2
POLYMERS
“Important things for easy life—through polymers”.
2.1 INTRODUCTION
In modern world, polymers are an integral part of an individual’s life. They have the most diverse structure and applications ranging from domestic articles to highly sophisticated instruments. Polymers are used in almost all fields such as medicine, industry, agriculture, construction and so on. In recent days, these materials are used to prepare nanomaterials.
The human body is built up and functions with polymers such as DNA, RNA, hormones, enzymes, proteins, lipids, phosphonitrilic acids and so on. Most of the food materials that we eat are polymers such as carbohydrates, starch and so on. In view of their importance, a proper understanding of polymeric materials is very essential.
The word polymer is derived from Greek word poly, which means many and meros which means units (or) parts. Polymers are macromolecules of high molecular masses built up by the linking together of a large number of small, repeated units by a covalent bond. The repeating unit present in the formation of a polymer is known as polymerisation.
For example,

2.2 DEGREE OF POLYMERISATION
The size of the polymer molecule is decided by the number of repeating units present in it. The number of repeating units (n) in a chain formed in a polymer is known as the “degree of polymerisation”.

In the figure, n is the degree of polymerisation. It is different from polymer and can be 104 or more.
Molecular weight of polymer = Molecular weight of repeating unit × degree of polymerisation
2.3 CLASSIFICATION OF POLYMERS
Polymeric materials can be classified into several ways:
2.3.1 Classification Based on Source
- Natural polymers: These polymers are isolated from natural material such as animals and plants.
For example, cotton, silk, wool, nucleic acids, proteins, starch, cellulose, natural rubber, etc.

- Synthetic polymers: Polymers synthesised from low molecular weight compounds are called synthetic polymers.
For example, polyethylene, polyvinyl chloride, nylon, terylene, etc.

2.3.2 Classification Based on Composition
- Univalent or homopolymers: These are formed with the same monomer units.
For example, polyethylene, polystyrene, polyvinyl chloride, etc.
- Copolymers: These are formed with two or more different monomer units.
For example,

Copolymers are classified into four categories depending upon the nature of the distribution of different monomers in the polymer chain.
- Random copolymer: These are formed by the random arrangement of monomer units in the chain.

- Alternating copolymers: Monomer units in a copolymer molecule are arranged in an alternate manner.

- Block copolymer: A block copolymer consists of one in which blocks of repeating units of one monomer alternate with blocks another monomer.

- Graft copolymer: This copolymer consists of a linear polymer chain of one monomer to which side chain of different monomer has been grafted.

- Random copolymer: These are formed by the random arrangement of monomer units in the chain.
2.3.3 Classification Based on Chemical Composition
- Organic polymers: A polymer whose backbone chain is essentially made of carbon atoms is termed as organic polymer.
For example, polyethylene, polystyrene, polyvinyl chloride, etc.
- Inorganic polymers: Polymers that are formed by non-carbon-carbon bonds are inorganic polymers.
For example, polysilanes, polygermanes, etc.
2.3.4 Classification Based on Structure
- Linear polymers: Here, monomeric units are joined in the form of long, straight chains.
For example, nylons, polyester, polyvinyl chloride, high-density polythene, etc.
- Branched chain polymers: These are mainly linear in nature but also possess some branches along the chain.
For example, glycogen, amylopectin, low-density polythene, etc.
- Cross-linked polymer: These polymers adjacent chains are joined one to another at various positions by covalent bonds.
For example, elastomers like rubber.
- Network polymers: Monomer units that have trifunctional groups form three dimensional networks. The cross-linked polymer is also called network polymer.
For example, Bakelite, urea formaldehyde resin, silicones, etc.
2.3.5 Classification Based on Mode of Polymerisation
- Addition polymers: Addition polymerisation takes place in compounds containing reactive double bonds. Chain polymerisation is characterised by a self-addition of the monomer molecules to each other very rapidly through a chain reaction.
For example, polyethylene, polypropylene, polyvinyl chloride, etc.
- Condensation polymers: This type of polymerisation was brought about by monomers containing two or more reactive functional groups condensing with each other to form a large condensed polymer and also loss of small molecules such as H2O, NH3, HCL and so on.
For example, polyester, nylon, polyamide, etc.
2.3.6 Classification Based on the Molecular Forces
- Thermoplastic polymers: These are linear, long chain polymers, which can be softened on heating and hardened on cooling reversibly. Thus, they can be processed several times.
For example, polythene, polypropylene, polyvinyl chloride, etc.
- Thermosetting polymers: These polymers get hardened during moulding, and once they have solidified, they cannot be softened. During moulding, such polymers acquire three-dimensional cross-linked structure with predominantly strong covalent bonds.
For example, polyester, Bakelite, urea formaldehyde, etc.
- Elastomer: These are rubber-like elastic polymers, which can be stretched to at least thrice its length, but return to their original shape and dimension as soon as the stretching force is released.
- Fibres: Fibres are polymers whose chains are held by strong intermolecular forces like hydrogen bonding. They are crystalline in nature and of high tensile strength, due to strong intermolecular forces.
For example, nylon, polyester, etc.
2.3.7 Classification Based on Tacticity
The stereo chemical arrangement of the monomer units in the main chain of a polymer is known as tacticity. The orientation of monomeric units in a polymer can take an orderly or disorderly twist with respect to main chain polymers that are mainly classified into isotactic, syndiotactic and atactic polymers.
- Isotactic polymers: In this polymer, all the side groups lie on the same side of the chain (cis arrangement), e.g., natural rubber.

- Syndiotactic polymer: In this polymer, side groups are arranged alternatively (trans arrangement), e.g., Gutta-percha.

- Atactic polymer: In this polymer, the monomers randomly arranged to the main chain, e.g., polypropylene.

2.4 TYPES OF POLYMERIZATION
Polymerisation is mainly of two types:
- Condensation polymerisation (or) step polymerisation
- Vinyl or addition polymerisation (or) chain polymerisation
2.4.1 Condensation Polymerisation or Step Polymerisation
Condensation is brought about by monomers containing two or more reactive functional groups condensing with each other to form large condensed polymer and also loss of small molecules such as H2O, NH3, HCl and so on.
- Polyester: Condensation between carboxylic acid and diol gives polyester.

- Polyethylene terephthalate (PET):

- Polyamide: Condensation reaction between diamine and dicarboxylic acid gives polyamide.

- Nylon 66: Condensation reaction between hexamethylenediamine and adipic acid gives nylon 66.

Reaction Mechanism
- In polymerisation, the monomers should have a minimum of two reactive functional groups for polymerisation.
- Polymerisation proceeds in a step-wise reaction between reactive functional groups. In this process, the first two monomer units condense to form the dimer. The dimer reacts with another dimer to form a tetramer or with monomer to form a trimer. In this process, small units such as NH3, H2O, HCl and so on, are also formed.
- Only one type of reaction between two functional groups is involved in polymer formation.
- The polymer formed still contains both the reactive functional groups at the end of the chain; hence it is active and not dead as in chain polymerisation.
- Reaction between two monomers contains two active functional groups. They will give straight chain polymers; otherwise, monomers containing more than two functional groups form cross-linked polymer.
Formation of Polyester

The process is continuous and forms polymers.
Formation of Polyethylene Terephthalate

The aforementioned reaction is continued and forms a large polymer.
n molecules of ethylene glycol react with n molecules of terephthalic acid.

Formation of Nylon 66

The aforementioned reaction proceeds that n molecules of hexamethylenediamine react with n molecules of adipic acid to form nylon 66.

Formation of Polyamide

n molecules of diamine and n molecules of dicarboxylic acid react to form polyamide.
![]()
2.4.2 Addition/Vinyl/Chain Polymerisation
Addition polymerisation takes place in compounds containing reactive double bonds. Chain polymerisation is characterised by a self-addition of the monomer molecules to each other very rapidly through a chain reaction. No by-product, such as HCl, NH3, H2O and so on, is formed. This polymerisation occurs in the presence of catalyst, light, or heat.
- For example, In the presence of oxygen or Ziegler–Natta catalyst, ethylene gives addition polymer, that is, polythene

- In the presence of azo compounds, vinyl chloride gives polyvinyl chloride.

- In the presence of metal, amide styrene gives polystyrene.

- In the presence of benzoyl peroxide, acrylonitrile gives polyacrylonitrile.

Reaction Mechanism
In the addition polymerisation, free radical, carbonium ion, or carbanium ions, act as active centres. Hence, polymerisation may occur in the following:
- Free radical
- Ionic (cationic or anionic)
- Coordination mechanisms
In addition, polymerisation, consists of the following steps:
- Chain initiation
- Chain propagation
- Chain termination
Free Radical Polymerisation
The initiation of the polymer chain is brought about by free radicals produced by the decomposition of monomers; thus, this reaction is called free radical polymerisation.
The decomposition of the initiation to form free radicals can be induced by heat energy, light energy or catalysts.
Three steps included in free radical polymerisation are as follows:
- Chain initiation:
- In the presence of catalyst, heat or light, the initiator converts into free radical.

Normally, H2O2, benzoyl peroxide, hydroperoxide, tertiary butyl peroxide and azobis-isobutyl nitriles (AIBN) act as initiators.
- Free radicals react with the monomer unit to form a new, active-centred free radical.

- In the presence of catalyst, heat or light, the initiator converts into free radical.
- Chain propagation:
- In this step, free radical attacks the fresh monomer unit to form another free radical.

- This process continues till the monomer units are present in the reaction mixture. Finally, it forms a chain propagate free radical.

Propagation reaction is a very fast reaction; in this reaction, no middle product is formed.
- In this step, free radical attacks the fresh monomer unit to form another free radical.
- Chain termination:
In this step, chain propagate polymer radical deactivates with coupling or disproportionation reaction to stop chain propagation and forms dead polymer.
- Coupling: Chain propagation free radicals meet to form polymer and terminate the reaction.

- Disproportionation reaction: Hydrogen atom shifts from one chain propagate free radical to another chain propagate free radical and ends the reaction.

For example, polymerisation of acrylonitrile in the presence of benzoyl peroxide.
- Coupling: Chain propagation free radicals meet to form polymer and terminate the reaction.
Reaction Mechanism
- Chain initiation:
- In this step, benzoyl peroxide dissociates to form phenyl free radicals.

