4
ALTERNATE ENERGY RESOURCES
Energy conservation is the foundation of energy independence
4.1 INTRODUCTION
Every day, a lot of carbon dioxide is being released due to different developmental activities that accumulates around the atmosphere. Such release of carbon dioxide is one of the causes of global warming. One answer to reduce the global warming effect is to replace and retrofit current traditional energy resources with alternative energy sources which perform comparably better without emitting carbon dioxide.
At the same time, with increasing demand for energy and with fast depleting conventional sources of energy such as coal, petroleum, natural gas and so on, the non-conventional sources of energy such as energy the sun, wind, biomass, tidal energy, geo-thermal energy and even energy from waste material are gaining importance. This energy is abundant, renewable, pollution-free and eco-friendly.
The importance of renewable energy was recognised in the country in the early 1970s. The renewable energy programme started with the establishment of the Department of Non-conventional Energy Sources (DNES) in 1982. The Indian Renewable Energy Development Agency (IREDA) was set up in 1987. In 1992, the DNES was converted into the Ministry of Non-conventional Energy Sources (MNES) which has taken several steps to create a suitable atmosphere for harnessing non-conventional sources of energy. Today, India has one of the largest programmes for renewable energy.
Depending on their usage, energy resources are broadly divided into two categories—conventional and nonconventional energy resources.
4.1.1 Conventional or Traditional Energy Resources
The energy resource that has been used from ancient period is known as conventional or traditional energy resources. A majority of these energy resources are non-renewable.
For example, coal, fuel wood, crude oil, natural gas, etc.
All conventional sources will become rare, endangered and extinct, as they produce lots of carbon dioxide that adds to the greenhouse effect in the atmosphere.
4.1.2 Nonconventional Energy Resources or Renewable Energy Sources
The energy sources which will not get exhausted even after continuous and excessive use and are abundantly available, continuously replenished by nature are nonconventional energy resources. These energy resources are environmentally and ecologically safe.
For example, solar energy, wind energy, tidal energy, biogas, geothermal energy, nuclear energy, etc.
4.1.3 Alternative Energy
Alternative energy is commonly referred to as any energy source that is an alternative to fossil fuel. According to previous reports, coal as an alternative to wood, petroleum as an alternative to whale oil, alcohol as an alternative to fossil fuels, coal gasification as an alternative to petroleum etc., were common; however, this is completely different. Depending on availability, demand and sustainability, the energy resources which are used as alternative energy source has changed considerably from time to time.
Alternative energy plays vital role to meet the future demands of energy and environment; hence, advance research in this area started in a multidisciplinary way. Let us examine the various definitions of alternative energy.
Oxford Definition
Energy fuelled into ways that does not use up natural resources or harm the environment.
Princeton WordNet
Energy derived from sources that does not use up natural resources or harm the nature.
Natural Resource Defence Council
Energy that is not popularly used and is usually environmental sound such as solar or wind energy.
Material and Management
Fuel source that are other than those derived from fossil fuels typically used inter changeable for renewable energy.
For example, wind energy, solar energy, biomass, wave and tidal energy.
Torridge District Council
Energy generated from alternatives to fossil fuel, but need not be renewable.
Climate Change 2007
Energy derived from nontraditional sources.
For example, compressed natural gas, solar energy, hydroelectric and wind energy.
Hence, common alternative energy resources are solar energy, water energy (hydroelectric power and tidal power), wind energy, geothermal energy, biomass energy, nuclear energy, etc.
4.2 NON-CONVENTIONAL ENERGY SOURCES AND STORAGE DEVICES
4.2.1 Solar Energy
The sun is a source of enormous energy. Solar energy originates with thermo-nuclear fusion in the sun, involving the fusion of hydrogen into helium either through the proton-proton chain or through the carbon-nitrogen cycle. The Earth receives solar energy as a radiant energy which reaches the top of the atmosphere at 1,366 watts per square meter. This energy ranges from ultraviolet, visible and infrared light. About half of this energy actually makes it to the Earth’s surface, 30% is reflected and 20% is absorbed by the atmosphere. Thus, when the sun is directly overhead, full sunlight can deliver about 700 watt per square meter and at that rate, the sun can deliver 700 MW of power to an area of 390 square miles.
The total amount of solar energy reaching the earth is vast and almost beyond belief. For an example, one year’s expenditure of fossil fuel in the USA is equivalent to just 40 minutes of sunlight striking the particular land surface of the USA.
The solar energy technology broadly comprises thermal conversion and photo conversion. Thermal conversion takes place through direct heating, ocean waves and currents and wind. Photo conversion includes photosynthesis, photochemistry, photo electrochemistry, photo galvanism and photovoltaics. Solar radiation is collected and converted by natural collectors such as the atmosphere, the ocean and plant life, as well as by man-made collectors of many kinds (Figure 4.1). There are a number of solar technologies by which it can be harnessed (Figure 4.2).

