Solutions to end-of-chapter problems
C.1 Chapter 2
P2.3. C1 (T: Met, Et, Prop; B: But); C2 (T: Met; B: Et, Prop); C3 (T: Et; B Prop)
P2.4. C1 (T: A; B: BCD); C2II (T: BC; B: D); C3 (T: B; B: C)
P2.5. C1(T: A; B: BC); C2 (T: B; B: C)
P2.10. C1 (AB, CDEF); C2(A, B); C3(CDE, F); C4 (CD, E); C5(C, D)
P2.11. C1 (AB, CDEF); C2(A, B); C3(CDE, F); C4 (C, DE); C5(D, E)
P2.12. QS=200 kW; QW=1600 kW; Matches: Above Pinch: H1-C1 (1000 kW); Heating C1 200 kW; Below Pinch: H2-C1 (2400 kW); Cooling H2 (1600 kW); H1-C1 (200 kW)
C.2 Chapter 3
P3.2. 190K; Win=2802 kcal/h; Wout=697 kcal/h
P3.3. P1=150 atm; T4=300K; q=2 kcal/kg
P3.8. f=0.35; W1=60 kcal/kg; W1=54 kcal/kg
P3.9. With precooling T=163K; q=15 kcal/kg; Isentropic expansion; T=282K
P3.10. 6 Trays; 81% molar fraction in N2
P3.12. Pdesign=200 bar; Poperation=150 bar; η=0.95
C.3 Chapter 4
P4.2. m=88.976 kg; Limestone: 11.024 kg;
| Component | % |
| CaO | 13.6 |
| Ca(OH)2 | 69.6 |
| CaCO3 | 16.8 |
5.18% excess of Ca(OH)2; Conversion 98%
| NaOH | 0.00296201 |
| Na2CO3 | 0.16254275 |
| H2O | 0.83449524 |
Limestone: 10.601 kg; 7.38% excess of Ca(OH)2; Conversion 97%
P4.7. Solution: 89.425 kg; Limestone: 10.574 kg
| Solution (kg) | 89.42577 |
| NaOH | 0.582368 |
| Na2CO3 | 14.89163 |
| H2O | 84.526 |
| Limestone (kg) | 10.57423 |
| CaO | 0.097362 |
| Ca(OH)2 | 0.790953 |
| CaCO3 | 0.111685 |
P4.8. Q=8246387 kcal/h; A=1645 m2; X=0.632
P4.9. 52% NaCl; 48% NH4HCO3; Required CaCO3, 860 kg; Production NaCl=1459 kg
P4.10. W=357,854 kW; No selection; 1200€/kW
P4.11. 0.28 kg/s H2; 2.23 kg/s O2; No selection; 1250€/kW
P4.13. A=21.68 m2; ΔP=7841 kPa.
P4.16. 0.41 kg/s H2 and 3.26 kg/s O2; Solar
P4.17. Electrolysis: 4.5 GW; Oxygen Comp: 43.5 MW; Hydrogen Comp: 108 MW
| H2O | 0.079 |
| CO | 0.316 |
| H2 | 0.509 |
| CO2 | 0.095 |
20% via Electrolysis: Electrolysis: 46 MW; Oxygen Comp: 443 kW; Hydrogen Comp: 687 kW
P4.19. T=115°C; W=7744 kg/s; A=90 m2; AIQ1=41 m2; AIQ2=8.2 m2
P4.20. T=111°C; A=283 m2; AIQ1=65 m2; AIQ2=23 m2
P4.21. MR=175 kg/h; MP=825 kg/h; xR=171,917 ppm; xP=0.063 ppm
C.4 Chapter 5
