10
SURFACE CHEMISTRY
10.1 INTRODUCTION
“Surface chemistry is the branch of chemistry which deals with the study of the phenomenon occurring at the surface or interface, that is, at the boundary separating two bulk phases.” We know that number of phenomena occur at the interface, for example, dissolution, crystallization, corrosion, electrode processes, etc.
10.2 ADSORPTION
It is observed that when certain solids like charcoal, silica, etc., are added into a closed vessel containing some gas or aqueous solution of a solute, the molecules of the gas or the solute are attracted towards the surface of the solid and then retained on the surface. As a result, the concentration of the gas or the solute on the surface of the solid increases. This is known as adsorption.
Hence, it may be defined as follows:
- Adsorption: The phenomenon of attracting and retaining the molecules of a substance on the surface of a liquid or a solid resulting into a higher concentration of the molecules on the surface is called adsorption.
- Adsorbate: The substance (gas or vapour or the solute) that is adsorbed on the surface (liquid or solid) is called adsorbate.
- Adsorbent: The substance (liquid or solid) that adsorbs either gas or vapour or the solute is called adsorbent.
- Desorption: The removal of adsorbed substance from the surface is called desorption. It is generally brought by heating or reducing the pressure.
- Occlusion: The adsorption of gases on the surface of metals is called occlusion.
As greater is the surface area of the adsorbent, greater is the adsorption, therefore, finely divided metals or substances having porous structure, for example charcoal, silica gel, clay, etc act as excellent adsorbents.
Examples of adsorption:
- Adsorption of solute by charcoal: When aqueous solution of raw sugar (which has yellowish brown colour) is shaken with animal charcoal and then filtered, the filtrate is colourless, which gives white crystals of sugar on crystallization. This is because the brown colouring substances are adsorbed by animal charcoal.
- Adsorption of moisture by silica gel: When silica gel is placed in a closed vessel containing moist air, the air becomes dry after some time. This is because the water molecules are adsorbed on the surface of silica gel.
10.2.1 Mechanism of Adsorption
We know that molecules in the interior of a liquid are completely surrounded by other molecules on all sides, and hence intermolecular forces of attraction are exerted equally in all directions. However, a molecule at the surface of a liquid is surrounded by large number of molecules (Figure 10.1). Because the unbalanced, inward forces of attraction on the surface of liquid or solid have the property to attract and retain the molecules of a gas or solute on their surfaces.

Figure 10.1 Molecules at the surface experiencing a net inward force of attraction in case of (a) liquid and (b) solid
10.2.2 Adsorption is Exothermic
During adsorption process, the residual forces on the surface of the adsorbent decreases; it means surface energy decreases. This decrease that appears in the form of heat is called heat of adsorption. Hence, adsorption is an exothermic process, that is, ΔHadsorption is always negative. “The amount of heat evolved when 1 mole of any gas is adsorbed on a solid adsorbent surface is called “heat of adsorption or enthalpy of adsorption.”
10.2.3 Difference between Adsorption and Absorption
Adsorption is different from absorption. The former refers to the attraction and retention of the molecules of a substance on the surface only, while the latter involves distribution of substance that is uniformly distributed over the liquid or the solid.
When both adsorption and absorption take place simultaneously, the process is called sorption. Representation of the three processes is shown in Figure 10.2, and the differences between adsorption and absorption are given in Table 10.1.

Figure 10.2 (a) Adsorption process (b) Absorption process and (c) Sorption process
Table 10.1 Differences between adsorption and absorption

10.2.4 Examples of Adsorption, Absorption, and Sorption
- When ammonia gas is placed in contact with charcoal, it gets adsorbed on the charcoal, whereas when ammonia gas is placed in contact with water, it gets absorbed into the water, giving NH4OH solution of uniform concentration.
- Dyes gets adsorbed as well as absorbed in the cotton fibers, that is, sorption takes place.
10.2.5 Positive and Negative Adsorptions
- When the concentration of the adsorbate is more on the surface of the adsorbent than in the bulk, it is called positive adsorption.
E.g.: When concentrated solution of KCl is shaken with blood charcoal, it shows positive adsorption.
- When the concentration of the adsorbate is less on the surface of the adsorbent than in the bulk, it is called negative adsorption.
E.g.: When a dilute solution of KCl is shaken with blood charcoal, it shows negative adsorption.
10.2.6 Classification of Adsorption
There are two main types of adsorption recognized:
- Physical adsorption or physisorption or van der Waal’s adsorption
- Chemical adsorption or chemisorption or Langmuir adsorption or activated adsorption
- Physical adsorption: In such type of adsorption, the gas molecules (adsorbed) are held on the surface of a solid by van der Waal’s forces without resulting into the formation of any chemical bond between adsorbate and adsorbent. It is called physical adsorption.
- Chemical adsorption: In such type of adsorption, the gas molecules are held on the surface of a solid by forces similar to those of a chemical bond (ionic or covalent). This type of adsorption results in the formation of chemical bond between adsorbate and adsorbent; it is called chemical adsorption. The differences between physical and chemical adsorptions are given in Table 10.2.
Table 10.2 Differences between physical and chemical adsorptions

10.2.7 Factors Affecting the Adsorption of Gases by Solids
The amount of gases adsorbed on the surface of solid depends upon the following factors:
- Nature and surface area of the adsorbent: It is observed that the extent of adsorption depends upon the nature of the adsorbent used at the same temperature. Moreover, the greater the surface area of the adsorbent, the greater is its adsorption capacity. Due to this reason, the substances like charcoal and silica gel are excellent adsorbent because they have high porous structures and hence have large surface areas. For the same reason, activated charcoal and finely divided solid substances are better adsorbents.
- Nature of the gas: Different gases are adsorbed to different extents by the same adsorbent at the same temperature. The easily liquefiable gases (like HCl, NH3, Cl2, etc.) are adsorbed more easily than the permanent gases (like H2, N2, O2, etc.). The ease of liquefaction of a gas depends upon its critical temperature (i.e., the minimum temperature above which a gas cannot be liquefied by applying even high pressure). The critical temperature of a gas is related to the intermolecular forces. Hence higher is the critical temperature, the more easily the gas is liquefied and hence more readily it is adsorbed.
- Temperature: As already discussed, adsorption is an exothermic process and it is accompanied by evolution of heat. Hence, according to Le Chatelier’s principle, it can be seen that increase of temperature decreases the rate of adsorption and vice versa.

In case of physisorption, the amount of adsorption decreases with increase of temperature in accordance with Le Chatelier’s principle. But in case of chemisorption, it is observed that the amount of adsorption initially increases with increase in temperature and then decreases (Figure 10.3). This is due to the fact that a chemisorption process requires some activation energy and hence the initial rise. Once the adsorption process commences, the amount of adsorption decreases due to its exothermic nature.

Figure 10.3 Adsorption isobars for (a) Physisorption and (b) Chemisorption
- Pressure: At constant temperature, the adsorption of a gas increases with increase of pressure according to Le Chatelier’s principle; since a dynamic equilibrium exists between the adsorbed gas and the adsorbent solid.
- Activation of adsorbent: In order to increase adsorption, activation of the adsorbing power of the adsorbent is very necessary.
This is done by increasing the surface area of the adsorbent, which can be achieved by the following ways:
- By making the surface of the adsorbent rough through mechanical rubbing.
- By subdividing the adsorbent into smaller pieces or grains.
- By removing the gases already adsorbed by strong heating; due to this, its pores are opened, hence the rate of adsorption activity increases.
10.2.8 Adsorption Isotherms
The extent of adsorption on a given surface increases with increase in pressure (for gases) and concentration (for solution) at constant temperature.
The extent of adsorption is usually expressed as x/m, where m is the mass of the adsorbent and x is the mass of the adsorbate when adsorption equilibrium is reached.
“A graph between the amount of the gas adsorbed per gram of the adsorbent (x/m) and the equilibrium pressure at constant temperature is called adsorption isotherm.”
Freundlich Adsorption Isotherm
To understand the effect of pressure on adsorption of a gas, we consider adsorption as an equilibrium process. When the adsorbent and adsorbate are enclosed in a closed vessel, after an initial decrease in the pressure of the gas, gas pressure as well as the amount of gas adsorbed reaches constant values. This is because when the equilibrium is attained, the rate of adsorption becomes equal to the rate of desorption (Figure 10.4).
From the graph, it is clear that the extent of adsorption ![]() increases with increasing pressure and becomes maximum at Ps, called the saturation pressure. At Ps, the rate of adsorption becomes equal to the rate of desorption (i.e., equilibrium is attained), and further increase in pressure does not alter the equilibrium.
increases with increasing pressure and becomes maximum at Ps, called the saturation pressure. At Ps, the rate of adsorption becomes equal to the rate of desorption (i.e., equilibrium is attained), and further increase in pressure does not alter the equilibrium.

