glycol polypropylene and glycol diethylene, etc.;
– methanolysis: with methanol;
– alcoholysis: with other alcohols, such as butanediol;
– hydrolysis: with water or acids;
– saponification: with alcali;
– aminolysis: with amines.
22.3.2.3.2. Thermolysis
Thermolysis processes depend on the application of high temperatures to produce the breakdown of polymer chains in the presence (or not) of catalysts. This can obtain basic hydrocarbons or synthesis gases such as volatilizable hydrocarbons, naphthas, liquid and waxy hydrocarbons, bituminous components, or mixtures of combustible gases with synthesis gases.
When the process of decomposition or breakdown is undertaken in the absence of air, it is called pyrolysis or steam cracking (at 800°C without catalyst, or 400°C with a catalyst). When it takes place in a hydrogen gas atmosphere, it is called hydrogenation or hydrocracking (at 400–450°C and 50–100 bars); if it is made in the presence of a controlled amount of oxygen, the process is known as gasification (at 850–950°C).
These recovery processes are the most suitable for addition polymers such as polyolefins, acrylic and vinyl polymers. The thermally stable pyrolysis is also possible. In that case, we can obtain mixtures of organic products with weak molecular mass which, subsequently, can be separated and purified.
The thermal processing of thermally stable composites can also lead to the recovery of large value inorganic components contained in the composite, such as fiberglass for example (see Figure 22.8).
Figure 22.8. Recovering process of composites through a fluidized bed. Source: “Recycling termoshet composites”. JET Composites n.17 May 2005

In a bed of fluidized sand, the warm air is at temperatures between 450 and 550°C; the polymer is decomposed in its lightest volatilizable fractions. The solid mineral fibers and charges, led by the gas, are separated for their re-use. The gases coming from the decomposition of the polymer are led to a combustion enclosure at high temperature for their complete oxidation and energy recovery.
The recovered amount of fiberglass and inorganic components (classical carbonate, aluminum oxide, etc.) present in the composites can represent 70% of the weight. These recovered materials can be used as replacement raw materials in furnaces for concrete production industries (1,480°C).
22.3.2.4. Incineration with energy recovery
In 2003 in Occidental Europe 21,150,000 tonnes of plastic residues were produced, among which 4,750,000 tonnes were incinerated with a view to recovering energy.
The aim of incineration is the thermal destruction of residues (900–1,000°C) through their complete oxidation by bringing oxygen in greater amounts than the stereochemical amount required for their own combustion. The heat of output gases in the combustion chamber can be used as thermal energy or transformed into mechanical and/or electric energy.
Figure 22.9. General diagram of an incineration process with energy recovery. Source: Incinérateur RSU de Txingudi, www.txinzer.com
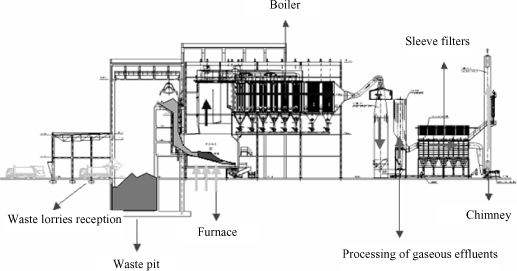
The energetic recovering or incineration with energy recovery is made in incineration power stations which behave as thermal power stations. The combustibles, in this case the residues, are burnt in a furnace with appropriate characteristics where we recover the heat of combustion gases to produce water vapor, which sets in motion a turbine for electrical energy production.
During incineration, three types of pollutants are produced: gases, flying ash and cinders which must be processed to reduce their environmental impact.
Since the calorific power of plastics is relatively high, plastic residues can be used as alternative combustible or mixed with other combustibles (coal, fuel, etc.) for use in industrial boilers, industrial furnaces for concrete production, etc. This allows a portion of the combustible required to produce heat, vapor or electrical energy to be saved.
Thermally stable polymers have a calorific power near that of good quality coal. The composites contain 25–35% of thermally stable resin and can be successfully burnt to obtain energy, for example mixed with urban solid residues (USW) for incineration.
Table 22.2. Energy content. Source: “Los Plásticos y la Gestión de sus Residuos” CEMAV Vicerrectorado de Metodología, Medios y Tecnología, UNED
| MATERIAL | MJ/Kg |
|---|---|
| Natural gas | 52 |
| Polypropylene | 44 |
| Polyethylene | 43 |
| Crude oil | 42 |
| Polystyrene | 40 |
| Polyamide | 37 |
| PET | 33 |
| Coal | 29 |
| PMMA | 25 |
| PVC | 20 |
22.3.3. Potential and limitations of recycling
The solutions, known as the 3 Rs, of the environmental issue of residues are, in order: reduce, re-use and recover. But it is not always possible to use this type of solution: there are associated technical, economical and social problems, such as:
– the difficulty of controlling the generation of residues when they result from the consumption of products with a short life-cycle or unique use (disposable products);
– the difficulty of separating residues in a selective manner and in sufficient quantities, depending on their nature and composition;
– the fact of finding a combination of incompatible products in the residues, which prevents good quality recycled material being obtained.
Also, sometimes recycling is not conceivable because of difficulties or restrictions with the technique, or because costs are excessive and the recycled materials are not competitive with the raw materials they could replace.
