1
WATER TECHNOLOGY
Water is the driving force in nature, we never know the worth of water till the river is dry.
1.1 INTRODUCTION
Water is a natural wonder and is the most common, important, useful thing for surviving of all the living beings. Without food, living beings can survive for some days but without water nobody can survive. Seventy percent of our body contains water, which regulates life processes such as digestion of food, transportation of nutrients, and excretion of body wastes. It regulates the body temperature by the process of sweating and evaporation. Water acts as a universal solvent; due to this reason, water is widely used in laboratories, irrigation, steam generator, industrial purpose, fire fighting, etc. Besides it is used for bathing, drinking, sanitary purposes, etc.
From an engineer’s point of view too, very important, without water nothing will happen. It is required in boilers for production of steam, which acts as a source of energy and a coolant in many power and chemical plants and many other industries.
1.2 SOURCES OF WATER
Water present on earth passes through a remarkable cycle of changes (as shown in Figure 1.1):
- Rain water is the purest form of natural water because it is obtained by evaporation from the surface water. But rain water during its downward path to the earth dissolved considerable amount of gases (e.g. CO2, NO, NO2, SO2, SO3 etc) and suspended solid particles, which are present in the atmosphere. So it becomes polluted.
- The water that comes to the surface through rain is in the form of river water and lake water. River water contains dissolved inorganic salts such as chlorides as well as dissolved impurities from the soil. In lake water, the main impurities are organic matter.
- A larger amount of rain water is percolated in the soil through permeable rocks, loose sand, gravel, etc. During its passage downward into the ground, the suspended matter is left behind, and organic matter is oxidized by bacteria. This water is extremely clear as a result of natural filtration through the sand bed.
- Sea water is the most impure form of natural water. River water joins the sea and thus gives its impurities to the sea. In addition, due to evaporation of water, sea is having about 3.5% of dissolved salts, and the maximum amount (2.6%) is due to sodium chloride. Hence sea water is salty in taste. This water is not directly useful to man because it is not palatable as it contains 2000 times more dissolved salt than fresh water.

Figure 1.1 Flow diagram of sources of water
1.3 TYPES OF IMPURITIES PRESENT IN WATER
Water may contain various impurities due to
- The ground or soil with which it comes in contact (e.g. garbage, soil particles, etc.)
- Its contact with sewage or industrial wastes
- The decomposition of dead plants and animals
- The growth of bacteria, algae, viruses, etc.
The common impurities present in natural water can be classified into four groups that are as follows and shown in Figure 1.2:
- Dissolved impurities
- Dissolved gases – NO2, CO2, SO2, etc., which are soluble in water and make it impure.
- Dissolved inorganic salts or ions
- Cations: Ca2+, Mg2+, Na+, K+, Fe2+, Al3+, Zn2+, etc.
- Anions:
 , etc.
, etc.
- Suspended impurities
- Inorganic – sand, clay, lime, etc.
- Organic – Plant and animal materials like discarded vegetables, dry leaves, dead materials, etc.
- Colloidal impurities
Finely divided silica, clay, organic products, colouring matter, etc.
- Microorganism
Various pathogenic microorganisms such as bacteria, fungi, virus, etc.
The various types of impurities present in the water impact certain properties in water.
- Presence of different chemicals impart colour, odour and taste to the water.
- Presence of dissolved salt makes the water hard.
- Excess quantities of metals and dissolved gases make the water corrosive in nature.
- Presence of pathogenic bacteria in water makes it unfit for drinking or domestic purposes.
- Suspended matter create turbidity to the water.

Figure 1.2 Types of impurities
1.4 HARD WATER AND HARDNESS
Depending on salts presents in water and reaction with soap, water is categorized into hard water and soft water. Hardness is the characteristic of water by which water does not produce lather with soap. It is due to presence of chlorides, sulphates and bicarbonates salts of magnesium, calcium and other heavy metals [CaCl2, CaSO4, MgCl2, MgSO4, Ca(HCO3)2, Mg(HCO3)2, etc]. When hard water is treated with soap, it does not produce lather, rather it forms a white scum. Soap is the sodium or potassium salt of higher fatty acids like stearic acid [C17H35 COONa – sodium stearate].

Water which can produces lather with soap easily is called soft water.
- Types of hardness
Depending on salts present in the water, hardness is of two types, i.e., temporary hardness and permanent hardness.
- Temporary or Carbonate Hardness: This is due to presence of dissolved bicarbonates of calcium and magnesium. By boiling water, temporary hardness can be removed.
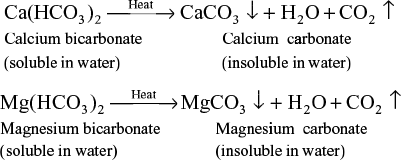
- Permanent or Non-Carbonate Hardness: This is due to presence of chlorides and sulphates of calcium and magnesium. Permanent hardness cannot be removed by boiling, therefore, special methods are followed.
- Temporary or Carbonate Hardness: This is due to presence of dissolved bicarbonates of calcium and magnesium. By boiling water, temporary hardness can be removed.
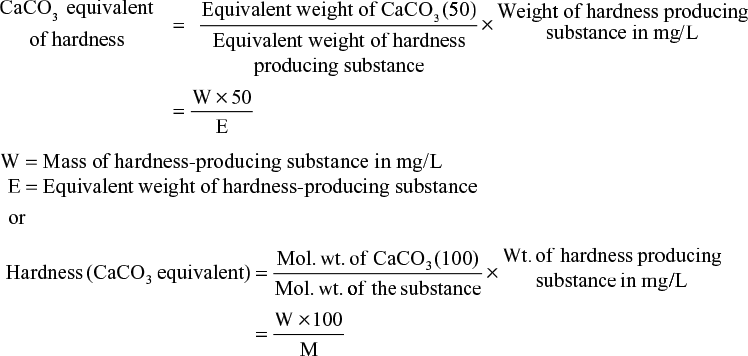
- Hardness is expressed in terms of CaCO3
We know that hardness of water is due to the presence of number of dissolved salts in water but for comparing the hardness of different samples of water of varying composition, it is necessary to choose a reference standard. For this purpose, hardness of water is expressed in terms of equivalents of calcium carbonate only. The following are the reasons for choosing CaCO3 as a standard for expressing the hardness:
- CaCO3 is a complete insoluble salt; thus it can be easily precipitated completely during water treatment.
- Its molecular weight is 100, and equivalent weight is 50, so the calculation becomes easy.
Hence whatever amount of dissolved salts is present in water, it is first converted into calcium carbonate equivalents by using the formula:
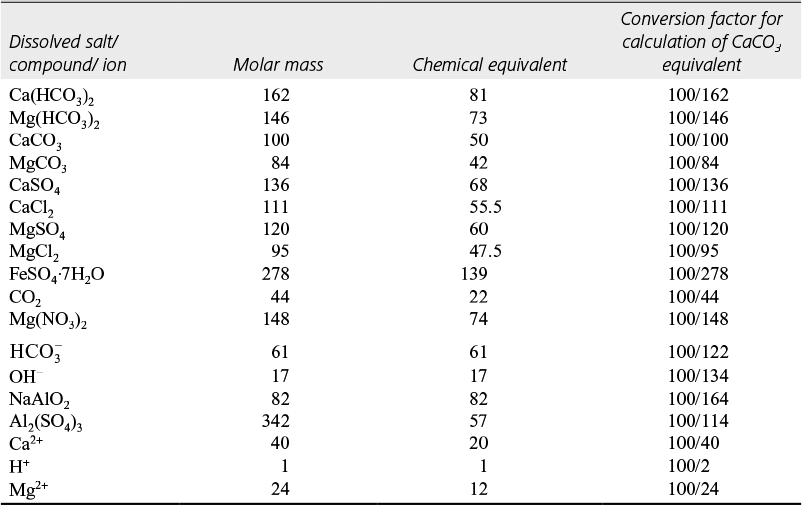
Salts responsible for hardness is given in Table 1.1 and method to calculate the hardness.
Table 1.1 Calculation of CaCO3 equivalent
- Units of hardness
Various units used for expressing hardness of water are given below:
- Parts per million (ppm)
- Milligrams per liter (mg/L)
- Degree French (°Fr)
- Degree Clark (°Cl)
- Parts per million (ppm): It is defined as the number of parts of calcium carbonate equivalent hardness present per 106 parts of water.

- Milligrams per liter (mg/L): It is defined as the number of milligrams of CaCO3 equivalent hardness present per liter of water.

- Degree French (°Fr): It is defined as the number of parts of CaCO3 equivalent hardness present per 105 parts of water.

- Degree Clarkes (°Cl): It is defined as the number of parts of CaCO3 equivalent hardness present per 70,000 parts of water.

- Parts per million (ppm): It is defined as the number of parts of calcium carbonate equivalent hardness present per 106 parts of water.
Relationship between various units of hardness is shown in Table 1.2.
Table 1.2 Relationship between various units of hardness

Solved Numerical Problems Based on Hardness of Water
- A water sample contains 204 mg of CaSO4/L. Calculate the hardness in terms of CaCO3 equivalent.
Solution

- Calculate temporary and permanent hardness of a sample of water, which on analysis is found to contain the following:

Solution



Now


- A sample of water upon analysis gave the following data:
MgCl2 = 0.143°Fr, MgSO4 = 0.572°Fr, CaSO4 = 0.286°Fr, and Ca(HCO3)2 = 2.316°Fr. Calculate the hardness in ppm.
Solution Since 1°Fr = 10 ppm

- Calculate hardness in terms of CaCO3 equivalent, if 100 ml of a hard water sample neutralizes exactly 12 ml of 0.12 N HCl by using methyl orange indicator.
Solution
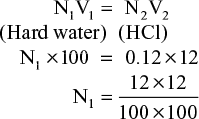
Strength of hardness in terms of a CaCO3 equivalent

- Calculate carbonate and non-carbonate hardness of water sample, if analysis of water sample gives CO2 = 22 ppm,
 = 305 ppm, Ca2+ = 80 ppm, Mg2+ = 48 ppm and total solids = 5000 ppm.
= 305 ppm, Ca2+ = 80 ppm, Mg2+ = 48 ppm and total solids = 5000 ppm.
Solution


1.5 DETERMINATION OF HARDNESS
We know that there are two types of hardness of water, i.e., temporary and permanent hardness. Temporary hardness is due to bicarbonate of calcium and magnesium, and permanent hardness is due to chlorides and sulphates of calcium and magnesium.
The hardness of water can be determined by complexometric titration by using ethylenediamine tetra acetic acid [EDTA] commonly known as EDTA method.
EDTA Method: It is the most important and more accurate method to determine the hardness of water. EDTA has limited solubility in water, Hence, disodium salt of EDTA is used which is soluble in water.
Principle: EDTA can from complex with salts (Ca2+ and Mg2+) which are present in hard water. Hence, it is known as complexometric titration. Calcium or magnesium ions present in the water sample with ammonical buffer solution form an unstable wine red colour complex with Eriochrome Black T (EBT) indicator. When it is titrated with EDTA solution the metal ions present in water give a stable deep blue colour (M-EDTA) complex and releases the free indicator.
The formula of EDTA is written as

Disodium salt of EDTA is

It is represented as Na2H2Y. It ionizes in aqueous solution to give 2Na+ ion and a strong chelating ion represented as H2Y2−.
![]()
It is a hexadentate ligand, and it forms complexes with bivalent cations (Mg2+, Ca2+, etc.), and these complexes are stable in alkaline medium (pH 8-10).
EBT may be represented as:

The EBT has two ionisable phenolic hydrogen atoms, and it is represented as Na+H2In−; indicator EBT gives different colours at different pH values.

End point: During titration, the colour of the solution changes from wine red to pure blue.
Reactions involved during titration:
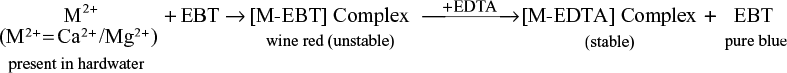
- The calcium and magnesium ion present in hard water combines with the indicator EBT at pH 9-10 to form less stable wine red complex.