P5.1. Air: 6 kmol per TM; Steam 8.34 kmol per TM; 8479 kcal/t
P5.2. H2O=1 kmol; CO=2 kmol; H2=3 kmol per kmol of C2H2; T=802K; alpha=0.75
P5.3. X=35% Recirculation (Syngas alone) 179.2; α=0.014
P5.4. Basis 1 kmol of Coal: Water gas 0.144 kmol; Generator gas 0.119 kmol; Qwater gas=4859 kcal; Qgenerator=1594 kcal; 91.5%
P5.6. P=1 atm; T=1215K; 0.2079 kmol of C
| Component | % |
| N2 | 73.82 |
| CO2 | 13.09 |
| CO | 13.09 |
| Total | 100.00 |
| Component | % |
| H2 | 48.39 |
| CO | 31.61 |
| H2O | 11.6 |
| CO2 | 0.08 |
| C | 0 |
| Total | 100.00 |
T=894 K; Qgenerator gas=1014 kcal; Qwater gas=5552 kcal
P5.8. 974 kg/h of water; 12,959 kW
P5.9. 0.7 mol of CH4 per mol of H2
P5.10. Steam: 0.96 mol per mol of methane; Oxygen: 0.48 mol per mol of methane
P5.11. α=64.4%; CO2 removed=20.5 kmol
| Comp Gas % | |
| H2 | 0.5027 |
| CO | 0.2514 |
| CH4 | 0.0492 |
| N2 | 0.1967 |
P5.12. α=64.4%; CO2 removed=20.5 kmol; T=200 °C
P5.13. Purge=0.022; Recycle gas=275 kmol; Recycle ammonia=15.18 kmol
| % Molar | |
| H2O | 9.90 |
| CO | 18.24 |
| CO2 | 6.76 |
| H2 | 65.10 |
P5.15. X=0.37; Purge=0.019; SG Recycle: 159.7 kmol/100 syngas fed; Methanol Recycle: 3.51 kmol/100 syngas fed
| Vapor | Liquid | |
| H2 | 0.61363282 | 0.02344087 |
| CO | 0.30362718 | 0.02069322 |
| CO2 | 0.07531264 | 0.00684493 |
| MetOH | 0.00742736 | 0.94902098 |
P5.21. X=0.35; 91% of the fed goes to 2nd Bed
P5.22. Efficiency=50%; 1.38 the minimum
P5.23. L=19.38 kmol/s with an excess of 34% with respect to the minimum
| Temp (K) | 573 | 254 | 254 |
| P (bar) | 50 | 50 | 50 |
| Vapor fraction | 1 | 1 | 0 |
| Enthalpy (MJ/h) | 141.8341 | −1147.413 | −3293.24 |
| Total flow | 352.69 | 306.0346 | 46.65541 |
| Total flow unit | kmol/h | kmol/h | kmol/h |
| Comp unit | kmol/h | kmol/h | kmol/h |
| Hydrogen | 211.11 | 211.0293 | 0.08069132 |
| Nitrogen | 70.37 | 70.30444 | 0.06556367 |
| Argon | 9.12 | 9.083407 | 0.03659212 |
| Ammonia | 62.09 | 15.61743 | 46.47256 |

P5.30. 27% of C3H8 is used as fuel; Syngas composition: 19% H2O; 19% CO; 4% CO2; 58% H2
