8
PHASE RULE
8.1 INTRODUCTION
In 1875, as a result of mathematical and thermodynamic studies, J. Willard Gibbs put forward a rule known as Phase Rule; without any exception, the rule is applicable to all heterogeneous system in equilibrium. By using Phase Rule, the effect of temperature, pressure and concentration can be predicted qualitatively on a heterogeneous system, which is in equilibrium by the Phase diagrams.
It is assumed that the equilibrium is influenced only by temperature, pressure and concentration, but not influenced by gravity, electrical or magnetic forces or by surface action. The maximum number of degree of freedom is taken as three; mathematically, Gibbs’ Phase Rule may be stated
as:
![]()

8.2 EXPLANATION OF THE TERMS INVOLVED IN PHASE EQUILIBRIA
8.2.1 Phase (P)
A heterogeneous system consists of various homogenous parts in contact with each other by distinct boundaries; any part of a system which is homogeneous, physically distinct and mechanically separable from other parts of the system is a Phase.
Essential Conditions for a Phase
- It should be homogeneous or may be a homogeneous part of a heterogeneous system.
- It should be physically distinct and is separated from other parts of a system by well defined boundary surface.
- A dynamic equilibrium between different phases of this system should be present to exchange of chemical species.
Examples
- A gas or a gaseous mixture is a single phase because there is no interface between one gas and another e.g. air which is a mixture of nitrogen, oxygen, carbon dioxide, water vapour, etc is composed of one phase only.
- Water exists in three forms-ice, water and vapour. It is a three phase system.

- Two or more completely miscible liquids present in a system constitute only one liquid phase as there will be no surface of separation between them when they are mixed. e.g.: water, alcohol, acetone etc.
- Two immiscible liquid forms two different phases. e.g.: water and ether, alcohol and ether etc. Both forms two different phase.
- A heterogenous mixture of solid substances consists of as many phases as there are substances present in that system.
e.g.: decomposition of CaCO3(s)

Here are three phases among those two phases are CaCO3(s) and CaO(s) and third phases is CO2(g) because all phases are separated by interface.
8.2.2 Components (C)
The smallest number of independently variable constituents by which the composition of each phase present can be expressed directly or in the form of a chemical equation is the number of components (C) of a system at equilibrium.
The number of components of a system may be or may not be the same as the actual number of substances or constituents present in the system; only those constituents of an equilibrium mixture, which can undergo independent variation, are known as components.
- When no reaction takes place, the number of components in equal to the number of
constituents. - When a reaction occurs, at that time, then the minimum number of species allowing for a reaction to prepare one species is known as the number of components.
Therefore, the number of components of a system


In case of Ionic Reaction
The number of component is calculated as
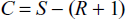
S = Total number of ionic species present in the system.
R = number of relation between ionic species.
Examples
- Water exists in three phases as
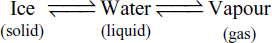
Each phase can be represented by H2O. Thus, the number of components is one.
- Consider aqueous solution of sugar. Here, the composition of this solution is described by specifying the presence of sugar and water. Thus, the number of components is two.
- Consider the decomposition of Ammonium chloride as

Actually this equilibrium exists as

- If the reaction is carried out in a closed vessel or vacuum then number of component is one because in the gaseous phase both HCl and NH3 are always present in equal amounts and represents NH4Cl(g).
- If the excess of either NH3 or HCl is introduced, the composition of gaseous phase can no longer be the same and the composition of this phase can no longer be represented by NH4Cl alone, but one more component is required. Hence, it becomes two component system.
- Consider the thermal decomposition of CaCO3 as

Hence, the number of component is two.
Here, three different constituents form three different phases, but the composition of each phase can be expressed in terms of any two of the constituents.
- If CaO and CO2 are chosen as the components, then

- If CaCO3 and CO2 are chosen as the components, then

- If CaCO3 and CaO are chosen as the components, then

Thus, in all these cases the smallest number of constituents which can fix the composition of the phase present at equilibrium is two; hence, dissociation of CaCO3 by heat is a two component system.
- If CaO and CO2 are chosen as the components, then
- Consider the system NaBr−KCl−H2O
The number of components is calculated by formula

Here number of species, S = 9 (NaBr, NaCl, KCl, KBr, H2O, Na+, Br−, K+, Cl−)

Number independent reactions (Relation)


Hence, it is a four compound system.
- Consider the dilute solution of sulphuric acid in water

∴ Hence, it is a two component system.
8.2.3 Degree of Freedom (F)
Degree of freedom is defined as the number of intensive variables (temperature, pressure and concentration) that can be changed independently without disturbing the number of phases in equilibrium. These variables describe the state of the system. On the basis of degree of freedom of the system, systems are classified as nonvarient(F = 0), monovarient (F = 1), bivarient (F = 2) etc.
Examples
- Consider the example of pure gas, the number of degree of freedom is two (F = 2). This
is because a pure gas satisfied the gas equation PV = RT. If the values of pressure (P) and temperature (T) are fixed, then the volume (V) automatically gets fixed. In fact if any two variables (out of P, V & T) are specified. The third one gets specified by itself. Hence a system consisting of pure gas has two degree of freedom i.e. it is a bivarient system. - Consider one component having two phase system.

This system consists of two phases of one component. The vapour pressure of water is definite at a definite temperature independent on the concentration. It follows, therefore, that if the temperature is fixed, the vapour pressure is also fixed and vice-versa. So, we cannot alter both the variables without disturbing the equilibrium. Hence, we have to mention only one variables without disturbing the equilibrium i.e. either temperature or pressure. Thus the system has one degree of freedom (F = 1). System is called as monovarient or univarient (F = 1).
- Consider one component, three phases in equilibrium

