9
PHOTOCHEMISTRY
9.1 INTRODUCTION
Photochemistry is the branch of chemistry which deals with the effect of light on chemical systems. A photochemical reaction is caused by the absorption of electromagnetic radiation, especially ultraviolet (UV)—visible radiations. Photochemical reactions proceed differently from thermal reactions; hence they have a lot of importance in organic and inorganic chemistry. The photochemical path offers advantages over thermal methods of forming thermodynamically disfavoured products by overcoming large activation barriers and the reaction will be completed in a short period of time. Hence, many thermal reactions have photochemical counterparts.
Some examples are photosynthesis, formation of Vitamin D with sunlight in the body and ozone formation when oxygen is exposed to sun light.
9.2 LIGHT SOURCE IN PHOTOCHEMISTRY
Photochemists typically work in only a few sections of the electromagnetic spectrum. The most widely used sections are UV and visible regions. The main source of light is the sun, mercury (high, medium and low pressure) lamps, sodium lamps, halogen lamps, LASER, etc. Different lamps and their intensity ranges are shown in Table 9.1.
Table 9.1 Different lamps and their intensity ranges

The common types of reactors, vessels and apparatus used for photochemical reactions are irradiated flasks (used for external irradiation), immersion-well reactors (here, the lamp is surrounded by the reaction solution), falling film apparatus and photo-microreactors (similar to falling films, but easy to handle). In all these cases, the lamp usually requires cooling to avoid its overheating.
Solid or liquid optical filters may be used to restrict the irradiation wavelength, the glass acts as a good solid filter. The different glasses used for irradiation at different wavelengths are as follows:
- Quartz glass to irradiate at 254 nm
- Pyrex glass to irradiate at 300 nm
- Normal lab glass to irradiate > 350 nm
9.3 LAWS OF PHOTOCHEMISTRY
Photochemical reaction is initiated by electronically excited molecules or atoms produced by the absorption of electromagnetic radiation, usually visible or near the UV region.
Photochemical reactions are governed by three basic principles. They are as follows:
- Grotthuss–Draper law
- Stark-Einstein law of photochemical equivalence
- Beer-Lambert law
9.3.1 Grotthuss–Draper Law or The First Law of Photochemistry
This law states that light must be absorbed by a chemical substance for a photochemical reaction to take place. Photoexcitation is the first step in the photochemical process, where the reactant is elevated to a state to higher energy an excited state. The Grotthuss–Draper law is also known as the first law of photochemistry.
When light pass through any substance, only a fraction of the incident light which is absorbed by the substance brings about a chemical change and the reflected and transmitted light do not produced any such affect. Hence, it is important to remark that in all light radiations, which are absorbed, the reaction systems do not cause an affect in producing the final product. In some cases, the absorbed light is reemitted as radiations of the same belong to a different frequency.
The Grotthuss–Draper law is purely qualitative assumption; it does not explain the relation between the amount of light absorbed by the system and the number of molecules which have reacted and give a final product.
9.3.2 Stark-Einstein Law or Photochemical Equivalence Law
According to this law, for each photon of light absorbed by a chemical system, only one molecule is activated for the subsequent reaction. This photoequivalence law was derived by Einstein during his development of the quantum theory of light; hence it is known as the Stark-Einstein law. No more than one molecule is activated for the photochemical reaction, as defined by the quantum yield.
According to this law, the part of a light induced on the system the primary process can occur this is the initial chemical change that results directly from the absorption of light. However, in most photochemical reactions, the primary processes are usually followed by a secondary process due to normal interactions between the reactants and they are not required to absorb any light radiation. As a result, such reactions do not obey the one quantum–one molecule reactant relationship.
The law is further restricted to conventional photochemical processes using the light source of moderate and high intensity sources; those used in flash photolysis and laser experiments are known as biphotonic processes—the absorption by a molecule of a substance of two photons of light.
9.3.3 Beer-Lambert Law
The Beer-Lambert law proposed that the absorption of light or single wave length is related to the exponential to the thickness of the absorbing compound and the optical path of light thus:
![]() (1)
(1)

Equation (1) is subsequently modified that thus the Beer-Lambert law of light absorbing is formulated as follows:

9.4 PHOTOPHYSICAL AND CHEMICAL PROCESSES
After absorbing the energy, the excited molecule may undergo different physical and chemical processes.
9.4.1 Photophysical Process
The excited or energised molecule may return to its initial state by any of the following physical processes:
- The molecule can release the excitation energy by emitting radiation through fluorescence or phosphorescence.
- The absorbed energy may transfer to some other molecule to colloids, without emitting light or giving product.
- An electron in the atom or molecule may absorb so much energy that it may escape from the atom or molecule, leaving behind the positive M+ ion by photoionisation.
Jablonski Diagram
The photophysical process can be easily explained by the Jablonski diagram. Once a molecule that has absorbed energy in the form of electromagnetic radiation goes to excitation state, while coming back to the ground state, a number of paths may follow.
Quantum mechanics explains internal conversion of energy as a transfer of excess electronic energy into excess vibrational energy of a lower electronic state, and followed by dissipation of vibrational energy into the surroundings as heat. New jabolonski diagram giving a glance about photo physical process shown in Figure 9.1.
Internal Conversion
Internal conversion is an intermolecular conversion of molecules which possess to a lower electronic state without emitting radiation. The higher excited singlet states (S1, S2, S3, … generally denoted as Sn) and the lowest energy triplet states (T1, T2, T3, …) perform a crossover of two states with the same multiplicity, that is, singlet to singlet or triplet to triplet state. The internal conversion is more efficient when two electronic energy levels are close enough that two vibrational energy levels can overlap in between S1 and S2.
Fluorescence
The internal conversion can also occur in between S1 and S0 (lowest energy or ground state) and is much slower, allowing time for the molecule to emit a photon or loss of energy from a higher excited state. This is known as fluorescence.
Inter-system Crossing
The internal conversion from S1 to S0 is due to the vibrational levels of the ground state overlap with the first excited state for some molecules, which leads to fast deactivation.
Inter-system crossing is a radiation-less process involving a transition between two different multiplicities—that is S1 (singlet) to T1 (triplet) electronic states. The probability of inter-system crossing is due to the overlapped vibration levels of the two singlet states. This is commonly observed in molecules containing heavy atoms such as iodine or bromine. The spin and orbital interaction increases, and the spin becomes more favourable; paramagnetic species also enhance inter-system crossing, which consequently decreases fluorescence.
Phosphorescence
The emission of photon by internal conversion of electron from T1 to S0 is known as phosphorescence. The triplet states (with parallel spins) interact more strongly than singlet states (with opposing spins), the energy difference of T1 – S0 is less than S1 – S2. Hence, phosphorescence occurs at longer wavelengths than fluorescence. Important photo physical processes and their transitions shown in Table 9.2.
Table 9.2 Photo physical processes and their transitions

9.4.2 Photochemical Process
A photochemical reaction is a chemical reaction initiated by the absorption of energy in the form of light.
Primary Photochemical Process
If the excited molecule (M*) reacts, it may undergo any of the following chemical processes:
- Photodissociation
- Inter-molecular rearrangement
- Reaction with another molecule

Figure 9.1 Jablonski diagram showing photophysical process
Photodissociation
Dissociation of the molecules of a substance is caused by absorption of radiation energy into atomic and/or molecular fragments.
![]()
Photoisomerisation
The conversion of a molecule into its isomer with the same number and types of atoms but a different structural arrangement is called photoisomerisation.
![]()
Inter-molecular Rearrangement
By irradiating with light, the molecule will absorb the light energy and will rearrange.
![]()
Secondary Photochemical Process
The secondary process may occur upon completion of the primary step.
Several examples of such process are described here:
- Ozone formation:

- Destruction of ozone in the upper stratosphere
- Chain reaction
9.5 QUANTUM YIELD AND QUANTUM EFFICIENCY
The efficiency of the photochemical reaction is expressed by the quantum efficiency or quantum yield.
It is a measure of the number of product molecules formed by the quantum of energy absorbed from each photon.
ϕ is defined as the number of molecules reacted for every number of quanta of light absorbed:

The concept of quantum efficiency was first introduced by Einstein as each quanta of light absorbed least formation of product. Though ϕ = 1, in practice, it can be observed from 10−2 to 107.
Low quantum is absorbed in cases where the deviation of the molecules takes place before they form the product. The deviation may take place by collisions of excited molecules split into other excited molecules or non-excited molecules in cases where the primary photochemical process gets reversed. The dissociation of molecule takes place and the dissociated fragments may be recombined to form the original molecule.
High quantum efficiency is observed in free radical reactions. Photochemical free radical energy is absorbed only in the chain initiation step to the formation of chain initiation-free radical, but will propagate the reaction in the propagation step without absorbing any energy. This process is continuous until the product is formed in the termination step.
9.6 PHOTOSENSITISATION
Photosensitisation is a process wherein an electronically excited molecule transfers its energy to another non-radioactive molecule. After transferring energy, the other molecule gets excited and undergoes photochemical change. This process is called photosensitisation. Photosensitisation may be inter-molecular or intra-molecular.
The initially excited molecule D* is designated as donor and the non–radioactive molecule is designated as the acceptor. This process is represented by this scheme:

There are two types of mechanisms postulated for non-radioactive energy as follows:
- Long-range transfer by dipole-dipole interaction
- Short-range transfer by change interaction
9.7 PHOTODYNAMIC THERAPY
Cancer can be defined as a class of disease characterised by uncontrolled growth of a group of cells beyond their normal limit and invasion to adjacent healthy tissues, which involves dynamic changes in the genome. Photodynamic therapy (PDT) has emerged as a promising non-invasive chemotherapeutic technique for the treatment of cancer which uses light to activate the drug molecule (photosensitiser) to produce reactive singlet oxygen species in the presence of molecular oxygen to damage the cancer cells. Here, cancer cells are selectively targeted and damaged by red light of 600–800 nm wavelength (photospectral window) by leaving the healthy cells unaffected.
In PDT, the drug is first injected into the patient’s body and sufficient time is given to accumulate the drug inside the tumour. Subsequently, light is selectively exposed to the tumour to activate the photosensitiser and to kill the cancer cells. Three essential components for the PDT are photosensitiser, light of a particular wavelength and molecular oxygen. A photosensitiser is a biocompatible light sensitive molecule which can be photoexcited by a particular wavelength of light. In the excited state, it can transfer energy to the molecular oxygen to generate reactive oxygen species like singlet oxygen.
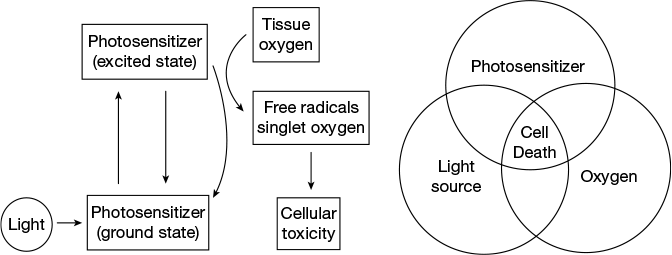
The photosensitiser used in PDT is mostly the organic molecule. An ideal photosensitiser suitable for PDT application must fulfil the following criteria. First, the dark toxicity should be as less as possible. Second, it should have an excited absorption band at visible wavelength, preferably in the range of 600–800 nm which is known as the PDT window. Shorter wavelength has less tissue penetration and often leads to skin photosensitivity. Longer wavelength of light lowers the quantum yield of triplet formation which hinders energy transfer to the ground state oxygen molecule to excite it to the singlet state. Third, the photosensitiser should have good aqueous solubility and its excretion from the patient’s body should be rapid to avoid side effects.
9.8 IMPORTANT PHOTOCHEMICAL REACTIONS
Some examples of photochemical reactions are as follows:
Photosynthesis
- Plants use solar energy to convert carbon dioxide and water into glucose and oxygen with the help of chlorophyll.
- Vitamin D is formed when the human body is exposed to sunlight.
Polymerisation
Many free radical polymerisation reactions start by photoinitiators, which decompose upon absorbing light to produce the free radicals.
Photodegradation
In many drug bottles, the first precaution is to preserve the drug in a cool and dark place. This is essential to avoid the drug from photodegradation. For example, poly vinyl chloride medicine bottles.
Photodynamic Therapy
Singlet oxygen is generated by the photosensitised drug when it is exposed to light; it destroys tumours without affecting normal healthy cells.
Organic Photochemistry
- Many organic reactions are initiated by light to give final products with low cost. For example, the formation of cyclo compounds from alkenes, Zimmerman’s di-pi-methane rearrangement, etc.
- Industrial preparation of benzyl chloride by the gas-phase photochemical reaction of toluene with chlorine.