P5.31. Flash: 0.625 M€; T (273K); Column 1.003 M€; 3 Stages; 321 kmol/s of liquid (Raoult’s law holds)
P5.32. Fresh feeds to beds 2nd=2.1 kmol/s; 3rd=2.3 kmol/s
| Stream No. | Feed | Out 1st bed | 2nd Feed | Exit HX to Bed 1 |
| Stream Name | ||||
| Temp K | 373.0000* | 867.9598 | 293.0000* | 673.0000 |
| Pres Pa | 29999999.6374* | 29999999.6374 | 29999999.6374* | 29999999.6374 |
| Enth MJ/s | 9.7083 | 47.443 | −0.31112 | 47.443 |
| Vapor mole frac. | 1.0000 | 1.0000 | 1.0000 | 1.0000 |
| Total kmol/s | 4.2000 | 3.7585 | 2.1000 | 4.2000 |
| Total kg/s | 42.0510 | 42.0511 | 21.0255 | 42.0510 |
| Total std L m3/h | 458.2150 | 405.7532 | 229.1075 | 458.2150 |
| Total std V m3/h | 338894.26 | 303273.80 | 169447.16 | 338894.26 |
| Flowrates in kg/s | ||||
| Nitrogen | 28.0140 | 21.8306 | 14.0070 | 28.0140 |
| Hydrogen | 6.0474 | 4.7126 | 3.0237 | 6.0474 |
| Ammonia | 0.0000 | 7.5184 | 0.0000 | 0.0000 |
| Argon | 7.9896 | 7.9896 | 3.9948 | 7.9896 |

| Stream No. | In 2nd bed | Out 2nd Bed | 3rd Feed | In 3rd Bed |
| Stream Name | ||||
| Temp K | 671.9910 | 814.7848 | 293.0000* | 670.2527 |
| Pres Pa | 29999999.6374 | 29999999.6374 | 29999999.6374* | 29999999.6374 |
| Enth MJ/s | 47.132 | 47.133 | −0.34076 | 46.793 |
| Vapor mole frac. | 1.0000 | 1.0000 | 1.0000 | 1.0000 |
| Total kmol/s | 5.8585 | 5.3825 | 2.3000 | 7.6825 |
| Total kg/s | 63.0766 | 63.0768 | 23.0279 | 86.1047 |
| Total std L m3/h | 634.8607 | 578.2836 | 250.9273 | 829.2109 |
| Total std V m3/h | 472720.96 | 434306.26 | 185585.00 | 619891.22 |
| Flowrates in kg/s | ||||
| Nitrogen | 35.8375 | 29.1691 | 15.3410 | 44.5101 |
| Hydrogen | 7.7363 | 6.2967 | 3.3117 | 9.6084 |
| Ammonia | 7.5184 | 15.6265 | 0.0000 | 15.6265 |
| Argon | 11.9844 | 11.9844 | 4.3753 | 16.3597 |

| Stream No. | Out 3rd Bed | Final product |
| Stream Name | ||
| Temp K | 789.4937 | 636.1416 |
| Pres Pa | 29999999.6374 | 29999999.6374 |
| Enth MJ/s | 46.794 | 9.0594 |
| Vapor mole frac. | 1.0000 | 1.0000 |
| Total kmol/s | 7.1454 | 7.1454 |
| Total kg/s | 86.1048 | 86.1048 |
| Total std L m3/h | 765.3866 | 765.3866 |