In this system, there are three phases of one component (H2O). These three phases can co-exist in equilibrium only at particular temperature (0.0098 °C) and under one particular pressure (4.58 mm of Hg). Any variation of these factors will result into disappearance of one or more of the phases. Hence this system has zero degree of freedom (F = 0) i.e.nonvariant or invariant.
8.2.4 True and Metastable Equilibrium
Under a given set of conditions, the same state of a system can be attained by approaching from either direction by any possible procedure; then, that system is said be in the state of true equilibrium.
According to thermodynamics, true equilibrium is attained when the free energy content of the system is at a minimum for the given values of the variables.
Example: At 1 atm pressure and 0 °C (273 K) ice and liquid water attain true equilibrium because it can be attained by either partial melting of ice or partial freezing of water.
![]()
Under a given set of conditions a state of a system can be attain by only one direction with careful changing of a system conditions; that system is said to be state of meta stable equilibrium.
Example: At 271 K (−2 °C) or at even lower temperature, it is possible to cool water very slowly and carefully without appearance of ice; hence, water at −2 °C is said to be in a state of metastable equilibrium. The metastable equilibrium state of the system may be preserved by not subjecting it to a sudden shock, stirring or seeding by the solid phase. Solidification sets rapidly as soon as a crystal of ice is introduced and the temperature rises to 0 °C (273 K).
8.2.5 Eutectic Mixture and Eutectic Point
Mixture of two or more components without reacting chemically in solution state and at a particular temperature having lowest freezing or melting point among all possible ratio of mixing of that component is known as eutectic mixture, and such a type of system is called the eutectic system and the corresponding lowest freezing or melting point of that eutectic mixture is called the eutectic point.
For example in a eutectic mixture of lead (Pb) and silver(Ag) forms a eutectic point at 303 °C having the lowest melting point of eutectic system.
8.2.6 Triple Point
Triple point is a point at which three phases of a system co-exists in equilibrium. The degree of freedom of one component system is zero. At triple point, system is in variant i.e. degree of freedom is zero (F = 0).
For example, consider a system of water having three phases
![]()
All these phase co-exist at a particular temperature (0.0098 °C) and a particular pressure (4.58 mm of Hg). At triple point F = 0 (invariant or non-variant)
![]()
8.3 PHASE RULE
The phase rule gives relationship between the numbers of phases, components and degree of freedom of a heterogenous system.
The Gibbs phase rule may be stated as follows:
In a heterogenous system in equilibrium, the number of degree of freedom plus the number of phases is equal to the number of components plus two.
Mathematically,
![]()
Where
F = number of degree of freedom
C = number of components
P = number of phases
Digit ‘2’ represents the temperature and pressure.
It is assumed that the equilibrium is not influenced by gravity, electrical or magnetic forces or by surface action and is influenced only by temperature, pressure and concentration. The maximum number of degree of freedom is taken as three.
8.3.1 Assumptions for the Validation of Phase Rule
- The system is in thermal and mechanical equilibrium; consequently, the pressure and temperature are the same in all the phases of system.
- Surface contribution as well contributions from any electric or magnetic field to the extensive properties of the system, are negligible.
- Inter phase surfaces are deformable and permeable to components.
8.3.2 Thermodynamic Derivation of the Phase Rule
Let us consider a heterogenous system having C components (C1, C2, C3, …, Cc) distributed between P phases (P1, P2, P3, …, PP) as shown in Figure 8.1.
As we know that the degree of freedom of a system is equal to the number of independent variables which must be fixed arbitrarily to define system completely.


Figure 8.1 A system showing C components distributed in P phases in equilibrium
- Total number of variable can be calculated as follows:
- Temperature: Same for all phase (one variable)
- Pressure: Same for all phase (one variable)
- Concentration: Suppose a phase contains two components (A and B). Then if molar concentration of one component is known, then that of other can be calculated by using formula xA + xB = 1.
Similarly, if a phase contains three components, only two concentration terms should be known (xA + xB + xC = 1).
Hence, in general if a phase contains C components, it can be defined completely by (C − 1) concentration terms or variable.
For a complete system having P phase and C components the total number of variable required = P(C − 1).
Total number of variables is given by

- The number of variables defined by the system itself i.e. number of relations of equilibrium:
When a heterogenous system is in equilibrium at constant temperature and pressure, the chemical potential of any component will have the same value in all the P phases.
Thus if a system consist of three phases,
Say α, β and γ, then for any component i,

If one of them is taken as standard value, then two equations are written as
- (μi)a = (μi)β = (μi)γ
- (μi)a = (μi)γ
Thus for a system of 3 phases, two equations are known for each component.
Similarly, for a system of 4 phases, three equations are known for each components.
In general, for a system of P phases, (P − 1) equations are known for each components.
Hence, for a system having P phases and C components.

Now, degree of freedom of the system having P Phases and C components will be given by

This is the statement of Phase Rule, where only three state variable (Temperature, pressure and concentration) are taken into consideration.
If one of these two variables (temperature and pressure) does not affect on equilibria then degree of freedom for such a system will be reduced by one and in this case phase rule is called as reduced phase rule and it is represented as

Reduced phase rule equation.
8.3.3 Utility of Phase Rule | Application of Phase Rule
The following are some of the advantages from the study of phase rule.
- Phase rule helps in classifying various equilibrium states of the system in terms of the number of components, number of phases or the number of degrees of freedom.
- The different systems which are having the same value of degree of freedom according to phase rule, they would behave in a similar fashion.
- By using phase rule, we can predict the behavior of any system with the changes in the variables such as temperature, pressure and concentration.
- Phase rule is applicable to physical and chemical equilibria irrespective of the nature or amounts of the substances.
- It finds extensive use in the study of heterogeneous systems. This rule helpful in metallurgy and provided us useful information about the complex formation among different components.
- By using phase rule, we can predict whether under a given set of conditions, a number of substances taken together would remain in equilibrium as such or would involve inter-conversion or elimination of some of them substances.
8.3.4 Limitations of Phase Rule
- Phase rule applies only to a single equilibrium state and does not tell us about the number of other possible equlibria present in the system.
- The phase rule equation (F = C − P + 2) is applicable only to system having three variables. When the numbers of variables change the equation shall have to be changed accordingly.
- Phase rule deals with macroscopic system only and it does not tell us anything about the molecular structure.
- The phase rule does not give any information regarding the time taken for the system to attain equilibrium.
- The phase rule also does not give any information regarding the amount of any phase under equilibrium. It only considers the number of phase present in the system at equilibrium.
8.4 PHASE DIAGRAMS
Phase diagram is the complete description of behavior of phases in equilibrium. When the system is in equilibrium, the number of phases that exist together depends upon the conditions of temperature and pressure concentration kept constant or conditions of temperature and composition, pressure being kept constant. The diagrams so obtained giving the conditions of equilibria between various phases of a substance are called as phase diagrams or equilibrium diagrams.
The phase diagram contains a number of lines, areas and point by intersection and by such diagrams we are able to know the conditions under which various phases will be present in the system.
Let us consider one component system with different number of phase
- Single phase: When a pure substance is in single phase and having one component.
Then according to phase rule equation

It means such a system can be completely describe by using two variables
- Two phase: When we are considering one component in two phase are in equilibrium
Then degree of freedom

All such type of systems can be completely described by stating only one variables either temperature or pressure.
- Three phase: When we are considering one component in three phase are in equilibrium, then the system has no degree of freedom