Figure 4.1 Natural and man-made collectors

Figure 4.2 Solar technologies
The earliest use of direct solar energy by mankind was drying the body or warming it in the sun during winters. Indeed, drying of clothes, fodder, timber, agricultural and animal products, salt water (to get salt) and passive space heating remained the most extensive form of use of direct solar energy in the history of mankind. All other active solar technologies or devices for harnessing direct solar energy have fairly recent origins (Figure 4.2). A variety of active solar collectors provide a broad range of applications as follows:
- Solar heating of water
- Solar space heating of buildings
- Solar air-conditioning
- Solar refrigeration
- Solar drying
- Solar cooking
- Solar green-houses
- Solar furnaces
- Solar desalination
- Salt production
- Solar electricity—thermal
- Solar electricity—photovoltaic
Solar Heating of Water
Solar water heating is very popular in warm, sunny climates. Flat plate collectors for heating water consists of a thin broad box with a glass or plastic top and a black bottom in which water tubes are embedded as shown in Figure 4.3. These collectors are faced towards the sun and the black bottom gets hot when it absorbs sunlight. Thus, water circulating tubes are heated and are conveyed to a tank where it is stored.
Solar heating system may be active or passive. The heated water is moved by means of pumps in an active system. However, in a passive system, the collector is lower than the tank. By natural convection, the heated water rises into the tank from the collector and the cool water descends into the collector from the tank. This is shown in Figure 4.4. The tank will usually have a source of auxiliary heat (electric or gas) in order to get the temperature to a desired level or to provide heat when solar energy is insufficient.
Solar Space Heating of Buildings
Flat-plate collectors such as those used in water heating with same concept and less expensive option can be used for space heating as only air circulation through the collector box is necessary.
In nonfreezing climates, simple water convection systems may suffice.
In freezing climates, an antifreeze fluid is circulated. Solar heat is augmented by an auxiliary heat source in the hot-water tank.
The efficiency of solar space heating can be enhanced by proper designing of a building to act as its own collector. Here, the basic principle is to have windows facing the sun. On cool days, it depends on sun’s angle of incidence and sunlight can heat the interior of the building; however, at nights, insulated drapes or shades can be pulled down to trap the heat inside. Excessive heat in hot days can be avoided by using an awning or overhang to shield the window from the sun.

Figure 4.3 Principle of a flat-plate solar collector
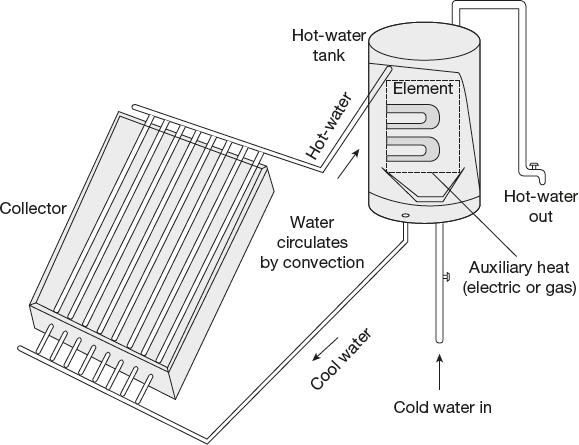
Figure 4.4 Solar water heaters
Solar Air-conditioning
Solar air-conditioning includes solar-powered refrigeration system of ranking cycle system, absorption refrigerator systems and solar-regenerated desiccant cooling systems. In such type of system, ambient air is adiabatically cooled, dehumidified, cooled both sensibly and evaporatively and then ducted to the living area. In the regenerative stage, air is evaporatively cooled, heated as it cools the supply air stream, heated again by solar collectors and humidified.
Solar Refrigeration
Solar refrigeration is closely related to air-conditioning. It is generally required for food preservation or for storage of medical and biological materials. Although most of the units/machines fabricated are simple in design, they are generally too complicated to operate and, therefore, are not usable by the people.
Solar Drying
Solar drying of agriculture and animal products is the most ancient, traditional and wide-spread method of utilising direct solar energy. Agricultural products such as grain, hay, copra, fruits, nuts and vegetables are still-dried with sun drying all over the world, including highly industrialised and well-developed countries. This method is followed because it is the cheapest and the simplest way to dry crops and abundant availability of sunlight is also ensured.
Solar Cooking
A solar cooker is a perfectly insulated shallow rectangular metal box, and inner side of this box is blackened and fitted with a flat glass cover. When this cooker is placed in sunlight, the solar energy penetrates the glass cover and is absorbed by the inner blackened surface. Thus, temperature will increase inside the box, and the food gets cooked quickly.
The collector area of such a solar cooker can be increased by providing a plane reflector mirror of size equal to the size of the box and is hinged on one side of the glass frame. With the help of reflector mirror, a temperature rise of 15°C–20°C can be achieved inside the solar cooker.
Solar Greenhouses
A greenhouse is a closed structure covered with transparent material (glass or plastic) which acts as a solar collector and utilises solar radiant energy for the growth of plants.
The incoming shortwave solar radiations can pass through the green house glazing; but the longwave thermal radiations emitted by the objects within the greenhouse cannot escape through the glazed surface. As a result, the radiations get trapped within the greenhouse and result in an increase in temperature. Further, the air inside the greenhouse gets enriched with carbon dioxide and the moisture loss is reduced due to restricted transpiration. All these factors help the plant growth to sustain during night and colder months.
Solar Furnaces
Extremely high temperatures with very clean condition of around 3,500°C can provide solar furnaces. This can be used to melt refractory material.
In a solar furnace, high temperature is obtained by concentrating the solar radiations on to a specimen using a number of heliostats (turnable mirrors) arranged on a sloping surface. The biggest advantage of a solar furnace is that heating can be accomplished without any contamination and temperature can be easily controlled by changing the position of the material in focus. It is anticipated that in future, solar furnaces can be utilised in the production of nitric acid and fertilisers from air.
Solar Desalination
In this method, solar radiation is admitted through a transparent air-tight cover of sloping sheets of glass into a shallow blackened pool containing brine. The water evaporates from the brine when solar radiations pass through the cover. The vapours produced get condensed as purified water on the cooling glass cover, flow down the sloping roof, collected into a water storage tank (as shown in Figure 4.5). The excess brine that has not evaporated is run to waste.

