6
SCIENCE OF CORROSION
6.1 INTRODUCTION
Corrosion can be defined as the slow degradation or deterioration of a metallic material from the metallic surface due to unwanted attack by the atmosphere gases, soil, chemical, or electrochemical reaction with its environment (gaseous or liquid medium).
Degradation or deterioration means reduction in the useful properties of the material which includes:
- Decaying of surfaces of metals
- Weakening of the material due to loss of cross sectional area.
- Loss of properties such as malleability, ductility.
- Cracking of polymer due to sunlight.
6.1.1 Causes of Corrosion
It has been found that most metals (exceptions noble metals like Au, Pt, etc.) exist in nature is combined forms as their oxides, carbonates, sulphates, sulphides, chlorides etc. Metals are extracted from their ores by using different extraction processes. Energy is required for the extraction of metals. So, consequently pure metals have higher energy than combined form which has lower energy. For this, metals easily undergo interaction with their environment either chemically or electrochemically to form a Stable compound by the process of corrosion.
Corrosion is an oxidation process in which metallic compound having lower energy is formed and energy liberates.

Examples:
- Rusting of iron:
When Iron exposed to the atmospheric conditions, a layer of reddish scale and powder of Fe3O4 is formed on the surface.
- Formation of green film on the surface of copper:
A green layer of basic carbonate consisting of [CuCO3 + Cu (OH)2] is formed on the surface of copper when exposed to moist air.
- Tarnishing of silver:
When silver is exposed to the atmosphere, a black coating of air is formed.
6.1.2 Types of Corrosion
Various types of corrosion processes along with their respective mechanism are given below:

Dry or Chemical Corrosion
In such a type, corrosion occurs due to direct chemical action of atmospheric gases such as oxygen, halogens, sulphur dioxide and hydrogen sulphide with metals resulting into the formation of compounds such as oxides, halides, sulphates and sulphides is known as chemical corrosion. The products which are formed are insoluble, soluble or liquid in nature.
Dry Corrosion is of types
- Oxidation corrosion (Corrosion by oxygen)
- Corrosion by other gases
- Liquid metal corrosion
- Oxidation corrosion:
Oxidation corrosion is due to the direct chemical attack of oxygen on the metal in the absence of moisture at low or high temperature leading to the oxidation of metal.
Alkali metals (Li, Na, K, etc) and alkaline earth metals (Be, Ca, Sr, etc) are oxidized at low temperatures, whereas all other metals (except Ag, Au, and Pt) are oxidized at high temperatures.
Mechanism
When a metal is exposed to air, absorption of oxygen takes place even at ordinary temperatures. This absorption is purely physical in nature and is due to vander Waal’s forces. However, due to climatic changes, the absorbed oxygen may gradually enter into chemical combination with the metal by electron transfer between the metal atoms and oxygen as shown below:

The metal oxide scale is formed at the metal surface. This scale acts as a barrier and tends to prevent the underlying metal atoms to come in contact with oxygen. The continuation of the oxidation process depends upon two factors.
- The nature of the oxide film formed
- The rate of diffusion of the metal ion and oxide ion through the layer formed.
Nature of the Oxide Film Formed on the Surface
When a metal is placed in atmosphere a thin layer of oxide film is formed at the surface of the metal which can be written as

This metal oxide layer can be
- Stable: When the oxide film is stable, impervious and highly adhering, such kind of layer forms a shield for metal surface. The layer consists of fine grain particles which tightly sticks to the metal surface and does not allow oxygen to diffuse into the metal surface and thus prevents metal from corrosion e.g. Al, Pb, Cu, Sn etc.
- Unstable: When the oxide film is unstable and has tendency to decompose back to metal and oxygen, it does not undergo in oxidation corrosion e.g. Au, Ag, Pt, etc.

- Porous: When the oxide layer having pores or cracks. In such a case, diffusion of cations (Mn+) and anions (O2-) take place smoothly then oxidation corrosion takes place continuously, till the entire metal is completely converted into its oxide.

The porous nature of oxide film may be explained by pilling-Bedworth rule.
Pilling Bedworth Rule
This rule describes the protective and non-protective nature of the oxide layer which is formed during corrosion.
According to this rule, the specific volume ratio is calculated as follow:

- If the specific volume ratio is smaller, the oxidation corrosion will take place because the oxide films will be sufficiently porous for diffusion of Mn+ and O2−.
- If volume of metal oxide ≥ volume of parent metal, then it will be non-porous or protective.
- If volume of metal oxide < volume of parent metal, then it will be porous or non-protective.
Examples: Alkali and alkaline earth metals (like Li, Na, K, Mg) form oxides of volume less than the volume of metal. So, oxide layer faces stress and strains, which result in development of cracks and pores in its structure. So, further corrosion continues till the whole metal is destroyed.
But in case of metal like Al forms oxide, whose volume is greater than volume of metal. So, non-porous, tightly adhering layer is formed, so rate of oxidation rapidly decreases to zero.
Rate of Diffusion of Metal Ion and Oxide Ion Through the Layer Formed
Metal and oxygen combine to form metal oxide which forms a thin film whose thickness is less than 300A°, and it’s called as scale, if its thickness exceeds this value.
This film or scale prevents further oxidation. But for oxidation to continue either the metal ion must diffuse outwards through the scale to the surface or oxygen ion must diffuse inwards through the scale to the underlying metal. Both transfers occur, but outward diffusion of metal ion is much easier because metal ions are smaller than oxide ion and of higher mobility as shown in Figure 6.1.

Figure 6.1 Oxidation mechanism of metals
- Corrosion by other gases:
Corrosion also occurs by other gases like SO2, Cl2, CO2, H2S, F2 etc. This depends upon the affinity of metals with these metals. The degree of attack depends on the formation of protective (non-porous) or non-protective (porous) film on the surface.
The extent of corrosion depends upon the following:
- Nature of the environment: The environment plays very important role in corrosion because it facilitates the affinity between metal and gases.
- Chemical affinity between metal and gas: If the affinity between metal and gas is more, then corrosion will be more and more
- Nature of the film formed on the metal surface:
- If the film formed is protective or non-porous, then the intensity of attack decreases

- If the formed is non–protective or porous, metal is destroyed rapidly

It evaporates and metal surface is exposed for corrosion.
- If the film formed is protective or non-porous, then the intensity of attack decreases
- Liquid metal corrosion:
This type of corrosion happens when liquid metal flows over solid metal or alloy at high temperature and solid metal or alloy usually gets weakened. This type of corrosion mainly occurs in nuclear powers devices.
There are two possibilities of liquid metal corrosion:
- Either the liquid dissolves the solid metal surface.
- Liquid penetrates into the solid surface and thus weakens the bond.
Wet or Electrochemical Corrosion
It is also known as immersed corrosion. It is more common than dry corrosion. It occurs mostly under wet or moist conditions through the formation of electrochemical cells, and is therefore, referred to as electrochemical corrosion.
Wet corrosion can be easily explained by electrochemical theory.
Electrochemical Theory of Corrosion
All metals have tendency to pass into solution. The tendency of metal to pass into solution when immersed in a solution of its salt is measured in terms of electrode potential. If a metal having lower reduction potential (higher electropositive) comes into contact with another metal having a higher electrode potential (higher electro negative) a galvanic cell is set up. The metal having lower electrode potential becomes anodic and get dissolved as corresponding metallic ions with the liberation of free electrons.
![]()
The metal with high electrode potential acts as cathode and gets protected during the process.
Mechanism of Electrochemical Corrosion
- The existence of separate cathodic and anodic areas between which current flows through the conducting solution.
- Oxidation (loss of electrons) takes place at the anodic area and the metal is destroyed by either dissolution or combination with oxygen. Hence, corrosion always takes place at the anode.

- Reduction (gain of electrons) takes place at the cathode. The electrons from the anode are accepted by the dissolved oxygen forming ions such as OH- or O2− ions.

