Production Technology for Bioenergy Crops and Trees
Tadashi Hirasawa, Taiichiro Ookawa, Shinya Kawai, Ryo Funada and Shinya Kajita
Abstract
New technologies for producing energy crops and trees based on fundamental studies have been developed to improve self-sufficiency in food and feed supplies in addition to achieving sustainable natural resources. Energy crops and trees with improved leaf growth, light interception of crop canopy, photosynthetic rate, lodging resistance, and saccharification efficiency of lignocellulose, among many other traits, need to be explored. DNA marker-assisted selection using genome information has been developed as a powerful tool for breeding new bioenergy crops and trees. In this chapter, the concept and basic technologies for producing biomass from herbaceous energy crops and trees, ecophysiological characteristics for high yield and biomass production, genetic analyses of the traits responsible for biomass production, and molecular breeding for improving these traits are discussed. The definitions of herbaceous energy crops for the first and second generations, agronomy and breeding technology for these crops are explained. Recent studies on woody cell wall formation and genetic improvements associated with biomass saccharification in energy crops and woods are introduced.
Keywords
Biomass production; breeding; DNA marker-assisted selection; energy crop; lignocellulose; photosynthesis; saccharification; second-generation bioenergy crops; wood; cambial activity
Chapter Outline
4.1. Photosynthesis and Biomass Production in Energy Crops
4.1.2. The Concept of Biomass Production
4.1.3. Rice as a Potential Plant for Energy Crops in Japan
4.1.4. Characteristics of the Rice Varieties Yielding Heavy Biomass
4.1.5. Further Increasing Production of Rice Biomass for Energy and Food
4.1.6. Genetic Analysis of the Traits Responsible for Biomass Production: Concluding Remarks
4.2. Agronomy and Breeding Technology for Bioenergy Crops
4.2.1. Types of Herbaceous Energy Crops
4.2.2. First-Generation Bioenergy Crops
4.2.3. Second-Generation Bioenergy Crops
4.2.4. Prospects: Future Research for the Development of Energy Crop Production Technologies
4.3. Plant Molecular Breeding to Energy Crops as Genetic Improvements of Biomass Saccharification
4.3.1. Importance of Plant Molecular Breeding
4.3.2. Reduction of Lignin Contents
4.3.3. Alternation of Lignin Composition
4.3.4. Decrease in the Degree of Polymerization of Lignin and Addition of Easily Hydrolyzable Linkages into the Lignin Polymer
4.3.5. Production of Cell Wall Degradation Enzymes in Plants
4.4. Improvement of Woody Biomass
4.4.1. Wood and Cell Formation
4.4.2. Effect of Tree Breeding on Wood Quality
4.1 Photosynthesis and Biomass Production in Energy Crops
Tadashi Hirasawa
4.1.1 Introduction
Annual crop plants that contain a large amount of oil, starch, and sugar have the potential to be converted into biomass energy; sunflower and rape seeds, as well as corn grain and sugar cane stem, are being used to produce oil and ethanol. The yield needs to be improved in order to lower the production cost of the biomass energy. The grain yield can be considered as a product of biomass yield and harvest index (i.e. yield/biomass). Modern, semi-dwarf varieties of crops such as rice and wheat generally have very high harvest index values and decent grain yield (Evans, 1993). However, harvest indices for these plants have approached the theoretical maximum level (Mann, 1999). Increasing the total biomass production is currently considered essential for further improving the grain yield of crop plants.
Recently, more crop land has been diverted to growing grain for producing fuel that causes an increase in food price. If non-edible plant parts such as rice or wheat straw, and corn stalk, among many others, can be used for producing ethanol effectively, plants can be used to produce both food and fuel. In other words, all the solar energy stored in plants and the biomass produced can be used more effectively and efficiently. Additionally, any land that is not feasible for growing food crops may be used for harvesting fuel crops so that problems caused by conflicting uses of grain as food and energy can be solved.
4.1.2 The Concept of Biomass Production
More than 90% of plant dry matter is derived from photosynthesis; hence the yield of plant biomass (BY) can be expressed as (Hay and Porter, 2006):
![]() (4.1)
(4.1)
where Q = the total quantity of incident solar radiation received over the growing period of the crop, Ic = the fraction of Q that is intercepted by the canopy, and ε = the overall photosynthetic efficiency of the crop.
For annual plants, the crop growth rate (CGR), expressed as dry matter accumulated per unit land area per unit time, is low right after planting, and it then increases rapidly because the leaf area index (LAI), defined as the leaf area per unit land area, increases exponentially (Figure 4.1A). It reaches maximum level when the leaf area index is equal to the critical LAI. Such changes of CGR with respect to time to increase biomass production conform to a sigmoid curve (Figure 4.1B). Subsequently, the growth rate decreases in proportion to lessening LAI. Based on equation (4.1) and Figure 4.1, images of the plant canopy and the individual leaves that contribute to biomass production for a given growing season can be constructed as shown in Table 4.1.
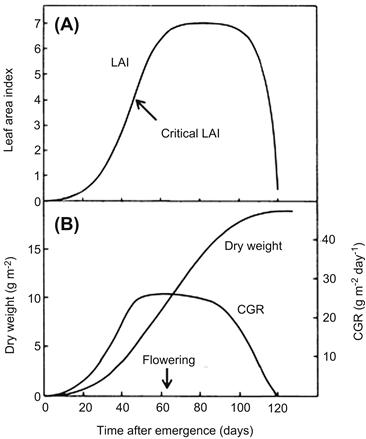
FIGURE 4.1 Diagram of the changes in leaf area index (LAI), crop growth rate (CGR), and above-ground dry weight of annual, determinate grain crop (Adapted from Gardner et al., 1985.)
TABLE 4.1
Characteristics Affecting Increased Dry Matter Production in High-Yielding Rice Varieties
∗Adapted from Ishihara (1996).
a Ic, the Fraction of Q that is Intercepted by the Canopy
If land is completely covered by a plant canopy, the interception of solar radiation increases because of high LAI (Gardner et al., 1985), resulting in a high crop growth rate (Horie and Sakuratani, 1985; Hay and Porter, 2006). The energy plant, i.e. Miscanthus, develops a leaf canopy earlier and maintains it later, which contributes to its prolific production of biomass as compared with maize (Dohleman and Long, 2010). The F1 rice varieties show vigorous growth during the early stages (Ishihara, 1996; Peng et al., 1998) so that biomass production of F1 is large, although the mechanism of its superior growth is presently unclear. This observation can be used to increase LAI during the early stage by increasing planting density (Box 4.1). However, increasing planting density sometimes causes severe lodging and deteriorations in canopy architecture that lead to reduction of biomass and grain production. Hence, crop plants with lodging resistance must be considered when increased planting density is practiced.
b ε, the Overall Photosynthetic Efficiency of the Crop
Once the land is completely covered by leaves, the canopy architecture becomes an important factor that affects light penetration and CO2 diffusion into the canopy, which in turn influence dry matter production. High rates of individual leaf photosynthesis from the seedling to the ripening stages are considered desirable in order to maximize biomass production. The maximum efficiency of the intercepted radiation is only 5–6% in many crop plants (Hay and Porter, 2006). If each of these parameters can be improved, the efficiency of crop plants to use solar energy will be improved significantly.
(i) Light Penetration into Canopy
The penetration of light into the canopy is quantified as the extinction coefficient k used in the Monsi and Saeki equation (Monsi und Saeki, 1953):
![]() (4.2)
(4.2)
where I0 = the irradiance above the canopy and I = the irradiance at a point in the canopy above which there is a leaf area index of L.
Parameter k is a useful indicator of the light-intercepting characteristics of the canopy. The leaf angle is a major factor governing k in a given species. Leaf surface properties affecting reflection, leaf properties including thickness, leaf size and shape, and characteristics affecting the three-dimensional arrangement of leaves also affect k (Hay and Porter, 2006).
(ii) CO2 Diffusion into Canopy
The CO2 concentration decreases to some extent in the canopy during daytime on a sunny day (Hay and Porter, 2006). This may induce the reduction in the rate of photosynthesis of a leaf in the canopy because of low ambient CO2 concentration as compared with the appropriate level for maximum photosynthesis, especially for C3 plants. When plant populations with similar LAI are compared, CO2 diffuses more effectively into a canopy consisting of taller plants than shorter plants because the former has smaller leaf area density (leaf area (m2) per unit volume of air space (m3)) (Kuroda et al., 1989). Increasing plant height sometimes causes severe lodging so that crop plants with lodging resistance should be used when increasing the height of canopy plants.
(iii) Rates of Individual Leaf Photosynthesis
We can consider the rate of individual leaf photosynthesis from three viewpoints: the capacity of photosynthesis, the rate of photosynthesis under senescence, and the rate of photosynthesis under abiotic stress.
The capacity of leaf photosynthesis is defined as the rate of photosynthesis measured for the fully expanded young leaf at optimum temperature with saturated light intensity and low vapor atmospheric pressure deficit without other abiotic stresses. The capacity of leaf photosynthesis increases with higher nitrogen content of a leaf (Makino, 2011). C4 plants have a higher capacity of photosynthesis than C3 plants due to the additional pathway of carbon assimilation in C4 plants. Various species of C3 plants have different photosynthetic capacity because of the properties of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) (Makino, 2011). Varietal differences in photosynthetic capacity are also observed for plants of the same species because of the different leaf nitrogen and Rubisco levels.
The rate of leaf photosynthesis decreases with senescence; the reduction differs among various plant species and varieties. Growth conditions also affect the rate of photosynthesis due to senescence. It is well known that there is a close correlation between the rate of leaf photosynthesis and leaf nitrogen content or Rubisco content during senescence (Hidema et al., 1991). The reduction in the rate of leaf photosynthesis is delayed in plants with a high capacity of cytokinin synthesis (Soejima et al., 1995).
The reduction in the rate of photosynthesis due to abiotic stress is usually remarkable during daytime on a sunny day. Plants sometimes show a midday and afternoon reduction in the rate of leaf photosynthesis even under the conditions of sufficient soil moisture (Hirasawa and Hsiao, 1999). Specific and varietal differences in the reduction in leaf photosynthesis have also been observed.
4.1.3 Rice as a Potential Plant for Energy Crops in Japan
Plants adapting well to the conditions of a region usually grow with relatively high productivity. Plants with high productivity can be considered as a candidate for energy crops. Although rice plants originated in tropical regions, it is one of the highest productive crops in Japan at present. Therefore, rice may become an important potential energy crop in Japan.
In Japan, brown rice yields have increased by approximately 1000 kg ha−1 over the 20-year period since the late 1940s; this is equivalent to the increase in the rate of rice yield in Asia during the “green revolution”. Since the 1970s, when Japan attained self-sufficiency in rice, further increase in grain yields has decreased in response to a shift in the eating habits of Japanese consumers so that growing rice crops emphasizes quality instead of quantity for consumption. During the same period, several high-yielding rice cultivars were released in Korea to produce more rice for human consumption. In the 1980s and 1990s, similar high-yielding rice cultivars were also released in Japan for various uses other than human consumption. These cultivars have approximately 20–30% higher yields than the regular cultivars grown in Japan for human consumption at that time (Ishihara, 1996). In the latter half of this section, characteristics of crops that can produce heavy biomass and methods to further increase their biomass production will be discussed using case studies.
4.1.4 Characteristics of the Rice Varieties Yielding Heavy Biomass
a Characteristics of the Improved Commercial Rice
When compared to the yields of several Japanese rice varieties under similar growth conditions (Figure 4.2), the cultivars, which were released about 50 years ago and are still grown for human consumption, have higher yields than the leading varieties that were released more than 100 years ago in the Kanto area. This increase in yield over time is the direct result of breeding. For the cultivars used in the experiment, the increase in yield resulted from an increase in dry matter production rather than from an increase in harvest index. Comparing rice cultivars currently grown in Japan with older cultivars, Kumura (1995) made the following observations:
1. The extinction coefficient of the canopy is smaller in currently grown cultivars than in the older cultivars.
2. With nitrogen top-dressing, the level of leaf nitrogen, and therefore the rate of leaf photosynthesis, increases significantly in currently grown cultivars compared with older cultivars.
3. Currently grown cultivars have a shorter culm and higher lodging resistance than the older cultivars.
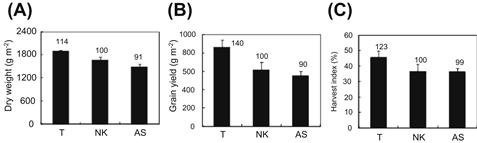
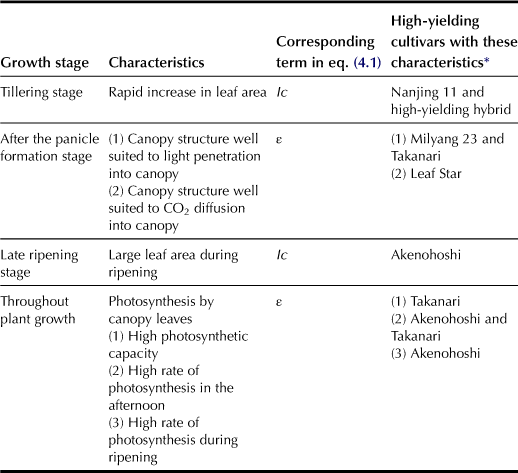
FIGURE 4.2 Comparisons of total dry weight of above-ground plant parts at harvest (A), grain yield (B), and harvest index (C) of varieties from different eras grown in a paddy field.
Data are averages of three years (2002, 2005, and 2006); error bars indicate standard deviations. T: Takanari, a high-yielding indica rice variety released in 1990. NK: Average values for Nipponbare and Koshihikari, japonica varieties currently grown in Japan for human consumption that were released in 1963 and 1956 respectively. AS: Average values for Aikoku and Sekitori, japonica varieties released in 1882 and 1848 respectively. The number above each bar represents the value for that trait relative to that of NK, which was set at 100. (Adapted from Taylaran et al., 2009.)
b Characteristics of the Most Productive Varieties
Compared with the varieties currently cultivated in Japan for human consumption, the high-yielding varieties that have been released in Korea and Japan since the 1970s have one or more superior characteristics that relate to canopy photosynthesis (Table 4.1). An indica variety “Takanari” is considered to be one of the most productive varieties in Japan, with higher grain yields and dry matter production consistently than any of the new or old commercial japonica varieties ever cultivated in Japan (Figure 4.2). Specifically, Takanari can produce 8–9 t grain and 19–21 t total dry matter per ha with the same rate of fertilizer application (San-oh et al., 2004; Taylaran et al., 2009). As shown in Table 4.1, the superior canopy characteristics of Takanari for increasing biomass are considered to be those aspects of canopy structure that affect light penetration into the canopy (Taylaran et al., 2009), photosynthetic capacity of the leaf (Hirasawa et al., 2010; Taylaran et al., 2011), and the rate of leaf photosynthesis at midday and in the afternoon as well. Compared with other high-yielding varieties, Takanari has many superior characteristics concerning the canopy photosyntheses that may explain why this strain can produce relatively higher dry matter than many of the other high-yielding varieties (Ishihara, 1996).
The superiority of Takanari with respect to dry matter production is most apparent after heading, when approximately 70% of the final carbohydrates in the rice grains are derived from the photosynthates. The larger dry matter production after heading will also increase the harvest index of Takanari compared with the japonica varieties that have been examined to date (Figure 4.2).
c Characteristics of the Highest Photosynthesis Capacity in the Most Productive Varieties
Higher levels of leaf nitrogen and larger leaf stomatal conductance in Takanari as compared with other Japanese cultivars can generally be attributed to the relatively higher rate of leaf photosynthesis (Hirasawa et al., 2010).
Takanari tends to accumulate a larger amount of nitrogen, as measured at harvest, even at the same rate of nitrogen application (Figure 4.3A). The currently grown commercial varieties fall in this ranking, whereas the old commercial cultivars have the lowest ranking. A large portion of nitrogen accumulates in Takanari during the period from heading to harvest (Figure 4.3B); other varieties after heading have not shown any larger relative partitioning of nitrogen to leaves as Takanari. These results indicate that the higher level of leaf nitrogen in Takanari may result from its ability to accumulate nitrogen more efficiently after heading than other varieties.