- Phenyl free radicals react with acrylonitrile and gives chain initiate free radical.

- In this step, benzoyl peroxide dissociates to form phenyl free radicals.
- Chain propagation:
- In this step, free radicals react with monomer units and give a chain propagate free radical. This reaction continues till the monomer units are present in reaction mixture.

- In this step, free radicals react with monomer units and give a chain propagate free radical. This reaction continues till the monomer units are present in reaction mixture.
- Chain termination:
- The chain terminates with the coupling reaction.

For example, polymerisation of methyl methacrylate in the presence of azobis-isobutyl nitrile.
- The chain terminates with the coupling reaction.
Reaction Mechanism
- Chain initiation:
- In the presence of ultraviolet rays, azobis-isobutyl nitrile homolysis gives cyanopropyl radical.

- Cyanopropyl radicals react with methyl methacrylate monomer to form chain initiate free radical.

- In the presence of ultraviolet rays, azobis-isobutyl nitrile homolysis gives cyanopropyl radical.
- Chain propagation:
- In this step, the chain initiates free radicals that react with monomer units; finally, it gives the chain propagate free radical.

- In this step, the chain initiates free radicals that react with monomer units; finally, it gives the chain propagate free radical.
- Chain termination:
- It terminates with a coupling reaction.

- It terminates with a coupling reaction.
Cationic Addition Polymerisation
The following are the important points in reaction mechanism:
- In this polymerisation, carbonium ion acts as the chain initiator.
- BF3, H2SO4 and AlCl3 like Lewis acids act as catalyst, water, carbonic acid, etc., act as co-catalysts.
- In this polymerisation monomer acts as an electron donor and reacts with catalysts (electron acceptor) to form carbonium ion. The formed carbonium ion reacts with monomer units and propagates the reaction.
For example, cationic polymerisation of isobutylene in the presence of BF3:

Reaction Mechanism
- Chain initiation:
- Carbonium ion is formed due to the following reactions.

- Carbonium ion is formed due to the following reactions.
- Chain propagation:
- Monomer molecules react one by one with carbonium ion and propagate the chain to form an addition polymer.

- Monomer molecules react one by one with carbonium ion and propagate the chain to form an addition polymer.
- Chain termination:
- Chain termination occurs with disproportionation reaction.
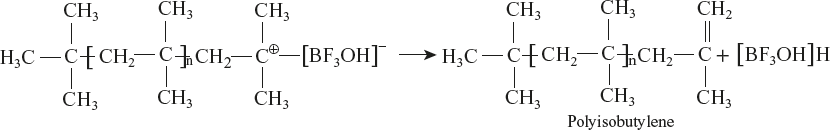
- Chain termination occurs with disproportionation reaction.
Anionic Addition Polymerisation
The following are the important points:
- Carbanion acts as chain initiator.
- Alkali metal amide, Grignard reagents, etc., act as catalysts and initiate the chain.
- Monomers containing electron-withdrawing groups participate in anionic polymerisation.
- In this polymerisation, there is no chain termination; hence anionic polymers are known as activated polymers.
Acrylonitrile, styrene and methyl methacrylate participate in anionic polymerisation. For example, Anionic polymerisation of styrene in the presence of alkali metal amide:

Reaction Mechanism
- Chain initiation:
- Carbanion is formed in the chain initiation.

- Carbanion is formed in the chain initiation.
- Chain propagation:
- Carbanion reacts with styrene monomers and propagates the reaction and forms chain propagate polymer ion.
- In this type of polymerisation, chain termination does not occur. Hence, anionic polymer is active till the monomer units are present in the reaction mixture; therefore, anionic polymers are also known as active polymers.

- When impurities are added to the reaction mixture, chain termination occurs. Therefore, chain termination reagent is added and gets dead polymers.

2.4.3 Coordination Polymerisation
Coordination polymerisation is invented by two Italian scientists, Ziegler and Natta. They shared the Nobel Prize for Chemistry in 1963 using the Ziegler–Natta catalyst to polymerise non-polar monomers.
Ziegler–Natta Catalyst
The mixture of titanium halides and trialkyl aluminium is known as Ziegler–Natta catalyst.
![]()
The reaction between titanium chloride and trialkyl aluminium forms Ziegler–Natta catalyst.
In this process, trialkyl aluminium adsorbs on the surface of titanium chloride, and forms electron deficient bridge structure.

In this structure, titanium chloride acts as catalyst and trialkyl aluminium acts as the co-catalyst.
Coordination polymerisation is a form of addition polymerisation in which monomer adds to a growing macro molecule through an organometallic active centre.
Reaction Mechanism
- Cossee–Arlman mechanism: The formation of C—C bonds in the polymerisation of alkenes are explained by Cossee–Arlman. In this pathway, an intermediate coordination complex contains both growing polymer chain and the monomer.
The coordination polymerisation of alkene can be proceeded by monometallic or bimetallic mechanism depending on the catalyst. Alkene or substituted alkene is polymerised by Ziegler–Natta catalyst.
The double bond of alkene will undergo cis addition with the empty orbital of Titanium catalyst to form a four-membered ring coordinate intermediate.
- Monometallic polymerisation mechanism:

- Bimetallic polymerisation mechanism: If the catalyst is made from aluminium and titanium compounds, the polymerisation proceeds through bimetallic mechanism. In this mechanism, first, a bridge structure is formed between two metal compounds. Then, the substituted alkene is coordinated with titanium compound to form a six-membered ring.

Importance
The Ziegler–Natta catalyst can control the linearity and tacticity of the polymer. Free radical polymerisation of ethylene produces a low density branched chain soft, rubbery polymer. However, the Ziegler–Natta catalyst produces more linear, rigid, high density, high tensile strength, harder and tougher isotactic polymer.
In the presence of the Ziegler–Natta catalyst, coordination polymerisation occurs and gives isotactic polymer of olefin.
For example, propylene undergoes coordination polymerisation in the presence of Ziegler–Natta catalyst at 50°C and gives isotactic polymer of polypropylene.

2.5 MOLECULAR MASS OF A POLYMER
Polymers are a mixture of different monomers with different molecular weights or masses. Hence, three kinds of molecular masses have been identified, which are as follows:
- Number average molecular mass (
 n): This is the total mass (w) of all the molecules in a polymer sample divided by the total number of molecules present. This can be determined by measuring colligative properties like freezing point depression, boiling point elevation, osmotic pressure, lowering of vapour pressure, etc.
n): This is the total mass (w) of all the molecules in a polymer sample divided by the total number of molecules present. This can be determined by measuring colligative properties like freezing point depression, boiling point elevation, osmotic pressure, lowering of vapour pressure, etc.

Where Ni = the number of molecules of mass Mi
The number average molecular mass is a good index for tensile strength, but not for flow.
- Weight average molecular mass (
 w): This can be determined from light scattering and ultra-centrifugation techniques and can measure molecular size as follows:
w): This can be determined from light scattering and ultra-centrifugation techniques and can measure molecular size as follows:

where wi = weight fraction of molecules of Mi

Polydispersity index or molecular mass distribution
This is a measure of the distribution of molecular mass of a polymer. This can be calculated using the weight average molecular weight divided by the number average molecular weight.

In a monodisperse system,
 w =
w =  N. However, the polydispersity index (PDI) value is always greater than one, that is, the weight of the average molecular mass is always greater than the number average molecular mass.
N. However, the polydispersity index (PDI) value is always greater than one, that is, the weight of the average molecular mass is always greater than the number average molecular mass. - Viscosity average molecular mass
 v: Viscosity average molecular mass can be determined by the measuring of viscosity of that particular polymer. This can be explained by the following formula:
v: Viscosity average molecular mass can be determined by the measuring of viscosity of that particular polymer. This can be explained by the following formula:

where a = constant.
When a = unity, the viscosity and weight average molecular masses are equal.
 v is almost less than
v is almost less than  w, a polydispersive polymer is represented as
w, a polydispersive polymer is represented as
2.6 PLASTICS
Plastics are mainly of two types:
- Thermoplastics: Thermoplastics are formed with straight chains; hence when heated, they soften, subsequently melt and when cooled, they become hard. Due to van der walls forces, two different chains slid over each other and the plasticity of such polymers is reversible. Hence, the materials can be moulded and remoulded without damage.
For example, PVC, nylon, polystyrene and polyethylene
- Thermosetting plastics: Thermosetting resins do not become soft on heating, and they never melt once they set. They are normally made from semi-fluid polymers with low molecular masses. In this case, the polymer chains are entangled with one another and hence cannot slide over each other. Deformation does not occur on heating, because only the primary covalent bonds are present in the entire structure, forming a three dimensional network. They have no scrap value.
For example, Bakelite

2.7 IMPORTANT POLYMERS—COMPOSITION, PREPARATION, PROPERTIES AND ENGINEERING USES
2.7.1 Thermoplastics
Polythene or Vinyl Resin
Polythene is the most widely used plastic. Polythene is obtained by high-pressure polymerisation of ethylene, making use of oxygen as initiator. The reaction takes place at 1,500 atmospheric pressure and 180°C–250°C temperature range. Ethylene polymerised into a waxy solid known as polyethylene.

By using free radical initiator, low density polythene (LDPE) is obtained, while by using ionic catalysts, high density polythene is obtained.
Properties
The properties of polythene are as follows:
- Polythene is a rigid, waxy, white, translucent non-polar material, exhibiting considerable chemical resistance to strong acids, alkalis and salt solutions at room temperature.
- It is a good insulator of electricity. However, it is swollen and permeable to most oils and organic solvents, particularly kerosene.
- Due to its highly symmetrical chain structure, polythene crystallises very easily. The degree of crystallinity may vary from 40%–95%, depending on the degree of branching in the polythene chain.
- Polyethylene produced by high-pressure process has a branched structure and therefore, is flexible and tough. On the other hand, the low-pressure process results in a completely linear polyethylene, having higher density and better chemical resistance.
- Commercial polythene can be subdivided into three groups:
- Low-density polythene (LDPE): It is polymerised under very high pressure of 1,000–5,000 atmospheres and temperature range of 80°C–250°C in the presence of O2 as the initiator. It has a density of 0.91–0.925 g/cm3 and M.P. 110°C –125°C.
- Medium-density polyethylene (MDPE): It is polymerised under medium pressure, having density 0.925–0.940 g/cm3 and M.P. 130°C–140°C.
- High-density polyethylene (HDPE): It is polymerised under atmospheric pressure (6–7 atmospheres) and temperature at 60°C in the presence of Ziegler–Natta catalyst [TiCl3 + Al(C2H5)3]. The HDPE, which is completely linear, has better chemical resistance and higher softening point but relatively brittle.

