3
FUELS AND COMBUSTION
“A revolution in humanity’s use of fossil fuel-based energy would be necessary sooner or later to sustain and to extend modern standards of living.”
3.1 INTRODUCTION
‘Fuel is the source of heat energy, it can be stored as potential chemical energy and can be released through combustion.’
‘Combustible matter having carbon as a major ingredient, produce large amount of heat energy on burning and can be used for heat generation in industry and domestic applications is known as a fuel.’
‘Any compound or a substance which can produce energy and can be used in the production of power is termed as a fuel.’
In the combustion process, a fuel reacts with oxygen and releases the energy.
![]()
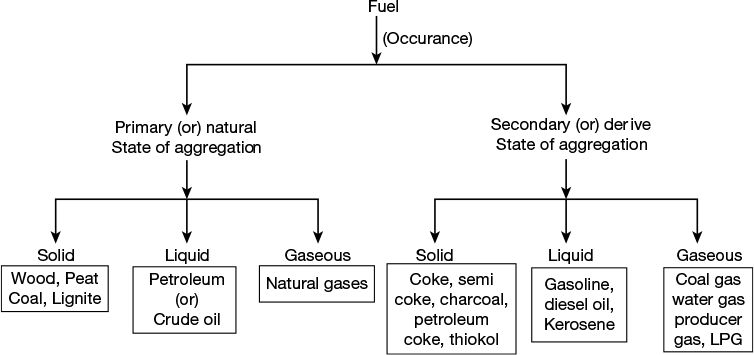
3.2 CLASSIFICATION OF FUELS
Fuels are broadly classified according to their occurrence and state of aggregation. According to the occurrence they are classified as primary (natural) and secondary (derived) fuels and based on the state of aggregation solid, liquid and gaseous fuels.
3.3 UNITS OF HEAT
- CGS system–calorie: The amount of heat required to raise the temperature of 1 g of water through 1°C at 15 to 16°C.
- MKS system–kilo calorie: The amount of heat required to raise the temperature of 1 kg of water through 1°C.

- British system (FPS system): British thermal unit (BTU): The amount of heat required to raise the temperature of 1 pound of water through 1°F at 60 to 61°F.

- Centigrade heat unit (CHU): The amount of heat required to raise the temperature of 1 pound of water through 1°C.
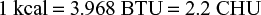
3.4 CALORIFIC VALUE
The total quantity of heat liberated by the complete combustion of one unit mass/volume of fuel in oxygen is known as calorific value. This is mainly divided into higher calorific value and lower calorific value.
- Higher (or) gross calorific value (HCV or GCV): The higher or gross calorific value is the amount of heat liberated when one unit mass/volume of the fuel is burnt completely and the combustible products are cooled to room temperature. i.e., 25°C or 77°F.
- Lower or net calorific value (LCV or NCV): Lower calorific value is defined as the amount of heat liberated when one unit of fuel is burnt completely but the combustible products are allowed to escape; hence, here lesser amount of heat is available.

This is because one part by mass of hydrogen gives nine parts by mass of water.

- Units of calorific value:

3.5 DETERMINATION OF CALORIFIC VALUE
A calorimeter is used for determining calorific value. For determining calorific value of solid and liquid fuels a bomb calorimeter is used and for gaseous fuel Junker’s calorimeter is used.
3.5.1 Bomb Calorimeter
Description
Bomb calorimeter consists of strong cylindrical stainless steel bomb with lid. The bomb carries the fuel, and the lid can be screwed to the body of the bomb and make a perfect gas tight seal. The lid has two stainless steel electrodes and an oxygen inlet valve, and among these a small ring is attached to one of the electrodes. A nickel or stainless steel crucible is supported by that right. The bomb is placed in a copper colorimeter, for preventing heat loss by radiation, it is surrounded by air and water jacket. Stirrer which can operated electrically and Beckmann’s thermometer, having sensitivity to read up to 0.01°C are provided. The set-up is shown in Figure 3.1.

Figure 3.1 Bomb calorimeter
Working
In the clean crucible, a weighted amount (0.5 to 1.0 g) of the fuel is taken and the crucible is supported by a ring, a fine magnesium wire touching the fuel sample is stretched across the electrodes. The bomb lid is tightly screwed, filled with oxygen to 25 atm pressure and then lowered into copper calorimeter, containing known mass of water, and the initial temperature (t1) is noted. Now, the circuit is completed by connecting the electrodes with a 6 V battery. The sample burns, liberates heat and absorbed by water. The water is stirred continuously for maintaining uniform temperature, and hence the final temperature (t2) is noted.
Observations and Calculation

3.5.2 Junker’s Calorimeter
Junker’s gas calorimeter (Figure 3.2) consists of a vertical cylindrical combustion chamber, and the pressure governor regulates the supply of gaseous fuel. Gasometer measures the volume of gas flowing in a particular time and combustion of fuel can be carried out by a Bunsen’s burner. The combustion chamber is surrounded by an annular water space, inside heat exchange coils and outer flues are fitted. Chromium plated outer jacket which prevent the radiative and convective heat loss from calorimeters because it contains air and acts as a very good insulator. Openings of annular space can circulate the water at the appropriate places at constant rate around the combustion chamber. Two thermometers placed at appropriate place can measure the temperatures of the inlet and outlet water.

Figure 3.2 Junker’s gas calorimeter
In the combustion chamber a known volume of gas is burned at a constant rate in excess of air, produced heat is absorbed by water. From the temperature difference, heat evolved from the gas can be calculated.
Observations and Calculation

Calculation of calorific value of a fuel can be made theoretically by using Dulongs formula.
Solved Numerical Problems Based on Calorific Value
- Calculate the gross and net calorific value of coal having the following compositions carbon –85%, hydrogen – 8%, sulphur – 1%, nitrogen – 2%, ash – 4%, latent heat of steam – 587 ca/g.
Solution Gross Calorific Value (GCV)


- A coal has the following composition by weight: C – 90%, O – 3.0%, S – 0.5%, N = 0.5% and ash = 2.5%. Net calorific value of the coal was found to be 8490.5 k cal/kg. Calculate the percentage of hydrogen and higher calorific value of coal.
 (i)
(i) (ii)
(ii)From (i) and (ii), we get
 (iii)
(iii) - 0.72 gram of a fuel containing 80% carbon, when burnt in a bomb calorimeter, increased the temperature of water from 27.3° to 29.1°C. If the calorimeter contains 250 gm of water and its water equivalent is 150 gm, calculate the HCV of the fuel. Give your answer in kJ/kg.
Solution Here x = 0.72 gm, W = 250 gm, ω = 150 gm, t1 = 273°C, t2 = 29.1°C
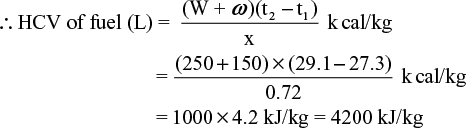
- On burning 0.83 g of a solid fuel in a bomb calorimeter, the temperature of 3500 g of water increased from 26.5°C to 29.2°C. Water equivalent of calorimeter and latent heat of steam are 385.0 g and 587.0 cal/g respectively. If the fuel contains 0.7% hydrogen, calculate its gross and net calorific value.
Solution Here, wt. of fuel (x) = 0.83 g; wt of water (W) = 3500 g; water equivalent of calorimeter (w) = 385 g; (t2 – t1) = 2.7°C; percentage of hydrogen (H) = 0.7%; latent heat of steam = 587 cal/g.

