11
THERMODYNAMICS
11.1 INTRODUCTION
Thermodynamics is the branch of science that deals with the conversion or transformation of heat energy into other forms of energy such as chemical, electrical, light energy, etc. It is also defined as the study of the flow of heat or any other form of energy into the system as it undergoes a physical or chemical transformation.
11.1.1 Thermodynamic Terms and Basic Concepts
System
A definite quantity of matter bounded by some enclosed surface is known as a system. A system is also defined as that part of the universe which is under thermodynamic study.
Examples: For a scientist, the gas enclosed in a cylinder is a system. For a mechanical engineer, the machine is a system.
Surrounding
All the matter which can influence the behaviour of the system is known as surroundings. In other words, except system, rest of the universe is surroundings.
Examples: For an egg placed on the table, the atmosphere around the egg is the surrounding.
Note: The choice of a system and its surrounding is arbitrary.
Boundary
The real or imaginary surface separating the system from its surroundings is called the boundary.
Homogeneous System
When a system is uniform throughout, it is called a homogeneous system.
Example: Pure single solid or liquid or gas system
Phase: Homogeneous, physically distinct and mechanically separable position of a system.
Heterogeneous System
Heterogeneous system is one which consists of two or more phases.
Examples: Ice in contact with water, ice in contact with water vapour, etc.
11.2 TYPES OF THERMODYNAMIC SYSTEMS
There are three types of thermodynamic systems depending on the nature of the boundary.
11.2.1 Isolated System
If a system may be exchanges neither matter nor energies with the surroundings, then it is known as an isolated system (Figure 11.1).
Example: A substance, say, boiling water, contained in a thermoflask.
Figure 11.1 Isolated system
11.2.2 Closed System
If a system exchanges energy but not matter with the surroundings, then it is called a closed system. In other words, a closed system is one which cannot transfer matter but can transfer energy in the form of heat, work and radiation to its surroundings.
Example: A specific quantity of hot water contained in a sealed tube is an example of a closed system while no water vapour can escape from this system; it can transfer heat through the walls of the tube to the surroundings (Figure 11.2).

Figure 11.2 Closed system
11.2.3 Open System
If a system exchanges both energy and matter with the surroundings, then it is called open system.
Example: Hot water contained in a beaker placed on laboratory table is an open system. The water vapour (matter) and also heat (energy) is transferred to the surroundings through imaginary boundary (Figure 11.3).

Figure 11.3 Open system
11.3 INTENSIVE AND EXTENSIVE PROPERTIES
11.3.1 Intensive Property
A property that does not depend on the quantity of matter present in the system is known as intensive property.
Examples: Pressure, temperature, density, EMF, etc.
11.3.2 Extensive Property
A property that depends on the quantity of matter present in the system is called extensive property.
Examples: Volume, number of moles, enthalpy, entropy and Gibbs free energy
Hence, macroscopic properties are divided as intensive and extensive properties.
11.3.3 State Variables
A thermodynamic system is said to be in a certain state when all its properties are fixed. Fundamental properties that determine the state of a system are pressure (P), temperature (T), volume (V), mass (M) and composition. Since a change in the magnitude of such properties alters the state of the system, these are referred to as state variables.
11.4 REVERSIBLE AND IRREVERSIBLE PROCESS
11.4.1 Reversible Process
The process in which all the changes that occurred in the direct process are repeated in the reverse direction is called reversible process.
In a thermodynamic system, in the change of the state of absorption of heat energy in the direct process, the system should release the same amount of heat energy. It changes the state in the reverse direction. This type of process is called reversible process.
For example, very slow process done at constant temperature, Carnot cycle process, etc.
If a certain amount of heat is given to ice through it melts and water is formed. If the same amount of heat is removed from water while the ice is formed.
11.4.2 Irreversible Process
The process in which all the changes that are accepted in the direct process were not repeated in the reverse direction is called irreversible process.
All the processes that irreversibly occur are examples of an irreversible process.
Examples: conduction, radiation, radioactive decay, electrical heating of a wire, etc.
If an electric current is passed in the wire, the heat is developed in the wire and this cannot be reversed.
11.4.3 Thermodynamic Processes
When a thermodynamic system changes from one state to another, the operation is called a process. The various types of thermodynamic processes are as follows:
Isothermal Process
The processes in which the temperature remains constant are termed isothermal processes.
![]()
Example: This is often achieved by placing the system in a thermostat.
Adiabatic Process
The processes in which no heat can flow in or out of the system are called adiabatic processes. For an adiabatic process,
![]()
Example: Adiabatic conditions can be approached by carrying the process in an insulated container such as a thermos bottle.
Isobaric Process
The processes which can take place at constant pressure are called isobaric processes.
![]()
Example: Heating of water to its boiling point and its vapourisation takes place at the same atmospheric pressure.
Isochoric Process
The processes which can take place at constant volume are known as isochoric processes.
![]()
Example: The heating of a substance in a non-expanding chamber is an example of isochoric process.
Cyclic Process
When a system in a given state goes through a number of different processes and finally returns to its initial state, the overall process is called a cyclic process.
![]()
Example: Carnot cycle
In thermodynamics, the state of a system can be represented by pressure, volume and temperature. These three parameters are known as thermodynamics variables.
Thermal Equilibrium
If there is no heat exchange between two bodies, the bodies are said to be on thermal equilibrium.
11.4.4 Isothermal Process or Isothermal Change
When a thermodynamic process occurs in such a way that its temperature remains constant, the process is called isothermal process. In an isothermal process, the heat generated or lost is given or taken from the surroundings.
Example: When a gas in a cylinder is slowly compressed, heat is generated and conducted to the surroundings, on other hand, when the gas is allowed to expand slowly a slightly cooled but heat is taken from the surrounding such that the temperature remains constant.
Heat
Every form of energy has its own method of transmission from one place to another. Heat is one of the forms of energy that can be transmitted over large distances in vacuum. The specialty of heat energy is that it can get transmitted by three distinct processes, totally different from one another. They are conduction, convection and radiation.
Conduction
The transmission of heat from the hotter to the colder part of a body without the transfer of the particles of the material medium is defined as conduction.
Convection
The transmission of heat from one part to another part of the body or surroundings by the transfer of particles of matter known as convection.
Radiation
Transmission of heat from a hot body to a cold body without the help of any material or medium.
Conduction and convection are the methods of heat transmission which can be carried out in the presence of only material medium.
Work
Force × distance [Joules]
When a force acts on a system such that it produces a displacement in the system, the work is said to be done.
Equivalence of Heat and Work
It is an established fact that heat is a form of energy; for example, when the brake is applied on a moving vehicle, the drum becomes hot. In this situation, friction is involved and heat is produced; when mechanical energy (work) disappears, heat is produced. This shows the equivalence between heat and work.
Internal Energy or Intrinsic Energy
Every thermodynamic system has a definite amount of energy within itself. The stored energy in a system which is not apparently shown is called the internal energy or intrinsic energy.
Example: Consider gas enclosed in a cylinder; according to kinetic theory of gases, gas molecules are in rapid motion and hence they possess kinetic energy. Due to intermolecular forces of attraction, the system possesses potential energy. The sum of kinetic energy and potential energy is equal to the internal energy of the system.
Note: The internal energy (U) is independent of the path followed in a cyclic path or process; the change in internal energy is dU = 0.
If the system can be transferred from the initial state to the final state, the work done by the system W and the accepted or rejected heat Q but practically the value of Q−W value is a constant. The value of Q−W can only depend on the initial and the final states of the work.
![]()
ΔV shows the difference between the initial and final states of the system. This difference is said as internal energy and is the function of P, V and T.
U in case of functions P, V

