Chapter 16
Insulating Oils for Transformers 1
16.1. Introduction
Insulating oils are used in electrical engineering as impregnators of cellulose insulations or as a filling product for various electrical equipment: transformers (power, distribution, traction, furnace, instruments, etc.), reactors, capacitors, cables, crossings, circuit-breakers, tap-changers, etc. [BER 97]. The volumes of oil which are used vary from a few liters for capacitors to several tens of thousand liters for power transformers. The main role of this oil is to remove the air and other gases so as to improve the dielectric behavior of the solid insulation. However, in many applications, they are used to ensure both the electrical insulation and the heat transfer of a component or a system, as in the case of transformers.
Oils can also be used for their capability of extinguishing electric arc (in the arcing chambers of switches and some circuit-breakers), thanks to their lubricating power for materials containing pieces in motion (plug selectors, immersion pumps) and in certain cases to improve resistance to fire, as is the case for distribution transformers near populations. Finally, they are used to slow down direct oxidation of cellulose insulation.
Mineral oil is the most commonly used liquid in power transformers, both for its physico-chemical properties and for its low cost [ROU 98]. Originally, the electric insulation of transformers was ensured by non-impregnated natural products in direct contact with air, which is a rapid source of oxidation. In 1854, the immersion of coils in turpentine [ABD 68] enabled their dielectric performance and their lifespan to be increased, and showed that we can remove solid insulation from direct oxidation. In 1891, petrol oil was used experimentally in the insulation of a transformer [BER 02] to replace air. It was only at the beginning of the 20th Century, with the multiplication of electric energy distribution networks and the increase of installed power, that air-insulated transformers (known as “dry”) became enormous in size. Since then, they have been substituted little by little for transformers filled with mineral oil (known as “immersed”); the mineral oil used as an electric insulator and fluid calocarrier ensuring heat transfer. One of the disadvantages of mineral oil lies in the fact that its resistance to fire is weak. To solve this problem, synthetic liquids such as polychlorobiphenyls (PCB) were developed in the 1930s. In the 1970s, the use of PCB was forbidden because of their toxicity; indeed they have been recognized as dangerous for populations, animals and the environment, notably by the fact that they could emit dioxins during incomplete combustion. Thus, new synthetic liquids of low flammability, such as silicone oils or synthetic esters were perfected to replace the PCB. However, the use of these latter remains limited to small transformers (distribution and traction) because of their higher cost. Nowadays, in the face of the increasing demand for the use of friendly products in industry, distribution transformers are more and more likely to be filled with vegetable oils instead of synthetic products.
This chapter gives a description of different insulator liquids used in transformers. After a general presentation of diverse insulating liquids destined for the electrical engineering industry, we describe in a more precise manner the five types of insulating liquids which can be found in transformers, i.e. mineral oil, synthetic ester oil, silicone oil, PCB and vegetable oil (natural ester). Finally, we give security instructions related to their use.
16.2. Generalities
There are three types of insulating liquids (Table 16.1): (i) vegetable oils (natural products), (ii) mineral oils (based on refined petroleum products), (iii) synthetic liquids.
This listing is related to their chronological order of their appearance in electrical engineering applications. Indeed, vegetable oils were the first used, then mineral oils were developed, then synthetic liquids were manufactured to solve the shortcomings of mineral oils in certain applications [BER 02].
Nowadays, with concerns related to the environment, the trend is oriented again towards vegetable oils (based on soya, colza, sunflower, etc.). Even though their use is restricted for the moment to distribution transformers, numerous ongoing projects try to extend their application to power transformers.
Table 16.1. Different types of insulating liquids [VUA 98A]
| Category | Type | |
|---|---|---|
| (i) | Vegetable oils | Castor oil, then soya, rapeseed, … |
| (ii) | Mineral oils | Naphthenics |
| Paraffinics | ||
| High molecular weight | ||
| (iii) | Unsaturated linear synthetic hydrocarbons | Polybutenes |
| Synthetic aromatic hydrocarbons | Alkylbenzenes | |
| Alkylnaphtalenes | ||
| Alkylbiphenyls | ||
| Chlorinated linear hydrocarbons | Perchlorinatedethylene | |
| Chlorinated aromatic hydrocarbons | Trichlorobenzenes | |
| Polychlorobiphenyls (PCB) | ||
| Polychlorophenylalkanes | ||
| Chlorofluoridated linear hydrocarbons | Trichlorotrifluoroethane | |
| Ethylenic aromatic hydrocarbons | Alkyldiarylethene | |
| Organic esters | Benzyl neocaprate | |
| Diotylphalate | ||
| Phosphate esters | ||
| Pentaerythritol esters | ||
| Ether-oxides | Diolylether | |
| Silicone liquids | Polydimethylsiloxanes (PDMS) | |
| Polymethylphenylsiloxanes |
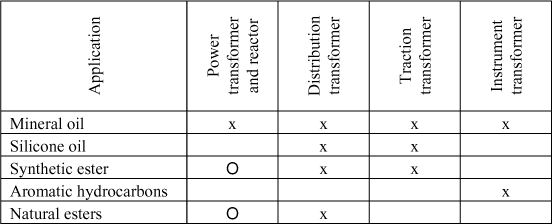
Table 16.2 presents the principal applications of different insulating liquids in electrical equipment. Three types of products are distinguished for their application in transformers:
– mineral oil: the most used insulating liquid in electrical equipment for its good dielectric and heat transfer properties, its good compatibility with cellulose insulators and its low cost; power transformers can contain between 40,000 and 80,000 liters of oil! It is principally for this technico-economical reason that this oil is the most commonly used in power transformers;
– synthetic oils: used every time some specific properties are searched for. This is notably the case when improving resistance to fire of transformers in the vicinity of populations (distribution and traction). These synthetic liquids are all obtained from different petrochemical processes. There are three principal types destined for transformers:
– vegetable oils: following the craze for the environment, have been and still are subject to numerous research [OOM 02] [DAR 07] [SAN 87] [MCS 02] [BER 04] to be developed in transformer-type applications. They are now commercially available and largely used in applications where fire resistance and biodegradable products are required.
In a transformer, the oil ensures several functions:
a) Insulation function
In the active part of a transformer, the insulation between the elements at different electric potentials is ensured:
– by the liquid dielectric only, when dealing with an insulation between two bare metallic pieces (switch contacts for example);
– by a solid layer (paper or pressboard) impregnated with liquid dielectric; this is the case, for example, for the insulation between two conductors neighboring the same coil;
– by a mixed insulation: combination of liquid dielectric films and barriers of solid insulators; this is the case for an insulation between two concentric coils at different voltages.