- Calculate the calorific value of a fuel sample of the coal with the following data.
Mass of the coal = 0.6 g
Water equivalent of calorimeter = 2200 gm
Specific heat of water = 4.187 kJ kg−1 °C−1
Rise in temperature = 6.52 °C
Solution Heat liberated by burning 0.6 g coal

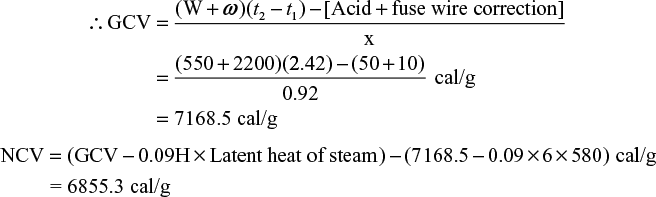
- A sample of coal contains C = 93% H = 6 % and ash = 1%. The following data were obtained when the above coal was tested in bomb calorimeter.
- Wt. of coal burnt – 0.92 gm
- Wt. of water taken – 550 gm
- Water equivalent of bomb & calorimeter – 2200 g
- Rise in temperature – 2.42 °C
- Fuse wire correction – 10.0 cal
- Acid correction – 50.0 cal
Calculate grass and net calorific values of the coal, assuming the latent heat of condensation of steam as 580 cal/g.
Solution Wt. of coal sample (x) = 0.92 g: wt. of water (W) = 550 g: water equivalent of calorimeter (w) = 2200 g: temperature rise (t2 – t1) = 2.42 °C; acid correction = 50.0 cal, fuse wire correction = 10.0 cal; latent heat of steam = 580 cal/g percentage of H = 6%
3.6 CHARACTERISTICS OF GOOD FUEL
Important characteristics of a good fuel are listed hereunder.
- HCV: The amount of heat released is dependent on high calorific value, hence fuel should possess more HCV.
- Low moisture content: Moisture content of fuel reduces the calorific value, hence fuel should possess low moisture content.
- Moderate ignition temperature: Minimum required temperature to preheat the fuel and starts burning is the ignition temperature. Fuel must have moderate ignition temperature, because low ignition temperature is dangerous for storage and transport due to fire hazard and for starting a fire, high ignition temperature is not suitable.
- Moderate velocity of combustion: For continuous supply of heat, fuel must burn with a moderate velocity.
- Low non-combustion matter and ash content: After combustion, non-combustible matter produces high ash content and also reduces the heating value. With this more heat loss, and loss of money for over storage, handling, disposal of ash, etc.
- Low cost: Good fuel should be available easily in bulk at low cost.
- High pyrometric effect: Pyrometric effect is the highest temperature obtained with the fuel, hence fuel should have high pyrometric effect.
- Less pollutants/environmental friendly: By-products of combustion like CO, SO2, NO2, etc. pollute the environment, so a good fuel should release less pollutants and should be environment friendly.
- Storage cost: Storage cost of a fuel in bulk should be low
- Easy transportation: Fuel should be easy to transport with low cost.
- Uniform particle size: In case of solid fuels, for easy combustion, the particle size should be uniform.
3.7 SOLID FUELS
Coal and coke are main solid fuels.
3.7.1 Coal
Coal is the primary and largest solid fuel used to produce electricity and heat through combustion. Black or brownish black sedimentary rock usually occur as coal beds, composed primarily of carbon along with other elements like hydrogen, oxygen, nitrogen and sulphur, also known as pulverised carbon.
Due to biogeological processes, from the dead plant matter and vegetation fossil fuel coal is formed, and is slowly converted into peat, lignite, bituminous coal and finally to anthracite.
According to carbon and hydrogen ratio, ranking of coal in increasing order is as follows
![]()
3.7.2 Analysis of Coal
Proximate and ultimate analysis is carried out to assess and determine the quality of coal.
3.7.2.1 Proximate Analysis of Coal
Practical utility of coal is determined by the proximate analysis. Here, information is obtained regarding moisture, volatile matter, ash and fixed carbon content.
- Moisture: In a crucible weighed about 1 g of finely powdered air dried coal sample is placed inside an electric oven at 105–110°C for 1 h. After that, the crucible is taken out from the oven, cooled in a desiccator and weighed. Difference in the weight gives information about weight loss as moisture.

- Volatile matter: Dried matter of coal left in crucible (a) is covered with a lid and heated up to 950°C for 7 min in a muffle furnace. The crucible is cooled first in air, next in a desiccator and then weighed. Loss of weight is due to the volatile matter present.

- Ash: The residual sample in the crucible (b) is repeatedly heated and cooled (air and desiccator) up to getting content weight in muffle furnace around 700–750°C and the remaining residue is responsible for ash.

- Fixed carbon: Fixed carbon is determined by the following equation

Good quality of coal has more fixed carbon.
3.7.2.2 Ultimate Analysis of Coal
Elemental analysis of coal is done by ultimate analysis, and with this analysis carbon, hydrogen, nitrogen, oxygen, sulphur and ash content are determined based on the following procedure.
- Carbon and hydrogen: In a combustion apparatus, accurately weighed 1–2 g of a coal sample is burnt in a oxygen current. The coal sample containing carbon and hydrogen is converted into carbon dioxide and water, and the formed gaseous products are absorbed by known weight of potassium hydroxide and calcium chloride tubes, respectively. From the weight difference of the tubes, percentage of carbon and hydrogen is determined as follows.

- Determination of Nitrogen content by Kjeldahl method: In a Kjedahl flask (long-necked flask), about 1g of accurately weighed powdered coal is heated with concentrated sulphuric acid and potassium sulphate as a catalyst. After getting a clear solution treated with excess KOH, liberated ammonia is distilled over and absorbed by known volume of standard acid solution. Unused acid is determined with standard NaOH by back titration. Nitrogen content in coal is calculated from the volume of acid used by liberated ammonia.

- Sulphur: Sulphur content in coal is determined from the washings obtained in the determination of calorific value by the bomb calorimeter. During the determination of calorific value, entire sulphur present in coal is converted into sulphate. The washings are treated with barium chloride solution, and the sulphate is precipitated as barium sulphate, then it is filtered, washed and heated for obtaining a constant weight.

- Ash: Ash content is determined as in proximate analysis.
- Oxygen: Oxygen content is determined by using the following equation

Solved Numerical Problems Based on Combustion of Fuel
- A sample of coal was analysed as follows: Exactly 2.500 g was weighed into a silica crucible. After heating for one hour at 110 °C, the residue weighed 2.415 g. The crucible next was covered with a vented lid and strongly heated for exactly seven minutes at 950 ± 20 °C. The residue weighed 1.528 g. The crucible was then heated without the cover until a constant weight was obtained. The last residue was found to weight 0.245 g. calculate the percentage results of the above analysis.

- Calculate the mass of air needed for complete combustion of 5 kg of coal contain; C – 80%, H = 15%, O = rest.
Solution 5 kg of coal contains: C = 4 kg; H = 0.75 kg; O = (5 – 4 – 0.75) kg = 0.25 kg

- A sample of coal was found to contain; C – 80%, H – 5%, O – 1%, N – 2% remaining being ash. Calculate the amount of minimum air required for complete combustion of 1 kg of coal sample.

∴ wt. of air reqd. = 2536 g (100/23) = 11026 gm = 11.026 kg.
- Calculate the weight and volume of air required for combustion of one kg of Carbon?
Solution Carbon undergoes combustion according to the equation.

- A gas has the following composition, by volume: H2 = 30%; CH4 = 5%; CO = 20%; CO2 = 6%; O2 = 5% and N = 34%. If 50% excess air is used find the weight of air actually supplied per m3 of this gas. [molecular weight of air = 28.97]
Solution In one m3 of the gas

Volume of air required for 1 m3 of gas using 50% excess air

Hence, weight of air actually supplied per m3 of the gas,

- A gaseous fuel has the following composition by volume; H2 = 20%; CH4 = 5%; CO = 20%; CO2 = 5%; N2 = 45%. If 50% excess of air is used find the weight of air actually supplied per m3 of this gas?
Solution Volume of components in 1 m3 of gaseous fuel and O2 needed for combustion can be calculated as:

∴ Volume of air required for 1 m3 of gas using 50% excess air

Hence, weight of air actually supplied per m3 of gas

- Calculate volume of air required for complete combustion of litres of CO, given percentage of oxygen in air 21.