- The metallic ions (at anodic area) and non-metallic ions (at cathodic area) diffuse towards each other through conducting medium and form a corrosion product somewhere between anode and cathode.
Depending on the nature of corrosive environment, the mechanism of electrochemical corrosion may be explained in terms of
- Evolving of hydrogen
- Absorption of oxygen
- Evolution of hydrogen:
The process of corrosion in which H2 is liberated is called evolution of hydrogen type corrosion. This mechanism of corrosion follows usually in acidic environment. Thus, in acidic medium (absence of oxygen) hydrogen ion acquire electrons with the liberation of H2 gas in cathodic reaction and the anode is the metal which undergo oxidation and looses electrons to the environment and pass into solution. This process is shown in Figure 6.2.
For example:
- If iron metal is used, the dissolution of iron as Fe2+

- These electrons flow through the metal from anode to cathode, Where H+ ions of acidic solution accept these electrons and get reduced in the form of H2 gas.

Overall Reaction

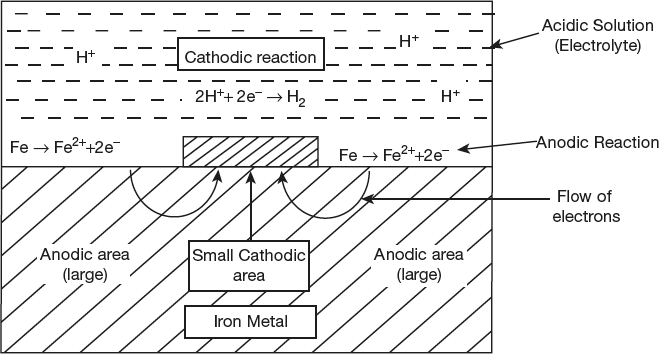
Figure 6.2 Mechanism of wet corrosion by hydrogen evolution
It is important to note that in hydrogen evolution type of corrosion, anodic areas are very large in comparison to cathodic areas. All the metals, above hydrogen in electrochemical series have a tendency to get dissolved in acidic solution with liberation of hydrogen.
- If iron metal is used, the dissolution of iron as Fe2+
- Absorption of oxygen:
This type of corrosion occurs in basic or neutral environment (such as NaCl solution used as electrolyte). The common example is corrosion of iron occurs by oxygen in the presence of aqueous solution of NaCl in the presence of oxygen. This process is shown in Figure 6.3.

Figure 6.3 Mechanism of wet corrosion of absorption of oxygen
- At anode, iron dissolves to form ions as
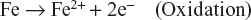
- At cathode, the electrons evolved by above reaction are accepted by oxygen in presence of water.

- The Fe2+ ions (at anode) and OH- (at cathode) diffuse and when they combine Fe(OH)2 is precipitate.

- In the presence of sufficient oxygen, Fe(OH)2 can be easily oxidized into ferric hydroxide [Fe(OH)3]

- If the supply of oxygen is limited then black anhydrous magnetite i.e. ferrousoferic oxide is formed as

- At anode, iron dissolves to form ions as

Types of Electrochemical Corrosion
Such type of corrosion takes place in following conditions:
- When two dissimilar metals or alloys are in contact with each other in the presence of a conducting medium (aqueous solution, moisture etc.)
- Separate anodic and cathodic areas between which the current flows the conducting medium.
- Oxidation takes place at anode and reduction takes place at cathode e.g. rusting of iron.
- Galvanic corrosion or bimetallic corrosion
The galvanic cell is formed if two different metals (e.g. zinc and copper) are electrically connected and exposed to an electrolyte. As a result, the less noble metal (i.e. the metal having a lower value of standard reduction potential or placed higher in the electrochemical series) gets corroded. This type of corrosion is called as galvanic corrosion e.g.: Zn-Cu, Zn-Ag, Fe-Cu etc.
In Zn-Cu galvanic cell, Zinc (E° = –0.76 V) with lower reduction potential than copper (E° = + 0.34 V) acts as anode and the electrons flow from anodic metal (Zn) to cathodic metal (Cu). The anodic metal is corroded, while cathodic metal remains protected. This process is shown in Figure 6.4.

Figure 6.4 Galvanic corrosion (The less noble metal zinc acts as anode and undergoes corrosion, whereas the most noble metal copper remains protected.)
- In acidic solution, the corrosion occurs by evolution of hydrogen

- In Neutral or slightly alkaline medium, the corrosion occurs by absorption of oxygen

Examples:
- Steel screw’s in a brass marine hardware
- A steel propeller shaft in bronz bearing.
- Steel pipe connected to copper plumbing.
- Galvanic corrosion or bimetallic corrosion
Control
Since Galvanic depends upon the following factors:
- Greater the potential difference between two metals, greater is the corrosion.
- Suitable medium for corrosion
- Surface area of the metal
Hence corrosion may be controlled by the following factors:
- Avoiding the suitable medium for corrosion.
- Minimizing the potential difference of metals i.e. avoiding the galvanic couples.
- By polishing the metals.
Concentration Cell Corrosion (Differential Aeration Corrosion)
When a metal is exposed to an electrolyte of varying concentrations or to varying aeration, it undergoes an electrochemical attack due to formation of miniature concentration cells on its surface and gets corroded.
Differential aeration corrosion is the most common type of concentration cell corrosion. This type of cell is formed when the metal is kept in different air concentration i.e. two ends of metal surface are at different concentration of air. The part of metal which is poorly oxygenated acts as anode and other part of the metal which is highly oxygenated acts as cathode. This develops electrode potential and hence the, metal gets corroded.
Let us consider the case of zinc (Zn) rod which is immersed in NaCl solution. A potential difference is developed between differently aerated areas. The part of the rod which is at greater depth acts as anode (less oxygenated) and that is which is above the surface acts as cathode (more oxygenated) and zinc corrodes due to electrode potential. This process is shown in Figure 6.5.
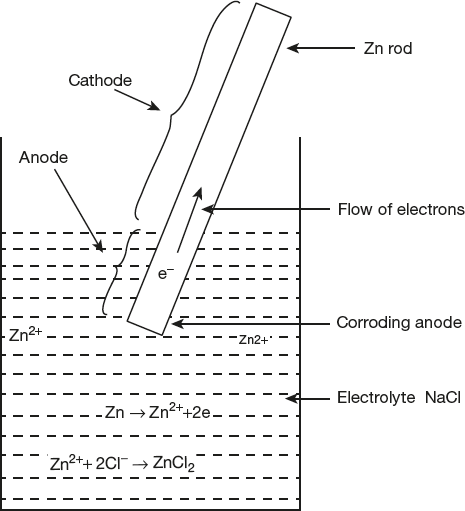
Figure 6.5 Differential aeration corrosion
At anode: less oxygenated part
![]()
At cathode; more oxygenated part
![]()
Overall Reaction
![]()
In a similar way, iron metal corrodes under drop of water (or salt solution). Areas covered by droplets, having no access of oxygen, it become anodic with respect to the other areas, which are freely exposed to air become cathodic. This process is shown in Figure 6.6.
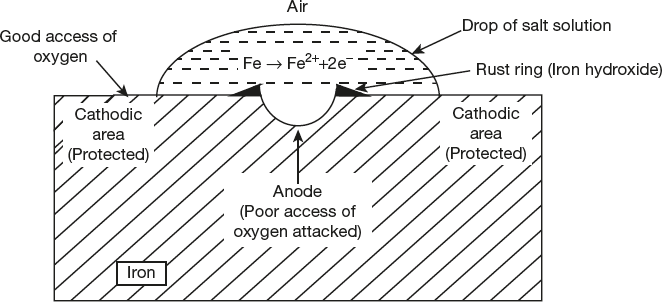
Figure 6.6 Differential aeration corrosion
At anode: Fe → Fe2+ + 2e- (Oxidation)
At cathode: ![]()
Overall reaction 
Important Characteristic about Differential Aeration Corrosion
- The metal having low oxygen concentration part act as anode and metal having high oxygen concentration act as cathode.
- Corrosion may be accelerated in apparently in accessible places, because of deficiency of oxygen at some part.
- This type of corrosion also accelerated under accumulation of dirt, sand, scale or other contamination, because such covered part act as anode due to difference in air concentration.
- It is a localized attack on some oxygen deficient areas such as metal exposed to aqueous media corrode under blocks of wood or pieces of glass, which screen that portion of metal from oxygen access, resulting into localized pitting.
Water-line Corrosion
It is the type of differential aeration corrosion, which occurs when a metal is partly immessed in water. The corrosion takes place just below the waterline and hence it is known as waterline corrosion.
It is an observed fact that when water is kept stagnant in a steel tank for a long time, corrosion takes place just below the water level, it is due to the concentration of dissolved oxygen at the water surface is greater than that under surface.It forms an oxygen concentration cell. The area above the waterline (highly oxygenated) acts as cathodic and corrosion takes place along a line just beneath the level of water meniscus (anodic area) as shown in Figure 6.7.

