CHAPTER 13
Renewable Gas and Fuel Cells
Generations of schoolchildren have been entertained by the oxyhydrogen reactions in chemistry class. When hydrogen is oxidized by oxygen from the air, the hydrogen gas explosively releases its stored energy. A spark is enough to ignite a mixture of hydrogen and normal air. In contrast to many other combustion processes, however, the reaction product is absolutely harmless from an environmental point of view. Hydrogen and oxygen simply react to produce pure water.
The proportion of electricity used in energy supply systems is increasing all the time. Fuel cells or gas-fired power plants can generate the much sought-after electricity from hydrogen. The only waste created is water. It is no wonder that the many people with a vision of a global hydrogen economy see it as the solution to our current climate problems. Hydrogen as a single energy source could at the same time help us to get rid of air pollution, acid rain, and other environmental problems caused by the use of energy.
Jules Verne saw the potential of hydrogen as early as 1874, and the question is why this vision has not already been developed. The answer is simple: hydrogen essentially does not occur in a pure form in nature. Energy and a complex technical process are needed before it can be burnt again. This makes hydrogen expensive, and some production processes involved even create high greenhouse emissions. However, with the aid of wind and solar energy, hydrogen, and other gases can be produced in a carbon dioxide-neutral manner.
 Jules Verne (1828–1905): ‘The Mysterious Island’
Jules Verne (1828–1905): ‘The Mysterious Island’
“And what will they burn instead of coal?” asked Pencroft. “Water” replied Harding. “I believe that water will one day be employed as fuel, that hydrogen and oxygen which constitute it, used singly or together, will furnish an inexhaustible source of heat and light, of an intensity of which coal is not capable. Water will be the coal of the future.”
Today, the production of hydrogen from renewable energies is sometimes referred to as power-to-gas technology. That sounds fascinating, for sure. Technically, the process of producing hydrogen from renewable energies is in fact quite basic. The electrolysis required for this is already known to many from chemistry lessons. The main problem is the use of hydrogen. Large hydrogen storage facilities or transport pipelines are practically non-existent.
 Step on the Gas
Step on the Gas
Natural gas is widely used, and many people are, therefore, familiar with it. However, natural gas is a fossil energy source, the combustion of which produces carbon dioxide. Natural gas consists of more than 90% methane. In a camping context we are familiar with propane or butane. Both gases come from natural gas production or petroleum processing and therefore also have a fossil origin.
Before natural gas began its triumphal advance in Germany and other countries, town gas was widespread. Town gas is produced from coal and consists only of around 20% methane and 50% hydrogen. Town gas has a lower calorific value than natural gas. For this reason, appliances had to be adapted during the conversion from town gas before they could be connected to natural gas. In West Berlin, the last consumers were not converted to natural gas until 1996.
Biogas consists of only 50–70% methane and also has a lower calorific value than natural gas. If biogas is to be fed into the natural gas grid, it must be conditioned accordingly.
Renewable gases are a new category of gases. Renewable gases are produced with the help of electricity from renewable power plants. Depending on the origin of the electricity, they are also referred to as wind gas or solar gas. Chemically, it is either hydrogen produced from renewable electricity by electrolysis or methane produced from hydrogen in a further process. The main advantage of methane is that it can directly replace natural gas. Combustion of renewably-produced methane also produces carbon dioxide – but only exactly as much as was previously extracted from the atmosphere during gas generation. The use of renewable gases is therefore carbon dioxide-neutral.
Today's gas industry is based on natural gas. In theory, hydrogen can also be added directly to the natural gas network. However, the problem is that the calorific value of hydrogen per cubic metre is significantly lower than that of natural gas. If a gas stove adjusted to natural gas were operated with hydrogen, the performance would decrease rapidly, and the time taken to heat up soup would feel like an eternity. This becomes even more problematic in industrial processes that use natural gas. Uncontrolled admixture of hydrogen could cause major damage. Therefore, the proportion of hydrogen admixture is limited to 2–5%.
In the case of the previously common town gas, around 50% hydrogen was already present in the gas network. Town gas was produced from fossil coal with the help of coal gasification. Since the 1960s, town gas has been successively replaced by natural gas. In Germany, town gas was finally phased out in the 1990s. During the conversion, all the nozzles of consumer appliances had to be readjusted to the changed calorific value. From today's perspective, the conversion is regrettable, as a gas network based on town gas would have been able to handle renewable hydrogen much more easily. A renewed conversion of all consumers to a gas mixture with a higher hydrogen content is not an acceptable alternative due to the high costs involved.
