16
STEREOCHEMISTRY
16.1 INTRODUCTION
The properties of molecules depend on the arrangement of atoms in a molecule. The arrangement of atoms or groups in a molecule is explained by stereochemistry. In Greek, “stereo” means solid/volume/space; hence, stereochemistry means chemistry in space.
Stereochemistry is the branch of chemistry which deals with three-dimensional (3D) structure of molecules and studies the physical, chemical and biological properties of organic molecules.
16.2 ISOMERISM
Organic compounds having the same molecular formula but differing from each other by some of physical or chemical properties or both are known as isomers and the phenomenon is known as isomerism. The term “isomer” was first introduced by Berzelius. In Greek, “iso” means equal and “meros” means parts.
The difference in the properties of isomers is due to the difference in the relative arrangement of various atoms or groups present in their molecules. There are two main types of isomerism as follows:
- Structural or constitutional
- Space or stereoisomerism
16.2.1 Structural Isomerism
The constituent atoms or groups are linked to one another within the molecule without any reference to space. Structural isomers are compounds that have the same molecular formula but have different structural formulae.
16.2.2 Space or Stereoisomerism
Stereoisomers have the same molecular and structural formulae but different spatial arrangement of atoms or groups. The spatial arrangement of atoms or groups is also referred to as configuration of the molecule.
16.3 CLASSIFICATION OF STRUCTURAL ISOMERISM
Structural isomerism is further classified into chain or nuclear isomerism, positional isomerism, ring or chain isomerism and functional group isomerism.
16.3.1 Chain or Nuclear Isomerism
This type of isomerism is due to the difference in the arrangement of carbon atoms constituting the chain, that is, straight or branched chain of carbon atom.
Example:

16.3.2 Position Isomerism
This is due to the difference in the position occupied by the particular atom or groups in the same carbon chain or due to different positions of = (or) ≡ bonds in alkene and alkynes.
![]()
16.3.3 Ring or Chain Isomerism
This type of isomerism is due to different modes of linking of carbon atoms, that is, the isomers possess either open chain or closed chain structures.
Example:

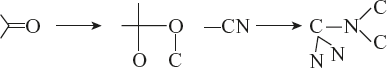
16.3.4 Functional Group Isomerism
Compounds having the same molecular formulae but different functional group in their molecule show functional isomerism known as functional group isomerism.
Example:

16.3.5 Metamerism
The different alkyl groups attached to the same polyvalent functional group or atom so that compounds having the same molecular formulae but different structural formulae due to different alkyl group on either side of the functional groups are called metamers and the phenomenon is known as metamerism.
Example:
![]()
16.3.6 Tautomerism
Isomers simultaneously exist in equilibrium with each other; this is a special type of functional isomerism. The term “tautomerism” comes from the Greek word “tauto” which means same, and “meros”, which means parts.
Tautomerism is a type of isomerism in which a substance exists in two readily interconvertible to different structures, leading to dynamic equilibrium.
It is caused by the wandering nature of the mobile hydrogen atom between two polyvalent atoms within the same molecule; it is also known as desmotropism (“desmos” means bond, and “tropos” means turn). If the hydrogen atom oscillates between two polyvalent atoms linked together, that system is a “dyad” and if the hydrogen atom moves from the first to the third in a chain, the system is a triad.
Dyad System
Example: Hydrocyanic acid; here, the hydrogen atom oscillates between carbon and nitrogen atoms.
![]()
Triad System
Example: Keto–enol system.

16.4 CLASSIFICATION OF STEREOISOMERISM
Stereoisomerism is further classified into three types—geometrical, optical and conformational isomerism.
16.4.1 Geometrical Isomerism
The phenomenon exhibited by isomers having the same molecular formula and a double bond in their special arrangements of atoms or groups with respect to the double bond is known as geometrical isomerism. The isomers are called geometrical isomers.
Example: Maleic acid and fumaric acid with the molecular formula C4H4OH.
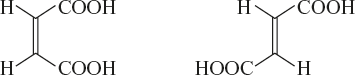
Geometrical isomerism is also known as “cis-trans” isomerism. If two carboxylic acid (same groups) groups are present on same side of the double bond, it is called “cis-isomers”. If they are present on the opposite side, it is called “trans-isomers”.