- When EDTA is added to the water sample, the free M2+ (metal ions) forms a stable complex of M-EDTA.

- At the end point, when all free metal ions get complexed with EDTA, then further addition of EDTA sets free the metal ion from metal indicator complex and forms more stable metal EDTA complex.

The metal-EDTA complex may be represented as:

Procedure
Step I: Standardization of EDTA solution: Rinse and fill the burette with EDTA solution. Pipette out 50 ml of standard hard water (S.H.W)/Standard MgSO4 solution in a conical flask. Add 10–15 ml of buffer solution and two drops of EBT indicator. Titrate the flask solution against the EDTA solution from the burette until the colour changes from wine red to pure blue, it is end point. Repeat the procedure to get two concordant readings. Let the volume of EDTA be consumed as V1 ml.
Step II: Determination of total hardness: Titrate 50 ml of unknown water sample with EDTA solution by addition of 10–15 ml of buffer solution and two drops of EBT indicator till the wine red colour changes to pure blue. Let the volume of EDTA be consumed as V2 ml.
Step III: Determination of permanent hardness: Take 250 ml of water sample in a 500 ml beaker and boil gently for half an hour. Cool, filter, and wash the precipitate with distilled water, collecting filtrate and washing in a 250 ml measuring flask, and make the volume up to the mark. Now titrate 50 ml of boiled water sample same as in step I. Let the volume of EDTA be consumed as V3 ml.
Observations and Calculations
1 ml of standard hard water = 1 mg of CaCO3
Step I: Standardization of EDTA solution:
Volume of S.H.W taken for titration = 50 ml
Concordant volume of EDTA used = V1 ml

Step II: Determination of total hardness:
Volume of unknown water sample taken for titration = 50 ml
Volume of EDTA used = V2 ml

Step III: Determination of permanent hardness:
Volume of hard water sample taken after boiling and filtering = 50 ml
Let concordant volume of EDTA used = V3 ml

Advantages of EDTA Method
- EDTA method shows the result with greater accuracy.
- This method is more convenient in comparison with other methods.
- Procedure of EDTA method is more rapid.
Solved Numerical Problems Based on EDTA Method
(vi) The 1 liter standard hard water (SHW) was prepared by dissolving 1.0 gm of pure and dry CaCO3 in liter distilled water. 50 ml of this SHW required 46 ml of EDTA solution while 50 ml of the given hard water sample consumed 20 ml of EDTA solution. After boiling, cooling, and filtering, the hard water sample consumed 10 ml of EDTA solution. Determine the total, permanent, and temporary hardness in ppm.
Solution
Step-I Standardization of EDTA solution

Step-II Determination of total hardness

Step-III Determination of permanent hardness

(vii) Calculate carbonate and non-carbonate hardness of water, if 20 mL of standard hard water which containing 1.5 g CaCO3 per liter, it required 25 mL EDTA solution for end point and 100 mL of water sample required 18 mL EDTA solution, while the same amount of water after boiling required 12 mL EDTA solution.
Solution 1000 ml H2O contains 1.5 g of CaCO3
1 ml of SHW = 1.5 mg of CaCO3
Step-I Standardization of EDTA solution

Step-II Determination of total hardness

Step-III Determination of permanent (non-carbonate) hardness

(viii) Calculate the amount of lime and soda required for the softening of 15000 liters of water, which is analyzed as follows:
Temporary hardness = 25 ppm
Permanent hardness = 20 ppm
Permanent Mg hardness = 15 ppm


(ix) Calculate the quantity of lime and soda required to soften 20,000 liters of water containing the following salts:

assuming the purity of lime as 90% and soda as 95%.
Solution


(x) Analysis of water gave the following results H2SO4 = 196 mg/L, MgSO4 = 24 mg/L, CaSO4 = 272 mg/L., and NaCl = 25 mg/L. Water is to be supplied to the town with the population of one lakh only. The daily consumption of water is 50 liter per head. Calculate the cost of lime and soda required for the softening of the hard water for the town for April 2008, if the cost of lime is Rs. 5 per kg and cost of soda is Rs. 8 per kg.
Solution


1.6 DISSOLVED OXYGEN (DO)
Amount of oxygen dissolved in water (mg/L) is known as dissolved oxygen. At ambient conditions of temperature and pressure, the solubility of oxygen is about 8 mg/L. The amount of dissolved oxygen measures the biological activity of the water bodies, and this is most essential for sustaining aquatic life. Estimation of DO content in a particular water body is of important significance of environmental as well as the industrial point of view. This serves as an indicator of the extent of pollution of water by oxidizable and organic impurities. Further, DO is also responsible for corrosion of boilers and requires to be eliminated.
The Winkler test is used to determine the concentration of DO in a water sample. Here the water sample is treated with a mixture of manganese sulphate and alkaline potassium iodide. Initially formed manganous hydroxide precipitate traps the dissolved oxygen and oxidizes manganous ion (Mn+2) to a brown-coloured precipitate of manganic oxide (MnO(OH)2).
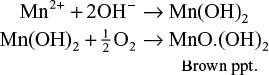
The formed manganic oxide precipitate is allowed to settle down for a few minutes and then 2 to 3 ml of concentrated H2SO4 is added to dissolved the precipitate. The liberated iodine is proportional to the dissolved oxygen content of water sample. This is estimated by titrating a standard sodium thiosulphate solution and using a starch solution as an indicator.
From the above equation, we can find that
![]()
Therefore, after determining the number of moles of iodine produced, we can calculate the number of moles of oxygen molecule present in the water sample. The oxygen content is usually presented as mg dm-3. The solubility of oxygen in water at ambient conditions of temperature, and pressure is about 8 mg/L.
1.7 DETERMINATION OF CHLORIDES IN WATER
Acidity is the ability of water to react with bases and certain metals. (or) An acid is a substance which can act as a proton donor (or) Quantitative capacity of water to neutralise the base. Chlorides are present in water as salts of calcium, magnesium, sodium and potassium [NaCl, CaCl2, KCl, MgCl2]. The salty taste of water is due to NaCl present in it. Chlorides are not considered harmful if their concentration is less than of 250 mg/L. Other salts such as MgCl2 in water undergo hydrolysis and cause problem in boilers.
Principle
When potassium chromate is added as an indicator to the water sample, it dissolves in water and the chromate ions give yellow colour to the sample. Sodium chloride is present in the dissolved state in the given sample of water. When this is titrated against silver nitrate, the silver ions react first with the chloride ions present in the sample and form silver chloride precipitate and sodium nitrate.
![]()
When all the chloride ions in the sample are precipitated, the excess silver nitrate present reacts with potassium chromate and forms a pale red precipitate of silver chromate.
![]()
The appearance of the pale red colour indicates that all chloride ions have been precipitated and indicates the end point of titration. From the titre values, the amount of chloride and salt present in the sample is calculated.
Indicator: Potassium chromate
End point: Yellow to brick red
Procedure
Take a 50 ml burette and wash it with tap water and distilled water and then rinse it with 0.005 N silver nitrate solution. Fill the burette with the 0.005 N silver nitrate solution and note down the initial reading. Pipette out 10 ml of the given water sample with a clean 10 ml pipette into a clean and dry conical flask. Add two to three drops of potassium chromate as the indicator. The solution in the conical flask turns to yellow colour. Titrate this solution against the 0.005 N silver nitrate solution taken in the burette. The appearance of a brick red colour is the end point of titration. Note down the final burette reading. Repeat the titration until consecutive concordant values are obtained. From the titre values, calculate the amount of chloride and salt present in the given water sample using the given formulae.
Calculations

1.8 DETERMINATION OF ACIDITY IN WATER
Dissolved carbon dioxide (CO2) in water contributes to the acidity of water by formation of carbonic acid. Water used for drinking purpose should not contain mineral acidity. Highly acidic water, i.e., having low pH affects aquatic life.
Principle
Acidity of water depends on the end point of indicator used. Hydrolysis or dissociation of acids release H+ ions which react with standard alkali (NaOH) during titrations.
The colour change of phenolphthalein indicator indicates neutralization of carbonic acid present in water sample.
Indicators
- Phenolphthalein
- Methyl orange
End Point
In case of methyl orange: Orange to yellow
In case of phenolphthalein: Appearance of pink colour
Procedure
Take a 50 ml burette and wash it with tap water and distilled water and then rinse it with sodium hydroxide solution. Fill the burette with 0.02 N sodium hydroxide solution and note down the initial reading. Take 100 ml of water sample into a conical flask. Add 4 to 5 drops of methyl orange indicator and colour changes to orange. Titrate the water sample against sodium hydroxide solution until the colour changes to yellow and note down the volume consumed as A ml. To the same solution, add 3 to 4 drops of phenolphthalein indicator and continue the titration until the appearance of pink colour. Note down the volume of sodium hydroxide consumed as B ml. Repeat the titration to get consecutive concordant readings.
Calculations

1.9 ALKALINITY OF WATER
The capacity of water for neutralizing an acid solution is known as alkalinity of water (or) the capacity of a water to accept protons is known as alkalinity of water.
The alkalinity of water is mainly dependent on the following factors:
- Due to the presence of ions like
 , etc. in water.
, etc. in water. - The presence of weak organic acid salts.
Because they consume or have a tendency to take up N+ ions, hence concentration of OH– ions in water increases.
Classification of Alkalinity
Depending on the ions present, alkalinity of water is broadly classified as
- Hydroxide (OH–)
- Carbonate
 and
and - Bicarbonate
 alkalinity
alkalinity
The alkalinity of a water sample may be due to
- OH−


- OH− and

 and
and 
But there is no possibility with OH− and ![]() , because they combine with each other to form carbonate.
, because they combine with each other to form carbonate.
![]()
Units
Alkalinity and hardness are expressed in terms of CaCO3 equivalents, ppm, mg/L, etc.
- Alkalinity < total hardness
Carbonate hardness in ppm = Alkalinity in ppm
- Alkalinity ≥ total hardness
Carbonate hardness in ppm = Total hardness in ppm
Non-carbonate hardness = Total hardness – Carbonate hardness
Determination
The type and extent of alkalinity of a water sample can be easily determined by volumetric method. A known volume of water sample is titrated against standard sulphuric acid by using phenolphthalein indicator. The end point is disappearance of pink colour. Further the titrated water sample is titrated against the same standard sulphuric acid by using methyl orange indicator. The end point is appearance of red colour and the volume of H2SO4 consumed is noted.