The principal properties which permit the aptitude of an oil to fulfill its function as a dielectric to be judged are the dielectric strength, the dissipation factor or the loss factor (tan δ), the dielectric contant and the resistivity. These four characteristics, which depend on the temperature and the frequency at a given voltage, are essential for the design of a transformer.
b) Heat transfer function
Although its efficiency is exceptionally high (99 to 99.9%), a transformer cannot escape the energy loss which accompanies any conversion. The energy lost gets dissipated in the form of heat, thus requiring the cooling of the apparatus. Because of the losses, the temperatures of each element rise until establishment of an equilibrium between the production and evacuation speeds of the heat.
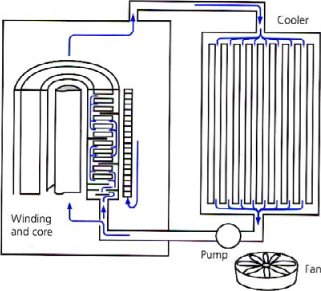
The heat to evacuate is conveyed by natural or forced circulation (streaming) of the oil, towards external radiators (Figure 16.1). A well-proportioned cooling device enables the formation of hot spots to be avoided, owing to important and well regulated circulation.
The cooling is made by convection and thermal conduction [PER 87], and the principal characteristics which allow the ability of an oil to evacuate heat to be judged are the viscosity, the thermal conductivity and the specific heat.
c) Other functions
Oils can also be used for their power to extinguish electric arcs (in cutting chambers of charge switches and certain circuit-breakers) because of their lubricating power for materials containing pieces in motion (plug selectors, immersion pumps), and in certain cases to improve the resistance to fire, as it is the case for distribution transformers near populations. Finally, we must not forget the insulating function of solid materials in the strict sense of the term, i.e. which is that of slowing down the direct oxidation of cellulose insulation.
16.3. Mineral oils
16.3.1. Composition
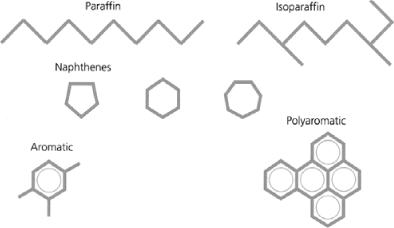
Mineral oils are obtained by refining of crude petroleum and are essentially constituted of carbon and hydrogen atoms. In each mineral oil, carbon is found (Figures 16.2 and 16.3) [NYN 04] in:
– paraffinic structure (CP): the molecules of this group are also known as saturated hydrocarbons in strait chain or alkanes, which can be linear (n-alkanes) or ramified (iso-alkanes) and whose general formula is CnH2n+2. The n-alkanes are also known as paraffins or waxes, and have bad flow properties at low temperatures;
– naphthenic structure (CN): the molecules of this group are also known as saturated cyclics or cycloalkane hydrocarbons. They correspond to closed carbonated chains of general formula CnH2n. The cycloalkanes present better properties at low temperatures and a better solvent power than n-alkanes;
– aromatic structure (CA): these molecules are cyclic components, also called unsaturated hydrocarbons, of general formula CnH2n−6. They are totally different to paraffinic and naphthenic molecules, but play a very important role in the properties of a mineral oil. We find them in two forms: the monoaromatics and the polyaromatics amongst which certain are considered carcinogenic. These aromatic components allow the oil to have a good performance with oxidation (production of phenols which destroy the radicals) and good gas properties (strong absorption capacity of the gases).
According to the original crude oil and the percentage of carbon in each structure, we distinguish two types of mineral oils: the naphthenic oils and the paraffinic oils. An infrared measurement permits the type of mineral oil to be defined with respect to percentage [NYN 04]:

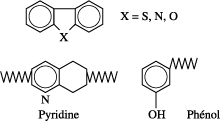
Mineral oils also contain a weak percentage of hydrocarbon molecules containing in their structure other elements such as nitrogen, sulfur and oxygen.
In most cases, these elements called heteroatoms (Figure 16.3), are related to the aromatic structures and we can find them, for example, in pyridine (C5H5N) or phenol (C6H5OH).
It is important to note that sulfur plays a natural inhibiting role to oxidation which destroys the peroxides, whereas the synthetic inhibitors which can be added (such as DBPC) destroy the radicals.
16.3.2. Implementation
Mineral oils are obtained according to a process which usually includes a distillation followed by a deparaffining operation, an extraction by solvent of undesirable molecules and a catalytic hydrogenation.
Distillation is the process during which a splitting is realized, i.e. the separation of the crude oil into distillates, having different boiling intervals, fitted to the dielectric application. The maximal splitting temperature is about 350°C; above this temperature, a thermal decomposition (cracking) of the oil starts to occur.
The naphtenic crudes practically contain no n-alkanes and do not require deparaffining. However, for paraffinic crudes, the deparaffining is necessary to obtain properties at acceptable low temperature. This process consists of mixing the oil with a solvent, then cooling down the whole. The n-alkanes then crystallize in long needles and the paraffin is taken out by filtering. After filtering, the solvent is removed from the oil by distillation.
The extraction process permits the removal of the undesirable distillate molecules in order to improve a desirable property such as stability for oxidation and gassing. These undesirable constituents include unsaturated hydrocarbons, nitrogen and sulfur components.
The hydrogenation process is realized to complete the solvent extraction. Indeed, this latter does not allow all undesirable components to be removed. Thus, the distillates undergo a hydrogen catalytic processing at high pressure and temperature (30 to 100 bars, 200 to 300°C) [DIM 69], which transforms the aromatic hydrocarbons into saturated hydrocarbons and notably decreases the rate of carcinogenic polyaromatic hydrocarbons (HPA), and the organosulfur and nitrogen in neutral chemical species. However, the strong decrease of the aromatic rate causes a degradation of the oxidation performance and the gassing.