Uses
The uses of LDPE are as follows:
- LDPE is used in making films and sheets. Pipes made of LDPE are used for agricultural irrigation and domestic water line connections. They are also used for making tubes, coated wires and cable wires.
- HDPE is used in the manufacture of toys, insulator parts, bottle caps, flexible bottles, kitchen and domestic articles.
Polyvinyl Chloride (PVC)
It is obtained by heating a water emulsion of vinyl chloride in the presence of a small amount of benzoyl peroxide or hydrogen peroxide in an autoclave under pressure.

Vinyl chloride so needed is generally prepared by treating acetylene at 1–1.5 atmospheres with hydrogen chloride at 60–80°C, in the presence of metal chloride as catalyst.
![]()
Properties
PVC is colourless, odourless, non-inflammable and chemically inert powder, resistant to light, atmospheric oxygen, inorganic acids and alkalis but soluble in hot chlorinated hydrocarbons such as ethyl chloride. Pure resin possesses high softening point and a greater stiffness and rigidity, but is brittle.
Uses
The uses of PVC are as follows:
- Rigid PVC or unplasticised PVC has superior chemical resistance and high rigidity but is brittle. It is used for making sheets, which are employed for tank linings, light fittings, safety helmets, refrigerator components, tyres, cycles and motor-cycle mudguards. It is also extruded in strip and tube form for use in place of nonferrous metals.
- Plasticised PVC is used for making continuous sheets, rain coats, table cloths and curtains, electric cables, toys, radio components, conveyor belts, etc.
Polystyrene (PS)
It is prepared by the polymerisation of styrene in the presence of benzoyl peroxide catalyst.

Properties
Polystyrene is a transparent, light-stable and moisture-resistant material. It is highly electric insulating and highly resistant to acids, and is a good chemical resistant. However, it has less softening and is brittle. It has the unique property of transmitting light through curved sections.
Uses
It is used in moulding of articles such as toys, combs, buttons, buckles, radio and television parts, refrigerator parts, battery cases, high-frequency electric insulators, lenses, indoor lighting panels, food containers, food packaging, umbrella handles and so on.
Teflon [Polytetrafluoroethylene (PTFE or FLUON)]
The presence of benzoyl peroxide catalyst and high pressure polymerisation of tetrafluoroethylene gives Teflon.

Properties
Teflon has a twisted, zigzag structure with fluorine atoms, packed tightly in a spiral around the carbon-carbon skeleton. Due to the presence of highly electronegative fluorine atoms, there are very strong attractive forces between different chains. These strong attractive forces give the material extreme toughness, high softening point, exceptionally high chemical resistance towards all chemicals, high density, waxy touch, very low coefficient of friction and extremely good electrical and mechanical properties. It can be machined, punched and drilled. The material cannot be dissolved and cannot exist in a true molten state. Around 350°C, it sinters to form a very viscous, opaque mass, which can be moulded by applying high pressure.
Uses
The uses of Teflon are as follows:
- It is used as an insulating material (motors, transformers, cables, wires, etc.) and for making gaskets, pickings, pump parts, tank lining, chemical-carrying pipes, tubings and tanks, etc.
- It is used for coating and impregnating glass fibres, asbestos fibres, cloths, in non-lubricating bearings, non-sticking stop-clocks, coating of frying pans, etc.
Nylon
These are polyamides. The word nylon is now accepted as a generic term for synthetic polyamides, which are characterised by a repeating acids linkage (—NHCO—). Nylon is formed with dicarboxylic acids and diamide under condensation process. It has been named on the basis of number of carbon atoms present in that two monomer units.
For example, nylon 6,6, nylon 6,10, nylon 6,11, etc.
Nylon 6,6 is formed with the condensation reaction of hexamethylenediamine and adipic acid.

Properties
They are translucent, whitish, horny and high melting polymers. They possess stability up to high temperature and good abrasion resistance. They are insoluble in common organic solvents and soluble in phenol and formic acid.
Properties of Nylon Fibres
The properties of nylon fibres are as follows:
- They are light, horny and high melting.
- They are insoluble in common solvents.
- They have good strength.
- They absorb little moisture and are thus “drip-dry” in nature.
- They are very flexible and retain original shape easily.
- They have resistances to abrasion.
- On blending with wool, the strength and abrasion resistance of the latter increase.
Uses
- Nylon 6,6 is primarily used for fibres and tyre cord, which find use in making rocks, ladies hoses, undergarments, dresses, carpets, ropes, etc.
- Nylon 6,6 and nylon 6,11 are mainly used for moulding purposes for gears, bearings, electrical mountings, etc. Nylon bearings and gears work quietly without any lubrication.
- They are also used for making filaments for ropes, bristles for tooth brushes, films, etc.
2.7.2 Thermosetting Plastics
Phenol-Formaldehyde Resins or Phenoplasts
These are condensation polymerisation products of phenolic derivatives with aldehydes, prepared by condensing phenol with formaldehyde in presence of acidic or alkaline catalyst. Depending upon catalyst and reactants mainly three kinds of resins are formed, they are classified as follows:
- Novalac resin: The presence of acid, phenol and formaldehyde condense to form novalac resin. At first, formaldehyde takes proton from acid and forms the carbonium ion.

Phenol react with carbonium ion to form ortho- and para-methylol phenol.

Ortho-methylol phenol condenses to form novalac resin.

- Resol resin: Phenol and formaldehyde are refluxed with ammonia at 100°C gives resol resin. In presence of ammonia, methylol phenol has greater reactivity with formaldehyde than phenol, hence it gives di- and tri-methylol products.

The polymethylol phenol condenses and forms resol resin.

- Bakelite: Bakelite was first prepared by Baekeland. In the presence of hexamethylenetetramine, phenol reacts with formaldehyde and forms cross linked resin, that is, Bakelite. It is a hard and insoluble solid.

Properties
Novalac resin is soluble and fusible solid. Resol resin is a hard and brittle solid. Bakelite is a rigid, hard, infusible, water resistant, and insoluble solid. It resist to non-oxidising acids, salts and organic solvents, but are attacked by alkali due to presence of free hydroxyl groups. All phenol formaldehyde resins possess excellent electrical insulating character.
Uses
The uses of resol resin are as follows:
- Making electric insulator parts like switches, plugs, switch-boards, heater-handles, etc.
- Preparing moulded articles like telephone parts, cabinets for radio and television.
- Impregnating fabrics, wood and paper.
- As adhesives for grinding wheels.
- Making bearings used in propeller shafts for paper industry and rolling mills.
- As hydrogen-exchanger resins in water softening.
Polyurethanes
Diisocyanate and diol give polyurethanes.
For example, a reaction between 1,4-butane diol and 1,6-hexane diisocyanate gives “Perlon - U”, a crystalline polymer.
Properties
The properties of polyurethanes are as follows:
- Polyurethanes are less stable than nylons.
- These have excellent resistance to abrasion and solvents.
Uses
These are used as coatings, films, foams, adhesives and elastomers.

2.8 RUBBER (ELASTOMERS)
Rubbers are high polymers, which have elastic properties. Thus, the rubber band can be stretched to four to 10 times its original length, and as soon as the stretching force is released, it returns to its original length. The elastic deformation in an elastomer arises from the fact that in the unstressed condition, an elastomer molecule is not straight chained, but in the form of a coil, it can be stretched like a spring consequently. The unstretched rubber is amorphous.
2.8.1 Processing of Natural Rubber
Isoprene is the basic molecule present in natural rubber. Dispersive form of isoprene units are known as latex. In the processing of natural rubber isoprene molecules polymerise and form long, coiled chains of cis-polyisoprene.

By making small incisions on the bark of rubber trees, like having a brasiliensis and guayule, the rubber latex can be collected into small vessels, as it oozes out. It contains 25%–45% of rubber in the form of milky colloidal emulsion, the remainder of which is made mainly of water and small amounts of protein and resinous material with time, the flow of latex from the incision made start decreases. Thus, at regular intervals, tapping is necessary throughout the life of the tree.
Latex is diluted to make 15%–20% of rubber and is filtered to eliminate any dirt present in it. It is then coagulated in a tank, fitted with irregular partitions by adding about one kg of acetic acid or formic acid per 200 kg of rubber, to a soft white mass. After washing and drying, the coagulated residue is treated as follows:
- Crepe rubber: It is prepared by adding a little sodium bisulphite to bleach the rubber and is then passed through a creping machine so that coagulum is rolled out into sheets of about 1 mm thickness. The sheet possesses an even rough surface resembling crepe paper. The sheet is then air-dried at 50°C.
- Smoked rubber: It is made by eliminating the bleaching with sodium sulphite and rolling the coagulum into thicker sheets, having ribbed pattern on its surface. The ribbed surface pattern on the sheet prevents them from adhering together on stacking. It also facilitates consequent drying as it exposes greater surface area of the sheet. The sheets are then dried in the smoke obtained from burning wood or coconut shells at about 50°C. The rubber sheet thus obtained is translucent and amber in colour.
2.8.2 Gutta–Percha
It is a trans-form of natural rubber. (In natural rubber, isoprene units are linked with cis-form). It is obtained from the matured leaves of Dichopsis gutta and Palaquium gutta trees, grown mostly in Malaya and Sumatra. Gutta–percha can be recovered by solvent extraction process, when insoluble resins and gums are separated. Alternatively, the matured leaves are grounded carefully and is treated with water at about 70°C for half an hour and then poured into cold water, when Gutta–percha floats on water surface it is removed.