The volume of the standard acid used up to phenolphthalein end point P marks the completion of reactions (i) and (ii), whereas the total volume of the standard acid used from the beginning up to methyl orange end point M corresponds to the completion of reactions (i), (ii) and (iii).
Solved Numerical Problems Based on Alkalinity of Water
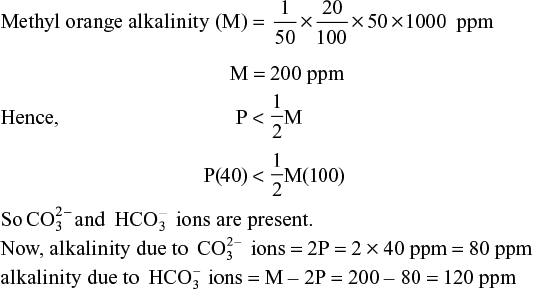
(xi) 100 mL of a water sample required 4 mL of ![]() H2SO4 for neutralization to phenolphthalein end point. Another 16 mL of same acid was needed for further titration to methyl orange end point. Determine the type and amount of alkalinity in terms of CaCO3 equivalent.
H2SO4 for neutralization to phenolphthalein end point. Another 16 mL of same acid was needed for further titration to methyl orange end point. Determine the type and amount of alkalinity in terms of CaCO3 equivalent.
Solution Volume of water sample for titration = 100 mL
Volume used to phenolphthalein end point (A) = 4 mL
Volume used to methyl orange end point (B) = 16 mL
Total volume used to methyl orange end point (A + B) = 20 mL
Phenolphthalein alkalinity (in terms of CaCO3 equivalent)

Similarly, for methyl orange alkalinity,

(xii) Calculate the alkalinity in CaCO3 equivalents, if 100 mL of a water sample on titration with 0.03 N HCl by using phenolphthalein indicator and end point is at 7.5 mL acid. Another water sample of same volume require 15 mL of same concentration acid by using methyl orange indicator to obtain complete neutralization.
Solution Volume of water sample = 100 mL
For Phenolphthalein alkalinity

For Methyl orange alkalinity,


(xiii) 50 ml of alkaline water sample required 20 ml of ![]() H2SO4 for phenolphthalein end point and another 5 ml for methyl orange indicator, i.e., complete neutralization. Describe the type and amount of alkalinity.
H2SO4 for phenolphthalein end point and another 5 ml for methyl orange indicator, i.e., complete neutralization. Describe the type and amount of alkalinity.
Solution For Phenolphthalein alkalinity,

For Methyl orange alkalinity,


1.10 DISADVANTAGES OF HARD WATER
Hard water contains large amounts of bicarbonates, sulphates, and chlorides of calcium and magnesium salts. It causes number of problems in domestic use, industrial use, and in boilers.
- Problems in Domestic Use:
- Cooking: Pulses and other vegetables do not cook well in hard water.
Tea, coffee and other drinks prepared with hard water gives an unpleasant taste.
- Drinking: Hard water causes bad effect on digestive system.
Due to formation of calcium oxalates, stones are formed in kidneys.
- Wastage of soap:
Washing: Hard water does not give much lather with soap, as most of the soap is consumed for removing calcium and magnesium salts present in water.
Bathing: It produces sticky scum on both tub and body.
- Damaging clothes: The Ca2+ and Mg2+ ions present in hard water combine with soap to form insoluble compounds, which sticks to the clothes. This is difficult to remove, and hence damages the clothes.
- Wastage of fuel: Much fuel is consumed for boiling hard water in kettles because salts form an incrustation inside the kettle due to formation of carbonates and hydroxides of calcium and magnesium.
After prolongedusage, kettle also gets damaged due to scale formation.
- Cooking: Pulses and other vegetables do not cook well in hard water.
- Problems in Industrial Use:
- Textile industry: Water is used in textile industry for cleaning, washing, and whitening of yarn. For such purposes, soap is required; if hard water is used, more amount of soap is wasted.
- Paper industry: The water that is used in paper industry should be free from hardness, suspended particles, iron, etc. Because hardness increases the ash contents of paper, suspended particles produce cracking tendency of paper, and the salt of iron decreases the brightness of paper.
- Iron industry: Hard water makes the iron of low quality. It corrodes the iron and its alloys.
- Dyeing industry: The water that is used for dyeing purpose should be free from hardness, because salts of calcium and magnesium spoil the desired shade.
- Sugar industry: Water should be free from hardness-suspended particles as well as pathogenic microorganisms because hard water causes difficulties in the crystallization of sugar from molasses.
- Problems in Boilers, Use:
For the generation of steam a huge quantity of water is used in boilers and is known as boiler feed water. If water used for boilers is hard, it may create number of problems like caustic embrittlement, corrosion, scale and sludge formation, priming and foaming, etc. This is very dangerous because at high pressure the same causes explosions. Hence water which is used in boilers should be softened and should be pure before feeding into the boilers.
Boiler-feed water should satisfy the following requirements:
- Hardness < 0.5 ppm
- Caustic alkalinity = 0.15 – 0.45 ppm
- Soda alkalinity < 1 ppm
- Excess soda ash < 0.55 ppm
1.11 QUALITY OF WATER FOR DOMESTIC USE
The potable water or drinking water should satisfy the following essential requirements:
- Water should be clear, clean, colourless, and odourless.
- Total dissolved solids (TDS) should be less than 500 ppm.
- It should be free from hardness, suspended particles, and pathogenic bacteria.
- Turbidity should be less than 10 ppm.
- Its pH should be about 7–8.
- It should be free from harmful, dissolved solids like arsenic, manganese, chromium, lead, etc.
- It should be free from harmful gases like H2S, SO2, etc.
- It should be neither too hard nor too soft. The recommended hardness is about 300 mg/L as CaCO3 equivalent.
- Its alkalinity should not exceed 600 mg/L.
- It should have an agreeable taste.
- Fluoride should be less than 3 ppm.
- Chloride and sulphate must be less than 250 ppm.
1.12 TREATMENT OF WATER FOR DOMESTIC USE
Purification of water for potable use involves mainly the following steps:
- Screening: Removes the floating materials like leaves.
- Sedimentation: Removes suspended impurities like sand, clay, etc.
- Coagulation: Removes finely divided suspended particles.
- Filteration: Removes colloidal impurities and large organisms.
- Disinfection: Kills the bacteria.
- Screening: The raw water is allowed to pass through screens having large number of holes, where floating impurities like rags, paper, leaves, etc., are held by them, and water is passed through the holes.
- Sedimentation: Sedimentation is a process for retention of water for certain period in a deep tank (≃5 meter) or to flow quietly at low velocities. Most of the suspended particles settle down due to the force of gravity. This process takes two to eight hours. This process removes 70%–75% of suspended impurities.
- Coagulation: Coagulation is the process by which the fine, suspended, and colloidal impurities are removed from the water by the addition of suitable chemicals (coagulants). The finely divided suspended inorganic matters do not settle down so easily, so these smaller particles are converted into larger ones, which have higher settling velocities.
The commonly used coagulants are the salts of iron and aluminium, e.g., alum (K2SO4 Al2(SO4)3 · 24H2O), ferrous sulphate (FeSO4 · 7H2O), sodium aluminate (NaAlO2), etc. These coagulants react with alkaline salts and form a thick gelatinous precipitate known as Flock. Flock has the property to attract finely suspended particles and form big flock, which settles down rapidly. This process is called flocculation.
A few commonly used coagulants and their reactions are as follows:
- Alum (Al2(SO4)3 K2SO4 · 24H2O)

- Sodium aluminate (NaAlO2)

- Ferrous sulphate (FeSO4 · 7H2O)

The precipitates obtained by using suitable coagulants in water get settled down during sedimentation.
- Alum (Al2(SO4)3 K2SO4 · 24H2O)
- Filteration: Almost all suspended impurities are removed through filteration process. During filteration, all types of insoluble colloidal and bacterial impurities are also removed by passing water through a bed of proper-sized material. Two types of filters are commonly used in domestic water treatment.
- Gravity sand filter
- Pressure filter
- Gravity sand filter: It consists of a large, shallow, rectangular tank made of concrete and a process medium, known as filter medium, which retains solid particles but allows the passage of water as shown in Figure 1.3.

Figure 1.3 Sand filter
It consists of three layers. The upper layer consists of fine sand (about 50 cm thick) and is a thick layer. The middle layer consists of coarse sand (about 20 cm thick), and the bottom layer consists of gravels (about 30 cm thick). It is provided with an inlet for sedimented water and an under drain channel at the bottom for the exit of filtered water. Sedimented water enters the sand filter from the top and is uniformly distributed over the fine sand layer. As the water percolates through the sand bed, finely suspended particles and most of the germs and bacteria are retained by the top layer. Clear, filtered water is collected in the under drain channel, from where it is drawn out.
The rate of filteration becomes slow after some time due to clogging of pores of the top sand layer by the impurities retained in the pores. Therefore, the portion of the top fine sand layer is scrapped off and replaced by a new sand layer. The filter is put to use again.
- Pressure filter: It consist of a cylindrical as shown in Figure 1.4, vertical steel tank containing three layers of filtering media, one above the other.
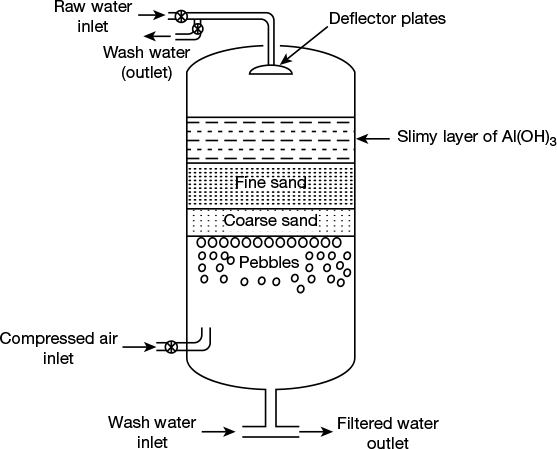
Figure 1.4 Vertical pressure filter
- Pebbles layer (10–35 mm grain size)
- Coarse sand layer (5–7 mm grain size)
- Fine sand layer (1–2 mm grain size)
Impure, sedimented water is mixed with a small amount of alum solution, and then water is forced through filter bed under pressure. Alum forms the slimy layer on the filter bed, and this helps in the removal of colloidal and bacteriological impurities. The function of deflector plate, which is provided at the top, is to distribute the slimy layer of alum uniformly over the top of the filter bed. Filtered water, as it comes out from the bottom of filter, is under pressure and can thus be pumped directly. These filters are widely used for industrial purposes.
- Disinfection/Sterilization: Sterilization of water means complete destruction of all living microorganisms (bacteria, virus, etc.) present in water. We know that water after passing through different processes such as sedimentation, coagulation, and filteration processes still contains a small percentage of pathogenic bacteria. Therefore, it is necessary to remove these bacteria and microorganisms from water. The chemicals used for sterilization are known as sterilizers or disinfectants.
Several methods have been adopted for sterilization of water. Some of them are given below:
- Boiling method
- Chlorination method
- Ozonolysis method
- UV-rays method
- Membrane technology method
- Boiling method: Water for domestic purposes on a smaller scale may be sterilized by simple boiling method. In this method, water is boiled for about 20–30 min. This method kills the harmful disease-causing bacteria and germs. But this method is useful only for household purposes because this process is very much expensive for municipal supply of water, and in addition, a large quantity of fuel is required to boil water on a large scale. It does not provide any protection for further contamination of water.
- Chlorination method: It is the most important method for sterilization of water. Chlorination is done by the following methods:
- By using chlorine gas or concentrated aqueous solution.
- By using bleaching powder.
- By using chloramine.
- By using chlorine gas or concentrated aqueous solution
Chlorine is a powerful germicide and most commonly used disinfectant. Chlorine used for this purpose can be used directly as a gas or as chlorine water.
It reacts with water to form hypochlorous acid and nascent oxygen, both of which are powerful germicides.

Apparatus: The apparatus used for disinfection by chlorine is known as chlorinator (Figure 1.5). It is a large tower containing number of baffle plates. From the top of the tower, proper dose of chlorine and water is introduced. They get thoroughly mixed during their passage through the tower, and treated water is taken out from the bottom.

Figure 1.5 Chlorinator
Advantages
- It is cheap and is an easily available disinfectant.
- At a low concentration, it is very effective bactericide.
- It can be used at high and low temperatures.
- It is stable and does not deteriorate on keeping.
- Chlorine residue can be maintained in treated water, which provides additional safety for preventing regrowth of bacteria.
Disadvantages
- Excess of chlorine produces an unpleasant taste and odour in water.
- It is less effective at higher pH value but more effective at lower pH value (below pH 6.5).
- By using bleaching powder (CaOCl2)
Bleaching powder is a strong oxidizing agent and is having 30 per cent available chlorine. When water is treated with bleaching powder, hypochlorous acid is formed. It releases nascent oxygen and the nascent oxygen thus released deactivates the enzymes of microorganisms; due to this, metabolic activities will stop and the microorganisms get killed.

About 1 kg of bleaching powder is sufficient for 1000 kilolitres of water, but allow the water to stand for several hours.
Disadvantages
- Excess of bleaching powder creates bad taste and odour to water.
- It introduces calcium hardness in water due to the formation of Ca(OH)2.
- It is unstable, so its storage is difficult.
- By using chloramines (NH2Cl)
By mixing of chlorine and ammonia in 2:1 ratio, chloramine is formed.