16.3.3. Characteristics
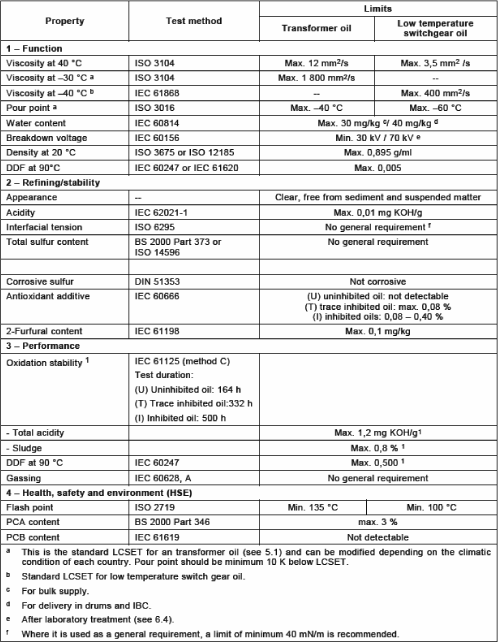
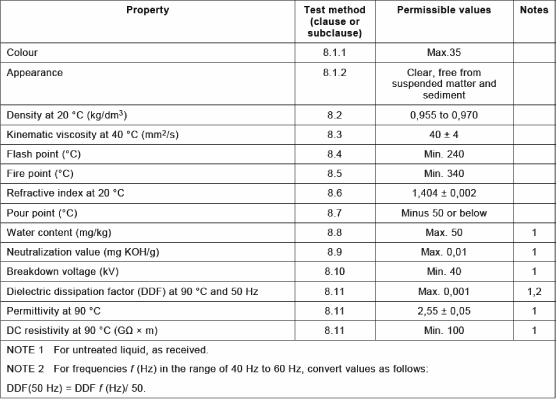
The classes and specifications of mineral oils of electrical use are defined in the publication IEC 60296 [STA 03] (Table 16.3). There is also a maintenance guide of mineral oils used in electrical apparatuses (IEC 60422 [STA 05B]).
Mineral oils are the only insulating liquids used in power transformers for their properties (which have never stopped improving since their first use at the beginning of the 20th Century), their availability and their low price. Even if, nowadays, their performances reach satisfactory levels, manufacturers of electrical devices still seek oils having:
– a better stability for oxidation, to prolong the lifespan of transformers and notably that of cellulose insulators;
– a better dielectric performance, to reduce the size of transformers or increase the security coefficients.
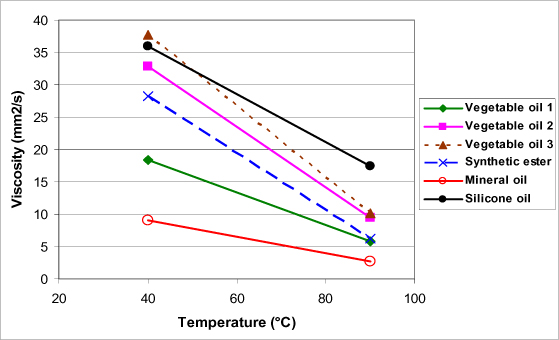
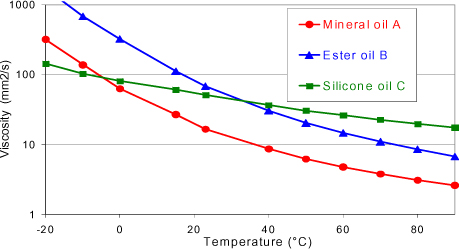
One of the advantages of mineral oils and, more particularly, naphthenic mineral oils, lies in the fact that they have a weak viscosity compared with other insulating oils for transformers (Figure 16.4). This allows a good evacuation of the heat and a correct cool start as well as a good impregnation of cellulose insulators.
One of the disadvantages of mineral oils is their flammability. These oils indeed possess a relatively low flash point (between 140 and 150°C), which poses a problem for apparatus installed near populations, such as traction or distribution transformers. This problem is less important for big power transformers which are generally installed far from the population. However, it still remains serious because of the fact that these latter can cause important damages to the factories they supply (nuclear power stations and other industries).
The principal problems regarding toxicity are related to the fact that certain polyaromatic molecules contained in mineral oils are listed as carcinogenic. Regarding ecotoxicity, mineral oils can be harmful to the environment when there are leaks at the transformer level, where they are potentially accumulable (through bioaccumulation, with possible contamination of the supply chain).
16.4. Synthetic esters or pentaerythritol ester
16.4.1. Composition and implementation
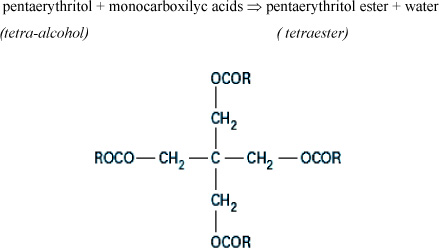
Ester oils for transformers are known as pentaerythritol ester or organic esters (as opposed to natural esters). They were developed following the banishment of PCB for the impregnation of fire resistant transformers. These esters are also known as tetraesters because they are obtained from a tetraalcohol (the pentaerythritol) and a mixture of monocarboxylic acids containing from 7 to 9 carbons (Figure 16.5). The esterification is brought to 100°C and followed by neutralization and distillation operations and a processing on activated ground.
16.4.2. Characteristics
The specifications of organic ester oils with electrical use are defined in IEC 61099 [STA 92A] (Table 16.4). There is also a maintenance guide of synthetic esters for transformers (IEC 61203 [STA 92B]).
One of the principal assets of this type of oil is the high concentration of water it can contain compared with other insulating oils. Indeed, they can contain up to 2,000 ppm of water at 20°C against 55 ppm for mineral oils (Figure 16.6, at 1/T ≈ 3.4.10−4). For this reason, humidity becomes a factor less limiting for dielectric properties. Numerous studies were made of the pentaerythritol esters, and notably on Midel 7131 oil [FOF 01] [BOR 91] [GOC 02] [DUM 95] [FOF 02A] [FOF 02B], principally stressing its high ability to absorb water (high solubility of water) and notably the assets which we can benefit from by mixing with mineral oil at the level of electrical properties.
Table 16.4. Specifications of synthetic ester oils according to standard CEI 61099 [STA 92A]
| Property | Test method (Subclause) | Permissible values |
|---|---|---|
| Physical | ||
| - Colour | 9.1 | Max. 200 |
| - Appearance | 9.2 | Clear, free from suspended matter and sediment |
| - Density at 20 °C (kg/dm3) | 9.3 | Max. 1,000 |
| - Kinematic viscosity at 40 °C (mm2/s) at −20 °C (mm2/s) |
9.4 | Max. 35,00 Max. 3 000 |
| - Flash-point (°C) | 9.5 | Min. 250 |
| - Fire-point (°C) | 9.6 | Higher than 300 |
| - Refractive index at 20 °C | 9.7 | (1) |
| - Pour-point (°C) | 9.8 | Max. −45 |
| - Crystallization | 9.9 | No crystals |
| Chemical | ||
| - Water content (mg/kg) | 9.10 | Max. 200 (2) |
| - Neutralization value (mg KOH/g) | 9.11 | Max. 0,03 |
| - Oxidation stability | 9.12 | |
| Total acidity (mg KOH/g) | Max. 0,3 | |
| Total sludge (% mass) | Max. 0,01 | |
| Electrical | ||
| - Breakdown voltage (kV) | 9.13 | Min. 45 (2) |
| - Dielectric dissipation factor tan δ at 90 °C and 50 Hz | 9.14 | Max. 0,03 (2)(3) |
| - D.C. resistivity at 90 °C (GΩm) | 9.14 | Min. 2 |
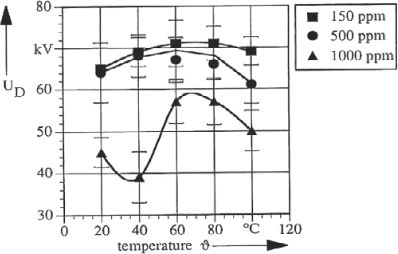
The Midel 7131 ester oil allows good breakdown properties to be kept despite a significant water content (Figure 16.7) by comparison with other oils, and notably with mineral oil. It also allows the degradation of paper at the electrical insulation level to be reduced.