U in case of functions P, T

U in case of functions T, V

11.4.5 Indicator Diagram
For an infinite small change in volume between states C, D, the pressure can be treated as almost constant.
![]()

It can be shown the area under the curve AB represents the total work done by the gas during expansions; it is shown with the shaded region in the graph. When the graph is traced from B to A, it represents the work done on the gas, that is, the gas is said to be compressed.
A graph between pressure and volume of a thermodynamic system is known as indicator diagram. These diagrams are used to discuss the theory and performance of heat engines (Figure 11.4).

Figure 11.4 Indicator diagram
Let us take the initial state (Pi, Vi) of the gas by a point A and the final state (Pf, Vf) by a point B as shown in the graph. The point A and B indicates to the state of change of gas from A to B and also called the indicator diagram.
Work Done in Isothermal Process
A process that takes place at constant temperature is known as an isothermal process (Figure 11.5).
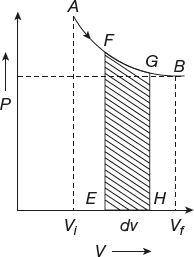
Figure 11.5 Isothermal process
Let one gram mole of an ideal gas expand isothermally. The initial stage A (Pi, Vi) and final stage are at B (Pf, Vf). In an isothermal process, the pressure and volume are changed but the temperature will be constant.
Work is done by the gas for a small expansion dW = PdV when it expands from initial value to final value.

![]()

The work done in an isothermal process is as follows:

11.4.6 Work Done by a System in an Adiabatic Process
An adiabatic system is a system with no exchange of heat between the system and its surroundings (Figure 11.6).

Figure 11.6 Work done in adiabatic process
One gram mole of an ideal gas expands adiabatically from the initial state A (Pi Vi) to a final state B (Pf Vf) as shown in the graph; during adiabatic expansion, the pressure and the volume change but the heat is constant.
Let P and V be at intermediate stage C for a small change in volume dV from A to B and C to D. The work done by the gas during the small expansion is PdV; this is equal to the shaded area CDC′D′ as shown in the graph.
The network done by the gas during the expansion from Vi to Vf is given as follows:

If Ti and Tf correspond to the initial and final of the stages,

Thermodynamics
The study of internal energy of any system is known as thermodynamics.
Indicator Diagram
A graph between pressure (P) on the Y-axis and volume (V) on the X-axis is the indicator diagram; this diagram is also called the P–V diagram (Figure 11.7).
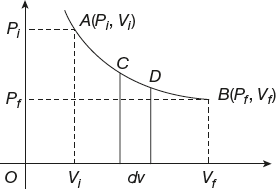
Figure 11.7 Graph between pressure and volume
Internal Energy
The ideal gas of internal energy can be dependent on the temperature.