For all such type of system, all variables must be specified fixed.
8.5 ONE COMPONENT SYSTEM
For a one component system,
According to phase Rule,

- When value of P = 1, then Degree of freedom, F = 2, it means both P and T can be varied independently without disturbing the equilibrium.
- When two phases are in equilibrium, value of P = 2. Degree of freedom (F) = 1, it means pressure can be changed freely if temperature is set or vice-versa.
- When three phases are in equilibrium (P = 3). The degree of freedom (F) is equal to zero it means system can only be non-variant in specific or definite conditions of temperature and pressure.
Water System
Water system is most typical example of one component system, in which the same chemical compound exists in the three phases in equilibrium as
![]()
These three phases may occur in the three possible combinations of the two phases in equilibrium as

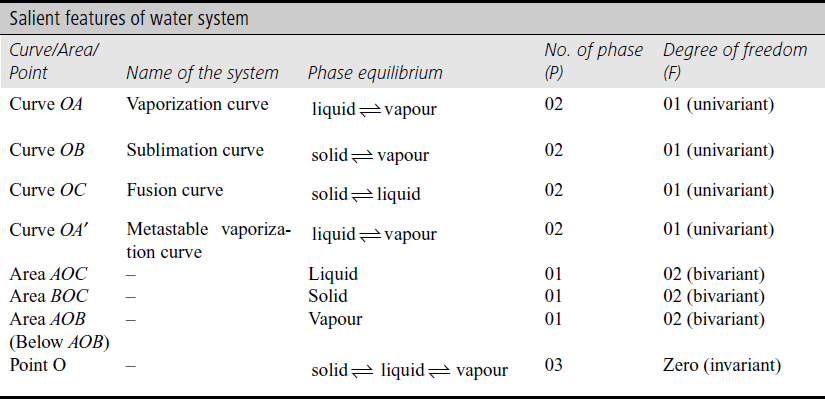
The conditions of temperature and pressure at which the various phases can exist have been determined experimentally and summed up in Figure 8.2.
These phase diagram of water system is represented as in Figure 8.2.

Figure 8.2 Phase diagram of water system
The phase diagram consists of:
- Three stable curves OA, OB and OC and a meta stable curve OA′
- Three areas AOC, BOC and below AOB
- Definite point, O
- Areas
- Area below AOB
In this area vapour phase of water exists,
According to phase rule

System is bivariant in this area. It means to show the conditions of existence of this phase two variables temperature as well as pressure are required.
- Area BOC
In this area solid phase (Ice) exists.

The system is bivariant and therefore to specify the conditions of as existence of this phase temperature and pressure are to be specified.
- Area AOC
This area consist liquid (water) phase

Due to bivariant nature of the system in this area both variable (T & P) are to be specified to explain the conditions of existence of liquid phase of water system.
- Area below AOB
- Curves
- Curve OA: It represents liquid-vapour equilibrium

This curve is called as vapourization curve or vapour pressure curve. This curve explain with increase of temperature, the vapour pressure increases.
This curve status from the point O the triple point of water (0.0098 °C at 4.58 mm) and extends upto critical temperature (374 °C) at critical pressure (218 atm), beyond which the two phases merge into each other.
Here P = 2 and C = 1

Since the degree of freedom is one, hence the system is univariant or monovariant. It means, for any give vapour pressure on curve there is only one value of temperature or vice-versa. At 100 °C, the vapour pressure of water equals the atmospheric pressure (760 mm). This is therefore, called as boiling point of water. Beyond the point ‘A’ (critical temperature) liquid phase of water does not exist.
The slope of the curve OA is positive i.e. the vapour pressure of water increases with temperature. It is also predicted by Clausius-Clapeyron equation as

- Curve OB: It is known as sublimation curve or vapour pressure curve of Ice.
It represents equilibrium between solid and vapour phase

It gives various values of temperature and pressure at which ice and vapour can exist together.
This curve starts from point O, the triple point of water and extends upto point B (absolute zero, −273 °C)

Since, the degree of freedom is one, hence the system is univariant i.e. for each temperature that can be one and only one pressure and vice-versa.
Here the slope of the curve OB is positive and this is also predicted by the Clausius-clapeyron equation

- Curve OC: It is called as melting point or fusion curve and it represents the equilibrium between ice and liquid water at various pressure.

Since, the degree of freedom in one, hence the system is univariant.
The slope of the curve OC is negative i.e. meeting point of ice is lowered by increases of pressure.
It is also predicted by Clasius-Clapeyron equation

Where ΔHf = change in molar heat of fusion.
Since the density of ice is less than that of water, so Vs > Vl,
Hence
 should have negative sign. This means that the increase of pressure must lower and decrease of pressure must increase the freezing point of water.
should have negative sign. This means that the increase of pressure must lower and decrease of pressure must increase the freezing point of water. - Metastable Curve OA′: It represents the liquid water and water vapour in metastable equilibrium.

It is vapour pressure curve of super cooled water, and it is possible to cool liquid water below its freezing point without the separation of ice and is shown by dotted line curve OA′; this state is known as metastable state. It is an unstable state because the curve OA′ lies above the curve OB. It means that at the same temperature, the vapour pressure of the metastable supercooled water is higher than the vapour pressure of the stable solid phase. If there is any disturbance in the process equilibrium may disturb and water may suddenly freeze.
The degree of freedom is one hence the system is univariant.
- Curve OA: It represents liquid-vapour equilibrium
- Point
The point at which all the three phases of water, that is, ice, water and vapour coexist in equilibrium is called triple point. Here, the curves OA, OB and OC intersect with each other at point ‘O’. This point is shown in the phase diagram at point ‘O’.