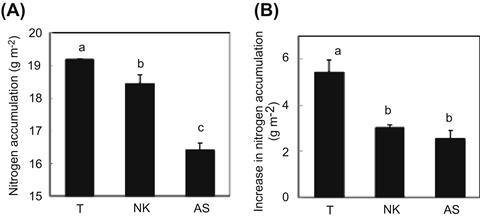
FIGURE 4.3 Comparisons of accumulated nitrogen at harvest (A) and from full heading to harvest (B).
T: Takanari, a high-yielding rice variety released in 1990. NK: Average values for Nipponbare and Koshihikari varieties currently grown in Japan for human consumption, released in 1963 and 1956 respectively. AS: Average values for Aikoku and Sekitori varieties released in 1882 and 1848 respectively. The same letters are not significantly different at the 5% level. (Adapted from Taylaran et al., 2009.)
The rate of leaf photosyntheses at an ambient CO2 concentration of 370 μmol mol−1 is closely correlated to leaf nitrogen content for all varieties (Figure 4.4A). However, Takanari is shown to have a higher rate of leaf photosynthesis than other current varieties based on the examination of leaf nitrogen content. The stomatal conductance at an ambient CO2 concentration of 370 μmol mol−1 in Takanari is also greater than that in other current varieties (Figure 4.4B). For both Takanari and other varieties, the rates of photosynthesis at an intercellular CO2 concentration of 260 μmol mol−1 in terms of leaf Rucisco content are quite similar, implying that these species have similar leaf photosynthetic activities with almost identical Rubisco contents in leaves (Figure 4.4C).
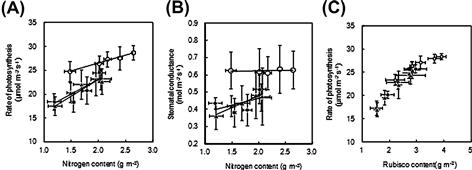
FIGURE 4.4 Relationships between leaf nitrogen content and the rate of photosynthesis at an ambient CO2 concentration of 370 μmol mol−1 (A), between leaf nitrogen content and stomatal conductance (B), and between leaf Rubisco content and the rate of photosynthesis at an intercellular CO2 concentration of 260 μmol mol−1 (C) of a flag leaf at full heading.
Open circles represent Takanari variety and filled symbols varieties currently grown in Japan for human consumption. (Adapted from Hirasawa et al., 2010.)
The water potential of the flag leaf in the currently grown varieties decreases significantly when compared with that in Takanari despite the fact that plants of all varieties are growing in submerged soil (Taylaran et al., 2011). This causes the larger stomatal conductance in Takanari (Figure 4.4B). Takanari has a far larger root surface area than other varieties. The hydraulic conductance from roots to leaves is thus much higher in Takanari whereas the hydraulic conductivity, defined as hydraulic conductance per unit root surface area, is not different among all varieties. Hence, the larger root surface area is suggested as the reason why Takanari has higher hydraulic conductance and higher leaf water potential.
The higher rate of photosynthesis in Takanari appears to result from both higher leaf nitrogen content and stomatal conductance than those in the currently grown cultivars even under similar nitrogen applications and even at the same levels of leaf nitrogen. The characteristics such as increased nitrogen uptake and hydraulic conductance may be related to the larger root surface area that leads to a higher rate of leaf photosynthesis in Takanari (Figure 4.5).
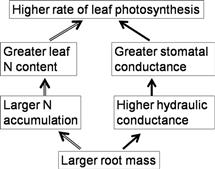
FIGURE 4.5 A schematic to illustrate how the high-yielding indica variety, Takanari, achieves a higher rate of leaf photosynthesis than other japonica varieties. (Adapted from Taylaran et al., 2011.)
4.1.5 Further Increasing Production of Rice Biomass for Energy and Food
Increasing parameters Ic and ε in equation (4.1) may be the key to increasing biomass production. Takanari is not superior to other high-yielding cultivars in all of the characteristics listed in Table 4.1. Consequently, if these non-superior characteristics in Takanari can be improved, its biomass production will be further enhanced.
a Light-Intercepting Characteristics of the Canopy
Compared with Japanese cultivars currently cultivated for human consumption, both Takanari and another high-yielding variety, Milyang 23, have erect leaves and small canopy extinction coefficients at the heading stage. However, because of its large and downward-pointing panicles, Takanari has a larger canopy extinction coefficient during the ripening stage than in Milyang 23.
b Rate of Photosynthesis in Fully Expanded Young Leaves
The maximum rate of rice leaf photosynthesis ranges from approximately 20 to 30 μmol m−2 s−1 when the ambient CO2 is between 370 and 400 μmol mol−1. The highest recorded rate of leaf photosynthesis is approximately 30–33 μmol m−2 s−1 as observed in Takanari, which has leaves with higher nitrogen content and larger stomatal conductance as mentioned above. Conversely, Koshihikari, the most popular rice variety in Japan, has a relatively low photosynthetic rate of 25–28 μmol m−2 s−1. Among backcrossed inbred lines derived from a cross between Takanari and Koshihikari (Koshihikari/Takanari//Takanari), rice lines with leaf photosynthesis values approximately 20% higher than that observed in Takanari have been identified (Adachi et al., 2013). These lines had mesophyll cells with large surface areas and unprecedented rates of leaf photosynthesis achieved by increasing CO2 diffusion from intercellular air spaces to chloroplasts, and from atmospheric CO2 to these intercellular spaces through high levels of stomatal conductance as well.
c Reduced Rates of Leaf Photosynthesis Associated with Senescence
Compared with Nipponbare, the high-yielding variety, Akenohoshi that maintains high rates of leaf photosynthesis during ripening is not observed in Takanari.
d Lodging Resistance and CO2 Diffusion into the Canopy
When above-ground biomass increases, the bending moment increases as well. Increasing the bending moment of the basal internode at breaking is considered to be important for lodging resistance in transplanted rice. This value is significantly larger in the high-biomass-producing variety Leaf Star than in Takanari owing to the larger section modulus in Leaf Star (Ookawa et al., 2010a). Additionally, because Takanari is shorter than Leaf Star, canopy leaf area density in Takanari is relatively larger. Consequently, shorter Takanari experiences the effect of decreasing CO2 diffusion into the canopy.
4.1.6 Genetic Analysis of the Traits Responsible for Biomass Production: Concluding Remarks
If the characteristics that affect biomass production as listed in Table 4.1 could be improved, biomass production in Takanari and other high-yielding varieties would increase in addition to enhancing biomass production in commercial varieties that are currently cultivated for human consumption. The major concern is how to improve these characteristics effectively. A marker-assisted approach is likely to become one of the most effective approaches for improving the traits as discussed in above sections. Indeed, the identification of the loci for numerous important quantitative traits that is currently being undertaken will be of great importance in this regard (Yamamoto et al., 2009; Ookawa et al., 2010b; Adachi et al., 2011). The capacity of biomass production for both food and fuel crops can be improved significantly in the near future.
4.2 Agronomy and Breeding Technology for Bioenergy Crops
Taiichiro Ookawa
Biofuel crops used as raw materials for producing bioenergy are grouped into herbaceous bioenergy crops and woody bioenergy crops. Herbaceous bioenergy crops grow faster than woody bioenergy crops in addition to having higher environmental adaptability, so that they grow in most environments on earth. In this section, the definition of herbaceous bioenergy crops as well as agronomy and breeding technology for these crops will be discussed.
4.2.1 Types of Herbaceous Energy Crops
Bioenergy crops can be classified into three development stages, i.e. the first generation, the second generation, and the third generation (Karp and Shield, 2008). The multiple use of these crops as feedstock often confuses their classification as bioenergy crops; the lignocellulose of the first-generation bioenergy crops (corn stover, bagasse, rice straw, and wheat straw) are considered as the second-generation crop.
a The First Generation
Energy conversion technologies have already been established in dealing with the first generation, such as sugar crops (sugar cane, sweet sorghum, sugar beet, etc.), starch crops (rice, wheat, barley, corn, sweet potato, potato, etc.), and oil crops (rapeseed, sunflower, soybean, etc.).
b The Second Generation
The process is to switch the raw materials for biofuels from food to non-food lignocelluloses consisting of the polysaccharides cellulose, hemicellulose and lignin; the energy conversion technologies are still under development.
c The Third Generation
Future technologies such as genetically modified crops or microbes, and conversion of organic matter into hydrogen gas, are proposed for third-generation biofuels.
4.2.2 First-Generation Bioenergy Crops
a Sugar Crops
(i) Energy Cane and Sugar Cane
Sugar cane (Saccharum officinarum) is a C4 perennial grass. Brazil is the leading sugar producer in the world, followed by India, China, and Thailand (Lichts, 2010). In Brazil, about half of the sugar cane crop is used for producing bioethanol.
Modern sugar cane varieties are interspecific hybrids among the thick culm species, S. officinarum, a high yielding plant originating in New Guinea, and fine culm species such as S. barberi or S. sinensis originating from India. Sugar cane and energy sugar cane are the same plant species; energy cane indicates that the sugar cane is used for producing energy like other forms of organic materials such as feedstock, and plant leaves and stems.
In first-generation energy crops, the accumulation of sucrose in the culm is used for producing fuel; energy sugar cane has the most efficient energy conversion in the process of biomass production and energy conversion to result in the highest output with the lowest input energy among all plants.
The plant height of sugar cane reaches 3–6 m with sugar accumulating in parenchyma cells of culms. The cane juice is squeezed from culms within one day after harvest to prevent decomposition. The juice is then heated and concentrated with the bagasse used as fuel to power the distillation of alcohol.
Cultivation and Breeding
Brazil
The sugar cane growing area in Brazil has been increased and expanded to about 500 million ha, with 79 t ha−1 average yield (FAOSTAT, 2010); about half of the cultivated area is used for growing the energy sugar cane.
Sugar cane can grow vigorously on fertile soils, hence applying the appropriate amount of fertilizer is important in getting high yields. Nutrient recycling is made possible by applying liquid fertilizers obtained from residues of bioethanol production. Crop rotation by growing soybean has been practiced in order to conserve the soil fertility of the field for growing energy sugar cane.
In Brazil, a number of private seed companies have bred and released many sugar cane varieties. The main breeding objective is to develop sugar cane with high biomass production, high sugar content and yield, pest resistance, and drought resistance.
The Brazilian government promotes the research on improving sugar yield by funding several biofuel projects (Ministry of Agriculture, 2006). Specific objectives of these studies include the maintenance of high sugar content from March to September through the entire growing period, high nutrient use efficiency, sugar cane genome research, and the development of DNA markers (Waclawovsky et al., 2010).
Japan
In Japan, sugar cane is grown in lowland and gently sloping alluvial fans in Kagoshima prefecture and Okinawa prefecture, where sandy soil is suitable for growing sugar cane because sugar cane roots need to be well ventilated.
In Japan, Sugimoto and other researchers of the National Agricultural Research Center in Kyushu Okinawa Region (NARO) have bred “monster cane” by crossing common sugar cane with Erianthus that is closely related to wild species of sugar cane (Figure 4.6). The new breed has higher biomass production than common sugar cane varieties. Bioethanol plants have been established for the demonstration of practical experiments in Ie Island, Okinawa prefecture (Kawamitsu et al., 2003), in collaboration with Asahi Beer Company in Japan.
(ii) Sugar Beet (Beta vulgaris L.)
Sugar beet is a C3 plant belonging to the Chenopodiaceae family; the cultivated species are F1 hybrid. France is a leading producer of sugar beet, and has been producing bioethanol using beet as the raw material just as sugar cane is used for biofuel elsewhere. In Japan, sugar beet has been grown in Hokkaido that is located in the northern part of Japan using the paper pot transplanting practice. Sugar beet has been introduced into the crop rotation with wheat, legumes, potato, and corn.
(iii) Sweet Sorghum
Sorghum is a C4 annual grass, and is classified in (Sorghum bicolor L.) the Poaceae family. Grain sorghum is mainly used as livestock feed concentrate, whereas sweet sorghum is used as a raw material for bioethanol in the USA, the EU, and China.
b Starch Crops
(i) Maize (Zea mays L.)
Maize is an annual C4 grass belonging to the Poaceae family. Similar to wheat and rice, maize is a major food crop and may also be utilized as a forage crop.
The USA is a major producer of maize, with harvest accounting for about one-half of the total production in the world (Lichts, 2010). In recent years, maize has been used as a raw material to produce bioethanol in the USA. The production of bioethanol from maize seed in the USA is the highest in the world. F1 hybrid maize varieties using hybrid vigor are easy to process for biomass and foodstuffs.
(ii) Rice (Oryza sativa L.)
Rice is an annual C3 grass belonging to the Oryza genus. Oryza sativa, which is the cultivated Asian native species, has two ecotypes: Indica and Japonica. Japonica is grown mainly in East Asia and Indica is distributed in the subtropical regions of Southeast Asia.
Cultivation and Breeding
In Asia, the International Rice Research Institute began the dwarf rice breeding program in 1962, and a dwarf selection from Dee-geo-woo-gen/Peta cross was released in 1966 as IR8. These short-culm varieties have a semi-dwarf gene sd1, which is recessive and inhibits the production of gibberellin biosynthetic enzyme, introduced into rice to reduce lodging at higher rates of fertilizer use.
In China, the F1 hybrid variety using hybrid vigor has been introduced in the southern parts of China and contributed to increased yields.
Since the 1960s in Japan, rice crops with high yield have been achieved by using some technologies with important innovations, i.e. controlling application of chemical fertilizer, mechanical transplanting, and planting semi-dwarf varieties. In recent years, direct seeding, which has been practiced in the USA and Australia, and has been widespread in Southeast Asia, has been introduced as a practical technology for reducing cultivation costs in Japan.
In Japan, the Ministry of Agriculture, Forestry and Fisheries started a research project in the 1980s to utilize rice as animal feed. One of the many objectives is to develop rice varieties with high yield and low cost production for forage use. High-yielding varieties have been developed, e.g. Indica type variety, and Takanari with the highest grain yield over 10 t ha−1. In recent years, rice varieties for biofuel have also been developed; bioethanol demonstration plants in Hokkaido and Niigata prefecture have been put into operation using the new high-yielding varieties developed for biofuel.
c Oil Crops
(i) Rapeseed (Brassica spp.)
Among the six species belonging to rapeseed Brassica (Brassicaceae), two species, Brassica napus L. and B. rapa L., are generally referred to as rapeseed.
The order of magnitude for nations with yield of rapeseed is China, Canada, India, France, and Germany. France and Germany in Europe have encouraged the use of rapeseed oil to replace biodiesel for automobiles. In Japan, the national “Nanohana Project” to process recycled cooking oil from rapeseed into synthesized biodiesel fuel has been implemented in many local towns.
4.2.3 Second-Generation Bioenergy Crops
a Current Status of the Technology for Producing Second-Generation Bioenergy Crops
(i) USA
The US Department of Energy (USDOE) and Department of Agriculture (USDA) started a joint development and demonstration project in 2005, to appropriate funds for USDOE to promote basic research on bioenergy. The research results were published in a report entitled “Breaking the Biological Barriers to Cellulosic Ethanol” that shows the roadmap for using cellulosic biomass biofuel (Houghton et al., 2006). The information on producing cellulosic biomass is taken from this report as follows.
Road map of research involved in the production of cellulosic biomass: Technology development phase within 10 years, 10–15 years individual system integration phase:
1. Development of sustainable biomass production technology:
• Development of next-generation energy crops with a high yield in sustainable agriculture (see Figure 4.7).
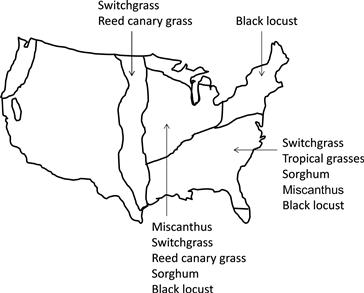
FIGURE 4.7 Geographic distribution of energy crops in the USA (US Department of Energy, 2006).