Figure 16.6. Saturation limit in water (WL) as a function of temperature for different insulating liquids [FOF 01]
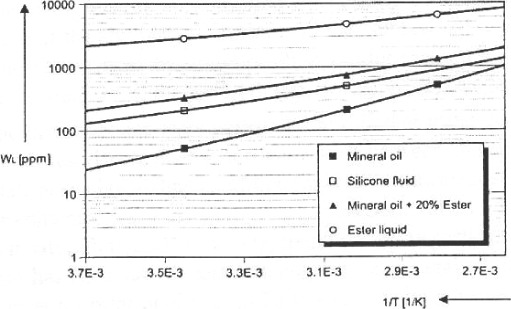
Figure 16.7. Breakdown voltage of the Midel 7131 ester oil as a function of temperature for different water contents (ppm) [DUM 95]
By studying the variation Δ of the relative dissipation factor, which is in fact the ratio of tan δ of aged paper on tan δ of non-aged paper, it was shown [FOF 02B] that the electrical insulation of paper was less altered in the presence of ester and notably in mixture with mineral oil (Figure 16.8).
The ester oils also allowed the degradation of paper at the mechanical level to be reduced. This is related to the fact that highly soluble oils, such as esters, absorb the humidity of the paper (Figure 16.9), thus reducing its alteration (Figure 16.10).
Figure 16.8. Evolution of the relative dissipation factor of a cellulose paper impregnated with different insulating liquids. 1: dried and impregnated paper (non-aged), 2: impregnated paper (non-aged), 3: dried and impregnated paper (aged) [FOF 02B]
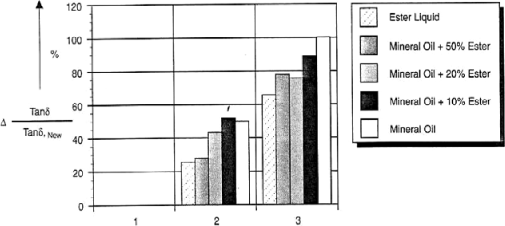
Figure 16.9. Evolution of the water content as a function of time, for Kraft paper aged in an ester oil and a mineral oil [MCS 02]
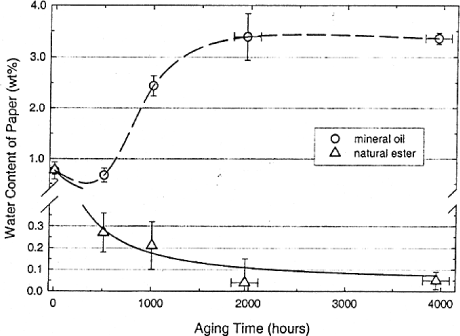
Figure 16.10. Evolution of the mechanical traction performance of paper, as a function of time, for Kraft paper aged in an ester oil and a mineral oil [MCS 02]
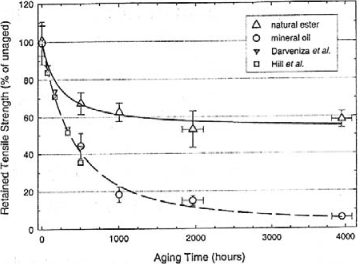
Figure 16.11. Biodegradability test (test OECD 301D) on a mineral oil, a silicone oil and a synthetic ester oil (7131 Midel) [MID XX]
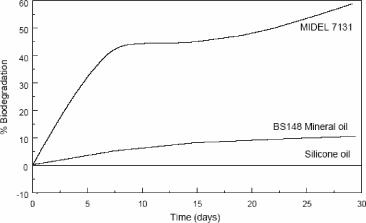
Another asset of organic ester oil is its high resistance to fire with a fire temperature higher than 300°C. Its final asset is its biodegradability compared with mineral or silicone oil (Figure 16.11).
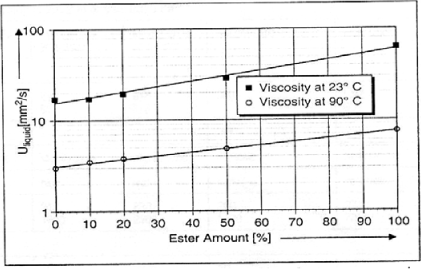
Its weak points are situated at the viscosity level which is a bit high and becomes very significant at low temperatures. This point constitutes one of the harmful effects which ester oil can have by mixing with mineral oil, as indicated in Figure 16.12.
Figure 16.12. Viscosity evolution of an ester/mineral oil mixture according to the percentage of ester, at different temperatures [FOF 02A]
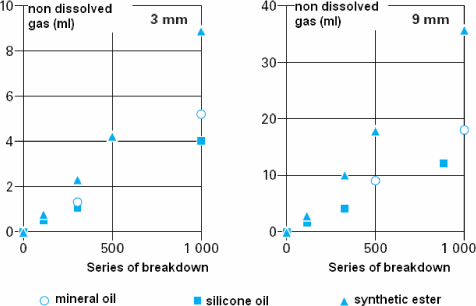
The other weak point concerns their instability for gassing. Indeed, a study made by DUMKE et al. [DUM 96] revealed their bad stability with a strong production of gas in comparison with other types of oil (Figure 16.13).
Figure 16.13. Volume of gas produced during successive breakdowns for two inter-electrode distances [DUM 96]
16.4.3. Application
Tetraesters are used for the filling of “fire resistant” distribution transformers. Their high fire point (> 300°C) with respect to mineral oils constitutes the primordial characteristic of these products. We thus find them in devices near populations, in buildings, tunnels, etc. However, their use being relatively new (only about 15 years), there is not yet enough hindsight to have a complete idea of their long-term behavior, knowing that the normal lifespan of a transformer is at least 25 years.