Since the degree of freedom is zero, hence the system is invariant. The temperature and pressure corresponding to this equilibrium are 0.0098 °C and 4.58 mm respectively.
The three phases can co-exist in equilibrium under only one set of conditions; even slight changes in the temperature and pressure will shift the equilibrium and the three phases cannot co-exist.
8.6 TWO COMPONENT SYSTEM
According to Phase Rule

For a system with two components and one phase degree of freedom will be three.
![]()
Therefore, three variables would be necessary to describe a system i.e. temperature, pressure and composition, all of these variables are required, therefore a three dimensional model is required for presentation which is more complex and difficult to understand we have a 2D planes and therefore we consider the one variable as constant, only two variables are considered. This reduces the degree of freedom of the equilibrium system and phase rule is written as
![]()
This is known as reduced or condensed phase rule. In general, most of experiments are conducted in open vessel where pressure in constant so, we generally expressed phase diagram of two components by using variable temperature and composition.
8.6.1 Eutectic System
Eutectic system is a binary system consisting of two substances that are miscible in all proportions in the liquid (solution) phase without reacting chemically. (The term eutectic means easy ‘to melt’.)
For example, bismuth–cadmium, lead–silver, KI–water systems, etc.
Eutectic Mixture
Eutectic Mixture is a solid solution of two or more substances having the lowest freezing point of all the possible mixture of the components, this is taken advantage of in “alloy of low melting point” which are generally eutectic mixture.
Eutectic Point
Two or more solid substances capable of forming solid solution with each other have the property of lowering each other’s freezing point, and the minimum freezing point attainable corresponding to the eutectic mixture is termed as the eutectic point. For example, in Pb-Ag system, eutectic point is achieved when the composition is 97.4% Pb and 2.6% of Ag.
Systems giving rise to eutectic point are known as eutectic systems. The eutectic composition and temperature of two metals and salt-water system is given in Table 8.1.
Characteristics of Eutectic Point
- Eutectic point represents the lowest or limiting temperature at which a liquid phase can exist in the system or it is the maximum temperature upto which a solid phase can exist.
- No other mixture containing the two components will have a melting point lower than the eutectic temperature.
Table 8.1 Some eutectic systems

- Eutectic point has precise values of temperature and composition and it represents an invariant system.
- If the liquid is cooled to just below the eutectic point, both the components of the eutectic simultaneously solidify without any change in the composition or temperature of the liquid phase.
- An eutectic system can maintain its temperature constant for long periods.
- When the liquid is cooled below the eutectic point, the components solidify in the form of small crystal intimately mixed with each other which fill in the spaces between the large crystals of the pure components which are already separated out.
- Eutectic mixtures appreciably contribute towards the strength of the solid structure in case of alloys.
- Eutectic are mixtures of the components but not their compounds.
Applications of Eutectic System
It has wide applications in industries, pharmaceutical science; medical science etc. Some important applications are the following:
- Freeze–drying
- Safety plugs or safety fuses
- Solders and
- Freezing mixture.
- Freeze–drying
The freeze–drying is the complete removal of water from the material, such as food. The following are two advantages of freeze–drying of food materials:
- The microorganisms require water to survive. If water is removed from food, then it won’t get spoiled for a long period of time due to non-survival of microorganisms. Enzymes also require water to react with food, so dehydrating of food will also stop ripening. Freeze-dried food materials such as fruits, vegetables etc. can be stored for years and can be completely revitalized with a little warm water. After the years, the taste and texture will be very much the same.
- Freeze-drying significantly reduce the total weight of the food. Freeze dried food can be easily transported to the military during war. It is also used by astronauts in a space craft.
Difference between Dehydration and Freeze Drying
Freeze drying is different from ordinary dehydration. In dehydration, water present in the material is removed by evaporation of water from liquid to vapour state by supplying heat energy. The food materials will be decomposed by the heat supplied for evaporation. Moreover the food will lose its taste, texture and vitality.
But in freeze drying, the liquid water present in the food material is freezed to solid ice, which is then sublimed under such conditions that the decomposition and volatilization of other constituents is avoided.
Principle of the Process
Sublimation is the fundamental principle in freeze–drying, that is, the conversion of a solid directly into a gas. Sublimation occurs when a molecule gains enough energy to break free from the molecules around it, just like evaporation. Water will sublime from solid form (ice) to gaseous form (vapour) when the molecules have enough energy to break free but the condition that the solid melting to its liquid form, which normally happens, does not take place.
- Safety Plugs or Safety Fuse
A safety fuse is a protective device made from a low melting alloy that melts under heat produced either by excess heating or by an excess current in the circuit.
They are used to ensure the safe working and avoid accidents. Most commonly used low melting alloys are wood metal, rose metal and fuse wires.
- Wood metal contain 50% Bi, 25% Pb, 12.5% Sn and 12.5% Cd. Its melting point is about 70 °C.
It is used for making safety plugs for cookers, fire alarms and for boilers and electric fuses
etc. - Rose metal contains 50% Bi, 28% Pb and 22% Sn. Its melting point is 88 °C. It is used for making fuse wires, fire arms and automatic sprinklers.
- Fuse wires for small current are made of Pb–Sn alloy and for high current are made of Pb, Sn, Zn, Sb, Cu, Al etc.
- Safety fuses as plugs are installed in building to protect them against any fire hazards. When a building catches fire, the heat melts fusible alloy plug and water rushes out from the pipe. This controls the fire automatically.
- They are fitted in the form of plug in steam boilers and pressure cookers. Whenever steam pressure exceeds the limiting value of pressure, safety plug gets over heated and melts, thereby permitting excessive steam to escape out of the boiler. Thus, accident due to overheating can be avoided.
- Safety fuse in the form of fuse wire is also used for protection the cable in an electrical circuit against damage from any excessive current than normal. This is because when current is exceeded than normal value, fuse wire gets heated upto the melting point of fuse wire thereby fuse wire gets melted and circuit is broken. As a consequence, wire in the circuit gets protected from over-heating.
- Wood metal contain 50% Bi, 25% Pb, 12.5% Sn and 12.5% Cd. Its melting point is about 70 °C.
- Solders
A solder is an alloy having lower melting point than that of the individual metals that are joined together by melting. It works based on the principle of eutectic mixture freezes sharply at its freezing point; hence, solders have somewhat different compositions from the eutectic so that the freezing occurs over a range of temperatures.
Table 8.2 Solders and their compositions and uses

The capacity of solders depends upon the formation of a surface alloy between the solder and the parts of metals being soldered. Based on desired melting point and the metals to be joined, the solder alloy can be selected. Solders usually contain Pb and Sn as the main components.
Important solders and their compositions are shown in Table 8.2.
A good solder has the following characteristics
- Its melting point should be lower than the soldering metals
- It should spread easily in liquid form and form homogeneous mixture with the soldering metals.
- It should possess good quality of wetting the soldering metals.
- Freezing Mixture
A mixture of ice and salt is known as freezing mixture; it has been observed that the addition of salt to ice results in considerable lowering of temperature.
A good freezing mixture should satisfy the following conditions:
- Salt must be cheap, highly soluble and have a low cryohydric temperature.
- The heat of solution of the salt should be high, that is, solubility of the salt should increase rapidly on increasing the temperature.
- Components used should form an intimate mixture on cooling.
Normally a mixture of ice and common salt is used as a freezing mixture because common salt is very cheap and easily available. However, it is not a good component for freezing mixture due to the heat of solution of the salt is very low and the heat absorbed is almost due to the heat of fusion of ice. Calcium chloride hexahydrate and ice give an excellent freezing mixture because of very low cryohydric point and high heat of solution.
Some important freezing mixtures, their eutectic temperatures and percentage composition are listed in Table 8.3.
Table 8.3 Freezing mixtures

8.6.2 Lead (Pb) – Silver (Ag) System
Both lead and silver are completely miscible in liquid state and form homogeneous mixture without formation of any compound by the mixing of lead and silver.
Melting point of pure lead and pure silver are 327 °C and 961 °C respectively. Melting point of both components i.e. solvent is lowered by the addition of solute. On addition of silver in lead, melting point of lead is lowered and vice-versa.
The phase diagram of Pb·Ag system consists of the following points
- Curves AO, BO
- Areas above AOB, below AO and below OB
- Eutectic point (O)
The phase diagram of lead-silver system is shown in Figure 8.3.