11.4.7 First Law of Thermodynamics and its Applications
The total energy of an isolated system remains constant though it may change from one state to another. Hence, the absorbed heat of any system is equal to the sum of the increase in internal energy and the work done by the system. According to this law, if a small amount of heat energy (dQ) is supplied to the system, then a part of the energy is used to increase the internal energy (dU) of the system and the remaining energy is used to do external work by the system (dW).
 (1)
(1)
This law explains the law of conservation energy. It means that thermal energy can be converted into mechanical energy. Energy can be neither created nor destroyed but it may change from one state to another. Whenever a quantity of some form of energy disappears, an exactly equivalent amount of some other form of energy must be produced.
Second Law of Thermodynamics
According to entropy the second law of thermodynamics can be written as
![]() (2)
(2)
Relation between (1) and (2)

Application of First Law of Thermodynamics
Isothermal Process
In an isothermal process, the temperature of the system remains constant;
that is, dT = 0
Hence, there is no change in the internal energy of the system: dU = mCdT = 0
∴ From the first law, dQ = 0 + dW
![]()
In an isothermal process, the heat supplied a system is used only to do external work.
Adiabatic Process
In an adiabatic process, there is no exchange of heat between the system and the surroundings;
that is, dQ = 0
∴ From the first law of thermodynamics,

Therefore, during adiabatic compression, work is done on the gas (dW − Vc) and hence the internal energy increases (du = +ve).
Cyclic Process
When a system undergoes a cyclic change, the dU = 0.
From the first law of thermodynamics,

Isochoric Process
In an isochoric process, there is no change of volume.
For an isochoric process, dV = 0
∴ From the first law of thermodynamics,

that is, the total heat supplied to the system is equal to the increase in internal energy of the system.
Isobaric Process
In an isobaric process, the pressure is constant.
Example: phase change process (water to stream)
Heat Capacity
Energy required to raise the temperature of a system is known as heat capacity. If a Q calorie of heat is absorbed by the system, the temperature of the system raises from T1 to T2.
Heat capacity:
![]()
Thus, the heat capacity of a system is the heat absorbed by unit mass in raising the temperature by 1°C. For gaseous systems, heat capacity is of two types.
Heat Capacity at Constant Volume (Cv )
In constant volume, the molar heat capacity of a system is defined as the amount of heat required to raise the temperature of one mole of the substance by 1°C. At constant volume (Qv) when the amount of heat absorbed by gaseous system (in calories) the temperature raises from T1 to T2.
Heat capacity:
![]()
According to the first law of thermodynamics,

Heat Capacity at Constant Pressure (Cp)
At constant pressure, the molar heat capacity of a system is defined as the amount of heat required to raise the temperature of one mole of the substance by 1°C. At constant pressure (Qp) when the amount of heat absorbed by the system (in calories) the temperature raises from T1 to T2.
![]()
According to the first law of thermodynamics,

11.4.8 Second Law of Thermodynamics
Clausius Statement
It may be defined as follows:
“It is impossible for any cylindrical machine to transfer heat continuously from a cold body to a hot body”.
“There is no perfect refrigerator”.
This statement implies that to transfer heat from one body to another body at a higher temperature, some work must be done by an external agent.
Kelvin–Plank Statement
It is impossible for any reversible heat engine to convert total heat into work.
There is no perfect heat engine
This statement implies that a heat engine should reject a part of heat to a body at lower temperature (sink) while converting the heat into work.
Importance of Second Law
The first law of thermodynamics says that heat and mechanical work is inter conversable; but it doesn’t give any limitation and condition for this conversion. The second law of thermodynamics specifies the direction of the flow of heat engine.
Carnot’s Theorem
In all reversible engines working with the same temperatures, limits will have the same efficiency.
No engine is more efficient than Carnot’s reversible engine working with the same temperature.
Consider two reversible engines A and B working with the temperature T1°C and T2°C. A is more efficient than B; A works as a heat engine and B as a refrigerator. Engine A observes an amount of heat energy Q1 from the source at T1°C. It does external work ‘W’. Q2 is rejected to the sink at temperature at T2°C (Figure 11.8).