Principally because of their higher cost (about 4 to 8 times more expensive than mineral oils), tetraesters are not well developed in power transformers. The most famous synthetic ester, destined for transformers and available on the market, is Midel 7131, manufactured by M&I Materials.
16.5. Silicone oils or PDMS
16.5.1. Composition and implementation
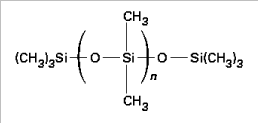
Silicone oils, like synthetic esters, were proposed as replacement liquids for PCB in transformers. The most commonly used product is the polydimethylsiloxane or PDMS. It is obtained from dimethylchlorosilane, which hydrolyse in silanol, an unstable component giving (by polycondensation) the chains:
![]()
The polymerization reaction is followed by a filtering and a passage in a vacuum-packed devolatilization column. The general formula is the following (Figure 16.14).
PDMS corresponds to chains where n ranges from 40 to 50 in order to obtain an acceptable viscosity and a good resistance to fire.
16.5.2. Characteristics
The specifications of silicone oils with electrical use are defined in the standard IEC 60836 [STA 05A] (Table 16.5). There is also a maintenance guide (IEC 60944 [STA 88]).
Owing to their very weak volatility, their chemical stability and their resistance to oxidation, silicone oils are capable of conserving their insulation properties during extended periods at high temperature, unlike mineral oils [BUR 74]. Below 150°C and in the presence of air, the oxidation of these oils is negligible [WIL 80] [CAN 81]. A study realized by Dow Corning shows that the dissipation factor of silicone oils stays relatively stable during ageing tests (Figure 16.15).
The other strong assets which characterize PDMS are its weak viscosity at low temperatures, its high viscosity index (weak viscosity variation with temperature), and its good performance with fire (high fire point).
Figure 16.15. Comparison of the dissipation factor evolution (tan δ) for silicone and mineral oils as a function of time, during an ageing at temperature [GOU 97]
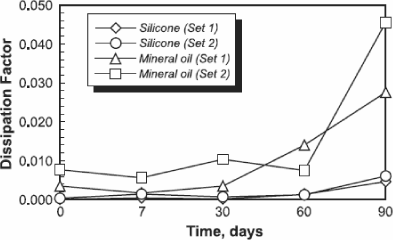
In a recent study, tetraesters, silicone oils and mineral oils were compared with regard to their aptitude to produce gas under the effect of breakdowns or partial discharges [DUM 96]. It appears that tetraesters produce more gas than other oils, while silicone oils produce less gas (Figure 16.13).
On the other hand, these oils present two disadvantages:
– having a good viscosity index, silicone oils certainly present a weak viscosity at low temperatures, but a high viscosity at positive temperatures in comparison with other types of oil (Figure 16.16), and more particularly at the functioning temperatures of a transformer (90°C);
– having a very high stability for oxidation, silicone oils are not very biodegradable (Figure 16.11).
16.5.2.1. Use
PDMS is essentially used for the filling of distribution and traction transformers, where a certain resistance to fire is sought. Their use is less widespread than those of organic esters because of their very high cost (8 times more expensive than mineral oil) and the difficulty to remove them after use because they are not at all biodegradable. Nevertheless, they are very used for traction transformers (embedded in trains) because they have a good thermal stability by supporting temperatures from 125 to 150°C.
The most famous silicone oils are Rhodorsil 604V50 manufactured by the Rhodia company, and 561 from Dow Corning.
16.6. Halogenated hydrocarbons or PCB
16.6.1. Composition and implementation
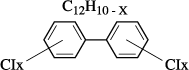
In the past, the use of chlorinated products (halogenated) by the electrical engineering industry was justified by the fact that these latter presented the advantage of not giving off flammable or explosive gases under the effect of temperature, partial discharges or during an electrical breakdown. The principle then amounts to replace part of the hydrogen atoms of the molecule by chlorine atoms which, under ionization or electric arc, form HCl molecules instead of gaseous hydrogen (Figure 16.17). Polychlorobiphenyls (PCB), still known as askarels (in Greek: fire resistant) or in France as pyralene, were widely used until their use was limited, then forbidden in 1985 because of their persistence in the environment and their toxicity (notably by emission of dioxins when they burn).
The big problem lies in the fact that numerous pieces of equipment are still filled with this liquid or contain traces of it. By 2010, all apparatus containing over 500 ppm of PCB should be removed. Apparatus containing between 50 ppm and 500 ppm will be declared slightly polluted in the European Union and will be removed at the end of their normal use. Finally, those containing less than 50 ppm will be declared un-contaminated.
16.6.2. Characteristics
The specifications of PCB are given in the IEC 60588-3 standard specification [STA 77], while the IEC 60588-5 [STA 79A] and 60588-6 [STA 79B] standard specifications describe the compatibility test with solid materials.
The volumic mass of PCB varies enormously with chlorine content and can range from 1,200 kg/m3 (dichlorinated) to 1,600 kg/m3 (hexachlorinated). Under partial discharges and electric arcs, PCB liberates chlorhydric acid, which can attack the cellulose. They must then contain acid fixative additives. The dielectric strength of PCB, at industrial frequency or lightning impacts, is less than that of mineral oils [VUA 98B].
16.6.3. Retro-filling
Considering the costs of transformers and to solve the banning of PCB, users of materials immersed in these latter wished to keep their transformers and replace the PCB by biodegradable liquids, at weak flammability and non-toxic risk. For this purpose, they realized a retro-filling operation which, however, poses a certain number of problems [0SB 84]:
– the efficiency of the retro-filling does not permit the limit value of 50 ppm to be reached in one go (notably because of residual liquid which remains in the cellulose);
– the replacement liquids do not present the same listing regarding fire, and apparatus must be delisted;
– the replacement liquids have lower electrical qualities and the apparatus cannot supply the same power.
16.7. Natural esters or vegetable oils
16.7.1. Composition and implementation
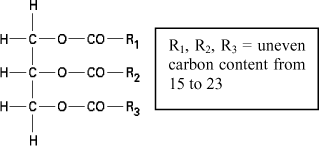
Vegetable oils were part of the first liquid insulators used for the manufacture of electrical apparatus [CLA 62]. They are essentially composed of triglycerides, which is in fact a triester (the formula of an ester is R-COO-R’), as represented in Figure 16.18. Vegetable oils are also known as natural or vegetal esters (as opposed to synthetic or organic esters) because they are naturally synthesized by any living organism and can stem from different seeds such as the castor oil plant, colza, sunflower, etc.