Figure 10.4 Freundlich adsorption isotherm
The following observations can be made from graph:
- At low pressure, the graph is almost straight line and sloping. This is represented by the following equation:
 (1)
(1) - At high pressure,
 is independent of pressure, that is, the graph becomes almost parallel to x-axis. This is represented by the following equation:
is independent of pressure, that is, the graph becomes almost parallel to x-axis. This is represented by the following equation:
 (2)
(2) - In the intermediate range of pressure,
 will depend on P raised to powers between 1 and 0, that is, fractions. So, for a small range of pressure values, we can write,
will depend on P raised to powers between 1 and 0, that is, fractions. So, for a small range of pressure values, we can write,
 (3)
(3)
Where n = positive integer and n and K are constants depending upon the nature of the adsorbate and adsorbent at a particular temperature.
This relationship was originally put forward by Freundlich in 1909 and is known as Freundlich adsorption isotherm.
To test the validity of this equation, taking logarithms on both sides, we get and compare with straight line equation
 (4)
(4)
A graph between ![]() against log P should, therefore, gives a straight line with slope equal to
against log P should, therefore, gives a straight line with slope equal to ![]() and ordinate intercept equal to log K (Figure 10.5).
and ordinate intercept equal to log K (Figure 10.5).

Figure 10.5 Linear graph between ![]() and log P
and log P
Limitations of Freundlich adsorption isotherm: When the experiment’s values are plotted, it shows some deviation from linearity, especially at high pressure. Hence, this relation is suitable only at low pressure.
Langmuir Adsorption Isotherm
In 1916, Irving Langmuir proposed another adsorption isotherm, which explained the variation of adsorption with pressure. Based on his theory, he derived Langmuir equation, which depicted a relationship between the number of active sites of the surface undergoing adsorption and pressure.
Assumption of Langmuir Isotherm
- Fixed number of vacant or adsorption sites are available on the surface of a solid.
- All the vacant sites are of equal size and shape on the surface of the adsorbent.
- Each site can hold maximum of one gaseous molecule, and a constant amount of heat energy is released during this process.
- The ability of a molecule to adsorb at a given site is independent of the occupation of the neighboring sites.
- Dynamic equilibrium exists between the adsorbed gaseous molecules and the free gaseous molecules.


- Adsorption is monolayer or unilayer.
Derivations of Langmuir Adsorption Equation
- Calculation of equilibrium constant: Langmuir proposed the dynamic equilibrium that exists between adsorbed gaseous molecules and the free gaseous molecules. Using the equilibrium equation, equilibrium constant can be calculated:


According to kinetic theory,
Rate of forward reaction = Ka[A][B]
Rate of backward reaction = Kd[A][B]
At equilibrium,

where K = adsorption constant or equilibrium constant
- Derivation of Langmuir equation: To derive Langmuir equation, a new parameter ‘θ’ is introduced. Let θ be the number of sites of the surface, which are covered with gaseous molecules. (1−θ) is the number of vacant sites.
Initially, the rate of adsorption is high because the number of vacant sites is quite large compared to the filled sites. As adsorption process increases, the number of vacant sites for adsorption increases. We know that the rate of adsorption depends upon the pressure P and the number of vacant sites (1−θ).
 (1)
(1)Rate of desorption under the same conditions depends on θ, the faction of surface covered.
 (2)
(2)At equilibrium,

Divide numerator and denominator on RHS by Kd.
We get

This is known as Langmuir adsorption equation.
- Freundlich adsorption equation: A special case of Langmuir equation: We consider Langmuir Equation

- At low pressure, KP < < 1
Therefore
 (1)
(1) - At high pressure,
 (2)
(2)Combining the result of equations (1) and (2),
 (3)
(3)Equation (3) is in agreement with Freundlich adsorption equation. Hence, we can say that Freundlich adsorption equation is a special case of Langmuir equation (Figure 10.6).
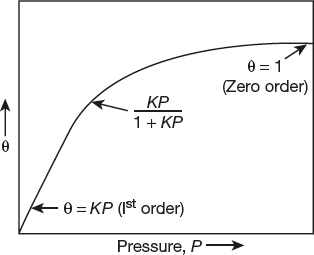
Figure 10.6 Langmuir variation of coverage with pressure for molecular adsorption
- At low pressure, KP < < 1
- Verification of Langmuir equation: The verification of Langmuir equation can be carried out as follows:
The amount of gas adsorbed per unit mass of absorbent is x ∝ θ
 (4)
(4)By substituting the value of θ,

where

Dividing both sides by P and then taking reciprocals, it gives

Since K and K2 are constants for a given system.
Plotting a graph between P/x against P should give a straight line with a slope
 and intercept
and intercept  . Figure 10.7 shows a linear relationship for same experimental data.
. Figure 10.7 shows a linear relationship for same experimental data.
Figure 10.7 Linear graph of P/x versus P
The Langmuir adsorption equation is only valid for unimolecular layer adsorption, not for multilayer adsorption in several solids.
BET Adsorption Isotherm
The concept of the theory is an extension of the Langmuir theory, which is a theory for monolayer molecular adsorption to multilayer adsorption. In 1938, Stephen Brunauer, Paul Hugh Emmett, and Edward Teller proposed this theory (BET theory) after their initials. BET Adsorption Isotherm is shown in Figure 10.8.
The following hypothesis of BET theory:
- Gas molecules adsorb on a solid in layer infinitely.
- There is no interaction between each adsorption layer.
- Langmuir theory can be applied to each layer.
The resulting BET equation is expressed as follows:

Figure 10.8 BET adsorption isotherm
 (1)
(1)
or
 (2)
(2)
Here P and P0 = equilibrium and saturation pressure of adsorbates at the temperature of adsorption
V = Volume of gas adsorbed
Vm = Volume of monolayer absorbed quantity
C = BET constant
 (3)
(3)
![]()
Verification of BET Equation
Equation (1) is an adsorption isotherm and can be plotted as a straight line (Figure 10.9). Plotting of ![]() against P/P0 should be a straight line.
against P/P0 should be a straight line.
The slope of the line is given as ![]() and intercept with the value of
and intercept with the value of ![]() . Thus, by knowing the value of slope and intercept, both Vm and C can be evaluated by using the following equation:
. Thus, by knowing the value of slope and intercept, both Vm and C can be evaluated by using the following equation:
![]() (4)
(4)
and
![]() (5)
(5)

Figure 10.9 Linear graph of P/P0 Vs P/V(P0-P)
Derivation of BET Equation
Consider a surface and molecules that are adsorbed on different layers, that is, adsorption on multilayer (Figure 10.10).
Consider θ0, θ1, θ2 ,……… θn = surface area covered by 0,1, 2,……, n layers of adsorbed molecules.


Figure 10.10 Adsorption of gas molecules on the surface of a solid
At equilibrium, θ0 must remain constant.
![]() (1)
(1)
Similarly, at equilibrium, θ1 must remain constant.