Hence, volume of air required

- A producer gas has following composition by volume: CH4 = 5%; CO = 30%; H2 = 20%; CO2 = 5%; N2 = 40%. Calculate the theoretical quantity of air required per cubic meter of the gas.
Solution volume of component in 1m3 of gaseous fuel and O2 needed for combustion can be calculated as:

∴ Volume of air required for 1 m3 of gas

- A coal sample gave the following analysis: C = 66.2%; H = 4.2%; O = 6.1%; N = 1.4%; S = 2.9%; moisture = 9.7% and ash = 9.5%. If one kg of coal is burnt with 25% excess air, determine the quantity of products of combustion?
Solution One kg of coal sample contains:
C = 662 gm; H2 = 42 gm; S = 29 gm; O = 61 gm; H2O = 97 gm

Hence, minimum weight of air required for complete combustion of 1 kg of coal
 (1)
(1)(Because the air has 23% (by oxygen weight))
And weight of air supplied for combustion using 25% excess air
 (2)
(2)Since, total weight of products of combustion
 (3)
(3)∴ We should first calculate individual weights of products.

- The percentage composition of a sample of bituminous coal was found to be as under: C = 75.4%; H = 5.3%; O = 12.6%; N = 3.2%; S = 1.3% and Ash = rest. Calculate the minimum weight of air necessary for complete combustion of 1 kg of coal and percentage composition of dry products of combustion by weight:
Solution Total weight O2 needed

Less O2 in coal = 126 gm
∴ Net O2 needed = 2321.7 gm
So, minimum weight of air necessary for complete combustion

Dry products of combustion

- The coal has following analysis:
C = 54%; H = 6.5%; O = 3%; N = 1.8%; moisture = 17.3 and remaining is ash. This coal on combustion with excess of air, gave 21.5 kg of dry flue gases per kg of coal burnt. Calculate percentage of excess air used for combustion.
Solution 1 kg of coal contains
C = 0.54 kg: H = 0.065 kg; O = 0.03 kg; N = 0.018 kg
Minimum weight of air required for combustion

Weight of dry products of combustion

∴ Total weight of dry products combustion = 1.98 + 6.478 = 8.458 kg
Given, the actual weight of dry flue gases is 21.5 kg. so balance must have come from excess air

- The percentage composition of a sample of coal by weight was found to be C = 76%; H = 5.2%; O = 12.8%; N = 2.7%; S = 1.2%. the remaining being ash. Calculate the minimum: (a) weight, and (b) volume at NTP of air necessary for complete combustion of 1 kg of coal. Also calculate percentage composition of dry products by weight, if 50% excess air is supplied.
Solution 1 kg of coal contains
C = 760 gm: H = 52 gm; S = 12 gm; O = 128 gm; N = 27 gm
∴ Net O2 needed for combustion = (O2 needed for combustion)-(O2 is fuel)

Now, weight of air necessary for complete combustion of 1 kg of coal
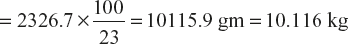
And volume of air necessary for complete combustion of 1 kg of coal

Weight and percentage of dry products of combustion are calculated below:

3.7.3 Metallurgical Coke
Coke used for metallurgy is called metallurgical coke, and it should have the following good characteristics.
- Purity: Low moisture content keeping down the heating expenses. Ash content hinders the heating, forms slag and also consumes excess coke for removal of ash. The sulphur and phosphorous produce undesirable products like SO2, P2O3, P2O5 etc. Which affect the quality of coke, and also sulphur make the coke brittle. Hence coke should have as low as possible moisture, ash, phosphorous and sulphur contents.
- Calorific value: Coke should have a high calorific value.
- Strength: It should be quite compact, strong, hard to withstand abrasion as well as pressure in furnace.
- Porosity: For complete combustion at high rate, coke should be porus, due to presence of pores oxygen can easily contact with carbon.
- Size: If coke is too big in size, uniformity of heating is not maintained, and if it is too small choking is observed. Hence, the size of the metallurgical coke should be medium.
- Cost availability and transportation: Coke should be easily available with cheap rate nearer the metallurgical plant, therefore, with this the transportation cost is also reduced.
- Combustibility: The combustibility of the coke mainly depends on nature of coal, carbonization temperature, reaction temperature, etc. Further, cokes obtained by high temperature carbonization process are less combustible when compared to coke obtained by low temperature carbonization at a given temperature. All cokes have equal reactivity at 800–900°C temperature. The rate of combustion depends on the rate of oxygen supply about 1000°C. Coke should burn easily.
- Reactivity of steam: Coke obtained from non-caking coals is more reactive to steam when compared with caking coals. Reactivity to steam of coke is directly proportional to reaction temperature and inversely proportional to carbonization temperature. Especially, the coke used for manufacture of water gas must be reactive to steam.
3.7.4 Manufacture of Metallurgical Coke
The coke, for metallurgical purposes, is mainly manufactured by two methods. They are (1) Beehive oven and (2) Otto Hoffman’s by-product oven method.
- Beehive oven: Schematic representation of Beehive oven is shown in Figure 3.3. This is the cheap and earliest method for manufacturing of metallurgical coke. The Beehive oven is the dome-shaped structure of bricks, with 4 m width and 2.5 m height. It is having two openings, and these can be opened and closed as desired. Thus, coal is charged from the top opening, and air supply as well as coke discharge from side opening is used.
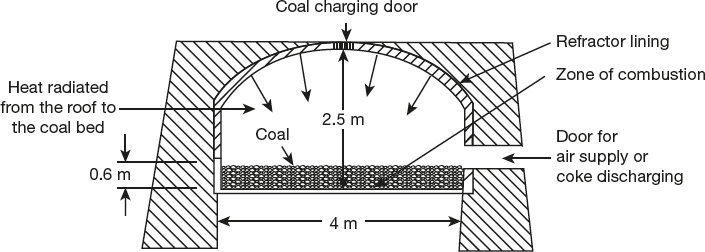
Figure 3.3 Beehive coke oven
Through the top opening, coal is charged about 0.6 m deep layer, air is supplied from the side opening and the coal ignited. For slow carbonization, combustion is allowed to proceed gradual diminish supply of air, and it will take to complete 3 to 4 days from the top to bottom layer and the volatile matter escapes inside the partially closed door. After completing and carbonization, the hot coke quenched with water and raked out through the side door, leaving the oven hot to start the next charge batch carbonization. The yield is 80 per cent of the charged coal. Many such ovens are arranged in series, and with this waste heat is utilized for heating. Hence, it saves energy, reduces the pollution and is economically beneficial.
- Otto Hoffman’s by-product oven: Schematic representation of the modern by-product coke oven, which is developed by Otto Hoffman is shown in Figure 3.4. It is mainly useful in (i) increased thermal efficiency of carbonization and (ii) recovery of valuable by-products like ammonia, coal gas, benzol air, tar etc., It is heated externally by coal gas produced itself or blast furnace gas or producer gas, and mostly heating is done by heat economy of regenerative system, i.e., utilization of flue gases for heating.

Figure 3.4 Otto Hoffman’s by-product coke oven with regenerators

Figure 3.5 Fractional distillation of crude petroleum
The oven consists of a number of narrow silica chambers about 10 to 12 m long, 3 to 4 m high and 0.40 to 0.45 m width. These chambers are erected side by side vertically; further, flues in between them form a sort of battery. Each chamber is provided at the top with a charging hole, at the end of chamber a gas off-take and refractory lined cast iron door for discharging coke.
A finely crushed coal is introduced through the charging holes, closed tightly on both the ends to prevent air access. The oven is heated to 1200°C by employing a regenerative principle, with burning of producer gas. During combustion, produced flue gases pass towards sensitive checker brick work until the temperature raises about 1000°C before escaping to chimney. The flow of heating gases is reversed, to serve in the preheat of inlet gases and the cycle goes on. The heating process is continued up to 11 to 18 h, till the carbonization and evolution of volatile matter ceases completely. After complete carbonization, a massive ram pushes the red hot coke into a truck and subsequently quenched.
3.8 LIQUID FUELS
Liquid fuels are those which are combustible, energy-generating substances and play vital role in transportation and economy. Most widely used liquid fuels are derived from fossil fuel/petroleum/crude oil. Some important liquid fuels are petrol, kerosene, diesel, etc.
3.8.1 Petroleum Refining
Petroleum is a complex mixture of organic liquids (hydrocarbons) also known as crude oil or fossill fuel. It is formed from the fossilized dead plants and animals by exposure to heat and pressure in the Earth’s crust, and was formed millions of years ago. It is a viscous dark coloured, foul-smelling liquid along with water and soil particles. Hence, it is necessary to separate these hydrocarbons into useful products, and this process is known as fractional distillation. In this process, products are separated depending on boiling points, known as refining of petroleum, and the plant set-up used here are oil refineries as shown in Figure 3.4.
Refining of petroleum involves the following 3 steps.