Figure 6.7 Water-line corrosion
Corrosion takes place at anodic part

This type of corrosion is accelerated when water is acidic in nature and presence of salts like chlorides, bromides, etc. When marine plants attach themselves to side of the ships, this type of corrosion is increased because of presence of different salts.
Prevention
- Water-line corrosion is reduced when the water is free from acidic impurities.
- Usage of special anti foaming paints minimizes such type of corrosion to some extent.
- By using anodic inhibitors like phosphates, carbonates, silicates, water-line corrosion can be retarded.
This type of corrosion is accelerated when water is acidic in nature and presence of salts like chlorides, bromides, etc. When marine plants attach themselves to side of the ships, this type of corrosion is increased because of presence of different salts. The use of special antifouling paints minimizes such type of corrosion to some extent.
Pitting Corrosion
Pitting corrosion is a non-uniform corrosion which is caused by localized accelerated attack on metal surface and forms pits, cavities and pin holes in the metal.
A pit is formed when the protective coating on the metal surface breaks at specific points. Once the pit is formed the process of corrosion becomes fast due to differential amount of oxygen in contact with metal surface. The portion with higher concentration of oxygen become cathode and that with lower oxygen concentration becomes anode. This causes corrosion of metal. This process is shown in Figure 6.8.
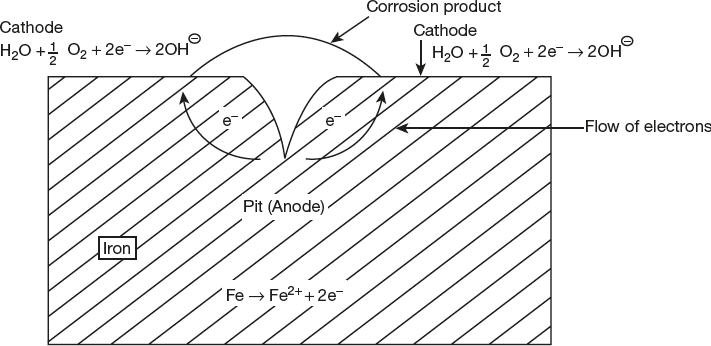
Figure 6.8 Pitting corrosion

Pitting corrosion may be caused by
- Surface roughness
- Scratches on metal surface
- Local strains of metal due to non-uniform stress
- Presence of extrageneous impurities (like sand, dust or scale)
- Presence of drop of salt solution
- Non-uniform polishing of metal etc.
Pitting corrosion may be prevented by
- Proper designing of metal
- Proper polishing of metal
- Use of pure metal
Stress Corrosion
Stress corrosion or stress cracking is the type of corrosion which occurs due to combined effect of tensile stresses and the corrosive environment on metal when metal is exposed to corrosive environment.
Pure metal generally does not undergo stress corrosion whereas fabricated metal components or an article of certain alloys like zinc and nickel brasses undergoes such types of corrosion.
Favorable Conditions for Stress Corrosion
- Tensile stress: The stress developed on metal surface may be internal or external. Internal stress is caused by manufacturing process (quenching, bending, annealing, etc.) or fabrication process or heat treatment process. In all such cases, metal under stress becomes more anodic and that area undergoes corrosion.
- Corrosive environment: The specific and selective environment play very important role in stress corrosion.
For example
- Mild steel undergoes stress corrosion in the presence of caustic alkalies and strong nitrate solution.
- Stainless steel in the presence of acid chloride solution.
- Brass in the presence of traces of ammonia.
Mechanism
Stress corrosion is localized electrochemical phenomenon. As we know that, the point or area under stress as well as grain boundaries act as electrochemical cell which occurs generally due to internal stresses due to metallurgical operations such as bending, pressing, rolling, quenching, annealing, etc. Due to presence of stress forms anodic areas in localized zones with respect to more cathodic areas at the metal surface. Such areas under stress act as anode and they become so chemically active that they are attacked, even by a mild corrosive environment, which result in the formation of cracks which propagate rapidly resulting in an unexpected failure of the metal surface. This process is shown in Figure 6.9.

Figure 6.9 Stress corrosion
In every type of corrosion there is formation of galvanic cells and corrosion takes place at the anodic part.

Stress corrosion takes place even in mild corrosive environment on the stressed metal part.
Types of Stress Corrosion
- Season cracking: This type of cracking is generally refers to the corrosion of copper alloys, particularly brass. Brasses are binary alloys of Cu and Zn which are electrochemically reactive in an environment of ammonia. Therefore, when brass is exposed in ammonical medium, both copper and zinc form complexes by losing electrons in ammonical solution. As a result, dissolution of brass occurs and forms cracks for stress corrosion.

This reaction is generally referred to a season cracking.
- Caustic Embrittlement: This type of corrosion generally occurs in mild steel, which undergoes stress corrosion in caustic alkalies at high temperature and pressure. It is very dangerous form of stress corrosion, generally occurs in steam-boilers and heat-transfer equipments in which water of high alkalinity attack the mild steel plants, particularly crevices near rivets, bends, joints etc.
The causes and methods of prevention of caustic embrittlement:
For water-softening purpose of Boiler-water, we generally added a certain proportion of sodium carbonate into it. In high pressure boilers, this breaks up to give sodium hydroxide and carbon dioxide.

This makes boiler-water alkaline in nature. This dilute alkaline boiler-water flows into the minute cracks and crevices by capillary action, where water evaporates and caustic soda concentration builds up. The area where metal is stressed and concentration of alkali is much higher than that in the body of the boiler, alkali dissolve metal as sodium ferrate in crevices, cracks etc. sodium ferrate is decomposes according to either of the following reactions:

Sodium hydroxide (NaOH) is regenerated and magnetite (Fe3O4) is precipitated, thereby enhancing further dissolution of iron.
Caustic embrittlement can be explained by considering the following electrochemical cell:

The iron surrounded by dilute NaOH is the cathodic area; while iron surrounded by concentrated NaOH (e.g. crevices, hair-cracks, rivets etc.) is the anodic area and undergoing corrosion and is thus dissolved the iron metal from that areas.
Prevention of Caustic Embrittlement
- Use of sodium sulphate in boiler-water.
- Use of tannin or lignin as additive boiler-water.
Both these methods prevent caustic cracking by blocking up the cracks and crevices with innocuous harmless substances, thereby preventing the sodium hydroxide from infiltrating into these areas.
- Corrosion fatigue: This type as corrosion cracking occurs due to repeated stresses caused by shaking, tapping, vibration etc. in the presence of corrosive environment. The repeated stress make same metal less elastic which on turn act as anode with respect to other part of metal. The corrosion take place in these region and cracks occurs. This type of corrosion occurs mostly in alloy steel.
6.2 GALVANIC SERIES
Electrochemical series is very helpful to understand the extent of corrosion on the basis of standard reduction potential. According to this series a metal placed at top in the series is more anodic and undergoes corrosion rapidly than the metal below in the series.
The rate and severity of corrosion depends upon the difference in their positions, greater is the difference, and the faster is the corrosion of metal. For example, Li corrodes faster than Mg; Zn corrodes faster than Fe, and so on.
However, some exceptions to this generalisation have been noticed. For example, position of titanium (Ti) is higher than silver (Ag) but Ti is less reactive towards corrosion. Similarly, aluminium (Al) is above zinc (Zn) but zinc corrodes faster. This is only due to formation of strongly adhering oxide layers on their surfaces, thereby making their effective electrode potential more positive (or less negative). Hence a new series came into exist which is based on relative oxidation potential in sea water. This series is known as galvanic series. Galvanic series is shown in Table 6.1.
Table 6.1 Galvanic series