- Mercaptan can be prepared by the photochemical addition of hydrogen sulphide to alpha olefins.
Inorganic and Organometallic Photochemistry
Many coordination and organometallic compounds are also photoactive and involve cis–trans isomerisation, dissociation of ligands in presence of light.
For example, tetra hydro furan solution of molybdenum hexacarbonyl gives the THF complex in presence of UV light, which is synthetically useful.
![]()
Like this reaction, the photolysis of iron pentacarbonyl affords diiron nonacarbonyl.
Luminescence
Emission of light by a substance, which is not due to heating of the substance, is called luminescence. Therefore, it is a form of cold body radiation.
Bioluminescence
In fireflies, an enzyme in the abdomen catalyses a reaction that produces light.
Chemical Luminescence
The light produced as a result of chemical reaction is called chemiluminescence.
For example, [A] + [B] → product + light
Luminol + H2O2 (B) → 3-aminophthalate + light
Due to the reaction of luminol with H2O2 in the presence of catalyst, it involves in excitation of molecule in electronic energy levels and produces 3-aminophthalate and light.
Photovoltaic Cell
Photovoltaic (PV) cells (or solar cells produce electricity by photoelectric effect; hence, PV cells are the building blocks of all PV systems because they are the devices that convert sunlight to electricity.
PV cells are made of semi-conducting materials with different sizes and shapes. They are connected together to form PV molecules that may be up to several feet long and a few feet wide.
Molecules, in turn, can be combined and connected to form PV arrays of different size and power output; such as electrical connections, mounting hardware, power-conditioning equipment and batteries that use solar energy when the sun is not shining.
When light passes on a PV cell, it may be reflected or absorbed; but only the absorbed light generates electricity. The energy of the absorbed light is transferred to electrons in the atoms of the PV cell semiconductor material, and these electrons escape from their normal position in the atoms and become a part of the electrical flow in an electrical circuit.
Molecular Photochemistry
This is the study of artificial assemblies of two or more molecules to understand the biological process and the design of artificial systems capable of performing of useful functions.
The main three types of supramolecular systems in the area of co-ordination chemistry are as follows:
- Second-sphere coordination compounds:
For example, hexacyano cobalt (III) anion with poly ammonium macro cyclic receptors.
The complex is associated with other species by electrostatic interaction, hydrogen bonds or other intra molecular forces.
- Cage-type co-ordination compounds:
This refers to complexes in which the metal ion is encapsulated in a single polydentate ligand.
For example, cage-type cobalt (III) complex.
- Molecular building blocks linked via bridging units by means of covalent or co-ordination bonds.
9.9 REVIEW QUESTIONS
9.9.1 Fill in the Blanks
- Crossover of electronically excited molecule two states with same multiplicity is called ___________.
[Ans.: Internal conversion]
- ________ can prevent the drugs from photodegradation.
[Ans.: Poly vinyl chloride medicine bottles]
- Vision is initiated by a photochemical reaction of ________.
[Ans.: Rhodopsin]
- When the excited molecule is breaks into its atomic or molecular fragment, it is called ____________.
[Ans.: Photodissociation]
- Who introduced the quantum efficiency concept?
[Ans.: Einstein]
9.9.2 Multiple-choice Questions
- _____________ is concerned with chemical effects of light.
- Photochemistry
- Photolysis
- (a) and (b)
- None of these
[Ans.: a]
- The law that explains thickness of absorbing light and optical path of light is
- Bear-Lamberts law
- Grotthuss–Draper law
- Stark-Einstein law
- None of these
[Ans.: a]
- Electronically excited molecules that return to ground state with same multiplicity are called
- Phosphorescence
- Fluorescence
- Photosensitisation
- Luminescence
[Ans.: b]
- Second-sphere co-ordination compounds belongs to
- Intra molecular photochemistry
- Inter molecular photochemistry
- Supramolecular photochemistry
- Super molecular photochemistry
[Ans.: c]
- Light formed from chemical reaction is called
- Luminescence
- Bioluminescence
- Chemiluminescence
- All the above
[Ans.: c]
- Fire flies are an example of
- Bioluminescence
- Photolysis
- Photosynthesis
- Photoisomerisation
[Ans.: a]
9.9.3 Short Answer Questions
- What are basic laws of photochemistry?
Ans.: The photochemical reactions are governed by the following two basic principles:
- Grotthuss–Draper law
- Stark Einstein law of photochemical equivalence.
- Define photochemistry.
Ans.: Photochemistry is a branch of chemistry which is concerned with the effect of chemical reactions caused by absorption photon from UV-visible and IR radiation.
- Explain inter-system crossing.
Ans.: Inter-system crossing is a radiation-less process involving transition between two electronic states with different multiplicities.
- Describe photosensitisation.
Ans.: It is a process wherein an electronically excited molecule transfers its energy to non-radioactive molecule to another type of molecule. After excitation, it undergoes a chemical change called photosensitisation.
- What is meant by photovoltaic cell?
Ans.: A photovoltaic cell or solar cell is a device in which electricity is produced by photoelectric effect.
- Name the types of supramolecular systems.
Ans.: Supramolecular systems are of three types as follows:
- Second-sphere co-ordination compounds
- Cage-type co-ordination compounds
- Molecular building blocks linked via bridging units by means of covalent or co-ordination bonds.
9.9.4 Descriptive Questions
Q.1 Explain the laws of photochemistry.
Q.2 Describe quantum yield and quantum efficiency of photochemistry.
Q.3 Explain the photochemical process in photochemistry.
Q.4 Explain the photosensitisation process in detail.
Q.5 Give an account of electronically excited states in photochemistry with an energy level diagram.
Q.6 Explain the photochemical reactions in photochemistry.
Q.7 Give a detailed account of photovoltaic cell.
Q.8 Describe supramolecular photochemistry.