Substituting in equation (1) gives
![]()
And so on. For other layers,
![]() (2)
(2)
According to the definitions, total surface area of the catalyst,
 (3)
(3)
Total volume of the gas adsorbed on the surface,
 (4)
(4)
Where V0 = Volume of the gas adsorbed on one square centimeter of surface when it is covered with a complete layer.
Then,
 (5)
(5)
Vm = Volume of the gas adsorbed when the entire surface is covered with a complete monolayer
From equation (1),

By assuming that the properties of 1st, 2nd, and nth layers are equivalent,
 (6)
(6)
Similarly,

Generally,

Substituting in equation (5),
 (7)
(7)
On solving,
![]()
At saturation, pressure of gas P0, an infinite number of adsorbate layers must build on the surface. This means at P0, X must equal 1.

Substituting in equation (7), we get BET isotherm equation

![]()
Calculation of Surface Area of Adsorbent by BET Method
BET method is widely used for the calculation of surface areas of solids by physical adsorption of gas molecules.
The total surface area STotal and a specific surface area S are evaluated by the following equations:
![]() (1)
(1)


For example, to determine the inner surface of a hardened cement paste, by plotting a graph between the quantities of adsorbed water at different levels of relative humidity, a BET plot is obtained.
From the slope A and intercept I, one can calculate Vm and C by using the following equation:

SBET can be calculated by using the equation,
![]()
The surface covered by one water molecule, S = 0.114 nm2
So,

Surface area of the hardened cement = 155.63 m2/g
It means the cement paste has inner surface of 155.63 m2 per g of cement.
10.2.9 Applications of Adsorption
Adsorption finds extensive applications both in industry and research laboratory. A few applications are briefly described below:
- In preserving vacuum: Adsorption process is used in the production of vacuum by using cultivated charcoal in Dewar’s flask.
- In clarification of sugar: Sugar is decolourized by treating sugar solution with charcoal powder.
- In gas masks: All gas masks are devices containing suitable adsorbent so that the poisonous gases present in the atmosphere are preferentially adsorbed and air for breathing is purified.
- In heterogeneous catalysis: Adsorption is useful in heterogeneous catalysis. This theory is called adsorption theory. According to this theory, gaseous reactants are adsorbed on the surface of the solid. As a result, concentration of reactant increases. Hence, the rate of reaction increases. For example, in the manufacturing of ammonia using iron as catalysts, V2O5 is used as catalyst in the manufacturing of H2SO4 by contact process.
- In softening of hard water: In water-softening process, hard water is passed through zeolite bed in which Ca2+ and Mg2+ ions of hard water are replaced by equal amount of Na+ ions. So by this process, water becomes soft.
- In adsorption indicator: Various dyes, which have the property of adsorption, have been intruded particularly in precipitation titration. For example, by the use of eosin as indicator, KBr is easily titrated with AgNO3.
- In dehydration process: Silica gel and alumina gel adsorbents are used to remove moisture and dehumidifies the air from rooms and instruments.
- In chromatographic analysis: The selective adsorption of certain substances from a solution by a particular solid adsorbent filled in a long and wide vertical tube: column chromatography.
Examples are as follows:
- Separation of isomeric aromatic or aliphatic hydrocarbons
- Separation of two or more components of mixture
- Purification of substances from their contaminants
- Identification of biochemicals such as amino acids, neutral lipids, carbohydrates, etc.
- In Froth floatation process: In this process, finely divided sulphide ores (PbS, ZnS, Cu2S, etc.) are mixed with pine oil and agitated with water containing detergent. Air is then bubbled through this mixture, and air bubbles stabilized by the detergent adsorb mineral particles, which float to the surface while the silica impurities settle down.
- In medicine: For the treatment of arsenic poisoning, colloidal Fe(OH)3 is administered as an antidote in which Fe(OH)3 adsorbs arsenic and retains it and thus can be removed from the body by vomiting.
- In dyeing: During the process of mordant dyeing, the metal ions like Al3+, Fe3+, and Cr6+ adsorb the dye, which otherwise does not adhere to the fabric.
- Ion exchange adsorption process is used to remove excess of sodium salt from the body fluids of a patient by providing him/her to a suitable cation exchanger to consume. Similarly, anionic resin is used to neutralize the excess acid in the stomach.
- By adsorption, we can easily remove polluting gases and control indoor pollution.
- By the use of surface active agents in detergents, paints, lubrication, surface cleaner, etc., surface active agents displace the adhering impurities from all surfaces by the phenomenon of wetting.
- Soil contains small amounts of colloidal fractions in the form of very fine particles of clay, which adsorb moisture in which essential plant nutrients N, P, and K dissolve and are taken up by the plants by capillary action.
10.3 COLLOIDAL STATE
The origin of the study of this field lies with Thomas Graham, who in 1861 studied the diffusion of liquids and solutions across the animal membranes, and as a result, he divided the soluble substances into two classes:
- Crystalloids diffuse readily in solution and those solution that can readily pass through animal or vegetable membranes, for example, urea, sugar, salts, acids, bases, and other crystalline substances.
- Colloids (from the Greek words “kola” and “eidos”, which mean glue and like, respectively), diffuse very slowly in solution and those solution that cannot pass through animal or vegetable membranes, for example, glue, starch, albumin, proteins, and other amorphous substances.
In recent years, this classification of substances has undergone a great change because distinction between crystalloids and colloids was not rigid. In fact, any substance, regardless of its nature, could be converted into a colloid by subdividing it into particles of small size (1–1000 nm).
10.3.1 Types of Solution
On the basis of the size of the particle, the solutions may be of the following three types:
- True solution: True solution is a homogeneous solution containing dispersed particles of molecular size (less than 10−9 m or 1 nm), for example, NaCl or glucose solution in water.
- Suspension solution: Suspension is a heterogeneous solution containing mixture of suspended insoluble particles of size (greater than 10000 A° or 1000 nm).
- Colloidal solution: Colloidal solution is a heterogeneous solution in which a substance is distributed in colloidal state (1 nm–1000 nm) in an insoluble medium.
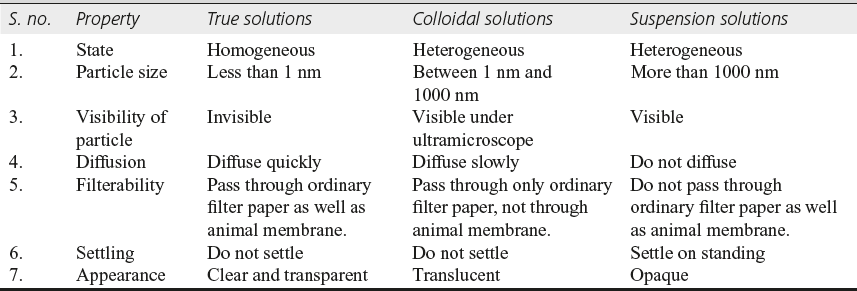
The common characteristics among the three solutions are given in Table 10.3.
Table 10.3 Characteristics of true solution, colloidal solution, and suspension
10.3.2 Classification of Colloids
In colloidal system, the terms dispersed phase and dispersion medium are used. Dispersed phase means the substance distributed in the dispersion medium in the form of colloidal particles, and dispersion medium means the medium in which the substance is dispersed in the form of colloidal particles. Just as in solution, where solute and solvent are used, in colloidal system, dispersed phase and dispersion medium are used.
Colloidal systems are classified in different ways:
- Based on the physical state of the dispersed phase and dispersion medium: Depending upon the physical state of the dispersed phase and dispersion medium, that is, solids, liquids, or gases, eight types of colloidal systems are possible (Table 10.4).
Table 10.4 Physical states of the dispersed phase and dispersed medium with examples