Figure 8.3 Phase diagram of lead-silver system
Curves
- Curve AO: This curve represents the melting point or freezing point curve of Pb by addition of small amount of Ag.
Along this curve solid Pb+ liquid melt are in equilibrium. Along this curve, the silver which is added goes into solution while the separation of solid Pb takes place.

The system is univariant along this curve. So, to specify the conditions of equilibrium between two phases only one variable either temperature or composition is to be specified.
- Curve BO: This curve represents the melting point or freezing point curve of Ag by addition of small amount of Pb. Along this curve the lead which is added goes into solution while separation of solid Ag takes place.
Along this curve two phases solid silver and liquid melt are in equilibrium.

The system is univariant along this curve as two phases solid Ag and liquid are in equilibrium. Only one variable either temperature or composition is to be specified for specify the conditions of equilibrium between two phases.
Areas
- Area above AOB
In this area only one phase i.e. liquid melt (solution of Ag and Pb) co-exists

The system is bivariant in this region. So, to show the existence of this phase in this region two variables temperature as well as composition are required.
- Area below AO or Area enclosed between AOC
In this area AOC, solid Pb and liquid melt co-exist
According to phase rule equation

The system is univariant in this region. Only one variable either temperature or composition is required to specify the equilibrium state.
- Area below BO or Area enclosed between BOD
In this area, solid Ag and liquid melt co-exist.
So, according to condensed phase rule equation

The system is univariant.
- Area below the line COD
- Area enclosed between COEF:
Solid Pb and eutectic phases are co-exist in this region.

The system is univariant in this region.
- Area enclosed between ODGE:
Solid Ag and Eutectic phases are co-exist in this region.

The system is univariant in this region.
- Area enclosed between COEF:
Points
- Point A and Point B: Point A represents the melting point of pure Pb (327 °C) and point B represents the melting point of pure Ag (961 °C)
At point ‘A’

At point ‘B’

- Eutectic Point ‘O’
It is a point where two curve AO and BO meet.
At this point, three phases are in equilibrium
At point ‘O’

Hence, system is invariant. This point ‘O’ represents the lowest possible temperature (303 °C) below which a liquid phase cannot exist and beyond which the liquid phase cannot be enriched in either component by freezing out the other component. Such type of liquid mixture of Pb and Ag which has the lowest freezing point corresponding to all other liquid mixtures is called eutectic mixture and corresponding temperature is known as eutectic temperature.
At Eutectic point Pb = 97.4%, Ag = 2.6% and Eutectic temperature = 303 °C.
Cooling of Melt in the Area Above AOB/Pattinson’s Process for Desilverization of Lead
When a liquid melt of certain composition at a point X (or X′) in the area above AOB is allowed to cool, it follows Newton’s Law of cooling, as follows the path XY(or X′Y′) in the phase diagram. Lead crystals start separating when point Y is reached and further follows the path YO as we continue the cooling of liquid melt and correspond silver crystals separate out along the path Y′O and point ‘O’ is reached, this point is known as eutectic point (low melting) and the solid mixture is called eutectic mixture which has a characteristic composition for each system.
8.7 HEAT TREATMENT OF STEEL
Heat treatment is the combination of heating and cooling of a metal or alloy in one or more temperature cycles to get desirable physical properties to the metals or alloys. Heat treatment of steel may be carried out under near equilibrium conditions to enhance the ductility or under non-equilibrium conditions to enhance the hardness. During heat treatment, the shape and size of the grains or the compositions of the phase undergoes changes with respect to the microconstituents..
Pure iron is not useful for fabrication of structure component because of its weak mechanical properties. So a non-metallic element i.e. carbon forms alloys with iron to give various type of steel and improves the mechanical properties of the base metal. Due to small size of carbon as compared with iron atom carbon interstitial positions in the lattice formed depends on the crystal structure of iron which in turn depends on the temperature, as pure iron exist in three allotropic modifications of α, γ and δ forms.
The low temperature allotropic form called the α-iron has a body-centered cubic (BCC) structure which is stable upto 910 °C. In the temperature range between 910 °C 1400 °C, γ-iron with a face centered cubic (FCC) structure is stable and the high temperature allotropic form δ-iron has a BCC structure and it is stable beyond 1400 °C and upto 1535 °C.
Plain carbon steel on heating to temperature more than 723 °C and maintained at this temperature for a long time allows the formation of the austenite phase and the dissolution of more carbon in the FCC structure. On slow cooling of the austenite phase transformation of FCC to BCC occurs and the excess carbon forms cementite.
Hardening
If the steel is quenched by plunging into water or oil to 204°C or a lower temperature, the carbon atom do not have sufficient time to form cementite but remain trapped in the BCC structure. The excess carbon precipitates out in the hot metal and prevents the slipping of the planes. Hence, quenched steel is quite hard and strong but has lower ductility; this heat treatment is called as transformation hardening. This involves the transformation of austenite to martensite or the bainite-phase making the steel hard.
Tempering
Due to its brittleness, the quenched steel is not useful for construction purpose; hence, quenching is always followed by another heat treatment process called tempering. The quenched steel is tempered by reheating to below the α-iron to γ-iron transition temperature. The residual stress and strain are relieved, and the excess of carbon is rejected in the form of ε-carbide (Fe2.4C). By tempering the steel becomes tougher and ductile. Tempering is carried out at about 200 °C to make hard steel resistant to abrasion or at higher temperature (~540 °C) to make tough steel capable of withstanding shock loads.
Annealing
This involves heating and holding the steel at a suitable temperature for some time to facilitate the dissolution of carbon in γ-iron in a furnace; steel is softened and becomes ductile and also machinable. However, annealing decreases the hardness and strength of the steel. Annealed hypereutectoid steel contains cementite. It is not soft but can be machined easily. In contrast, annealed hypo-eutectoid steel contains ferrite and is relatively soft and malleable.