The distillation chamber is a steel cylindrical tube about 31 m height and 3 m in diameter, and inside, the chamber trays are fitted at short distances. Every tray is having many holes and an up going short tube with a bubble cap. At different heights of chamber, the vapours go up, begin to cool and condense in fractions. Fractions which are having higher boiling point condenses first and lower boiling fractions one after other. Various products obtained in distillation are given in Table 3.1.
Table 3.1 Fractions by distillation of crude

3.8.2 Important Petroleum Products and their Uses
- Gasoline (or) petrol (or) motor spirit: In North America, gasoline is often shortened as gas, while petrol is the common name in the United Kingdom. It is a transparent petroleum derived oil obtained between 40 to 120°C as mixture of hydrocarbons C5H12–C8H8. Its calorific value is about 11,250 kcal/kg, with 84 per cent of carbon, 15 per cent of hydrogen and 1 per cent of nitrogen, sulphur and oxygen as its composition. It is highly volatile inflammable oil, primarily used as a fuel for internal combustion engines of automobiles.
- Kerosene oil: Kerosene is the fraction obtained between 180 to 250°C, as a mixture of hydrocarbons C10H22–C16H34 in petroleum distillation. Its calorific value is about 11,000 kcal/kg, with 84 per cent of carbon, 16 per cent of hydrogen and less then 0.1 per cent of sulphur as its composition. It is widely used fuel for cooking, also used as an jet engine fuel and for making oil gas.
- Diesel oil: In a petroleum distillation, it is a fraction obtained between 250 to 320°C as a mixture of hydrocarbons of C15H32–C18H38. Its calorific value is about 11,000 kcal/kg, and is mainly used as a diesel engine fuel.
- Liquefied petroleum gases (LPG): This is obtained from cracking of heavy oils (or) natural gas and named as bottled gas. It consist of n-butane, isobutene, butylene, propane and less ethane, and supplied under pressure containers with the trade name of Indane gas, Bharat gas, etc. LPG is dehydrated, desulphurized and added trace amounts of mercaptans for giving warning of gas leak, and its calorific value is about 27,800 kcal/m3. It is widely used as a domestic, industrial and motor fuel.
3.9 SYNTHETIC PETROL
Petrol can be synthesized by the following methods.
- Cracking
- Fischer–Tropsch method and
- Bergius method
3.9.1 Cracking
The process of breakdown of high molecular weight hydrocarbons of high boiling points into simple, lower molecular weight hydrocarbons of low boiling points is known as cracking.

With these we can prepare different fuels having high quality.
Cracking is mainly two types: thermal and catalytic cracking.
3.9.1.1 Thermal Cracking
In this cracking heavy oils are subjected to high temperature and pressure in the absence of catalyst. In this cracking, the bigger hydrocarbon molecules break down to give smaller paraffins and olefins.
Mechanism of Cracking Process
Cracking processes invoke free radical and carbonium ion intermediates. Thermal cracking mainly goes through the free radical mechanism. In this mainly of three steps they are as follows: Example: Thermal cracking of nonane.
Initiation: Nonane undergoes homolytic cleavage at high temperature to give free radicals.
![]()
Propagation: The formed free radicals undergo further fissions up to thermally more stable radical is formed.
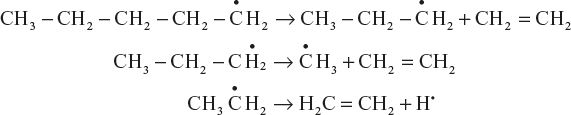
Termination: Coupling of unstable free radical intermediates gives final products in termination reaction.

3.9.1.2 Catalytic Cracking
In catalytic cracking, higher molecular weight hydrocarbons breakdown in the presence of catalyst like alumina (or) aluminium sulphate via carbonium ion intermediate. Here, quality and quantity of gasoline can be increased, and it is mainly of two types. They are as follows:
- Fixed bed catalytic cracking
- Moving bed catalytic cracking
Mechanism of Catalytic Cracking
This reaction proceeds via carbonium–ion intermediates.
- Fixed-bed catalytic cracking: A simple sketch of fixed bed catalytic cracking is shown in Figure 3.6. Here, heavy oil charge is passed through a pre-heater, having a temperature of about 425–450°C. The formed hot vapours of oil is passed over a fixed bed of catalyst chamber also having the temperature of about 425–450°C.

Figure 3.6 Fixed-bed catalytic cracking
In a catalytic chamber, 40 per cent of oil is converted into petrol and 2–4 per cent of carbon formed is absorbed on the catalyst bed. Catalyst stops function after 8–10 h, and due to carbon deposition it deactivates. This is re-activated by burning off the deposited carbon, During re-activation, the vapours are directed through another catalyst chamber.
Cracked vapours enter into the fractionating column from the catalyst chamber, and different gases are cooled and collected.
- Moving-bed catalytic cracking: A schematic representation of a moving bed catalytic cracking is shown in Figure 3.7. Here, feed oil is passed through a pre-heater, oil vapours formed here along with very finely powdered catalyst are passed to a reactor, which maintain about 500°C temperature. The cracked oil vapours are then passed through fractionating column, and heavy oil is separated. Formed vapours are sent to a cooler, gasoline condenses along with gases, and is separated from gases as a purified petrol.

Figure 3.7 Moving-bed type catalytic cracking
3.9.2 Fischer–Trapsch Method
Oven-heated coke is mixed with hydrogen and passed steam through it, and water gas (CO + H2) is formed. It is purified by passing through first Fe2O3, here H2S is removed, next a mixture of Fe2O3 + Na2CO3, removes organic sulphur compounds. The purified gas is then compressed to 5–25 atm and is sent through a converter containing catalyst. Catalyst is the mixture of 100 parts of cobalt, 8 parts of magnesia, 5 parts of thoria and 200 parts of keiselgur at 200–300°C temperature. A mixture of saturated and unsaturated hydrocarbons is formed.

This reaction is highly exothermic. Hence, formed hot gaseous mixture is sent to a cooler. Here, liquid like crude oil is formed, and passed through a fractionating column. From the column, petrol and heavy oil are formed. Cracking of heavy oil gives and petrol. Schematic diagram of Fisher-Tropsch method is shown in Figure 3.8.

Figure 3.8 Fischer–Trapsch method
3.9.3 Bergius Method
A paste of finely powdered low ash coal, heavy oil and tin or nickel oleate (catalyst) is heated with hydrogen at 450°C temperature, and 200–250 atm pressure for about 1.5 h. Here, hydrogen reacts with coal to give saturated hydrocarbons, these are send to condense. Liquid like crude is formed and sent to fractionating column. From the column petrol, middle oil and heavy oil are formed. Heavy oil is used further for making paste with fresh coal. The middle oil is hydrogenated in presence of a solid catalyst in vapour phase to give petrol. The schematic diagram of Bergius process is shown in Figure 3.9.