If one wishes to avoid building a hydrogen infrastructure parallel to the natural gas network, a further chemical process can be used to produce methane gas from renewable hydrogen. Since natural gas consists almost entirely of methane, renewable methane can then simply be fed into the natural gas grid. All natural gas storage facilities, pipelines, and consumer appliances can be used directly for renewable methane without conversion. Since Germany has huge natural gas storage facilities, there is also an immediate option to use these facilities for a renewable energy industry. No wonder that power-to-gas technology has become an important source of hope for a carbon-dioxide-free energy supply.
13.1 Hydrogen as an Energy Source
Hydrogen is by far the most common component in our solar system and constitutes around 75% of the mass and more than 90% of all atoms. Our sun and the large gas planets Jupiter, Saturn, Uranus, and Neptune consist primarily of hydrogen (Figure 13.1).

Figure 13.1 Hydrogen is the smallest and lightest atom. Hydrogen was used as energy source for the Space Shuttle.
Graphic/Photo: NASA.
Here on Earth hydrogen occurs much less frequently. Its share of the total weight of the Earth is only about 0.12%. Although hydrogen occurs more frequently in the Earth's crust, it practically never occurs even there as a pure gas. Hydrogen is almost always chemically bonded. The most frequent compound is water.
Hydrogen is the smallest and lightest atom. As an extremely light gas, hydrogen was used to fill the gas bags of airships like the Zeppelins during the first half of the nineteenth century. The Hindenburg disaster, where an electrostatic charge supposedly caused the hydrogen to ignite, brought a tragic end to the prospects of hydrogen use.
The main application of hydrogen today is in the chemical industry. As an energy source it is currently used on a large scale mainly in the aviation sector and in space travel. Hydrogen has occasionally been used to drive the jet engines of aeroplanes. In space travel liquid hydrogen is used as rocket fuel. For example, the launch of a space shuttle consumes about 1.4 million litres of liquid hydrogen weighing more than 100 tons. This is burnt along with the 0.5 million litres of liquid hydrogen that the shuttle carries with it. The combustion temperature is up to 3200 °C.
Hydrogen must first be produced in a pure form before the energy from it can be used. This requires an easily available inexpensive raw material containing hydrogen. Aside from water (H2O), which consists of hydrogen (H) and oxygen (O), hydrocarbon compounds can also be an option. This is primarily natural gas, or methane (CH4). Heating oil and coal consist of hydrogen (H) and carbon (C) but have a much higher proportion of carbon than natural gas (Figure 13.2).
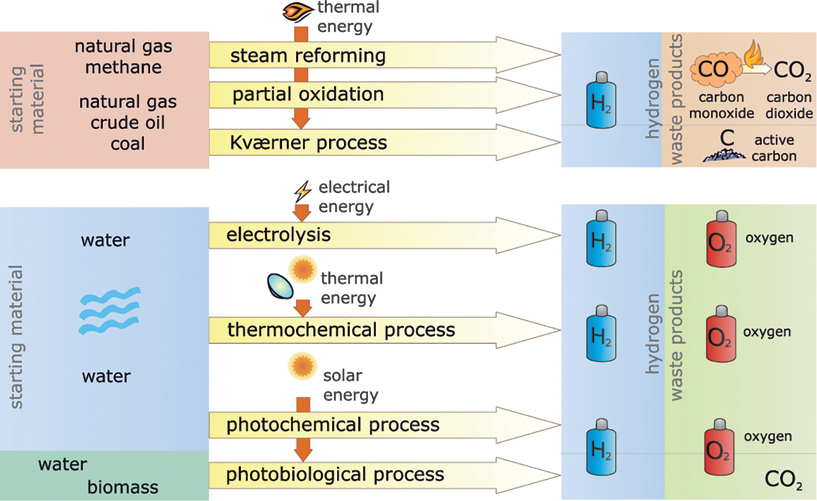
Figure 13.2 Procedures for producing hydrogen.
Current industrial methods for producing hydrogen almost exclusively use fossil fuels, such as natural gas, crude oil, or coal, as the raw material. Methods such as steam reforming or partial oxidation to produce hydrogen from fossil hydrocarbons chemically separate the carbon. It then reacts to form carbon monoxide (CO), which can be utilized. The end product is carbon dioxide (CO2). These methods for producing hydrogen are therefore not real options for actively protecting the climate.