Figure 11.8 Working of reversible engines
Engine B observes the amount of heat Q2′ from the sink at temperature T2°C, and ‘W’ work is done on the work substance and gives the heat Q1′ to the source at the T1°C.
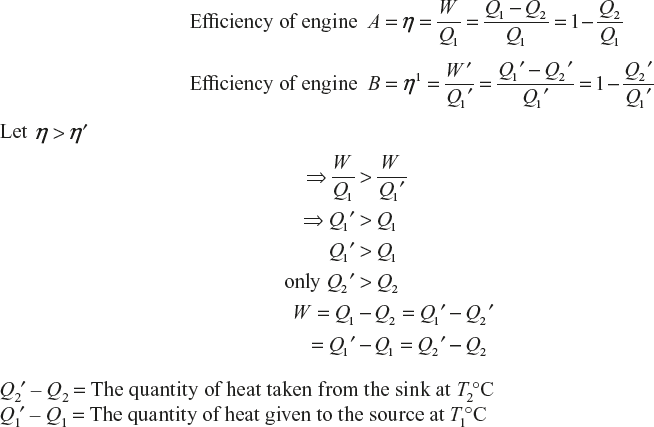
The heat flows from the sink at the temp T2°C [lower] to the source at temperature T1°C (high temperature) without any external work
∴ n = n′ its define the second law of thermodynamics
11.4.9 Carnot’s Engine Efficiency
A Carnot’s engine is a heat engine which can transfer heat energy from a body having higher temperature to lower temperature while transforming the heat energy from one body to another. The Carnot’s engine loss of heat energy is very minimum.
A Carnot’s engine has mainly four parts:
- Source
- Sink
- Working substance
- Insulating stand
Source
A source is the part of the Carnot’s engine which supplies the heat energy with high temperature T1K; it has infinite thermal capacity. Since it has infinite thermal capacity, its temperature does not change when some amount of heat energy is drawn from heat.
The source is made up with an adiabatic wall at the bottom and the sides are closed with a diathermic wall at the top as shown in Figure 11.10.
Sink
A sink is the part of Carnot’s engine which receives the heat energy, with low temperature T2K. The sink similar to the source as shown in Figure 11.9.
Working Substance
It is the part of Carnot’s engine which cross heat from the source and is supplied to the sink. The working substance to heat generally uses ideal gas. The work substance is taken in a cylinder having movable piston (P) as shown in Figure 11.9. The bottom surface of the cylinder is made with the adiabatic wall.
Insulating Stand
It is a part of the Carnot’s engine which can be used for transferring heat from the source to the sink without loss of heat. This is made with adiabatic material as a block as shown in Figure 11.9.

Figure 11.9 Carnot’s engine

Figure 11.10 Working of Carnot’s engine
11.4.10 Working of Carnot’s Engine
The working of Carnot’s Engine is explained by four cyclic operations; these are shown in Figure 11.10.
Step 1
Let a cylinder having work substance be placed on the source. Let the initial state of gas on the system be A (P1, V1, T1). It allows the expansion of gasses, slowly moving the piston in the upward direction whose state reaches B (P2, V2, T1).
∴ The temperature remains constant in this expansion. It is an isothermal expansion.
In this expansion, the working substance can cross heat energy from the source with work done, W1. The change of state of process is representation in P–V diagram.
The work done in this process is equal to the area of ABCD.
 (1)
(1)
Step 2
Let the cylinder detached from the source be placed on the insulating stand. It allows the adiabatic expansion whose temperature changes to T2. In this adiabatic expansion, the change of state is observed from B (P2, V2, T1) to from position C (P3, V3, T2). This is shown in the indicator diagram.
Let the work done in this process be W2.

Step 3
Let the cylinder from the stand and place it into the sink to allow the isothermal contract. In this process, the change of state is from C (P3, V3, T2) to D (P4, V4, T2). The isothermal contraction is represented in the indicator diagram as CD.
Let the work done in this process be W3 and the heat supplied to the Q2 be

Step 4
Let the cylinder be detached from the sink and place it on the insulating stand. Allow the adiabatic contraction until the temperature of the system reaches the T1 element in this process. The change of state from D (P4, V4, T2) to A (P1, V1, T1) is represented in the indicator diagram DA.
Let the work done in this process be
![]()
∴ The total work done W = W1 + W2 + W3 + W4
 (5)
(5)
According to adiabatic relations from the curves A, D
 (6)
(6)
Adiabatic from BC curves relation
 (7)
(7)
![]() (8)
(8)
Substitute equation (8) in equation (5) we get,

The efficiency of adiabatic Carnot’s engine defines the ratio of work done to the heat energy drawn from the source.

The efficiency of the Carnot’s engine depends on the temperature of the sink and the source. The efficiency of the Carnot’s engine does not depend on the physical properties of the working substance.
11.4.11 Absolute Zero
The efficiency of the Carnot’s engine in terms of the temperatures in Kelvin scale is written as n = ![]()
If T2 = 0°K, then n = 1.
Absolute zero in Kelvin scale is defined as the temperature of the sink for which the efficiency of Carnot’s engine is equal to unity; it converts total heat into work.
Thermodynamics Scale of Temperature or Kelvin’s Absolute Scale of Temperature
Thermodynamic or Kelvin scale of temperature is based on the efficiency of Carnot’s reversible engine. Carnot’s engine depends on the temperature of the source (T1) and sink (T2), and it is independent of the working substance.
Consider a reversible heat engine absorbing an amount of heat Q1 at T1 rejecting heat Q2 at T2.
∴ The efficiency is only the function of two temperatures:

Where f (T1, T2) is a function of T1 and T2.
 (1)
(1)
Where F (T1, T2) is another function of T1 and T2.
Now consider another reversible heat engine which absorbs heat Q2 at T2 and rejected Q3 at T3.
![]() (2)
(2)
![]() (3)
(3)
 (4)
(4)
We observe that there is no T2 on the left hand side of the equation. This is possible only if
![]() (5)
(5)
where ϕ is another function
![]() (6)
(6)
∴ From equation (1) and (5)
![]()
Since Q1 > Q2, ϕ (T1) > ϕ (T2) provided T1 > T2
i.e. ϕ (T) is linear function of a T

where T1, T2 are the temperature in Kelvin scale.
The aforementioned equation defines the Kelvin scale as follows.
“Kelvin scale is that scale in which the ratio of any two temperatures is the same as the ratio of heat absorbed and heat rejected by a reverse Carnot’s engine working between the two temperatures”.
11.4.12 Numerical Problems Based on Carnot’s Cycle
- Calculate the efficiency of a reversible heat engine working between 72°C and 187°C.