Figure 3.9 Bergius method
3.10 POWER ALCOHOL
Power alcohol is one of the most important non-petroleum fuels. The first four aliphatic alcohols, methanol, ethanol, propanol and butanol, can be synthesized chemically or biologically and used as a fuel for internal combustion engines. These are not used as a prime fuel, but used in blends as additives.
Chemical formula of power alcohol is CnH2n+1 OH.
3.10.1 Manufacture of Power Alcohol
Methanol can be prepared from biomass. Ethanol is commonly prepared from various biological organic substances through fermentation process. However, widely it is manufactured from molasses. It is a viscous semisolid material, left after crystallization of sugar from sugar cane juice. It is a mixture of sucrose, glucose and fructose.
The molasses are diluted with water, to reduce sugar concentration from about 50–60 per cent to 10–12 per cent. Nutrients like ammonium sulphate, ammonium phosphate, and some amount of sulphuric acid is added to maintain pH value around 4–5. Right proportions of yeast are added and maintain the temperature of about 30°C. The invertase enzyme of yeast converts entire sucrose into glucose and fructose.
![]()
The zymase enzyme of yeast converts entire glucose and fructose into ethyl alcohol and releases carbon dioxide. During this process CO2 produces lot of froth, hence this process is known as fermentation process.
![]()
The fermentation process may be completed in about 36–38 h. Depending on the concentration of alcohol, it is named as wash or rectified spirit or absolute alcohol.
Wash: The fermented liquid containing 18–20 per cent of alcohol is known as wash.
Rectified spirit: Fractional distillated wash contains 90–95 per cent alcohol, and it is known as rectified spirit.
Absolute alcohol: The rectified spirit is digested with lime for about 2 days and then distilled to get 100 per cent alcohol which is known as absolute alcohol.
Advantages
- These are prepared from waste, hence it is a good non petroleum alternative source of energy and also reduces the pollution.
- It can burn completely, thereby increasing combustion efficiency.
- It has an octane value of about 90, but petrol is having about 60–70. When alcohol mixes with petrol, it tends to increase the octane rating, and these blended petrol possesses better antiknock property and reduces the carbon monoxide emission.
- Petrol is blended with alcohol, and it can absorb traces of moisture.
Disadvantages
- Alcohol may cause corrosion due to easy oxidation with acids.
- Due to low calorific value of alcohol, more fuel is required for each mile driven.
- Particularly at low temperatures, alcohol is difficult to atomize, due considerable surface tension.
3.11 KNOCKING
Knocking is the metallic sound produced by a spark ignition petrol engine under certain conditions. The following terms can explain the knocking in better way.
- Ignition temperature: It is the minimum temperature at which the combustion is self-supporting. This is also referred to as spontaneous ignition temperature (SIT).
- Compression ratio (CR): The power output and efficiency of an IC engine depends on a factor called CR. It is defined as the ratio of gaseous volume (V1) in the cylinder at the end of suction-stroke to the volume (V2) at the end of compression-stroke of the piston.

The CR obviously indicates the extent of compression of fuel–air mixture by the piston.
The fuel–air mixture gets heated to a temperature greater than its ignition temperature as a result of compression. This leads to spontaneous combustion even before sparking.
It is also possible that the last portion of the fuel–air mixture undergoes self-ignition after sparking. It is due to the heating and compression of the unburned fuel, by the spreading flame-font sweeping across the cylinder.
The resulting shock wave dissipates its energy by hitting the cylinder walls and the piston. In view of the characteristic rattling sound emitted, this is called knocking. The CR at which fuel tends to knock is called critical CR.
To summarise: With the increase in CR, the efficiency of IC engine also increases but after critical CR, tendency to knock also increases.
Consequences of knocking:
- Decreased power output
- Mechanical damage by overheating of the cylinder parts.
Probable mechanisms of chemical reactions that lead to knocking are the following:
- Free radical chain reaction leading to cracking and oxidation of the hydrocarbons is probably the mechanism of chemical reactions that lead of knocking.
Factors on which knocking depend are the following
- Engine design
- Running conditions and
- Chemical structure of the fuel hydrocarbons. For instance:
- Knocking tendency decreases in the following order: n-alkanes > mono-substituted alkanes > cycloalkanes > alkenes > poly-substituted alkenes > aromatics. And for straight chain hydrocarbons, the tendency to knock increases with molecular weight and boiling point. Example: n-hexane > n-pentane > n-butane.
Aromatic hydrocarbons have higher anti-knocking properties than paraffins and olefins.
3.12 DIESEL ENGINE, CETANE AND OCTANE NUMBER
In the diesel engine, air is first drawn into the cylinder and compressed to a pressure of about 500 psi (3.52 × 105 kg/m2). This compression is accompanied by a rise in temperature to about 500 °C.
Towards the end of the compression, stroke is injected in the form of finely divided spray into air in the cylinder heated to about 500 °C by compression. The oil absorbs the heat from the air and it ignites spontaneously as it attains ignition temperature. This raises the temperature and pressure. The piston is pushed by expanding gases in the power stroke.
In a diesel engine, combustion of fuel is not instantaneous, as the ignition delay is caused. Ignition delay is the interval between the start of fuel injection and its ignition. This is due to the time taken for the vaporization fuel droplets and attaining of the vapour to its ignition temperature. It depends on the (a) engine design; (b) efficiency of mixing of the spray and air; (c) the injector design; and (d) mostly on the chemical nature of the fuel. Example: Ignition delay is shorter for paraffinic fuel than that of olefinic, naphthalenic and aromatic fuels.
If the ignition delay is long, it will lead to fuel accumulation in the engine even before the ignition. When ignited, an explosion results as the combined effect of increased temperature and pressure. This is responsible for diesel knock. The diesel fuel should have a SIT less than the temperature produced by compression.
As the temperature to which air can be heated by compression is limited by various constraints, it is desirable to have fuels with short ignition delay but the ignition delay must be long enough for the compression stroke to be completed. In order to grade the diesel fuels, cetane rating is employed.
Cetane number: It is used for diesel engines to measure the ease of with which a fuel will ignite under compression

Cetane number of fuel primarily depends on the nature and composition of its hydrocarbons.
For instance, consider the following series: n-alkanes > cycloalkanes > alkenes > branched alkanes > aromatics (i.e. cycloalkanes):
- ignition delay increases from left to right
- ignition quality increases from right to left
- cetane no. increases from right to left
As straight chain alkanes such as n-cetane have low ignition delay (high ignition quality) and ignite readily on compression, while aromatics do not ignite readily on compression, so that high cetane number fuels eliminate diesel knock. The cetane number of diesel fuel may be raised by the addition of pre-ignition dopes such as alkyl nitrites such as ethyl nitrite, amylnitrite, etc., 2,2,4,4,6,6,8, 8-hepta methyl nonane (HMN).

With a cetane rating of 15 is now considered as the low-quality diesel in the view of its easy availability and purity. On the revised scale (HMN reference), the cetane number (CN) represents the % cetane, in the blend with HMN plus 15/100 of the % HMN. Thus, a blend of 50% cetane and 50% HMN has a following cetane rating:
![]()
Octane number: The resistance offered by gasoline to knocking cannot be defined in absolute terms. It is generally expressed on an arbitrary scale, known as octane rating.

The % of iso-octane in the n-heptane iso-octane blend which has the same knocking characteristics as the gasoline sample under the same set of conditions is known as octane number.
Additives for improving anti-knock properties: Tetra ethyl lead (TEL) and diethyl telluside (C2H5)2Te are the most commonly used additives. TEL gives rise to Pb of PbO during combustion. These particles act as free-radical chain inhibitors as they arrest the propagation of the explosive chain reactions responsible for knocking.
The efficiency of TEL decreases in the presence of sulphur hence desulphurised gasoline is preferred. Pb and PbO2 decrease engine life hence they must be removed along with exhaust gases by adding ethylene dibromide.
![]()
Because PbBr2 formed is volatile its escape into atmosphere. But pollution problem still exists. Another cause of pollution is incomplete combustion leading to the formation of CO, NO, NO2, SO2, SO3, etc. Hence, catalytic converters based on Pt are employed which will catalyse combustion reaction to completion. Example: CO–CO2.
But Pt is poisoned by Pb, so unleaded petrol should be used. Benzene is added for decreasing knocking. Since benzene is carcinogenic, very low concentration of benzene should be used.
3.13 GASEOUS FUELS
Important gaseous fuels are natural gas, producer gas, water gas, coal gas, bio gas, etc.
3.13.1 Natural Gas
Natural gas obtained along with petroleum in oil wells is called wet gas. It is purified and removed. Propane, propene, butane, butene, etc. are used for preparing LPG. If the gas is associated with crude oil, it is called dry gas. It is having some of the objectionable ingredients like water, H2S, N2, CO2, etc. and hydrocarbons like propane, butane, propene, butene, etc. are removed.
Composition
Natural gas consists of 70–90 per cent of methane, 5–10 per cent of ethane, 3 per cent of hydrogen and rest of carbon monoxide and carbon dioxide, approximately. Calorific value is about 12,000–14,000 kcal/m3.
Uses
- Used as a domestic fuel, also conveyed over large distances through pipelines.
- Used as a raw material in carbon block manufacture.
- Used for manufacture of different synthetic chemicals.
- Used in the preparation of synthetic products by microbiological fermentation of methane.
- Used in the preparation of compressed natural gas.
- Due to less pollution, it is a good substitute for petrol and diesel.
3.13.2 Producer Gas (or) Suction Gas
Composition
Producer gas is the mixture of about 20–22 per cent carbon monoxide (CO), 11–13 per cent carbon dioxide (CO2), 20–22 per cent hydrogen (H2), 2.5–3.5 per cent methane (CH4) and 40–42 per cent nitrogen (N2). Hence main composition is CO + N2.
Manufacture
Air is passed through a red hot coal or coke in a gas producer, and maintained temperature is about 1100°C. Producer gas is formed with oxidation and reduction reactions. Initially, oxidation of carbon gives carbon monoxide and carbon dioxide.