Vegetable oils are naturally synthesized by all living organisms and are obtained by esterification of a simple tri-alcohol, the glycerol, with three fatty acids. These acids are monocarboxylics (of formula R-COO-H), with unplugged linear chains containing an even number of carbon atoms (between 8 and 24). They can be saturated (without double bonding) or unsaturated and sometimes hydroxyled. The esterification reaction of a vegetable oil is the following:
![]()
The tryglycerides are obtained by trituration (grinding and pressure) of seeds (soya, corn, colza, sunflower, castor oil plant, etc.).
16.7.2. Characteristics
There are no specifications defined by the IEC today for vegetable oils, and it is therefore difficult to use this type of oil at the industrial level in transformers. Nevertheless, Technical Committee 10 (insulating fluids) from the IEC is actively working on the topic.
One of the big assets of these oils is their excellent biodegradability, which unfortunately confers a high sensitivity for oxidation on them [OOM 02] [DAR 07] [SAN 87] [MCS 02] [BER 04] with a higher increase in the dielectric dissipation factor, acidity and viscosity.
For this reason, their use is limited to sealed electrical equipment (i.e. without any communication with the atmosphere), where this sensitivity is compensated by antioxidants whose environmental properties can be harmful (“non-green” products).
Their other assets are that they are not very flammable and have a high water solubility (like synthetic esters), compared with mineral oils.
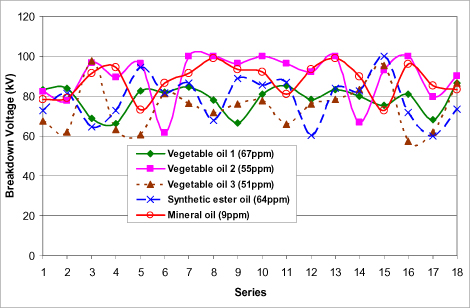
Figure 16.19. Breakdown voltage of different insulating liquids in accordance with IEC 60156 test (60 Hz and 2.5 mm gap) [DAR 07]
Apart from these two advantages, this type of oil presents a higher viscosity than mineral oils (Figure 16.4) and notably a fairly high pour point (in the neighborhood of −20°C), which limits heat transfer and restrains their use in countries where the climate is not too cold.
Oils presenting a high percentage of unsaturated fatty acids present lower viscosities and lower pour points, whereas oils in which saturated fatty acids are in the majority turn out more viscous and are known for having a better resistance to oxidation [BER 04].
Finally, new vegetable oils have electrical properties near those of mineral oils for small gaps, in accordance with IEC 60156 [STA 95] test (Figure 16.19); the gap indicated by IEC 60156 specifications is equal to 2.5 mm. Several studies are running to define vegetable oil’s behavior for larger gaps (of tens of mm). Their resistivity is lower (and, inversely, their dissipation factor is higher).
16.7.3. Use
As indicated in Table 16.2, this type of oil is mainly used in small transformers. However, and notably with the recent interest in biodegradable products, numerous power transformers were made or retrofilled in order to replace mineral oils by vegetable oils for impregnation [DAR 07] [STA 07]. We can thus find Envirotemp FR3 marketed by the Cooper Power Systems company, Midel eN manufactured by M&I, or Biotemp oil marketed by ABB.
16.8. Security of employment of insulating oils
The security of employment of insulating liquid concerns the risks of fire and explosion which they could be the cause of, as well as the influence they can have on the health of individuals or on the environment. The fire hazard lies in the flammability characteristics (flash point, fire point, auto-inflammation temperature) and certain combustion characteristics (oxygen index, emitted heat quantity) [FOF 01] [MIL 78].
The explosion hazard is related to the nature of gas produced by decomposition of liquids under electric discharges and hot spots. Hydrogen, methane and acetylene are the principal gases concerned.
The dangers concerning health and the environment, respectively related to toxicological and eco-toxicological characteristics, are subject to public organization research, such as that undertaken by the National Institute of Scientific Research, and are published in the form of biochemical studies, toxicity profiles, etc. [EEC 92].
16.8.1. Characteristics related to fire
In certain applications, where the risk in case of a fire is considered unacceptable, we use fire resistant liquids. The fire hazard is estimated from flammability characteristics such as flash point, fire point, and auto-inflammation temperature as well as certain combustion characteristics such as the oxygen index or amount of emitted heat.
PCBs (polychlorobiphenyls) were considered non-flammable, i.e. they could not be burned without an energy supply from an external fire. Their banishment and the banning of the use of chlorinated products no longer allows such flammable liquids to be used. We currently use less flammable liquids which are defined as having a fire point greater than 300°C.
16.8.1.1. Flash point, fire point and auto-inflammation
The gradual heating of a liquid causes the emission of vapors. Once the concentration of these vapors in the atmosphere surmounting the liquid becomes sufficient to form a flammable mixture, they burn near a flame. The corresponding temperature is called the flash point of the liquid.
There are two types of methods to determine the flash point:
– the open-cut method, such as the Cleveland method (ISO 2592 [STA 00A]);
– the closed-cut method, such as the Pensky–Martens method (ISO 2719 [STA 02B]).
For the open-cut method, as indicated by its name, the liquid is in contact with free air and the vapors can freely mix with the surrounding air.
In the other method, the volume above the cupel is closed and the vapors remain in the neighborhood of the liquid surface. Closed-cut methods generally give weaker values than open-cut methods.
If we carry on heating, experiments show that a permanent combustion settles in the presence of a flame, from a certain temperature corresponding to the fire point of the liquid. Non-flammable liquids such as PCB do not have a fire point.
We generally consider that, for the inflammation hazard to become negligible, the temperature must remain 25 to 30°C cooler than the flash point [BER 02].
The auto-inflammation temperature of a liquid is the minimal temperature at which an instantaneous combustion occurs. This temperature is much higher than that of the flash point and therefore is of less interest. It can be determined by the IEC 60079-4 method [STA 75], which consists of injecting a weak volume of liquid in an enclosure containing air and determining the temperature at which a flame appears in a fixed period of time.
The auto-inflammation temperature corresponds to the behavior of a liquid in a fire, while the fire point characterizes thermal limits of service.
16.8.1.2. Combustion characteristics
Since the banning of PCBs, there are no more non-flammable liquids (without fire point) for sale. These products were replaced by liquids less flammable than mineral oils, with a fire point greater than 300°C.
However, they still burn just as much and their behavior in a fire poses a problem. To address this issue, combustion characteristics such as the oxygen index and the lower calorific power (LCP) are studied.
The oxygen index corresponds to the minimal oxygen content in an air/nitrogen mixture, which allows the combustion to be maintained in defined conditions (ASTM D 2863 [STA 00B]).
The LCP is the net heat quantity liberated by the total liquid combustion; it is determined by the ASTM D 240 [STA 02A] method.