When geometrical isomerism is caused due to the presence of one double bond and if the two groups are identical, it is easy to name the geometrical isomers.
When a geometrical isomer contains more than one double bond, the compound is considered a derivative of the longest chain which contains the maximum number of double bonds. The prefix “cis” and “trans” being placed before the numbers indicate the position of the double bonds to describe the relative positions of the carbon atoms in the main chain.

Geometrical isomerism is also exhibited by the cumulenes provided the number of adjacent double bonds is in odd number.
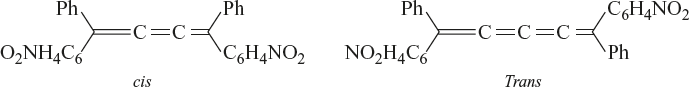
16.4.2 Optical Isomerism
Optical isomerism is a type of stereoisomerism in which the isomers are identical in molecular weight, molecular formula; physical and chemical properties but differ in the arrangement of substituents about an asymmetric carbon atom and their effect on the rotation of beam of plane polarized light.
Optical Activity
Ordinary light consists of rays of varying wave length vibrating in all directions perpendicular to the direction of propagation. When ordinary light is passed through a Nicol prism, the vibration are adjusted in a single plane only. The light whose vibrations occur only in one plane is termed as plane polarised light. The devise that brings polarisation in the light is called a polariser.
Substances have the ability to rotate the plane polarised light either towards right (clockwise) (or) left (anticlockwise); such substances are termed optically active substances and the property is called optical activity. The apparatus which measures the extent rotation of the plane polarised light is called a polarimeter.
The angle of rotation depends on the following factors:
- Nature of substance
- Wave length of light used (λ)
- Concentration of the solution (C) in g cm−3
- Nature of the solvent
- Length of the path through which polarised light possess.
- Temperature at which the measurements are made
The measurement of optical activity is done in terms of specific rotation which is defined as the rotation produced by a solution of 10 cm and unit concentration for the given wavelength of light at a given temperature.


Compound having the same physical and chemical properties but differing only in behaviour towards polarised light is called optical isomers. This phenomenon is known as optical isomerism.
Optical isomers which rotate the plane polarised light to the right are known as “dextrorotatory”, “d-form” or +ve sign.
The optical isomer which rotates the plane polarised light to the left (anticlockwise) is known as “laevorotatory”, “l-form” or is indicated by —ve sign.
The optical power of the two aforementioned isomers is equal in magnitude but opposite in sign. An equimolar mixture of the two forms, therefore, will be optically inactive. This mixture is termed “racemic mixture”, dl-form or (t) mixture.
Molecular Symmetry and Chirality
Stereochemistry is primarily concerned with molecular geometry and is best described in terms of molecular symmetry. In order to study the symmetry of a molecule, certain operations such as rotation, reflection, etc., are required. By doing so if indistinguishable arrangement from original is obtained; the operation is called “symmetry operation” and the molecule said to possess an element of symmetry.
A symmetry element is a mathematical entity present in a molecule. It may be point, an axis or a plane. A symmetry element is a label given to a molecule that transforms in a given way. A symmetry operation is a way of interchanging the molecule to form two geometrically equivalent parts.
There are four symmetry operations namely, rotation, reflection and inversion and a combination of the first two. The symmetry of a molecule can be generally attained by four symmetry operations and four corresponding elements of symmetry. Any organic molecule can be described by four symmetry elements as follows:
- Axis of symmetry, rotational axis of symmetry or simple axis of symmetry (Cn)
- Plane of symmetry or reflection of symmetry (σ)
- Centre of symmetry (i)
- Rotational and reflection axis of symmetry (Sn)
Axis of Symmetry (Cn)
![]()
If an imaginary line can be drawn through the centre of the molecule by rotation about that axis by an angle of 360/n, it gives a structure indistinguishable from the original. Then, the molecule is said to possess the simple axis of symmetry (Cn). The axis is called n-fold or proper axis of symmetry and the operation is called a Cn operation.

Plane of Symmetry (σ)
Plane of symmetry can divide the molecule into two halves which are mirror images of each other or a reflection of two halves of molecules across the plane; this makes it indistinguishable from the original. The plane is called σ and the operation is called σ operation.

Centre of Symmetry (i)
If a line is drawn from any part of the molecule to that point and is extended on equal distance beyond it, an analogous part will be encountered and the molecule shows the centre of symmetry
- This symmetry element is sometimes called the “point of inversion”.