Reduction reaction gives producer gas:

Formed gas is distilled and purified. The calorific value of producer gas is about 1300 kcal/m3.
Uses
- Used in the manufacture of steel, glass, etc. for heating of open-hearth furnace.
- Used in the manufacture of coke and coal gas for heating of muffle furnace.
- Used as a reducing agent in metallurgical operations.
3.13.3 Water Gas (or) Blue Gas
Composition
Water gas is the mixture of carbon monoxide (40–42 per cent), hydrogen (50–52 per cent), nitrogen (3–4 per cent and carbon dioxide (3–4 per cent).
Manufacture
Steam and little air are passed alternatively through a red hot coal or coke in a reactor maintained at about 1000°C temperature and water gas is formed in the following reactions.

The calorific value of water gas is about 2800 kcal/m3.
Uses
- Used as an illuminating gas, fuel gas, source of hydrogen gas etc.
Carbonated water gas: It is a mixture of producer gas and hydrocarbons. Calorific value is about 4500 kcal/m3, and used for illuminating and heating purpose.
Semi-water gas: It is a mixture of water gas and producer gas. Calorific value is about 1700 kcal/m3. Used as a fuel and a source of N2 and H2 in the manufacture of ammonia.
3.13.4 Coal Gas
Coal gas is mainly used as an illuminant in cities and towns; hence, it is known as town gas or illumination gas.
Composition
It is a mixture of carbon monoxide (27–29 per cent), carbon dioxide (2.4 per cent), hydrogen (16–18 per cent), nitrogen (49–51 per cent) and methane (0.5–1 per cent).
Manufacture
It is manufactured by destructive distillation of coal in the absence of air, at about 1300°C temperature.
![]()
The calorific value of coal gas is about 4900 kcal/m3.
Uses
- It is used as a fuel and illuminant.
- Used for maintaining reducing atmosphere in metallurgical operations.
3.13.5 Biogas
Composition
Biogas is the mixture of methane (50–60 per cent), carbon dioxide (30–40 per cent), hydrogen (5–10 per cent), nitrogen (2–6 per cent) and trace amount of hydrogen sulphide.
Manufacture
It is produced by the degradation of biological matter like animal dung, poultry waste, vegetable waste, waste paper, plant waste, human excreta, birds’ excreta, etc. by the anaerobic bacterial action in the absence of free oxygen.
Uses
- Used for cooking food.
- Used as a fuel to run engines.
- Used as an illuminant.
3.14 FLUE GAS ANALYSIS BY ORSATS APPARATUS
Flue gas is the mixture of CO2, CO and O2 gases, exhausted from the combustion chamber. Analysis of flue gas gives an idea about efficiency of combustion. Suppose the flue gas contains considerable amount of CO, it indicates incomplete combination and short supply of oxygen, and this will lead to wastage of fuel. If the flue gas contains considerable amount of oxygen, this indicates excess supply of oxygen and results in loss of heat.
With the help of Orsat’s apparatus flue gas analysis is carried out, as is shown in Figure 3.10. The set-up consists of a horizontal tube, with a three-way stopcock at one end and another end is connected with a graduated burette. For maintaining constant temperature during the experiment, the burette is surrounded by a water jacket. The burette is connected as a set of three absorption bulbs in a series, through a separate stopcock. The lower end of the burette is further connected to a water reservoir through a rubber tube.