The Kværner method also uses hydrocarbons as the base material. However, the waste product that it produces is activated carbon, i.e. pure carbon. A direct formation of carbon dioxide can be prevented with this method if the carbon is not burnt further.
Basically, all the methods mentioned to produce hydrogen from fossil energy sources are run at high processing temperatures. This requires large amounts of energy. If this energy comes from fossil sources, this will again lead to the emission of carbon dioxide. For climate protection it is usually better to burn natural gas or oil directly than to take the circuitous route of producing hydrogen and then using it as a supposedly environmentally friendly fuel.
Other methods are therefore necessary to produce hydrogen, so that it is environmentally safe. Electrolysis is the ideal method for this. The German chemist Johann Wilhelm Ritter first used electrolysis to produce hydrogen as early as 1800. Using electric energy, electrolysis decomposes water directly into hydrogen and oxygen. If the energy comes from a renewable energy power plant, the hydrogen can be extracted free of carbon dioxide.
Alkaline electrolysis is an example (Figure 13.3). With this method two electrodes are dipped into a conductive watery electrolyte. This can be a mixture of water and sulphuric acid or potassium hydroxide (KOH). The anodes and cathodes conduct direct current into the electrolytes. There they electrolyse water into hydrogen and oxygen.
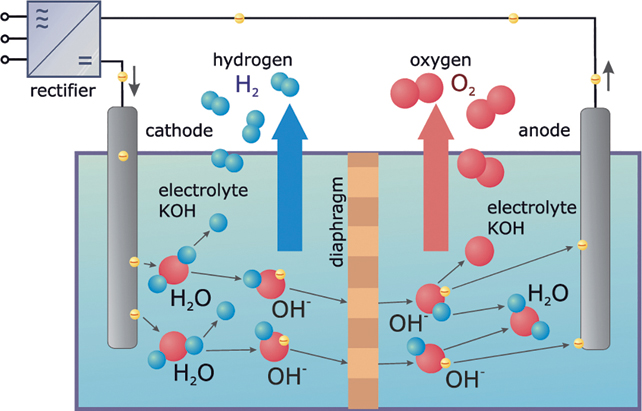
Figure 13.3 Principle of alkaline electrolysis.
Although electrolysis has already reached a high state of technical development as an environmentally compatible option for oxygen production, other alternative methods are also in development.
Thermo-chemical methods are an example. At temperatures above 1700 °C water decomposes directly into hydrogen and oxygen. However, these temperatures require expensive heat-resistant facilities. The required temperature can be reduced to below 1000 °C through different coupled chemical reactions. For example, concentrating solar thermal power plants can produce these temperatures, and this has already been successfully proven.
Other procedures include the photochemical and photobiological production of hydrogen. With these procedures, special semiconductors, algae or bioreactors use light to decompose water or hydrocarbons. These methods are also still at the research stage. The main problem is developing long-term stable and reasonably priced facilities.
13.2 Methanation
In order to replace fossil-based gas directly with renewable gas, methane must be produced from hydrogen generated with renewable electricity (Figure 13.4). Methane has the chemical formula CH4 and consists of four hydrogen atoms and one carbon atom. Carbon dioxide can serve as carbon source. This can come from fossil power plants, biogas plants, or biomass power plants, for example. In principle, it is also possible to extract carbon dioxide from the atmosphere. However, as the concentration here is very low, the carbon dioxide would have to be separated. Various technologies are being developed for this purpose, but these are still energy-intensive and expensive.
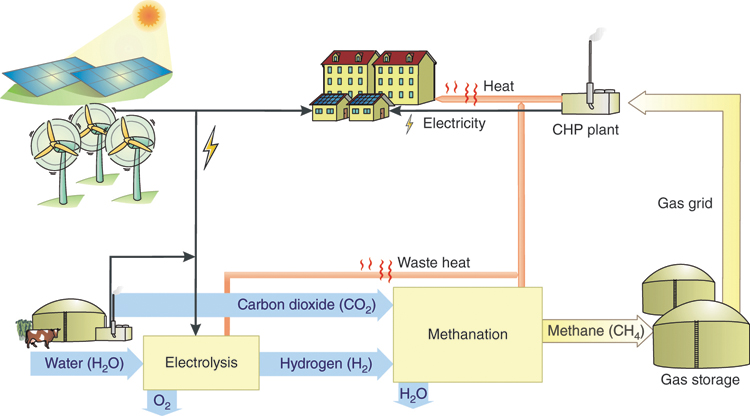
Figure 13.4 Generation, storage, and re-conversion to electricity of renewable methane [Qua13].