Rotational–Reflection Axis of Symmetry (Sn)
It is a combination of rotation axis and reflection symmetry. The Sn operation can be achieved by rotation, followed by reflection—identical ⊥ C2 = Sn.

Chirality
Organic molecules are classified into two types. Based on the chirality of a molecule, they are classified into chiral molecules and achiral molecules.
Chiral Molecule
A molecule containing an asymmetric carbon is called “chiral”. It can also be defined as a molecule that lacks reflection symmetry or exhibits optical rotation. It may also be a molecule that lacks Sn-axis of symmetry.
Achiral Molecule
A molecule may contain any kind of symmetry operation, that is, plane of symmetry, axis of symmetry, rotational axis of symmetry and reflexion axis of symmetry.
Molecule Containing an Asymmetric Carbon
A tetragonal carbon containing four different atoms/groups are called asymmetric carbon (or) chiral carbon.

Lacks Reflection Symmetry
If a molecule is superimposable on its images, it is said to possess reflection symmetry. On the other hand, if it is not superimposable, the molecule lacks reflection symmetry. The molecule that possesses reflection symmetry is achiral.
Molecule with One Chiral Centre
Molecule with tetra coordinate centre

Molecule with two chiral centres
Dissimilar chiral centre

Similar chiral centre

Stereochemical Terms
- Homomers: Molecules which have the same molecular formulae and are superimposable on each other are called homomers.
- Isomers: Molecules which have the same molecular formulae and are not superimposable on each other are called isomers.
- Superimposability: When one molecule is placed on another molecule, and if all the atoms, groups, bond length and connectivity coincide with the corresponding ones in each other, they are said to be superimposable.
- Constitutional isomers: Isomers which have different molecular connectivity are called constitutional isomers.
Example: chain, positional, functional, metamers, tautomers and ring chain isomers.
- Stereoisomers: Those isomers which have the same molecular connectivity are called stereoisomers.
- Enantiomers: Stereoisomers which are non-superimposable mirror images are represented by (+ & −). Compounds which have asymmetric carbon show optical isomerism, but, sometimes, it may not exhibit due to the presence of meso compound.
- Diastereomers: Stereoisomers which are neither non-superimposable nor mirror images are called diastereomers.
Example:
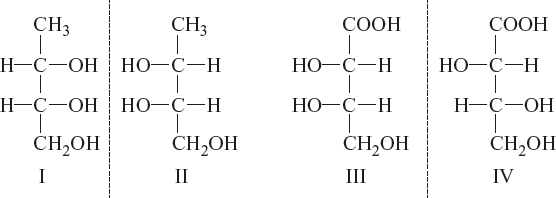
I and II; III and IV are enantiomers and I and III; I and IV; II and III; and II and IV are diastereomers.
Properties of Enantiomers and Diastereomers
The properties of enantiomers and diastereomers are as follows:
- A molecule can have only one mirror image and hence exists only in one enantiomeric pair; on the other hand, structural conditions permitting a molecule can have many diastereomers.
- No two stereoisomers can be enantiomers and diastereomers at the same time, that is, two enantiomeric and diastereomeric relationships are mutually exclusive.
- Enantiomers have identical physical and chemical properties such as boiling point, densities, refractive index, etc. They differ only in their action on plane polarised light. One of the enantiomers rotates the plane polarised light to the right or the clockwise direction and is known as dextrorotatory (d) and the other to the left or the anti-clockwise direction is known as laevorotatory (l). A mixture of two equal amounts of dextro and laevo enantiomers is called racemic mixture; it is optically inactive due to equal and opposite direction rotation of the plane polarised light.
- Diastereomers have different physical and chemical properties.
Meso Compounds
A meso compound possesses a plane of symmetry even though it contains chiral centres. A meso compound is “optically inactive as it contains an internal plane of symmetry; whatever relation is caused by one chiral carbon is compensated by the rotation caused in the opposite direction but to the same magnitude by the second chiral carbon.
Example: Two isomers of tartaric acid are optically active, due to the presence of a plane of symmetry as shown in Figure 16.1. However, on mono esterification of tartaric acid, similar chiral centres become dissimilar. Therefore, there is no plane of symmetry and it is optically active and will give four isomers.