- Based on the nature of the dispersion medium: Depending upon the nature of the dispersion medium, the sols (solid in liquid) are given special names as in the following Table 10.5:
Table 10.5 Nature of the dispersed medium and the names of the sols

- Based on the nature of interaction between dispersed phase and dispersion medium: On this basis, colloidal sols are divided into two categories, namely, lyophilic and lyophobic. If water is the dispersion medium, the terms used are hydrophilic and hydrophobic.
- Lyophilic sols are those in which the dispersion medium possesses great affinity (liquid-loving) for the dispersed phase. Examples are starch, glue, gelatin, agar sols in water, etc.
Characteristics:
- Lyophilic sols are directly formed colloidal sols; that is why they are also called intrinsic colloids.
- The dispersion medium is easily separated from the dispersed phase (by evaporation), and the sol can be made by simply remixing with dispersion medium and shaking. That is why, these sols are also called reversible sols.
- These sols are quite stable and cannot be easily precipitated.
- Lyophobic sols are those in which there is no affinity or interaction between the dispersion medium and the dispersed phase. Examples are gold sol, silver sol, and metal sulphide sol in water.
Characteristics:
- Their colloidal sols are generally prepared by indirect methods; that’s why they are called extrinsic colloids.
- Such types of colloidal sols are not precipitated out once they formed. These sols are also called irreversible sols.
- These sols are easily precipitated (or coagulated), and hence are not stable.
The points of difference between lyophilic sols and lyophobic sols are given below in Table 10.6:
Table 10.6 Differences between lyophilic sols and lyophobic sols

- Lyophilic sols are those in which the dispersion medium possesses great affinity (liquid-loving) for the dispersed phase. Examples are starch, glue, gelatin, agar sols in water, etc.
- Based on the type of particles of the dispersed phase: Depending upon the different size of the colloids, colloidal dispersion may be divided into the following three categories:
- Multimolecular colloids: Multimolecular colloids are colloidal solutions in which colloidal particles (or dispersed phase) consist of aggregates of atoms or molecules, each having diameter less than 1 nm. In these aggregates, the atoms or molecules are held together by weak force of attraction. They are lyophobic in nature. For example,
- A gold sol in water consists of dispersed particles of various sizes made up of several atoms of gold.
- A sulphur sol in water consists of dispersed particles containing a large number of S8 molecules.
- Macromolecular colliods: Macromolecular colloids are colloidal solutions in which the dispersed particles are very large molecules of high molecular mass (called macromolecules). These particles contain a large number of atoms (joined together through covalent bonds), each having dimensions compared to those of colloidal state. They are lyophilic sols and possess gold number.
For example,
- Naturally occurring macromolecules such as starch, cellulose, proteins, enzymes and gelatine, etc.
- Man-made macromolecules like polyethylene, nylon, polystyrene, synthetic rubber, etc.
- Associated colloids–micelles: Associated colloids are the colloidal solutions in which the colloidal particles behave as normal, strong electrolytes when dissolved in a medium at low concentrations, but at higher concentrations, they form aggregated particles of colloidal dimensions, called micelles.
The formation of micelles takes places only above a particular temperature called Kraft temperature (Tk) and above a particular concentration called Critical micelle concentration (CMC).
For example, most common associated colloids are soaps and synthetic detergents. For soaps, the CMC is 10−4–10−3 mol L−1. Each micelle contains at least 100 molecules.
Mechanism of micelle formation: When soap is dissolved in water, the negative ions aggregate to form an ionic micelle, which is of colloidal size (Figure 10.11).
We know that soap is sodium salt of higher fatty acid RCOONa; when dissolved in water, it dissociates into RCOO− and Na+ ions.

RCOO− ions consist of two parts, that is, non-polar, long hydrocarbon chain R, called the tail, which is hydrophobic and polar group COO−, called the head, which is hydrophilic. At higher concentration, ions (RCOO−) forms an aggregate of spherical shape with their hydrocarbon chains pointing towards the centre and COO− parts outward on the surface of the sphere.

Figure 10.11 A micelle in a soap solution
A few common features among multimolecular, macromolecular, and associated colloids are compared in Table 10.7.
- Multimolecular colloids: Multimolecular colloids are colloidal solutions in which colloidal particles (or dispersed phase) consist of aggregates of atoms or molecules, each having diameter less than 1 nm. In these aggregates, the atoms or molecules are held together by weak force of attraction. They are lyophobic in nature. For example,
Table 10.7 Comparison of multimolecular, macromolecular, and associated colloids

10.3.3 Properties of Colloidal Solutions
Colloidal solutions possess some distinct characteristics. Some of them are discussed as follows:
- Physical properties:
- Heterogeneous character: Colloidal particles are heterogeneous in nature, having two distinct phases, that is, dispersed phase and dispersion medium.
- Stability: Colloidal sols are quite stable. Only a few colloidal particles of comparatively larger size may settle but very slowly.
- Filterability: Colloidal solutions can readily pass through ordinary filter paper, because the pore size of the filter paper is much larger than that of colloidal particles. But colloidal particles cannot pass through semi-permeable membrane.
- Visibility and colour: The particles of the colloidal solution are not visible to naked eye or under ordinary microscope. The colour of the colloidal solution depends upon the wavelength of light scattered by the colloidal particles.
- Colligative properties: Colloidal particles have a very high molecular weight, so the number of moles present in a solution is extremely low. Since colligative properties depend only on the number of effective moles present per litre, the value of any of the colligative properties—osmotic pressure, elevation in boiling point, depression in freezing point, and lowering of vapour pressure of specific substance—will be quite low, compared to its value, when it is in the form of a true solution.
- Mechanical properties: When colloidal solution is viewed through an ultramicroscope, colloidal particles are seen continuously moving in a zigzag way. This phenomenon was first observed by Robert Brown in 1827, and hence, it is called Brownian movement. Thus, “Brownian movement is defined as continuous, rapid and random zigzag movement of the colloidal particles in a colloidal sol.”
Cause of Brownian movement: The molecules of dispersion medium due to their kinetic motion strike against the colloidal particles from all sides with different forces, causing them to move. However, colloidal particles, being comparatively higher, move with a slower speed (Figure 10.12).

Figure 10.12 Brownian movement is due to the unbalanced imparts of molecules of the dispersion medium on colloidal particles
- Optical properties: It was observed that when colloidal solution is placed in a dark room and a strong converging beam of light is passed through it, a path of the beam gets illuminated with a bluish light when viewed at right angles to the direction of the passage of light. The path of light becomes visible due to scattering of light by the colloidal particles as shown in Figure 10.13. This phenomenon was observed by Tyndall in 1869 and is called Tyndall effect. Thus, “Tyndall effect may be defined as the scattering of light by the colloidal particles present in a colloidal solution.”
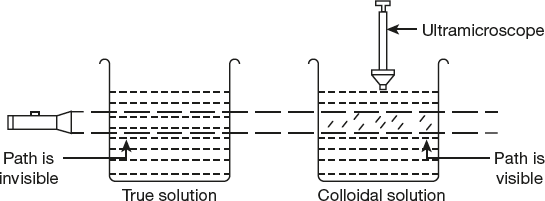
Figure 10.13 Tyndall effect exhibited by a colloidal solution, but not by true solution
Causes of Tyndall effect: Tyndall effect is shown by colloidal particles due to scattering of light by colloidal particles. The colloidal particles absorb the incident light energy, become self-luminous, and scatter this absorbed light from their surfaces. As the intensity of scattering is maximum in the plane at right angles to the direction of incident beam, the path becomes visible. The molecules of true solution do not scatter light, as their size is comparatively very small.
The importance of Tyndall effect is that it helps to confirm the heterogeneous nature of the colloidal solutions.
- Electrical properties:
- Stability of colloidal sols: The colloidal particles in a colloidal solution carry either positive or negative charge. So due to electrically charged colloidal particles, colloidal solution is stable. The particles, due to the same charge, repel one another and do not come close together to form large, non-colloidal particles. The dispersed phase in a colloidal solution carries the same charge while the dispersion medium has an equal and opposite charge.
Table 10.8 Charge on particles of some common sols using water as dispersion medium