Figure 3.10 The orsats apparatus
The water level in the burette can be changed by raising or lowering the reservoir water. One end of the tube, which is connected to a three-way stopcock, is further connected to a U-tube. For drying flue gas and avoiding the incoming smoke particles, the U-tube is packed with fused CaCl2 and glass wool.
Among the three absorption bulbs, first bulb has potassium hydroxide solution and absorbs only CO2. The second bulb contains alkaline pyrogallic acid absorbs only O2 and CO2. The third bulb has ammonical cuprous chloride and can absorb CO2, O2 and CO. For proper analysis of flue gas, first it is passed through potassium hydroxide containing bulb, and here CO2 is absorbed. Then, it is passed through alkaline pyrogallic acid containing bulb, and here O2 is absorbed and it can also absorb CO2, but already it is removed by KOH. Finally, flue gas is passed through the third bulb containing ammoniacal cuprous chloride, and here CO is absorbed; however, it can absorb CO2 and O2 also but these are already removed.
The entire apparatus is thoroughly cleaned, the steppers are greased, tested for air tightness, the absorption bulbs are filled with their respective solution and the stopcocks are closed. The water reservoir and water jacket are filled with water, and air is excluded from the burette by the raising of reservoir water level till the burette is completely filled with water. For the exclusion of air, the three-way stopcock is opened, next the lowering of water level is done and the fuel gas supply is connected to the three-way stopcock.
Further, 100 ml of the flue gas is carefully sent to the burette with closing of the three-way stopcock. The fuel gas is forced through the first bulb by opening its stopcock and raising the water level in the reservoir. Here, potassium hydroxide absorbs the CO2 flue gas is sent repeatedly 2 or 3 times to the first bulb for complete absorption of CO2. The remaining gas is taken back in the burette and the stopcock of the first bulb is closed. The levels of water in the reservoir and burette are equalized and decreasing volume of gas is noted. This decrease in volume gives the volume of CO2 in 100 ml of flue gas. Similarly, the volumes of O2 and CO are determined by passing the flue gas through the second and third bulbs, respectively. The remaining gas in the burette after absorption of CO2, O2 and CO is nitrogen.
The decrease in volume of flue gas by first bulb = volume of CO2
The decrease in volume of flue gas by second bulb = volume of O2
The decrease in volume of flue gas by third bulb = volume of CO.
3.15 REVIEW QUESTIONS
3.15.1 Fill in the Blanks
- _______ is the C.G.S unit of heat.
[Ans.: Calorie]
- 1 K Cal = 3.968 B.T.H = _______ C.H.U.
[Ans.: 2.2]
- _______ is used for determining the calorific value of solid and liquid fuels.
[Ans.: Bomb calorie meter]
- Latent heat of steam is _______.
[Ans.: 588 cal/gm]
- The lightest temperature obtained with the fuel is known as _______.
[Ans.: Pyrometric effect]
- KJeldahl method is used for determination of _______.
[Ans.: Nitrogen]
- Aromatic hydrocarbons have _______ anti knocking properties than paraffins and olefins.
[Ans.: Higher]
- In flue gas analysis by orsat’s method _______ containing bulb is absorbed by CO2.
[Ans.: KOH reduction]
3.15.2 Multiple-choice Questions
- Which of the following fuels possesses the maximum calorific value?
- C = 84%, H = 6%, S = 4% and O = 6%
- C = 84%, H = 12%, S = 1% and O = 1%
- C = 90%, H = 5%, S = 2% and O = 3%
- C = 95%, H = 2%, S = 1% and O = 2%
[Ans.: b]
- A good fuel should posses
- High ignition temperature
- Moderate ignition temperature
- High calorific value
- Both b and c
[Ans.: d]
- Ignition temperature of a fuel is the
- Temperature at which the fuel can be stored safely
- Lowest temperature at which the fuel must be preheated so that it starts burning
- Temperature attained with the fuel is burnt
- Temperature at which the fuel ignites for a moment, but doesn’t burn after then
[Ans.: b]
- Which of the following is not an advantage of gaseous fuels over solid and liquid fuels
- They can easily be conveyed through pipelines to the actual place of use
- They can be lighted at moments notice
- They cannot be preheated by the heat of the hot waste gases
- Their combustion can readily be controlled
[Ans.: c]
- Which of the following statements is true
- Coke possesses better strength than coal
- Coke burns with a long flame
- Coke burns with a short flame
- Sulphur content of coke is higher than that of coal from which it is obtained
[Ans.: c]
- Which of the following fuel gases possesses the highest calorific value
- Water gas
- Coal gas
- Producer gas
- Natural gas
[Ans.: b]
- Petrochemicals can be used to prepare:
- PVC plastics
- Polystyrene plastics
- Terylene fibers
- None of these
[Ans.: d]
- The maximum temperature reached, when the coal is completely burnt in the theoretical amount of air is called:
- Fusion temperature
- Calorific intensity
- Ignition temperature
- None of these
[Ans.: b]
- The calorific value of a coal sample is higher, if its:
- Moisture content is high
- Ash content is high
- Volatile matter is high
- Fixed carbon is high
[Ans.: d]
- Which of the following in coal decreases its calorific value:
- Carbon
- Hydrogen
- Oxygen
- Sulphur
[Ans.: c]
- Which of the following is not a characteristic of progressive transformation of wood to coal during coalification?
- Fixed carbon content increases
- Moisture content decreases
- Volatile matter increases
- Oxygen content decreases
[Ans.: c]
- Peat is:
- Soft brown coloured
- Brown jelly like mass
- Pitch black coal
- Last stage of coalification
[Ans.: b]
- Which of the following contain highest percentage volatile matter?
- Peat
- Lignite
- Bituminous coal
- Anthracite
[Ans.: a]
- In orsats apparatus, potassium hydroxide is used to absorb:
- Oxygen
- Carbon dioxide
- Carbon monoxide
- Sulphur dioxide
[Ans.: b]
- Orsats apparatus is used to obtain:
- Specific heats of components
- Molecular weights of components
- Gravimetric analysis of a gas mixture
- Volumetric analysis of flue gases
[Ans.: d]
- Higher calorific value of fuel assumes that is:
- Contains H2O in liquid form
- Contains H2O in vapour form
- Ignores the presense of H2O
- Contain H2O in liquid and vapour forms
[Ans.: d]
- Stoichiometric quantity of air is the quantity of air required for complete combustion of fuel with
- some excess oxygen
- non oxygen left unused
- 50% excess air
- 100% excess air
[Ans.: b]
- Analysis of flue gages is done by:
- Boy’s gas calorimeter
- Orsat apparatus
- Retort
- Bomb calorimeter
[Ans.: b]
- Bomb calorimeter is used for determining the calorific value of
- Solid fuel
- liquid fuel
- gaseous fuel
- both a and b
[Ans.: d]
- Proximate analysis of fuel is determination of percentage of
- C, H, N, S, H2O
- C, H2O, ash, volatile matter
- C only
- useful heat evolved
[Ans.: b]
- Ultimate analysis of fuel is determination of percentage of
- C, H, N, S, H2O
- C, H2O, ash, volatile matter
- sulphur only
- fixed carbon only
[Ans.: a]
- Bomb calorimeter is used to determine:
- HCV at constant pressure
- LCV at constant pressure
- HCV at constant volume
- LCV at constant volume
[Ans.: c]
- Incomplete combustion can be best judged by
- smoky chimney exit
- excess air in flue gases
- measuring CO in flue gases
- measuring O2 in flue gases
[Ans.: c]
- Gas with least calorific value is:
- coal gas
- water gas
- producer gas
- natural gas
[Ans.: c]
- Main constituent of natural gas is
- carbon monoxide
- methane
- Hydrogen
- ethane
[Ans.: b]
- The process of splitting bigger hydrocarbons into smaller hydrocarbons is called
- pyrolysis
- thermal decomposition
- cracking
- combustion
[Ans.: c]
- Iso-octane (2,2,4-trimethy pentane) has an octane rating of:
- 100
- Zero
- 50
- above 100
[Ans.: a]
- Which of the following possess zero octane number:
- Iso-octane
- petrol
- n-heptane
- LPG
[Ans.: c]
- Suitability of a diesel fuel is determined by:
- Octane rating
- percentage of carbon
- length of hydrocarbon chain
- Cetane number
[Ans.: d]
- For good performance, the hydrocarbon molecules in a diesel fuel should be
- Branch-chained
- side-chained
- straight–chained
- aromatic
[Ans.: c]
- The cetane rating of hexadecane is:
- 100
- 0
- 50
- none of these
[Ans.: a]
- Which of the following is used as a jet engine fuel:
- LPG
- Kerosene
- Power alcohol
- coal
[Ans.: b]
- Main constituent of LPG is
- Methane
- Propane
- Benzene
- Butane
[Ans.: d]
- Alcohol has an octane number of about
- 50
- 60–70
- 90
- 25
[Ans.: c]
- Alcohol-blended petrol possesses
- Better calorific value
- Better anti knock properties
- Poorer-antiknock properties
- None of these
[Ans.: b]
- In Bergius process of preparing synthetic petrol by
- Passing water gas over heated powdered coke under pressure
- Catalytic hydrogenation of coal
- Heating coal along under pressure
- Cracking of heavy oil
[Ans.: b]
- A good fuel should possess:
- Low calorific value
- High ignition temperature
- High calorific value
- High ash content
[Ans.: c]
- Anthracite:
- is lowest rank coal
- contains high percentage of carbon
- contains high percentage of volatile matter
- high calorific value and high carbon percentage
[Ans.: d]
- An example of primary fuel is
- Natural gas
- Petrol
- Wood charcoal
- Coke
[Ans.: a]
- Lignite is:
- Lowest rank coal
- Highest rank coal
- Used in metallurgy of iron
- Contains no moisture
[Ans.: a]
3.15.3 Short Answer Questions
- Define fuel and give some examples.
Ans.: Fuel is a combustible substance containing carbon as the major constituent which on proper burning gives large amount of heat that can be used economically for domestic and industrial purposes.
Examples are coal, petrol, diesel, etc.
- Give classification of fuels according to the occurrence.
Ans.: According to the occurrence, the fuels are classified into natural (primary) and secondary (derived) fuels.
- What are the units of heat and their inter-conversions?
Ans.: Units of heat are calorie, kilo calorie, British thermal unit and centigrade heat unit.
1 kcal = 1000 cal = 3.968 BTU = 2.2 CHU
- Define calorific value and give relation between higher and lower calorific values.
Ans.: Calorific value is the total quantity of heat liberated by the complete combustion of one unit mass/volume of a fuel in oxygen.
LCV = HCV – latent heat of water vapour formed
- What kinds of calorimeters are used for determining calorific value of solid, liquid and gaseous fuels?
Ans.: Bomb calorimeter is used for determining the calorific value of solid and liquid fuels. Junker’s calorimeter is used for determining the calorific value of gaseous fuels.
- What are the characteristics of a good fuel?
Ans.: High calorific value, moderate ignition temperature, low moisture content, low non-combustible matter content, etc.
- Name some of the petroleum products.
Ans.: Liquified petroleum gas, gasoline, petrol, kerosene, diesel oil, heavy oil, etc.
- What are the ovens used for preparation of metallurgical coke?
Ans.: Beehive oven and Otto Hoffman’s by-product oven.
- Explain cracking with a suitable example.
Ans.: The process of breakdown of high molecular weight hydrocarbons of high boiling points into simple, lower molecular weight hydrocarbons of low boiling points is known as cracking.