- A refrigerator works under a reversible cycle between the temperature 300°K and 400°K. Calculate the coefficient of performance.

- In a steam turbine, steam heated to 750°C enters the turbine and its temperature is 110°C. What is the maximum theoretical efficiency of the turbine?

- A Carnot’s engine works between 227°C and 27°C. Its efficiency is 50%. What is the percentage of actual efficiency of theoretical efficiency?

- A reversible engine works between two temperatures whose difference is 100 K. If it absorbs 746 J of heat from source and rejects 546 J to sink, calculate the temperatures of source and sink.
We know that

Actually

11.4.13 Solved Numerical Problems Based on Isothermal and Adiabatic Process
- A gas at 27°C is compressed to half its volume suddenly calculate the resulting pressure and temperature (given r = 1.41).
In an adiabatic process,

- The initial temperature of a gas is 27°C; to calculate the rise in temperature when the gas is compressed suddenly to eight times its original pressure (r = 1.5),
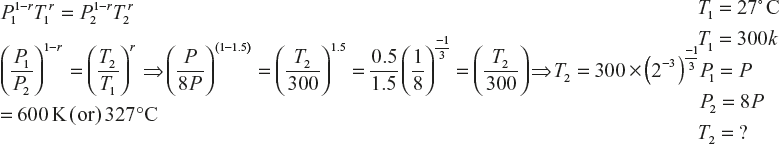
- A quantity of an ideal gas at 17°C is adiabatically compressed to one-tenth of its initial volume. Calculate the final temperature of the mono atomic and diatomic gas (given r for mono atomic gas is 1.6, diatomic gas is 1.4)
- Monoatomic gas

- Diatomic gas r = 1.4

- Monoatomic gas
- Calculate the work done in if one mole of an ideal gas expands isothermally at 127°C to double its original volume, given R = 8.314 J/m/K.
Work done in isothermal expansion process:

- Calculate the work done when a gram mole of a perfect gas expands isothermally at 27°C to double its original volume [R = 8.314 JK−1mole−1]

- Two moles of oxygen (O2) at 0°C are compressed until the volume becomes one-fourth of the volume at the same temperature. Calculate the work done.

‘–‘denotes compression
- Deduce the change in the boiling point of water when the pressure changes by 1 cm of Hg. Given L = 2.268 × 105 J/kg, V1 = 10−3 m3/kg, V2 = 1.674 m3/kg
According to Clausius–Clapeyron, the change in boiling point of water is

- A tyre pumped to a pressure of 3 atm bursts suddenly. Calculate the fall in temperature due to adiabatic expansion. The temperature of air before expansion is 27°C and r = 1.4.

- Entropy
The thermal property of a body which remains constant during an adiabatic process is called entropy; it remains constant between two adiabatic lines. The entropy denoted by S.
The entropy value is not estimated at a particular point of process. It is calculated between any two points on adiabatic lines (Figure 11.11).

Figure 11.11 Calculation of entropy value
Change of entropy generally expressed as

where dQ is the change in heat energy at the temperature (T) between the two points which are much closed to each other; thus the total entropy can be written as
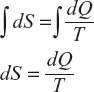
Explanation
Let a Carnot engine be at working between the temperature T1 and T2 as the Carnot’s cycle of ABCDA.
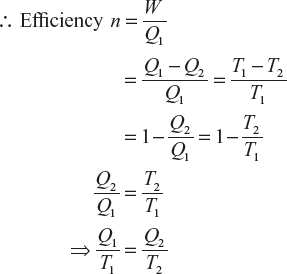 (1)
(1)If the Carnot engine is working between the temperature T2 and T3 from Carnot cycle of DCEFD, ∴ Efficiency of Carnot engine
 (2)
(2)By the Carnot engine’s working, temperature T3 and T4 be:
 (3)
(3)The quantity of
 which remains constant between the two adiabatic lines L and M is called entropy.
which remains constant between the two adiabatic lines L and M is called entropy.
- Entropy disorder
According to Boltzmann’s theory, the disorder of a material increases from solid to liquid and liquid to gasses. The disorder of gasses is maximum and the disorder of the solid is minimum. As a result the change of entropy is maximum for gasses and the change of entropy is minimum for solids.
Hence, entropy is directly proportional to the disorder.
Calculation of entropy
Change in entropy for a reversible process is shown in Figure 11.12.

Figure 11.12 Calculation of entropy
Consider a reversible process in the Carnot cycle ABCDA; working with two adiabatic processes and two isothermal processes, this adiabatic process is shown in the indicator diagram.
Let the total change in entropy reversible process be S.
Total change in entropy = sum of change in entropy of all process Q.
 (1)
(1)Here, δ1 is the change in entropy of the system. The state is isothermal and expanded from A to B with a transformation of heat energy Q1.

δ2 is the change of entropy of the system. This state is adiabatically expanded from B to C without transformation of heat energy.