Whenever water is treated with chloramine, hypochlorous acid is formed and with release of hypochlorous acid it provides greater safeguard from recontamination.

So, HOCl + germs → germs are killed.
Advantages
- Excess dose of ClNH2 does not create bad odour and taste in water.
- It provides a greater lasting effect than chlorine.
- Ozonolysis method: Ozone is used for this method. Ozone is a highly unstable and excellent disinfectant. It breaks down and gives nascent oxygen.

The nascent oxygen is very powerful oxidizing agent, which kills all the bacteria and germs present in water.
Apparatus: The reaction of ozone and water is carried out in ozone sterilizer (Figure 1.6). During the treatment of water, water is allowed to enter from top to bottom, and ozone is allowed to enter from bottom to top, which kills the germs when they come in contact with each other. Sterilized water is collected at the bottom of the tank. The contact time for ozone and water is about 10–15 minutes.

Figure 1.6 Ozone sterilizer
Advantages
- It removes colour and odour from water.
- It improves the taste of water.
- The excess dose of ozone is not harmful, because it releases O2 on decomposition.
- UV-rays method: When water is exposed to UV-rays from electric mercury lamp that is immersed in water, most of the pathogenic bacteria are destroyed. This method is widely used for the disinfection of swimming pool water.
Advantages
- It does not require any chemicals.
- It has not any bad effect during treatment.
- It does not produce any odour in water.
Disadvantages
- It is very expensive, so it is not widely used on a large scale.
- Membrane technology method: Disinfection by this method is generally used for drinking water. In this method, water purifies most of the contaminant ions, molecules, and small particles including viruses and bacteria by passing them through a membrane having uniform microscopic-size pores. Membrane processes include microfilteration, ultrafilteration, nanofilteration, and reverse osmosis.
In all these methods, water is forced through membranes made of synthetic polymers, cellulose acetate, or even ceramics by the application of high pressure in the range of 10 to 50 atm. pressure. Microfilteration and ultrafilteraton membranes with pores of 0.002 to 10 μm in diameter can filter off most bacteria and colloidal particles but not viruses and ions. Nanofilteration soften water by removing hardness causing metal ions, and reverse osmosis is used for desalination of sea water.
1.13 BREAK-POINT CHLORINATION
Chlorination of water is done carefully in a controlled manner with the dip or break is called break-point chlorination. Added chlorine consumed for different reactions such as
- Oxidation of reducing substance
- Chlorination of organic substance
- Oxidation of ammonia and disinfection of bacteria
With this method not only living organisms but also organic impurities and free NH3 present in water are destroyed. The point at which free residual chlorine begins to appear is called break-point chlorination. It is also known as free residual chlorination.
Break-point chlorination shows four stages of sterilization as shown in Figure 1.7:
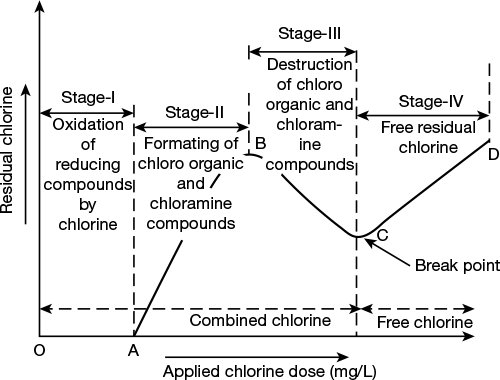
Figure 1.7 Break-point chlorination curve
Stage I: Initially, with the lower dosage of Cl2, there is no free residual chlorine since all the added Cl2 gets consumed in complete oxidation of reducing substances present in water.
Stage II: As the amount of Cl2 dose is increased, the amount of residual Cl2 also shows steady increase. This stage corresponds to the formation of chloro-organic and chloramines compounds without undergoing oxidation.
Stage III: As more amount of Cl2 is applied, the amount of free residual chlorine also decreases, due to oxidation of chloro-organic and chloramines. When the oxidation destruction is complete, it reaches a minima.
Stage IV: After minima, the added Cl2 is not used in any reaction. Thus, the residual Cl2 keeps on increasing in direct proportion to added Cl2.
The point ‘C’ is called break point. It is a point at which free residual chlorine begins to appear. Thus, break-point chlorination helps in eliminating disagreeable odour and bad taste in water.
Advantages
- It prevents the presence of excess chlorine, which may impart bad odour and taste to water.
- It ensures complete destruction of disease-producing bacteria.
- It prevents the development of any weeds in water.
- It helps to calculate the sufficient amount of chlorine for adding in water.
Dechlorination: Excess of chlorine after the break-point chlorination gives unpleasant taste and odour in water. The excess of Cl2 may be removed by filtering the treated water over activated carbon. Over chlorination may also be removed by treating the water with SO2, Na2SO3, and Na2S2O3.

Superchlorination: Superchlorination is the addition of excess amount of chlorine for disinfection of water. It destroys the pathogenic microorganisms as well as organic impurities by oxidation.
Prechlorination: Prechlorination is the treatment of water with chlorine before filtration. In this process high chlorine is required to satisfy the chlorine demand of filterable matter. With prechlorination the quality of water is superior because unpleasant tastes and odours due to chlorinated products may be absorbed during filtration. This process is highly expensive.
Post-chlorination: Post-chlorination is the treatment of chlorine with water after filtration. In this method treated water may have unpleasant taste and odour, but it is cheaper than prechlorination due to lower chlorine demand.
1.14 BOILERS AND BOILER TROUBLES
In all the industries, boilers are used for generating steam. Boiler-feed water is the water required for generation of steam and with the safety, economy and efficiency concerns it should be of very good quality.
Depending upon the operating pressures, boilers are classified into low-pressure (10–15 kg/cm2), medium-pressure (15–35 kg/cm2), high-pressure (50–140 kg/cm2), very high-pressure (150–225 kg/cm2) and supercritical boilers (>225 kg/cm2).
Depends upon the quality of the feed water, so many problems may arise in the boilers. Some of them are listed hereunder.
- Scale and sludge formation
- Priming and foaming or carry over
- Boiler corrosion
- Caustic embrittlement
- Scale and Sludge formation
In the boilers, when water is vaporized to steam gradually the concentration of dissolved salts increases. When the concentration of salts reaches their saturation, they are thrown out in the form of precipitates. Sludge is the soft, slimy and non-adherent layer of precipitate inside the boiler and also called mud. Hard adhering coating of precipitate inside the boiler walls is called scale. Scale and sludge are shown in Figure 1.8.

Figure 1.8 Sludge and scale
- Sludge: Sludge is a soft, loose, and slimy precipitate formed within the boiler sludge. It can easily be scrapped off with a wire brush. It is formed at comparatively colder portions of the boiler and collects in areas of the system, where the flow rate is slow or at bends. Sludges are formed by substances that have greater solubility in hot water than cold water.
Composition: The main composition of sludge includes MgCO3, MgCl2, CaCl2, MgSO4, etc.
Disadvantages
- Sludges are poor conductor of heat, so they tend to waste a portion of heat generated.
- Sludges decrease the efficiency of the boiler.
- Since sludges settle in areas of poor water circulation such as joints, bends, etc., therefore choking of pipes takes place.
Removal of Sludge
- By taking small precautions, like using of soft water, prevent the formation of sludge
- Scrapping of sludge with hard brush
- With frequent ‘blow-down operation’
i.e., replacement of concentrated water with fresh water.
- Scale: Scale is a hard, adhering and sticky deposit. They stick very firmly on the inner walls of the boilers and very difficult to remove even with a chisel and hammer. These are formed with CaCO3, Ca(OH)2, Mg(OH)2, CaSO4, CaSiO3, MgSiO3, etc.
Formation of Scales: Due to the following reactions, scales are formed.
- Decomposition of calcium bicarbonate
In low pressure boilers, calcium bicarbonate decomposes and gives calcium carbonate.

At high pressure boiler formed CaCO3 is soluble and gives calcium hydroxide, whose solubility decreases with the temperature and deposit as scale.

- Deposition of calcium sulphate as a scale
With increase of temperature the solubility of calcium sulphate decreases, and consequently gets precipitated as hard scale. This scale is quite adherent and difficult to remove.
- Hydrolysis of magnesium chloride
At high temperatures, the magnesium salts undergo hydrolysis and give magnesium hydroxide.

- Formation of silicates
Minute amounts of silica present in water form and deposit as calcium or magnesium silicates and stick very firmly to the inner side of the boiler surface.
Disadvantages
- Fuel wastage: As scales have a low thermal conductivity to provide a continuous supply of heat to water, overheating is done, which results in the wastage of fuel.
- Decrease in efficiency: Scales get deposited in the valves and condensers of the boilers, thereby choking them partially. It results in decrease in efficiency of the boiler.
- Danger of explosion: Sometimes at high pressure, the scales crack and water suddenly comes in contact with overheated iron plates. This results in the sudden formation of large amount of steam, which may cause explosion.
- Lowering of boiler safety: Super heating of boiler makes the boiler material softer and weaker, which causes distortion of boiler tube.
Removal
- For soft scale: Soft scale is loosely adhering, so it can be removed with the help of wire brush or blow-down operation.
- For brittle scale: Brittle scale can be removed by giving thermal shocks to the boiler, i.e., heating and cooling suddenly.
- For hard and adhering scale: They can be removed by adding chemicals.
Example:
- CaCO3 scale can be dissolved by using 5%–10% HCl.
- CaSO4 scale can be dissolved by adding EDTA since Ca-EDTA complex is highly soluble in water.

- Decomposition of calcium bicarbonate
- Sludge: Sludge is a soft, loose, and slimy precipitate formed within the boiler sludge. It can easily be scrapped off with a wire brush. It is formed at comparatively colder portions of the boiler and collects in areas of the system, where the flow rate is slow or at bends. Sludges are formed by substances that have greater solubility in hot water than cold water.
- Priming, carry over and foaming
- Priming: Priming is the carrying of small droplets of water along with steam while boiling the water. This is also known as wet steam.
Causes of Priming
- Improper design of the boiler
- Presence of large amount of finely divided particles in the boiling water
- Presence of large amount of dissolved solids
- Very high level of water in the boiler
- High steam generation velocities
- Sudden increase in steamproduction rate
- Presence of foam on the surface
Precautions to Reducing Priming
- Maintaining low level of water
- Fitting of mechanical steam purifiers
- Ensuring efficient softening and filtration of boiler-feed water
- Avoiding of rapid change in steam generation
- Removal of scale and sludge frequently
Carryover: Carrying of suspended and dissolved solids along with wet steam is called carryover.
- Foaming: Foaming is the formation of persisted form (or) stable bubbles in the boilers, which do not break easily. This is due the concentration difference of suspended solid between the film and the bulk of water. It is also due to the presence of oil and alkalies in boiler-feed water. Oily substances and alkalies react to form soaps, which reduce the surface tension of water and thus increase the foaming tendency of water.
Causes of Foaming
The following are the causes of foaming:
- Due to the presence of oil or grease in water
- Due to the presence of finely divided sludge in water
- Due to the presence of some chemicals, which reduce the surface tension.
Prevention of foaming
- By the addition of antifoaming agents such as castor oil or polyamides in low-pressure boilers
- By using soft and filtered water
- By removing oil from boiler water by adding coagulants like ferrous sulphate or sodium aluminate, etc.
Disadvantages of Priming and Foaming
- Due to the presence of foaming, boiling point of water is increased; hence wastage of fuel occurs.
- It is very difficult to maintain the constant pressure of steam.
- Due to the excess formation of foaming, the bubbles entered into the engine along with the steam, which lowers the efficiency of engine.
- Due to priming and foaming, corrosion takes place in the part of the engine.
- Due to the presence of foam, water level is not identified.
- Priming: Priming is the carrying of small droplets of water along with steam while boiling the water. This is also known as wet steam.
- Boiler Corrosion
It is the disintegration or decay of boiler material either due to chemical or electrochemical reaction with its environment.
Factors Causing the Boiler Corrosion
- Formation of rust with dissolved oxygen: Dissolved oxygen present in the water attacks the boiler material and easily forms rust.