We present below a few potentialities and limitations for the recovery of plastic residues in general and, in particular, for residues coming from electrical installations:
– today, the recovery of materials contained in all plastic residues is fundamentally made via the mechanical recycling of the main consumer thermoplastics: PE, PP, PVC, etc. Mechanical recycling is a processing which is only applied to plastic residues of one selected and homogenous material. The plastics thus recovered represent 15% of the total residues;
– there are residues which contain plastics with other types of materials (metals, ceramic-glass, etc.) that are grinded together, which prevents them from being easily dismantled or separated: insulated cables, small size components, etc. In these cases, there are effective processes to separate recyclable plastics from the rest of the materials (magnetism, extraction with solvents, gravity, flotation, etc.);
– the current processes of mechanical recycling are not efficient to recover complex plastics or multimaterials since the recovered material has no thermal and homogenous rheological properties, and the material which would be obtained could not be processed, or would give a material of weak quality;
– plastic residues are materials which might have been subjected to a degradation during their useful life, such as thermal degradation, chemical degradation, etc. However, they can still be used for less demanding products; we can combine them with the original material or improve their properties by adding corresponding additives before their reprocessing;
– the recycling of thermohardenable composites is not very developed. From a technical point of view, it has been verified that the recovery of these materials, in the form of grinded powder or by separating the strengthening fiber, is possible without any loss of mechanical properties for the new products. Others possibilities to recover the material are the combustion and use of organic scraps as subsidiary raw material in the production of concrete;
– chemical recycling, with which we claim to make residual plastic cost-effective by transforming it into a basic raw material or into a combustible, starts to appear as a real alternative owing to the improvement of separation processes and the development of new technologies. Chemical recycling is currently in an advanced experimental phase;
– the elements of electric grids: cables, insulating materials, capacitors, etc. are long life (>20 years) products. The ratio between the generation and the total volume of plastic in this type of residue is much lower than in other consumer products in the electrical–electronic sector which have a shorter life cycle. However, the residues coming from the dismantling or the renewing of large lattices and electrical installations could represent an advantage in recovering residues in large quantities;
– the thermoplastics used for the electrical insulation of cables are potentially recyclable (by mechanical recycling), except in the case of crossed-linked polyethylene (XLPE) which can only be recovered by chemical depolymerization or energetic recovery;
– in the case of unipolar cables with only one type of plastic as an insulator, recycling could be made by means of a mechanical process. For more complex cables, in which there are different types of plastics, we could use separation processes with selective solvents such as the Vinyloop process for PVC;
– the economical value of cable which conducts copper, aluminum, etc. is a decisive factor for the recovery of cable by using separation processes for the plastic which covers it. Consequently, the plastic could also be recycled;
– the high calorific power of plastics makes them potentially usable as alternative combustibles in certain industrial processes (e.g. in furnaces for concrete production). They can also be burned to recover the thermal energy or be transformed into electricity;
– the dielectric films (PP) of capacitors, impregnated with oil, would require a previous cleaning and depollution, which complicates and increases the recovery cost of the material. It is therefore more likely that the incineration of the residue is preferred in installations with systems processing pollutants present in gases and fly ashes.
22.4. Bibliography
[AGU 99] AGUADO J., SERRANO D., Feedstock Recycling of Plastic Wastes. RSC Clean Technology. Monographs, Ed. Royal Society of Chemistry, Cambridge, 1999.
[ANS 00] ANSEMS A.M.M., DE GROOT J.L.B., VAN DER VLUGT M., Best Practices for the mechanical recycling of post-user plastics. Association of Plastics Manufacturers in Europe (APME), 2000.
[APME] Association of Plastics Manufacturers in Europe, Plastics in Europe – An analysis of plastics consumption and recovery in Europe 2002, 2003 and 2004. www.plasticseurope.org
[PLASTIC EUROPE] The Compelling Facts About Plastics 2007 – An analysis of plastics production, demand and recovery for 2007 in Europe. www.plasticseurope.org
[EPIC] ENVIRONMENT AND PLASTICS INDUSTRY COUNCIL. Plastics Recycling Overview. Summary Report by Environment and Plastics Industry Council – A Council of the Canadian Plastics Industry Association. www.plastics.ca/epic
[EuPR] EUROPEAN PLASTIC RECYCLERS: http://www.eupr.org/
[MAR 04] MARTÍ-FERRER F., SANZ-BOX C., BENEDITO-BORRÁS A., TEROL-MARTÍ M.L. “Propiedades mecánicas de mezclas de HDPE con residuos de poliéster reforzado con fibra de vidrio”, VIII Congrés National de Materiaux, Espagne, June 2004.
[PIC 05] PICKERING, S. “Recycling termoshet composites”, JET Composites, no. 17, May 2005.
[PWMI] PLASTIC WASTE MANAGEMENT INSTITUTE, An Introduction to Plastic Recycling in Japan 2004. Plastic Waste Management Institute, Tokyo, Japan, May 2004. www.pwmi.or.jp/
[SCH 98] SCHEIRS J., Polymer Recycling, p.1, Wiley, Chichester, UK, 1998.
[VERT 05] EU Project G7RT-CT-2002-05073, “Setting-up of European Virtual Institute for Recycling”, Technological Reference Paper on Recycling Plastics, May 2005.
1 Chapter written by Pilar MARTINEZ and Eva VERDEJO.