 Methanation of Hydrogen
Methanation of Hydrogen
The Sabatier process, named after the French chemist Paul Sabatier, converts hydrogen, and carbon dioxide into methane:
A catalyst based on nickel or ruthenium is required. During the reaction, heat is released, which can be utilized.
A 25 kW prototype plant that uses carbon dioxide from the atmosphere was built in 2009 at the Centre for Solar Energy and Hydrogen Research Baden-Württemberg. A 250 kW test plant followed in 2012. However, the efficiency of the first plant for the conversion of renewable electricity to methane was still very low, at around 40%. A 6 MW plant with an efficiency of 54% went into operation at Audi AG in 2013. In 2016, a 1.25 MW plant was built by the Haßfurt municipal companies in collaboration with Greenpeace Energy, which converts excess electricity from renewable energies into hydrogen and feeds it into the public gas grid.
13.3 Transport and Storage of Renewable Gas
13.3.1 Transport and Storage of Hydrogen
Once pure hydrogen has been produced and is not to be immediately converted into methane, it has to be stored and transported to the consumer. In principle, we are familiar with the storage and transport of combustible gases from the use of natural gas. Hydrogen is an extremely lightweight gas with very minimal density but has a relatively high calorific value. Compared to natural gas, hydrogen with the same energy content requires much larger storage volumes, although the stored hydrogen is much lighter.
Hydrogen can either be compressed and stored under high pressure or liquefied in order to reduce the necessary storage volumes. Under normal pressure hydrogen condenses but not until it reaches extremely low temperatures of minus 253 °C. Liquid hydrogen is abbreviated as LH2. A large amount of energy is needed to achieve such low temperatures. Around 20–40% of the energy stored in the hydrogen is used to liquefy it.
In principle, the same technologies used in the natural gas sector can be used for the liquidization, transport, and storage of hydrogen. Hydrogen can be transported either in pipelines or in special tankers and freighters. Whereas pipelines usually transport the gaseous form, tankers are preferred for liquid hydrogen to reduce the volume. In contrast to hydrogen, natural gas already becomes liquid at minus 162 °C and is abbreviated as LNG (liquefied natural gas) (Figure 13.5).

Figure 13.5 Experience from the natural gas sector can be used for storing and transporting hydrogen. Left: LNG tanker. Right: Pipeline.
Source: BP, www.bp.com.
Compressed gas or liquid gas tanks are used to store small quantities. Another disadvantage of hydrogen is the very small atomic portion, which makes it extremely volatile. Large quantities can be lost if it is stored in metal tanks for long periods of time, because it diffuses through the storage walls. Underground storage facilities similar to those already used for natural gas are also suitable for storing large quantities of hydrogen. Hydrogen is injected into underground cavities at high pressure and can be removed again as required.
13.3.2 Transport and Storage of Renewable Methane
Renewable methane can be fed directly into the natural gas grid. This means that the existing natural gas infrastructure can be used without modification. Transport over long distances takes place through high-pressure pipelines with a diameter of 1–2 m. Compressor stations located every 100–200 km maintain the high pressure of up to 100 bar. Smaller medium- and low-pressure supply lines distribute the gas locally to the end users. The pressure at the consumer appliances is only around 20 mbar. Gas pipelines with a total length of over 400 000 km have been laid in Germany.
Large quantities of natural gas are stored underground (Figure 13.6). A distinction is made between pore or adsorptive storage of natural gas and cavern storage. Depleted natural gas or oil reservoirs are already being used as pore storage facilities. Pores and fissures of underground layers of lime and sandstone absorb the gas. The geological condition of pore reservoirs is generally well-known and the tightness has been proven by the natural gas or oil originally stored for millions of years.