δ3 is the change of entropy of the system whose isothermal expansion lies between C and D with transformation of heat energy Q2.

δ4 is the change of entropy of the system whose state is adiabatic contraction between D to A without transformation of heat energy
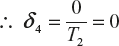
All these values are substitute in equation (1)
 (2)
(2)From the efficiency of the reversible process,

 (3)
(3)Substitute equation (3) in equation (2) and we get the change of entropy in the reversible process:

- Change in entropy for irreversible process
From the efficiency of irreversible process


Substitute equation (3) in equation (2) we get the change of entropy in irreversible process

∴ The change of entropy in irreversible process is positive
- Change of entropy of the universe
The change of entropy for reversible process is zero and the irreversible process is positive. However, in the universe, all the processes are irreversible.
∴ The change of the entropy of the universe should increase with the time.
Let a reversible process in the universe which initially the heat energy Q1 from the source at the temperature to available of energy in the universe which as reversible process equal to Q1 – Q2.
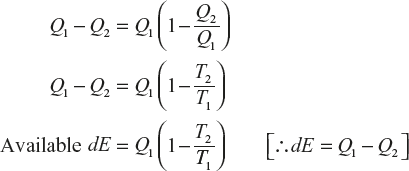
(The available energy of the universe becomes the maximum as the sink temperatures becomes T0.)
The maximum available energy at the initial state of the universe is

The maximum available energy at the final state of the universe is

∴ The net available energy dE = Q1 – Q2

Here, dS is the change of entropy of the universe. In the universe, the number of irreversible processes increase and the available energy decreases; so the entropy of the universe increases with the time.
- Entropy–temperature diagram
The curves formed by the variation of temperature with the entropy of the system known as T-S diagram. In the T-S diagram, the change in entropy of the system is taken on the X-axis and the temperature of the system is taken on the Y-axis as shown in Figure 11.13. It is similar to indicator or P-V diagram.
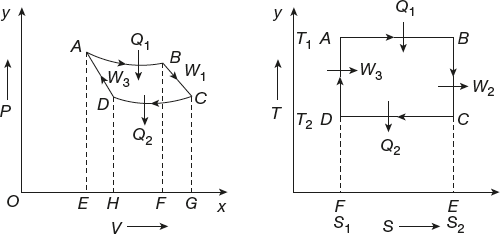
Figure 11.13 Entropy–temperature diagram
In an isothermal process, the temperature is constant but entropy can change. In adiabatic process, entropy is constant.
The efficiency of Carnot engine

 (1)
(1)Let a system undergo the change of state isothermally from A → B. In this process, the change of entropy is

From the graph, the change of entropy dS = S2 – S1
 (2)
(2) (3)
(3)The system undergoes adiabatic process from B to C with the change of temperature T1 → T2.
∴ Change of entropy = 0
The system undergoes a change from C to D in the isothermal process.
Change of entropy


From the graph, change of entropy is dS = S2 – S1
 (4)
(4) (5)
(5)Substitute equation (2) and (4) in equation (1), we get

Substitute equation (3) and equation (5) in equation (1)we get

Substitute equation (3) and equation (5) in equation (1)

Boyle’s law is valid for isothermal process.
PV = RT (for 1 mole of gas)
- Adiabatic process or adiabatic change
When a thermodynamic process occurs in such a way that no change of heat takes places a system and surroundings, the process is known as adiabatic process. In other words, there is change in temperature in an adiabatic process.
Example:
Boyle’s law is not valid for adiabatic process.
∴ For an adiabatic process

Adiabatic relations between P, V, T of ideal gas or we show that PVr = constant:
Consider 1 mole of an ideal gas enclosed
Let its thermodynamic coordinates be P, V, T
If the gas under goes adiabatic expansion, work done by the gas dW = PdV
Decrease in internal energy dU = CvdT
∴ From the first law of thermodynamics

For an adiabatic process, dQ = 0
 (1)
(1)For 1 mole of ideal gas,
 (2)
(2)Substitute equation (2) in equation (1)
On integration,
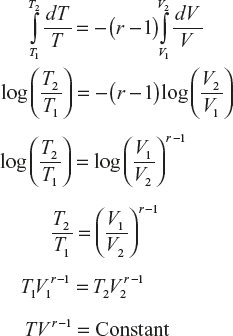 (1)
(1) (2)
(2) (3)
(3)Equations (1), (2) and (3) are known as adiabatic relations.
- Coefficient of performance of a refrigerator
The coefficient of performance of a refrigerator is defined as the ratio of heat taken from sink to the work needed to run the refrigerator (Figure 11.14).