8.8 REVIEW QUESTIONS
8.8.1 Fill in the Blanks
- A homogenous and physically distinct and mechanically separable part of system is called as ____________.
[Ans.: Phase]
- Number of phases when CaCO3(s) heated is ____________.
[Ans.: 3 (Three)]
- The smallest number of independent variable constituents by which the composition of each phase can be expressed in the form of chemical equation is ____________.
[Ans.: Components]
- The number of component when NH4Cl heated in a closed vessel is ____________.
[Ans.: One]
- The point at which the gaseous, liquid and solid phases of the system co-exist in equilibrium is called as ____________.
[Ans.: Triple point]
- Degree of freedom is zero at triple point is called as ____________.
[Ans.: Invariant system]
- At Invariant system, value of F is ____________.
[Ans.: Zero]
- In the expression C = S − R, R is number of ____________.
[Ans.: Independent chemical reactions]
- In case of ionic reaction, the formula for calculating number of component is ____________.
[Ans.: C = S − (R + 1)]
- The point which refers to the temperature and pressure where a liquid and its vapour become identical is called as ____________.
[Ans.: Critical Point]
- The degree of freedom at the triple point of one component system is ____________.
[Ans.: Zero]
- The existence of a solid substance in more than one crystalline form is called as ____________.
[Ans.: Polymorphism]
- In a phase diagram, the crossing of a two-phase equilibrium curve is called as ____________.
[Ans.: Transition]
- The melting point of ice can be lowered by an increase of ____________.
[Ans.: Pressure]
- Eutectic temperature of Pb-Ag system is ____________.
[Ans.: 303 °C]
- Phase diagram of Pb-Ag system is plotted between temperature and ____________.
[Ans.: Composition]
- Mathematical statement of reduced phase rule is ____________.
[Ans.: [F′ = C − P + 1]]
- At Eutectic point the composition of Pb = ____________ and Ag = ____________.
[Ans.: = 97.4%, 2.6%]
- Metals essential for solders are ____________ and ____________.
[Ans.: Lead and Tin]
- In order to get a two metal low melting alloy, ____________ composition is preferred.
[Ans.: Eutectic]
- The fundamental principle of freeze-drying is ____________.
[Ans.: Sublimation]
- Iron –Carbon alloys containing 1.7% of carbon are classified as ____________.
[Ans.: Steel]
- The percentage of carbon in cast iron is ____________.
[Ans.: 3%]
- The most important low melting alloys are ____________ and ____________.
[Ans.: Wood metal, Rose metal]
- A ____________ is a low melting alloy which is used to join two metal pieces together.
[Ans.: Solder]
- Soft solders mainly contain ____________ and ____________.
[Ans.: Sn, Pb]
- An ____________ equilibrium diagram indicates the phase changes during heating and cooling and the nature and amount of structural component of steel and cast-iron exist and any temperature.
[Ans.: Iron-carbon]
- The solubility or carbon in iron depends on the ____________ of iron.
[Ans.: Crystal structure]
- The curve above which the system consist of liquid phase only is ____________.
[Ans.: Freezing curve]
- The curve below which the system consist of solid phase only is ____________.
[Ans.: Melting curve]
8.8.2 Multiple-choice Questions
- The Gibb’s phase rule is given as
- F = C − P + 2
- F = C − P + 1
- F = C + P − 2
- F = C + P + 2
[Ans.: a]
- The condensed phase rule is given as
- F = C − P + 2
- F = C − P + 3
- F = C + P + 1
- F = C − P + 1
[Ans.: d]
- Which of the following has higher vapour pressure
- super-cooled water at -5 °C
- Ice at -5 °C
- Vapour at -5 °C
- None of these
[Ans.: a]
- Dissociation of NH4Cl in a closed vessel is a
- one component, one phase system
- one component, two phase system
- Two component, one phase system
- Two component, two phase system
[Ans.: b]
- A system consisting of rhombic sulphur, monoclinic sulphur and vapour in equilibrium is
- Non variant
- univariant
- bivariant
- trivariant
[Ans.: a]
- In the lead-silver system, the percentage of silver present at the eutectic point is
- 0%
- 2.6%
- 97.4%
- 100%
[Ans.: b]
- In water system, the three phases exist in equilibrium at
- 0 °C, 1 atm
- 0 °C, 4.58mm
- 0.0098 °C, 1 atm
- 0.0098 °C, 4.58mm
[Ans.: d]
- Water system is non variant at
- Melting point
- Boiling point
- Triple point
- Critical point
[Ans.: c]
- Number of phases present in a mixture of N2 and H2 are
- 1
- 2
- 3
- 4
[Ans.: a]
- Number of component present in the following reaction CaCO3(s) → CaO(s) + CO2(g)
- 1
- 2
- 3
- 4
[Ans.: b]
- At the eutectic point, a system has
- The lowest melting point
- The highest melting point
- Only two phases
- Uncertain composition
[Ans.: a]
- What is degree of freedom at eutectic point
- 1
- 0
- 2
- 3
[Ans.: b]
- At eutectic point, composition of Pb-Ag system
- 100% Pb + 0% Ag
- 0% Pb + 100% Ag
- 50%Pb + 50% Ag
- 97.4%Pb + 2.6% Ag
[Ans.: d]
- For C = 1 and P = 3, the degree of freedom is
- 0
- 1
- 2
- 3
[Ans.: a]
- For water system, the value of component is
- 0
- 1
- 2
- 3
[Ans.: b]
- Two miscible liquids such as water and alcohol has how many phases
- 1
- 2
- 3
- 4
[Ans.: a]
- Two inmiscible liquids such as water and mercury has how many phases
- 1
- 2
- 3
- 4
[Ans.: b]
- A system of NaCl-KCl-H2O has how many components
- 1
- 2
- 3
- 4
[Ans.: c]
- A binary system consisting of two substances, which are miscible in all proportions in the liquid phase, but do not react chemically is known as
- Invariant system
- Univariant system
- Metastable system
- Eutectic system
[Ans.: d]
- The hardest structure that appears on the iron-carbon equilibrium is
- Ledeburite
- Pearlite
- Cementite
- Ferrite
[Ans.: c]
- In Pb-Ag system, the euetectic temperature is
- 298 °C
- 300 °C
- 303 °C
- 310 °C
[Ans.: c]
- The melting point of pure Pb and pure Ag is
- Pb = 100 °C, Ag = 100 °C
- Pb = 373 °C, Ag = 298 °C
- Pb = 327 °C, Ag = 961 °C
- Pb = 961 °C, Ag = 327 °C
[Ans.: c]
- A system consists of water ⇋ vapour, the degree of freedom is
- 0
- 1
- 2
- 3
[Ans.: b]
- With increase of pressure, melting point of ice is
- Increase
- decreases
- remains unchanged
- doesn’t show any behaviour
[Ans.: b]
- For one component system, the maximum number of degree of freedom is
- 0
- 1
- 2
- 3
[Ans.: c]
- A binary alloy system having value of component is
- C = 1
- C = 2
- C = 3
- C = 4
[Ans.: b]
- Soft or Tinner’s solder consists of mainly
- Pb and Sn
- Pb and Ag
- Sn and Ag
- Pb and Bi
[Ans.: a]
- Wood metal alloy containing metal is
- 50% Bi + 20% Pb + 20% Sn + 10% Cd
- 20% Bi + 50% Pb + 20% Sn + 10% Cd
- 50% Bi + 25% Pb + 12.5% Sn + 12.5% Cd
- 25% Bi + 50% Pb + 12.5% Sn + 12.5% Cd
[Ans.: c]
- Plumber solder used for soldering consist of
- 67% Pb + 33% Sn
- 50% Pb + 50% Sn
- 33% Pb + 67% Sn
- 25% Pb + 75% Sn
[Ans.: a]
- The principle of freeze-drying is
- Evaporation
- condensation
- sublimation
- Fusion
[Ans.: c]
8.8.3 Short Answer Questions
- Define or state phase rule.
Ans.: Phase rule can predict qualitatively the effect of temperature, pressure and concentration on a heterogenous system which is in equilibrium and it is assumed that equilibrium is not influence by gravity, electrical or magnetic forces, or by surface action. The degree of freedom is related to number of components (C) and of phases (P) by the phase rule equation.
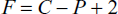
- What is condensed phase rule?
Ans.: When the pressure of the system remains constant, then the phase rule equation becomes.