- Due to the presence of dissolved CO2: The source of CO2 in water, either dissolved CO2 gas or bicarbonates, on heating gives CO2 and is also responsible for boiler corrosion.

Carbon dioxide (CO2) dissolves in water to form a weak carbonic acid.

- Due to the formation of acids from dissolved salts: Chlorides of some inorganic salts like MgCl2, CaCl2 etc., which present in water can produce hydrochloric acid and can corrode the boilers.

The liberated HCl reacts with boiler material in chain-like reaction.

- Due to the presence of oil: Oil undergoes hydrolysis, releasing free fatty acids leading to corrosion of the boiler.
Prevention of Boiler Corrosion
- By removal of DO
- Preheating: As solubility of gases decreases with increases in temperature, at approximate 65°C, complete DO is removed.
- Chemical treatment: The DO is removed through the addition of Na2SO3 or Na2S or hydrazine (N2H4).

Hydrazine is found to be an ideal compound for removing DO because the products are water and nitrogen gas, which do not form hard products, while due to sodium sulphite (Na2S) and sodium sulphide (Na2SO3), there is a formation of sodium sulphate (Na2SO4), which decomposes and gives SO2, and it forms sulphurous acid (H2SO3) in steam condensate.
- Mechanical deaeration: As shown in Figure 1.9, the water passes through the perforated plates and undergoes deaeration at high temperature and low-pressure dissolved oxygen and carbon dioxide escapes. The solubility of a gas in water is directly proportional to pressure and inversely proportional to temperature, hence the water gets deaeration.

Figure 1.9 Mechanical deaeration of water
- By removal of dissolved carbon dioxide (CO2)
- Preheating: By increasing the temperature, solubility decreases.
- Chemical treatment: By adding calculated quantity of ammonia

- Mechanical deaeration: It removes DO as well as CO2 from feed water.
- Addition of alkali: Corrosion by acids may be prevented by adding some alkalies from outside so that product acid may be neutralized.
- By using soft water in the boiler for steam generation.
- By removal of DO
- Formation of rust with dissolved oxygen: Dissolved oxygen present in the water attacks the boiler material and easily forms rust.
- Caustic Embrittlement
Caustic embrittlement is the special type of boiler corrosion caused by the use of highly alkaline water. With this phenomena boiler material becomes brittle with the accumulation of caustic substances.
During the softening of water by lime soda process, usually small amount of free Na2CO3 is present. In high-pressure boilers, sodium carbonate decomposes and gives sodium hydroxide and this makes the boiler water ‘caustic.’

The concentration of NaOH is increased by evaporation of water, and attacks the boiler material by giving sodium ferroate (Na2FeO2), which decomposes and forms rust.

This is an electrochemical phenomenon and can be explained on the basis that a concentration cell is formed due to concentration difference of sodium hydroxide in the boilers particularly at highly stressed parts like joints, rivets, etc. The dilute NaOH region in the boiler acts as a cathode and the concentrated NaOH region acts as an anode and undergoes corrosion.

Preventions
- By using sodium phosphate as a softening agent instead of sodium carbonate.
- By adding certain chemicals such as lignin and tannin to boiler water because they block the hair cracking inside the boiler.
- By adding sodium sulphate to boiler water, which blocks the minute cracks thereby preventing the entry of sodium hydroxide solution.
1.15 SOFTENING OF WATER
In water, there is a formation of scale-like impurities in the boiler. This scale formation may be minimized by the following treatments:
- Internal treatment
- External treatment
- Internal Treatment
In this method, chemicals are added to the water in the boiler, which is hard in nature. The added chemicals may function as precipitating agents or sequestering agents to form more soluble complex compounds with metal ions. In this method, hard deposit scale is changed into loose deposits, which are easily removed by blow-down operation. Internal treatment of boiler water depends on the nature of feed water and the type of the boiler.
Some important internal treatment methods are:
- Colloidal conditioning: In this method, scale formation can be reduced by introducing organic substances like kerosene, tannin, agar-agar, etc. They surround the minute particles of scale-forming salts, thereby yielding non-sticky and loose deposits, which can easily be removed by blow-down operation.
- Carbonate conditioning: In low-pressure boilers, scale formation is prevented by adding sodium carbonate (Na2CO3) to boiler water to prevent the precipitation of scale-forming calcium sulphate (CaSO4).
When calcium sulphate is converted into calcium carbonate by the addition of sodium carbonate, CaCO3 acts as a loose sludge, which can be removed by blow-down operation.
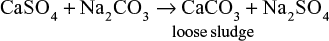
Carbonate conditioning is not used in high-pressure boilers because excess of Na2CO3 might be converted into NaOH due to hydrolysis, which causes caustic embrittlement.
- Phosphate conditioning: Phosphate conditioning involves conversion of scale-forming calcium and magnesium salts into the most insoluble compound of calcium phosphate (Ca3(PO4)2) and magnesium phosphate (Mg3(PO4)2), which form easily removable non-adherent soft sludge, which can be removed by blow-down operation.

The three sodium orthophosphates may be used depending upon the alkalinity of the boiler-feed water.
- In acidic medium, sodium dihydrogen phosphate (NaH2PO4)
- In weakly alkaline medium, disodium hydrogen phosphate (Na2HPO4)
- In alkaline medium, trisodium phosphate (Na3PO4)
- Calgon conditioning: Addition of calgon or sodium hexameta phosphate (NaPO3)6 to boiler water converts calcium salts into soluble complex compound thereby preventing scale or sludge formation.

- Sodium aluminate treatment: When we add sodium aluminate (NaAlO2) in boiler-feed water, it gets hydrolyzed and form sodium hydroxide (NaOH) and gelatinous precipitate of aluminium hydroxide Al(OH)3.

The sodium hydroxide reacts with magnesium salt and converts it into magnesium hydroxide Mg(OH)2 precipitates.

The gelatinous precipitate of Al(OH)3 and Mg(OH)2 entraps colloidal and finely suspended impurities along with oil drops and silica. The loose slimy precipitate can be easily removed by blow-down operation.
- Complexometric conditioning (EDTA conditioning): When EDTA is added to boiler-feed water having pH 8.5, then EDTA binds with the scale-forming cations to form stable and soluble complex. Hence, scale and sludge formation in boiler is prevented.
- Electrical conditioning: In this method, sealed glass bulbs, containing mercury connected to a battery, are set rotating in the boiler. As water boils, mercury bulb emits electrical discharges, which prevent scale-forming particles to adhere together to form scale.
- Radioactive conditioning: Radioactive salts containing tablets are placed inside the boiler-feed water at a few points. Energy radiated from radioactive substances prevents the scale and sludge formation.
- External treatment
Hard water causes a number of harmful effects when used for domestic, industrial, and boiler purposes. So we have to remove or reduce hardness-causing impurities present in water before using it for any purpose.
The most common methods for softening of water are given below:
- Lime soda process
- Zeolite process/permutit process/base exchange process
- Demineralization/ion-exchange process/de-ionization
- Lime Soda Process
It is a very important and popular process for softening of water.
Principle: This method involves the treatment of water sample with calculated quantities of lime [Ca(OH)2] and soda (Na2CO3), which react with calcium and magnesium salts to form insoluble precipitates as calcium carbonate (CaCO3) and magnesium hydroxide (Mg(OH)2). To accelerate the precipitation of CaCO3 and Mg(OH)2, certain substances are added, known as “coagulants” or “flocculants.”
Functions of lime: For removing temporary hardness, permanent magnesium hardness, free mineral acids, iron and aluminium salts, dissolved CO2 and H2S in water, lime acts as a good agent.
- Removal of temporary hardness: Here lime converts bicarbonates into carbonates

- Removal of permanent magnesium hardness: Lime can remove the permanent magnesium hardness and converts the magnesium hydroxide

In this case, permanent magnesium hardness is converted to permanent calcium hardness.
- Removal of dissolved iron and aluminium salts: Lime can convert iron and aluminium salts to the corresponding hydrates.

- Removal of dissolved CO2 and H2S: Lime can remove the carbon dioxide as calcium carbonate and hydrogen sulphide as calcium sulphide.

Functions of soda: When lime is used to remove the hardness or mineral acids, it has been found that permanent calcium hardness (CaCl2) and (CaSO4) is introduced in water. Soda is very effective to remove permanent calcium hardness as follows:

Important Points about Calculation of Lime and Soda
- There is no consideration of substances like NaCl, KCl, Na2SO4, SiO2, Fe2O3, etc., for calculating lime and soda requirement as they do not impart any hardness.
- Equivalent weight as NaAlO2 is equal to its molar mass.
- When the impurities are given as CaCO3 and/or MgCO3, they should be considered due to bicarbonates of calcium and/or magnesium, respectively.
- When the impurities are presented in the form of ions such as Ca2+ and/or Mg2+ ion, they are considered as a permanent hardness.
- If there are OH- and
 ions present in the treated water, it indicates that excess of lime and soda, which are added for the treatment, and hence these excess amount should be added (in terms of CaCO3 equivalent) to the calculation.
ions present in the treated water, it indicates that excess of lime and soda, which are added for the treatment, and hence these excess amount should be added (in terms of CaCO3 equivalent) to the calculation.
Requirement of lime and soda for the constituents responsible for hardness is given in Table 1.4.
Table 1.4 Calculation of lime and soda requirement

Formula for Lime and Soda Requirement
100 parts by mass of CaCO3 are equivalent to
- 74 parts of Ca(OH)2
- 106 parts of Na2CO3


Process: Lime soda process is of two types:
- Cold lime soda process (at room temperature)
- Hot lime soda process (at 90°C–100°C temperature)
- Cold lime soda process
- Calculated quantity of lime and soda is mixed with hard water at room temperature.
- At room temperature, the precipitates formed are finely divided, so they do not settle down easily.
- So it is essential to add a small amount of coagulant, which hydrolyzes to form flocculent and gelatinous precipitate of aluminium hydroxide or ferric hydroxide, which entraps the fine precipitates (as shown in Figure 1.10).

Figure 1.10 Cold lime soda softener
Hard water (containing Ca2+, Mg2+, or other heavy metals) + lime + soda
- Addition of coagulants or flocculent
- Proper setting time

- Use of sodium aluminate (NaAlO2) as coagulant also helps in the removal of silica as well as oil, if present in water.

- The residual hardness after cold lime soda process is 50 to 60 ppm.
- Hot lime soda process
- In this method, softening of water by lime and soda at temperatures close to the boiling temperature of water (100°C).
- The chemical reactions proceed at a faster rate, because the viscosity of water is low at higher temperature and precipitates sludge settle down easily.
- No coagulants are required for hot lime soda process.
- The hot L-S plant consists of three parts (as shown below in Figure 1.11):

Figure 1.11 Hot lime soda process
- A ‘reaction tank’ in which feed water, chemicals, and steam are thoroughly mixed.
- A ‘conical sedimentation vessel’ in which sludge settles down.
- A ‘sand filter’ that ensures complete removal of sludge from the water.
In the hot process, sodium carbonate (Na2CO3) is used for softening because it decomposes into sodium hydroxide under high pressure and temperature.