Properties
The properties of gutta percha are as follows:
- At room temperature, gutta percha is horny and tough, but softens and becomes tacky at about 100°C.
- It is soluble in aliphatic hydrocarbons, but insoluble in aromatic and chlorinated hydrocarbons.
Uses
It is used in the manufacturing of golf ball covers, submarine cables, adhesives and tissues for surgical purposes.
2.8.3 Vulcanisation of Rubber
Drawbacks of Raw (Natural) Rubber
The drawbacks of raw (natural) rubber are as follows:
- It is plastic in nature. Crude rubber becomes soft and sticky in hot summer, while in cold weather, it becomes hard and brittle. Its usage is limited to a particular temperature range of about 10°C–60°C.
- It is weak, due to low tensile strength (200 kg/cm2).
- It has large water absorption capacity.
- It is non-resistant to non-polar solvents such as vegetable and mineral oils, gasoline, benzene and carbon tetra chloride.
- It is attacked by oxidising agents (HNO3, H2SO4, etc.). It perishes due to the oxidisation in air.
- In organic solvents, it undergoes swelling and gradual disintegration.
- It possesses tackiness, which means that under pressure, two fresh raw rubber surfaces coalesce to form a single piece.
- It possesses very less durability.
- When stretched to a great extent, some molecular chains undergo sliding or slipping over each other, hence it suffers from permanent deformation.
To improve the properties of rubber, it is compounded with some chemicals such as sulphur, hydrogen sulphide, benzoyl chloride and the rubber mix is prepared for vulcanisation. The addition of compounding agents is facilitated by the process of mastication. Mastication of rubber means that it is subjected to severe mechanical working. Oxidative degradation accompanied by a marked decrease in the molecular weight of the rubber occurs and converts rubber into a soft and gummy mass.
Vulcanisation
Heating of raw rubber with sulphur at around 100°C –140°C is known as vulcanisation. Sulphur combines chemically at the double bonds of rubber chains and forms cross links. With these crosslinks, the rubber becomes stiff and the percentage of sulphur determines the stiffness of rubber.
For example, a rubber tyre contains 3–5% of sulphur.

Advantages of Vulcanisation
The advantages of vulcanisation are as follows:
- The tensile strength of vulcanised rubber is very good and has extensibility about 10 times the tensile strength of raw rubber; when a tensile force is applied, it can bear a load of 2000 kg/cm2 before it breaks.
- After the removal of deforming force, the articles made from vulcanised rubber regain their original shape, so that it has excellent resilience.
- It has broader useful temperature range (–40°C–100°C) compared to raw rubber’s temperature range (10°C–60°C).
- It has much high resistance to moisture, oxidation and abrasion.
- It has much higher resistance to wear and tear as compared to raw rubber.
- It is a better electrical insulator, although it tends to absorb small amounts of water.
or example, ebonite is a better insulator.
- It has resistance to organic solvents like benzene, carbon tetrachloride, petrol, etc., but it swells in them.
- It is very easy to manipulate vulcanised rubber to produce the desired shapes.
The superior properties of vulcanised rubber compared to raw rubber are summarised in Table 2.1.
Table 2.1 Raw rubber vs. vulcanised rubber

2.8.4 Compounding of Rubber
Compounding is “mixing of the raw rubber with other chemicals so as to impart the product-specific properties suitable for particular job”. The following substances are generally mixed with raw rubber:
- Antioxidant: Natural rubber has a tendency to “perish” due to oxidation, retarding the deteriorating of rubber by preventing its oxidation by light and air; about 1% of the antioxidants are used. These are phenolic substances, phosphites and complex organic amines like phenyl naphthylamine.
- Colouring agents: Colouring agents impart the desired colour to the rubber product.
For example, for white products, titanium dioxide (TiO2) is the usual pigment. For colour products, the following pigments are used:

- Vulcanising agents: The main vulcanising agent is sulphur. Among these, sulphur monochloride, hydrogen sulphide and benzoyl chloride are also used as vulcanisers. Depending on the nature of the product required, the percentage of S added varies between 0.15% and 32%. The process of vulcanisation brings about excellent changes in the properties of crude rubber.
- Accelerators: They are positive catalysts for vulcanisation process as they drastically shorten the time required for vulcanisation. Generally, benzothiazole, 2-mercaptol, zinc alkyl xanthate and thiocarbamates are used in 0.5%–1% instances as the most usual accelerators.
- Reinforcing agents: These agents give rigidity, strength and toughness to the rubber and are present up to 35% of the rubber compound. Commonly used reinforcers are carbon black, zinc oxide, CaCO3, MgCO3, etc.
For example, addition of carbon black in the elastomer is used in the manufacture of automobile tyres.
- Plasticisers and softness: They impart greater tenacity and adhesion to the raw rubber. The important plasticisers are stearic acid, waxes, vegetable oils, rosin, etc.
- Inert fillers: They lower the cost of the product and alter the physical properties of the mix for simplifying the subsequent manufacturing operations.
2.8.5 Synthetic Rubbers or Artificial Rubber
The landmark discovery of rubber is the greatest achievement in polymer industry and with the efforts of scientists and technologists the first useful synthetic rubber Buna-S was prepared.
Advantages of Synthetic Rubbers
Due to better performance properties of synthetic elastomers, natural rubber failed to give stiff competition.
- They are produced from petrochemical raw materials in abundant amounts.
- They are economically beneficial.
- They are not only replacements but are superior to natural rubber in certain cases.
- They are tailor-made elastomers with diverse applications.
- They have high abrasion resistance and high tensile strength.
- Certain elastomers like silicones have low temperature (–80°C), flexibility and high temperature stability.
- Silicones are valuable in surgical prosthetic devices.
2.8.6 Important Artificial Rubbers
Styrene Butadiene Rubber (GR-S or Buna-S)
It is a copolymer of styrene (25% by weight) and butadiene (75% of weight). The monomers are emulsified in water using soap or detergent. The reaction is initiated by peroxide initiators. Polymerisation is carried out at 5°C, and therefore, the product is known as cold SBR.

Properties
Styrene rubber is slightly inferior to natural rubber in its physical properties. It possesses high abrasion resistance, high load-bearing capacity and resilience. However, it gets readily oxidised, especially in the presence of traces of ozone present in the atmosphere. It swells in oils and solvents. It can be vulcanised in the same way as natural rubber, but it requires less sulphur and more accelerators for vulcanisation.
Uses
The uses of styrene rubber are as follows:
- It is mainly used for the manufacture of motor tyres, (passenger car tyres, motor cycle tyres and scooter and cycle tyres.) but not used for truck tyres.
- It is also used for floor tiles, shoe soles, gaskets, footwear, conveyor belts, wire and cable insulations, carpet backing, adhesives, tank linings, etc.
Buna-N, Nitrile Rubber or GR–A
It is a copolymer of a 1,3-butadiene and acrylonitrile. They are also prepared in emulsion systems. They are noted for their oil resistance but not suitable for tyres.

Properties
It possesses excellent resistance to heat, sunlight, oils, acids and salts, but it is less resistant to alkalis than natural rubber because of the presence of cyano groups. As the proportion of acrylonitrile is increased, the resilience to acids, salts, oils, solvents, etc., increases, but the low temperature resilience suffers. Vulcanised rubber is more resistant to heat and ageing than natural rubbers and may be exposed to high temperature.
Uses
The uses are as follows:
- They are extensively used for fuel tanks, gasoline hoses, creamery equipment, conveyor belts and high-altitude aircraft components.
- They are also used as adhesives, latex, gaskets, printing rollers, leather and textile.
Poly Sulphide Rubber, Thiokol or GR–P
Thiokols are those elastomers in which sulphur forms a part of the polymer chain. It is a copolymer of sodium poly sulphide (Na2S4) and ethylene dichloride.
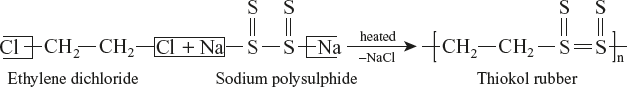
Properties
Thiokols have outstanding resistance to swelling and disintegration by organic solvents, mineral oils, fuels, solvents, oxygen, ozone, gasoline and sunlight. Thiokol films have low permeability to gases. They have the following limitations:
- It tends to flow or lose shaped under continued pressure. As it cannot be vulcanised, it does not form hard rubber.
- Its tensile strength is lesser than that of natural rubber.
- It cannot be vulcanised.
Uses
The uses of Thiokols are as follows:
- Thiokols are used for barrage balloons, life rafts and jackets, which are inflated by CO2.
- It is also used for lining hoses for conveying gasoline and oil, in paints, for gaskets, diaphragm and seats in contact with solvents, and for printing rolls.
Polyurethanes (Isocyanate Rubber)
These polymers are formed by the reaction between diisocyanates and polyalcohols.

Properties
Polyurethane elastomers have outstanding abrasion resistance and hardness combined with good elasticity and resistance to oils, greases, chemical, weathering and solvents.
Uses
They are used in applications where extreme abrasion resistance is required such as in heel lifts, surface coatings, manufacture of foams, spandex fibres and small industrial wheels.
Silicone Rubbers
Silicones are organic silicone polymers. They have alternate Si-O-bonds.
Preparation: For preparation of silicones, dialkyl-substituted silanes are used as raw materials.
They undergo hydrolysis and condensation polymerisation to form silicone polymers.
Silicones are of mainly two types:
- Straight-chain polymers: Dialkyl-substituted silanes are subjected to hydrolysis and undergo condensation polymerisation to form straight-chain thermoplastic silicones.

- Cross-linked polymers: Mono alkyl-substituted silanes are subjected to hydrolysis and proceeds condensation reaction gives cross-linked polymers.

Alkyl chlorosilanes are prepared by this process.