Figure 4.5 Basin type solar-still
The cost of distilled water per litre obtained by this process is cheaper than distilled water obtained by other electrical energy-based processes.
Salt Production
It is the most widely used method for salt production in many developing countries of the world. The basic concept is that in areas where evaporation exceeds rainfall, a shallow pool of brine is exposed which results in evaporation of water, leaving behind the salt.
Solar Electricity—Thermal
Solar energy may be used to heat a fluid, which then generates electricity through a conventional heat engine. To obtain an adequate working temperature, some form of concentration of solar energy is required, so that there is little contribution from diffused sunlight for most designs.
Broadly, the systems fall into two categories:
- Systems in which individual mirrors track the sun continuously
- Systems in which mirrors are fixed
In continuous tracking systems, a large number of plane or curved mirrors is used. Each mirror is steered to reflect sunlight onto a single tower mounted boiler and gives a high temperature with high efficiency. However, mirrors require complex, rugged and accurate mechanisms.
Non-tracking systems consist of assemblies of trough-shaped collector, aligned east to west. Above each collector, the absorber is fitted in the form of a tube. A general plan of a solar-thermal electric power plant is shown in Figure 4.6. It is expected that cost-effective and utility scale solar-thermal power plants might be built in the near future.
Solar Electricity—Photovoltaic
A solar cell—more properly called a photovoltaic, or a PV cell—directly converts incident solar radiation to electrical current. A PV cell looks like a simple wafer of material with one wire attached to the top and one to the bottom. As sunlight shines on the wafer, it puts out an amount of electric current roughly equivalent to that emitted by a flashlight battery. Thus, PV cells collect light and convert it to electric power in one step. However, several cells can be connected together to obtain large amount of power.

Figure 4.6 General plan of a solar-thermal electric power plant
Working Principle
PV cells consist of two very thin layers of semiconductor materials separated by a junction layer. The lower layer has atoms with single electrons in their outer orbit which are easily less. The upper layer has atoms lacking electrons in their outer orbit; these atoms readily gain electrons. When the kinetic energy of light photons striking the two-layer “sandwich” dislodges electrons from the lower layer, it creates a current that can flow through a motor or some other electrical device back to the upper side.
The major material used in PV cells is silicon. PV cells consist of a single crystal of p-type silicon with a surface layer of n-type silicon. When light falls on the junction, the electrons and holes move in opposite directions across the p-n junction and electric current will flow if an external circuit is connected. This is shown in Figure 4.7.
Cost
The cost of PV power (cents per kilowatt-hour) is the cost of the PV cells divided by the total amount of power they may be expected to produce over their lifetime currently around 25 cents per kilowatt-hour while the cost of power from other power alternative is 6–12 cents per kilowatt-hour for residential electricity. PV power had its first significant application in the 1950s in the solar panels of space satellites. The cost comes down dramatically if more efficient cells with less expensive production techniques are evolved.
Uses
The uses of PV cells are as follows:
- PV cells are widely used in watches, calculators, toys, etc.
- The panels of PV cells provide power for traffic signals, radio transmitters, light houses, irrigation pumps, earth orbiting satellites, etc.

Figure 4.7 Semiconductors functioning as solar cells
Future of Solar Energy
Solar electricity is growing at a phenomenal rate due to adverse environmental effects and logging of non-renewable energy resources. The sun provides power only during the day, but 70% of electrical demand occurs during the day for industries, offices and stores that are in operation. Thus, remarkable savings can be achieved by using solar panels for daytime requirements and continuing to rely on conventional sources at night.
4.2.2 Wind Energy
The research pertaining to wind energy dates back several decade to the 1970s when the NASA developed an analytical model to predict wind turbine power generation during high winds. Today, both Sandia laboratory and national renewable energy have programmes dedicated to wind research. The Field Laboratory Operator is Optimised Wind Energy (FLOWE) at Caltech was established to research alternative approaches to wind energy.
The sun is the origin of wind energy. Wind is a form of solar energy and is caused by the uneven heating of the atmosphere by the sun, the irregularities of the earth surface and rotation of the Earth. Wind flow patterns are modified by the Earth’s terrain bodies of water and vegetation cover. Kinetic energy in the wind can be used to run wind turbines but the output power depends upon the wind speed. Turbines generally require a wind in the range of 20 km/h. Wind turbine converts kinetic energy in the wind into mechanical power. This mechanical power can be used for a specific task or the generator converts mechanical power into electricity to power homes, businesses, schools and so on.

Figure 4.8 Wind turbines
Like air draft propeller blades, wind turbines (Figure 4.8) turn in the moving air and power an electric generator that supplies an electric current. Simply stated, a wind turbine is the opposite of a fan. Instead of using electricity to make wind, it is using wind to make electricity. The wind turns the blades, which spin a shaft, which connects to a generator and makes electricity. Wind energy is a free, renewable resource; the wind turbines set-up is a bit expensive as well.
4.2.3 Geothermal Energy
Geothermal energy is produced by tapping into the thermal energy created and stored within the Earth’s crust (Figure 4.9). It is considered sustainable because thermal energy is constantly replenished. Several entities such as the National Renewable Energy Laboratory and Sandia National Laboratories are conducting research towards the goal of establishing a proven science around the geothermal energy.
Geothermal energy comes from the heat within the earth. The word geothermal comes from the Greek words geo meaning earth and thermal meaning heat. Geothermal energy has been used for thousands of years in some countries for cooking and heating. People around the world use geothermal energy to produce electricity to heat buildings, green houses and for other purposes.

Figure 4.9 Geothermal plant
There are four kinds of geothermal sources—hydrothermal, geo pressured, hot dry rock and magma.
4.2.4 Water Power
The energy of falling or fast-running water can be harnessed and used in the form of motive energy or temperature difference. Since water is about a thousand times heavier than air, even a slow flowing stream of water can yield great amount of energy.
Hydroelectric Energy
Hydroelectric energy is a term usually referred to in the context of hydroelectric dams. Hydroelectric power stations capture the kinetic energy of moving water and give mechanical energy to turbines. The moving turbines then convert mechanical energy into electrical energy through generators. This energy has been exploited for centuries. Farmers since the ancient Greeks have used water wheels to grind wheat into flour. Placed in a river, a water wheel picks up flowing water in buckets located around the wheel. The kinetic energy of the flowing river turns the wheel and is converted into mechanical energy that runs the mill (Figure 4.10).
In the late 19th century, hydropower became a source for generating electricity. The first hydroelectric power plant was built at Niagara Falls in 1879. In 1881, street lamps in Canada were powered by hydropower. In 1882, the world’s first hydroelectric power plant began operating in Appleton, Wisconsin.
A typical hydro plant is a system with three parts—an electric plant where the electricity is produced, a dam that can be opened or closed to control water flow and a reservoir where water can be stored. The water behind the dam flows through an intake and pushes against blades in a turbine, causing them to turn. The turbine spins a generator to produce electricity. The amount of electricity that can be generated depends on how far the water drops and how much water moves through the system. The electricity can be transported over long-distance electric lines to homes, factories and businesses.
Generation of electricity from a small-sized hydropower source is a low-cost, environment friendly and renewable source of energy. Small and mini hydel projects have the potential to provide energy in remote and hilly areas where extension of grid system is uneconomical.