For example, arsenious sulphide particles are negatively charged, whereas the dispersion medium (water) is positively charged. Ferric hydroxide particles are positively charged, whereas the dispersion medium (water) is negatively charged (Table 10.8).
Origin of electrical charge on colloidal particles: The various reasons for the origin of electrical charge on the colloidal particles are as follows:
- Preferential adsorption of ions from solution: It may be either positive or negative ion on its surface. For example, if colloidal sol of AgI is prepared by adding KI solution to AgNO3 solution till KI is in slight excess, iodide ions (I−) will be adsorbed on the surface of AgI particles, thereby giving them negative charge:
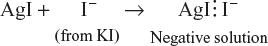
or if AgI is prepared by adding AgNO3 to KI solution, till AgNO3 is in slight excess,

In either case, the left-out ions will remain in the dispersion medium (e.g., K+ ions in the first case and
 ions in the second case), thereby giving equal and opposite charge to the dispersion medium.
ions in the second case), thereby giving equal and opposite charge to the dispersion medium.
Figure 10.14 Formation of electrical double layer
- Dissociation of molecules followed by aggregation of ions: For example, in case of soap, the RCOO− group gets dissociate from Na+ ions and have a tendency to aggregate into a cluster, carrying negative charge.
- Dissociation of the molecular electrolytes adsorbed on the surface of particles: For example, H2S molecules get adsorbed on colloidal particles of As2S3 during precipitation in which H+ ions are lost, giving negative charge to colloidal particles.
The theory of electrical double layer was proposed by Helmholtz, and he explained that charge on colloidal particles is due to the preferential adsorption of common ions on their surface. So when one type of ion of the electrolyte is adsorbed on the surface of the colloidal particles, it forms a fixed layer; it attracts the counter ions from the medium, forming a second layer, which is mobile and is called diffused layer. The double layer of opposite charges thus formed is called Helmholtz electrical double layer. As a result, a potential difference is set up between the fixed layer and the diffused layer, and this is known as electro kinetic potential or zeta potential (Figure 10.14).
- Preferential adsorption of ions from solution: It may be either positive or negative ion on its surface. For example, if colloidal sol of AgI is prepared by adding KI solution to AgNO3 solution till KI is in slight excess, iodide ions (I−) will be adsorbed on the surface of AgI particles, thereby giving them negative charge:
- Electrophoresis or cataphoresis: The colloidal particles are electrically charged either positive or negative, and the existence of electrical charge can be shown by the process known as “electrophoresis,” which is also called “cataphoresis.”
It consists of a U-tube, partly filled with a colloidal solution and rest of the tube is filled with distilled water. So when electric field is applied, the electrically charged colloidal particles move towards one or the other electrode, depending on the charge present on colloidal particles (Figure 10.15). Thus, “The movement of colloidal particles under the influence of an electric field is called electrophoresis or cataphoresis.”

Figure 10.15 (a) Before electrophoresis (b) After electrophoresis (for negatively charged colloidal particles)
On applying potential gradient within a U-tube, which is filled with colloidal solution and the rest with distilled water, the colloidal particles move towards oppositely charged electrode. On reaching the electrode, they lose their charge and coagulate. If the movement of colloidal particles is towards cathode, it is called cataphoresis, as in the case of positively charged sols like Fe(OH)3.
However, the term electrophoresis is preferred because colloidal particles may migrate towards anode or cathode depending upon the charge on the colloidal particles.
- Electro-osmosis: When an electric field is applied on colloidal solution, the particles of the dispersion medium (which are also electrically charged) move towards oppositely charged electrode, provided the colloidal particles are not allowed to move as shown in Figure 10.16. This phenomenon is called electro-osmosis. Thus, “Electro-osmosis may be defined as a phenomenon in which molecules of dispersion medium are allowed to move under the influence of an electric field whereas colloidal particles are not allowed to move.”

Figure 10.16 Electro-osmosis
- Coagulation or flocculation or precipitation: “Coagulation or precipitation is a process of aggregating together the colloidal particles so as to change them into large sized particles which ultimately settle as precipitate.”
- By addition of electrolyte: Coagulation is generally brought about by the addition of electrolytes. Because when an electrolyte is added to a colloidal solution, the particles of the sol take up the ions, which are oppositely charged and thus get neutralized. The ions responsible for neutralization of charge on the colloidal particles are called the coagulating ion or flocculating ion. The neutral particles then start accumulating to form particles of large size, which settle down.
“The minimum amount of an electrolyte (millimoles) that must be added to one liter of a colloidal solution so as to bring about complete coagulation is called the coagulation or flocculation or precipitation value of the electrolyte. Thus, smaller is the coagulation value of an electrolyte, greater is its coagulating or precipitating power.”
The observation about coagulation of colloidal sol was studied by Hardy Schulze law. The main points of which may be stated as follows:
- • The effective ions of the electrolyte in bringing about coagulation are those which carry charge opposite to that of the colloidal particles. These ions are called coagulating ions or flocculating ions.
- • Greater is the valency of the coagulating or flocculating ion, greater is its power to bring about coagulation.
Thus, for coagulation of negatively charged As2S3 sol, the flocculating power decreases in the order.
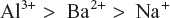
Similarly, for coagulation of positively charged Fe(OH)3 sol, the coagulating powers are in the order of
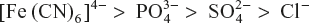
The coagulating power is inversely proportional to coagulation/flocculation value. Comparing the relative coagulating powers of two electrolytes for the same colloidal sol, we have

- By electrophoresis: Coagulation is also brought about by electrophoresis process, in which particles of the dispersed phase move towards oppositely charged electrode and get neutralized. These neutral particles unite and grow in size and then settle down.
- By mutual precipitation: In this process, oppositely charged sols are mixed in proper proportions to neutralize the charges of each other, which result in the coagulation of both the sols.
For example, if positively charged Fe(OH)3 and negatively charged As2S3 sols are mixed, both the sols get coagulated.
- By prolonged dialysis: The stability of a colloidal sol is due to the presence of a small amount of the electrolyte. On prolonged dialysis, the electrolyte is completely removed. As a result, the colloidal sol becomes unstable and gets coagulated.
- By boiling or cooling: In some cases, heating the sols results in coagulation, for example, coagulation of butter. During boiling, the adsorbed layer is disturbed because the number of collisions on them by the molecules of dispersion medium increases. As a result, charge on the particles decreases. Hence the stability decreases, leading to their settling down as a precipitate.
Similarly, in some cases, cooling the sol results in coagulation, for example, coagulation of milk, that is, on cooling milk, fats start floating on the surface.
- By addition of electrolyte: Coagulation is generally brought about by the addition of electrolytes. Because when an electrolyte is added to a colloidal solution, the particles of the sol take up the ions, which are oppositely charged and thus get neutralized. The ions responsible for neutralization of charge on the colloidal particles are called the coagulating ion or flocculating ion. The neutral particles then start accumulating to form particles of large size, which settle down.
- (e) Isoelectric point: The charge present on lyophilic colloids like proteins, amino acids, polypeptides, etc., is a function of pH of the medium. They are positively charged in strongly acidic solution and negatively charged in alkaline medium. However, at a certain pH of the dispersion medium, the dispersed particles are neutral, and hence that point is called isoelectric point. The molecules at the isoelectric point exist as zwitter ions. The dispersed particles are neutral, and hence they do not migrate when subjected to an electric field. So, at isoelectric point, coagulation of colloidal particles starts.