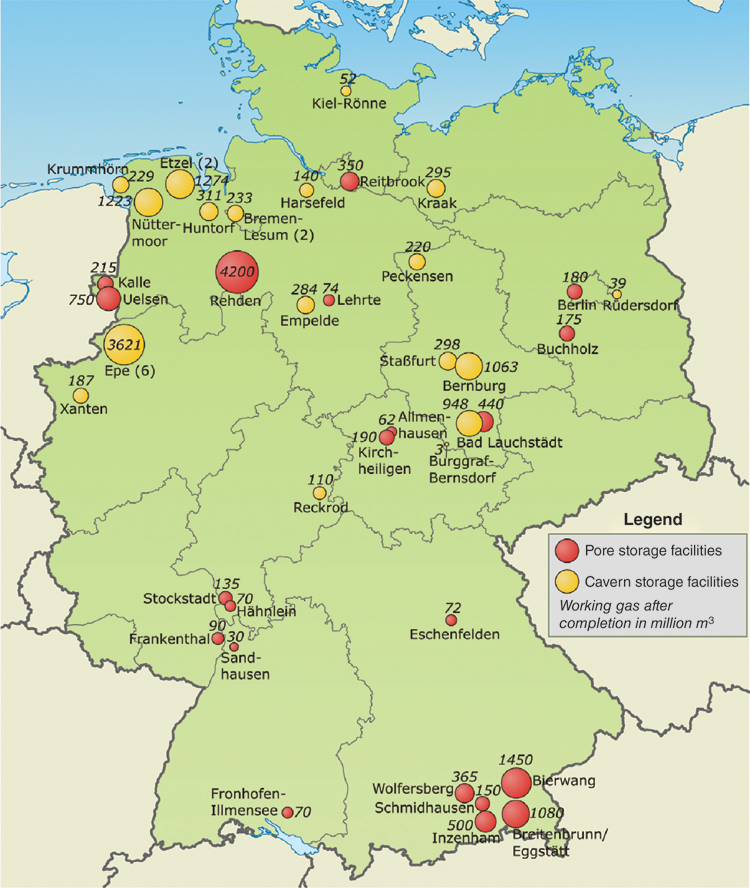
Figure 13.6 Underground natural gas storage facilities in Germany.
Source: Map of Germany: NordNordWest, www.wikipedia.de, Data on storage facilities: LBEG Lower Saxony D [LBEG12].
For cavern storage facilities, artificial caverns are created in salt domes. Salt is washed out, through a well, with water at a depth of several hundred metres, creating large cavities of up to 100 million cubic metres. The underground caverns reach heights of up to 500 m. Several individual caverns can be combined to form an even larger cavern storage facility. Due to the salt above the cavity, the reservoir is naturally tight and can only be filled and discharged through the existing boreholes. Not all of the gas can be taken out again. About one third of the cavity must remain filled with so-called cushion gas to ensure the pressure and stability of the reservoir. The remaining storage volume can be continuously charged and discharged with the working gas.
In total, Germany has 51 pore and cavern storage facilities with a working gas volume of 24.3 billion cubic metres. A further 7 storage facilities with a storage volume of 4.0 billion cubic metres are being planned or under construction (Table 13.1). Cavern storage facilities can also be used to store hydrogen. All existing or planned cavern storage facilities could then absorb hydrogen with an energy content of 56 billion kilowatt hours. With methane, the storage capacity is even greater. The total storage potential amounts to 283 billion kilowatt hours.
Table 13.1 Capacities for the storage of hydrogen and methane in Germany [LBEG16]
| Storage capacity in billion kWh for | |||
| Working gas volume in billion m3 |
Hydrogen | Methane | |
| 20 pore storage facilities in operation | 9.8 | — | 98 |
| 31 cavern storage facilities in operation | 14.5 | 44 | 145 |
| 7 cavern storage facilities in planning or construction | 4.0 | 12 | 40 |
| 58 storage facilities in operation, planning, or construction | 28.3 | 56 | 283 |
Total natural gas consumption in Germany in 2016 was around 850 billion kilowatt hours. This would allow the storage facilities to cover Germany's current natural gas demand for around three months. Gas demand could increase even further if, in future, gas storage facilities are to be used increasingly for the long-term storage of surpluses from solar and wind power plants. But even then, with the existing and planned gas storage facilities, there is sufficient capacity to guarantee a secure supply based on renewable energies.
13.4 Fuel Cells: Bearers of Hope
Fuel cells are considered a key technology for the future energy use of hydrogen, because they can convert hydrogen directly into electric energy. Theoretically at least, this results in higher efficiency levels than with combustion in conventional thermal power plants.
The principle of fuel cells has been known for a very long time. There is some controversy about who actually invented the technology. The German-Swiss chemist Christian Friedrich Schönbein conducted the first tests in fuel cell technology in 1838. The English physicist Sir William Robert Grove built the first fuel cell in 1839. Well-known scientists like Henri Becquerel and Thomas Edison were subsequently involved in its further development. A sufficiently advanced stage of development was finally reached in the mid-twentieth century, enabling NASA to make major use of fuel cells by 1963.