Figure 4.10 Hydroelectric power plant
Tidal Energy
Tidal energy is a form of hydropower that converts the energy obtained from tides into useful forms of power, mainly electricity. Sea water keeps rising and falling alternatively twice a day under the influence of gravitational pull of moon and sun. This phenomenon is known as tides. Tidal power refers to capturing energy from the tides in horizontal direction. Tides come in, raise water levels in a basin and tides roll out. The water is made to pass through turbine to get out of the basin. Therefore, power generation through this method has a varying degree of success (Figure 4.11).
There are two types of tidal energy systems that can be used to extract energy—kinetic energy, the moving water of rivers, tides and open ocean currents; and potential energy from the difference in height between high and low tides. The first method—generating energy from tidal currents—is becoming more popular because people believe that it does not harm the environment as much as barrages or dams. Many coastal sites worldwide are being examined for their suitability to produce tidal energy.

Figure 4.11 Tidal energy
4.2.5 Biomass
Biomass refers to all plant material and animal excreta when considered as an energy source. Some important kinds of biomass are inferior wood, urban waste, farm animal and human waste. Biomass as an energy source can be either used directly via combustion to produce heat or indirectly after converting it into various forms of biofuels. Conversion of biomass to biofuels can be achieved by different methods such as thermal, chemical and bio chemical methods. Wood remains the largest biomass energy source.
Since biomass includes plant or animal matter that can be converted into fibers or other industrial chemicals including biofuels, industrial biomass can be grown from numerous types of plants. Biomass, biofuel and bio gas are burned to produce heat/power and consequently, sulphur oxide, nitrous oxide and particular matter that pollute the environment are produced from this combustion.
Biogas
Biogas is the gas resulting from an anaerobic digestion process (Figure 4.12). A biogas plant can convert animal manure, green plants, waste from agro industry and slaughterhouses, paper production, sugarcane production, sewage and so forth into combustible gas.
Biogas is a mixture of methane, carbon dioxide, water and hydrogen.

Figure 4.12 Flow chart of biogas power generation
Biofuel
Biofuel is any fuel that derives from biomass; this may be recently living organisms or their metabolic byproducts, such as manure from cows, buffaloes etc., Typically, biofuel is burnt to release its stored chemical energy. Biomass can be directly used as fuel or to produce liquid biofuel. Agriculturally produced biomass fuels such as biodiesel, ethanol and bagasse (byproduct of sugarcane cultivation) can be burnt in internal combustion engines or boilers.
Algae Fuel
Algae fuel is a biofuel derived from algae. During photosynthesis, algae and other photosynthetic organisms capture carbon dioxide and sunlight and convert it into oxygen and biomass. This is usually done by placing the algae between two panes of glass. The algae create three forms of energy fuel—from its growth cycle, heat can be generate, the oil derived from the algae is used as biofuel and on maturity, algae can give biomass.
The heat can be used to power building systems such as heating of water or to produce energy. Biofuel is oil extracted from algae upon maturity and is used to create energy similar to the use of biodiesel. Biomass is the matter left over after extracting oil and water and can be harvested to produce combustible methane for energy production.
Biomass Briquettes
Biomass briquettes are developed as an alternative to charcoal. Almost any kind of plant matter compressed into briquettes has about 70% the calorific value of charcoal.
4.2.6 Nuclear Energy
With growing realisation that the supply of fossil fuels is depleting fast and is very limited, nuclear energy has gained importance and has become essential as an alternative source of energy. Due to rapid increase in industry and urbanisation, along with booming increase in population, it is estimated that the present fossil fuels (coal and petroleum) will not last more than a few decades to cater to the demand for electric power. Therefore, attention has been focused mainly on nuclear energy, which seems to offer an infinite source of energy. This field began with the discovery of radioactivity. The release of tremendous amount of energy through nuclear reaction became possible with the development of artificial radioactivity.
Nuclear energy is the energy released when the nuclei of certain atoms undergo induced reactions such as fission and fusion. The materials that make such energy available are called nuclear fuel. Nuclear fuels release energy by an entirely different mechanism as compared to chemical reactions. In nuclear fuel, different elements are produced, and some of the binding energy of the nucleus is released, during bombardment of neutron on radioactive element such as uranium-238, etc. Therefore, a large amount of energy is released in less than a millionth of a second. For example, complete fission of one kg of uranium provides the energy equivalent of about 2 × 107 kWh; such amount of energy can be obtained by burning of about 3,000 tonnes of high grade coal.
Mass Defects and Nuclear Binding Energy
The mass of an atomic nucleus has been found to be always less than the sum of the masses of the constituent nucleons (i.e., protons, neutrons and electrons). This difference is called mass defect. The mass thus lost appears in the form of energy in accordance with Einstein’s mass-energy relationship; the energy emitted is called the binding energy of the nucleus.
Consider an isotope having atomic number ‘Z’ and mass number ‘A’. Evidently, its atom contains protons = Z, electrons = Z, Neutrons = (A – Z).
Let mp, me and mn respectively represent the masses of proton, electron and neutron.
Thus, the calculated mass of this isotope is