• Low-input and no tillage cultivation using perennial crops.
• Introduction of biological diversity by crop rotation and the mixing of a wide variety of plant species.
• Effects of the removal of crop residues on nitrogen and carbon recycling and soil microorganisms.
2. Low-cost harvest and transportation technology:
• Developments of the harvester and the packaging machinery for corn stover, etc.
3. Development of next-generation energy crops with a high conversion efficiency:
• Improvements of the lignin composition and the structure of cellulose involved in the conversion efficiency, cell wall-related genes and genome analysis.
4. Technology development of the bioenergy system that is applicable to the characteristics of each local region.
• System integration of the sustainable agriculture in local regions, the conversion technology of bioenergy, the evaluation of economic values of bioenergy.
In the USA, three companies have operated bioethanol plants using lignocellulose such as corn stover, corn cobs and bagasse of energy cane, and another 16 companies have made plans to use switchgrass and wheat straw as raw materials for bioenergy (Jessup, 2009).
(ii) EU
In the EU, the Renewable Energy Road Map was proposed in 2006. The bioethanol derived from wheat grain (and straw) and the BDF from rapeseed will be produced in order to increase the proportion of bioenergy in energy consumption, whereas bioethanol derived from energy cane and soybean and the BDF from oil palm are scheduled to be imported from overseas.

FIGURE 4.8 Gold hull phenotype in Leaf Star (Ookawa et al., 2010a).
(iii) China
China established the Renewable Energy Law in 2005. Because productions of biomass from corn stover, rice straw, and wheat straw are enormous, direct combustion for power generation and indirect use as bioethanol from these lignocelluloses have been proposed (Wang Q., 2011). China is divided into the following five regions of “green oil fields”: Northeast (sweet sorghum), Northwest (sweet sorghum, shrub), North (sweet sorghum), Southwest (sweet sorghum, shrub), and Southeast (trees, grass).
(iv) Japan
Japan is a highly industrialized country that depends on almost 100% of imported energy with high total GHG emissions. Six ministries collaborated to start the “Biomass Nippon Strategy” program in 2002 with the object to alleviate global warming. The Biomass Nippon Strategy is expected to develop a low-carbon and “circular” economy that utilizes renewable resources in a highly efficient manner. The motivations behind the program include: (1) preventing climate change; (2) creating a recycling-oriented society to use limited resources in an effective manner by utilizing renewable biomass; (3) fostering new strategic industries; (4) activating agriculture, forestry, and fishery, as well as uniting rural communities. In this plan, the utilization of unused portions of farm crops such as rice straw or husk for biofuel will become visible by around 2010. By around 2020, energy crops will be widely cultivated so that they can be utilized as an energy source, and by around 2050 newly developed crops such as marine plants and genetically modified crops will contribute to an increased production of biomass.
In 2007, the Biomass Research Center was founded by the Japan Ministry of Agriculture, Forestry and Fisheries as a virtual research organization to start the project “Development of biomass utilization technology for regional revitalization”. In this project, the following tasks will be undertaken: (1) Development of biofuel production technology using the first-generation energy crops (e.g. sugar cane, sugar beet, potato, sweet potato, and sorghum); and (2) Development of technology to efficiently convert lignocellulose such as rice straw to bioethanol.
b Second-Generation Energy Crops
(i) Miscanthus (Miscanthus spp.)
Miscanthus is a perennial C4 grass originating in East Asia; Miscanthus sacchariflorus (2n = 76) genotypes are more adapted to warmer climates whereas Miscanthus sinensis (2n = 38) genotypes grow well in winter. Miscanthus × giganteus, which is an indeterminate type in triploid (2n = 57) (Karp and Shield, 2008), has been considered as a model plant for second-generation bioenergy crops in the EU. It was introduced to Denmark from Yokohama, Japan in 1935, and has now spread throughout Europe. This plant is a natural hybrid of M. sacchariflorus and M. sinensis (Lewandowski et al., 2000). The details of its origin are unclear, but the possibility of its origination from “Ogisusuki” is high (Adati, 1958). These two species are of interest for breeding genotypes for bioenergy. Interspecific hybrids show more vigorous growth than parental plants.
In Europe except Scandinavia, Miscanthus × giganteus can be grown in most countries, and the biomass yield is higher in warm climates such as Southern European countries, Italy and Greece.
Miscanthus × giganteus is exceptional among C4 species for its high productivity in cold climates because it can maintain photosynthetically active leaves at temperatures below the minimum for maize (Zea mays) so that it has a longer growing season in cool climates than most other plants (Naidu et al., 2003; Wang D. et al., 2008).
Cultivation and Breeding of Miscanthus × giganteus
The sterile hybrid Miscanthus × giganteus has to be propagated vegetatively using rhizome (macro-propagation) or tissue culture (micro-propagation).
Because Miscanthus × giganteus is a perennial grass, weed control during the first two years is critical but the nutrient requirements are minimal for its growth; 50 kg nitrogen fertilizer, 20 kg phosphate, and 100 kg of potassium oxide per ha are sufficient for a satisfactory yield. In Europe, the harvest is carried out in February or March when large amounts of nutrients are stored in rhizomes to promote spring regeneration.
The biomass production in miscanthus is high even in low nitrogen conditions (Danalatos et al., 2007) with no changes of biomass production even if the nitrogen fertilizer were reduced by half to 50 kg-N ha−1. The highest biomass production was also obtained at a sparse planting density of 1 plant m−2.
In Europe, many genotypes of Mischantus × giganteus have been bred and widely used for productivity trails since its introduction in Denmark in 1930. Lewandowski et al. (2000) reported the following current situations and problems for cultivating and breeding miscanthus:
1. To improve biomass productivity and cold tolerance, many strains have been developed by crossing genetic resources of M. sinensis and M. sacchariflorus in the European Mischanthus Improvement Project (EMI).
2. From reports in European countries, the yields are 30 t ha−1 for irrigated fields and 10–25 t ha−1 for fields without irrigation in Southern Europe.
3. Results of case studies show that 30–35 cm young seedling or 10 cm cut rhizome is transplanted to fields in Denmark and Germany, whereas in the UK seedling or tissue culture using a piece of rhizome is transplanted in March or April.
4. High costs to construct and operate nursery facilities have become a serious problem.
5. Mischanthus cannot overwinter in Denmark, Ireland, and Germany.
6. In low fertilized fields, the retranslocation of nutrients to the rhizome becomes significant (N: 21–46%).
7. Weed control should be carried out thoroughly in the first year.
8. The yield limiting factors are soil type and soil moisture.
In the USA, strains from the Miscanthus × giganteus “Illinois” clone are selected for their high biomass yield based on studies conducted at Illinois State University. The amounts of bioethanol production and biomass production of this energy crop were estimated as shown in Table 4.3 (Heaton et al., 2008). In this estimation, mischanthus yields 260% ethanol production per unit land area compared with that from corn grain.
(ii) Switchgrass (Panicum virgatum L.)
Switchgrass is a perennial C4 grass originating from central Mexico and has spread to 55° north latitude; it is classified into two ecotypes of lowland and upland. The upland ecotype, which is octoploid (2n = 72) or hexaploid (2n = 54), has adapted to dry land. On the other hand, the lowland ecotype is tetraploid (2n = 36), and has adapted to swamps (Karp and Shield, 2008).
The breeding of switchgrass as a livestock feed was started in the 1930s, and the technology for breeding and cultivating switchgrass as feed has been established (Bassam, 1998). In recent years, evaluating switchgrass as energy crops has become a model US DOE project for using herbaceous crops in the Biofuel Feedstock Development Program undertaken by Oak Ridge National Laboratory.
For the quantitative trait locus (QTL) analysis of yield traits, the DOE Great Lakes Bioenergy Research Center and the University of Wisconsin have jointly established a research center. The breeding program has been carried out to select switchgrass of low lignin content by using marker-assisted selection (MAS). In addition, the syntenies of sorghum genome is expected to be applied to the same grasses. Heterosis of 32% in biomass production has been obtained from the F1 in both upland and lowland types (Vogel et al., 2002).
Switchgrass reproduces by seed propagation so that the biomass yield can be maintained even when grown in fields of less fertile soil. In order to reuse the carbon and nitrogen for growing next year’s crop, resources are recycled into rhizomes, thus the fertilizer requirement can be reduced (McLaughlin and Walsh, 1998). Typically, the application of P and K is not necessarily as frequent as once a year in the cropping system (McLaughlin and Adams, 2005). Although switchgrass has lower biomass yield than miscanthus, it requires less fertilizer; 50 kg-N ha−1 of nitrogen fertilizer is sufficient for switchgrass with other advantages by keeping the low cost of chemical fertilizer application in swithchgrass cultivation.
(iii) Rice (Whole Crop, Straw)
In Japan, fallow and abandoned paddy fields have been caused by the overproduction of rice. On the other hand, the proportion of self-sufficient food supply is only 40% on a calorie basis in Japan. Furthermore, 75% of the domestic demand of feed for livestock is imported from overseas. To increase the self-sufficient feed supply and establish the recycling system between rice cultivation and the livestock industry, the cultivation of forage crops in fallow and abandoned paddy fields has been conducted since the 1970s. Whole crop silage (WCS) is one of the ways to achieve self-efficiency in livestock feed, and breeding rice varieties for WCS have been developed for feed.
High biomass production, lodging resistance and digestibility are the main targets for the breeding of WCS rice varieties. These varieties can be applied as a raw material for second-generation biofuel (Box 4.2).
4.2.4 Prospects: Future Research for the Development of Energy Crop Production Technologies
Many researchers have developed new technology for second- and third-generation bioenergy (Sarath et al., 2008; Vega-Sanchez and Ronald, 2010). In the USA, the research has been promoted toward using energy crops such as switchgrass, which can be grown widely in this country, to expand biofuel production from lignocellulose in the future. To utilize the biomass for both bioethanol and BDF, new GM graminaceous crops that can accumulate sugar and oil in straw are proposed. On the other hand, Tilman et al. (2006) suggested that achieving sustainable bioenergy production is important to maintain biodiversity in a form close to natural vegetation from the viewpoint of plant ecology.
In countries with limited land space such as Japan, improving the self-sufficiency of food and feed and recycling natural resources depends on the development of a recycling-based high biomass production system. The ecophysiological properties associated with sustainable biomass production, such as expansion of leaf area, photosynthetic rate, nitrogen use efficiency, lodging resistance, etc., need to be improved in order to breed new energy crops capable of adapting to this system. DNA marker-assisted selection using genome information and crop physiology is a powerful tool for breeding new bioenergy crops (Ookawa et al., 2010b). Further research is required to develop the sustainable production technology of energy crops.
4.3 Plant Molecular Breeding to Energy Crops as Genetic Improvements of Biomass Saccharification
Shinya Kawai
4.3.1 Importance of Plant Molecular Breeding
This review is written based on prior excellent reviews carried out by previous researchers (Sticklen, 2006; Torney et al., 2007; Weng et al., 2008; Simmons et al., 2010) and recent research. Most bioethanol produced in the world is derived from starch of maize seeds and fermentable sugars (e.g. sucrose) of molasses from sugar canes. But starch and sucrose are also important sources of nutrients for both humankind and livestock. Therefore, the development of biofuels including bioethanol from non-food crops and agricultural residues has been strongly promoted.
Projects of fermentable sugars for ethanol production made from non-utilized biomass (such as agricultural residues, timber, switchgrass, and Miscanthus) have been undertaken in various parts of the world. However, saccharification (decomposition of polysaccharides into monosaccharides or fermentable sugars) of lignocellulosic materials contained in the plant cell wall is expensive, and the converted products are difficult to convert into ethanol due to its rigidity, complexity, and tolerance to cellulolytic enzymes. Addtionally, lignin restricts the availability of polysaccharides to enzymes, therefore limiting the enzymatic degradability and digestibility of biomass. In brief, lignin is an effective barrier against enzymatic saccharification. The improvement in pulping and bioconversion efficiencies of the wood seems useful to improve biomass for saccharification (Leple et al., 2007). Pretreating the lignocellulosic materials prior to the enzymatic saccharification process with cell wall degradation enzymes, microbial ligninases, cellulases, hemicellulases, etc. becomes necessary. Although efficient delignification is accomplished by treating lignocellulosic materials with organic solvents, ionic liquids, and Kraft pulping, these methods are costly. Therefore, other less expensive methods such as treating the fibers with dilute acid, ammonia fiber expansion, or heat are also applied. Although dilute acid and heat pretreatments are effective in decomposing the cell wall matrix, allowing enzymes to access cellulose, these methods require high temperatures (Hamelinck et al., 2005).
Accordingly, developing plant materials that are suitable for converting the contained cellulose into glucose cost-effectively provide a better solution to this problem. Recent plant molecular breeding research has aimed to increase the digestibility of the cellulose by modifying the lignin content and composition, and the accumulation of “redesign” lignin in the plant cell wall because the structures of lignin contribute to the resistance to degradation. Unlike cellulose and amylose, lignin is not a linear polymer of identical and repetitive monomers; it is a three-dimensional phenolic polymer linked by several types of carbon–carbon and ether bonds. Lignin monomers consist of three major monolignols: (1) coniferyl alcohol, (2) sinapyl alcohol and p-coumaryl alcohol, and (3) minor monolignols (Figures 4.9 and 4.10A). Lignin is mainly present only in certain types of mature plant cells such as xylem and fibers.
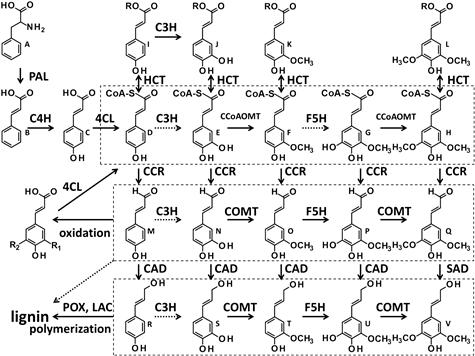
FIGURE 4.9 The grid model of the partial pathway for lignin biosynthesis.
Enzymes: 4CL, 4-coumarate:coenzyme A (CoA) ligase; C3H, coumarate 3-hydroxylase; C4H, cinnamate 4-hydroxylase; CAD, hydroxycinnamyl alcohol dehydrogenase; CCoAOMT, S-adenosyl-methionine caffeoyl-CoA/5-hydroxyferuloyl-CoA O-methyltransferase; CCR, hydroxycinnamoyl-CoA reductase; COMT, caffeate/5-hydroxyferulate O-methyltransferase; F5H, ferulate 5-hydroxylase; HCT, hydroxycinnamoyl-CoA shikimate/quinate hydroxycinnamoyl transferase; LAC, laccase; PAL, phenylalanine ammonia-lyase; POX, peroxidase; SAD, sinapyl alcohol dehydrogenase. Substrates: A, phenylalanine; B, trans-cinnamic acid; C, p-coumaric acid; (p-hydroxycinnamoyl-CoAs: D, E, F, G, and H); D, p-coumaroyl-CoA; E, caffeoyl-CoA; F, feruloyl-CoA; G, 5-hydroxyferuloyl-CoA; H, sinapoyl-CoA; I, p-coumaroyl shikimic acid/quinic acid; J, caffeoyl shikimic acid/quinic acid; K, feruloyl shikimic acid/quinic acid; L, sinapoyl shikimic acid/quinic acid; (p-hydroxycinnamyl aldehydes: M, N, O, P, and Q); M, p-coumaraldehyde; N, caffeylaldehyde; O, coniferaldehyde; P, 5-hydroxyconiferaldehyde; Q, sinapaldehyde; (p-hydroxycinnamyl alcohols: R, S, T, U, and V; major monolignols: R, T, and V); R, p-coumaryl alcohol; S, caffeyl alcohol; T, coniferyl alcohol; U, 5-hydroxyconiferyl alcohol; V, sinapyl alcohol. –ORs of I, J, K, and L represent shikimate/quinate ester bonds. p-Hydroxycinnamyl aldehydes: M, N, O, P, and Q are highly oxidizable, and they are often oxidized to p-hydroxycinnamic acids: C, caffeic acid, ferulic acid, 5-hydroxyferulic acid, and sinapic acid respectively. These p-hydroxycinnamic acids are substrates of 4CL and are recovered to p-hydroxycinnamoyl-CoAs: D, E, F, G, and H respectively.