Figure 16.1 Meso compounds
Racemic Mixture
A mixture of two equal amounts of dextro and laevo enantiomers is called racemic mixture; it is optically inactive due to equal and opposite direction rotation of the plane polarised light.
Resolution of Racemic Mixture
The synthesis of an optically active compound produces a mixture of both (+) and (−) isomers in equal amounts. Such mixtures are called racemic mixture or racemate. The separation of a racemic mixture into its two optical active components is known as resolution.
The following methods are used in racemic mixtures.
Chemical Resolution
Optically active isomers of the same compound resemble one another so closely in all properties except optical isomers that are not possible to separate them by ordinary laboratory. If, however, the racemic mixture is made to combine with another optically active compound, the difference is set up which can be exploited for separation.
Example: Racemic lactic acid is allowed to combine with the optically active base (−) strychnine.
The salt formed will be as follows:
![]()
These salts are not true enantiomers. They differ considerably in properties and, in particular, in solubility in various solvent. By fractional crystallisation from a suitable solvent, they can be separated. The treatment with dilute mineral acid removes optically active base and leaves two separate samples of (+) lactic acid and (-) lactic acid.
Biochemical Resolution
When certain bacteria or moulds are added to a solution of racemic mixture, they bring about the decomposition of the optically active forms more rapidly than others.
Example: Penicillium glaucum decomposes (+) tartaric acid more readily than (-) isomers.
Racemisation
Conversion of an optically active compound into a racemic mixture is called racemisation.
Thus, racemisation can be accomplished by heat and light.
Example: The conversion of either the optically active lactic acid into a racemic mixture by heating its aqueous solution may proceed through an end intermediate.

When the proton migrates back to the carbon atom, the process can involve the opening of either of two linkages of the double bond that can produce either the original configuration or in it.
Molecule with Helical Shape
The helical, propeller or screw-shaped molecular entity shows chirality. A right-hand side helix is described as a plus or p, a left hand side one as m or minus.
Erythro and Threo System
This nomenclature is used only for two adjacent chiral carbon compounds and has the following structure:
![]()
Out of six substituents, the two substituents on two chiral centres should be the same.
When fisher projection are drawn for stereoisomers with two adjacent chirality centres, the pair of enantiomers with similar groups on the side of carbon vertical chain is called erythro form. The pair of enantiomers with similar groups on the opposite side is called threo form.

16.4.3 Conformational Isomers
Stereoisomers whose energy barrier for interconversion is less than 40 kJ/mole are called conformational isomers.
Configurational Isomers
Stereoisomers whose energy barrier for interconversion is more than 60 kJ/mole are called configuration isomers.
16.4.4 R–S Nomenclature or CIP Nomenclature
The R–S nomenclature system is based on a 3D structure of molecule. It was first introduced by Chan and Ingold and was subsequently developed by Chan, Ingold and Prelog, and hence the system is known as CIP nomenclature. The assignment of configuration is done by applying two rules; the first is the sequence rule and the second is the chirality rule.
The sequence rule arranges four ligands of chiral centre with priority sequences as 1 > 2 > 3 > 4 (or) a > b > c > d.
The chiral centre is then viewed from the side remote from the lowest ranking group 4 or d and the assigned name to the chiral centre as ‘R’ or ‘S’.
Sequence Rule
- The ligand with a higher atomic number will get higher priority and is numbered from 1 to 4; the decrease in the priority is 1 > 2 > 3 > 4.
Example: Br > Cl> F > H
- When two atoms attached to the chiral centre is the same, take the next atoms in the group and so on.
Example: —C2H5>—CH3
- If group has multiple bond, do duplication and triplication.
Example:
- Heavier isotopes will have more priority over lighter isotopes.
Example: 13C > 12C, D > H
- When the chiral centre atom is a part of the ring system, the sequence rule is exactly the same. Each branch of the ring is followed until a difference can be detected.
- Give more priority to R configuration than S. Once the priority order of the ligand is identified, the configuration assignment is made by chirality rule. According this rule, put these least priority group, (4 (or) d) away from the viewer and determine order of 1 → 2 → 3 from this point of view of the arrangement. 1 → 2 → 3 appears in clockwise direction the configuration is R, and the arrangement appears in the anti-clockwise direction the configuration is ‘S’.
Example: lactic acid

The priority sequence for the most common groups and atoms is given as follows.