- Protective action of lyophilic colloids: Lyophobic sols are less stable and hence more easily coagulated than lyophilic colloids. This is because the stability of lyophilic sols is due to two factors:
- Same charge on all the colloidal particles
- Solvation of the colloidal particles
Thus to bring about coagulation, both these factors have to removed. This is done by
- • By adding electrolyte
- • By adding suitable electrolyte.
But the stability of lyophobic sol is only due to charge. This factor can be removed by adding only electrolyte. Hence they can be easily coagulated.
However, it is observed that the addition of certain lyophilic colloids like gums, soaps, gelatine, etc., to lyophobic colloids (like a metal sol of Ag, Au, etc.) render lyophobic colloids difficult to coagulate by the addition of electrolytes. The process is known as “protection” and the lyophilic colloids are termed as protective colloids. The protective action of the lyophilic colloids is due to the covering up of the particles of the lyophobic colloid by those of the lyophilic colloids as shown in Figure 10.17.

Figure 10.17 Protection of colloids
This explanation is not fully correct because the particles of protecting substance have almost the same size as those of the substance being protected.
To compare the protective action of different lyophilic colloids, Zsigmondy (in 1901) introduced a term called gold number. It is defined as follows:
“Gold number of a protective colloid is the minimum weight of it in milligrams which is just sufficient to prevent the coagulation of 1 ml of standard gold sol (i.e., containing 0.0053–0.0058% gold) by the rapid addition of 1 ml of 10% of sodium chloride solution.” The coagulation of a gold sol is indicated by the change in colour from red to violet. Thus, the smaller the gold number of a protective colloid, the greater is its protective action. The gold numbers and reciprocals of few colloids sols are listed in Table 10.9.
Table 10.9 Gold number of a few protective colloids

- Stability of colloidal sols: The colloidal particles in a colloidal solution carry either positive or negative charge. So due to electrically charged colloidal particles, colloidal solution is stable. The particles, due to the same charge, repel one another and do not come close together to form large, non-colloidal particles. The dispersed phase in a colloidal solution carries the same charge while the dispersion medium has an equal and opposite charge.
10.3.4 Applications of Colloids
- In everyday life
- Colloids play an important role in our daily life. Protoplasm and blood are all colloidal in nature.
- A number of food particles that we eat are colloidal in nature, for example, milk, butter, ice creams, fruit juices, halwa, etc.
- The clothes and shoes that we wear are based on colloidal nature. So generally all products in everyday life that we are using depend on colloids, for example, inks, paints, rubber, cement, synthetic plastic, etc.
- In analytical chemistry
- Traces of noble metals in solution can be detected by microanalysis in which the colour of colloidal solution formed is analyzed. For example, cassius purple test for gold is due to the production of colloidal gold.
- Silica and alumina gels are used as adsorbent for gases and as drying agent in laboratory.
- In quantitative and qualitative analysis technique, colloidal sols are formed during analysis process.
- In agriculture
- Humus acts as protective colloids in good colloidal soils, which retain moisture for emergency.
- The soil retains the moisture; this can be reduced by using calcium salts, which results in coagulation of soil and makes them less hydrophilic.
- In medicine
Medicines in the form of colloids are easily absorbed by the body tissue due to large surface area and hence are more effective, for example,
- Argyrol is a silver sol used as an eye lotion.
- Colloidal antimony is used for curing kala azar.
- Colloidal gold is used as an intramuscular injection.
- Milk of magnesia, which is an emulsion, is used as an antacid for reducing acidity in the stomach.
- Blood from minor cuts/wounds is stopped, by coagulation with alum or ferric chloride. The trivalent Al3+ or Fe3+ ions neutralize the negative charge on the albuminoid particles (which constitute the blood) and thus coagulate it.
- In industry
- Sewage disposal: Colloidal particles of dirt, mud, etc., carry electric charge. Hence, when sewage water is passed through the plates kept at a high potential, the colloidal particles are coagulated due to electrophoresis, and the suspended matter gets removed.
- Purification of water: Impure water containing bacteria or suspended, negatively charged clay particles can be purified by the removal of these substances by their precipitation with the help of alum in which Al3+ ions neutralize negatively charged colloidal impurities and settle down, and pure water can be decanted off.
- Smoke precipitation: Smoke particles are actually electrically charged colloidal particles of carbon in air. Precipitation of smoke particles is carried out by Cottrell precipitator, which is based on the principle of electrophoresis. In this process, smoke is allowed to pass through a chamber having a number of metal plates attached to a metal wire connected to a source of high potential as shown in Figure 10.18. Charged particles of smoke get attracted by oppositely charged electrode, and get precipitated after losing their charge and hot air that passes out through the chimney. The dust particles are also removed in this process. Thus, the nuisance of smoke in big industrial cities can be avoided.

Figure 10.18 Cottrell precipitator
- Electroplating of rubber: Latex is a colloidal solution of negatively charged rubber particles. From latex, rubber can be obtained by coagulation. Rubber-plated articles are prepared by depositing negatively charged rubber particles over the articles to be rubber plated, making that article an anode in a rubber-platting bath.
- Leather tanning: The process of hardening of leather is known as tanning. The tanning of leather is based on the mutual coagulation of oppositely charged colloids. Tannin, which is obtained from plants (barks, wood, leaves, etc.) is a mixture of derivatives of polyhydroxy benzoic acids. It contains negatively charged colloidal particles. Animal hides (raw skin) are also colloidal in nature and contain positively charged particles. When they are soaked in tannin, mutual coagulation takes place, and leather becomes hard. Chromium salts are also used in place of tannin, and it is called chrome tanning.
- In photography: A colloidal solution of silver bromide (AgBr) in gelatine is applied on glass plates or celluloid films or paper to form sensitive plates in photography.
- Cleansing action of soap: Soap solution is colloidal in nature. It helps in the removal of dirt or dust sticks on the greasy or oily materials on the cloth. We know that soap is an emulsifier and soaps are sodium or potassium salts of higher fatty acids, for example, sodium palmitate (C15H37COONa), sodium sterate (C17H35COONa), etc. It consists of two parts: the hydrocarbon part (C15H31-, C17H35-, etc), which is soluble in oil and the polar group (COO− Na+), which is soluble in water.

Thus, if a drop of oil is surrounded by soap solution, the R-part of soap remains in the oil and the COO− Na+ part remains in water as shown in Figure 10.19. Thus soap molecules are concentrated over the surface of the drop of oil. As a result, the interfacial tension between oil and water decreases, and hence they are intermixed into each other to form the emulsion. Thus micelles are formed.

Figure 10.19 Role of soap as an emulsifier
- Fiber dyeing: The dyeing process means absorption of the colour on fiber. Various dyes are colloidal in nature. So, there is diffusion of colloidal particles of dye into the fabric.
- In warfare: In warfare, smoke screens are used, which are nothing but colloidal dispersion of certain substances (like titanium oxide) in the air.
- Artificial rain: Artificial rain can be caused by spraying charged colloidal dust or sand particles over a cloud. The colloidal particles (water particles) present in the cloud will get neutralized and coagulate to form bigger water drops, causing artificial rain.
- In nature
- Blue colour of the sky: This is due to the scattering of light by colloidal dust particles present in air (Tyndall effect). Similarly, sea water looks blue due to the scattering of light by the colloidal impurities present in the sea water.
- Tail of comets: Comets are celestial bodies, which fly with very high velocity and leave behind solid particles in its path, and due to the scattering of light, it is seen as a Tyndall cone-like structure, which we call the tail of comets.
- Fog, mist, and rain: In winters, at night, the moisture of the air condenses on the surface of dust particles, forming fine droplets. These droplets are colloidal in size and hence continue to float in the air in the form of fog or mist.
- In the upper atmosphere, where the temperature is low, clouds are suspended in air. They condense together to form bigger drops, which come down in the form of rain. Rain is also caused when two oppositely charged clouds meet each other.
- Formation of delta: River water contains charged colloidal particles of clay, sand, and many other materials. Sea water is a very big storehouse of a variety of electrolyte dissolved in it. As river water reaches the sea water, the electrolytes present in sea water coagulate the suspended colloidal particles, which ultimately settle down at the point of contact. Thus, the level of the river bed rises. As a result, the water adopts a different course, and delta is formed (Figure 10.20), so we can simply say that delta is formed due to silting of estuary (i.e., deposit of sediments at the mouth of the river).