According to this series the metal or alloys higher up the position in the series is more anodic and undergoes corrosion very rapidly. For example, the position of Zn is higher than Al; hence Zn undergoes corrosion rapidly not aluminium Al.
6.2.1 Factors Affecting Corrosion
The rate and extent of corrosion of a metal depends upon the following factors:
- Nature of the metal and
- Nature of the environment.
- Nature of the metal:
The various factors such as its purity, position in galvanic series, physical state, overvoltage etc. which decide the nature of a metal from the view point of corrosion are as follows.
- Purity of the metal: The presences of impurities in a metal accelerate its corrosion. This is because impurities form minute electrochemical cells with the metal under suitable environmental conditions, and the anodic parts get corroded.
For example: Zinc metal containing impurities (such as Pb or Fe) undergoes corrosion of zinc, due to formation of local electrochemical cells.
- Position in galvanic series: The extent of corrosion depends upon the position of metal in galvanic series. The metal or alloy which is placed at higher up in the series are more reactive and has greater tendency to undergo corrosion. The rate and severity of corrosion, depends upon the difference in their positions, and greater is the difference, the faster is the corrosion of the anodic metal alloy.
- Over Voltage: The dissolution of metals in acids may also be considered as a corrosion reaction. Metals like Zn, Cd, Sn and Pb dissolve rather slowly in acids when they are pure. However, these metals dissolve rapidly when they contain impurities which are relatively more noble and also have a low overvoltage.
The difference between the potential of the electrode (voltage) when gas evolution is actually required and expected theoretical value for the same evolution is called over voltage.
For example, the presence of copper in small amounts as an impurity in zinc increases the rate of dissolution of zinc by anodic oxidation. This may be explained on the basis of the hydrogen voltage of the two metals. Pure zinc with higher hydrogen over voltage of 0.70V dissolves slowly and hydrogen evolution is also slow. Copper with low hydrogen over voltage of 0.25V also dissolves but redeposit, on the zinc surface and functions as an efficient cathode rendering the zinc anodic. Since the hydrogen over voltage is lower at the copper cathode, the rate of hydrogen evolution increases, since this cathodic reaction is favoured, the anode reaction, namely, the oxidation (corrosion) of zinc is also favoured.
- Physical state of the metal: The rate of corrosion is influenced by physical state of the metal such as grain size, orientation of crystals, stress etc. The smaller the grain size of the metal or alloy, the greater will be its solubility in corroding medium and hence greater will be its corrosion. Moreover, areas under stress, even in a pure metal, tend to be anodic and corrosion takes place at these areas.
- Relative areas of the anode and cathode:
Rate of corrosion is less when cathodic area is less and anodic area is more.

It is clear that, due to small cathodic part, the demand for electrons will be less and this results in less dissolution of metal at the anodic part and rate of corrosion is also less.
- Reactivity of metals: Some metals are passive in nature, Good examples are Al, Ti, Mg, Ni, Co, etc. These metals react with oxygen to form non-porous oxide layer that protects the material from further corrosion. This oxide layer on metal is also self-healing in nature, i.e., heals itself if scratched on metal surface.
- Nature of oxide film: In aerated atmosphere, particularly all metals get covered with a thin surface film of metal oxide having a thickness of few Angstromes. Whether the metal oxide layer is protective or non-protective is decided by pilling-Bedworth rule. This rule decides the rate of corrosion in a metal.
Greater the specific volume ratio, lesser is the oxidation corrosion rate eg., the specific volume ratio of Ni, Cr and W are 1.6,2.0 and 3.6 respectively, which indicates that the rate of oxidation at elevated temperature is least for Tungsten(W).
- Solubility of the corrosion product: In electrochemical corrosion, the solubility of the corrosion products in the corroding medium is an important factor in deciding the extent and the rate of corrosion. If the corrosion product is soluble in the corroding medium, corrosion of metal will take place at a higher rate, But if the corrosion product is insoluble in the corroding medium (e.g. PbSO4 in case of Pb in a medium of H2SO4) it forms a protective layer on the metal surface and inhibits further corrosion of the metal.
- Volatility of the corrosion product: If corrosive product is volatile in nature, they volatile as soon as they are formed. Hence, the underlying metal surface is exposed for further attack, resulting rapid and continuous corrosion.
- Purity of the metal: The presences of impurities in a metal accelerate its corrosion. This is because impurities form minute electrochemical cells with the metal under suitable environmental conditions, and the anodic parts get corroded.
- Nature of the environment:
- Effect of the temperature: The extent and rate of corrosion usually increases with rise in temperature. This is because an increase in temperature increases the rate of a chemical reaction as well as the rate of diffusion and decreases polarisation.
- Effect of pH: It has been observed that the corrosion takes place more in acidic media (PH < 7) than neutral or alkaline media (PH ≥ 7). Thus, corrosion of metals can be reduced by increasing the PH of the environment contrary to it; amphoteric metals like Al, Zn and Pb are more corroded in alkaline media because they form complex ions in alkaline media and pass into solution.
- Effect of moisture: Moisture or humidity of air is an excellent medium of corrosion. Moisture present in the atmosphere acts as a solvent for oxygen, other gases (O2, SO2 etc.) and salts and forms electrochemical cell. Hence, presence of moisture accelerates the rate of corrosion of a metal. For example, rusting of iron is quite slow in dry air but increases rapidly when the humidity of air is 60–80%.
Critical humidity is the humidity of the air above which the rate of atmospheric corrosion of metal increases sharply and depends on the nature of the metal and the nature of the corrosion products.
- Effect of corrosive gases present in air: The gases like CO2, SO2, H2S etc. present in the atmosphere or fumes of HCl, HNO3, H2SO4 etc. forms the medium more acidic above the metal surface because these gases are soluble in water to form acids and make it more conducting. This increases the rate of corrosion due to an increase in the corrosion current flowing in the miniature electrochemical cells on the metal surface.
- Effect of corroding medium: Corroding medium plays an important role in deciding the rate of corrosion. Rate of corrosion is increased in the conductive corroding medium. For example, the conductance of clay and mineralised soil is much higher than those of dry sandy soils.
- Effect of concentration of oxygen: Differential aeration concentration cell is setup due to change in the concentration of oxygen. Rate of corrosion increases with increase in concentration of oxygen. The region where oxygen concentration is lesser becomes anodic and oxygen concentration rich portion becomes cathodic. The anodic portion suffers corrosion. Rate of corrosion increases due to formation of differential aeration cell.
- Effect of suspended particles in atmosphere: Two types of suspended particles are present in atmosphere viz., chemically active and chemically inactive. The chemically active suspended particles like NaCl. (NH4)2 SO4 absorb moisture and thus act as strong electrolytes thereby enhance corrosion rate. Whereas chemically inactive suspended particles like charcoal, absorb both moisture as well as sulphur gases and thus slowly enhances corrosion rate.
- Effect of the nature of the presence of electrolyte: Electrolyte presence in the medium is also responsible for deciding rate of corrosion. For example, chloride ions (Cl−) present in the medium increase the rate of corrosion by destroying the passive film on metal surface; on the other hand, silicate (
 ) forms an insoluble layer which prevents corrosion of metal.
) forms an insoluble layer which prevents corrosion of metal.
6.3 PROTECTION FROM CORROSION (PREVENTIVE MEASURES FOR CORROSION CONTROL)
Protection against corrosion means not allowing corrosion reactions to take place. Noble metals do not corrode but they cannot be used for common purposes, because of their high cost. We have to use other metals or alloys in the fabrication of many kinds of machinery and equipments and adopt measures to protect these from corrosion.
- Material selection:
- The chosen metal should be as pure as possible because the presence of impurities enhances the rate of corrosion.
- The choice of noble metals are preferable because they are highly resistant to corrosion.
- Avoid the contact of dissimilar metals in the presence of a corroding environment.
- If two dissimilar metals in contact have to be used, they should be as close as possible to each other in the electrochemical series.
- Proper designing:
- When anodic and cathodic materials are used together, then the area of anodic material should be large.
- The anodic part should not be painted or coated because any damage in coating would cause rapid localized corrosion.
- Whenever the direct joining of dissimilar metals, is unavoidable, an insulting fitting may be applied in-between them to avoid direct metal-metal electrical contact.
- Angles, corners, edges etc. should be avoided in construction.For this reason L, T and U shaped structures should be avoided as far as possible some better shapes of L, T and U structure are given below:

- The material should not have sharp corners and recesses because they help in accumulation of impurities. It should be avoided by proper designing as show in figure.

- Always prevent the occurrence of in homogeneities in metal and in the corrosive environment. Thus a proper design should avoid the presence of crevices between adjacent parts of the structure, even in case of the same metal, since crevices permit concentration differences. Hence bolts and rivets should be replaced by a butt-weld as shown in figure.

- Whenever possible, the equipment should be supported on legs to allow free circulation of air and prevent the formation of stagnant pools or damp areas.