| Total std V m3/h | 576555.86 | 576555.86 |
| Flowrates in kg/s | ||
| Nitrogen | 36.9874 | 36.9874 |
| Hydrogen | 7.9845 | 7.9845 |
| Ammonia | 24.7733 | 24.7733 |
| Argon | 16.3597 | 16.3597 |
C.5 Chapter 6
P6.1. Kp=5.78 atm−1; T=27.25°C
| Composition | % |
| N2 | 0.47660706 |
| O2 | 0.0466796 |
| H2O | 0.28999495 |
| NO | 0.1867184 |
P6.4. T=784K; T=505K; L=5.98kg H2O and 0.153 kg HNO3
| Pfin (atm) (20 °C) | |
| O2 | 0.03249854 |
| N2 | 0.59247339 |
| NOini | 0 |
| NO2 | 0.08901046 |
| N2O4 | 0.08048442 |
| Total | 0.7944668 |
| Pfin (atm) (10 °C) | |
| O2 | 0.03138935 |
| N2 | 0.57225205 |
| NOini | 0 |
| NO2 | 0.06218873 |
| N2O4 | 0.08962934 |
| Total | 0.75545947 |
| Pfin (atm) (40 °C) | |
| O2 | 0.03471692 |
| N2 | 0.63291624 |
| NOini | 0 |
| NO2 | 0.15585831 |
| N2O4 | 0.05559242 |
| Total | 0.87908389 |
| % Molar | |
| N2 | 0.80685892 |
| O2 | 0.05068866 |
| H2O | 0.002514 |
| NO | 0 |
| NO2 | 0.13976608 |
| N2O4 | 0.00017234 |
P6.7. Sol1: 0.168; Sol2: 0.606
P6.8. 30 °C—200 s; 50 °C—300 s; 100 °C—450 s; 200 °C—t>1000 s
| kmol | |
| N2 | 0.45054664 |
| O2 | 0.01559242 |
| H2O | 0.002156928 |
| NO | 0 |
| NO2 | 0.013797762 |
| N2O4 | 0.01790625 |
C.6 Chapter 7

| After 1st Bed | After 2nd Bed | |
| SO2 | 0.01856078 | 0.0033564 |
| N2 | 0.79 | 0.79 |
| O2 | 0.09928039 | 0.09204708 |
| SO3 | 0.06143922 | 0.01446663 |
| S→SO2 | SO2→SO3 | ||||
| Initial | Final | Initial | 1st Bed | 2nd Bed | |
| SO2 | — | 0.08 | 0.08 | 0.01856078 | 0.0033564 |
| N2 | 0.79 | 0.79 | 0.79 | 0.79 | 0.79 |
| O2 | 0.21 | 0.21–0.08 | 0.21–0.08 | 0.09928039 | 0.09204708 |
| SO3 | — | 0.06143922 | 0.01446663 | ||

| S→SO2 | SO2→SO3 1st Bed | SO2→SO3 2nd Bed | ||||
| Initial | Final | Initial | Final | Initial | Final | |
| SO2 | — | 0.1 | 0.01 | 0.03119672 | 0.03119672 | 0.0039281 |
| N2 | 0.79 | 0.79 | 0.79 | 0.79 | 0.79 | 0.79 |
| O2 | 0.21 | 0.21–0.1 | 0.21–0.1 | 0.07559836 | 0.07559836 | 0.06930267 |
| SO3 | — | 0.06880328 | 0.00688033 | 0.02726863 | ||

P7.4. Raw Material 0.5% H2O; 88.1% FeS2; 11.4% Slag; Losses 83,689 kcal/100 kg Pyrite
| kmol | |
| N2 | 15.56 |