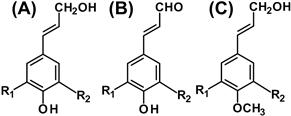
FIGURE 4.10 Monolignols and related molecules.
(A) p-Hydroxycinnamyl alcohols: R1 = H, R2 = H, p-coumaryl alcohol; R1 = H, R2 = OCH3, coniferyl alcohol; R1 = OCH3, R2 = OCH3, sinapyl alcohol; R1 = H, R2 = OH, caffeyl alcohol; R1 = OCH3, R2 = OH, 5-hydroxyconiferyl alcohol. (B) p-Hydroxycinnamyl aldehydes: R1 = H, R2 = H, p-coumaryl aldehyde; R1 = H, R2 = OCH3, coniferaldehyde; R1 = OCH3, R2 = OCH3, sinapaldehyde; R1 = H, R2 = OH, caffeyl aldehyde; R1 = OCH3, R2 = OH, 5-hydroxyconiferaldehyde. (C) 4-O-methylated monolignols: R1 = H, R2 = H, 4-O-methylated p-coumaryl alcohol; R1 = H, R2 = OCH3, 4-O-methylated coniferyl alcohol; R1 = OCH3, R2 = OCH3, 4-O-methylated sinapyl alcohol.
Several of the energy crops, including rice, maize, wheat, sugar cane, sorghum, switchgrass, and sugar beet, can be practically transformed. A direct method for altering saccharification efficiencies of plant materials is through these transformation technologies to modify the expression of the enzymes and transcription factors involved in lignin biosynthesis, and to introduce lignin degradation enzymes.
This review covers the recent research on improving plant biomass characteristics through plant genetic engineering for bioethanol production. The following strategies for modifying the lignin to breed transgenic plants with characteristics of easy saccharification have been examined by researchers using many plant species.
4.3.2 Reduction of Lignin Contents
Firstly, transgenic plants with reduced lignin have been developed. Lignin is the second most abundant biomass after cellulose on Earth. For example, maize stover (leaves and stalks) constitutes a large portion of agricultural biomass. Lignocellulosic materials of maize stover are composed of 30% hemicellulose, 44% cellulose, and 26% lignin (US Department of Energy, 2006). Lignocellulosic materials of plant cell walls consist of crystalline cellulose embedded in the lignocarbohydrate complex (LCC), which is a complicated compound of high molecular weight made of lignin and hemicellulose. Pretreatments, such as acid and heat, prior to the saccharification process disrupt the structures of the lignocellulosic materials of the cell wall and remove lignin to allow easy access to the cellulose by cellulases (Hamelinck et al., 2005). Plant molecular breeding has been adapted to decrease the lignin contents and/or change the compositions of lignin. In previous studies, reducing lignin content was examined as a tool for enhancing sugar recovery.
Decreases in lignin contents through down-regulation of several genes for lignin biosynthetic enzymes (Figure 4.9) and transcriptional factors have been reported. These enzymes are involved in the biosynthesis of monolignols, which are the direct precursors of lignin, e.g. phenylalanine ammonia-lyase (PAL), cinnamate 4-hydroxylase (C4H), 4-coumarate:coenzyme A ligase (4CL), hydroxycinnamoyl-CoA shikimate/quinate hydroxycinnamoyl transferase (HCT), coumarate 3-hydroxylase (C3H), S-adenosyl-methionine caffeoyl-CoA/5-hydroxyferuloyl-CoA O-methyltransferase (CCoAOMT), hydroxycinnamoyl-CoA reductase (CCR), hydroxycinnamyl alcohol dehydrogenase (CAD, including sinapyl alcohol dehydrogenase (SAD)), ferulate 5-hydroxylase (F5H), and caffeic acid/5-hydroxyferulic acid O-methyltransferase (COMT). Furthermore, monolignol polymerizing enzymes, peroxidase and laccase during the last step of lignin biosynthesis have also been manipulated. The genes for these enzymes have often been adapted to alter the lignin content and/or composition in the transgenic plants. It is very important to note that, according to the types of artificially regulated enzymes, the adopted specific genes in the gene family for the enzyme, plant species and transgenic lines, and various phenotypes in transgenic plants appear in either independent or combinational manner such as reduced lignin content, altered lignin composition, dwarfism, collapsed vessels, etc. Therefore, in some cases, the down-regulation of one gene led to the reduction of lignin content, but in some other cases the same manipulation caused different phenotypes.
Transgenic aspen (Populus tremuloides Michx.) trees in which expression of Pt4CL1 (encoding 4-coumarate:coenzyme A ligase) had been down-regulated were bred (Hu et al., 1999). Trees with suppressed Pt4CL1 expression exhibit up to 45% reduction of lignin, but this reduction is offset by a 15% increase in cellulose. As a result, the total lignin–cellulose mass remains essentially unchanged. The growth of leaf, root, and stem is substantially enhanced, and the structural integrity is maintained both at the cellular and whole-plant levels in the transgenic lines. These results indicate that lignin and cellulose deposition can be regulated in a compensatory fashion that may contribute to metabolic flexibility and a growth advantage for sustaining the long-term structural integrity of woody perennials.
There are similar examples of 4CL down-regulation in grasses. Pv4CL1 encodes 4CL in switchgrass (Panicum virgatum). RNA interference (RNAi) of Pv4CL1 reduces extractable 4CL activity by 80%, leading to a reduction in lignin content with decreased guaiacyl (G) unit composition (Xu et al., 2011). Altered lignification patterns in the stems of RNAi transgenic plants were observed with phloroglucinol-HCl staining. The dilute acid pretreatment significantly increases the saccharification efficiency of the low-lignin transgenic biomass. In spite of 4CL down-regulated aspen, the transgenic switchgrass plants also had uncompromised biomass yields. Additionally, transgenic plants with a genetically modified lignin biosynthetic pathway often show dwarfism (Reddy et al., 2005). Therefore, transgenic plants with undesirable agronomic phenotypes such as dwarfism, and the collapse of vessel elements in the xylem, must be excluded.
Other genes for lignin biosynthesis have also been down-regulated. COMT catalyzes the methylation of 5-hydroxyconiferaldehyde, which is converted from coniferaldehyde by F5H. Maize brown midrib (bm) mutations are known for their naturally reduced lignin content and higher digestibility (Vignols et al., 1995). bm3, one of the bm mutations, is the recessive mutation of a gene for COMT. Therefore, the COMT antisense gene construct was introduced into maize, and the transgenic maize plants had decreased COMT activity and lignin content (He et al., 2003). Similar results using a sorghum COMT antisense construct in maize were also obtained (He et al., 2003); comparable experiments have also been done using many other plant species. A COMT down-regulated alfalfa (Medicago sativa L.) with reduced lignin content and altered lignin composition has been developed. Down-regulation of COMT brings about the decrease of lignin content by losing syringyl (S) units, whereas down-regulation of CCoAOMT caused a decrease of lignin content without reducing S units (Guo et al., 2001). The digestibility of forage from COMT down-regulated alfalfa plants is better than that of wild alfalfa plants, but increasing digestibility is also observed in CCoAOMT down-regulated alfalfa plants (Guo et al., 2001). The results indicate that both lignin content and composition affect the digestibility of alfalfa forage that is closely related to the saccharification efficiency (Guo et al., 2001). The CCoAOMT down-regulated transgenic alfalfa with low lignin content has been on the market with the proprietary name KK179 or the registration name OECD UI:MON-ØØ179-5.
To modify lignification more efficiently in plants, gene expressions for transcriptional factors related to lignin biosynthesis have been altered. Several transcription factors, which control genes for enzymes involved in lignin biosynthesis, have been identified; they belong to members of the NAC or MYB families. For example, several R2R3-MYB transcription factors have been identified to control lignin biosynthesis, such as Arabidopsis thaliana MYB61 (AtMYB61), Pinus taeda MYB4 (PtMYB4), Antirrhinum majus MYB308 (AmMYB308), and Eucalyptus gunnii MYB2 (EgMYB2). The down-regulation of these transcription factors may lead to the reduction of carbon flux into the lignin biosynthesis in the down-regulated transgenic plants (Besseau et al., 2007).
4.3.3 Alternation of Lignin Composition
Although reduction of lignin content is an important factor in improving digestibility, other alternatives may also achieve the same objective. The modification of lignin composition can significantly alter its degradation tendency but also leads to undesired agronomic features (Pedersen et al., 2005; Reddy et al., 2005) or undesirable phenotypes for bioenergy crops such as dwarfing, the collapse of vessel elements in the xylem, and increased susceptibility to fungal pathogens. Hence, another strategy has been suggested to alter lignin composition but not the content.
Lignin principally consists of p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) units, which are derived from p-hydroxycinnamyl alcohols, and the three major monolignols (p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol) (Figure 4.10A). Their distributions are different among species, individual plants, and even cell types. In gymnosperms, the lignin is constructed with only G and H units; in dicotyledons, G and S units are predominant. Grass lignin of monocotyledons contains all three units. Monolignols are secreted to the extracellular space, then radicalized by plant peroxidases and laccases within the secondary plant cell walls, and then polymerized.
A higher G unit content creates a highly condensed lignin composed of a greater portion of biphenyl and other carbon–carbon linkages, whereas S units are commonly linked through more labile ether bonds at the 4-hydroxyl position (Figure 4.11). S units assist in lignin degradation; therefore, materials and energy production from angiosperms are more efficient than those from gymnosperm wood. To ensure the efficient degradation of the cell wall biomass in agriculture, paper making, and biofuel production, the lignin content should be lowered or the ratio of the more chemically labile S units in lignin should be increased. However, in some case studies, increasing G units in lignin was observed to cause more efficient digestion of cell wall materials.
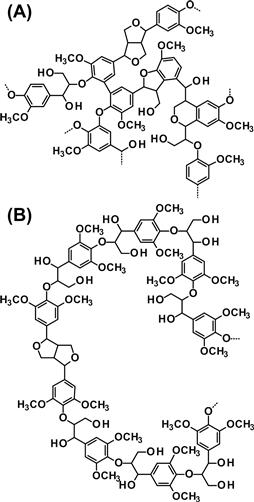
FIGURE 4.11 Partial structures of G lignin and S lignin. (A) In the G lignin, there are many kinds of bonds, carbon–carbon bonds and ether bonds, among the coniferyl alcohol residues. The condensed interunit linkages, which are 5–5 bonds, β-5 bonds, β-1 and β–β bonds, are abundant. The G lignin is a random, three-dimensional network polymer. (B) The S lignin basically contains β-O-4 ether bonds and β–β bonds that link between sinapyl alcohol residues, and it has a linear structure.
Enrichment of the G unit composition without causing total lignin content alteration has been reported to increase alfalfa digestibility (Chen and Dixon, 2007). G units can be enriched by reducing the enzyme activities specific for S unit biosynthesis such as COMT, F5H, and SAD. SAD is a group of CAD that prefers sinapaldehyde and sinapyl alcohol to coniferaldehyde using coniferyl alcohol as the substrate. Therefore, both F5H and COMT are required to supply carbon flux from coniferyl alcohol precursors to sinapyl alcohol, and these hydroxylation and methylation steps determine the ratio of G and S units (S/G) of lignin (Humphreys et al., 1999; Osakabe et al., 1999).
Down-regulation of COMT leads to a dramatic reduction of S unit content in transgenic alfalfa plants that are related to wild-type alfalfa plants but without changing the G unit content or reducing biomass yields (Guo et al., 2001). Similar results were obtained in the transgenic sugarcane (Jung et al., 2012). Down-regulation of COMT brings about another alteration of lignin composition. In COMT down-regulated plants, 5-hydroxyconiferaldehyde is difficult to convert to sinapaldehyde due to the reduced COMT activity; it is a substrate of CAD and can be reduced to 5-hydroxyconiferyl alcohol by CAD. In the presence of peroxidases and/or laccases, 5-hydroxyconiferyl alcohol can also be oxidized as the three major monolignols, and can be linked with major monolignols to be incorporated into the lignin polymer (Marita et al., 2003). As a result, lignin in the COMT down-regulated plants has novel characteristics that partially contribute to the improvement of digestibility for COMT down-regulated alfalfa in which the G unit composition is increased without altering the total lignin content (Chen and Dixon, 2007).
Conversely, the increase of S unit composition may improve the digestibility of lignin. The overexpression of F5H was examined in order to breed S-unit-rich plants that have improved degradability and better saccharification efficiency. In transgenic poplar plants, a significant increase of the S/G value in lignin has been found (Stewart et al., 2009). Lignin with a high S/G value is more flexible than lignin with a low S/G value.
Reduction of the CAD gene expression in lignifying tissues improves pulping efficiency (Baucher et al., 1996) and digestibility (Baucher et al., 1999) but sometimes leads to reduction of lignin or plant growth, whereas CAD reduction in some plants does not cause lignin reduction. CAD reduction, CAD-deficient spontaneous, and artificial mutants in some plant species such as gh2 in rice (Zhang et al., 2006), bm1 in maize, bmr6 in sorghum and cad-n1 in loblolly pine have been reported (MacKay et al., 1997). Lignin composition in these mutants shows dramatic modifications, including increased incorporation of p-hydroxycinnamyl aldehydes (Figure 4.10B) as the substrates of CAD/SAD. These observations indicate that CAD/SAD may modulate lignin composition (Ralph et al., 2001). The modification is seen as a reddish-brown color in the modified lignin that causes visual phenotypes in mutant plants. Rice plants with gh2 show the phenotype of gold hull and internode; maize with bm1 and sorghum with bmr6 have characteristic reddish-brown to tan colored midribs of mutant leaf blades from accumulating reddish-brown to yellow pigment in stalks and roots that is in contrast to the pale green midrib of wild-type leaf blades. The heart wood of CAD-deficient loblolly pine with cad-n1 is brown whereas the wood of wild types is nearly white (MacKay et al., 1997). These similar phenotypes have also been found in some transgenic plants with reduced CAD activity. While no difference in lignin content was reported in maize bm1 lines, bmr-6 mutations in sorghum cause both a decrease in lignin and an increase in cinnamyl aldehydes (Pillonel et al., 1991). Similarly, the rice gh2 mutant and the loblolly pine cad-n1 mutant have decreased lignin contents (MacKay et al., 1997; Zhang et al., 2006). Artificial CAD reduced switchgrass transformants that are saccharified effectively have already been developed (Saathoff et al., 2011).
Furthermore, down-regulation of C3H in alfalfa changes lignin compositions and structures dramatically, and the accumulated lignin in the transgenic plants significantly enhances the digestibility (Reddy et al., 2005).
Results of catalyzing the last step of the monolignol biosynthesis with CCR show that down-regulation of CCR in transgenic poplar (Populus tremula × P. alba) is associated with up to 50% reduced lignin content to result in an orange–brown, often patchy, coloration of the outer xylem (Leple et al., 2007). In the reduced lignin of the transgenic poplar plants, S units are relatively more reduced than G units. The cohesion of the walls is affected particularly at sites that are generally richer in the S units contained in wild-type poplar. Ferulic acid residues incorporated into the lignin via ether bonds have been detected from the lignin in the transgenic poplar, and the xylem coloration is due to the presence of ferulic acid residues in the lignin. The reduced lignin and hemicelluloses levels are associated with an increased proportion of cellulose. Finally, chemical pulping of wood derived from 5-year-old field-grown transgenic lines shows improved pulping characteristics, but the growth is affected in all transgenic lines tested (Leple et al., 2007).