- Uniform flow of corrosion liquid is desirable, since both stagnant areas and highly turbulent flow and high velocities can cause accelerated corrosion.
- Cathodic protection (Electrical protection):
The principle involved in this method is to force the metal to be protected to behave like a cathode, thereby corrosion does not occur. Cathodic protection is carried out by two methods:
- Sacrificial anodic protection (Galvanic protection)
In this method, the metallic structure (to be protected) is connected by a wire to a more anodic metal, so that corrosion occurs at that anodic metal and metallic structure is protected. This method is generally used for the protection of underground pipes and tanks.
In this method, the more active metal like Mg is used as anode and this metal used is called as “sacrificial anode”. A block piece or plate of a more reactive metal (Zn or Mg) is buried beside the iron pipe and connected to it by a wire as shown in Figure 6.10.

Figure 6.10 Sacrifice anodic protection
Since more reactive metal (e.g., Mg) has a greater tendency to get oxidised, it undergoes oxidation in preference to iron. Thus more active metal acts as anode.
At anode:

The electrons thus released migrate to the iron object which starts acting as cathode. At cathode released electrons reduce O2 into OH− as:
At cathode:

Thus cathode (iron etc.) gets protected. Since the reactive metals (Mg, Zn etc.) scarify itself during the protection of other metal. The corroded sacrificial metal block is replaced by a fresh one, when consumed completely. Hence it is termed as sacrificial anode protection.
- Impressed current cathodic protection
In this method, an impressed current from an external source is applied in the opposite direction to neutralize the corrosion current. This is done to convert corroding metal from anode to cathode. Once the metal becomes cathodic, it is protected from corrosion. Usually, the impressed current is derived from a DC source (like battery or rectifier on a.c. line) in which negative terminal of a DC source is connected with the object to be protected is made the cathode of an electrolytic cell and positive terminal of the DC source is connected to scrap iron, platinum, graphite, nickel or lead anode (insoluble anode) and buried or immersed in a conducting medium adjacent to the metal to be protected. The anode is, usually, taken in a backfill (composed of coke, breeze or gypsum) so as to increase the electrical contact with the surrounding soil. This type of cathodic protection has been applied to protect buried structures, pipes, water-tanks etc. This process is shown in Figure 6.11.
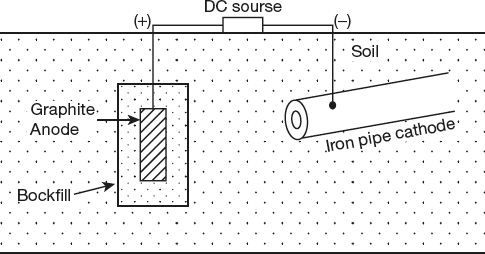
Figure 6.11 Impressed current cathodic protection
- Sacrificial anodic protection (Galvanic protection)
- Surface coatings: Protecting the surface of an object by the application of coating by different methods. A brief description of two important protective coatings is given below.
- Anodic coatings:
In this process, the base metal (i.e. which is to be protected) is coated with more active metal (i.e. having lower electrode potential) such as Zn, Al and Cd coating on steel surface. If any pores, breaks or discontinuities occur in such an anodic coating, a galvanic cell is formed between the coating metal and the exposed part of the base metal, i.e. steel object. For example, in case of galvanized steel, zinc, the coating metal is attacked, leaving the underlying cathodic metal unattacked. Zinc act as anode with respect to iron, which act as cathode zinc dissolves anodically and iron metal is protected. Zinc has first corroded in the vicinity of the exposed iron spot. So, zinc coating protect iron “sacrificially”.
Due to oxidation, zinc layer may be converted to basic zinc carbonate, ZnCO3. Zn (OH)2 by the action of oxygen, CO2 and moisture. This layer protects the exposed part further. This process is shown in Figure 6.12.

Figure 6.12 Anodic coating i.e. galvanized steel
- Cathodic coatings:
In this process, base metal is coated with a more noble metal (i.e. having higher electrode potential). For example, coating of tin on iron, coating of copper on iron because both Sn, Cu having higher electrode potential than iron. This type of coating provides effective protection to the base metal only when the base metal is completely continuous and free from pores, cracks or discontinuities. If the coating develops scratches or cracks, iron is not protected any more; the tin becomes the cathode, while the exposed iron acts as anode. This is because the standard reduction potential of iron is less than that of tin.

- Anodic coatings:
A galvanic cell is set up and an intense localized attack at the small exposed part i.e. iron metal occurs, which results into severe pitting and perforation of the base metal. In such a case the rusting is much more rapid as compared to that in case of an unprotected iron piece. This process is shown in Figure 6.13.

Figure 6.13 Cathodic coating i.e. Tin – plated steel
Method of Application of Metal Coatings
- Hot dipping:
In this process, metal or metal alloys such as iron, copper or steel having a high melting point is coated with a low melting metals such as tin, zinc, lead or aluminum is known as hot dipping. This process involves dipping or immersing the base metal article in a molten bath of the coating metal and covered by a molten flux layer (usually ZnCl2). The flux cleans the base metal surface and prevents the oxidation of the molten coating-metal.For good adhesion; the base metal surface must be very clean; otherwise it cannot be properly wetted by the molten metal. The most commonly used hot dipping methods include
- Galvanizing
- Tinning or Tin plating
- Galvanizing:
The process of coating a layer of zinc on iron or steel is called galvanizing. This protects iron object from rusting. The steel article first pickled with dilute sulphuric acid to remove traces of rust, dust or any other impurities etc.; at 60–90 °C for about 15 to 20 minutes. Then the metal is dipped in a molten zinc bath at 430 °C. The surface of the bath is covered with ammonium chloride flux to prevent oxide formation on the molten zinc. When the article is taken out, it is found to have been coated with a thick layer of zinc. It is then passed through a pair of hot rollers. This process removes any excess of zinc and produces a thin film of uniform thickness. The coated article is annealed at a temperature of 650 °C and cooled to room temperature slowly. Galvanized articles cannot be used under acidic conditions and galvanized containers cannot be used to store acidic foods. This process is shown in Figure 6.14.

Figure 6.14 Galvanisation of steel sheet
- Tinning:
The coating of tin on iron is called tin plating or tinning. In tinning, the base metal is first pickled with dilute sulphuric acid to remove surface impurities. Then it is passed through molten tin covered with zinc chloride flux. Then tin coated article is passed through a series of rollers immersed in a palm oil bath to remove the excess tin. The palm oil protects the hot tin-coated surface against oxidation. This process produces a thin film of uniform thickness on the steel sheet.
Because of non-toxic nature of tin, tinning is widely used for coating steel, copper and brass sheets which is used for manufacturing containers for storing food stuffs, ghee, oils, kerosene’s and packing of food materials. This process is shown in Figure 6.15.