Let M = actual mass of the element

Thus, the mass defect (ΔM) is the loss of mass in the formation of nucleus from its constituents. This loss of mass represents the amount of energy, which would be released if an atom of mass number A were synthesised from its constituents. This form of energy is in accordance with Einstein’s mass-energy relationship.
Thus, the binding energy of the nucleus may be defined as the energy released during the formation of a nucleus from its constituents nucleons.
Thus, if ΔM is the mass defect,
![]()
The binding energy per nucleon is calculated by dividing the binding energy of a nucleus by the number of nucleons.
The binding energy corresponding to one atomic mass defect is 931.5 MeV (million electron volts)
![]()
The greater is B.E./nucleon, the greater is the stability of the nucleus.
The energy equivalent to one atomic mass unit (1 amu) is given by,

Example to Calculate the Binding Energy per Nucleon
The formation of helium atom can be written as follows:
![]()
Change in mass is given as follows:
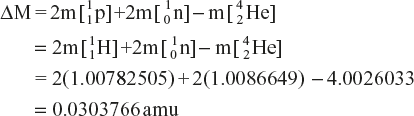
Since the energy equivalent to 1 amu of mass is 931.5 MeV, the energy change during the formation ![]() He of atom is
He of atom is

Further, since there are four nucleons in ![]() He nucleus, the binding energy per nucleon (EB/A) is obtained by dividing the total binding energy with the number of nucleons, that is,
He nucleus, the binding energy per nucleon (EB/A) is obtained by dividing the total binding energy with the number of nucleons, that is,
![]()
The binding energy per nucleon is a direct measure of the stability of the nucleus.
Binding Energies and Stability of Nuclei
Mean binding energies, that is, the binding energies per nucleon, drastically increase with increasing atomic mass in lighter elements, reach a maximum at mass numbers 55–58, and gradually decrease in the case of heavier elements. The mass number 55–58 corresponds to iron and nickel, the most stable elements, and this stability is also reflected in the abundant presence of these elements in the inner core of the earth.
From Figure 4.13, it is seen that the maximum occurs at about mass number 56 (iron). Thus, the nucleus of iron is thermodynamically most stable. It is also seen that the points for helium (mass number = 4), carbon (mass number = 12) and oxygen (mass number = 16) lie quite high in the graph. This shows that the nuclei of these elements are exceptionally stable. The maximum binding energy is seen to be about 8.7 MeV. This is the energy required to remove a proton or a neutron from the most stable nucleus.
4.2.7 Nuclear Reactions
Nuclear reactions involve changes in the number of nucleons present in the nucleus. Hence, there is a formation of new atomic species. Thus, nuclear reactions lead to atomic transformations. In addition, a huge amount of energy is released in nuclear reaction, involving a small but measurable loss in mass. This mass is transformed into energy in accordance with Einstein’s equation (E = mc2).

Figure 4.13 Mean binding energies of various nuclei
Rutherford carried out the first nuclear reaction by bombarding nitrogen (target nucleus) with α-particles (projectile) to produce an oxygen isotope.

Types of Nuclear Reactions
- Nuclear fission:
For example,

- Nuclear fusion:
For example,

Nuclear Fission
Very high nuclei have a lower binding energy per nucleon than the nuclei with intermediate mass. Thus, the former are less stable than the latter. During 1934–1938, Otto Hahn and F. Strassmann observed that when 235U is bombarded with slow-moving thermal neutrons (energy = 0.025 eV), it undergoes fission giving barium (z = 56) as one of the products of fission. In order to explain this observation, L. Meither and O.R. Frisch suggested that after the capture of a neutron, the uranium nucleus gets excited and then splits into two fragments of approximately equal mass, which is known as nuclear fission.
There is an invariably mass defect during fission, that is, the total mass of products of fission is less than the total mass of neutron and the 235U atom. The loss of mass appears in the form of energy according to Einstein’s mass-energy relation, E = mc2.
The fission reaction is represented as follows:

The splitting of a heavier atom like Uranium-235 into a number of fragments of much smaller mass by suitable bombardment with sub-atomic particles with liberation of huge amount of energy is called nuclear fission.
In this reaction, in addition to the release of enormous amount of energy, the most significant feature of nuclear fission is that more neutrons are produced than those consumed in the reaction. This process is schematically represented in Figure 4.14.
Mechanism of Nuclear Fission—the Liquid Drop Model
In the fission process, a heavy nucleus splits apart into nearly two equal fragments of more stable nuclei of intermediate mass. This fission reaction that occurs due to absorption of neutron may be treated as analogous to the behaviour of a liquid drop as it contracts, elongates and eventually splits apart into two droplets as shown in Figure 4.15. According to this liquid-drop model, the absorption of neutron by a nucleus causes the nucleus to oscillate like a liquid drop and then split’s it into two small, stable nuclei.

Figure 4.14 A schematic diagram representing the uranium fission process

Figure 4.15 Schematic diagram representing the fission process by the Bohr-Wheeler liquid drop model for heavy nuclei
There are 14 isotopes of uranium, and their mass number ranges from A = 227 to A = 240. The most important isotopes are 235U and 238U whose natural relative abundance is 0.72% and 99.28%, respectively. Both 235U and 238U undergo fission upon absorbing a neutron. 238U undergoes fission with ‘fast’ neutrons while 235U with both ‘fast’ and ‘slow’ neutrons.
Among naturally occurring nuclides, only 235U undergoes fission, but 238U and 232Th are converted into 239Pu and 233U by neutron capture and two successive β-decay. These two converted nuclides then undergo fission. Both 235U and 233U can be excited to fissionable state by slow neutrons more easily than 238U as both are less stable.
The division of 235U occurs in different ways and nearly 34 elements were identified in fission products. Enormous amount of energy is also liberated. In any single reaction, two particular nuclides are produced along with two or three secondary neutrons. These neutrons have about 200 MeV of kinetic energy. The daughter nuclei produced in fission reactions have different Z and A values.
Some of many pathways through which 235U undergoes fission with fast neutrons are mentioned here.