Since the 1990s, fuel cell development has been moving ahead at full speed. Car manufacturers and heating companies have adopted the technology and are looking to profit from a positive image as a result.
Fuel cells basically involve a reversal of electrolysis. A fuel cell always contains two electrodes. Depending on the type of fuel cell, pure hydrogen (H2) or a fuel containing hydrocarbons, is fed through the anode and pure oxygen (O2) or air as an oxidation material, is fed through the cathode. An electrolyte separates the anode and cathode (Figure 13.7). As a result of this, the chemical reaction is controlled. Electrons flow over a large circuit and emit electric energy. The remaining positively charged ions diffuse through the electrolyte. The waste product is water.
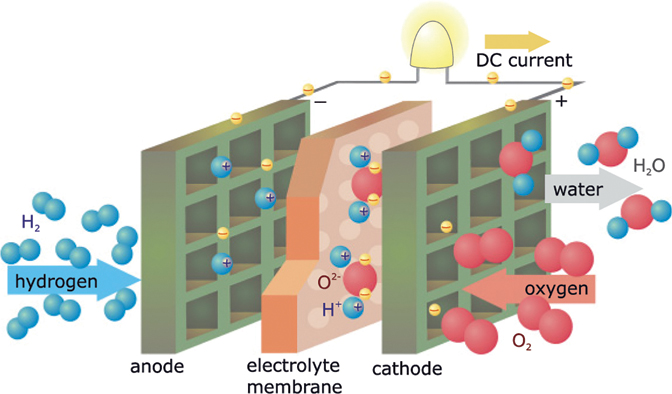
Figure 13.7 Operating principle of a fuel cell.
There are different types of fuel cells that essentially differ from each other based on electrolytes, the permissible fuel gases and operating temperatures. In practice, the following abbreviations are used to identify the fuel cell types:
| AFC | alkaline fuel cell |
| PEFC | polymer electrolyte fuel cell |
| PEMFC | proton exchange membrane fuel cell |
| DMFC | direct methanol fuel cell |
| PAFC | phosphoric acid fuel cell |
| MCFC | molten carbonate fuel cell |
| SOFC | solid oxide fuel cell |
Figure 13.8 shows the respective fuel gases and oxidation materials as well as the electrolytes and operating temperature ranges for the different types of fuel cells.
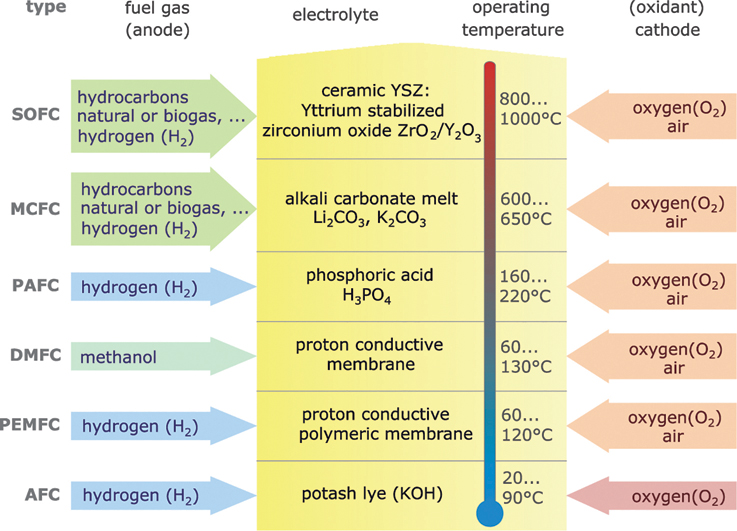
Figure 13.8 Differences between fuel cell types.
The PEMFC is the one most frequently used today. In this fuel cell the electrolyte consists of a proton-conductive polymer film. The fuel gases flow through carbon or metal substrates, which serve as electrodes. The substrates have a platinum coating that acts as the catalyst. The typical operating temperature is about 80 °C. These cells do not require pure oxygen for operation but can also work with normal air.
Because hydrogen as an energy source is only available in limited quantities today, there is an interest in using fuel cells directly with energy sources like natural gas and methanol that are relatively easily available. At a preliminary stage a reformer uses a chemical process to break down hydrocarbons such as natural gas into hydrogen and other components. This process creates a hydrogen-rich reformate gas, after which a gas purification stage is still needed to eliminate harmful carbon monoxide (CO) for the fuel cell.
MCFC and SOFC work at much higher temperatures. This enables gases containing hydrocarbons, such as natural gas and biogas, to be used directly without any previous reforming. The disadvantage of high temperatures is the long time required for starting up and switching off.