| O2 | 1.96 |
| H2O | 1.41 |
| SO2 | 1.19 |
| NO | 0.16 |
P7.7. X=0.975; 61.65 kg Water; Q=−93,500 kcal
P7.11. H2SO4 (99%)=9.19 kg; H2SO4 (98%)=174.6 kg; Water added=22.25 kg; H2SO4 (Absorption)=182 kg
P7.12. y=0.0095 kg Water/kg as;
| kmol | |
| N2 | 21.15 |
| O2 | 2.81 |
| H2O | 0.41 |
| SO2 | 2.81 |
| N2 | 21.15 |
| O2 | 1.49 |
| H2O | 0.27 |
| SO2 | 0.14 |
| SO3 | |
| NO | 0.014 |
| Stream No. | Feed | Exit 1st Bed | Feed 2nd bed | Exit 2nd bed |
| Temp K | 750.0000 | 918.8530 | 673.0000 | 774.9077 |
| Pres Pa | 1.0000 | 101325.0000 | 101325.0000 | 101325.0000 |
| Flowrates in kg/h | ||||
| Sulfur Dioxide | 640.6501 | 264.5776 | 264.5776 | 43.2041 |
| SO3 | 0.0000 | 469.9894 | 469.9894 | 746.6465 |
| Oxygen | 351.9890 | 258.0692 | 258.0692 | 202.7838 |
| Nitrogen | 2213.1060 | 2213.1060 | 2213.1060 | 2213.1060 |

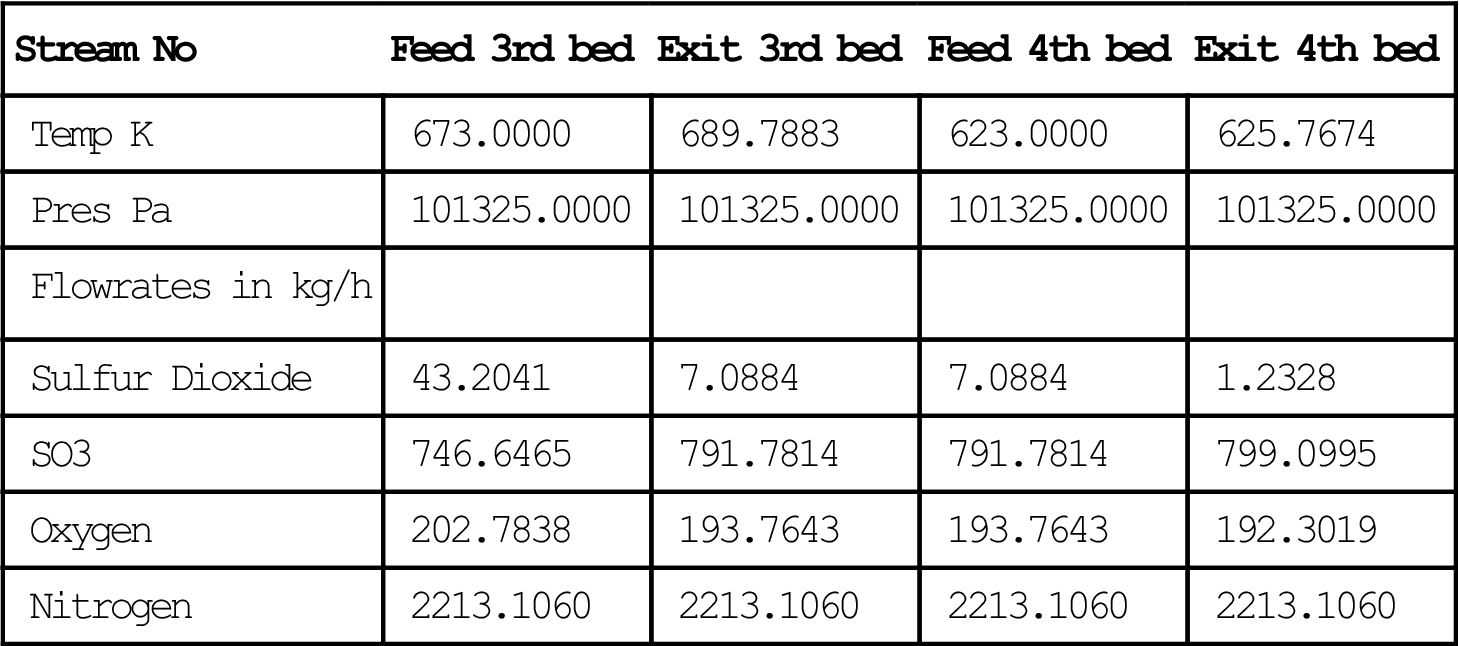
| Stream No | Feed 3rd bed | Exit 3rd bed | Feed 4th bed | Exit 4th bed |
| Temp K | 673.0000 | 689.7883 | 623.0000 | 625.7674 |
| Pres Pa | 101325.0000 | 101325.0000 | 101325.0000 | 101325.0000 |
| Flowrates in kg/h | ||||
| Sulfur Dioxide | 43.2041 | 7.0884 | 7.0884 | 1.2328 |
| SO3 | 746.6465 | 791.7814 | 791.7814 | 799.0995 |
| Oxygen | 202.7838 | 193.7643 | 193.7643 | 192.3019 |
| Nitrogen | 2213.1060 | 2213.1060 | 2213.1060 | 2213.1060 |
C.7 Chapter 8
P8.1. MetOH added 2.9 kmol per kmol of Oil
P8.2. D=88 kg/s; R=5.12 kg/s; XD=0.8
P8.3. 42.2% Cellulose; 42.2% Hemicellulose; 15.6% Lignin; Xxylose=0.66; 18.6 kmol of ethanol
P8.4. MetOH=308 kmol; Purge=0.057
P8.7. D=0.75 kg/s; R=3.75 kg/s; L/D=1; Number of trays 9
P8.8. C1HaOa/2; 13.4% of the biogas is used as fuel
| HX1 | Col Water–Ethanol | Col 1 (Met–Eth/Prop/But) | Col 2 (Eth–Prop/But) | |
| Thermodynamics | UNIFAC | UNIFAC | UNIFAC | UNIFAC |
| Q (MJ/s) | 11.8 | 18.9 | 19.3 | 5.8 |
| Reflux ratio | 2.8 | 8.2 | 0.15 | |
| Tin (K) | 305 | 363 | 348 | 355.5 |
| Tout (K) | 363 | 351 (T) – 372 (B) | 337.6 (T) – 355.5 (B) | 351.1 (T) – 375 (B) |
| Ethanol prod (kg/s) | 4.85 | 4.82 |

P8.11. C1H3.14O1.36; 45% CO2; 55% CH4