Figure 11.14 Coefficient of performance of a refrigerator

- Relationship between the efficiency (n) of a Carnot heat engine and the efficient of performance (K) of a refrigerator
Consider a Carnot heat engine’s efficiency is ‘n’ and a Carnot refrigerator’s coefficient of performance as ‘K’. The working between temperature T1 and T2 is as follows:

This is the relation between n and K.
- Calculation of change in entropy when ice converted into steam
The process of conversion of m gram of ice at T1°K into steam at T2°K involves three processes.
- First, ‘m’ gram of ice converts into water; this is a phase change process occurring at constant temperature T1.
Heat absorbed during the process dQ = mL1
L1 = Latent heat of fusion

- Second, ‘m’ gram of water at T1°K converts into water at T2°K
Heat absorbed for dT change in temperature = mcdT
where ‘C’ specific heat of water

- Finally, ‘m’ gram of water at T2°K is converted into steam at T2°K. This is also a phase at which the process occurs at constant temperature, T2.
Heat observed during the process dQ = mL2
L2 = Latent heat at vapourisation

∴ Total change in entropy when ice converts into stream

- First, ‘m’ gram of ice converts into water; this is a phase change process occurring at constant temperature T1.
Solved Numerical Problems Based on Entropy
- Find the increase in entropy when 10 g of ice at 0°C is converted into water at the same temperature given latent heat of fusion of ice = 80 cal/g.
0°C ice —0°C water (phase change)
Change in entropy



11.5 THERMODYNAMIC POTENTIALS AND MAXWELL EQUATIONS
11.5.1 Thermodynamic Potential
The thermo function which gives the energy of thermodynamics systems as defined in P, T, V and S is called thermodynamic potentials. There are four thermodynamic potentials which represent the energy of the systems.
These are as follows:
- Internal energy (U)
- Helmholtz function (F)
- Total heat energy or Enthalpy (H)
- Gibb’s function or Gibb’s free energy (G)
11.5.2 Internal Energy (U)
It is the thermodynamics potential of the system which represents its total internal energy. The change in internal energy of the system is derived as follows.
From first law of thermodynamics,
![]()
From the second law of thermodynamics, dQ = TdS
External work done: dW = PdV
![]() (1)
(1)
Now equation (1) can be partially differentiated with respect to variables S and V

Equation (1) can be written as perfect differentiation
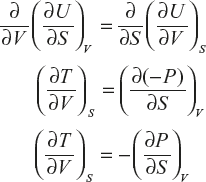
This is the first Maxwell thermodynamics equation.
![]()
∴ This condition for exactness is given by

11.5.3 Total Heat Function (H)
It is the thermodynamics potential of the system which represents about the total heat energy of the system. The total heat energy of the system is equal to the sum of internal heat energy and external energy. Thus, the enthalpy can be written as
![]()
Differentiate the equation
 (2)
(2)
Equation (2) is the form of exact differential equation and hence it can be partially differentiated with respect to P and S.

Equation (2) can be written as exact differential equation as

This is the Maxwell third equation.

11.5.4 Helmholtz Function (F)
It is the thermodynamic potential of the system which uses the internal energy of the system of constant temperature. The Helmholtz function in terms of P, V, T and S is derived as follows.
According to the first and second laws of thermodynamics, the equation of change in internal energy is as follows:

Here, U – TS = F is called the Helmholtz function; differentiate the above function.
 (2)
(2)
Equation (2) is the form of the exact differential form hence it can be partially differentiated as with respect to V and T.

Equation (2) can be written as perfect differential equation.

This equation is the Maxwell second equation.
11.5.5 Gibb’s Free Energy or Gibb’s Function (G)
It is the T.D potential of the system which gives the total heat of the system at constant temperature and pressure.
Gibb’s function is mathematically defined as follows:
G = H – TS on differentiation

∴ H – TS called Gibb’s function
![]()
Differentiate the above Gibb’s function
 (4)
(4)
Equation (4) is the form of exact differential equation and hence it can be partially differentiated with respect to P, T.

Equation (4) can be written as an exact differential equation.
 (5)
(5)
or 
This is called Maxwell’s fourth equation.
11.5.6 Maxwell’s Equations

Maxwell’s First Equation
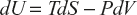 (1)
(1)
Equation (1) can be written as dZ = Mdx + Ndy
M → T ; x → S
N → –P; y → V
 (1)
(1)
- dH = TdS + VdP can be written as dZ = Mdx + Ndy

- dF = −SdT - PdV this equation can be written as dz = Mdx + Ndy

- dG = −SdT + VdP, this equation can be written as G = H − TS

Specific Heat (C)
The quantity of heat required to raise the temperature of one gram of a substance through 1°C is known as specific heat.
![]()
For gases, there are two types of specific heat:
- Specific heat at constant pressure (CP):
At constant pressure, the amount of heat required to raise the temperature of a gas at unit mass through 1°C is known as specific heat at constant pressure.

- Specific heat at constant volume (CV):
At constant volume, the amount of heat required to raise the temperature of a gas at unit mass through 1°C is known as specific heat of constant volume.

11.5.7 Clausius–Clapeyron Equation
Clausius–Clapeyron’s latent heat equation relates the change in melting and boiling point with change in pressure.
From Maxwell’s second equation,

Multiplying both sides with ‘T’

TdS = dQ
![]() (1)
(1)
Now, assume that the unit mass of a substance undergoes a phase change (from solid to liquid or gas), the heat observed at constant temperature is given by
![]()
Let V1, V2 be the specific volumes in first state and second state respectively. (Specific volume = volume per unit mass)

which is the latent heat of Clausius–Clapeyron.
Use
Clausius–Clapeyron is used to study the change in melting and boiling point with change in pressure.
Relation between CP and CV
Derivation of CP – CV = R
 (1)
(1)
 (2)
(2)
Clausius equation dQ = TdS
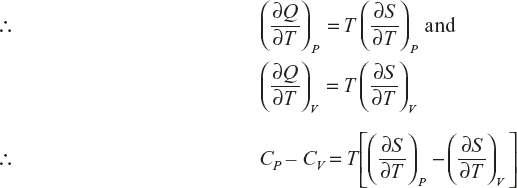 (3)
(3)
If the change in entropy at system is assumed as a function in terms of T, V,

The above equation divided by ∂T.