Another strategy proposed for altering lignin composition is to target the plant peroxidase that catalyzes the polymerization of monolignols along with laccases. These enzymes consist of many isozymes and the substrate specificities of plant peroxidases related to lignin biosynthesis are generally broad. Most plant peroxidase can efficiently polymerize coniferyl alcohol but not sinapyl alcohol. However, many S units are contained in natural angiosperm lignin. It is suggested that coniferyl alcohol radicals can oxidize sinapyl alcohol molecules to sinapyl alcohol radicals through a radical mediation mechanism (Sasaki et al., 2004) (Figure 4.12).
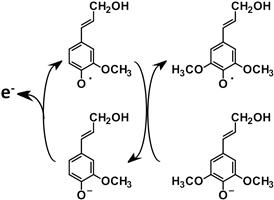
FIGURE 4.12 Coniferyl alcohol radical as a radical mediator to sinapyl alcohol. O• represents the radical atom.
On the other hand, several plant peroxidases, e.g. cationic cell wall-bound peroxidase (CWPO-C) from poplar, can also catalyze oxidization of sinapyl alcohol as a preferable substrate (Sasaki et al., 2004). Where coniferyl alcohol and sinapyl alcohol coexist, sinapyl alcohol molecules are also polymerized through coniferyl alcohol radicals as the radical mediators. Sinapyl alcohol-specific peroxidases are needed to improve lignin degradation through F5H up-regulation. Therefore, these enzymes would be potential targets for lignin engineering in order to control the S/G value of lignin. Peroxidases, which can efficiently oxidize sinapyl alcohol, are thought to have additional substrate-oxidizing sites on the protein surfaces except the heme pockets. PrxA3a, an anionic peroxidase from the hybrid aspen, Populus kitakamiensis, is one such typical plant peroxidase (Osakabe et al., 1995) that can polymerize coniferyl alcohol efficiently but not sinapyl alcohol. Down-regulation of PrxA3a results in decreased G unit content whereas the S unit content is not affected (Li et al., 2003). However, PrxA3a with substituted amino acid residues has additional substrate-oxidizing sites on the protein surface (Yoshinaka and Kawai, 2012). The mutated enzymes of PrxA3a can oxidize and polymerize sinapyl alcohol into high-molecular-weight compounds (see Box 4.3). Therefore, manipulating monolignol-specific peroxidases will lead to new approaches for genetic engineering to modify lignin content and composition.
4.3.4 Decrease in the Degree of Polymerization of Lignin and Addition of Easily Hydrolyzable Linkages into the Lignin Polymer
In addition to down-regulation and up-regulation of lignin biosynthetic enzymes and transcription factors, the incorporation of altered monolignols into lignin biosynthesis has been shown to be a more effective method to create “redesigned” lignin with better saccharification of the plant cell wall.
Polymerization of lignin is initiated by the dehydrogenation of monolignols by peroxidases and/or laccases at the para-hydroxyl (4-OH) site of the aromatic rings (Figure 4.13). By subsequent coupling of the phenoxy radicals to one another or to the growing polymers, low-molecular-weight polymers become high-molecular-weight lignin. In brief, the generation of radical intermediates and formation of ether linkages between the phenylpropane units require the 4-hydroxyl groups of monolignols. Therefore, methylation of the 4-hydroxyl groups of monolignols may lead to low-molecular-weight lignin. To reduce the degree of polymerization in lignin, a novel monolignol 4-O-methyltransferase is created through amino acid residue substitutions on the active site of the original enzyme, i.e. isoeugenol 4-O-methyltransferase (Bhuiya and Liu, 2010). The mutated enzyme can also methylate the 4-hydroxy residue of monolignols, and the resulting 4-O-methylated monolignols (Figure 4.10C) cannot be catalyzed by peroxidases and laccases so that carbon flux to lignin polymerization is prevented. This enzyme will be a useful tool to reduce lignin content and the degree of polymerization of lignin (Bhuiya and Liu, 2010).
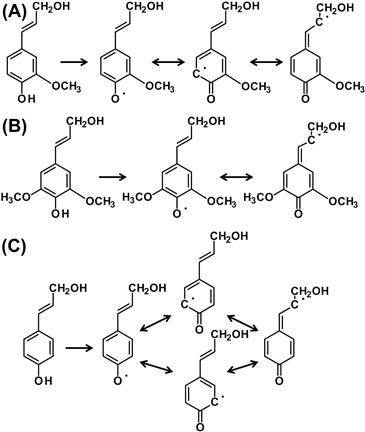
FIGURE 4.13 Radicalization of monolignols.
(A) Dehydrogenation of coniferyl alcohol and the resulted radicals. (B) Dehydrogenation of sinapyl alcohol and the resulted radicals. (C) Dehydrogenation of p-coumaryl alcohol and the resulted radicals. O• and C• represent the radical atoms.
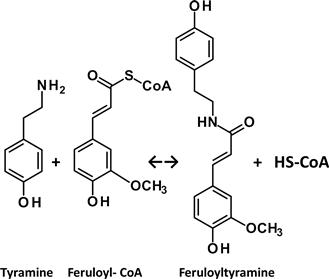
To increase linkages that are easy to hydrolyze in the lignin polymer, amide and ester interunit linkages are introduced into the lignin polymer. The number of amide interunit linkages in a lignin structure can be increased by up-regulating the synthesis of minor monomer units of lignin, e.g. hydroxycinnamic acid amides (HAAs) (Figure 4.14A). The synthesis of HAAs is initiated when plants are wounded or subject to pathogen attack. HAAs are then secreted into the extracellular space, and radicalized like monolignols by peroxidases and laccases (McLusky et al., 1999), and then cross-coupled to lignin in the cell wall. The amide bonds derived from HAAs in the HAA-incorporated lignin become easily hydrolyzable sites, thus improving lignin degradability. The enzyme involved in the last step of HAA biosynthesis is hydroxycinnamoyl-CoA:tyramine N-(hydroxycinnamoyl) transferase (THT; EC 2.3.1.110) (Figure 4.15). The genes for synthesizing THTs have been isolated from various plant species (McLusky et al., 1999). These genes are related to tyrosine decarboxylase (TYDC; EC 4.1.1.25), which has been isolated; tyrosine decaroxylase is involved in another step of HAA biosynthesis, to catalyze tyrosine to tyramine. Transgenic tobacco plants with higher THT and TYDC activities than wild-type plants have been characterized (Hagel and Facchini, 2005). For the addition of ester interunit linkages to the lignin polymer, coniferyl ferulate (Figure 4.14B), a methoxylated analog of coniferyl p-coumarate, is introduced into the maize cell walls (Grabber et al., 2008). Coniferyl ferulate moderately reduces lignification and cell wall ferulate copolymerization with monolignols; cell walls lignified with coniferyl ferulate are easier to hydrolyze with cellulase with and without alkaline pretreatment (Grabber et al., 2008).
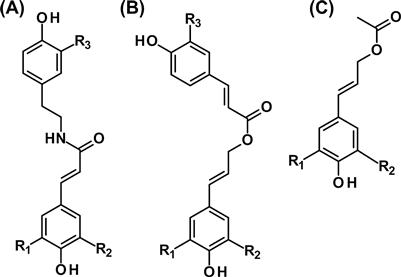
FIGURE 4.14 Monolignol analogs containing easily hydrolyzable bonds.
(A) Hydroxycinnamic acid amides (HAAs): R1 = H, R2 = H, R3 = H, coumaroyltyramine; R1 = H, R2 = H, R3 = OCH3, coumaroyl-3′-methoxytyramine; R1 = OCH3, R2 = H, R3 = H, feruloyltyramine; R1 = OCH3, R2 = H, R3 = OCH3, feruloyl-3′-methoxytyramine; R1 = OCH3, R2 = OCH3, R3 = H, feruloyltyramine; R1 = OCH3, R2 = OCH3, R3 = OCH3, feruloyl-3′-methoxytyramine. (B) Acylated monolignols: R1 = H, R2 = H, R3 = H, coumaryl p-coumarate; R1 = H, R2 = H, R3 = OCH3, p-coumaryl ferulate; R1 = OCH3, R2 = H, R3 = H, coniferyl p-coumarate; R1 = OCH3, R2 = H, R3 = OCH3, coniferyl ferulate; R1 = OCH3, R2 = OCH3, R3 = H, sinapyl p-coumarate; R1 = OCH3, R2 = OCH3, R3 = OCH3, sinapyl ferulate. (C) Acetylated monolignols: R1 = H, R2 = H, p-coumaryl acetate; R1 = H, R2 = OCH3, coniferyl acetate; R1 = OCH3, R2 = OCH3, sinapyl acetate.
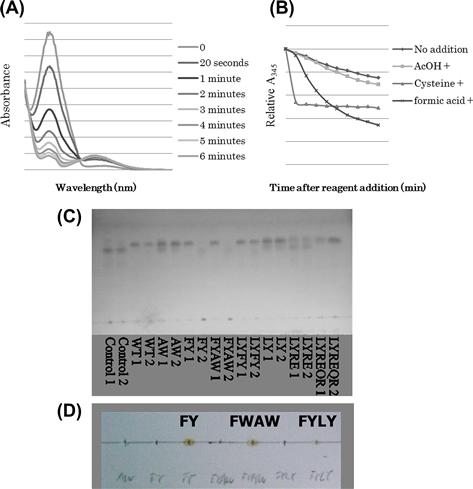
FIGURE 4.16 Sinapyl alcohol polymerizing activities.
Sugar chains of enzymes were removed. Abbreviations [FYAW; PrxA3a F77Y A165W, LY; PrxA3a L182Y, FYLY; PrxA3a F77Y L182Y, LYRE; PrxA3a L182Y R245E, LYREQR; PrxA3a L182Y R245E S178Q N181R]. (A) The peak of A285 decreased rapidly and the peak of A345 increased temporally when the PrxA3a excessive amount (2 μg mL−1) was added. (B) The peak of A345 was derived from syringyl-type quinone methide intermediate because it decreased rapidly by adding the acid, the oxidant, and the reducing agent. (C) 1: After a 6-minute reaction the A285 decrease was not measured. 2: Reaction mixtures incubated overnight were developed on TLC. Spots from reaction mixtures of PrxA3a F77Y, PrxA3a F77Y A165W, and PrxA3a F77Y L182Y were different from the others because those acquired sinapyl alcohol polymerizing activity. The sinapyl alcohol polymerizing activity of PrxA3a F77YL182Y is weaker than those of PrxA3a F77Y and PrxA3a F77Y A165W. (D) Expanded picture for the reaction mixture spotted points of PrxA3a F77Y, PrxA3a F77Y A165W, and PrxA3a F77Y L182Y in (C).
Acylated lignin units including mono- and eudicotyledons were found in the milled wood lignins of angiosperms, but were absent in the gymnosperms analyzed (Del Río et al., 2008). The structure of the lignins from sisal (Agave sisalana), kenaf (Hibiscus cannabinus), abaca (Musa textilis) and curaua (Ananas erectifolius) is remarkable; they are extensively acylated at the γ-carbon of the lignin side chain (up to 80% acylation) with acetate and/or p-coumarate groups and preferentially over S units. Whereas lignins from sisal and kenaf are γ-acylated exclusively with acetate groups, lignins from abaca and curaua are esterified with acetate and p-coumarate groups. The structures of all these highly acylated lignins are characterized by a very high S/G ratio, a large predominance of β-O-4 linkages (up to 94% of all linkages), and a strikingly low proportion of traditional β–β linkages, which indeed are completely absent in lignins from abaca and curaua. The occurrence of β–β homocoupling and cross-coupling products of sinapyl acetate (Figure 4.14C) in the lignins from sisal and kenaf indicates that sinapyl alcohol is acetylated at the monomer stage, and that sinapyl acetate should be considered as a real monolignol involved in the lignification reactions (Del Río et al., 2008). β-O-4 linkages in lignin can be easily cleaved, thereby the highly acylated lignin is thought to be more digestible than the non-acylated lignin.
4.3.5 Production of Cell Wall Degradation Enzymes in Plants
a Production of Lignin Degradation Enzymes in Plants
The introduction of genes for producing microbial lignin degradation enzymes in biomass crops in order to enhance the decomposition of cell walls during and before the saccharification process has been studied. White-rot fungi can oxidize lignin to carbon dioxide because they contain many peroxidases, laccases, and other oxidases responsible for generating reactive molecules to degrade lignin. The genome sequence of a white-rot fungus (Phanerochaete chrysosporium) has been determined that provides much insightful information on biofuel production (Vanden Wymelenberg et al., 2006).
b Cellulase Production in Plants
Another approach is the heterogeneous expression of microbial cellulose degradation enzymes in plants. Cellulases are classified into three groups: endoglucanases, exoglucanases, and β-glucosidases. One suggested method is to produce microbial cellulases in plant cells. The catalytic domain of Acidothermus cellulolyticus thermostable endoglucanase gene (encoding for endo-1,4-β-glucanase enzyme or E1) is constitutively expressed in rice, tobacco, and maize (Biswas et al., 2006; Oraby et al., 2007) that are healthy and develop normally as compared with the wild-type (WT) plants. After thermochemical pretreatment and enzyme digestion, the transformed plants are clearly more digestible than WT and require lower pretreatment severity to achieve comparable conversion levels. Furthermore, the decreased recalcitrance is not due to post-pretreatment residual E1 activity and cannot be reproduced by the addition of exogenous E1 to the biomass prior to pretreatment. This indicates that the expression of E1 during cell wall construction alters the inherent recalcitrance of the cell wall (Oraby et al., 2007). Other solutions have also been suggested. For example, the use of expansin, a hydrogen bond-breaking protein, to loosen the cell wall during plant growth, as a candidate for plant endogenous enzymes to promote cell wall degradation, is being studied.
4.3.6 Conclusions
Recently, breeding of renewable lignocellulosic plant materials that can be saccharified more efficiently and less expensively to produce bioethanol has been studied by altering the content and structure of lignin in the cell walls of these energy crops so that the lignocellulosic materials can be easily decomposed into glucose and pentose. Several approaches have been attempted, and some mutant and transgenic plants are available on the market. Most research efforts have been directed toward up-regulating or down-regulating the enzymes involved in lignin biosynthesis. Some success has been obtained in altering both lignin content and composition to ease lignin decomposition, but many transgenic plants have shown undesirable characteristics such as dwarfing or vascular collapse. Therefore, recent research on the transcriptional factors involved in lignin biosynthesis has focused on controlling the lignin biosynthetic system in addition to modifying lignin for efficient degradation. The addition of new or rare linkages that are easier to hydrolyze to the interunits of lignin polymer has been attempted. Also, there have been several approaches to use in planta production of microbial lignin degradation enzymes in the energy crops to decompose the cell walls during and before the saccharification process.
4.4 Improvement of Woody Biomass
Ryo Funada and Shinya Kajita
4.4.1 Wood and Cell Formation
a Wood Formation by Cambial Activity
Wood has been used for thousands of years as a raw material for timber, furniture, pulp and paper, chemicals, and fuels. In addition, since wood is a major carbon sink, it is expected to play an important role in removing the excess atmospheric CO2 that is generated by burning fossil fuels. Moreover, wood has recently been used as a resource for bioethanol. Therefore, there is still great demand for woody biomass as a renewable source of biomaterial and bioenergy.
Wood is produced by the vascular cambium of trees (Catesson, 1994; Larson, 1994; Funada, 2000, 2008). Cambium is defined as the active layer of active and delicate meristematic tissue between the inner bark or phloem and the wood or xylem that produces the secondary phloem on the outside and the secondary xylem on the inside (Figure 4.17). Cambium consists of fusiform cambial cells and ray cambial cells. The length of fusiform cambial cells varies depending on the species and the age of the cambium; it ranges from 1100 to 4000 μm in conifers and from 170 to 940 μm in hardwoods (Larson, 1994). The periclinal division of cambial cells leads to an increase in the stem diameter of trees. The amount of secondary xylem cells produced is usually much higher than the amount of secondary phloem cells. Thus, mature xylem cells are usually used as wood.