16.8.2. Toxicology and ecotoxicology
16.8.2.1. The toxicological properties
These properties concern all tests relative to the harmful effects which an oil can have on mankind. These tests are realized in a laboratory on animals (rats, rabbits, guinea-pigs).
We find [EEC 92]:
– the acute toxicity estimated from the DL50 (lethal dose) which is the dose or concentration which causes the death of 50% of individuals in 24 to 48 hours;
– the acute irritating aspect for skin or eyes;
– the sensitization power, which permits the allergic reactions that the product can cause to be estimated;
– the genotoxicity which indicates whether the liquid can alter genetic material, but also indicates the carcinogenic potential of the substance;
– the subacute toxicity, which is determined by administering substance doses for 28 days. The highest concentration which does not lead to any effect is defined as the dose without effect.
16.8.2.2. The ecotoxicological properties
These properties concern all tests relative to the harmful effects which an oil can have on the environment. We find [EEC 92]:
– the acute toxicity, is determined from tests on fish, daphnia and seaweed. According to the species, we establish respectively the CL50 (lethal atmospheric concentration for 50% of individuals) to know if a product is very toxic, the CE50 (atmospheric concentration having an effect on 50% of individuals) to define a product as toxic, and the Cl50 to know if the product is more or less harmful.
– for substances not very soluble in water (such as insulating oils), the tests are realized at the limit of solubility.
– the biodegradability permits the potential persistence of the product in the environment to be estimated. The normalized tests were originally developed for products very soluble in water.
– these tests then allow “easily biodegradable” products to be distinguished from “not easily biodegradable” products.
– other tests were developed, notably for not very soluble products like insulating oils. They then allow “potentially biodegradable” products to be distinguished from “non-biodegradable” products.
– the accumulative potential permits the risk of accumulation in the food chain to be estimated. The water-sharing coefficient (W)/octanol (O), called lg Pow, indicates the solubility difference of the substance in water and fat. A product is considered potentially accumulable if 3 < lg Pow < 6.
– the bioconcentration factor (BCF) permits the accumulative potential of a product to be estimated by tests, most often on fish. The products having a BCF > 100 are generally recognized as potentially accumulable.
– the long-term toxicity is established on fish or daphnia by 21–day tests. Almost insoluble products are tested at the limit of their solubility.
16.9. Conclusion and perspectives
It emerges from the observations made in this chapter that ester oils distinguish themselves with their strong ability to absorb humidity (high water solubility). The difference between synthetic esters and natural esters lies at the level of their stability for oxidation and their pour point. The natural esters, which are easily biodegradable, have a weak performance to oxidation and get fixed at much higher temperatures. Silicone oils have a very good stability for oxidation but are not at all biodegradable.
Mineral oil, which has been used for over a century, still remains the reference insulating liquid in the transformer industry for:
– its good physico-chemical characteristics;
– its good compatibility with cellulose insulators;
– its low viscosity;
– its relative low cost.
Nevertheless, numerous recent problems, related to the corrosivity of mineral oils, challenge its reign and little by little drives transformers manufacturers to choose esters, which in addition to their good properties, do not have this problem and are less harmful for the environment and to humans.
Growing interest in vegetable oil-based dielectric fluids is also due to (1) their excellent fire safety characteristics which ensure better safety in operation, handling, storage and transportation and thus the operational safety of transformers using such liquids; and (2) the alarming predictions concerning the shortage of petroleum oils by the middle of this century; one can expect a serious crisis for petroleum oils and very significant and rapid increases in their price.
16.10. Bibliography
[ABD 68] ABDY C.A., “The early years of the power transformer”, Electronics and Power, vol. 14, no. 8, p. 335–338, August 1968.
[BER 97] BERGER N., RANDOUX M., OTTMANN G., VUARCHEX P., “Revue des isolants liquides”, Electra (f), no. 171, p. 32–57, 2002.
[BER 02] BERGER N., “Liquides isolants en électrotechnique – Présentation générale”, Techniques de l’ingénieur, Techniques de l’ingénieur, Génie Electrique, ISSN 0992-5449, vol. D3, no. D2470, pp. 2470.1–2470.40, 2002.
[BER 04] BERTRAND Y., HOANG L.C., “Vegetable oils as substitute for mineral insulating oils in medium-voltage equipments”, CIGRE, Paper D1-202, 2004.
[BOR 91] BORSI H., “Dielectric behavior of silicone and ester fluids for use in distribution transformers”, IEEE Transactions on Electrical Insulation, vol. 26, no. 4, p. 755–762, August 1991.
[BUR 74] BUROW R.F., VINCENT G.A., IEEE PES Winter Meeting, New York, N.Y., January 27–February 1, 1974.
[CAN 81] CANDILLON C., BOSS P., “Utilisation des fluides silicones dans les transformateurs de distribution, en vue du remplacement des askarels”, Bull. ASE/UCS, p. 142–146, 7 February 1981.
[CLA 62] CLARK F. M., Insulating materials for design and engineering practice, John Wiley & Sons, 1962.
[DAR 07] DARWIN A., PERRIER C., FOLIOT P., “The use of natural ester fluids in transformers”, 3rd European Conference on HV and MV Substation Equipment: Evolution, Trends and Perspectives, Lyon, France, Paper 0036, 15–16 November, 2007.
[DIM 69] DIMELER G. R., MILLS J. W., MELCHIOR J. J., “The scope of hydrogenation as a refining tool for manufacture of transformer oils”, IEEE Trans. Elect. Insul., EI-4, no. 1, p. 7–12, March 1969.
[DUM 95] DUMKE K., BORSI H., GOCKENBACH E., “A synthetic insulating liquid for applications in transformers”, 9th ISH, Graz, subject 1, p. 1025, August–September 1995.
[DUM 96] DUMKE K., BORSI H., GOCKENBACH E., “Experimental investigation on the behaviour of decomposition gases in insulating liquids caused by partial discharge and breakdown”, Conference Record of the ICDL′96, 12th Intern. Conf. Conduction and Breakdown in Dielectric Liquids, Rome, Italy, p. 300–303, July 15–19, 1996.
[EEC 92] “Directive 92/69/CEE de la Commission, du 31 juillet 1992, portant dix-septième adaptation au progrès technique de la directive 67/548/CEE du Conseil concernant le rapprochement des dispositions législatives, réglementaires et administratives relatives à la classification, l’emballage et l’étiquetage des substances dangereuses”, Journal officiel no. L 383A, p. 235, 29 December 1992.