Since emission of more than one neutron occurs in the fission process, there is a possibility of a chain reaction in which the releases of energy increases in geometric fashion. The neutrons released in one fission reaction cause a second fission reaction and so on, and a chain reaction continues, which, if goes unchecked, would quickly lead to the release of enormous amount of energy. A chain reaction is possible only when the amount of fissionable material exceeds its critical mass (Figure 4.16). The critical mass is defined as the amount of fissionable material, which is just large enough to recapture one neutron, on an average, for every fission reaction. If the amount of fissionable material is less than the critical mass, less than one neutron is recaptured; the rate of fission events does not grow, and the rate of energy release is low. In addition, if the amount of fissionable matter is more than the critical mass, the number of fission events increases, and a chain reaction is set up, which quickly grows into explosive proportions.
Release of Fission Energy
On this basis, nuclear fission reactions may be categorised into two types:
- Uncontrolled nuclear fission used for construction of nuclear weapons, the atomic bombs
- Controlled nuclear fission, which is exploited for the controlled generation of energy in nuclear reactors

Figure 4.16 Self-propagating nuclear chain reactions
- Uncontrolled nuclear fission—the atom bomb: In a fission reaction, two or three neutrons are produced. These neutrons can collide with other fissionable atoms to sustain and multiply the fission reactions. If the amount of fissionable material exceeds the critical mass, an uncontrolled explosive chain reaction may result. An enormous amount of energy is produced in the chain reaction. A nuclear bomb is a frightening example of the enormous amount of energy released by nuclear fission, which is not controlled.
The central feature of a fission explosion is a growing chain of fission reactions. For this, there are three requirements as follows:
- The fissionable nuclide must be concentrated enough so that it becomes critical.
- The sub-critical portions of this fissionable nuclide must be combined into a critical mass.
- The critical mass must be held together for a long period so that the chain multiplies to immense size.
The potential of uncontrolled nuclear fission was first realised in the atomic bomb (Figure 4.17). It contains two sub-critical portions of fissionable materials.
One portion is propelled into another to form supercritical mass by carefully designed detonation of an ordinary chemical explosive such as trinitrotoluene (TNT). The fission chain multiplies, and then a nuclear fission explosion occurs. Tremendous amount of heat energy and many other radio nuclides are also released; their effects are disastrous to life and environment. The radioactive dust and debris are called fall-out.
In 1945, the United States started the nuclear age by dropping two nuclear bombs on Hiroshima and Nagasaki, Japan. Both these bombs were fission weapons of tremendous power. The bomb, which was dropped on Hiroshima, contained 235U (Uranium-235) while the Nagasaki bomb had 239Pu (Plutonium-239) as fissionable materials.

Figure 4.17 A simple design of atomic bomb
- Controlled nuclear fission—nuclear reactor: For controlled release of fission energy, the chain reaction is carried out in a device called a nuclear reactor. The fission is controlled in such a manner that on an average, only one neutron is left from each fission; to excite further fission, the large amount of energy released in nuclear fission can be used to generate electrical power. This requires a delicate balance between neutron generation and neutron loss, and this is achieved by proper use of moderator and control rods in the nuclear reactors.
Two types of nuclear reactors are in common use. They are as follows:
- Thermal reactors: Most thermal reactors in the United States are ‘light water reactors’ in which ordinary water is used as moderator to slow the neutrons. However, heavy water reactors had been developed in Canada in which heavy water, D2O is used as moderator in place of ordinary water.
Light water reactor (LWR), which is a type of thermal reactor, uses normal water as its coolant as well as neutron moderator.
There are three varieties of LWRs:
- Pressurised water reactor (PWR)
- Boiling water reactor (BWR)
- Supercritical water reactor (SCWR)
- • Reactor design: The LWR produces heat by controlled nuclear fission. The nuclear reactor core is the major portion of a nuclear reactor where the nuclear reactions take place (Figure 4.18).
It consists essentially of the following parts:
- • Fuel: The fissionable material used in the reactor is called fuel. The fuel used is enriched uranium-235 (in the form of U3O8). This is obtained from the naturally occurring U-235. The solid fuel is made into the form of rods or pellets, shielding by placing them in stainless steel tubes.
- • Moderator: The most efficient fission reactions occur with slow neutrons. Thus, moderators, which are atoms of comparable mass, slow down the fast neutrons produced in fission and do not absorb them. Light water reactor uses ordinary water, also called light water, as its neutron moderator (Figure 4.18).
- • Control rods: To control the fission process, rods made of cadmium or boron are suspended between the fuel rods. These rods can be raised or lowered and control the fission process by absorbing neutrons. Therefore, they are called control rods.
Cadmium and boron are good neutron absorbers.
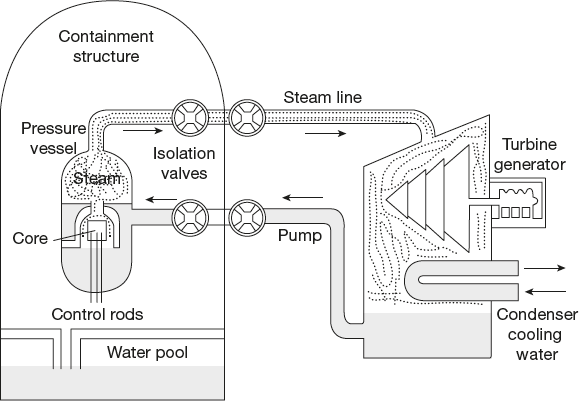
Figure 4.18 Light water nuclear reactor
- • Cooling system: The LWR uses ordinary water to keep the reactor cooled. The light water is circulated in the reactor core to absorb the heat, which is generated due to fission reaction. This transfers the heat to a steam generator, which converts water into steam, and this steam is then taken to turbines, which drive generators to produce electricity.
- • Shielding: To prevent loss of heat and protect the persons operating the reactor from radiation and heat, the entire reactor core is enclosed in a steel containment vessel. This vessel is housed in a thick-walled concrete building. The operating people are protected by a thick layer of organic material made of compressed wood fibres, which absorb the neutrons, β particles and γ-rays.
- (b) Breeder reactors: Considering the rate at which U-235 is being used to produce power, the stocks are likely to exhaust very soon. Therefore, scientists have been actively engaged in investigating other fissionable materials. They have found plutonium-239 (Pu-239) and uranium-233 (U-233) to be quite suitable. These are produced by bombardment of more abundantly available U-238 and Th-232 with neutrons.
A breeder reactor is a nuclear reactor that breeds fuel. It consumes fissile and fertile material at the same time as it creates new fissile material. In this reactor, neutrons produced from fission of U-235 are partly used up for carrying on fission of U-235 and partly to produce Pu-239 or U-233. Such reactors produce more fissionable materials (as Pu-239 or U-233) than they consume (as U-235).
The sequence of reactions that take place when U-238 and Th-232 are bombarded with fast neutron producing the fissionable nuclei of Pu-239 and U-233 are represented as follows:
- Fissionable Pu-239 by neutron bombardment followed by two successive β-decays.
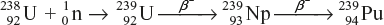
- Fissionable U-233 by neutron bombardment on Th-232 by two successive β-decays.