Axial Chirality
The term used to refer to stereoisomerism resulting from the non-planar arrangement of four groups in pair about a chirality axis is exemplified by allenes, abc=c=c=d and by the atropisomerism of ortho-substituted biphenyls. The configuration in molecular entities possessing axial chirality is specified by the stereo descriptors, Ra and Sa (or by P or M).
Example: Spiro compounds biphenyls

16.4.5 E–Z Nomenclature
The configurational stereoisomers of the kind shown above need an additional nomenclature in order to specify the spatial orientations of the group attached to the double bond. But description of cis-trans configuration is ambiguous when all the four substituents attached to the double bond are different. This is explained by E–Z nomenclature. Here, Z stands for “zusammen”, which means together and E stands for “entagegen”, which means opposite in German.
This system also follows the sequence rules of R–S nomenclature, to assign the configuration of geometrical isomers.
Select the atom or group with higher priority on each carbon if the atoms or group of the higher priority on each carbon come on the same side of the double bond, the isomer is assigned ‘Z’ configuration. On the other hand, if the atoms are groups of higher priority are present on each carbon atoms on the opposite side of the double bond, the isomer is assigned the configuration ‘E’.
Example:

16.5 MOLECULAR REPRESENTATION
In 1871, van’t Hoff suggested that carbon has tetrahedral geometry. Since then, stereochemistry gained importance. Originally, organic molecules were 3D, but 3D structures cannot be represented in the original form on two-dimensional (2D) planes like board or paper. Stereochemistry can explain the conversion of 3D molecular structure into 2D molecular structure on plane or paper.
In stereochemistry, four kinds of representation are available to represent the 3D structure into 2D structure. They are as follows:
- Wedge and Dash projections
- Fisher projections
- Sawhorse projections
- Newman projections
16.5.1 Wedge and Dash Projections
In this representation, the bonds are represented with three types of lines. One is with wedge shaped thick line (—), another is the dashed line (||||) and the remaining are normal solid lines (—). The wedge-shaped thick line indicates that the corresponding group is projecting towards the viewer, or above the plane or in front of the viewer. The dashed line indicates that the corresponding group is projecting away from the viewer or below the plane and the normal solid line indicates that the corresponding group is in the plane of the black board or paper. If stereochemistry is unknown, this can be indicated explicitly by the away line ![]() .
.
These wedge formulae will give complete information, including the basic molecular formula, geometry and bond angles as well.
Example: lactic acid

16.5.2 Fisher Projections
- In Fisher representation, each asymmetric carbon (chiral centre) is represented with cross (+).

- Fisher representation can have only one vertical line and one or more horizontal lines ≢.
- The maximum number of carbons should be on the vertical line or select the longest carbon chain as a vertical line and one or more horizontal lines, depending on the number of asymmetric carbon in the molecules.
- Put the highly oxidised group at the top of the vertical line.
- (v)
- If any of the Fisher representation may rotate 180° in plane it produces initial representation only.
- If any of the Fisher representation may rotate 90° in plane it produces mirror image of initial representation.
- Each Fisher representation is eclipsed.
- In the Fisher representation,
- The groups on the vertical line are below the plane.
- The groups on the horizontal line are in front of the plane.
- The Fisher representation gives easier way to represent the molecule having more number of carbons.
Example: n-pentane

16.5.3 Sawhorse Representation
- In sawhorse representation, the molecule must be see in side way. Therefore, the observer can see both front and back carbon.
- In this projection, draw the C—C bond as a diagonal line.
- At the termini of the diagonal axis, place the three bonds with 120° representation each.
- In the Sawhorse representation, the groups must be with the eclipse.

16.5.4 Newman Representation
In Newman projection formulae, the molecules are seen along the C—C bond.
- The front carbon is represented by the dot and the three other bonds are attached with
 .
. - The back carbon is represented as a circle and the three other bonds are attached with 120°
 .
. - The C—C bond cannot be represented on the plane of the paper.
- It is good for easy observation of groups present on two adjacent carbons; as the C—C bond allows rotating to change orientation of groups in space. Therefore, this representation is very useful for conformational analysis.
Example: CH3—CH3

16.6 MOLECULAR ISOMERISM
Molecules with the same molecular formulae but with different arrangement of atoms in space are called isomers. The criterion for determining isomeric relation is shown in Figure 16.2.