- Point out the effect of increase of pressure on the melting point of ice.
Ans.: Melting point of ice decreases with rise of pressure as shown in the water system diagram that curve is inclined towards pressure axis.
- Define phase
Ans.: A phase may be defined as any part of a system which is homogeneous in itself, physically distinct, and mechanically separable from other parts of the system.
- Calculate the, degree of freedom of the following systems
- Unsaturated solution of NaCl in equilibrium with its vapour.
- Na2SO4 in water a closed contained at 32.4 °C
Ans.: (i) The system has two phases, solution and vapour and it is a two component system.
Then degree of freedom

So, the system is bivariant,
(ii) The system has two phases, solution and vapour and it is a two component system. Since Temperature is fixed at 32.4 °C. So we will apply condensed phase rule equation to evaluate degree of freedom

So, the system is univariant.
- What is the number of phases when CaCO3(s) is heated,
Ans.:

Three phases i.e., two solids and one gaseous.
- How many components are present when NH4Cl is heated in closed vessel.
Ans.: One.
- Give the number of phases, components and degree of freedom of following
- Mixture of N2 and H2 contained in a vessel.
- Ice, water and vapour in equilibrium.
- An unsaturated sugar solution.
- A system consisting of NaCl, KCl, and H2O.
- Dissociation of NH4Cl in a closed vessel.
- Dissociation of NH4Cl in a closed vessel containing NH3 also.
Ans.:
- P = 1 (gaseous phase)
C = 2 (N2, H2)
F = C − P + 2 = 2 − 1 + 2 = 3
- P = 3 (ice, water, vapour)
C = 1 (water)
F = C − P + 2 = 1 − 3 + 2 = 0
- P = 2 (solid, solution)
C = 2 (sugar, water)
F = C − P + 2 = 2 − 2 + 2 = 2
- P = 1 (solution)
C = S − R = 5 − (2 + 1) = 2
F = 2 − 1 + 2 = 3
- P = 2 (solid, gas)
C = 1 (NH4Cl)
F = C − P + 2 = 1 − 2 + 2 = 1
- P = 2 (solid, gaseous)
C = 2 (NH4Cl, NH3)
F = C − P + 2 = 2 − 2 + 2 = 2
- Classify the following into true and metastable equilibria
- Ice and liquid water at atmospheric pressure and 0 °C
- water at -3 °C
- water and its vapours at 20 °C
- SR and monoclinic SM below 95.5 °C
Ans.:
- True equilibrium
- Metastable equilibrium
- True equilibrium
- True equilibrium
- Metastable equilibrium
- What is the criterion of multiphase equilibrium?
Ans.: Chemical potential of a component must be same in all phases in equilibrium.
- What is an invariant system.
Ans.: A system in which degree of freedom is zero i.e., no condition is required to be specified to define the system.
- Give an example of invariant system.
Ans.: A system consisting of ice, water and water vapour in equilibrium.
- Give a mathematical statement of phase rule in the following cases:-
- When temperature, pressure and concentration are variables.
- When temperature and concentration are variables
- When concentration and pressure are variables.
- When concentration, pressure, temperature and magnetic force are variables.
Ans.:
- F = C - P + 2
- F = C - P + 1
- F = C - P + 1
- F = C - P + 3
- Give the degree of freedom of one component system in the following cases
- When a single phase exists.
- When two phases are in equilibrium.
- When three phases are in equilibrium.
Ans.:
- Two
- One
- Zero
- What is the minimum number of phases that can co-exist in a one component system.
Ans.: Three
- What is triple point.
Ans.: A point at which the gaseous, liquid and solid phases of the system co-exist in equilibrium
- What is the triple point of water system.
Ans.: When system is at 0.0098 °C and 4.58 mm Hg pressure.
- What is the difference between a phase and a state of matter.
Ans.: There are three states of matter-solid, liquid and gas. A phase is a sample of matter with definite composition and uniform properties throughout the sample.
- What is meant by polymorphism.
Ans.: The existence of a solid substance in more than one crystalline form.
- What is meant by transition?
Ans.: The crossing of a two-phase equilibrium curve in a phase diagram.
- What is difference between critical point and triple point,
Ans.: Critical point refers to the temperature and pressure where a liquid and its vapour become identical while triple point is the condition of temperature and pressure under which three phases of a substance co exit in equilibrium.
- What is metastable state.
Ans.: The state of super cooled or super saturated solution in which the phase, which is normally stable under the given conditions, does not form, under normal conditions.
- What is meant by the term eutectic?
Ans.: A solid solution of two or more substances having the lowest freezing point of all the possible mixture of the components.
- What is eutectic composition of Pb-Ag system
Ans.: 97.4%Pb and 2.6% Ag.
- What is the degree of freedom at eutectic point?
Ans.: zero
- State the condition in which two substances can form a simple eutectic.
Ans.: The two substance must
- Be completely miscible in the liquid state, but immiscible in the solid state
- Not chemically react with each other
- Justify the statement the eutectic is a mixture and not a compound.
Ans.: Eutectic is a mixture of two solids, which exists at the lowest melting point. Since eutectic is completely immiscible in the solid state, so it is a mixture and not a compound.
- What is the eutectic temperature of Pb-Ag system.
Ans.: Euetctic temperature: 303 °C
- Name two systems which form eutectic mixtures,
Ans.:
- NH4Cl +ice
- KNO3 + ice
- Which metals form the essential constituents of solders and what is the composition of tinman’s solder?
Ans.: Lead and Tin are the essential constituents of solders,
Composition of tinman’s solder is
66% Sn + 34% Pb
- What is a phase diagram
Ans.: Phase diagram is obtained by plotting concentration versus temperature.
- Why do study phase diagram.
Ans.: To predicts whether a triple point or eutectic alloy or solid solution is formed on cooling a homogenous liquid mixture containing two metals.
8.8.4 Solved Numerical Problems
- Calculate the number of phases, components and degree of freedom in the following systems
- CaCO3(s) → CaO(s) + CO2(g)
- H2O(s) → H2O(g) + H2O(l)
- An aqueous solution of NaCl and Na2SO4
Solution
- CaCO3(s) → CaO(s) + CO2(g)
Number of phases = 3 [i.e. CaCO3(s), CaO(s), CO2(g)]
Number of component, C = S − R
S = 3 (number of species)
R = 1 (Number of relation)
C = S − R = 3 − 1 = 2 Component system
So, according to phase rule
F = C − P + 2 = 2 − 3 + 2 = 1 (univariant)
- H2O(s) → H2O(g) + H2O(l)
Number of component = 1 (H2O only)
Number of phases = 3 (solid, liquid, vapour)
So, degree of freedom
F = C − P + 2
= 1 − 3 + 2 = 0 (Invariant)
- An aqueous solution of NaCl and Na2SO4
Number of phases, P = 1 (i e solution)
Number of component, C = 3 (i.e NaCl, Na2SO4, H2O)
So, degree of freedom
F = C − P + 2 = 3 − 1 + 2 = 4
- Write down the number of component, number of phases and calculate the degree of freedom for the following equilibria:-
- N2(g) + 3H2(g) → 2NH3(g)
- Fe(S) + H2O(g) → FeO(s) + H2(g)
- H2O(g) → H2(g) + ½O2(g)
Solution
- Chemical species present are
N2(g), H2(g) and NH3(g)
Number of component C = S − R
S = 3 [Number of species N2, H2 and NH3]
R = 1 [Number of relation]
C = S − R = 3 − 1 = 2