FIGURE 4.17 Light micrograph of a transverse section showing active cambium and differentiating secondary xylem of the main stem in hybrid poplar (Populus sieboldii × P. grandidentata). Arrows: cell division; Ca: cambium; Ph: secondary phloem; R: ray parenchyma cell; V: vessel element; Xy: secondary xylem. (Courtesy of Dr S. Begum.)
The cambial activity of trees exhibits seasonal cycles of activity and dormancy, which are known as annual periodicity, in temperate and cool zones. This periodicity plays an important role in the formation of wood and reflects the environmental adaptivity of trees, i.e. their tolerance to cold in winter in cool and temperate zones. The quantity and quality of wood depend on the division of cambial cells and the differentiation of cambial derivatives. Therefore, details of the cell biological and physiological aspects of the regulation of cambial activity in trees are of considerable interest.
Cambial activity ceases in autumn or winter and the cambial dormancy can be considered to consist of two stages, i.e. rest followed by quiescence (Catesson, 1994; Larson, 1994). The resting stage is maintained by conditions within the tree whereas the quiescent stage is controlled by environmental conditions. The resting stage of dormancy is a physiological state wherein the cambium cannot divide even under favorable growth conditions. By contrast, during the quiescent stage of cambial dormancy, the cambium is able to divide when exposed to appropriate environmental conditions. The transition from rest to quiescence involves structural, histochemical, and functional changes in cambial cells (Lachaud et al., 1999; Samuels et al., 2006).
Cambial activity generally resumes in the spring with a change from the quiescent dormant state to the active state (cambial reactivation). It is regulated by internal factors, such as plant hormones, and environmental factors including temperature, rainfall, and photoperiod. However, with respect to plant hormone auxins, no increase in the level of indole-3-acetic acid (IAA) is detected in the cambial region of conifers at the onset of cambial reactivation. This suggests the absence of a clear relationship between the timing of cambial reactivation and endogenous levels of IAA (Sundberg et al., 1991; Funada et al., 2001a, 2002). Therefore, other factors appear to be involved in cambial reactivation. Recent studies have demonstrated that the timing of cambial reactivation is controlled by ambient temperature, which influences both the quantity and quality of wood (Oribe and Kubo, 1997; Oribe et al., 2001, 2003; Gričar et al., 2006; Begum et al., 2007, 2008, 2010, 2013).
b Process of Cell Wall Formation
As soon as a cambial cell loses the ability to divide, it will start to differentiate into secondary phloem or xylem cells. The stages in the development of secondary xylem cells can be categorized into the following steps: cambial cell division, cell enlargement, cell wall thickening, cell wall sculpturing (formation of modified structure), lignification, and cell death (Panshin and de Zeeuw, 1980; Thomas 1991; Funada, 2000). Fusiform cambial cells differentiate into longitudinal tracheids (tracheids), vessel elements, wood fibers, and axial parenchyma cells, while ray cambial cells differentiate into ray parenchyma cells. In some conifers, such as Pinus, ray cambial cells differentiate into ray tracheids. Cells derived from fusiform cambial cells increase in length and diameter as they approach their final shape during differentiation. For example, tracheids in conifers increase only slightly in length but they increase considerably in radial diameter. Vessel elements do not increase significantly in length but their increases in radial and tangential diameter are conspicuous.
The major component of the very thin (often less than 0.1 μm thick) and plastic cell wall that is characteristic of the stage of cell enlargement is called the primary wall; it is cellulose that is highly crystalline and has very high tensile strength. Thus, cellulose microfibrils form a framework in the cell wall, and the primary wall is considered to consist of loose aggregates of cellulose microfibrils (Harada, 1965; Harada and Côté, 1985). This structure allows relatively unimpeded expansion of the cells derived from the cambium. In addition, the primary wall of cambial cells has a characteristic histochemical composition that allows for considerable extensibility (Catesson, 1990, 1994).
When cell expansion is almost complete, the well-ordered cellulose microfibrils are deposited on the inner surface of the primary wall to form the so-called secondary wall (Harada, 1965). Once the formation of the secondary wall has begun, no further expansion of cells will occur. The secondary xylem cells of woody plants, such as tracheids and wood fibers, have cell walls with a highly organized structure. Continuous deposition of the secondary wall increases the thickness of the cell wall. The thickness of the cell wall varies depending on cell function, cambial age, and the season in which the cell is formed (early wood or late wood; Figure 4.18). In general, cells that function to support the tree form thick secondary walls. The cell wall supports the heavy weight of the tree itself and allows the transport of water from roots to leaves, which can sometimes reach more than 100 m in height. In addition, the cell wall prevents microbial and insect attack, thereby protecting the tree during its very long life that in some cases can exceed thousands of years. The thickness of the cell wall of wood fibers varies depending on species, showing different amounts of fixation of CO2 in cell walls (Figure 4.18). Thus, the ultrastructure of tracheids and wood fibers is of great importance to define the mechanical properties of wood.
FIGURE 4.18 Scanning electron micrographs of transverse section showing tracheids of Chamaecyparis obtusa (A) and wood fibers of Ochroma lagopus (B). (Courtesy of Dr Y. Sano.)
During formation of the secondary wall in tracheids or wood fibers, the cellulose microfibrils change their orientation progressively from a flat helix to a steep Z-helix in a clockwise rotation when viewed from the lumen side. They are oriented at about 5–20° with respect to the cell axis. No cellulose microfibrils with an S-helix are observed during formation of the S2 layer. This shift in the angles of cellulose microfibrils is considered to generate a semi-helicoidal structure (Prodhan et al., 1995a; Abe and Funada, 2005). The concept of a helicoidal pattern has been proposed in the cell walls of numerous plants (Roland and Vian, 1979; Roland et al., 1987). This pattern consists of a series of planes in which the direction of cellulose microfibrils changes progressively. The arc-shaped or bow-shaped patterns observed by transmission electron microscopy in oblique ultrathin sections of tracheids or wood fibers correspond to the helicoidal structure (Roland and Mosiniak, 1983; Prodhan et al., 1995b).
The cellulose microfibrils of the S2 layer are closely aligned, with a high degree of parallelism. The continuous deposition of cellulose microfibrils in one direction produces a thick cell wall layer with a consistent texture. When the rotational change in the orientation of cellulose microfibrils has been arrested, a thick cell wall layer is formed as a result of the repeated deposition of cellulose microfibrils (Roland and Mosiniak, 1983; Roland et al., 1987; Abe et al., 1991; Prodhan et al., 1995a). The thickness of the secondary wall is important in terms of the properties of wood because it is closely related to the specific gravity of wood (Panshin and de Zeeuw, 1980; Zobel and van Buijitenen, 1989). The duration of the arrest in the orientation of cellulose microfibrils seems to determine the thickness of the S2 layer and, thus, the thickness of the secondary wall.
The average microfibril angle in the S2 layer differs among species, and between the radial and tangential wall; it also depends on the time of cell formation. For example, the angles in differentiating early-wood tracheids with respect to the cell axis are 3–14° for Abies sachalinensis, 9–21° for Larix kaempferi, and 17–32° for Picea jezoensis (Abe and Funada, 2005). In addition, the microfibril angles in the S2 layer can vary within the stem; they are usually large in the growth ring near the pith (juvenile wood), and decrease outwards to the bark side with increases in the age of cambium. Moreover, tracheids of compression wood, which is a reaction wood of conifers formed on the lower side of inclined stems in conifers, have large microfibril angles of about 45° with respect to the cell axis (Timell, 1986; Yoshizawa, 1987). These variations in microfibril angles are due to differences in the pattern of orientation of cellulose microfibrils. For example, while the direction of cellulose microfibrils in normal wood tracheids rearranges into a steep Z-helix oriented at about 5–20° from the tracheid axis, the orientation of cellulose microfibrils in compression wood tracheids becomes oblique until the cellulose microfibrils are oriented in a Z-helix about 45° from the tracheid axis. Such a rotation of cellulose microfibrils controls the microfibril angle in the S2 layer, and thus it is one of the most important factors in determining the wood’s properties.
At the final stage of the formation of secondary wall, the orientation of newly deposited cellulose microfibrils changes from a steep Z-helix to a flat helix, with counterclockwise rotation from the outer towards the inner part of the layer when viewed from the lumen side. This corresponds to a directional switch in the orientation of the cellulose microfibrils from clockwise to counterclockwise (when viewed from the lumen side) during formation of the secondary wall. The deposition of cellulose microfibrils in a flat helix results in the formation of an S3 layer to bundle the deposited cellulose microfibrils (Abe et al., 1991, 1992, 1994). This texture differs from that of the S2 layer in which the cellulose microfibrils have a high degree of parallelism. When transverse sections are observed with polarization microscopy, the S3 layer exhibits birefringence, as does the S1 layer. The shift in angles of cellulose microfibrils is more abrupt during the transition from the S2 to the S3 layer as compared to the transition from the S1 to the S2 layer (Harada and Côté, 1985; Abe et al., 1991). The rate of change in the orientation of cellulose microfibrils determines the structure of the cell wall layer.
A model of the orientation of newly deposited cellulose microfibrils in a tracheid is shown schematically in Figure 4.19. The direction of orientation of cellulose microfibrils changes progressively with changing speed of rotation during the formation of the secondary wall (Funada, 2000, 2008).
4.4.2 Effect of Tree Breeding on Wood Quality
a Targets of Tree Breeding on Wood Quality
As mentioned above, wood is produced by the vascular cambium of living trees. Therefore, the wood quality among various species or even within a single species varies significantly depending on environmental conditions such as climate, soil, and the spacing of growing trees, as well as cambial age, stem position, and distance from the crown. The tree-to-tree variability within members of a species under identical growth conditions shows that genetic factors also influence the wood structure. The wood quality as indicated by its mechanical properties is largely due to differences in wood structure. These observations indicate the strong possibility that the wood quality may be improved not only by silvicultural treatments but also by breeding to select genetically desirable trees (Panshin and de Zeeuw, 1980; Zobel and van Buijtenen, 1989; Zobel and Jett, 1995). The molecular biology also has the potential to improve wood quality (Mellerowicz et al., 2001).
The secondary xylem cells of woody plants, such as tracheids, vessel elements, and wood fibers, have cell walls with a highly organized structure. The orientation of cellulose microfibrils in the primary wall determines the direction of cell elongation and expansion, thereby controlling the shape and size of xylem cells. In addition, the orientation of cellulose microfibrils is one of the most important characteristics that determine the physical properties of wood. In particular, microfibril angles of the S2 layer have a significant influence on the strength of wood, and it is possible to control the microfibril angles genetically. The microfibril angles differ significantly among clones of different origin. Thus, detailed screens to select plus trees with low microfibril angles should be included in future breeding programs. In addition, cortical microtubules, which are part of the cytoskeleton, are parallel to the newly deposited cellulose microfibrils and change their orientation progressively during the formation of secondary wall in a similar manner as the formation of cellulose microfibrils (Funada, 2000, 2002, 2008; Funada et al., 2001b; Chaffey, 2002). Therefore, cortical microtubules control the orientation of cellulose microfibrils in the secondary wall of tracheids or wood fibers. The manipulation of cortical microtubules might control microfibril angles of cell walls.
b Woody Biomass is One of the Present Energy Sources
The total forest area of the world is over 4 billion hectares, covering about 30% of the world’s land. Although the total carbon stock of the forest biomass in the world decreased by about 10 Gt from 1990 to 2010, it still amounts to 600 Gt (Global Forest Resources Assessment, 2010). According to the report from the Food and Agriculture Organization of the United Nations (FAO), the global wood production in 2005 was estimated to reach 3.4 billion m3 (Figure 4.20). Fuel wood and industrial roundwood each account for nearly 50% of the total wood production, with about 70% and 90% of wood used as fuel wood in Asia and Africa respectively. In these developing regions, wood is either burned directly or processed into charcoal for fuel. Although burning fuel wood or charcoal directly is the most widespread and typical application of wood, the total heating value stored in fuel wood is not fully and efficiently used. Thus, alternative strategies for using woody biomass more efficiently such as gasification, pyrolysis, and cellulosic ethanol are desirable for the sustainable development of the future world.
FIGURE 4.20 Wood production in the world.
Values are 5-year averages for 2003–2007 (Global Forest Resources Assessment 2010, FAO).
c Lignocellulose: The Main Part of Woody Biomass
In woody plants, about 60–80% of the biomass is made up of cell walls of woody tissue of stems. For example, approximately 75% of the biomass contained in poplars is present in terrestrial parts of stems and branches. Secondary cell wall composed of cellulose, hemicelluloses, and lignin constitutes the main part of the woody biomass. Cellulose consists of polymer with β-O-4-linked glucose units that are bonded to one another via hydrogen bonds to form microfibrils with crystalline properties. Cellulose microfibrils consist of bundles of around 36 cellulose chains that are embedded in a matrix of hemicelluloses and lignin. This chemical complex of cellulose, hemicellulose, and lignin is commonly designated as “lignocellulose”. In recent years, lignocellulose is considered as an alternative source for heat, electricity, fuels, and chemicals in the future. Because the carbon contained in wood has been derived from atmospheric carbon dioxide during the growth of trees, using lignocelluloses as fuel will cycle the carbon between the atmosphere and wood. In other words, using forest resources to replace fossil fuels as energy sources will not increase atmospheric carbon dioxide as does burning fossil fuels. Thus, there are currently significant efforts to improve the productivity and characteristics of woody biomass (trees) by using emerging silvicultural systems and new biotechnology.
4.4.3 Molecular Breeding for Tailoring Lignocellulose
a Strategy for Selection of Desirable Woody Biomass
Since woody biomass has huge potential as raw material for cellulosic ethanol, its saccharification efficiency is becoming one of the target traits for new tree breeding. Unlike corn grains, rice and sugar cane juice, which are typical sources of fermentable sugars for bioethanol production, lignocellulose in woody biomass consists of a larger quantity of lignin and highly crystallized cellulose that are difficult to convert into fermentable sugars (Table 4.4). Although these characteristics favor woody biomasses for combustion, pyrolysis, and gasification, they are less favorable as feedstocks for producing bioethanol. They have recalcitrant characteristics that resist enzymatic, chemical, and physical breakdown. Thus, pretreatments of the woody mass before the saccharification process are necessary in order to enhance the recovery of fermentable sugars from lignocelluloses. Toxic acids, alkalis, and high temperature along with mechanical size reduction are applied to pretreat the biomass to make it susceptible to enzymatic hydrolyses for recovery of fermentable sugars. However, the pretreatment step is the major bottleneck for reducing the cost of bioethanol production. Thus, new types of woody biomass, which are easy to break down into simple components (simple sugars and phenolics), should be developed.
TABLE 4.4
Chemical Composition of Biomasses
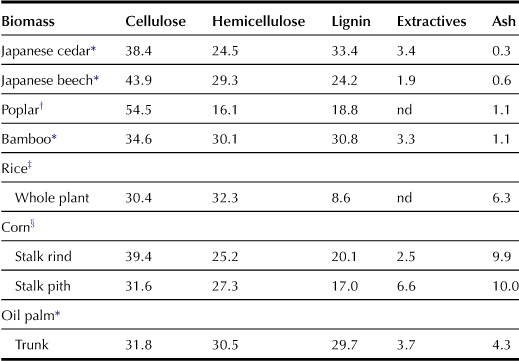
Values are all expressed as percentages (w/w). nd: not determined.