Properties
The properties of silicones are as follows:
- Silicones have resistance to heat, cold, oxidation, etc.
- They obtain as liquids, resins and elastomers.
- Silicon liquids are having repulsion with water. They have also resistance with chemicals.
- Silicone rubber may form reaction between silicone resins, silica and alumina.
Uses
The uses of silicones are as follows:
- Silicones are used as anti-forms in the preparation of food stuff.
- They are used as elastics, insulators, gascatoles and ceiling joints, and also used in medicine.
- They are used as surface-coating agents.
- Silicones are used for lamination.
Reclaimed Rubber
Reclaimed rubber is rubber obtained from waste rubber articles such as worn-out tyres, tables, gaskets, hoses, foot wear and so on. The process of reclamation of rubber is carried out as follows:
The waste is cut to small pieces and powdered by using a cracker, which exerts powerful grinding and tearing action. The ferrous impurities, if any are removed by the electromagnetic separator. The purified waste powdered rubber is then digested with caustic soda solution at about 200°C under pressure for 8–15 hours in a “steam-jacketed autoclave”. By this process, the fibres are hydrolysed. After the removal of fibres, reclaimed agents (like petroleum and coal tar based oils) and softeners are added and sulphur gets removed as sodium sulphide and rubber becomes devulcanised. The rubber is then thoroughly washed with water sprays and dried in hot air driers. Finally, the reclaimed rubber is mixed with small proportion of reinforcing agents (like clay, carbon black, etc.).
Properties
The reclaimed rubber is of less tensile strength lower in elasticity and possesses lesser wear-resistance than natural rubber. However, it is much cheaper, uniform in composition and has better ageing properties. Moreover, it is quite easy for fabrication.
Uses
For manufacturing tyres, tubes, automobile floor mats, belts, hoses, battery containers, mountings, shoes and heals, etc.
2.9 REINFORCED OR FILLED PLASTICS
Polymers, generally of low strengths and moduli of elasticity, are needed for structural purposes. For these reasons, polymers are combined with fillers (which are primary silicates) to get better products. The fillers are solid additives, which modify the physical properties, particularly, the mechanical properties of basic polymeric materials. For example, they improve: (i) thermal stability, (ii) mechanical strength, (iii) insulating characteristics, (iv) water resistance, (v) external appearance, (vi) rigidity, (vii) finish, (viii) hardness, (ix) opacity and (x) workability, besides reducing (xi) cost and (xii) shrinkage on setting and brittleness.
Usually, specific fillers are added to a polymeric compound to impart special characters to the final products. Some examples are as follows:
- To improve hardness, fillers such as carborundum, quartz and mica are added.
- To make the plastic impervious to x-ray, barium salts are fillers.
- To provide heat and corrosion resistances, asbestos is added.
The combination of polymeric substance with solid fillers, is called filled or reinforced plastics. The filler acts as a reinforcing material while the polymer acts as binder, which links the filler particles. The polymer serves as stress transforming agent from filler to filler particles.
Most commonly used fillers are as follows:
(i) wood flour, (ii) saw dust, (iii) ground cork, (iv) asbestos, (v) marble flour, (vi) china clay, (vii) paper pulp, (viii) corn husk, (ix) mica, (x) pumice powder, (xi) carbon, (xii) cotton fibres, (xiii) boron fibres, (xiv) silicon carbide, (xv) silicon nitride, (xvi) graphite, (xvii) alumina, (xviii) glass fibres, (xix) Kevlar fibres, (xx) cotton fibres, (xxi) metallic oxides such as ZnO, PbO and so on, and (xxii) metallic powder such as Al, Cu, Pb and so on.
2.9.1 Composition
Fillers are usually employed in a sizable weight percentage. The percentage of filler can be up to 50% of the total moulding mix.
2.9.2 Nature of Polymers Used
Polymers used are thermoplastics, thermosetting polymers as well as rubber (elastomers) such as polyethylene, polypropylene, nylon-6, PET, polystyrene, melamine, silicone, natural and synthetic rubbers, epoxy among others.
Some examples of filled plastics are as follows:
- Carbon black on addition to natural rubber brings about 40% increase in tensile strength and such filled plastic is used in automobile tyres.
- The addition of china clay to PVC enhances the electrical insulation characteristics of PVC. Therefore, this filled plastic is used for electrical insulation purposes.
- PVC and calcium carbonate combination is used for general purposes such as tubings, seat covers, wires and cable insulations.
- Fibrous fillers such as wood flour, short length of synthetic fibres, cotton flock, macerated paper and cloth etc., are used in thermosetting polymers, phenol-formaldehyde, melamine, formaldehyde, melamine urea, etc., to get filled plastics of higher impact strength.
- Asbestos-filled plastics are employed in electrical appliances.
- Fibre-reinforced plastics possess good thermal and shock resistance, good dimensional stability, good process ability and moulded articles can be repaired easily.
2.9.3 Application of Filled Plastics (Reinforced Plastics)
Filled or reinforced plastics find numerous applications. Some examples are as follows:
- In automobiles for making door handles battery cases, engine cooling fans, etc. (using base polymers polyethylene, polypropylene and nylon-66).
- In defence for making nose cones, pistol grips and riffle bullets (using base polymers polystyrene and nylon-66).
- In textiles for making shuttle (nylon-66 as base polymer).
- In electrical/electronic industry for making exhaust fan, computer tape, insulators, wire and cable insulation, switch gear parts, spools, etc., (using polypropylene, PET, nylon and SAN as base polymers.)
- In consumer goods like door handles, and window, hinge, chair shells, camera housing etc. (using polypropylene and ABS as base polymers).
- Miscellaneous like water meter housings, chemical pump housings, tubings, seat coverings etc., (using nylon-66, PVC and polypropylene as polymers).
2.10 BIOPOLYMERS
Biopolymers are macromolecules that occur in nature from plants, trees, bacteria, algae or other sources that are long chains of molecules linked together through a chemical bond. They are often degradable through microbial processes such as composting. For example, cellulose, proteins, starch, collagen, casein, polyesters, etc.
“Sustainable biopolymers” are sourced from sustainably grown and harvested cropland or forests, manufactured without hazardous inputs and impacts, healthy and safe for the environment during use and designed to be reutilised at the end of their intended use through recycling or composting. The potential for using these materials to make synthetic polymers was identified in the early 1900s, but they have only recently emerged as a viable material for large-scale commercial use.
2.10.1 Major Feed Stocks for Biopolymers
While biopolymers can be made from an almost unlimited range of bio-based materials, most of the currently marketed biopolymers are made from starch. Corn is currently the primary feedstock, with potatoes and other starch crops also used in lower amounts.
2.10.2 Preparation Methods
At present, either renewable or synthetic starting materials may be used to produce biodegradable polymers. Two main strategies may be followed in synthesising a polymer. One strategy is to build up the polymer structure from a monomer by a process of chemical polymerisation. The alternative is to take a naturally occurring polymer and chemically modifying it to give it the desired properties. A disadvantage of chemical modification is that the biodegradability of the polymer may be adversely affected. Therefore, it is often necessary to seek a compromise between the desired material properties and biodegradability.
2.10.3 Important Biodegradable Polymers
Biodegradable polymers can be easily degradable by biological activities of decomposers. The natural raw materials are abundant, renewable and biodegradable, making them attractive feedstock for a new generation of environmentally friendly bioplastics. Even if only a small percentage of the biopolymers already being produced were used in the production of plastics, it would significantly decrease our dependence on non-renewable resources.
- Lactic acid is now commercially produced on a large scale through the fermentation of sugar feedstock obtained from sugarcane, from the conversion of starch from corn, potato peels or other starch source. It can be polymerised to produce poly (lactic acid).
Poly (lactic acid) has become a significant commercial polymer, useful for recyclable and biodegradable packaging, such as bottles, yogurt cups and candy wrappers. It has also been used for food service ware, lawn and food waste bags, coatings for paper and cardboard and fibres for clothing, carpets, sheets and towels and wall coverings. In biomedical applications, it is used for prosthetic materials, drug encapsulation, biodegradable medical devices and materials for drug delivery.
- Triglycerides is another promising raw material for producing plastics from a large part of the storage lipids in animal and plant cells such as soybean, flax and rape seed. Triglycerides have recently become the basis for a new family of composites. With glass fibre reinforcement, they can be made into long-lasting durable materials with applications in the manufacture of agricultural equipment, the automotive industry, construction and other areas. Fibres other than glass can also be used in the process, such as fibres from jute, hemp, flax, wood and even straw or hay. If straw could replace wood in composites now used in the construction industry, it would provide a new use for an abundant, rapidly renewable agricultural commodity and at the same time conserve less rapidly renewable wood fibre.
- Starch is found in corn (maize), potatoes, wheat, tapioca (cassava) and some other plants. Starch-based bioplastics are important not only because starch is the least expensive biopolymer but because it can be processed by all of the methods used for synthetic polymers, like film extrusion and injection moulding. Utensils, plates, cups and other products have been made with starch-based plastics. The annual world production of starch is well over £70 billion, with much of it being used for non-food purposes, such as making paper, cardboard, textile sizing and adhesives.
Starch-protein compositions have the interesting characteristic of meeting nutritional requirements for farm animals. Hog feed, for example, is recommended to contain 13%–24% protein, and complemented with starch. If starch-protein plastics were commercialised, used food containers and service ware collected from fast food restaurants could be pasteurised and turned into animal feed.
- The interest in soybeans has been revived, recalling Ford’s early efforts. In research laboratories, it has been shown that soy protein, with and without cellulose extenders, can be processed with modern extrusion and injection moulding methods and also used for making adhesives and coatings for paper and cardboard.
- Many water-soluble biopolymers such as starch, gelatine, soy protein and casein form flexible films when properly plasticised. Although such films are regarded mainly as food coatings, it is recognised that they have potential use as non-supported stand-alone sheeting for food packaging and other purposes.
- Casein, commercially produced mainly from cow’s skimmed milk, is used in adhesives, binders, protective coatings and other products.
- Polyesters are now produced from natural resources such as starch and sugars, through large-scale fermentation processes, and are used to manufacture water-resistant bottles, utensils and other products. Polyesters are produced by bacteria, and can be made commercially on a large scale through fermentation processes. They are now being used in biomedical applications.
- Collagen is the most abundant protein found in mammals. Gelatine is denatured collagen, and is used in sausage casings, capsules for drugs and vitamin preparations and other miscellaneous industrial applications including photography.
2.10.4 Importance of Biopolymers in Sustainable Development
There are enormous societal benefits that result from a shift to bio-based plastics. Bio-based materials have the potential to produce fewer greenhouse gases, require less energy and produce fewer toxic pollutants over their lifecycle than products made from fossil fuels. They may also be recyclable or composted (depending on the biomaterial and how it is produced), reducing waste streams to already crowded landfills or to incinerators.
As the cost of petroleum increases, making products with bio-based materials is increasingly attractive. Increased demand for agricultural and forest-based feedstock also offers new resource-based economic development opportunities for farmers, struggling rural communities and manufacturing sectors.
However, many of these advantages are not inherent in the material. They depend on ensuring that bio-based products meet minimal standards for the safe production, use and end-of-life disposition.
Making the transition from a petroleum-based to a bio-based economy also gives us an opportunity to ensure that the impact of product standards on the environment, health and society are included.
The widespread use of these new plastics will depend on developing technologies that can be successful in the marketplace. This, in turn, will partly depend on how strongly society is committed to the concepts of resource conservation, environmental preservation and sustainable technologies. There are growing signs that people indeed want to live in greater harmony with nature and leave a healthy planet to the future generation. If so, bioplastics will find a place in the current age of plastics.
2.11 CONDUCTING POLYMERS
Due to non-availability of free electrons, most normal polymers are insulators. Scientists have taken this property as an advantage, and with their curiosity and challenging nature, they prepared conducting polymers as promising materials.
“Conducting polymer is an organic polymer having highly delocalised π-electron system and electrical conductance”.
Conducting polymers are broadly classified into two categories as intrinsically conducting polymers and extrinsically conducting polymers.
2.11.1 Intrinsically Conducting Polymer (ICP) or Conjugated π-Electrons Conducting Polymer
Polymers which contain conjugated π-electron backbone or delocalised electron pairs act as intrinsic conducting polymers.
For example,
- Polyacetylene polymers like Poly-p-phenylene, polyquinolone, etc.
- Condensed aromatic polymers like polyaniline, polyphenanthrylene, etc.
- Heteroaromatic and conjugated aliphatic polymers like polypyrrole, polyazomethine, etc.
If the conductivity of intrinsically conducting polymers is less, they are doped with positive or negative charges and this process is known as doping.
It is mainly oxidative or p-doping, reductive or n-doping and protonic acid doping.
- Oxidative or p-doping: Treating of intrinsically conducting polymers with Lewis acid like I2, Br2, FeCl3, CCl4, HBF4, etc., is known as p-doping.