- Chemical species present are
Fe(s), FeO(s), H2O(g), H2(g)

- Number of species present are
H2O(g), H2 (g), and O2(g)
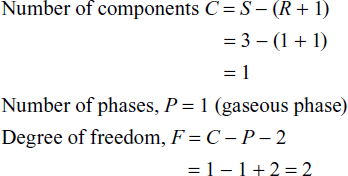
- Explain KCl-NaCl-H2O is a three component system while NaBr-KCl-H2O is a four component system.
Solution
First system KCl-NaCl-H2O
Total Number of species, S = KCl, NaCl, H2O, K+, Cl−, Na+ (dissociation of water is neglected)
S = 6
Number of independent relations, R = 2
KCl ⇌ K+ + Cl−
Nacl ⇌ Na+ + Cl−
Thus, C = S − (R + 1) = 6 − (2 + 1) = 3
In the second system,
Total number of species, S = 9
KCl, NaBr, H2O, NaCl, KBr, Na+, K+, Cl−, Br−
The number of independent reactions, R = 4
KCl ⇌ K+ + Cl−
NaBr ⇌ Na+ + Br−
Na+ + Cl− ⇌ NaCl
K+ + Br− ⇌ KBr
C = S − (R + 1)
= 9 − (4 + 1) = 4
It is a four component system.
8.8.5 Descriptive Questions
Q.1 State phase rule and explain the significance of the term involved. Illustrate with suitable examples.
Q.2 Define the terms with suitable example.
- Phase
- Component
- Degree of freedom
- Triple point
- Eutectic mixture
- Condensed phase rule
Q.3 Draw a phase diagram for one component water system. Label it and discuss the importance of various points, lines and areas at equilibrium.
Q.4 What is meant by triple point of water?
Why is it different from the normal melting point of ice?
Q.5 Differentiate between True equilibrium and Metastable equilibrium?
Q.6 Derive Gibb’s phase Rule equation.
Q.7 Justify the statement ‘The Eutectic is a mixture and not a compound.”
Q.8 What is meant by eutectic point? Explain how can the eutectic point be calculated ? Discuss the Pb – Ag system.
Q.9 What is the number of phases in the following systems?
- Saturated solution of NaCl
- Mixture of rhombic and monoclinic sulphur
- Mixture of O2 and N2
- Mixture of benzene and water
Q.10 Water system is a respresentative system for explaining phase rule and phase equilibria. Explain.
Q.11 Discuss a typical one-component system from the stand point of phase rule.
Q.12 Explain why KCl-NaCl-H2O should be regarded as a three component system: whereas KCl-NaBr-H2O should be regarded as a four component system.
Q.13 What is condensed phase rule? When is it applied?
Q.14 Define and explain the term components of a system with suitable examples.
Q.15 Discuss the salient feature of Fe-C system.
Q.16 Define the various curves involved in water system with a neat and sketched diagram, why is the fusion curve in the phase diagram of water system inclined towards the pressure axis? Explain
Q.17 What is a triple point? Explain triple point with reference to water system.
Q.18 Explain the terms
- Stable equilibrium,
- Metastable equilibrium,
Q.19 Derive phase rule equation viz. F = C − P + 2, why reduced phase rule is applicable in Pb-Ag system.
Q.20 Calculate P, C and F in the following cases
- NH3(g) at 42 °C
- An emulsion of oil in water at 2 atm and 70 °C
- SR ⇋ SM at the transition temperature
- Pure crystal of CuSO4 5H2O
- Water system at 4.578 mm of Hg and at 0.0098 °C