In addition to hydrolysis conditions, efficiency of the saccharification is affected by many other biomass-related factors including cellulose content, degree of cellulose polymerization, chemical heterogeneity of hemicelluloses, content and chemical compositions of lignin. Substantial variations in chemical composition such as cellulose and lignin have been identified within the species of woody plants (Brereton et al., 2010; Studer et al., 2011). These results suggest that identification of low-recalcitrance variants of lignocelluloses for different end-uses can be selected from natural and cultivated forests in the near future. In order to breed improved woody biomasses, several technologies to assist in the selection by using markers such as DNA/RNA, transcripts, and metabolite variations have been developed recently. Mapping populations of willow (138 different genotypes) were used to characterize their variation in enzymatic glucose release from stem and to identify quantitative trait loci (QTL) associated with the saccharification yield (Brereton et al., 2010). Glucose yields after enzymatic hydrolysis varied significantly among the population, and the four QTLs that influence this trait have been identified. Another study examined the effect of lignin characteristics on sugar release from 1100 individual undomesticated Populus trichocarpa trees and concluded that the sugar release is positively affected by lignin content but not by lignin composition (ratio of syringyl to guaiacyl residues) (Studer et al., 2011). These results indicate that there are opportunities to improve tree breeding programs for increasing enzymatic saccharification yields and production of bioethanol.
b Molecular Breeding is an Attractive Tool for Tailoring Lignocellulose to Fitting Saccharification
The approach to modify gene expression such as antisense RNA, RNA interference, and overexpression of any genes will provide easier ways for genetic modification of cell wall biosynthetic pathway to develop new species of woody plants with higher saccharification characteristics. Three main cell wall components, i.e. cellulose, hemicelluloses, and lignin, should be targeted for the modification of the cell wall architecture.
Cellulose microfibrils have high crystallinity; therefore, their surface area is restricted for hydrolysis enzymes to act upon. On the other hand, the cellulose microfibrils also have paracrystalline regions that have a greater surface area exposed to degrading enzymes. Thus, increasing the portion of paracrystalline regions in cellulose microfibrils may improve the efficiency of saccharification of the woody biomass. Kaida et al. (2009a) overexpressed a gene for poplar cellulase in a woody species, sengon (Paraserianthes falcataria). Although the crystallinity of cellulose molecules had not been characterized, one of the resultant transgenic plants exhibits 1.4-fold more efficiency in cellulose hydrolysis than a wild-type plant. This modification also contributes to increasing the subsequent ethanol production from the transgenic plants.
Modification of the hemicellulose is another option for improving the saccharification characteristics of woody biomass. This cell wall component plays a key role in the attachment of cellulose and lignin to the surface of the microfibrils for building up the massive complex. Kaida et al. (2009b) introduced three different genes for hemicellulases, xyloglucanase, xylanase, and galactanase independently into poplar plants. Among the genes tested, the expression of the genes for xyloglucanase and xylanase exhibits a positive effect of saccharification in the transgenic poplar plants. The amount of glucose released from the transgenic biomasses increases significantly to reach 1.8- (xyloglucanase) and 1.7-fold (xylanase) higher than the wild-type plant. Increased susceptibility of cellulose in the xyloglucanase-expressing poplar may be due, at least in part, to enlargement of the cellulose microfibrils in the cell walls. Recently, 24–44% reduction of above-ground biomass of the xyloglucanase-expressing poplar has been revealed in field trials carried out in Japan. Cell wall modification without any detrimental effects to plant growth and development should be future tasks to be achieved for the genetic engineering of cell wall polysaccharides.
Lignin, the third component of the secondary cell wall, is a hydrophobic biopolymer that provides a barrier for enzymes to access cellulose. Reduction of lignin content and modification of its molecular structure is expected to increase the capability of cellulytic enzymes to attack the cell wall structure. Numerous studies on the increment of saccharification efficiency of lignocelluloses via the lignin modification have already been reported. However, most of these studies concern grass and herbaceous plants (see Section 4.3). A successful example of the transgenic woody biomass with modification of lignin for improving saccharification efficiency has been reported by Wang H. et al. (2012). In this study, the biomass characteristics were analyzed with stem prepared from 5-year-old transgenic poplar plants cultivated in a field. Transgenic plants with down-regulation of caffeoyl CoA 3-O-methyltransferase (CCoAOMT) gene exhibit 7–10% reduction in lignin content as compared to wild-type poplar. The yield of total sugar released from the transgenic biomasses by enzyme saccharification shows significant increase after pretreatments both with and without 1% sodium hydroxide solution. These results suggest that lignin modification contributes to both increase of sugar recovery from woody biomass and decrease of chemical consumption in the pretreatments.
References
1. Abe H, Funada R. The orientation of cellulose microfibrils in the cell walls of tracheids in conifer: A model based on observations by field emission-scanning electron microscopy. IAWA Journal. 2005;26:161–174.
2. Abe H, Ohtani J, Fukazawa K. FE-SEM observation on the microfibrillar orientation in the secondary wall of tracheids. IAWA Bull New Series. 1991;12:431–438.
3. Abe H, Ohtani J, Fukazawa K. Microfibrillar orientation of the innermost surface of conifer tracheid walls. IAWA Bull New Series. 1992;13:411–417.
4. Abe H, Ohtani J, Fukazawa K. A scanning electron microscopic study of changes in microtubule distributions during secondary wall formation in tracheids. IAWA Journal. 1994;15:185–189.
5. Adachi S, Nito N, Kondo M, et al. Identification of chromosomal regions involving in the rate of photosynthesis of rice leaves by using a progeny from japonica and high-yielding indica varieties. Plant Production Science. 2011;14:118–127.
6. Adachi S, Nakae T, Uchida M, et al. The mesophyll anatomy enhancing CO2 diffusion is a key trait for improving rice photosynthesis. Journal of Experimental Botany. 2013;64(4):1061–1072.
7. Adati S. Studies on the genus Miscanthus with special reference to the Japanese species for breeding purpose as fodder crops. Bulletin of the Faculty of Agriculture, Mie University. 1958;17:1–112.
8. Bassam NEI. Enery plant species. London: Science Publishers; 1998.
9. Baucher M, Chabbert B, Pilate G, et al. Red xylem and higher lignin extractability by down-regulating a cinnamyl alcohol dehydrogenase in poplar. Plant Physiology. 1996;112:1479–1490.
10. Baucher M, Bernard-Vailhe MA, Chabbert B, et al. Down-regulation of cinnamyl alcohol dehydrogenase in transgenic alfalfa (Medicago sativa L.) and the effect on lignin composition and digestibility. Plant Molecular Biology. 1999;39:437–447.
11. Begum S, Nakaba S, Oribe Y, Kubo T, Funada R. Induction of cambial reactivation by localized heating in a deciduous hardwood hybrid poplar (Populus sieboldii × P. grandidentata). Annals of Botany. 2007;100:439–447.
12. Begum S, Nakaba S, Bayramzadeh V, Oribe Y, Kubo T, Funada R. Responses to ambient temperature of cambial reactivation and xylem differentiation in hybrid poplar (Populus sieboldii × P. grandidentata) under natural conditions. Tree Physiology. 2008;28:1813–1819.
13. Begum S, Nakaba S, Oribe Y, Kubo T, Funada R. Changes in the localization and levels of starch and lipids in cambium and phloem during cambial reactivation by artificial heating of main stems of Cryptomeria japonica trees. Annals of Botany. 2010;106:885–895.
14. Begum S, Nakaba S, Yamagishi Y, Oribe Y, Funada R. Regulation of cambial activity in relation to environmental conditions: Understanding the role of temperature in wood formation of trees. Physiologica Plantarum. 2013;147(1):46–54.
15. Besseau S, Hoffmann L, Geoffroy P, Lapierre C, Pollet B, Legrand M. Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. Plant Cell. 2007;19:148–162.
16. Bhuiya MW, Liu CJ. Engineering monolignol 4-O-methyltransferases to modulate lignin biosynthesis. The Journal of Biological Chemistry. 2010;285:277–285.
17. Biswas GCG, Ransom C, Sticklen M. Expression of biologically active Acidothermus cellulolyticus endoglucanase in transgenic maize plants. Plant Science. 2006;171:617–623.
18. Brereton NJB, Pitre FE, Hanley SJ, Ray MJ, Karp A, Murphy RJ. QTL mapping of enzymatic saccharification in short rotation coppice willow and its independence from biomass yield. Bioenergy Research. 2010;3:251–261.
19. Catesson AM. Cambial cytology and biochemistry. In: Iqbal M, ed. The vascular cambium. Taunton: Research Studies Press; 1990;63–112.
20. Catesson AM. Cambial ultrastructure and biochemistry: changes in relation to vascular tissue differentiation and the seasonal cycle. International Journal of Plant Sciences. 1994;155:251–261.
21. Chaffey N. Immunolocalisation of the cytoskeleton in the secondary vascular system of angiosperm trees and its visualization using epifluorescence microscopy. In: Chaffey N, ed. Wood formation in trees: Cell and molecular biology techniques. London: Taylor & Francis; 2002;113–142.
22. Chen F, Dixon RA. Lignin modification improves fermentable sugar yield for biofuel production. Nature Biotechnology. 2007;25:759–761.
23. Danalatos NG, Archontoulis SV, Mitsios I. Potential growth and biomass productivity of Mischanthus × giganteus as affected by plant density and N-fertilization in central Greece. Biomass and Bioenergy. 2007;31:145–152.
24. Del Río JC, Rencoret J, Marques G, et al. Highly acylated (acetylated and/or p-coumaroylated) native lignins from diverse herbaceous plants. Journal of Agricultural and Food Chemistry. 2008;56:9525–9534.
25. Dohleman FG, Long SP. More productive than maize in the midwest: How does Miscanthus do it? Plant Physiology. 2010;150:2104–2115.
26. Evans LT. Crop evolution, adaptation and yield. Cambridge: Cambridge University Press; 1993.
27. FAOSTAT. FAO. 2010; In: <http://faostat.fao.org/site/567/default.aspx#ancor>; 2010.
28. Funada R. Control of wood structure. In: Nick P, ed. Plant microtubules. Berlin: Springer; 2000;51–81.
29. Funada R. Immunolocalisation and visualisation of the cytoskeleton in gymnosperms using confocal laser scanning microscopy (CLSM). In: Chaffey N, ed. Wood formation in trees: Cell and molecular biology techniques. London: Taylor & Francis; 2002;143–157.
30. Funada R. Microtubules and the control of wood formation. In: Nick P, ed. Plant microtubules. Berlin: Springer; 2008;83–119.
31. Funada R, Kubo T, Tabuchi M, Sugiyama T, Fushitani M. Seasonal variations in endogenous indole-3-acetic acid and abscisic acid in the cambial region of Pinus densiflora stems in relation to earlywood–latewood transition and cessation of tracheid production. Holzforschung. 2001a;55:128–134.
32. Funada R, Miura H, Shibagaki M, et al. Involvement of localized cortical microtubules in the formation of modified structure of wood. Journal of Plant Research. 2001b;114:491–497.
33. Funada R, Kubo T, Sugiyama T, Fushitani M. Changes in levels of endogenous plant hormones in cambial regions of stems of Larix kaempferi at the onset of cambial activity in springtime. Journal of Wood Science. 2002;48:75–80.
34. Gardner FP, Pearce RB, Mitchell RL. Physiology of crop plants. Ames: Iowa State University Press; 1985.
35. Global Forest Resources Assessment. Food and Agriculture Organization of the United Nations (FAO). 2010; In: <http://www.fao.org/forestry/fra/fra2010/en/>; 2010.
36. Grabber JH, Hatfield RD, Lu F, Ralph J. Coniferyl ferulate incorporation into lignin enhances the alkaline delignification and enzymatic degradation of cell walls. Biomacromolecules. 2008;9:2510–2516.
37. Gričar J, Zupančič M, Čufar K, Koch G, Schmitt U, Oven P. Effect of local heating and cooling on cambial activity and cell differentiation in the stem of Norway spruce (Picea abies). Annals of Botany. 2006;97:943–951.
38. Guidi W, Tozzini C, Bonari E. Estimation of chemical traits in poplar short-rotation coppice at stand level. Biomass and Bioenergy. 2009;33:1703–1709.
39. Guo D, Chen F, Wheeler J, et al. Improvement of in-rumen digestibility of alfalfa forage by genetic manipulation of lignin O-methyltransferases. Transgenic Research. 2001;10:457–464.
40. Hagel JM, Facchini PJ. Elevated tyrosine decarboxylase and tyramine hydroxycinnamoyl transferase levels increase wound-induced tyramine-derived hydroxycinnamic acid amide accumulation in transgenic tobacco leaves. Planta. 2005;221:904–914.
41. Hamelinck CN, van Hooijdonk G, Faaij APC. Ethanol from lignocellulosic biomass: techno-economic performance in short-, middle- and long-term. Biomass and Bioenergy. 2005;28:384–410.
42. Harada H. Ultrastructure and organization of gymnosperm cell walls. In: Côté Jr WA, ed. Cellular ultrastructure of woody plants. New York: Syracuse University Press; 1965;215–233.
43. Harada H, Côté Jr WA. Structure of wood. In: Higuchi T, ed. Biosynthesis and biodegradation of wood components. Orlando, FL: Academic Press; 1985;1–42.
44. Harris JM, Meylan BA. The influence of microfibril angle on longitudinal and tangential shrinkage in Pinus radiata. Holzforschung. 1965;19:144–153.
45. Hay R, Porter J. The physiology of crop yield. Oxford: Blackwell Publishing; 2006.
46. He X, Hall MB, Gallo-Meagher M, Smith RL. Improvement of forage quality by downregulation of maize O-methyltransferase. Crop Science. 2003;43:2240–2251.
47. Heaton EA, Dohleman FG, Long SP. Meeting US biofuel goals with less land: The potential of Miscanthus. Global Change Biology. 2008;14:2000–2014.
48. Hidema J, Makino A, Mae T, Ojima K. Photosynthetic characteristics of rice leaves aged under different irradiances full expansion through senescence. Plant Physiology. 1991;97:1287–1293.
49. Hirasawa T, Hsiao TH. Some characteristics of reduced leaf photosynthesis at midday in maize growing in the field. Field Crops Research. 1999;62:53–62.
50. Hirasawa T, Ozawa S, Taylaran RD, Ookawa T. Varietal differences in rates of leaf photosynthesis in rice plants, with special reference to the nitrogen content of leaves. Plant Production Science. 2010;13:53–57.
51. Horie T, Sakuratani T. Studies on crop-weather relationship model in rice (1) Relation between absorbed solar radiation by the crop and the dry matter production. Journal of Agricultural Meteorology. 1985;40:331–342.
52. Houghton J, Weatherwax S, Ferrell J, eds. Breaking the biological barriers to cellulosic ethanol: a joint research agenda A Research Roadmap Resulting from the Biomass to Biofuels Workshop. Rockville, MD: US Department of Energy Office of Science and Office of Energy; 2005; December 7–9, 2005.
53. Hu WJ, Harding SA, Lung J, et al. Repression of lignin biosynthesis promotes cellulose accumulation and growth in transgenic trees. Nature Biotechnology. 1999;17:808–812.
54. Humphreys JM, Hemm MR, Chapple C. New routes for lignin biosynthesis defined by biochemical characterization of recombinant ferulate 5-hydroxylase, a multifunctional cytochrome P450-dependent monooxygenase. Proceedings of the National Academy of Sciences USA. 1999;96:10045–10050.
55. Ishihara K. Eco-physiological characteristics of high yielding cultivars in crop plants – a case study of the rice plant. Japanese Journal of Crop Science. 1996;65i(Extra issue 2):321–326.
56. Jessup RW. Development and status of dedicated energy crops in the United States. In Vitro Cellular & Developmental Biology. 2009;45:282–290.
57. Jin SY, Chen HZ. Structural properties and enzymatic hydrolysis of rice straw. Process Biochemistry. 2006;41:1261–1264.
58. Jung JH, Fouad WM, Vermerris W, Gallo M, Altpeter F. RNAi suppression of lignin biosynthesis in sugarcane reduces recalcitrance for biofuel production from lignocellulosic biomass. Plant Biotechnology Journal. 2012;10:1067–1076.
59. Kaida R, Kaku T, Baba K, Hartati S, Sudarmonowati E, Hayashi T. Enhancement of saccharification by overexpression of poplar cellulase in sengon. Journal of Wood Science. 2009a;55:435–440.
60. Kaida R, Kaku T, Baba K, et al. Loosening xyloglucan accelerates the enzymatic degradation of cellulose in wood. Molecular Plant. 2009b;2:904–909.