Figure 6.15 Tinning of steel sheet
- Metal cladding:
In this process, the base metal is protecting from corrosion by coating of a thin uniform homogenous layer of a coating metal on the base metal. In this method, base metal sheet is sandwiching between thin sheets of corrosion resisting metals such as nickel, copper, lead, silver or platinum and bonded either on one side (e.g. copper clades in cooking vessels) or on both sides (e.g. duralumin is sandwiched between two layers of Pure Aluminium) permanently by the application of heat and pressure. Metal cladding is generally practiced in the air craft industry in which a sheet of duralumin is sandwiched between two layers of pure aluminum to produce a sheet.
The basis requirement for this specification that base metal and the cladding metal should have similar working characteristics for effective cladding.
In some cases we are also used metal oxide powders in a revolving heating drum in which base metal is thoroughly immersed. This is known as diffusion or cementation of the base metal to protect from corrosion, when ZnO is used, it is known as sherardizing. When Al2O3 and Cr2O3 are used, it is known as chromizing and when only Al2O3 is used, it is known as colorizing. In all this method, we protect the base metal from corrosion by coating of thin film of different metal.
- Electroplating:
In this process, noble metal is coated over more reactive metal. Most commonly used are tin plating and nickel plating. In electroplating, the object to be plated is made as cathode and suspended in an electroplating bath containing the metal ions to be plated by electro deposition. The anode may be of the metal to be deposited or it may be an inert electrode (such as graphite) with good electrical conductivity. During this process, the variables such as voltage, temperature, pH, current and density are kept constant so that electroplating process remains unchanged (i.e., rate of deposition of metal on cathode and rate of dissolution on anode).
For example, iron can be protected from corrosion by coating the metal with chromium or nickel by electroplating process.
- Electro less plating:
In this process, we immersed the base metal article in a bath of a noble metal salt which is used for coating. The noble metal forms a layer on the base metal article by displacement of base metal by noble metal. This process is also called as ‘immersion plating’ or ‘displacement plating.’
For example, nickel coating on base metal, In this process, base metal article is dipped in a bath of nickel sulphate and sodium hypophosphite kept at temperature of 100°C and at pH from 4.5 to 5.0. Nickel ion from solution reduces to nickel and nickel phosphide, which forms a strong adherent thin film.
- Organic surface coatings:
Organic coatings are useful for the protection of metal surface by providing inert barrier on the surface from corrosion as well as corrosive environment. Organic coating also helpful in decoration of metal surface. Organic coatings commonly used include paints, varnishes, lacquers and enamels.
Paints
Paint is a term which has been used to signify a uniform dispersion of finally divided solids in a liquid called “vehicle” or “medium”. The solid comprises of pigments, driers and fillers. Volatile solvent is mixed with a non-volatile forming a film on metal surface. Example of non-volatile is drying oil and volatile solvent is thinner.
Constituents of paints and their function
The various constituents of paint include
- Pigment
- Vehicle or drying oil
- Thinner
- Drier
- Filler or extender
- Plasticizers and
- Anti-skinning agent
- Pigment: It is an essential constituent of paint. It provides color and opacity, in addition of that imparting strength and aesthetic appeal to the paint. Pigments increase the life of paint film because they prevent the penetration of UV rays which deteriorate the oil film.
Many properties need to be looked for a pigment. It should be opaque, chemically inert, non-toxic and miscible with the vehicle. The pigment should have good hiding power i.e., it should be opaque so that the surface underneath is not visible; otherwise all the dirty spots, surface defects etc. would be seen. Opacity of the paint is due to the difference between the refractive indices of the pigment and the vehicle and also on the fine size of the pigment particles. Pigments commonly used in paints are inorganic solids with high refractive index either naturally occurring minerals or synthetic chemicals.

- Vehicle or Drying oil: It is a liquid which binds the pigment to the surface and protects pigment from decay. Common example of such oil as linseed oil, dehydrated castor oil, perilla oil or tung oil or a mixture of drying and semidrying oils. When paint is applied on a metallic surface, the unsaturated fatty acids in oil undergoes oxidation and forming oxides, peroxides and hyperoxides at the double bond and further undergo polymerisation and forming a protective, tough and insoluble film of the polymer on surface.

By adding phenolic and alkyl resin into drying oil, hardness and glossiness of the film can be improved.
- Thinner: Thinner is a volatile solvent, which is often added to paint which helps to adjust the consistency of the paint. Other functions of thinner area
- To increases the penetrating power of the vehicle
- To increases the elasticity of the paint film on surface
- It helps in retaining the constituent solids into vehicle
Examples of thinness are turpentine, petroleum fractions such as benzene, naphtha, white spirit, toluol, etc.
- Driers: Main function of a drier is to increase the drying power of the vehicle. In addition to this, driers work as oxygen-carrying catalysts which accelerates the drying of the oil film by oxidation, polymerization and condensation. Examples of common driers are borates, tungstates, resinates, linoleates of metals such as Ni, Zn, Co and Mn.
- Filler or extender: These are often colourless inorganic substances like aluminium silicate, barium carbonate, barium sulphate, asbestos, gypsum, calcium carbonate; clay, magnesium silicate etc. are added to the paints.
The function of addition of filler in paint is that it improves the properties of the paint and mainly to reduce the cost. It also acts as carriers for the pigment colour, also fill the voids in the paint film, reduce the cracking of the paint film and improve the durability of the film.
- Plasticizer: They remain permanently in paints and varnishes. They improve the elasticity of the paint film which prevents cracking of the film. Commonly used plasticizers are tricrecyl phosphate, triphenyl phosphate, di butyl phthalate etc.
- Anti-skinning agent: Anti-skinning agents like polyhydric phenols are added to the paint so that getting or skinning of paint can be prevented and can be used for a long period.
- Use of inhibitors:
Inhibitors are chemical substances which on adding in small portion to the corrosive medium decreases the corrosion rate.
Inhibitors are mainly of following two types
- Anodic inhibitors: This type of inhibitors stifles the corrosion reaction, occurring at the anode by forming a sparingly soluble compound with a newly produced metal ion. Anodic inhibitors such as chromates, tungstates, phosphates of transition metal react with ions at the anode and form an insoluble precipitate. These precipitates formed are absorbed on metal surface by forming a protective film on the metal and prevent corrosion.
This type of control method is effective, but it may be dangerous because if certain areas are left unprotected by depletion of the inhibitor which causes severe local attack occur on the metal surface.
- Catholic inhibitor: This type of inhibitors slow down the corrosion reaction by considerably decreasing the diffusion of hydrated H+ ion to the cathode and can be used in acidic as well as in neutral medium.
In acidic solution, the corrosion process involves the following catholic reaction.

The corrosion of a metal can be reduced by slowing down the rate of diffusion of H+ ions through the cathode. It can be done by using organic compounds such as mercaptans, amines, substituted ureas, heavy metal soaps, heterocyclic nitrogen compounds, etc. They adsorb to the metal surface and act as cathodic inhibitors. Antimony and arsenic oxides deposit adherent film of metals at the cathode and slow down the overvoltage for hydrogen evolution.
In a neutral solution, cathodic reaction is written as