As the electric voltage of a single cell with values of around 1 Volt is too low for most applications, a number of cells are usually connected in series to form a so-called stack (Figure 13.9).
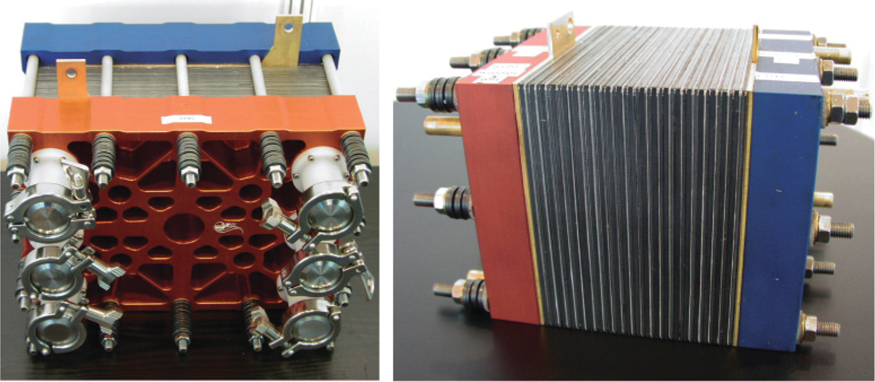
Figure 13.9 Fuel cell prototypes.
The electric efficiency of fuel cells today is usually in the order of 40–60%. Values of more than 60% can be reached in individual cases. A fuel cell can therefore only convert a part of the energy contained in hydrogen into electric energy. Basically, the waste heat of fuel cells is also usable. Power-heat coupling, or the simultaneous generation of power and heat, raises the overall efficiency of a fuel cell and can increase it to over 80%.
Major advances have been made in fuel cell technology in recent years, and many companies are already offering commercial units. However, the number of units currently being sold is still relatively low. The price of fuel cell systems is still fairly high compared to other energy supply units. Furthermore, it will be necessary to increase the sometimes quite short service life of fuel cells if they are to have a broader appeal.
13.5 Economics
It currently costs about 4 cents to produce a kilowatt hour of hydrogen through the steam reforming of natural gas – assuming that natural gas prices are relatively reasonable. One litre of petrol has a calorific value of about 10 kW h. Added to this would be the equivalent of hydrogen to 1 l of conventional petrol at about 40 cents. At this point the hydrogen would not yet even have reached the tank of the consumer. Including liquidization, transport, and storage, the cost rises threefold from the given price to well over €1. This makes it more than double the net petrol price in Europe in 2017.
Producing hydrogen in an environmentally compatible way through electrolysis using renewable energy is even more expensive. With a prototype facility using wind power for electrolysis, the equivalent of hydrogen to 1 l of conventional petrol would cost €5. With large-scale technical plants, a price of €2 could easily be achieved at the refuelling pump. Taxes and duties would have to be added to this.
The medium-term hope is that hydrogen at prime renewable sites using electricity from wind turbines, hydropower plants, or solar power plants will be available for delivery at the equivalent of less than €2 l−1 of petrol. If the petrol price then rises well above €2 l−1, hydrogen at the pump would become competitive. Presumably it will be some time before we get to that stage.
In addition to its use as a fuel, hydrogen is also considered an option for the large-scale storage of electrical energy. Relatively large losses occur during the electrolysis, storage, and re-conversion to electricity of hydrogen or methane (Figure 13.10). Therefore, this only makes sense if the electricity is available at a very reasonable price, for example due to a very high supply of solar and wind power at times. The use of waste heat in the production of hydrogen or methane and in power recovery could significantly improve the economic efficiency. From today's perspective, some technological progress and, above all, an expansion of combined heat and power generation are still necessary to make the use of renewable hydrogen or methane in the electricity industry meaningful from an economic point of view. As seasonal storage will not yet be necessary on a large scale within the next 10 years, it can be assumed that the necessary progress may still be made in good time.
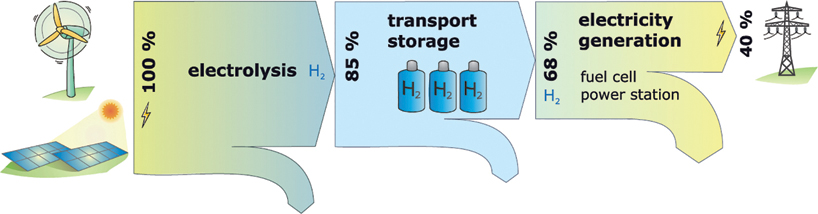
Figure 13.10 Losses when hydrogen is used to store electric energy, based on the current state of the art.