- The residual hardness after hot lime soda process is 15–30 ppm.
Advantages of Lime Soda Process
- It is very economical.
- Hot L-S process is much faster than cold lime soda process.
- During lime soda process, pH value of water is increased; hence corrosion of pipe is reduced.
- The alkaline nature of treated water controls the amount of pathogenic bacteria in water.
- This process also helps to remove Fe and Mn to some extent.
- It removes not only hardness-causing salts but also other minerals.
Disadvantages of Lime Soda Process
- Soft water contains 15–30 ppm residual hardness.
- It requires careful operation and skilled supervision for efficient softening.
- Sludge disposal is different and poses a problem.
Difference between cold and hot lime soda process is given in Table 1.5.
Table 1.5 Difference between cold and hot lime soda process

- Removal of temporary hardness: Here lime converts bicarbonates into carbonates
- Zeolite or permutit process
Zeolite is known as permutit, i.e., boiling stone. Zeolite process is widely used to soften water. Zeolites are hydrated alumino silicate minerals. or
Sodium aluminium orthosilicate, and it is represented as Na2O·Al2O3·xSiO2·yH2O (x = 2 – 10, y = 2 – 6) represented as Na2Z.
Zeolites are of two types:
- Natural zeolite: It is non-porous and derived from green sand.
Example: Natrolite (Na2OAl2O3·3SiO2·2H2O)
- Synthetic zeolite: It is porous and possesses a gel structure. It is prepared by heating china clay, feldspar, and soda ash together. They have higher exchange capacity as compared to natural zeolite. So it is more common in use.
Softening Process
In the zeolite process for softening hard water, the raw water is percolate through a bed of zeolite (Na2Z), which is packed in a vertical cylindrical tank as shown in the Figure 1.12. The zeolite bed retains the Ca2+ and Mg2+ ions from hard water by exchanging with Na+ ions thereby the out-flowing water contains sodium salts.
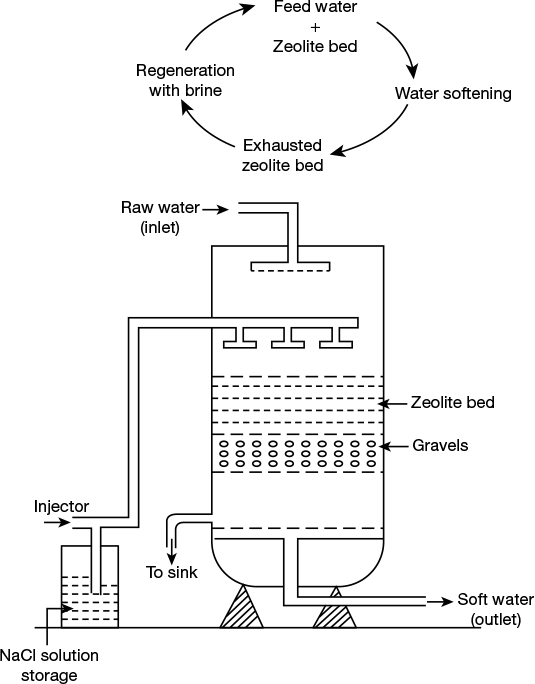
Figure 1.12 Zeolite softener

This process removes both temporary and permanent hardness. After long use, the zeolite bed gets exhausted. It can be regenerated by using chemicals.
Regeneration: When the zeolite bed is completely converted into calcium and magnesium zeolite, it no longer works as softener. It gets exhausted. At this stage, supply of feed water is stopped, and the exhausted zeolite is regenerated by treating with a concentrated (10%) brine (NaCl) solution.

The regenerated zeolite bed thus obtained is used again for softening operation. Zeolite process reduces hardness to 0–15 ppm.
Limitations
- Hard water should be turbidity free otherwise impurities will clog the pores.
- Mineral acids must be removed if present because they destroy the zeolites.
- If Mn2+ or Fe2+ ions are present in feed water, it must be removed, otherwise they form MnZ or FeZ, which cannot be removed easily during regeneration.
Advantages of Zeolite Process
- Hardness-causing ions are completely removed with a very low residual hardness of about 10 ppm in the softened water.
- Zeolite process automatically adjusts for any variation in hardness of incoming water.
- It is a clean process because it does not produce any sludge.
- Zeolite equipment requires less area.
- It requires less time for softening the water.
- It requires less skill for operation as well as maintenance.
Disadvantages of Zeolite Process
- Zeolite process cannot tolerate acidity as the zeolite disintegrates.
- Turbid water cannot be treated satisfactorily.
- Treated water contains more sodium salts.
- This process replaces Ca2+ and Mg2+ ions by Na+ ions, and hence softened water contains more sodium and also more dissolved salts.
- Anions such as
 ,
,  remain in water as sodium salts, which contributing to the alkalinity causes corrosion and caustic embrittlement of the boiler.
remain in water as sodium salts, which contributing to the alkalinity causes corrosion and caustic embrittlement of the boiler.
Difference between zeolite and lime soda process is given in Table 1.6.
Table 1.6 Differences between zeolite and lime soda process

- Natural zeolite: It is non-porous and derived from green sand.
- Demineralization process/Ion exchange process
In this process the cations and anions present in water and which can produce hardness are removed by ion-exchange resins. Resins are long, cross-linked organic polymers with a porous structure. Ion-exchange resins are mainly (1) cation-exchange resins and (2) anion-exchange resins.
- Cation-exchange resins: These are styrene-divinylbenzene copolymers. These resins have acidic functional groups such as -COOH, -SO3H, etc., which are capable of exchanging the cation by their hydrogen ions. Hence, they are also called cation exchangers. They can be represented as R-H, where R is the insoluble polymeric heavy part.

- Anion-exchange resins: These are copolymers of styrene and divinylbenzene containing basic functional groups such as amine, substituted amine, quaternary ammonium groups, etc. They can be represented as R′-OH.

Process: Both cation exchanger and anion exchanger are inter-connected with a pipe as shown in the Figure 1.13. The hard water is first passed through cation-exchange resin chamber, which removes all the cations (e.g., Ca2+ and Mg2+) from it, and equal amount of H+ ions are released from its column to water.


Figure 1.13 Demineralization by ion exchangers
After passing through cation-exchange chamber, the hard water is now pumped to ‘anion-exchange resin’ chamber where all anions like Cl-,
 , etc., are removed, and equal amount of OH- ions are released from this resin bed to water.
, etc., are removed, and equal amount of OH- ions are released from this resin bed to water.
H+ and OH– ions released from reactions in equivalent amount get combined to produce water molecules.

Thus, the treated water is completely free from cations as well as anions, so it is known as demineralized or deionized water.
Regeneration of Resins
After some time of usage (depending on water) of cation/anion exchange resins will exhaust, and it is most important to regenerate.
Regeneration of cation-exchange resins: The cation-exchange resins are regenerated by addition of dil. HCl or H2SO4:

Regeneration of anion-exchange resin: The anion-exchange resins are regenerated by addition of dil. NaOH:

After regeneration of both resins, columns are washed with deionized water, and the washed product is passed to sink.
Advantages
- It produces water of very low hardness (≈2 ppm).
- Highly acidic or highly alkaline water also can be softened.
Disadvantages
- The equipment is very costly.
- Expensive chemicals are required for regeneration.
- Turbid water decreases the efficiency of resins.
Mixed-bed deionizer: As shown in Figure 1.14, mixed-bed deionizer contains a single cylindrical vessel with a mixture of a strong cation exchanger and a strong anion exchanger, and is the most efficient process than separate column exchanger process. Hard water which pass through the mixed bed contacts number of times with both exchangers and purifies the water. Purified water is having less than 1 ppm hardness and also this is a most widely used convenient method.

Figure 1.14 Mixed-bed deionizer
Regeneration of resins: When the resins get exhausted, the mixed bed is backwashed with water. The lighter anion exchangers get displaced to form an upper layer above the heavier cation exchangers. Then the anion exchangers are regenerated by passing NaOH solution from the top and then rinsed with deionized water. The lower layer of cation exchangers is generated by passing H2SO4 solution and is finally rinsed with deionized water. The two beds are then mixed again by forcing compressed air through it. Now the regenerated bed is ready for use again.
- Cation-exchange resins: These are styrene-divinylbenzene copolymers. These resins have acidic functional groups such as -COOH, -SO3H, etc., which are capable of exchanging the cation by their hydrogen ions. Hence, they are also called cation exchangers. They can be represented as R-H, where R is the insoluble polymeric heavy part.
1.16 DESALINATION
Desalination or desalting involves the removal of dissolved salts (e.g., NaCl) from water. The salinity of water is due to dissolved NaCl and to a smaller extent of other inorganic salts.
Natural saline water such as sea water contains more than 35,000 ppm while brackish water contains dissolved salts in the range 1000–3500 ppm.
Desalination of saline water may be achieved by any of the two approaches:
- Separating water from the saline water
As in
- Distillation or evaporation
- Freezing
- Solvent extraction
- Reverse osmosis
- Separating the salt from the saline water
As in
- Osmionic process
- Electrodialysis
Reverse osmosis and electrodialysis are more important in large-scale operations, and operation and principle involved in reverse osmosis are discussed.
Reverse Osmosis (RO) (Hyper Filteration)
This technique worksbased on the principle of osmosis. Reverse osmosis is a process by which a solvent such as water is purified of solutes by being forced through a semipermeable membrane through which the solvent, but not the solute may pass. It is exactly opposite of osmosis and hence it is known as reverse osmosis.
Generally the tendency of a fluid, i.e., water, to pass through a semipermeable membrane into a solution where the solvent concentration is higher, thus equalizing the concentrations of materials on either side of the membrane is known as osmosis. But when pressure is applied on the concentrated side, the solvent will flow in the reverse direction. Reverse osmosis uses 100–150 micron thick membrane made from cellulose acetate or polymeric membranes having pores in the range of 0.0001–0.001 μm in diameter; it allows only water to pass through it and not to the salt. The water molecules diffuse through the membrane while the contaminants get concentrated in the effluent stream and are discharged.
Process: In this process, a high pressure (≈15–40 kg cm–2) is applied to the sea water or brackish water, which is to be treated (as shown in Figure 1.15). The semi-permeable membrane allows only the solvent molecule (pure water) to pass through it. Thus dissolved ionic and non-ionic solvents are left behind, and water get purified from salt. Generally, we use membrane made up of polymethacrylate and polyamide polymers for this process.

Figure 1.15 Reverse osmosis unit
Advantages
- It removes ionic as well as non-ionic salts present in saline water.
- It is economical, compact, and very simple.
- It is effective in removing colloidal matters also.
- It is suitable for converting sea water into drinking water.
- It has long life and is easy to replace the membrane within two minutes.
- The water obtained from the process can be used in high-pressure boilers.
Electrodialysis
Electrodialysis is another efficient technique used for the desalination of saline water and is a membrane process.
Principle: Under the influence of an electric potential across a salt water solution, charged ions move towards respective electrodes through ions and selective membrane.
The membranes are cation or anion selective, which basically means that either positive ions or negative ions will flow through cation-selective membrane consisting of sulphated polystyrene, which allows only cations to flow through and rejects anions. However, anion-selective membrane consists of polystyrene with quaternary ammonia, which allows only anions and rejects cations. Multiple membranes alternatively allow cation or anions to flow through. Hence, with this method we can get fresh water from saline water.
Process: The process is carried out in a special type of the cell called electrodialysis cell (as shown in Figure 1.16). It consists of two electrodes and ion selective membranes which are permeable to either cation or anion. The anode is placed near anion-selective membrane while the cathode is placed near the cation-selective membrane. The anion selective membrane has positively charged functional groups such as R4N+ and therefore allows negatively charged ions only to pass through them. Similarly, a cation-selective membrane has negatively charged functional groups such as RCOO− and allows only positively charged ions to pass through it. Saline water under a pressure of around 5−6 kg/m2 is passed from the top of the cell and it passes between membrane pairs.