- What is meant by flue gas?
Ans.: The mixture of gases like CO2, CO and O2 exhaust of the combustion chamber is called flue gas.
- What is the importance of analysis of flue gas?
Ans.: The analysis of flue gas either from a furnace or from an engine’s exhaust would give an idea about the efficiency of the combustion process. If the flue gas contains considerable amount of CO, it indicates that incomplete combustion is occurring and it also indicates the short supply of O2 for combustion, and this will lead to wastage of fuel.
- What happens if the flue gas contains considerable amount of O2?
Ans.: It indicates that the O2 supply is very much in excess, and it results in loss of heat.
- Which apparatus is used in the analysis of flue gas?
Ans.: Orsat’s apparatus.
- In Orsat’s apparatus, which gases are absorbed by which solutions?
Ans.: Potassium hydroxide solution – only CO2
Alkaline pyrogallic acid – CO2 and O2
Ammonical cuprous chloride – CO, O2 and CO2
3.15.4 Descriptive Questions
Q.1 a. What do you understand by the term knocking in IC engines? Explain its relationship with chemical constituents of fuels.
b. A sample of coal contains 60% carbon, 33% oxygen, 6.0% hydrogen, 0.5% sulphur, 0.2% nitrogen and 0.3% ash. Calculate GCV and NCV of coal.
Q.2 Distinguish between the followings:
- Gross and net calorific values
- Octane number and centane number
Q.3 a. How coal is graded? Explain its calorific value of coal.
b. Give the advantages and disadvantages of coal over gaseous fuels.
Q.4 a. Explain the proximate analysis of coal and its significance.
b. Distinguish between low-temperature carbonisation and high-temperature carbonisation.
Q.5 a. What is metallurgical coke? How it is superior than coal? Explain the manufacture of the metallurgical coke by Otto Hoffman’s by-product coke oven method. List the various by-products obtained.
b. Define octane number of gasoline. Why is ethylene dibromide added, when tetra ethyl lead is used as an anti-knock?
Q.6 A fuel containing 92% C and 4% H2 by mass was burnt in 90% of air of that required for complete combustion. Find out the % of composition of dry product of combustion by mass of H2 is burnt completely and no carbon is left behind.
Q.7 Give brief note on the following:
- Explain how fuels are classified with suitable examples.
- Give the comparison between solid, liquid and gaseous fuels.
- What are the characteristics of a good fuel?
Q.8 Explain the significance of the following constituents present in coal:
- Moisture
- Volatile matter
- Ash
- Fixed carbon
Q.9 a. Discuss the relative merits and demerits of solid, liquid and gaseous fuels.
b. Explain the significance of the following constituents present in coal.
Q.10 a. How a calorific value of a gaseous fuel is determined by Junker’s gas calorimeter? Describe the experiment with a neat diagram.
b. Calculate gross and net calorific value of a gaseous fuel from the following data. Vol. of gaseous fuel burnt at STP -0.09 m3, weight of water used for cooling 25.0 kg, temperature of inlet water 25 °C, temperature of the outlet water 30.0 °C, weight of water produced by steam condensation 0.02 kg latent heat of steam 587 kcal/kg.
Q.11 a. What are the constituents of petroleum? Describe the origin of petroleum.
b. Give an account of production of petrol from crude oil.
Q.12 The analysis of a gas has the following composition: H2 = 14%, CH4 = 2%, CO = 22%, CO2 = 5%, N2 = 55%, O2 = 2%. Find the air required for the combustion of 1 m3 of the gas. If 45% excess air is supplied, find the volume analysis of the products.
Q.13 a. Define a fuel? How chemical fuels are classified and give examples for each?
b. What is meant by calorific value of a fuel? Define calorie and kilocalorie.
Q.14 Explain the significance of the following constituents present in coal:
- Total carbon
- Hydrogen
- Nitrogen
Q.15 a. What are chemical fuels? Give complete classification of chemical fuels with examples.
b. What are different types of fuels? What are the characteristics of a good fuel?
c. Mention the criteria for selecting good fuel.
d. Distinguish between solid, liquid and gaseous fuels.
Q.16 a. What is meant by calorific values of a fuel?
b. Describe how the calorific value of a solid fuel is determined using a bomb calorimeter.
c. What are the fuels used for determination of water equivalent of bomb calorimeter and why?
Q.17 a. Differentiate proximate and ultimate analysis of coal.
b. Discuss the importance of ultimate analysis of coal.
Q.18 a. What a good fuel must have low ash content? Or what is role of ash on coal?
b. How is nitrogen determined in a solid fuel?
c. What is the significance of a volatile matter in coal?
d. How is ranking of coal make based on ultimate analysis?
Q.19 a. What are the advantages of liquid fuels over solid fuels?
b. Differentiate between coal and coke.
c. Explain carbonisation of coal.
d. Why is coke preferred to coal in metallurgical purposes?
e. Why are gaseous fuels more advantageous than solid fuels?
Q.20 a. Write short note on Beehive coke oven.
b. Why is peat not considered as an economical fuel?
Q.21 What is crude oil? Write short note on refining of crude petroleum. What are the various fractions obtained from petroleum? Mention the industrial uses to which they are put.
Q.22 a. What are the structural features of hydrocarbons in unlead petrol and diesel? What are the structural factors that promote its high value?
b. What is the significance of octane number and cetane number and for which these are used? How these can be improved?
c. Why is C2H4Br2 added, when TEL is used as an anti-knock?
d. What types of compounds nowadays are being added to petrol to improve octane rating?
Q.23 a. What is meant by cracking of petroleum? Explain? Fluidised-bed catalytic method of obtaining gasoline. Give its mechanism.
b. What are the advantages of catalytic cracking process? Describe, with a neat diagram, the fixed-bed catalytic cracking process.
c. Differentiate between thermal and catalytic cracking.
d. What are the advantages of catalytic cracking over thermal cracking?
e. What is meant by knocking? How is it related to chemical constitution? Describe the functions of TEL. Explain octane number and cetane number.
Q.24 a. What is LPG? What are the advantages of LPG over gaseous fuels?
b. Write the approximate compositions and calorific values of water gas and producer gas.
Q.25 a. Define flue gas. How the analysis of flue gas is done by the Orsat apparatus? What conclusion can be drawn from the experiment? What is the significance of this analysis?
b. How distinction can be made between complete and incomplete combustion of fuel?
c. What is leaded petrol?
Q.26 Write short notes on the following:
- Catalytic converter
- Flue analysis and its significance
Q.27 a. What is the principle of bomb colorimeter?
b. How gross calorific value of a solid fuel determined by Bomb Calorimeter? Write Dulong’s formula for calculating calorific value of fuel from its ultimate combustion data.
c. Discuss Beehives oven method for the manufacture of coke.
Q.28 a. Describe the fractional distillation of petroleum.
b. What do you understand with the knocking of fuel? Report the ways to improve the anti-knocking characteristic of a fuel.
Q.29 Write short note on metallurgical coke.
Q.30 A coal sample has the following composition: C = 90%; H = 3.5%; O = 3%, S = 0.5%; and N2 = 1%; the remaining being ash. Calculate the theoretical volume of air required at 27 °C and 1 atm pressure when 100 kg of the coal is burnt.
Q.31 The composition by weight of a coal sample is C = 81%; H = 5%; O = 8.5%; S = 1%; N = 1.5%; Ash = 3%:
- Calculate the amount of air required for the complete combustion of 1 kg of the coal.
- Calculate the gross and net calorific values of the coal sample. Given that the calorific values of C, H and S are 8,060 kcal/kg; 3,400 kcal/kg and 2,200 kcal/kg, respectively.
Q.32 A producer gas has the following composition by volume: CO = 30%; H2 = 12%; CO2 = 4%; CH4 = 2% and N2 = 52%. When 100 m3 of the gas is burnt with 50% excess air used, what will be the composition of the dry flue gases obtained?
Q.33 A hydrocarbon fuel on combustion gave the flue gas having the following composition by volume: CO2 = 13%, O2 = 6.5% and N2 = 80.5%. Calculate (a) the composition of fuel by weight and (b) the percentage of excess air used.
Q.34 Calculate the approximate calorific value of a coal sample having the following ultimate analysis: C = 80%; H = 3.5%; S = 2.8%; O = 5.0%; N = 1.5% and ash = 7.2%.