Mechanism of conduction: The removal of an electron from the polymer П-back bone using a suitable oxidising agent leads to the formation of delocalised radical ion called polarion.
A second oxidation of a chain containing polarion, followed by radical recommendation, yields two charge carriers on each chain. The positive charges sites on the polymer chains are compensate by anions formed by the oxidising agent.
The delocalised positive charges on the polymer chain are mobile, not the dopant anions.
Thus, these delocalised positive charges are current carriers for conduction. These charges must move from chain to chain as well as along the chain for bulk conduction.
- Reductive (or) n-doping: Treating of intrinsically conducting polymers with Lewis bases such as Li, Na, tetrabutyl ammonia, naphthylamine, etc., is known as n-doping.

Mechanism of conduction: The addition of an electron to the polymer П-back bone by using a reducing agent generates a polarion. A second reduction of chain containing polarion, followed by the recombination of radicals, yields two negative (-ve) carriers on each chain. These charge sites on the polymer chains are compensated by cations formed by the reducing agent.
- Protonic acid doping: The synthesis of conducting polyaniline is a typical example of this type of doping technique. In this technique, current-carrying charged species (-ve/+ve) are created by the protonation of imine nitrogen.
Polyaniline is partially oxidised first, with a suitable oxidising agent, into a base form of aniline, which contains alternating reduced and oxidised forms of aniline polymer backbone. This base form of aniline when treated with aqueous HCl (IM) undergoes protonation of imine nitrogen atom, creating current due to +ve sites in the polymer backbone. These charges are compensated by the anions (Cl−) of the doping agent, giving the corresponding salt. This doping results increase conductivity up to 9–10 orders of magnitude.
Applications
Conducting polymers are the most important materials to be used in electric and electronic applications. Some of the uses are as follows:
- As electrode material for commercial rechargeable batteries, for higher power-to-weight ratio.
- As conductive tracks on printed circuit boards.
- As sensors—gas sensor, radiation sensor, humidity sensor, bio sensor for glucose and galactose, etc.
- As film membranes for gas separations.
- As light-emitting diode.
- In electrochemical display windows.
- In fuel cells, as the electro catalytic materials.
- In information storage devices.
2.11.2 Conducting Polyaniline
In 1985, Alan MacDiarmid investigated polyaniline as an electrically conducting polymer.

Properties
The properties of polyaniline are as follows:
- Except specific conductivity, all other properties of polyaniline are considered as that of an organic metal.
- It is transparent in thin layers.
- When heated, it is stable in air.
- It is a highly reactive, redox active material.
- In conducting state, it is green and changes its colour in different media.
For example, under reducing condition, it becomes yellow and under oxidising or basic condition, it becomes blue.
Advantages
The advantages are as follows:
- It is the foremost air-stable conducting polymer.
- It has a wide and controllable range of conductivity.
- It shows a number of interesting properties such as multicolour, electrochromism, chemical sensitivity and so on.
- It is considered to be unique among all the conducting polymers as it can be synthesised chemically or electrochemically as a bulk powder or film.
Disadvantages
The disadvantages are as follows:
- It is insoluble in common solvents except strong acids and n-methyl pyrrolidine.
- It has poor mechanical properties.
- It decomposes prior to melting, and hence it is difficult to process.
Uses
The uses of intrinsically conducting polymers are as follows:
- Due to its reversible electrochemical response during anodic oxidation and cathodic reduction, it is useful as a secondary electrode in rechargeable batteries and electrochromic display devices.
- It is used for coating of films and semi-finished articles.
- It is also used for corrosion protection, sensors, smart windows, printed circuit boards, conductive pipes for explosives, and conductive fabrics.
2.11.3 Extrinsically Conducting Polymers
Polymers whose conductivity is due to externally added ingredient are known as extrinsically conducting polymers. They are conductive element filled polymers and blended conducting polymers.
- Conductive element-filled polymers: In this kind of polymers, conducting materials like carbon black, metal oxides, etc., are added to the polymers.
- Blended conducting polymers: In this kind of polymer, conducting polymers are mixed with conventional polymer.
Application of Conducting Polymers
Conducting polymers have many uses because they are light weight, easy to process and have good mechanical properties. They are used in the following:
- In chargeable light-weight batteries based on perchlorate-doped polyacetylene lithium systems. These are approximately 10 times lighter than conventional, lead storage batteries. They have flexibility to fit into different designs.
- In optical display devices, based on polythiophene and polyaniline. When the structure is electrically biased, the optical density of the film and its colour changes. Such electrochromic systems produce colour displays with faster switching time and better viewing than conventional liquid display devices. Due to redox activity in conducting state, polyaniline is green; under reducing condition, it is in yellow colour, whereas under oxidising condition, it appears blue.
- In telecommunication systems
- In electromagnetic screening materials
- In antistatic coatings for clothing
- In electronic devices such as transistors and diodes
- In wiring in aircrafts and aerospace components
- In solar cells and as biosensors in metabolic reactions and drug delivery system for the human body
- In photo voltaic devices
- In non-linear optical material
- In molecular wires and molecular switches
2.12 POLYPHOSPHAZENES/PHOSPHONITRILIC POLYMERS
Polyphosphazenes are hybrid inorganic organic polymers with a number of different skeletal structures that contain a backbone of alternating phosphorous and nitrogen atoms, and are interesting, commercially promising materials. A variety of substituents can substitute the basic backbone and hence we can get a variety of products. The basic backbone of polyphosphazene is as follows:

Preparation: The most popularly used method for preparing polyphosphazenes is ring opening and substitution method. Allcock and co-workers discovered that cyclic trimer (hexachlorocyclo triphosphazene) can be thermally ring opened and can give high molecular weight soluble poly (dichlorophosphazene). After the replacement of the chlorine atoms in poly (dichlorophosphazene) by reaction with organic/organometallic nucleophiles, they give a variety of polyphosphazenes.
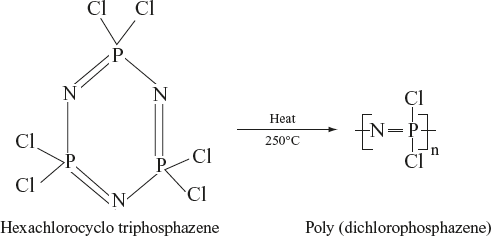
Substitution Reactions
- Reaction with sodium alkoxide: Poly (dichlorophosphazene) reacts with sodium alkoxide and gives poly (dialkoxyphosphazene).

- Reaction with amine: Poly (dichlorophosphazene) reacts with amine and gives poly (dialkylaminephasphazene).

- Reaction with metal alkyd: Poly (dichlorophosphazene) reacts with metal alkyd and gives poly (dialkylphasphazene).