The neutrons produced by fission reactions are absorbed in a blanket of uranium or thorium. Since higher temperature is required to operate, water cannot be used as a coolant. Liquid sodium is used as a coolant. This type of reactor uses fast neutrons; therefore, no moderator is required.
The nuclides such as U-238 and Th-232, which can be converted into fissionable nuclides, are called fertile nuclides, whereas nuclides such as U-235 and Pu-239, which are fissionable and are called fissile nuclides.
- Fissionable Pu-239 by neutron bombardment followed by two successive β-decays.
- Thermal reactors: Most thermal reactors in the United States are ‘light water reactors’ in which ordinary water is used as moderator to slow the neutrons. However, heavy water reactors had been developed in Canada in which heavy water, D2O is used as moderator in place of ordinary water.
Nuclear Fusion
Nuclear fusion may be defined as a process of combination of two lighter nuclides to form a heavier nuclide with the release of energy.
The following reactions have been successfully investigated as fusion reactions:

These processes are generally known as thermonuclear reactions, because they require that the colliding nuclei must possess very high kinetic energies before they are initiated. The high kinetic energies of the reacting nuclei overcome the coulombic repulsion between positive particles of reactants. This is only possible of extremely high temperature of millions of degrees (≈4 × 106°C), so these processes generally occur in the sun and other stars because of tremendous high temperatures that are nearly impossible to achieve and contain on earth. It is, therefore, believed that in the sun, the following process takes place:
![]()
There is evolution of 26 MeV of energy, and this energy is available to us from the sun and keeps the sun at extremely high temperature.
The fission reactions take place only with a few rare and extremely heavy nuclides. On the other hand, fusion reactions are possible for light nuclides such as 1H, which are abundant. Furthermore, fusion reactions release more energy per unit mass than fission reactions. For example, fission of U-235 yields 7.9 ×107 KJ/g energy while fusion of two isotopes such as hydrogen, deuterium and tritium releases 3.4 ×108 KJ/g energy. Another advantage of fusion reaction is that the fusion reaction produces radio nuclides of very short half lives, so there would be no long-term waste disposal problem.
There are several ways to study fusion reactions as follows:
- By using particle accelerators.
- Through stellar nuclear reactions, the process occurs in the sun and other stars.
- By fusion bombs, which use a fission bomb to produce a temperature high enough for fusion.
Fusion Bombs (Uncontrolled Nuclear Fusion Reactions)
A hydrogen bomb uses nuclear fusion. A conventional explosive first triggers a fission bomb, which then induces the fusion reaction. These bombs are more powerful than fission bombs because they can incorporate large masses of nuclear fuel to produce unlimited energy.
A hydrogen bomb has an arrangement of nuclear fission in the centre, which is surrounded by a mixture of deuterium (![]() ) and lithium 6 isotope (
) and lithium 6 isotope (![]() ).
).
Nuclear fission provides heat and neutrons. The neutrons are used up for converting lithium isotope into tritium (![]() ), and the heat liberated is required for the fusion between
), and the heat liberated is required for the fusion between ![]() and
and ![]() to start.
to start.
The fusion reactions are then accompanied by the liberation of a large amount of energy.
Thus the reactions taking place in a hydrogen bomb may be represented as follows:
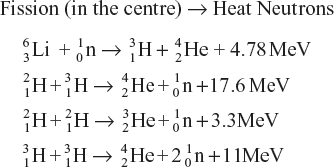
All these processes occur in an extremely short time to release an immense amount of energy, and the bomb blasts.
Controlled Nuclear Fusion
The major problem in a nuclear fusion reaction is the attainment of high temperature required for the purpose. So far, attempts to maintain such a high temperature to generate energy on a large scale have been successful for only a very small fraction of a second, because all known containers would vapourise at such a high temperature. At this temperature, the atoms become fully ionised, and the ions (i.e., nuclei and electrons) form the plasma state in which the particles move about independently but in the form of inter penetrating gases. The plasma is electrically neutral.
Lastly, it can be mentioned that the attempts to make fusion reactors have not met with any success so far because of many technical difficulties involved, for example, that of the container material, which can withstand very high temperatures as required for the fusion to start. However, if a solution is found, it will place at our disposal a tremendous source of energy at a very cheap rate because deuterium is present in huge amount in sea water (in the form of D2O).
The differences between nuclear fission and nuclear fusion are listed in Table 4.1.
Table 4.1 Differences between nuclear fission and nuclear fusion