61. Karp A, Shield I. Bioenergy from plants and the sustainable yield challenge. New Phytologist. 2008;179:15–32.
62. Kawamitsu Y, Fukuzawa Y, Ueno M, Komiya Y, Sugimoto A, Matsuoka M. Nutrient uptake characteristics and photosynthetic rate in Monster cane. Japanese Journal of Tropical Agriculture. 2003;47(Extra 2):49–50.
63. Kumura A. Physiology of high-yielding rice plants from the viewpoint of dry matter production and its partitioning. In: Matsuo T, Kumazawa K, Ishii R, Ishihara K, Hirata H, eds. Science of the rice plant Vol 2 Physiology. Tokyo: Food and Agriculture Policy Research Center; 1995;691–696.
64. Kuroda E, Ookawa T, Ishihara K. Analysis on difference of dry matter production between rice cultivars with different plant height in relation to gas diffusion inside stands. Japanese Journal of Crop Science. 1989;58:374–382.
65. Lachaud S, Catesson AM, Bonnemain JL. Structure and functions of the vascular cambium. Comptes Rendus de l Acadmie des Sciences – Series III – Sciences Vie (Paris). 1999;322:633–724.
66. Larson PR. The vascular cambium: development and structure. Heidelberg: Springer; 1994.
67. Leple JC, Dauwe R, Morreel K, et al. Downregulation of cinnamoyl-coenzyme A reductase in poplar: Multiple-level phenotyping reveals effects on cell wall polymer metabolism and structure. Plant Cell. 2007;19:3669–3691.
68. Lewandowski I, Clifton-Brown JC, Scurlock JMO, Huisman W. Mischanthus: European experience with a novel energy crop. Biomass and Bioenergy. 2000;19:209–227.
69. Li Y, Kajita S, Kawai S, Katayama Y, Morohoshi N. Downregulation of an anionic peroxidase in transgenic aspen and its effect on lignin characteristics. Journal of Plant Research. 2003;116:175–182.
70. Li Z, Zhai H, Zhang Y, Yu L. Cell morphology and chemical characteristics of corn stover fractions. Industrial Crops and Products. 2012;37:130–136.
71. Lichts FO. Industry statistics: 2010 world fuel ethanol production. Renewable Fuels Association 2010.
72. MacKay JJ, O’Malley DM, Presnell T, et al. Inheritance, gene expression, and lignin characterization in a mutant pine deficient in cinnamyl alcohol dehydrogenase. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(15):8255–8260.
73. Makino A. Photosynthesis, grain yield, and nitrogen utilization in rice and wheat. Plant Physiology. 2011;155:125–129.
74. Mann CC. Crop scientists seek a new revolution. Science. 1999;283:310–314.
75. Marita JM, Ralph J, Hatfield RD, Guo D, Chen F, Dixon RA. Structural and compositional modifications in lignin of transgenic alfalfa down-regulated in caffeic acid 3-O-methyltransferase and caffeoyl coenzyme A 3-O-methyltransferase. Phytochemistry. 2003;62:53–65.
76. McLaughlin B, Samuel B, Kszos LA. Development of switchgrass (Panicum virgatum) as a bioenergy feed stock in the United States. Biomass and Bioenergy. 2005;28:515–535.
77. McLaughlin SB, Walsh ME. Evaluating environmental consequences of producing herbaceous crops for bioenergy. Biomass and Bioenergy. 1998;14:317–324.
78. McLusky SR, Bennett MH, Beale MH, Lewis MJ, Gaskin P, Mansfield JW. Cell wall alterations and localized accumulation of feruloyl-3′-methoxytyramine in onion epidermis at sites of attempted penetration by Botrytis allii are associated with actin polarisation, peroxidase activity and suppression of flavonoid biosynthesis. Plant Journal. 1999;17:523–534.
79. Mellerowicz EJ, Baucher M, Sundberg B, Boerjan W. Unravelling cell wall formation in the woody dicot stem. Plant Molecular Biology. 2001;47:239–274.
80. Ministry of Agriculture. Brazilian Agroenergy Plan 2006–2011. Brasilia: Embrapa Publishing House; 2006.
81. Monsi M, Saeki T. Über den Lichtfaktor in den Pflanzengesellschaften und seine Bedeutung für die Stoffproduktion. The Journal of Japanese Botany. 1953;14:22–52.
82. Mukouyama T, Motobayashi T, Chosa T, et al. Dry matter production and grain yield of high yielding rice cultivar, Takanari, directly seeded in the paddy field with an “Air-assisted Drill”: Effects of planting pattern on ecophysiology of direct seeded rice. Japanese Journal of Crop Science. 2012;81:414–423.
83. Naidu SL, Moose SP, Al-Shoaibi AK, Raines CA, Long SP. Cold tolerance of C4 photosynthesis in Miscanthus × giganteus: Adaptation in amounts and sequence of C4 photosynthetic enzymes. Plant Physiology. 2003;132:1688–1697.
84. Ookawa T, Yasuda K, Seto M, et al. Biomass production and lodging resistance in ‘Leaf Star’, a new long-culm rice forage cultivar. Plant Production Science. 2010a;13:58–66.
85. Ookawa T, Hobo T, Yano M, et al. New approach for rice improvement using a pleiotropic QTL gene for lodging resistance and yield. Nature Communication. 2010b;1:1–11.
86. Oraby H, Venkatesh B, Dale B, et al. Enhanced conversion of plant biomass into glucose using transgenic rice-produced endoglucanase for cellulosic ethanol. Transgenic Research. 2007;16:739–749.
87. Oribe Y, Kubo T. Effect of heat on cambial reactivation during winter dormancy in evergreen and deciduous conifers. Tree Physiology. 1997;17:81–87.
88. Oribe Y, Funada R, Shibagaki M, Kubo T. Cambial reactivation in the partially heated stem in an evergreen conifer. Abies sachalinensis Planta. 2001;212:684–691.
89. Oribe Y, Funada R, Kubo T. Relationships between cambial activity, cell differentiation and the localization of starch in storage tissues around the cambium in locally heated stems of Abies sachalinensis (Schmidt) Masters. Trees. 2003;17:185–192.
90. Osakabe K, Koyama H, Kawai S, Katayama Y, Morohoshi N. Molecular cloning of two tandemly arranged peroxidase genes from Populus kitakamiensis and their differential regulation in the stem. Plant Molecular Biology. 1995;28:677–689.
91. Osakabe K, Tsao CC, Li L, et al. Coniferyl aldehyde 5-hydroxylation and methylation direct syringyl lignin biosynthesis in angiosperms. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8955–8960.
92. Panshin AJ, de Zeeuw C. Textbook of wood technology. 4th ed. New York: McGraw-Hill; 1980.
93. Pedersen JF, Vogel KP, Funnell DL. Impact of reduced lignin on plant fitness. Crop Science. 2005;45:812–819.
94. Peng S, Yang J, Garcia FV, et al. Physiology-based crop management for yield maximization of hybrid rice. In: Virmani SS, A.Siddiq E, Muralidharan, eds. Advances in hybrid rice technology. Los Banos: IRRI; 1998; (pp. 157–176).
95. Pillonel C, Mulder M, Boon J, Forster B, Binder A. Involvement of cinnamyl-alcohol dehydrogenase in the control of lignin formation in Sorghum bicolor (L.) Moench. Planta. 1991;185:538–544.
96. Prodhan AKMA, Funada R, Ohtani J, Abe H, Fukazawa K. Orientation of microfibrils and microtubules in developing tension-wood fibers of Japanese ash (Fraxinus mandshurica var japonica). Planta. 1995a;196:577–585.
97. Prodhan AKMA, Ohtani J, Funada R, Abe H, Fukazawa K. Ultrastructural investigation of tension wood fibre in Fraxinus mandshurica Rupr var japonica Maxim. Annals of Botany. 1995b;75:311–317.
98. Rabemanolontsoa H, Ayada S, Saka S. Quantitative method applicable for various biomass species to determine their chemical composition. Biomass and Bioenergy. 2011;35:4630–4635.
99. Ralph J, Lapierre C, Marita JM, et al. Elucidation of new structures in lignins of CAD- and COMT-deficient plants by NMR. Phytochemistry. 2001;57:993–1003.
100. Reddy MSS, Chen F, Shadle G, Jackson L, Aljoe H, Dixon RA. Targeted down-regulation of cytochrome P450 enzymes for forage quality improvement in alfalfa (Medicago sativa L.). Proceedings of the National Academy of Sciences of the United States of America. 2005;102:16573–16578.
101. Roland JC, Mosiniak M. On the twisting pattern, texture and layering of the secondary cell walls of lime wood proposal of an unifying model. IAWA Bull New Series. 1983;4:15–26.
102. Roland JC, Vian B. The wall of the growing plant cell: Its three dimensional organization. International Review of Cytology. 1979;61:129–166.
103. Roland JC, Reis D, Vian B, Satiat-Jeunemaitre B, Mosiniak M. Morphogenesis of plant cell walls at the supermolecular level: Internal geometry and versatility of helicoidal expression. Protoplasma. 1987;140:75–91.
104. Saathoff AJ, Sarath G, Chow EK, Dien BS, Tobias CM. Tobias: Downregulation of cinnamyl-alcohol dehydrogenase in switchgrass by RNA silencing results in enhanced glucose release after cellulase treatment. PLoS One. 2011;6:e16416.
105. Samuels AL, Kaneda M, Rensing KH. The cell biology of wood formation: From cambial divisions to mature secondary xylem. Canadian Journal of Botany. 2006;84:631–639.
106. San-oh Y, Mano Y, Ookawa T, Hirasawa T. Comparison of dry matter production and associated characteristics between direct-sown and transplanted rice plants in a submerged paddy field and relationships to planting patterns. Field Crops Research. 2004;87:43–58.
107. Sarath G, Mitchell RB, Sattler SE, et al. Opportunities and roadblocks in utilizing forages and small grains for liquid fuels. Journal of Industrial Microbiology and Biotechnology. 2008;35:343–354.
108. Sasaki S, Nishida T, Tsutsumi Y, Kondo R. Lignin dehydrogenative polymerization mechanism: a poplar cell wall peroxidase directly oxidizes polymer lignin and produces in vitro dehydrogenative polymer rich in β-O-4 linkage. FEBS Letters. 2004;562:197–201.
109. Simmons BA, Loqué D, Ralph J. Advances in modifying lignin for enhanced biofuel production. Current Opinion in Plant Biology. 2010;13:313–320.
110. Soejima H, Sugiyama T, Ishihara K. Changes in the chlorophyll contents of leaves and in levels of cytokinins in root exudates during ripening of rice cultivars Nipponbare and Akenohoshi. Plant Physiology. 1995;36:1105–1114.
111. Stewart JJ, Akiyama T, Chapple C, Ralph J, Mansfield SD. The effects on lignin structure of overexpression of ferulate 5-hydroxylase in hybrid poplar. Plant Physiology. 2009;150:621–635.
112. Sticklen M. Plant genetic engineering to improve biomass characteristics for biofuels. Current Opinion in Biotechnology. 2006;17:315–319.
113. Studer MH, DeMartini JD, Davis MF, et al. Lignin content in natural Populus variants affects sugar release. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6300–6305.
114. Sundberg B, Little CHA, Cui K, Sandberg G. Level of endogenous indole-3-acetic acid in the stem of Pinus sylvestris in relation to the seasonal variation of cambial activity. Plant, Cell & Environment. 1991;14:241–246.
115. Taylaran RD, Ozawa S, Miyamoto N, Ookawa T, Hirasawa T. Performance of a high-yielding modern rice cultivar Takanari and several old and new cultivars grown with and without chemical fertilizer in a submerged paddy field. Plant Production Science. 2009;12:365–380.
116. Taylaran RD, Adachi S, Ookawa T, Usuda H, Hirasawa T. Hydraulic conductance as well as nitrogen accumulation plays a role in the higher rate of leaf photosynthesis of the most productive variety of rice in Japan. Journal of Experimental Botany. 2011;62:4067–4077.
117. Thomas RJ. Wood: formation and morphology. In: Lewin M, Goldstein IS, eds. Wood structure and composition. New York: Marcel Dekker; 1991;7–47.
118. Tilman D, Hill J, Lehman C. Carbon-negative biofuels from low-input high-diversity grassland biomass. Science. 2006;314:1598–1600.
119. Timell TE. Compression Wood in Gymnosperms. (Vol. 1). Berlin: Springer; 1986.
120. Torney F, Moeller L, Scarpa A, Wang K. Genetic engineering approaches to improve bioethanol production from maize. Current Opinion in Biotechnology. 2007;18:193–199.
121. US Department of Energy. Breaking the biological barriers to cellulosic ethanol A research roadmap resulting from the biomass to biofuels workshop. Rockville, MD: US Department of Energy; 2006.
122. Vanden Wymelenberg A, Sabat G, Mozuch M, Kersten PJ, Cullen D, Blanchette RA. Structure, organization, and transcriptional regulation of a family of copper radical oxidase genes in the lignin-degrading basidiomycete Phanerochaete chrysosporium. Applied and Environmental Microbiology. 2006;72:4871–4877.
123. Vega-Sanchez ME, Ronald PC. Genetic and biotechnological approaches for biofuel crop improvement. Current Opinion in Biotechnology. 2010;21:218–224.
124. Vignols F, Rigau J, Torres MA, Capellades M, Puigdomènech P. The brown midrib3 (bm3) mutation in maize occurs in the gene encoding caffeic acid O-methyltransferase. Plant Cell. 1995;7:407–416.
125. Vogel KP, Bredja JJ, Walters DT, Buxton DR. Switchgrass biomass production in the Midwest USA: Harvest and nitrogen management. Agronomy Journal. 2002;94:413–420.
126. Waclawovsky AJ, Sato PM, Lembke CG, Moore PH, Souza GM. Sugarcane for bioenergy production: An assessment of yield and regulation of sucrose content. Plant Biotechnology Journal. 2010;8:263–276.
127. Wang D, Portis Jr AR, Moose SP, Long SP. Cool C4 photosynthesis: Pyruvate Pi dikinase expression and activity corresponds to the exceptional cold tolerance of carbon assimilation in Miscanthus × giganteus. Plant Physiology. 2008;148:557–567.
128. Wang H, Xue Y, Chen Y, Li R, Wei J. Lignin modification improves the biofuel production potential in transgenic Populus tomentosa. Industrial Crops and Products. 2012;37:170–177.
129. Wang Q. Time for commercializing non-food biofuel in China. Renewable and Sustainable Energy Reviews. 2011;15:621–629.
130. Weng JK, Li X, Bonawitz ND, Chapple C. Emerging strategies of lignin engineering and degradation for cellulosic biofuel production. Current Opinion in Biotechnology. 2008;19:166–172.
131. Xu B, Escamilla-Treviño LL, Sathitsuksanoh N, et al. Silencing of 4-coumarate:coenzyme A ligase in switchgrass leads to reduced lignin content and improved fermentable sugar yields for biofuel production. New Phytologist. 2011;192:611–625.
132. Yamamoto T, Yonemaru J, Yano M. Towards the understanding of complex traits in rice: Substantially or superficially? DNA Research. 2009;16:141–154.
133. Yoshinaka K, Kawai S. Mutagenesis, heterogeneous gene expression, and purification and amino acid substitution analyses of plant peroxidase, PrxA3a. Journal of Wood Science. 2012;58:231–242.
134. Yoshizawa N. Cambial responses to the stimulus of inclination and structural variation of compression wood tracheids in gymnosperms. Bulletin of the Utsunomiya University Forests. 1987;23:23–141.
135. Zhang K, Qian Q, Huang Z, et al. GOLD HULL AND INTERNODE2 encodes a primarily multifunctional cinnamyl-alcohol dehydrogenase in rice. Plant Physiology. 2006;140:972–983.
136. Zobel BJ, Jett JB. Genetics of wood production. Berlin: Springer; 1995.
137. Zobel BJ, van Buijtenen JP. Wood variation: Its causes and control. Berlin: Springer; 1989.