Important Characteristics of Polyphosphazenes
polyphosphazenes have many important properties; biocompatibility, high dipole moment, flexibility, chemical inertness, broad range of glass transition temperature (Tg), elastomeric property and impermeability are the most important of them.
Applications
Based on their wide range of unique properties, polyphosphazenes have countless and advanced applications. They have potential for formation of new compounds. The applications include in challenging areas of biomedical research such as tissue generation, macromolecules and so on. These are also used as ion-conductive membranes for rechargeable lithium batteries and fuel cell membranes. These are advanced materials of elastomers for aerospace engineering. Polyphosphazenes are good photonic materials and fire-resistant polymers.
2.13 COMPOSITES
Composites are multiphase materials that exhibit a significant proportion of the properties of both the constituent materials.
![]()
Composite materials composed of at least two distinctly dissimilar materials act in harmony. A judicious combination of two or more distinct materials can provide better combination of properties or an artificially prepared multiphase material in which the chemically dissimilar phases are separated by a distinct interface.
For example, wood is the composite of cellulose and lignin, bone is the composite of a soft, strong protein collagen, and brittle, hard apatite material.
Packing paper impregnated with bitumen or wax, rain-proof cloth (cloth impregnated with waterproof material), insulating tape, reinforced concrete, etc.
2.13.1 Constituents of Composites
Composite material mainly comprises of the following:
- Matrix phase: Matrix phase is the continuous body constituent enclosing the composite and given in its bulk form. Depending upon the matrix phase, composites are known as ceramic matrix composites, metal matrix composites, polymer matrix composites, etc.
Functions of Matrix Phase
- Matrix phase binds the dispersion phase, act as a medium, applied stress is transmitted and distributed uniformly.
- It protects the surface from damage due to chemical reactions, mechanical abrasion, etc.
- It prevents the propagation of brittleness, cracking, etc.
Hence, a good matrix phase should be ductile, having corrosion resistant and possess high binding strength.
- Dispersed phase: Dispersed phase is the structural constituent of composite. Fibres, flakes, whiskers, etc., are some important dispersed phases.
2.13.2 Classification of Composites
Composites are broadly classified into three categories:
- Particle-reinforced composites: In these composites, the dispersed phase is equiaxed, that is, the dimensions of the particles are nearly the same in all directions. They are subdivided into the following:
- Large-particle composites
- Dispersion-strengthened composites
- Fibre-reinforced composites: In these composites, the dispersed phase is in the form of fibres. These are subdivided into (a) continuous aligned (b) discontinuous.
Discontinuous composites are further divided into (a) aligned (b) randomly oriented.
- Structural composites: In these composites, the properties depend not only on the constituent material but also on this geometrical design. These are subdivided into (a) laminates and (b) sandwich panels.
Among these, fibre-reinforced polymer composites are widely used.
Fibre-reinforced Polymer Composites
These are prepared by reinforcing a plastic matrix with a high-strength fibre material.
Fibre-reinforced composites involve three components, namely filament, a polymer matrix and an encapsulating agent (which ties fibre filaments to polymer). Glass fibres and metallic fibres are commonly employed for this purpose. The fibres can be employed either in the form of continuous lengths, staples or whiskers. Such composites possess high specific strength (tensile strength/specific gravity) and high specific modulus (elastic modulus/specific gravity), stiffness and lower overall density.
Characteristics
The fibre-reinforced composites possess superior properties such as higher yield strength, facture strength and fatigue life. The fibres prevent slip and crack propagation and inhibit it, thereby increasing mechanical properties. When a load is applied, there is a localised plastic flow in the matrix, which transfers the load to the fibres embedded in it. When a soft phase is present in hard matrix, the shock resistance of the composite is increased. On the other hand, if hard-reinforcing fibres are present in a soft matrix, the strength and modulus of the composite are increased. To obtain composites having the maximum strength and modulus, it is essential that there should be maximum number of fibres per unit volume, so that each fibre takes its full share of the load. The fibre-reinforced composites are, generally, anisotropic (i.e., having different directions), and the maximum strength is in the direction of alignment of fibres. For getting isotropic properties, the fibres are oriented randomly within the matrix, for example, ordinary fibre glass. It may be pointed here that the cost of laying fibres aligned in a particular direction is much higher than that for random orientation. For preparing fibre-reinforced composites, the following are essential:
- The coefficient of expansion of the fibre matches closely that of the matrix.
- The fibre and matrix should be chemically compatible with each other and no undesirable reaction takes place between them.
- The fibre should be stable at room temperature and should retain a good percentage of strength at elevated temperatures.
Some important reinforced composites are described here.
- Glass fibre-reinforced polymer composites: For improving the characteristics of nylon, polyester, etc., containing polymer matrices, glass fibres are employed. These have lower densities, higher tensile strengths and resistance to corrosion and chemicals.
Limitations: The limitations are as follows:
- Since the most polymeric matrices start deteriorating or flow or melt at high temperatures, they find application with limited temperature service conditions.
- They cannot be employed as structural components, since these materials do not possess the desired stiffness and rigidity.
Applications: They are used in automobile parts, storage tanks, floorings (industrial), transportation industries, plastic, pipes, etc.
- Carbon fibre-reinforced polymer composites: They are also known as advance polymer matrix composites or high performance composites and are employed in situations requiring (i) excellent resistance to corrosion, (ii) lighter density and (iii) retention of desired properties, even at elevated temperatures. However, the general use is limited due to their higher costs.
Applications: They are used as structural components (like wing, body and stabiliser) of aircrafts (military and commercial) and helicopter’s recreational equipment (fishing rod), sport materials (golf clubs), etc.
2.13.3 Advantages of Composites over Conventional Materials
Composites have the following advantages over conventional materials such as metals, polymers, ceramics and so on.
- They have higher specific strength and stiffness. They can maintain strength up to higher temperatures.
- They have lower specific gravity, electrical conductivity and thermal expansion.
- They have better toughness, impact, thermal shock resistance, fatigue strength, corrosion and oxidation resistance.
2.13.4 Applications of Composites
- In automobile industries, transportation industries, turbine engines, wire drawing dies, valves, pump parts, spray nozzles, storage tanks, fabrication of roof and floors, furniture, sport goods (lawn, tennis rackets), high-speed machinery, etc.
- Marine applications like propellers, shafts, hulls, spars (for racing boats) and other ship parts.
- Aeronautical applications like components of rockets, aircrafts (business and military), helicopters, missiles, etc.
- Communication antennae and electronic circuit boards
- Safety equipment like ballistic protection
2.14 REVIEW QUESTIONS
2.14.1 Fill in the Blanks
- Repeating units present in polymer is _______.
[Ans.: monomer]
- When two or more different monomer units participate in polymerisation, _______ polymers are formed.
[Ans.: copolymers]
- _______ plastics cannot be remoulded.
[Ans.: thermosetting]
- Monomer units that participate in the formation of Fluon/Teflon are called _______.
[Ans.: tetrafluoroethylene]
- Bakelite is _______ plastic.
[Ans.:hermosetting]
- _______ is the basic unit present natural rubber
[Ans.: Isoprene]
- _______ monomers are involved in formation of nylon 66.
[Ans.: Hexamethylenediamine and Adipic acid]
- Monomers possess the double bonds undergo _______ polymerisation mechanism.
[Ans.: addition]
- An organic polymer with a highly delocalised pi-electron system, having electrical conductance is called a _______.
[Ans.: conducting polymer]
- _______ is an organic conducting polymer
[Ans.: Polyaniline]
- The polymers which occur in nature from plants, bacteria and algae are called _______.
[Ans.: Bio polymer]
- Triglycerides are produced from _______.
[Ans.: soya bean]
2.14.2 Multiple-choice Questions
- High polymers are
- Liquids
- Gases
- Solids
- Colloidal
[Ans.: d]
- Polymerisation in which two or more chemically different monomers takes part, is called
- Addition polymerisation
- Co polymerisation
- Chain polymerisation
- None of these
[Ans.: b]
- Structural units of high polymers, are called
- Fibres
- Thermo units
- Monomers fabrics
- Fabrics
[Ans.: c]
- GR-S rubber is an example of
- Condensation polymerisation
- Co polymerisation
- Addition polymerisation
- Cross-linked polymer
[Ans.: b]
- A thermoplastic is formed by the phenomenon of
- Chlorination
- Condensation polymerisation
- Nitration
- Chain polymerisation
[Ans.: d]
- A copolymer is formed by the union of two or more
- A molecules of some monomer by the elimination of small molecules like H2O
- Monomer molecules in a chain without the elimination of H2O etc.
- Monomer molecules with the elimination of small molecules like H2O
- Thermosetting and thermoplastic resins
[Ans.: b]
- Phenol-formaldehyde resin is commercially known as
- PVC
- Elastomer
- Bakelite
- Nylon
[Ans.: c]
- Polymer commonly used for making fibre/cloth is
- Rubber
- PVC
- Nylon
- Bakelite
[Ans.: c]
- Bakelite is prepared by the condensation of
- Benzene and formaldehyde
- Phenol and formaldehyde
- Phenol and acetaldehyde
- Glycerol and phthalic acid
[Ans.: b]
- A high molecular weight material that can easily be moulded in any desired shape is
- Graphite
- Resin
- Jelly
- Grease
[Ans.: b]
- A plastic which can be softened on heating and hardened on cooling is called
- Thermoelastic
- Thermoplastic
- Thermosetting
- Thermite
[Ans.: b]
- Phenol-formaldehyde (Bakelite) is an example of
- Thermoelastic
- Thermoplastic
- Thermosetting
- Thermite
[Ans.: c]
- Natural rubber is basically a polymer of
- Propylene
- Isoprene
- Ethylene
- Propane
[Ans.: b]
- Which one of the following is an elastomer?
- PVC
- Bakelite
- Natural rubber
- Nylon
[Ans.: c]
- Raw rubber on vulcanisation becomes
- Plastic
- Rocky
- Soft
- Less elastic
[Ans.: d]
- The most commonly used vulcanising agent is
- Graphic
- Carbon black
- Sulphur
- Dry ice
[Ans.: c]
- Ebonite is
- Polyethylene
- Highly vulcanised rubber
- Natural
- Synthetic rubber
[Ans.: b]
- One of the important uses of Bakelite is for making
- Cables
- Electrical switches
- Cloth
- Hose pipe
[Ans.: b]