Figure 16.2 Determination of molecular isomerism
16.7 REVIEW QUESTIONS
16.7.1 Fill in the Blanks
- Organic compounds having the same molecular formula but differ in some physical or chemical properties are called __________.
[Ans.: Isomer]
- Non-superimposable mirror images are __________.
[Ans.: enantiomers]
- The stereoisomers which are interconvertible at room temperature >60 kJ mole are called __________.
[Ans.: configurational isomers]
16.7.2 Multiple-choice Questions
- __________ is a staggered form.


- Both (a) and
- None of these
[Ans.: b]
- Which of the following is an achiral carbon?
[Ans.: a]
- Alcohol and ethers exhibit __________ isomerism.
- Metamerism
- Tauto isomerism
- Functional group
- Chain isomerism
[Ans.: c]
- Which substance is optically active?
[Ans.: a]
- The same molecular formula and superimposable are used in __________.
- Stereoisomers
- Homomers
- Optical isomers
- All of these
[Ans.: b]
- Detect the isomer.
[Ans.: a]
- CH3 CH2=CH2 and CH3CH=CH3 is an example of __________.
- Chain isomerism
- Position isomerism
- Metamerism
- Tautomerism
[Ans.: b]
- Isomers with the same molecular formula but different structural formula are called __________.
- Structural isomers
- Stereoisomers
- Optical isomers
- None of thesew
[Ans.: a]
- The different alkyl groups attached to the same polyvalent functional group are called (b)
- Tautomerism
- Metamerism
- Mesomerism
- Ring chain isomerism
[Ans.: b]
- A tetragonal carbon containing four different groups are called __________.
- Symmetric carbon
- Asymmetric carbon
- Both (a) and (b)
- None of these
[Ans.: b]
- A molecule containing an asymmetric carbon is called __________.
- Achiral
- Chiral
- Enantiomer
- Diastereomer
[Ans.: b]
- Cis-tran isomerism belongs to __________.
- Optical isomerism
- Conformational isomerism
- Geometrical isomerism
- Structural isomerism
[Ans.: c]
- Eclipsed and staggered representations are observed in __________.
- Newman molecular representation
- Fisher representation
- Sawhorse representations
- Wedge and Dash representation
[Ans.: a]
- The ability of a substance which rotates the plane polarised light is called __________.
- Optical activity
- Optical inactive
- Both (a) and (b)
- None of these
[Ans.: a]
 are __________.
are __________.
- Structural isomers
- Geometrical isomers
- Optical isomers
- Stereoisomers
[Ans.: a]
- Keto–enols __________ are isomers.
- Meta isomers
- Geometrical
- Stereoisomers
- Tauto isomers
[Ans.: d]
- Erythro and threo isomers belong to __________.
- Optical isomers
- Stereoisomers
- Structural isomers
- Configuration isomers
[Ans.: a]
16.7.3 Short Answer Questions
- Define isomerism.
Ans.: Organic compounds having the same molecular formula but differing from each other at least in some physical properties or chemical properties or both are known as isomers and the phenomenon is called isomerism.
- Write a note on structural isomerism.
Ans.: The constituent atoms or groups are linked to one another within the molecule without any reference to space structural isomers. These are compounds that have the same molecular formula but different structural formulae.
- Write the types of structural isomers.
Ans.:
- Chain isomerism
- Position isomerism
- Ring chain isomerism
- Functional group isomerism
- Metamerism
- Tautomerism
- Explain stereoisomerism.
Ans.: Stereo means space, “iso” means equal and “mers” means parts.
It has the same chemical formula but differs with spatial arrangement of atoms or groups and are called stereoisomers and the phenomenon is called stereoisomerism.
- Describe optical activity and optional isomerism.
Ans.: Substance that has the ability to rotate the plane polarised light either to the right or left are termed as optical active compounds and that property is called optical activity.
Compound having the same physical and chemical properties but differs only in the behaviour towards plane polarised light is called optical isomerism.
- Write the types of molecular representations.
Ans.:
- Wedge and Dash representation
- Fisher representation
- Sawhorse representation
- Newman representation
- Differentiate between enantiomers and diastereomers.
Ans.:

16.7.4 Descriptive Questions
Q.1 Give a detailed sketch on molecular representations and the types of molecular representation.
Q.2 Write a note on stereochemical terms and the properties of enantiomers and diastereomers.
Q.3 Describe configuration isomerism.
Q.4 Write a brief note on geometrical isomerism.
Q.5 Explain the E–Z nomenclature with a neat sketch.
Q.6 Write about isomerism and its significance.