4.3 REVIEW QUESTIONS
4.3.1 Fill in the Blanks
- In wind, _________ is converted into mechanical energy or electrical energy.
[Ans. kinetic energy]
- _________ is used to produce biofuel.
[Ans. algae]
- Geo thermal resources are _________.
[Ans. hydrothermal, geopressure, hot dry rocks and magma]
- Complete the following reactions:
 _________
_________
[Ans.:
 ]
] - ______is used as a coolant in nuclear reactor.
[Ans.: light water]
- Atom bomb is based on ______.
[Ans.: nuclear fission]
- ______isotope of uranium is used in nuclear reactor.
[Ans.: U235]
- Nuclear reaction is produced large quantities of energy according______ to equation.
[Ans.: Einstein energy mass E = mc2]
- A fission reactor which produces more fissionable material than its consumed in its operation is ______ reactor.
[Ans.: breeder]
- ______changes alter the number of protons and neutrons in the nuclei of atoms consumed while simple chemical changes involve in reorganization of ______ only.
[Ans.: nuclear, electrons]
- Sun’s energy is given by fusion of ______ nuclei.
[Ans.: hydrogen]
- Photovoltaic cell converts directly incident solar radiations to ______.
[Ans.: electric current]
- The major material used in pv cell is ______.
[Ans.: silicon]
4.3.2 Multiple-choice Questions
- Generating mechanical power by using wind is called
- Solar energy
- Tidal energy
- Wind energy
- Biofuels
[Ans.: d]
- Geothermal energy comes from
- Heat from within earth
- Heat from atmosphere
- Heat from organic matter
- None of the above
[Ans.: a]
- Bio-fuel producing pollutes are
- H2 and O2
- Oxides of sulphur and nitrogen
- H2O
- None of the above
[Ans.: b]
- Energy which is not harm to the environment is
- Solar energy
- Wind energy
- Alternative energy
- None
[Ans.: c]
- Among the following, which one belongs to alternative energy?
- Tidal Energy
- Wind energy
- Solar energy
- All of the above
[Ans.: c]
- The source of energy in nuclear fuel is due to
- Chemical reaction
- Nuclear reaction
- Nuclear fission
- Nuclear fusion
[Ans.: c]
- Binding energy of a nucleus is related with
- ΔMc2
- Mc2
- ΔMc
- hv
[Ans.: a]
- Fissionable material used in nuclear reactor is
- U235
- U238
- Th232
- Pu239
[Ans.: a]




- None of these
[Ans.: c]
- Control rods used in nuclear reactor is made of
- Na
- B
- CO2
- U235
[Ans.: b]
- Atom bomb is based on principle of
- Nuclear fusion
- Nuclear fission
- Chemical reaction
- None of these
[Ans.: b]
- Uncontrolled nuclear fusion reaction takes place in
- Atom bomb
- H-bomb
- Nuclear bomb
- Radium bomb
[Ans.: b]
- Solar energy originates from the reaction taking place in the sun
- Nuclear fusion
- Nuclear fission
- Chemical reaction
- Nuclear reaction
[Ans.: a]
- Photovoltaic cells are commonly known as
- Primary cell
- Solar cell
- Secondary cell
- Fuel cell
[Ans.: b]
- Example of indirect solar energy is
- Nuclear energy
- Wind energy
- Chemical energy
- Surface energy
[Ans.: b]
4.3.3 Short Answer Questions
- Give definitions of alternative energy from different sources.
Ans.: Oxford definition: Energy fueled into ways that do not use up natural resources or harm the environment.
Princeton WordNet: Energy derived from sources that do not use up natural resources or harm the nature.
Natural resource defense council: Energy that is not popularly used and is usually environmental sound such as solar or wind energy.
Material and management: Fuel source other than those derived from fossil fuels typically interchangeably used for renewable energy.
For example, wind, solar, biomass, wave and tidal energy.
Torridge District Council: Energy generated from alternatives to fossil fuel; but need not be renewable.
Climate Change 2007: Energy derived from nontraditional sources.
For example, compressed natural gas, solar, hydroelectric and wind energy
- Write a short note on wind energy.
Ans.: The term ‘wind energy’ or ‘wind power’ describes the process by which the kinetic energy of wind is used to generate mechanical power or electricity with turbines.
- Explain geothermal energy.
Ans.: Geothermal energy comes from the heat within the Earth. Geothermal is a Greek word; geo–earth and thermal–heat. Geothermal energy is used to produce electricity, green houses and to heat building for other sources.
- Write a short note on biofuels.
Ans.: Biofuel is any fuel derived from biomass of recently living organisms or their metabolic byproducts, such as manure from cows. Algae fuel is a biofuel which is derived from algae. During photosynthesis, algae and other photosynthetic organisms capture carbon dioxide and sunlight and convert them into oxygen and biomass. This is usually done by placing the algae between two panes of glass. The algae create three forms of energy fuel: they are from its growth cycle heat can generate, the oil derived from the algae used as biofuel, and on maturity algae can give biomass.
4.3.4 Descriptive Questions
Q.1 Write a brief note on geothermal energy.
Q.2 Write a note on wind energy.
Q.3 Explain the importance of biofuels.
Q.4 Explain any three alternative energy resources.
Q.5 Define alternative energy resources and explain water power.
Q.6 Discuss the theoretical principles involved in the generation of power by nuclear fission and nuclear fusion.
Q.7 Describe the various components of a nuclear power reactor and their functions.
Q.8 Discuss the environmental aspects of nuclear power generation.
Q.9 Write informative notes on the following:
- Breeder reactors
- Energy from nuclear fusion.
Q.10 Write notes on nuclear fission and fusion.
Q.11 (a) Explain how fission grade U235 is obtained.
(b) Write short note on nuclear binding energy.
Q.12 What are the functions of following in a nuclear reactor?
- 235U
- Cadmium rods
Q.13 Define nuclear reaction.
Q.14 Calculate the binding energy in Mev of ![]() , if its experimentally determined mass is 4.00390 amu. The masses of a proton, an electron and a neutron are respectively 1.007825, 0.0005852 and 1.008668 amu.
, if its experimentally determined mass is 4.00390 amu. The masses of a proton, an electron and a neutron are respectively 1.007825, 0.0005852 and 1.008668 amu.
Q.15 Explain how binding energy is useful for the stability of a nucleus.
Q.16 Write the difference between nuclear fusion and nuclear fission.
Q.17 Write the principle involved in atomic bomb and its reactions.
Q.18 Write the reaction taking place in sun and stars.