The hydroxide (OH−) ions are formed due to presence of oxygen. The corrosion can be controlled by either eliminating oxygen from the corroding environment or by retarding its movement to the cathodic areas. The oxygen is eliminated by adding reducing agents like Na2SO3 or by dearation and diffusion of oxygen to the catholic areas can be retarded by the use of Mg, Ni or Zn salts. These salts react with hydroxide ions to form corresponding insoluble hydroxides which deposit on the cathodic areas and form an almost impermeable barrier. This method is also helpful for the protection of metal surface by corrosion by slow down the corrosion process.
- Anodic inhibitors: This type of inhibitors stifles the corrosion reaction, occurring at the anode by forming a sparingly soluble compound with a newly produced metal ion. Anodic inhibitors such as chromates, tungstates, phosphates of transition metal react with ions at the anode and form an insoluble precipitate. These precipitates formed are absorbed on metal surface by forming a protective film on the metal and prevent corrosion.
6.4 REVIEW QUESTIONS
6.4.1 Fill in the Blanks
- The gradual loss of a metal by chemical or electrochemical action of environment is called ________.
[Ans. corrosion]
- The formula for rust is ________.
[Ans. Fe2O3 · xH2O]
- The wet corrosion involves the flow of ________ from anodic area to cathodic area through a conducting solution.
[Ans. electrons]
- When the oxide film is volatile in nature, rate of corrosion of underlying metal is ________.
[Ans. increases]
- In galvanic corrosion, the metal having ________ value of reduction potential gets corroded.
[Ans. lower]
- Larger the potential difference between two metals, ________ is the extent of corrosion.
[Ans. greater]
- In differential aeration corrosion, the poor oxygenated part acts as ________, and undergoes corrosion.
[Ans. Anode]
- ________ and ________ are important factors for stress corrosion.
[Ans. Tensile stress, corrosive environment]
- The rate of corrosion of a metal is inversely proportional to the ________ areas.
[Ans. anodic]
- The rate of corrosion is more in presence of oxygen when pH value is ________.
[Ans. below 7.0]
- The conductance of clay and mineralised soil is much higher than those of ________ soils.
[Ans. dry sandy]
- In tinning, iron is protected with a coating of ________ metal.
[Ans. tin]
- ________ is an example of anodic inhibitors.
[Ans. Chromates]
- Cathodic inhibitors slow down the corrosion reaction by decreasing the diffusion of ________ ions to the cathode.
[Ans. H+]
- In sacrificial anodic protection, the more active metal is used as ________.
[Ans. anode]
- An example of anodic coating is ________.
[Ans. galvanization]
- An example of cathodic coating is ________.
[Ans. tinning]
- In metal cladding, ________ is sandwiched between two layer of pure aluminium.
[Ans. duralumin]
- Oxidation corrosion is an example of ________.
[Ans. dry corrosion]
- The immersion of base metal article in a bath of a noble metal salt which is used for coating is called as ________ plating.
[Ans. Electroless]
- ________ is used to bind the pigment to the surface and protects pigment from decay.
[Ans. Vehicle/Drying oil]
- Commonly used thinner in paint is ________.
[Ans. turpentine or petroleum]
- Commonly used plasticizers in paint is ________.
[Ans. Tricresyl phosphate or Triphenyl phosphate]
- Brass and copper utensils are usually coated with ________.
[Ans. Tin(Sn)]
- ________ are providing desired colour and protection to paint film.
[Ans. Pigments]
- In chromizing, the base metal is thoroughly mixed in revolving drum containing metal oxides of ________ and ________.
[Ans. Al2O3, Cr2O3]
- ________ is non-toxic in nature, so it is widely used for coating steel, copper sheets which is used for storing food stuffs and packing of food materials.
[Ans. Tinning]
- In galvanizing, molten zinc bath is covered with ________ flux.
[Ans. Ammonium chloride]
- ________ coating is most preferrable than ________ coating.
[Ans. Anodic, cathodic]
- In sacrificial anodic protection, commonly ________ metal block is connected with underground pipes.
[Ans. Mg]
6.4.2 Multiple-choice Questions
- Corrosion is an example of
- Oxidation
- Reduction
- Electrolysis
- Erosion
[Ans. a]
- Chemically, the rust is
- Fe2O3
- FeO · Fe2O3
- Fe2O3 · xH2O
- FeO · xH2O
[Ans. c]
- The metal which is protected by a layer of its own oxide
- Cu
- Fe
- Au
- Al
[Ans. d]
- The corrosion caused by the direct chemical action of environmental gases or anhydrous liquids on metal surface is called
- Dry corrosion
- Wet corrosion
- Pitting corrosion
- Electrochemical corrosion
[Ans. a]
- Which of the following factors does not govern the rusting of iron?
- Presence of air
- Presence of moisture
- Presence of electrolytes in water
- Presence of impurities of more electropositive metals in iron
[Ans. d]
- In galvanic corrosion
- More metal gets corroded
- Less noble metal gets corroded
- The metal having a higher standard reduction potential gets corroded
- The metal placed lower in the electrochemical series get corroded
[Ans. b]
- In electrochemical corrosion
- Anode undergoes oxidation
- Cathode undergoes oxidation
- Both undergo oxidation
- None undergoes oxidation
[Ans. a]
- In differential aeration corrosion
- Poor oxygenated part acts as anode
- Rich oxygenated part acts as anode
- Poor oxygenated part acts as anode
- Metal as a whole acts as cathode
[Ans. a]
- The localised attack of a corroding environment leading to the formation of holes in an otherwise relatively unattacked surface of a metal is called
- Water-line corrosion
- Pitting corrosion
- Concentration cell corrosion
- Wet corrosion
[Ans. b]
- Water-line corrosion is enhanced by the presence of
- Hydroxides
- Chlorides
- Carbonates
- Silicates
[Ans. b]
- Caustic embrittlement is a particular case of
- Pitting corrosion
- Dry corrosion
- Stress corrosion
- Wet corrosion
[Ans. c]
- To protect buried pipeline from corrosion is connected to Mg piece through a wire. This process is called as
- Impressed current cathodic protection
- Galvanic protection
- Sacrificial anodic protection
- Sacrificial cathodic protection
[Ans. c]
- In an electrochemical series, the metal at the top is
- Most noble
- Most stable
- Most active
- Most protective
[Ans. c]
- Galvanizing is the process of coating iron with
- Mg
- Cu
- Zn
- Ni
[Ans. c]
- Corrosion of zinc metal containing an impurity of copper is called
- Water line corrosion
- Moist corrosion
- Stress corrosion
- Galvanic corrosion
[Ans. d]
- Anodic coating protects underlined metal
- Due to its higher reduction potential
- Due to its lower reduction potential
- Due to its noble nature
- Due to its higher oxidation potential
[Ans. d]
- Addition of hydrazine-hydrate to corrosive environment
- Retard anodic reaction
- Retard cathodic reaction by consuming dissolved oxygen
- Prevents diffusion of protons to cathode
- Increases hydrogen overvoltage
[Ans. b]
- In general, corrosion is maximum when the pH of the corroding medium is
- Above 7.0
- Equal to 7.0
- Below 7.0
- Equal to 1.0
[Ans. c]
- The process of covering steel with zinc to prevent it from corrosion is called
- Galvanizing
- Tinning
- Electroplating
- Electroless plating
[Ans. a]
- Acid pickling of steel is carried out by dipping the steel in
- Dilute HCl
- Dilute H2SO4
- Conc H2SO4
- Dil HNO3
[Ans. b]
- During galvanization, the function of flux ammonium chloride is
- To prevent oxide formation, on molten zinc
- To prevent reduction of molten zinc
- To acts as a barrier
- None of these
[Ans. a]
- In electroplating, the object to be protected from corrosion is made as
- Anode
- Cathode
- Both anode and cathode
- None of the above
[Ans. b]
- The oxygen carrier of the paint is called
- Drier
- Pigment
- Thinner
- Extenders
[Ans. a]
- In Electroless plating, the base metal article is immersed in a solution of
- More active metal salt
- More noble metal salt
- Any one of these
- None of the above
[Ans. b]
- An inhibitor which when added in small quantities to aqueous corrosive environment
- Effectively decreases the corrosion of a metal
- Increases the corrosion of a metal
- No effect on corrosion of metal
- Increases the corrosion nature of the environment
[Ans. a]
- The cathodic inhibitors slow down the corrosion reaction by decreases
- Diffusion of hydrated H+ ion to the cathode
- Diffusion of cl− ions to the cathode
- Diffusion of hydrated H+ ion to the anode
- None of the above
[Ans. a]
- In cathodic coating, base metal is coated with
- More noble metal
- Less noble metal
- More active metal
- Having more reduction potential
[Ans. a]
- In Impressed current cathodic protection, anode is placed in backfill because
- To slow down the rate of corrosion reaction
- To increases the rate of reaction
- To increase the electrical contact with the surrounding soil
- None of the above
[Ans. c]
- The rate of corrosion is more when
- Anodic area is large
- Anodic area is small
- Athodic area is small
- None of the above
[Ans. b]
- According to pilling-Bedworth rule, Greater is the specific volume ratio
- More is the oxidation corrosion
- Lesser is the oxidation corrosion
- More is the reduction corrosion
- None of the above
[Ans. b]
6.4.3 Short Answer Questions
- Define corrosion.
Ans.: Any process of deterioration and consequent loss of solid metallic materials through an unwanted chemical or electrochemical attack by its environment, is called as corrosion.
- What is meant by rusting of iron.
Ans.: The attack of atmospheric gases on iron or steel, formation of a layer of reddish scale of hydrated ferric oxide fe2O3 · 3H2O on its surface is known as rusting of iron.
- What is dry corrosion.
Ans.: Dry corrosion takes place due to the direct chemical action of atmospheric gases like CO2, SO2, O2, H2 etc or anhydrous liquids on the metal surfaces.
- Formation of which types of metal oxide film cause rapid and continuous corrosion.
Ans.: Volatile oxide film and porous oxide film.
- Formation of which types of metal oxide film prevents corrosion.