Figure 10.20 Formation of delta
10.4 REVIEW QUESTIONS
10.4.1 Fill in the Blanks
- Adsorption is essentially __________ phenomenon.
[Ans.: Surface]
- The solid on whose surface adsorption takes place is called __________.
[Ans.: Adsorbent]
- The substance (a gas or a liquid) that is adsorbed on the surface of a solid (adsorbent) is known as __________.
[Ans.: Adsorbate]
- The process of removing an adsorbate from the surface of an adsorbent is referred to as __________.
[Ans.: Desorption]
- When the concentration of the adsorbate becomes more at the surface of an adsorbent than in bulk phase, the phenomenon is known as __________.
[Ans.: Adsorption]
- The adsorption of H2 or O2 on charcoal is __________.
[Ans.: Physical adsorption]
- The heat of adsorption in case of physical adsorption is 20–40 KJ/mole while in case of chemical adsorption, it is __________ KJ/mole.
[Ans.: 40–400]
- At low pressure, the amount of gas adsorbed is __________ proportional to pressure.
[Ans.: Directly]
- All gas masks contain __________ as adsorbent.
[Ans.: Activated charcoal]
- A true solution is transparent while a __________ is opaque in nature.
[Ans.: Colloidal solution]
- A colloidal solution essentially consists of two phases namely (i) __________ and (ii) __________.
[Ans.: (i) Dispersed phase (ii) Dispersion medium]
- Soap is an example of __________.
[Ans.: Associated colloids]
- The pH of a lyophilic sol at which the dispersed particles are neutral is called __________.
[Ans.: Isoelectric point]
- Fe(OH)3 sol is __________ charged while As2S3 sol is __________ charged.
[Ans.: Positively, Negatively]
- The process of purifying a colloidal solution by placing it in a parchment bag kept in water is known as __________.
[Ans.: Dialysis]
- The process of converting a precipitate into colloidal solution on the addition of an electrolyte is known as __________.
[Ans.: Peptization]
- Owing to the phenomenon of Tyndall effect, the colloidal particles can be observed as points of light with the help of __________.
[Ans.: Ultramicroscope]
- The phenomenon of zigzag motion of colloidal particles is known as __________.
[Ans.: Brownian movement]
- The migration of colloidal particles under the influence of an electric field is known as __________.
[Ans.: Electrophoresis]
- The lyophilic colloids that stabilize a lyophobic sol are referred to as __________.
[Ans.: Protective colloids]
- An aerosol is a colloidal system consisting of __________ dispersed in __________.
[Ans.: Liquid, Air]
- The size of colloidal particles lies in the range of __________.
[Ans.: 10–1000 A°]
- The colloidal solution of a solid in a liquid is called __________.
[Ans.: Sol]
- Process of separating colloids from crystalloids is called __________.
[Ans.: Dialysis]
- The movement of colloidal particles in a colloidal sol is called __________.
[Ans.: Brownian movement]
- The movement of colloidal particles under the influence of an electric field is called __________.
[Ans.: Electrophoresis]
- The movement of dispersion medium under the influence of an electric field is called __________.
[Ans.: Electro-osmosis]
- The addition of an electrolyte to a colloidal sol results in __________.
[Ans.: Coagulation]
- Smoke is a colloidal solution of __________ in the __________.
[Ans.: Carbon particles, Air]
- Milk is a colloidal solution of__________ in __________.
[Ans.: Fats, Water]
- The adhering of the molecules of a gas on the surface of a solid is called __________.
[Ans.: Adsorption]
- The protective action of different colloids is compared in terms of __________.
[Ans.: Gold number]
- Liquid–solid dispersions are known as__________.
[Ans.: Gels]
- A true solution __________ scatter light, whereas a colloidal solution __________ light.
[Ans.: Does not, Scatters]
- Freundlich adsorption isotherm is a plot of mass versus __________.
[Ans.: Pressure]
10.4.2 Multiple-choice Questions
- Adsorption is a phenomenon in which a substance:
- Goes into the body of the other substance
- Remains close to the other substance
- Accumulates on the surface of the other substance
- None of these
[Ans.: c]
- In adsorption of oxalic acid on activated charcoal, the activated charcoal is known as
- Adsorbent
- Adsorbate
- Adsorber
- Absorber
[Ans.: a]
- Physical adsorption is essentially quite appreciable
- At room temperature
- At higher temperature
- At lower temperature
- None of these
[Ans.: c]
- In physical adsorption,
- Van der Waal’s forces play a key role.
- Chemical bonding plays a key role.
- Density of the adsorbing materials plays a key role.
- None of these
[Ans.: a]
- In chemisorption,
- Chemical bonding is involved.
- Physical attractive forces are involved.
- Van der Waal’s forces are involved.
- None of the above
[Ans.: a]
- Freundlich adsorption isotherm is given by the simple empirical relationship
[Ans.: b]
- The size of the colloidal particle ranges from
- 10−2 cm to 10−3 cm
- 10−3 cm to 10−5 cm
- 10−5 cm to 10−7 cm
- 10−7cm to 10−8 cm
[Ans.: c]
- How many phases are present in a colloidal system?
- 1
- 2
- 3
- 4
[Ans.: b]
- The empirical relationship for Langmuir adsorption for moderate pressure is
[Ans.: c]
- At high pressure, the Langmuir adsorption relationship is
[Ans.: b]
- At low pressure, the Langmuir adsorption relationship is