13.6 Ecology
The broad public perception of hydrogen as an energy source and fuel cells is favourable, mainly because water is the waste product when hydrogen is used.
However, what is important for the environmental balance is not what comes out at the end but what is put in at the beginning. When steam reforming is used to produce hydrogen from natural gas, around 300 g of carbon dioxide is created per kilowatt hour of hydrogen (g CO2/kWhH2); with partial oxidation of heavy oil this rises to even 400 g CO2/kWhH2 [Dre01]. This is clearly more than is created through the direct use of natural gas and crude oil. If hydrogen is extracted by electrolysis using average electricity in countries like Britain or Germany, the carbon dioxide figure rises to more than 600 g CO2/kWhH2. Hydrogen as an energy source then ends up being far from ideal for the environment. For the greenhouse gas balance, it ultimately makes more sense to continue driving petrol and natural gas-powered cars than to change them over to run on hydrogen.
Hydrogen only offers a true alternative if it is extracted using pure renewable energy sources, such as through electrolysis using energy from wind turbines and solar power plants. However, as long as hydrogen is produced using methods that can create carbon dioxide, it will, at best, be suitable for testing prototypes.
Many manufacturers are developing products that allegedly rely on clean hydrogen as an energy source and fuel cells for generating electricity. They owe us an explanation of how they intend to make sufficiently large quantities of reasonably priced carbon-free hydrogen available soon.
In recent years, prices for solar and wind power plants have fallen significantly and the trend is continuing. As a result, the production costs for hydrogen produced with renewable electricity have dropped. In the foreseeable future, this will enable hydrogen and fuel cells to become an interesting component of sustainable energy supply. Therefore, it is already a good idea to encourage the development of these technologies.
The production of methane is only climate-neutral if the energy required for it comes from renewable power plants. Furthermore, strict care must be taken to ensure that no unused methane escapes into the atmosphere along the entire process chain, as methane has a significantly higher global warming potential than carbon dioxide. Here the risk of direct use of hydrogen is considerably lower.
13.7 Markets, Outlook, and Development Potential
Hydrogen production is currently at a total of 45 million tons worldwide. In comparison, crude oil consumption at 4418 million tons worldwide in 2016 was higher by several orders of magnitude. As the chemical industry uses the bulk of the available hydrogen, a market for it does not yet exist in the energy sector. The capacity for hydrogen production using renewable energies is very low, and there is also no infrastructure for the transport and storage of large volumes of hydrogen.
Only a small number of hydrogen refuelling stations currently exist to supply hydrogen for filling the tanks of hydrogen test vehicles. The cost of developing a comprehensive network of stations that could provide hydrogen fuel is estimated in the billions of euros in countries like Germany and Britain alone. In larger countries such as the USA this sum would be even higher. As long as hydrogen is still much more expensive than conventional fuels, the chances of this kind of investment are very low (Figure 13.11).

Figure 13.11 Many car makers and energy companies are banking on hydrogen. However, the network of hydrogen fuel stations is still extremely small and is only capable of filling up a few prototypes that use hydrogen.
Photos: BP, www.bp.com.
Whereas electric drives with batteries charged using renewable energies offer an alternative for cars, climate-neutral concepts are still lacking for the powering of aeroplanes. Hydrogen could be the solution in this area. However, as planes operating on hydrogen also emit water vapour and produce condensation trails, which in turn contributes to the greenhouse effect, air traffic based on hydrogen would not be totally climate-neutral.
In recent years, a few prototype plants have been built for the production of renewable methane. However, the quantities of methane that can be produced are so small that they are insignificant from an energy economy perspective.
If the share of solar and wind energy in the electricity supply increases noticeably to more than 50%, the importance of long-term storage facilities will also increase. Although renewable hydrogen or methane is a very promising option, it is expected to take at least another 10 years before it can be used on a larger scale.
Technical developments in fuel cell technology are progressing at a much slower pace than the product announcements in the 1990s may have led us to believe. A few commercial projects exist at the moment, but the number of units sold is still relatively low. Technical advances are also needed in the fuel cell arena before a larger market can be exploited. Above all, a functioning renewable hydrogen or methane industry is needed before fuel cells can become a truly climate-compatible alternative.