Figure 1.16 Electrodialysis of sea water
When an emf is applied across two electrodes the cations (Na+) present in salt water move towards cathode through cation selective membrane and anions (Cl−) move towards the anode through anion selective membrane. As a result, the concentration of ions in alternate compartments 2, 4, 6 etc. decreases, while it increases in the alternate compartment 1, 3, 5 etc. Thus water in the even number compartments becomes pure and is collected from the bottom of the cell. Similarly, water in the odd number compartment becomes rich in the saline water i.e. it becomes concentrated saline water. It is collected from a separate outlet at the bottom of the cell.
Advantages
- It is economical.
- It is convenient and may be applied at room temperature.
- It is most compact in size and requires only electricity for operation.
1.17 REVIEW QUESTIONS
1.17.1 Fill in the Blanks
- Hard water prevents lathering of soap due to the presence of the dissolved salts of _______ and _______.
[Ans. calcium, magnesium]
- _______ water has high quantity of organic matter.
[Ans. Lake]
- Hardness is expressed in terms of _______ equivalent.
[Ans. CaCO3]
- 1 ppm = _______ mg/L = _______ °Fr = _______ °Cl.
[Ans. 1, 0.1, 0.07]
- _______ indicator is used in EDTA titration.
[Ans. EBT]
- Caustic alkalinity in water is due to _______ and _______ ions.
[Ans. OH-,
 ]
] - Solubility of _______ in water decreases with increase of temperature.
[Ans. CaSO4]
- The phenomenon of carrying of water along with impurities by steam is called _______.
[Ans. carry-over]
- _______ is an ideal chemical for the removal of dissolved oxygen.
[Ans. Hydrazine]
- The presence of residual _______ in boiler water causes caustic embrittlement.
[Ans. caustic, NaOH]
- By adding _______ to boiler water, caustic embrittlement can be prevented.
[Ans. sodium sulphate]
- Sodium aluminated is added as a _______ in purification of water.
[Ans. coagulant]
- During lime soda process, calcium and magnesium ions impurities precipitate into _______ and _______.
[Ans. CaCO3, Mg(OH)2]
- Name of natural zeolite is _______.
[Ans. natrolite, Na2O · Al2O3 · 4SiO2 · 2H2O]
- In zeolite process, calcium and magnesium ions are replaced by _______ ions.
[Ans. sodium]
- Exhausted cation-exchange column is regenerated by passing a solution of _______.
[Ans. dilute HCl or dilute H2SO4]
- Cation exchange resin have acidic functional groups like –SO3H, –COOH, –OH capable of exchanging cation by their _______ ions.
[Ans. hydrogen, H+]
- Anion-exchange resins are having _______ ions, which are capable of exchanging anions in water.
[Ans. hydroxide, OH–]
- _______ process produces least residual hardness in water.
[Ans. Ion-exchange]
- _______ and _______ are used for colloidal conditioning.
[Ans. Tannin, agar-agar]
- Water having no ions is called as _______ water.
[Ans. demineralized or deionized]
- Calgon having chemical name is _______.
[Ans. sodium hexameta phosphate, Na2[Na4(PO3)6]]
- For domestic use, pH of water should be in the range of _______.
[Ans. 7–8]
- _______ flocculent is precipitated out when Al2(SO4)3 alum is added as a coagulant in water.
[Ans. Al(OH)3]
- Chemical formula of alum is _______.
[Ans. K2SO4 · Al2(SO4)3 · 24H2O]
- _______ is the best way for disinfection of water in comparison with chlorine or bleaching powder.
[Ans. chloramines, ClNH2]
- In electrodialysis process, _______ can be separated from brackish water.
[Ans. NaCl]
- Electrodialysis consists of _______ selective membranes.
[Ans. ions]
- Cation selective membrane is allowed to pass _______ and move towards cathode, and anion selective membrane is allowed to pass _______ and move towards anode.
[Ans. cations, anions]
- Flow of solvent from a region of low concentration to high concentration when two solutions are separated by semi permeable membrane is called _______.
[Ans. osmosis]
1.17.2 Multiple-choice Questions
- The purest form of natural water is
- River water
- Sea water
- Underground water
- Rain water
[Ans. d]
- The alkaline hardness of water is due to the presence of the following salts of calcium and magnesium in water.
 only
only and
and  only
only only
only ,
,  , OH− only
, OH− only
[Ans. d]
- A sample of water contains 120 mg of Mg2+ per liter. The hardness of the sample of water in terms of CaCO3 equivalent is
- 120 mg/L
- 500 mg/L
- 250 mg/L
- 1000 mg/L
[Ans. b]
- The total hardness of a sample of water is 1.88°Cl eq. CaCO3. Its hardness in ppm would be
- 26.88
- 18.8
- 0.188
- 34.65
[Ans. a]
- The colour obtained by adding EBT indicator to a sample of water containing Ca2+ and Mg2+ at pH = 9–10 is
- Blue
- Wine red
- Pink
- No colour
[Ans. b]
- On boiling and filtering hard water, water sample contains
- Temporary hardness
- Permanent hardness
- Both
- None of the above
[Ans. b]
- Blow-down operation causes the removal of
- Scales
- Sludges
- Acidity
- Basicity
[Ans. b]
- Scale formation in boiler-feed water is due to
- Metallic deposition
- Corrosion in boilers
- Deposition of hard water
- All the above
[Ans. c]
- Scale formation is mainly due to which of the following salt present in boiler-feed water?
- CaSO4
- MgCO3
- Na2SO4
- KCl
[Ans. a]
- Solubility of CaSO4 salt present in water
- Increases with increase in temperature
- Decreases with increase in temperature
- Remain unchanged with increase in temperature
- Not having any definite change with increase in temperature.
[Ans. b]
- EDTA method is used for determining
- Temporary hardness
- Permanent hardness
- Temporary and permanent hardness
- Alkalinity
[Ans. c]
- When phenolphthalein alkalinity, P = M then alkalinity is due to
- OH-


 and
and 
[Ans. b]
- Permanent hardness of water cannot be removed by
- Lime soda process
- Permutit process
- Boiling
- Demineralization process
[Ans. c]
- Permutit is chemically
- Sodium silicate
- Hydrated sodium alumino silicate
- Aluminium silicate
- All the above
[Ans. b]
- Hard water is not suitable for use in boilers because
- It has a higher boiling point
- It leads to scale formation in the boiler
- It consumes more fuel in steam generation
- The quality of steam generated is not good
[Ans. b]
- Which of the following substances is capable of removing dissolved oxygen from water?
- Cl2
- N2H4
- Na2SO4
- CaOCl2
[Ans. b]
- Sterilization of water can be done by using
- H2O2
- O2
- Cl2
- NaOH
[Ans. c]
- Coagulants help in settling of
- Suspended impurities only
- Finely suspended impurities only
- Colloidal particles only
- Both the suspended and colloidal particles
[Ans. d]
- 1 ppm of K+ present in a sample of demineralized water is equal to
- 4.3478 × 10–8 mol L–1
- 2.564 × 10–10 mol L–1
- 2.564 × 10–5 mol L–1
- None of the above
[Ans. c]
- Calgon is a name given to
- Sodium silicate
- Sodium hexameta phosphate
- Sodium meta phosphate
- Calcium phosphate
[Ans. b]
- Permutit exchanges Ca2+ and Mg2+ ions present in hard water with
- Zeolite ions
- H+ ions
- Na+ ions
- None of these
[Ans. c]
- The exhausted zeolite can be regenerated by treating it with
- 10% NaCl solution
- 50% NaCl solution
- 10% HCl solution
- 50% HCl solution
[Ans. a]
- Brackish water contains dissolved
- CaSO4
- MgCl2
- NaCl
- Na2SO4
[Ans. c]
- Which of the following samples of water cannot be softened by zeolite process?
- Water containing temporary hardness
- Water containing permanent hardness
- Water containing excess of alkalinity
- Water containing excess of dissolved salts
[Ans. c]
- The cation-exchange resins possesses
- Acidic groups
- Basic groups
- Amphoteric groups
- None of these
[Ans. a]
- Priming and foaming process in boiler-feed water is due to
- The formation of air bubbles and production of wet steam
- The formation of scales
- The formation of sludges
- None of these
[Ans. a]
- The cation and anion resins are made up of the basic polymer unit of
- Polyvinyl chloride
- Poly acrylate
- Poly styrene
- Polybutadiene
[Ans. b]
- By ion-exchange process the hardness of water can be reduced up to
- 0 ppm
- 5 ppm
- 10 ppm
- 15 ppm
[Ans. a]
- Boiler corrosion caused by using highly alkaline water in boiler is called
- Corrosion
- Boiler corrosion
- Caustic embrittlement
- Erosion
[Ans. c]
- Desalination is the process of removing
- Common salt from sea water and making it potable
- Hard salts from sea water
- NaOH from hard water
- None of the above
[Ans. a]
- An exhausted anion-exchange resin can be regenerated by treating it with
- Conc. HCl solution
- Conc. NaOH solution
- Dilute brine solution
- Conc. brine solution
[Ans. b]
- Tannins and agar-agar are used for
- Phosphate conditioning
- Colloidal conditioning
- Radioactive conditioning
- Calgon conditioning
[Ans. b]
- Alum is commonly used in the treatment of municipal water for
- Filteration
- Sedimentation
- Coagulation
- Flocculant
[Ans. d]
- The chemical formula of alum is
- K2SO4 · Al2 (SO4)3 · 20H2O
- KNO3 · Al2 (SO4)3 · 24H2O
- K2SO4 · Al2 (SO4)3 · 24H2O
- K2SO4 · Al2 (SO4)3 · 21H2O
[Ans. c]
- Liquid chlorine is most effective
- Disinfectant
- Coagulant
- Flocculant
- Sterilizing agent
[Ans. a]
- The soft, loose, and slimy precipitate formed within the boiler is called
- Scale
- Sludge
- Flocculant
- Coagulant
[Ans. b]
- In reverse osmosis (RO) the flow of solvent is due to
- Potential gradient
- Vapour pressure gradient
- Concentration gradient
- None of the above
[Ans. c]
- In RO process, the membrane used is
- Polysulfone
- Polysulfone amide
- Poly amide
- All above
[Ans. d]
- Chemical formula of bleaching powder is
- Cl2
- HOCl
- NH2Cl
- CaOCl2
[Ans. d]
- The acid responsible for disinfection of germs and bacteria is
- HCl
- HNO3
- HOCl
- H2CO3
[Ans. c]
1.17.3 Short Answer Questions
- Name the main sources of water.
Ans.: Sea water, rain water, ground water, and surface water.
- What is the cause for alkalinity of natural water?
Ans.: Due to the presence of dissolved bicarbonates of Ca and Mg in water.
- Define hardness of water.
Ans.: Hardness is the characteristic property, which produces white scum on treating with soap solution.
- Why does not hard water give lather with soap?
Ans.: Because hard water produces insoluble white precipitate on treating with soap.

- How is hardness of water expressed?
Ans.: The concentration of hardness-producing salts is expressed in terms of calcium carbonate (CaCO3) equivalent.
- How hardness is determined in terms of CaCO3 equivalent.
Ans.:

- Define ppm, mg/L, Clarke’s degree, and French degree.
Ans.: ppm: 1 part of CaCO3 equivalent hardness present in 106 parts of water.
mg/L: Number of mg of CaCO3 equivalent hardness present in 1L of water.
Clarke’s degree: Number of parts of CaCO3 equivalent hardness present in 70,000 parts of water.
French degree: Number of parts of CaCO3 equivalent hardness present in 105 parts of water.
- What is the relationship between ppm, mg/L, °Cl, and °Fr.
Ans.: 1 ppm = 1 mg/L = 0.07°Cl = 0.1°Fr
- Explain why water containing Ca2+ (aq) and
 (aq) ions is said to be hard.
(aq) ions is said to be hard.
Ans.: The Ca2+ ions give precipitates with soaps. On heating
 ions, they are converted to
ions, they are converted to  ions, which precipitate in kettles/boilers with Ca2+ ions.
ions, which precipitate in kettles/boilers with Ca2+ ions. - Why do we express hardness of water in terms of CaCO3 equivalent?
Ans.: Because addition and subtraction of concentration of hardness-causing constituents are easy. Its molecular mass is 100.
- What are the salts responsible for the temporary and permanent hardness of water?