Ans.: Highly unstable oxide film and finely grained tightly adhering, impervious oxide film.
- State the two conditions for wet corrosion to take place.
Ans.: (i) Immersion or partial dipping of two dissimilar metals or alloy in a solution.
(ii) A metal in contact with the conducting liquid.
- Bolt and nut made of the same metal is preferred in practice. Why?
Ans.: Because such a combination will not permit galvanic corrosion to take place.
- What is wet corrosion.
Ans.: Wet corrosion is due to the flow of electrons from metal surface anodic area towards cathodic area through a conducting solution. It is also known as electrochemical corrosion.
- What is galvanic corrosion.
Ans.: When two dissimilar metals are electrically connected and exposed to an electrolyte, the metal higher in electrochemical series undergo corrosion.
- The rate of metallic corrosion increases with increase in temperature. Give reason.
Ans.: With increase of temperature of the environment, the rate of reaction as well as rate of diffusion increases, thereby corrosion rate increases.
- Iron corrodes faster than aluminium, even though iron is placed below aluminium in the electrochemical series, why?
Ans.: This can be explained by the fact that aluminium forms a non-porous, very thin, highly adhering protective oxide film (Al2O3) on its surface and this film does not permit corrosion to occur.
- Wire mesh corrodes faster at the joints, why?
Ans.: The joints of wire mesh are stressed due to welding, so that part acts as anode, Hence oxidation takes place easily at such joints leading to faster corrosion at the joints of wire mesh.
- Impure metal corrodes faster than pure metal under identical conditions. Why?
Ans.: Because presence of impurities in metal cause heterogeneity and form minute electrochemical cells at the exposed parts, and anodic parts get easily corroded.
- How is galvanization different from cathodic protection.
Ans.: In galvanization, the iron object is protected from corrosion by coating it with a layer of zinc, whereas in cathodic protection, the iron object in made cathodic by connecting it with a more anodic metal like Al, Mg, etc.
- Where the electrochemical corrosion takes place.
Ans.: At the anodic area.
- Rusting of iron is faster in saline water than in ordinary water. Give reason.
Ans.: Due to presence of sodium chloride in saline water, it leads to increased conductivity of water, so when saline water comes in contact with the iron surface, corrosion current increases and rusting is speeded up.
- Why does part of a nail inside the wood undergoes corrosion easily?
Ans.: Corrosion is due to differential aeration, Because part of nail inside the wood is not exposed to atmospheric conditions, whereas the nail outside is exposed to atmospheric air. Thus nail inside the wood becomes anodic while remaining part acts as cathodic. So due to differential aeration, a differential current starts flowing, and the anodic parts gets corroded easily.
- Why should nickel plated steel articles be free from pores and pin holes.
Ans.: with respect to nickel, steel is anodic and if there are pin holes and pores in nickel plated steel article, they will expose the anodic steel to atmosphere. A galvanic cell is set up and an intense localized corrosion at these small exposed parts occur.
- Corrosion of water filled steel tanks occurs below the water line. Why?
Ans.: This is because, the area above the waterline is highly oxygenated and acts as cathodic, while the part below the waterline is poorly oxygenated and acts as anodic. So due to differential aeration, an electrochemical cell is set up which result in corrosion of steel tanks below the waterline.
- What is meant by the term passivity?
Ans.: It is the phenomenon by which a metal or alloy shows higher corrosion resistance due to formation of a highly protective, very thin and quite invisible surface film on metal surface.
- What is effect of pH on corrosion.
Ans.: The lower the pH (or more acidic), greater is the corrosion.
- Can we use aluminium in place of zinc for cathodic protection of rusting of iron, comment.
Ans.: Standard electrode potential of
Al3+/Al = -1.66V
Zn2+/Zn = -0.76V
In cathodic protection, the metal (iron) to be protected from corrosion is connected by a wire to a more anodic metal (like Al, Zn etc.), so that all the corrosion occurs at this more active metal. Thus, the parent metal is protected while the more active metal gets corroded slowly. As the standard potential of aluminium is more than zinc, so Al is more anodic than Zn, so we can better use aluminium in place of zinc for cathodic protection of rusting of iron.
- Why are galvanized utensils not used?
Ans.: Because galvanized articles gets dissolved in dilute food acids and forms highly toxic compounds. So, galvanized utensils cannot be used for preparation and storing food stuffs.
- Why are brass utensils usually tinned?
Ans.: Because Tin (Sn) is a noble metal and protects the brass utensils from corrosion, moreover, tin is non-toxic in nature. Hence, it is widely used for coating copper and brass utensils.
- Galvanization of iron article is preferred to tinning, why?
Ans.: Galvanization (coating iron with zinc) is preferred to tinning (coating iron with tin) due to the following reason: zinc(Zn) is more electropositive than iron, so zinc coating acts as anode; while the exposed iron portions of coating acts as cathode, If by chance, the zinc coating is broken at some place, the zinc (being more anodic than iron), undergoes corrosion, protecting iron from rusting. So, zinc coating protects iron sacrifically.
On the other hand, tin is a noble metal (i.e. having higher reduction potential than iron), so it protects the iron due to its higher corrosion resistance than iron, If by chance, the tin coating is broken at some place, much more corrosion of iron takes place because small exposed part of iron cuts as anode and tin acts as cathode, a galvanic cell is set up, thereby an intense corrosion at the small exposed iron part occurs.
- What is chromizing?
Ans.: The process of heating a mixture of chromium powder, alumina and iron/steel article in a revolving drum at 1300-1400 °c for 3-4 hours, It increases the corrosion resistance of the article.
- What are the main constituents of oil varnish.
Ans.: synthetic resin, drying oil and volatile solvent
- Give two functions of plasticizers.
Ans.: (i) It provides elasticity to the paint film.
(ii) To minimize the cracking of dried paint film.
- Give three functions of drier in paints.
Ans.: (i) It acts as a carrier of pigments
(ii) It helps in forming a thin, homogeneous and protective film.
(iii) It supplies to paint film adhesion, toughness, durability and water-proofness.
- Give two functions of extenders or fillers
Ans.: (i) It reduce the cost of paint.
(ii) It reduce the cracking of the paint film.
- What is an enamel?
Ans.: enamel is an intimate dispersion of pigment in a varnish.
- Give three function of thinner in a paint.
- To suspend pigment particles
- To dissolve film-forming materials.
- To reduce the consistency of paint for getting smooth finish.
6.4.4 Descriptive Questions
Q.1 Define corrosion of metals. Explain the electrochemical theory of wet corrosion with mechanism.
Q.2 Give reasons for the following:
Silver and copper do not undergo much corrosion like iron in moist atmosphere.
Q.3 Write short notes on the following:
(i) Wet corrosion (ii) Dry corrosion (iii) pitting corrosion
Q.4 Explain the mechanism of galvanic corrosion and differential aeration corrosion.
Q.5 What are the factors affecting corrosion? How is it prevented?
Q.6 Explain how can corrosion be controlled by proper designing.
Q.7 What is the role of sacrificial anode in corrosion control.
Q.8 What are the effects of temperature, pH, overvoltage and reactivity of metal influences the corrosion.
Q.9 Write short notes on the following:
(a) Sacrificial anodic protection (Galvanic protection)
(b) Impressed current cathodic protection
(c) Galvanization
(d) Galvanic series
(e) Caustic embrittlement
Q.10 Discuss the role of nature of oxide formed in oxidation corrosion. State and explain pilling-Bedworth rule.
Q.11 Explain the mechanism of hydrogen evolution and oxygen absorption in electrochemical corrosion.
Q.12 Describe the following methods of corrosion control
(i) Tinning (ii) hot dipping (iii) proper designing (iv) electroplating (v) metal clading.
Q.13 What is meant by corrosion inhibitors. Give two examples.
Q.14 Explain the importance of tinning in corrosion control.
Q.15 Give reasons for the following:
(i) corrosion of water filled tank occurs below the waterline.
(ii) A copper equipment should not possess a small steel bolt.
Q.16 Discuss the importance of design and material selection in controlling corrosion.
Q.17 Explain (i) pitting corrosion (ii) Bi-metallic (Galvanic) corrosion.
Q.18 Outline the difference in the use of anodic and cathodic coatings for corrosion prevention.
Q.19 Describe the process of galvanization of iron. How does it prevents the corrosion of iron.
Q.20
(i) Give the requirements of a good paint.
(ii) Write brief account on pigments.
Q.21 Explain the principle involved in
(i) anodic protection (ii) Cathodic protection (iii) galvanization (iv) cementation
Q.22 What happens and why?
(i) Iron sheet riveted with copper rivets.
(ii) An iron pole is partly burried under earth.
(iii) Zinc plate fixed below the ship.
Q.23 Iron corrodes faster than aluminium, even though iron is placed below aluminium in the electrochemical series, why?
Q.24 Explain rusting of iron with the help of electrochemical theory of corrosion.
Q.25 Discuss the differences between varnishes and paints.
Q.26 What are the constituents of paints and what are their function.
Q.27 What are the important factors that influence the corrosion phenomenon.
Q.28 Write short notes on
(i) Sacrificial anode (ii) Corrosion inhibitors (iii) Electroplating (iv) Electroless Plating
Q.29 How does the nature of metal influence rate of corrosion.
Q.30 How are the metals protected against corrosion by modifying the environment?