- The fibre obtained by condensation of hexamethylenediamine and adipic acid is
- Decorn
- Nylon
- Rayon
- Terylene
[Ans.: b]
- Nylon is
- A polythene derivative
- A polyester fibre
- A polyamide fibre
- None of these
[Ans.: c]
- Monomer units involved in the formation of Bakelite are
- Urea and formaldehyde
- Phenol and formaldehyde
- Urea and phenol
- Butadiene and ethylene
[Ans.: b]
- Buna-s is a co polymer of
- Vinyl chloride and vinyl alcohol
- Butadiene and acrylonitrile
- Butadiene and styrene
- Butadiene and ethylene
[Ans.: c]
- Which one is not a macro molecule?
- Protein
- Insulin
- Ice
- Cellulose
[Ans.: c]
- Which one is a co polymer?
- Nylon-66
- Teflon
- PVC
- Polybutadiene
[Ans.: a]
- An example of chain-growth polymer is
- Nylon-66
- Bakelite
- Terylene
- Teflon
[Ans.: d]
- What is the repeating unit in Teflon?
- F2C = CF2
- H2C = CH2
- H2C = CHCl
- H2C = CHC6H5
[Ans.: a]
- Which of the following is not a thermoplastic?
- Bakelite
- Terylene
- Polystyrene
- Polythene
[Ans.: a]
- Which one is a step-growth polymer?
- Teflon
- PVC
- Polybutadiene
- Bakelite
[Ans.: d]
- Which of the following is a synthetic polymer?
- Cellulose
- PVC
- Proteins
- Nucleic acids
[Ans.: b]
- Which of the following is a linear polymer?
- Nylons
- Bakelite
- Low density polythene
- Formaldehyde polymer
[Ans.: a]
- Which of the following has the largest molecular mass?
- Monomer
- Dimer
- Oligomer
- Polymer
[Ans.: d]
- Isoprene is monomer of
- Starch
- Synthetic rubber
- Natural rubber
- PVC
[Ans.: c]
- Which of the following is thermosetting polymer?
- Linear
- Branched chain
- Cross-linked
- Thermoplastic
[Ans.: c]
- Which of the following is an addition polymer?
- Terylene
- Nylon
- Polyethylene
- All of these
[Ans.: c]
- Which of the following is thermosetting polymer?
- Nylon
- Polyethylene
- Bakelite
- PVC
[Ans.: c]
- The monomer of PVC is
- Ethylene
- Vinyl chloride
- Tetrafluoroethylene
- Styrene
[Ans.: b]
- Chloroprene is the repeating unit in
- Polystyrene
- Neoprene
- PVC
- Polythene
[Ans.: b]
- The process of vulcanisation makes rubber
- Soluble in water
- Hard
- Soft
- More elastic
[Ans.: b]
- A common catalyst used in addition polymerisation is
- Nickel
- Y-zeolite
- Ziegler–Natta catalyst
- Platinum
[Ans.: c]
- Functionality of phenol is
- One
- Two
- Three
- Six
[Ans.: c]
- _______ polymer is formed from the fermentation of sugar feed stocks obtained from sugarcane.
- Lactic acid
- Steric acid
- Triglycerides
- None of these
[Ans.: d]
- Adhesive, binders and protective coating characteristics exhibiting polymers is
- Lactose
- Galactose
- Starch
- Casein
[Ans.: d]
- Water soluble bio-polymers is/are
- Starch
- Polyester
- Both (a) and (b)
- None of these
[Ans.: a]
2.14.3 Short Answer Questions
- What is a monomer?
Ans.: The repeating unit present in the formation of a polymer is known as a monomer.
- What is a polymer?
Ans.: Polymers are macromolecules of high molecular masses built up by the linking together a large number of small, repeated units by a covalent bond.
- What is meant by polymerisation?
Ans.: The chemical process leading to the formation of a polymer is known as polymerisation.
- What is meant by degree of polymerisation?
Ans.: The number of monomer units in a polymer is known as the degree of polymerisation.
- Differentiate between homopolymer and copolymer.
Ans.: Homopolymers are formed with same monomer units.
For example, PE, PS, PVC, etc.
Copolymers are formed with two or more different monomers.
For example, styrene butadiene rubber (styrene + butadiene)
- Differentiate between addition and condensation polymerisation.
Ans.: Addition polymerisation is the process of polymerisation by the addition of monomer units which have unsaturated double or triple bonds.
For example, polyethylene, polyvinyl chloride, etc.
Condensation polymerisation takes place where the monomer units have two or more reactive functional groups.
For example, polyester, nylon, polyamide, etc.
- Define thermoplastics.
Ans.: Linear, long chain polymers which can be softened on heating and hardened on cooling are known as thermoplastics.
For example, polythene, polyvinyl chloride, etc.
- What are the monomers present on nylon 66?
Ans.: Hexamethylenediamine and adipic acid.
- What is meant by vulcanisation of rubber?
Ans.: Vulcanisation is heating of the raw rubber at 100–140°C with sulphur.
- What is natural rubber?
Ans.: Cis-polyisoprene is a natural rubber.
- What is gutta percha?
Ans.: Trans-polyisoprene is gutta percha.
- What is crepe rubber?
Ans.: Rubber with sodium bisulphite is passed through a creping machine and the coagulum is rolled into sheets. The sheet is hence having the surface like crepe paper; hence, it is known as crepe rubber.
- What is an elastomer?
Ans.: An elastomer is vulcanisable rubber like polymer, which can be stretched to at least twice its length and returned to its original shape and dimensions as soon as stretching force is released.
- Why does raw rubber need vulcanisation?
Ans.: In vulcanisation, sulphur combines chemically at the double bonds of different rubber spring and provides cross-linking between the chains. Hence, for stiffening the rubber needs vulcanisation.
- Give examples for natural polymers.
Ans.: Cotton, silk, wool, nucleic acid, proteins, starch, cellulose, etc.
- Give examples for inorganic polymers.
Ans.: Polyphosphazenes, polysilanes, polygermanes, etc.
- What are the substances mixed in the compounding of rubber?
Ans.: Antioxidants, colouring agents, vulcanising agents, accelerators, plasticisers and inert fillers are adding in the compounding of raw rubber.
- Give note on Bio-polymers?
Ans.: Bio-polymers are macromolecules that occur in nature from plants, tress, bacteria, algae or others source that are long chains linked together through a chemical bond. They are often degradable through microbial process such as compositing.
For example, cellulose, proteins, starch, collagen, casein and polyesters
- Write important bio-degradable polymers?
Ans.: (a) Lactic acids: It is obtained from fermentation of sugar feed stocks conversion of starch from corn, potato peels, etc.
(b) Triglycerides: It is produced from soya bean, fax and rape seed.
(c) Starch: It is found in corn, potatoes, wheat, tapioca and some other plants.
(d) Collagen: It is found in mammals. It is used in capsules for drug.
- Explain the importance of bio polymers?
Bio-based materials have the potential to produce fewer greenhouses gases, require less energy and produce fewer toxic pollutants.
- They may also be recyclable or composted.
- They reduce waste stream.
2.14.4 Descriptive Questions
Q.1 (a) Write a note on the properties and uses of Teflon.
(b) Differentiate between natural polymer and synthetic polymer.
(c) Write a note on silicone rubbers.
Q.2 (a) Distinguish between addition and condensation polymerisation.
(b) Explain the differences between thermoplastics and thermosetting plastics with examples.
(c) What is meant by degree of polymerisation?
Q.3 Write the structures of four addition polymers and four condensation polymers with their respective monomers.
Q.4 (a) Describe the preparation, properties and engineering uses of polyethylene.
(b) What is meant by fabrication of plastics? Mention the different fabrication techniques.
Q.5 (a) What are elastomers? Give examples.
(b) What are the ingredients used in the compounding of plastics? What are their functions?
Q.6 (a) What is a plastic?
(b) Write the merits and demerits of using plastics in the place of metals.
Q.7 (a) Identify the thermosets and thermoplastics among the following:
- PVC
- Polyethylene
- Silicone
- Polyester fibre
- Bakelite
(b) What is Bakelite? How is it manufactured? Mention its uses.
Q.8 (a) Explain the process of extrusion moulding with a neat diagram.
(b) How are the following polymers prepared? Mention their properties and uses.
- PVC
- LDPE
Q.9 (a) What are elastomers? Give the preparation, properties and uses of Buna-S.
(b) Describe a method for moulding of thermoplastic resin.
Q.10 (a) Explain the preparation, properties and uses of Bakelite.
(b) Describe the process of compression moulding with a neat sketch.
Q.11 (a) Why are silicones called inorganic polymers? Discuss the synthesis of linear chain silicones.
(b) Why can Bakelite not be remoulded? Write its repeating unit.
(c) Describe condensation polymerisation with an example.
Q.12 (a) What is a homochain polymer? Give examples.
(b) What is polymerisation? Explain the different types of polymerisation with examples.
Q.13 (a) How is HDPE prepared? Give its properties and uses.
(b) Explain the injection moulding process with a neat diagram. Mention its advantages.
Q.14 Write informative notes on the following:
- Classification of polymers
- Mechanism of radical polymerisation
- Anionic and cationic polymerisation
- Thermodynamics of a polymerisation process
Q.15 What are the common constituents of plastics and what are their functions?
Q.16 Write short notes on the following:
- Vulcanisation of rubber
- Polyvinyl chloride
- Compounding of rubber
- Reclaimed rubber
Q.17 Write an essay on the preparation, properties and uses of the following:
- Polyvinyl acetate
- Cellulose acetate
- Phenol formaldehyde resins
- Teflon
- Polyethylene
- Polystyrene
- Polymethylmethacrylate
Q.18 Discuss the various polymers related to natural rubber with emphasis on their preparation, properties and uses.
Q.19 Write an account of application of polymers in bio-medical electronic fields.
Q.20 Write an essay on the various types of synthetic rubber with brief description of the preparation, properties and uses of any three of them.
Q.21 Write an essay on fibre-reinforced plastics.
Q.22 What type of rubber would you recommend for the following?
- For a flexible connection to a steam line
- As a gasket for a pipe containing a chlorinated solvent
- In a solvent to form an adhesive
Q.23 Write short notes on the following:
- Molecular weights of polymers
- Conductive polymers
- Ionic polymers
- Liquid crystal polymers
- Engineering polymers
- Photo conductive polymers
- Polymer structure and properties of polymers
Q.24 Define the following terms and give examples: monomer, polymer, polymerisation and degree of polymerisation.
Q.25 Distinguish clearly between the following:
- Thermoplastic and thermosetting plastic
- Natural and synthetic rubber
- Addition and condensation polymerisation
Q.26 Discuss the addition polymerisation reaction mechanism.
Q.27 Explain condensation polymerisation with an example.
Q.28 Write informative notes on the following:
- Vulcanisation of rubber
- Compounding of rubber
- Gutta percha
- Silicone rubber
Q.29 Give the preparation, properties and engineering uses of the following:
- Teflon
- Polystyrene
- Polyethene
- Polyurethane
- HDPE
- Conductive polymers
Q.30 Discuss the various polymers related to natural rubber with emphasis on their preparation, properties and uses.
Q.31 Write an essay on the various types of synthetic rubbers.
Q.32 What are elastomers? Write the structure for natural rubber and gutta percha.
Q.33 What is vulcanisation of rubber? Mention its uses. Explain why natural rubber needs vulcanisation. How is it carried out?
Q.34 How is crepe rubber obtained from latex?
Q.35 What is latex? How is natural rubber isolated from it?
Q.36 Write short notes on silicones. How are they prepared?
Q.37 Write a short note on conducting polymers.
Q.38 Give the structural unit of gutta percha.
Q.39 Give detailed notes bio-polymers and its importance.
Q.40 Explain the preparation methods of bio-polymer and the importance of bio-degradable polymers.