[FOF 01] FOFANA I., BORSI H., GOCKENBACH E., “Fundamental investigations on some transformer under liquids under various outdoor conditions”, IEEE Transactions on Dielectric and Electrical Insulation, vol. 8, no. 6, p. 1040–1047, December 2001.
[FOF 02A] FOFANA I., BORSI H., GOCKENBACH E., WASSERBERG V., “Challenge of mixed insulating liquids for use in high-voltage transformers, Part 1: Investigation of mixed liquids”, IEEE Electrical Insulation Magazine, vol. 18, no. 3, p. 18–31, May–June 2002.
[FOF 02B] FOFANA I., BORSI H., GOCKENBACH E., WASSERBERG V., “Challenge of mixed insulating liquids for use in high-voltage transformers, Part 2: Investigation of mixed liquids impregnated paper insulation”, IEEE Electrical Insulation Magazine, vol. 18, no. 4, p. 5–16, July–August 2002.
[GOC 02] GOCKENBACH E., BORSI H., “Performance and new application of ester liquids”, Proceedings of 14th ICDL, Graz (Austria), p. 203–206, 7–12 July 2002.
[GOU 97] GOUDIE J.L., “Silicones materials in new high temperature liquid transformer designs”, Electrical Insulation Conference and Electrical Manufacturing and Coil Winding, vol. 23, p. 455–458, September 1997
[MCS 02] MCSHANE C.P., RAPP K.J., GAUGER G.A., LUKSICH J., CORKRAN J.L., “Aging of Kraft paper in natural ester dielectric fluid”, IEEE Proceedings of 14th International Conference on Dielectric Liquids (ICDL), Graz (Austria), p. 173–177, 7–12 July 2002.
[MID XX] http://www.midel.com/English/PDFFiles/EnvironmentalBehaviour.pdf
[MIL 78] MILLER D.B., “Tests and standards to evaluate the fire safety of electrical insulating fluids”, IEEE Transactions on Electrical Insulation, EI-B, no.5, p. 378–381, October 1978.
[NYN 04] Transformer oil handbook, Copyright Nynas Naphtenics AB, Sweden, 2004.
[OOM 02] OOMMEN T.V., “Vegetable oils for liquid-filled transformers”, IEEE Magazine, vol. 18, no. 1, p. 6–11, January–February 2002.
[0SB 84] OSBORN S. W., “PCB removal from transformers”, EPRI Report EL-3345, Project 2028–2 final report, May 1984.
[PER 87] PERRET J., PARIS M., “Les huiles silicones pour transformateurs”, E.D.F Bulletin des etudes et recherché, Série B, Réseaux électriques, matériels électriques, no. 2, 1987, p. 5–13, 1987.
[PER 04] PERRIER C., BEROUAL A., BESSEDE J-L., “Experimental investigations on different insulating liquids and mixtures for power transformers”, IEEE International Symposium on Electrical Insulation (ISEI 2004), paper 11–3, Indianapolis, Indiana, USA, 19-22 September (2004).
[ROU 98] ROUSE T.O., “Mineral insulating oil in transformers”, IEEE Electrical Insulation Magazine, vol. 14, no. 3, p. 6–16, May–June 1998.
[SAN 87] SANKARALINGAM S., KRISHNASWAMY K.R., “New dielectric liquids from vegetable origin – A feasibility study on indian rape seed oil”, CIGRE Symposium, Section 5, paper 500–01, Vienna, 1987.
[STA 75] Standard IEC 60079-4 (Ed. 2), Matériel électrique pour atmosphère explosives – Partie 4: Méthode d’essai pour la détermination de la température d’inflammation, January 1975.
[STA 77] Standard IEC 60588-3 (Ed. 1), Askarels pour transformateurs et condensateurs – Partie 3: Spécifications pour askarels neufs, January 1977.
[STA 79A] Standard IEC 60588–5 (Ed. 1), Askarels pour transformateurs et condensateurs – Partie 5: Essai éliminatoire pour déterminer la compatibilité des matériaux avec les askarels pour transformateurs, January 1979.
[STA 79B] Standard IEC 60588-6 (Ed. 1), Askarels pour transformateurs et condensateurs – Partie 5: Essai éliminatoire pour déterminer les effets des matériaux sur les askarels, January 1979.
[STA 88] Standard IEC 60944 (Ed. 1), Guide de maintenance des liquides silicones pour transformateurs, September 1988.
[STA 92A] Standard IEC 61099 (Ed. 1), Spécifications pour esters organiques de synthèse neufs à usages électriques, May 1992.
[STA 92B] Standard IEC 61203 (Ed. 1), Esters organiques de synthèse à usages électriques – Guide de maintenance des esters pour transformateurs dans les matériels, December 1992.
[STA 95] Standard IEC 60156, Insulating liquids – Determination of breakdown voltage at power frequency – Test method, 1995.
[STA 00A] Standard ISO 2592, Détermination des points d’éclair et de feu – Méthode Cleveland en vase ouvert, September 2000.
[STA 00B] Standard ASTM D 2863, Standard test method for measuring the minimum oxygen concentration to support candle-like combustion of plastics (oxygen index), 2000.
[STA 02A] Standard ASTM D 240, Standard test method for heat of combustion of liquid hydrocarbon fuels by bob calorimeter, 2002.
[STA 02B] Standard ISO 2719, Détermination du point d’éclair – Méthode Pensky-Martens en vase clos, November 2002.
[STA 03] Standard IEC 60296 (Ed. 3), Fluides pour applications électrotechniques – Huiles minérales isolantes neuves pour transformateurs et appareillages de connexion, November 2003.
[STA 05A] Standard IEC 60836 (Ed. 2), Spécifications pour liquides isolants silicones neufs pour usages électriques, May 2005.
[STA 05B] Standard IEC 60422 (Ed. 3), Huiles minérales isolantes dans les matériels électriques – Lignes directrices pour la maintenance et la surveillance, October 2005.
[STA 07] Technical report no. B900-04062, E-FR3 Large and medium Power transformer users list (Retrofill and new installations), Cooper Power Systems, December 2007.
[VUA 98A] VUARCHEX P.J., “Caractéristiques des diélectriques liquides”, Techniques de l’ingénieur, Traité K 714, p. 3, January 1998.
[VUA 98B] VUARCHEX P.J., “Les isolants liquides dans les condensateurs et les transformateurs: emploi, nature et recherche”, Journée électrotechnique, 10–11, p.21, March 1998.
[WIL 80] WILSON A.C.M., Insulating liquids: Their uses, manufacture and properties, Peter Peregrinus, Stevenage, UK, p. 124, 1980.
1 Chapter written by Abderrahmane BEROUAL, Christophe PERRIER, Jean-Luc BESSEDE.