- Name the gases that dissolve in water and cause corrosion.
Ans.: Oxygen, carbon dioxide, and sulphur dioxide.
- What happens when hard water is boiled?
Ans.: On boiling, temporary hardness is removed by precipitating as

- Name any three substances that are used for sterilization of water.
Ans.: (i) Liquid chlorine (ii) Bleaching powder (iii) Chloramine
- Why is chlorination is better than chlorine or bleaching powder for sterilization of water.
Ans.: Because chloramine
- is quite stable
- does not impart bad taste to treated water
- imparts good taste to treated water
- What is break-point chlorination?
Ans.: It involves addition of sufficient amount of chlorine to water in order to
- oxidize organic matter
- reduce substance and
- free ammonia
and leaves behind mainly free chlorine for disinfecting disease-producing bacteria.
- What are the advantages of break-point chlorination?
Ans.: (i) It oxidizes organic matter, NH3, and reducing substances completely.
(ii) It removes colour in water.
(iii) It destroys all the disease-producing bacteria completely.
(iv) It removes odour from water.
(v) It prevents any growth of weeds in water.
- Mention the impurities present in natural water.
Ans.: (i) Suspended impurities
(ii) Colloidal impurities
(iii) Dissolved impurities
- What is standard hard water.
Ans.: Usually it is a solution containing 1 g of CaCO3 equivalent hardness in 1 liter, i.e., 1000 ppm of hardness water.
- What is sedimentation with coagulation?
Ans.: The process of removing of finely suspended impurities as well as colloidal impurities by adding requisite amount of coagulant to water before sedimentation.
- What is colloidal conditioning?
Ans.: Scale formation can be avoided in low-pressure boilers by adding substances like kerosene, tannin, agar-agar, etc., which get adsorbed over the scale-forming precipitates, thereby yielding non-sticky and loose deposits, which can be removed by blow-down operation.
- What is the indicator used in EDTA method? What is the end-point?
Ans.: Indicator: EBT
End point: Wine red to pure blue
- Why is NH4OH-NH4Cl buffer solution added during the determination of hardness of water by EDTA method?
Ans.: The indicator used in this titration (EBT) shows colour change at a pH value of about 10. So alkaline buffer (NH4OH-NH4Cl) is used.
- Soft water is not demineralized, whereas demineralized water is soft. Why?
Ans.: Soft water may contain
 ions, so it is not demineralized, whereas demineralized water does not contain any cation and anion.
ions, so it is not demineralized, whereas demineralized water does not contain any cation and anion. - Why is water softened by zeolite process fit for use in boilers?
Ans.: Because zeolite-softened water contains large quantities of sodium salts like NaCl, Na2SO4, etc., which avoids caustic embrittlement.
- CO2 should not be present in boiler-feed water. Why?
Ans.: Because CO2 forms carbonic acid (H2CO3) on reacting with water. So boiler’s wall material can be attacked slowly by carbonic acid and becomes weaker and weaker progressively.
- What is meant by softening of water?
Ans.: Softening of water means removing hardness-producing salts from water.
- Why is water softened before using in boilers?
Ans.: Water should be softened before using in boilers otherwise it may cause various boiler problems like
- scale and sludge formation
- priming and foaming
- boiler corrosion.
- What is meant by disinfection of water by UV method.
Ans.: When water is irradiated by UV radiations, microorganisms and bacteria are killed. This so-called disinfection of water by UV radiation.
- What is zeolite?
Ans.: It is hydrated sodium alumino-silicate having formula Na2O · Al2O3 · xSiO2 · yH2O,

It is represented as Na2Z, and Na+ ions are capable of exchanging by M2+ (Ca2+ or Mg2+) present in water sample.
- If silica is present in water, what harmful effects it can cause to boilers?
Ans.: If silica is present in water, it causes formation of very firmly sticking deposits of calcium silicate (CaSiO3) and magnesium silicate (MgSiO3) scales in the boilers, which are very difficult to remove.
- Why is the presence of NaAlO2 in water equivalent to the presence of equivalent to Ca(OH)2?
Ans.:

- Alkalinity of water cannot be due to presence of (a) OH-,
 and
and  or (b) OH-,
or (b) OH-,  in water. Give reasons.
in water. Give reasons.
Ans.:

- Why does magnesium bicarbonate require double amount of lime for softening?
Ans.: Mg(HCO3)2 + 2Ca(OH)2 → 2CaCO3↓ + Mg(OH)2↓ + 2H2O
Thus from the above equation, mole of Mg(HCO3)2 ≡ 2 mol of Ca(OH)2
- Are coagulants also used in hot lime soda process? Give reasons.
Ans.: No, because reaction proceeds faster in hot lime soda process, and the precipitate and sludge formed settle down rapidly. Thus, no coagulants are required in hot lime soda process.
- Water should not be soft for drinking purposes. Why?
Ans.: Water should not be soft for drinking purposes because soft water is plumbosolvent, i.e., it attacks lead used in plumbing.
- What is the main advantage of reverse osmosis over ion-exchange process?
Ans.: Reverse osmosis removes all ionic, non-ionic, colloidal, and high molecular weight organic matter.
- Why does the water softened by lime soda process cause boiler troubles?
Ans.: The treated water still contains some residual hardness.
- Why can caustic embrittlement be controlled by adding Na2SO4 to boiler-feed water?
Ans.: When Na2SO4 is added to boiler-feed water, it blocks hair cracks, thereby preventing infilteration of caustic soda solution in these areas. So by this way, caustic embrittlement is prevented by using Na2SO4 in boiler-feed water.
- Why is calgon conditioning better than phosphate conditioning?
Ans.: In calgon conditioning, the added calgon forms soluble complex compound with CaSO4, thereby it prevents the scale and sludge formation in water.

This soluble complex does not cause any problem in the boilers.
On the other hand, in phosphate conditioning, sodium phosphate is added to boiler water so that precipitate of calcium phosphate is formed. Although this precipitate is non-adherent and soft, it has to be removed by frequent blow-down operation.

Hence, calgon conditioning is better than phosphate conditioning.
1.17.4 Descriptive Questions
Q.1 Complete the following equations:
- 1 ppm = ______mg/L = ______°French = ______°Clark

- Mg(HCO3)2 + 2Ca(OH)2→
- Na2[Na4(PO3)6] + 2CaSO4→
Q.2 Explain the following:
- Scale and sludge formation and their disadvantages
- Caustic embrittlement
- Boiler corrosion
Q.3 What is hardness of water? How is it determined by EDTA method?
Q.4 Describe the continuous lime soda process of softening hard water. Compare continuous cold lime soda process with hot lime soda process.
Q.5 How is true exhausted zeolite bed regenerated? Give the merits and demerits of zeolite process.
Q.6 What are the requirements of water for domestic use?
Q.7 A water sample contains Ca(HCO3)2 = 32.4 mg/L, Mg(HCO3)2 = 29.2 mg/L, and CaSO4 =
13.5 mg/L. Calculate the temporary and permanent hardness.
[Ans. 40 ppm,10 ppm]
Q.8 Calculate the hardness of water containing the following salts:
CaSO4 = 28 mg/LMg(HCO3)2 = 22 mg/L
MgCl2 = 30 mg/LCaCl2 = 85 mg/L
Ans.: Temporary hardness = 15.07 ppm
Permanent hardness = 128.7 ppm]
Q.9 1 g of CaCO3 was dissolved in dil. HCl, and the solution was diluted to 1 liter. 50 ml of this solution required 45 ml of EDTA solution. 50 ml of hard water required 18 ml of EDTA solution during titration in ammonia buffer using EBT indicator. On the other hand, 50 ml of boiled water sample required 9 ml of EDTA solution under the same condition. Calculate each type of hardness in ppm.
Ans.: Temporary hardness = 15.07 ppm
Permanent hardness = 128.7 ppm]
Q.10 0.28 g of CaCO3 was dissolved in HCl and the solution made up to 1 liter with distilled water. 100 ml of the above solution required 28 ml of EDTA solution on titration. 100 ml of a hard water sample required 33 ml of same required solution on titration. After 100 ml of this water, cooling and filtering and then titrated 10 ml of EDTA solution. Calculate the temporary and permanent hardness.
[Ans. 230 mg/L, 100 mg/L]
Q.11 Explain the ion-exchange method of purifying the water. Discuss their use and regeneration, giving the reaction involved.
Q.12 Write a short note on break-point chlorination.
Q.13 Pure soft water is not fit for drinking purpose. Why?
Q.14 Write the principle of lime-soda process? Why should we use coagulants along with lime and soda? Why is water softened by zeolite process that is unfit for use in boilers?
Q.15 Explain reverse osmosis process for desalination of sea water.
Q.16 A water sample contains the following impurities: Ca2+ = 20 ppm, Mg2+ = 18 ppm, ![]() = 183 ppm, and
= 183 ppm, and ![]() = 24 ppm. Calculate the amount of lime and soda needed for softening.
= 24 ppm. Calculate the amount of lime and soda needed for softening.
Ans.: Lime = 185 mg/L
Soda = Zero mg/L]
Q.17 Water sample on analysis gave the following results: Mg(HCO3)2 = 70 mg/L, CaCl2 = 220 mg/L, MgSO4 = 120 mg/L Ca(NO3)2 = 164 mg/L. Calculate the quantity of lime (80% pure) and soda (90% pure) needed for softening the 10,000 liters of water.
Ans.: Lime = 1.81 kg
Soda = 4.68 kg]
Q.18 A water sample contains the following constituents in ppm: Mg(HCO3)2 = 73, MgCl2 = 95, MgSO4 = 12, CaSO4 = 68, Ca(HCO3)2 = 81, and NaCl = 4.8. Calculate the cost of chemicals required for softening 20,000 liters of water, if purity factor for lime is 95% and soda is 90%. The costs per 100 kg each of lime and soda are Rs. 75 and Rs. 2480, respectively.
Ans.: Lime cost = Rs. 3.03;
Soda cost = Rs. 93.44]
Q.19 What do you mean by pre-chlorination, post-chlorination, and superchlorination. Write the significance of break-point chlorination.
Q.20 What do you mean by screening, sedimentation, and coagulant sedimentations? How are colloidal impurities removed from water?
Q.21 What are the factors that cause alkalinity in water? How is alkalinity of water determined by titrimetric method ?
Q.22 Write a short note on the followings:
- Phosphate conditioning
- Calgon conditioning
- Colloidal conditioning
- Carbonate conditioning
- EDTA conditioning
1.17.5 Problems for Practice
- 200 ml of water sample require 25 ml of N/50 H2SO4 for neutralization to phenolphthalein end point. After that methyl orange was added to this, and further acid required was 35 ml
 H2SO4. Calculate the type and amount of alkalinity of water as CaCO3 in ppm.
H2SO4. Calculate the type and amount of alkalinity of water as CaCO3 in ppm.
Ans.:
 = 250ppm
= 250ppm = 50 ppm]
= 50 ppm] - Calculate the amount of lime (88.3% pure) and soda (99% pure) required to soften 24,000 liters of water per day, which contains the following:
CaCO3 = 1.85 ppm CaSO4 = 0.34 ppm MgCO3 = 0.42 ppm
MgCl2 = 0.76 ppm MgSO4 = 0.90 ppm NaCl = 2.34 ppm
Ans.: Lime = 88.49 kg
Soda = 46.25 kg]
- Calculate the amount of lime and soda needed for softening 106 liters of water sample, which contains Mg2+ = 36 ppm, Ca2+ = 20 ppm, and
 = 183 ppm.
= 183 ppm.
Ans.: Lime = 222 kg
Soda = 53 kg]
- A water sample contains the following: Ca2+ = 120 ppm, Mg2+ = 120 ppm, CO2 = 132 ppm,
 = 122 ppm, and K+ = 40 ppm. Calculate the amount of lime 80% pure and soda 90% pure for softening 106 liters of water sample.
= 122 ppm, and K+ = 40 ppm. Calculate the amount of lime 80% pure and soda 90% pure for softening 106 liters of water sample.
Ans.: Lime = 832.5 kg
Soda = 824.4 kg]