- None of the above
[Ans.: a]
- When a beam of light is passed through a colloidal solution, it
- Is reflected
- Is scattered
- Passes through undeviated
- Is completely absorbed
[Ans.: b]
- The process of separation of crystalloids by using a semi-permeable membrane is referred to as
- Filtration
- Dialysis
- Ultrafiltration
- Electrophoresis
[Ans.: b]
- The amount of heat evolved when 1 mole of any gas is adsorbed on a solid adsorbent surface is called
- Entropy
- Enthalpy
- Heat of reaction
- Enthalpy of adsorption
[Ans.: d]
- The random or zigzag motion of the colloidal particles in the dispersion medium is referred to as
- Electro-osmosis
- Electrophoresis
- Brownian movement
- Tyndall effect
[Ans.: c]
- Soap essentially forms a colloidal solution in water and removes the greasy matter by
- Absorption
- Emulsification
- Coagulation
- None of the above
[Ans.: b]
- The presence of electric charge on the colloidal particles is indicated by
- Osmosis
- Dialysis
- Electrolysis
- Electrophoresis
[Ans.: d]
- The protective action of different colloids is expressed in terms of
- Oxidation number
- Atomic number
- Gold number
- Avogadro’s number
[Ans.: c]
- The coagulating power of an electrolyte for arsenious sulphide (As2S3) decreases in the order
[Ans.: c]
- The smoke is a colloidal sol of:
- Solid dispersed in liquid
- Gas dispersed in solid
- Solid dispersed in gas
- Gas dispersed in liquid
[Ans.: c]
- The best coagulant for the precipitation of ferric hydroxide is
- Na3PO4
- NaNO3
- KCl
- MgSO4
[Ans.: a]
- Fog is an example of colloidal system of
- Liquid dispersed in gas
- Gas dispersed in gas
- Solid dispersed in gas
- Solid dispersed in liquid
[Ans.: a]
- The colloidal sols are purified by
- Peptization
- Coagulation
- Dialysis
- Flocculation
[Ans.: c]
- Crystalloids differ from colloids mainly in respect of
- Electrical behavior
- Particle size
- Particle nature
- Solubility
[Ans.: b]
- At the critical micelle concentration (cmc), the surface molecules
- Decompose
- Dissociate
- Associate
- Become completely soluble
[Ans.: c]
- Alum purifies muddy water by
- Dialysis
- Adsorption
- Coagulation
- Forming a true solution
[Ans.: c]
- Movement of dispersion medium under the influence of electric field is
- Electrodialysis
- Electrophoresis
- Electro-osmosis
- Cataphoresis
[Ans.: c]
- Colloidal sol is
- True solution
- Suspension
- Heterogeneous sol
- Homogeneous sol
[Ans.: c]
- Blood may be purified by
- Dialysis
- Electro-osmosis
- Coagulation
- Filtration
[Ans.: a]
- Blue colour of water in the sea is due to
- Refraction of blue light by impurities in sea water
- Scattering of light by water
- Refraction of blue sky by water
- None of the above
[Ans.: a]
- Which of the following acts as a protective colloids?
- Hydrophilic
- Hydrophobic
- Gold sol
- Both hydrophilic and hydrophobic
[Ans.: a]
- Finely divided metal and porous substances provide
- Large surface area
- Small surface area
- Moderate surface area
- No surface area
[Ans.: a]
10.4.3 Short Answer Questions
- Define adsorption.
Ans.: A process in which molecules of a gas (or liquid) adhere to the surface of solid through van der Waal’s forces or chemical bond.
- What is an adsorbent? Give two examples.
Ans.: The solid or liquid surface on which adsorption takes place is called adsorbent. E.g.: Silica gel and charcoal.
- What is the effect of increase of temperature on the adsorption of a gas on a solid surface?
Ans.: In case of physical adsorption, rise in temperature decreases the adsorption, but in case of chemical adsorption, it first increases and then decreases.
- State one difference between physisorption and chemisorption.
Ans.: Physisorption is non-specific, whereas chemisorption is specific in nature.
- What is meant by sorption?
Ans.: When adsorption and absorption take place simultaneously, the process is called sorption. E.g.: Ammonia passed through water in contact with charcoal.
- What is meant by desorption?
Ans.: Removal of the adsorbed species from the surface of the adsorbent is called desorption.
- What is an adsorption isotherm?
Ans.: It is a plot of amount of gas adsorbed per unit mass of adsorbent (x/m) on a solid adsorbent versus the gas pressure at a constant temperature.
- What is an adsorption isobar?
Ans.: It is a graph between the magnitude of adsorption and temperature at constant pressure.
- Give any two examples each for hydrophilic and hydrophobic adsorbents.
Ans.: Hydrophilic adsorbents: Silica gel and zeolites
Hydrophobic adsorbents: Charcoal, polymer adsorbent like polystyrene divinylbenzene
- Which colloidal system is represented by butter?
Ans.: Butter represents the type, emulsion, that is, liquid fat dispersed in water.
- Define emulsion.
Ans.: Emulsion is a colloidal suspension of one liquid in another, for example, milk.
- Give one example for each of the following: (i) solid sol, (ii) solid emulsion and (iii) liquid aerosol.
Ans.: Solid sol: Ruby glass
Solid emulsion: Cream
Liquid aerosol: Fog
- What do you understand by electrophoresis?
Ans.: Motion of the colloidal particles of a sol under the influence of an electric field is electrophoresis.
- How do you distinguish a colloidal solution from a true solution?
Ans.: When a beam of light is passed through both the solutions, then only colloidal solution shows Tyndall effect, whereas true solution does not.
- What substances do the gold numbers possess?
Ans.: Lyophilic sols, which act as protective colloids, possess gold numbers.
- Why are Brownian movement and Tyndall effects shown by colloidal solutions only?
Ans.: Large-sized particles in a colloidal solution do not settle down, because the molecules of the dispersion medium bombard them continuously.
- What happens when a beam of light is passed through a colloidal solution?
Ans.: When a beam of light is passed through a colloidal solution, placed in a dark room, the path of the beam becomes illuminated and visible when viewed through a microscope placed at right angle to the path of light. This phenomenon is called Tyndall effect.
- What is observed when an electrolyte (NaCl) is added to ferric hydroxide sol?
Ans.: When NaCl is added to ferric hydroxide sol, the Cl- ions of NaCl neutralize the positive charge on ferric hydroxide sol particles and coagulate it. Thus, coagulation of sol occurs.
- Explain which one of the following electrolysis is most efficient in coagulating a ferric hydroxide sol: (i) KCl, (ii) FeCl3, (iii) K4 [Fe(CN)6], and (iv) K3 [Fe(CN)6].
Ans.: Ferric hydroxide sol is positively charged, and it is coagulated by negative ions. Since, negatively charged ion [Fe(CN)6]4− is furnished by K4[Fe(CN)6], it is the most efficient in coagulating a ferric hydroxide sol.
- Which one of these will be the most efficient for the precipitation of As2S3 sol and why? (i) NaCl (ii) BaCl2 (iii) AlCl3
Ans.: Arsenic sulphide (As2S3) sol is negatively charged and is coagulated by positively charged ions furnished by the added electrolyte. Since the highest positive valency Al3+ is furnished by AlCl3, so, according to Hardy-Schulze law, AlCl3 is the most efficient in the precipitation of As2S3 sol.
- Gold number of gelatin and starch are 0.01 and 0.10, respectively. Which of the two possesses a higher protective power and why?
Ans.: Gelatin, because the smaller the gold number, the greater is the protective power of lyophilic colloids.
- Deltas are formed at a place where the rivers pour their water into sea. Give reason.
Ans.: The negative-charged, fine, sand particles present in the river water are precipitated by the positive-charged Na+, K+, Mg2+, and Ca2+ ions present in the sea water, thereby leading to the formation of deltas at the place of their meetings.
- Potash alum is used for removing impurities from water. Give reason.
Ans.: When alum is added to impure water containing fine, suspended, negatively charged clay and sand particles, bacteria, etc., the Al3+ ions precipitate them and coagulate them, and the purified water is left behind.
- The colloidal particles precipitate on adding electrolyte. Why?
Ans.: Because, when electrolyte is added to a colloidal sol, the charge on the colloidal particles is neutralized by the oppositely charged ions furnished by the electrolyte. So these particles coagulate and change into bigger particles, which finally settle down under the influence of gravity.
10.4.4 Long Answer Questions
- Distinguish between physisorption and chemisorption with examples.
- What are the various factors affecting adsorption of a gas on a solid adsorbent?
- Derive Langmuir’s adsorption isotherm for monolayer adsorption and show under what conditions it becomes identical to Freundlich adsorption isotherm.
- Define the terms adsorption, occlusion, adsorption and sorption. How does pressure affect the adsorption of a gas over activated charcoal at constant temperature?
- State and explain BET equation for multilayer adsorption.
- What is a colloidal sol? How are colloidal sols prepared? Explain the meaning of the following terms:
- Tyndall effect
- Eletrophoresis
- Brownian movement
- Give a brief account of the optical and electrical properties of colloids.
- Write a brief account on the following.
- Origin of charge on the colloidal particles
- Zeta potential and electrical double layer
- Gold number
- Cataphoresis
- What is meant by coagulation of colloidal sol? Bring out clearly the role of electrolytes to cause flocculation of colloidal sols.
- When a beam of light is passed through a colloidal solution, the path of the beam becomes visible. Give reasons.
- Write an account on electro-osmosis and also the significance of electro-osmosis?
- Explain the natural applications of colloids.
- Explain the method for determining the surface area of absorbents.
- Differentiate the following:
- Crystalloids from colloids
- Lyophilic from lyophobic sols
- Chemisorption from physiorption
- Discuss the various applications of colloid chemistry.
- Discuss in detail the important applications of colloids in:
- Food industry
- Chemical industry
- Purification of air
- Pharmaceutical industry