Multiply with T, on both sides


Ideal gas equation PV = RT

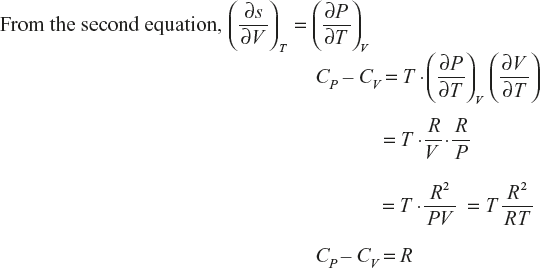
11.5.8 Derivation of the Stefan–Boltzmann Law using Maxwell’s Equations
According to Stefan’s law, the total radiant energy E emitted per second from unit surface area of a black body is proportional to the fourth power of its absolute temperature T.
Thus, 
where σ is a constant called Stefan’s constant. In the Meter-Kilogram-Second (MKS) system, its value is 5.672 × 10−8J.sec−1.m−1.k−4.
Consider a block body having volume V, total internal energy U and energy density as E, then internal energy of the system can be written as ![]()
From the first law of thermodynamics, dQ = dU + dW
From the second law of thermodynamics, dQ = TdS
From Maxwell’s (2) equation
 (1)
(1)
 (2)
(2)
or
![]() (3)
(3)
Substitute equation (2) in equation (3):
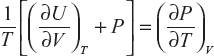 (4)
(4)
- According to Maxwell’s electromagnetic theory, the black body radiation in an enclosure exerts a pressure P on the walls which is equal to one third of energy density E.
 (5)
(5) - If V is the volume of the enclosure and E is the energy density of variation, total internal energy U is expressed as follows:
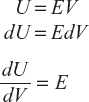 (6)
(6) (7)
(7)
Equations (5) and (6) are substituted in equation (7).


For perfect gas
![]()
For van der Waals gas:
 (1)
(1)
 (2)
(2)

Differentiating with respect to ‘T’ at constant pressure since v << b, replace (v – b) by v,

From equations (3) and (4),

The coefficient of volume elasticity E is defined as

 (1)
(1)
 (2)
(2)

By using Maxwell’s equations, we get

The ratio of specific heat of the substance at constant pressure and at constant volume is equal to the ratio of adiabatic and isothermal electricity.
11.5.9 Joule–Thomson Effect or Joule–Kelvin Effect
The phenomena of cooling of gas allowing through the porous plug from high pressure region to low pressure region at any point of temperature is called the Joule–Thomson effect.
Explanation
Let some amount of the gas on the system pass through the porous plug; enthalpy remains constant.
∴ Enthalpy ![]()
Differentiate the function,
![]()
Here, enthalpy is constant dH = 0
![]() (1)
(1)
 (2)
(2)
![]() (3)
(3)
 (4)
(4)
 (5)
(5)
The above equation is called differential Joule–Thomson or the Joule–Kelvin effect.

Case I
Joule–Kelvin effect for perfect gas or ideal gas
The ideal gas equation PV = nRT
 (1)
(1)
 (2)
(2)
In case of perfect gas, the Joule–Kelvin effect is zero.
∴ In the Joule–Kelvin expansion for ideal gas, there is no change in heating, cooling and no change in temperature.
Case II
Joule–Kelvin effect for van der Waals’ gas
Let one gram mole of gas of the state of gas equation be
 (1)
(1)
At constant pressure, differentiate the equation (1) with respect to temperature:
 (2)
(2)
Multiplying ‘T’ on both sides

(V–b) Multiply N and D

Now V >> b
Here, b2 and 2b are neglected.

According to binomial theorem,

Solution
![]()

At constant voltage, dV = 0.


Solution

11.6 REVIEW QUESTIONS
11.6.1 Fill in the Blanks
- Energy in transition due to temperature difference is _____________.
[Ans. Heat]
- Change in enthalpy is equal to _____________ at constant pressure.
[Ans. Heat transfer]
- The specific heat at constant pressure is _____________ than the specific heat at constant volume.
[Ans. Greater]
- The difference between two specific heats is _____________.
[Ans. Gas constant]
- When two bodies are in thermal equilibrium, then they are at _____________ temperature.
[Ans. Same]
- Thermometric property in thermocouple is _____________.
[Ans. Electro Motive Force]
- The statement that energy can be neither created nor destroyed but only converted from one form to another is known as _____________.
[Ans. Law of conservation of energy]
- Thermodynamics is the study of the effects of _____________ on a system.
[Ans. work, heat and energy]
- Thermodynamics can be expressed in terms of _____________ quantities.
[Ans. temperature (T), internal energy (U), entropy (S) and heat (Q)]
- The first law of thermodynamics is an extension of the _____________.
[Ans. Law of conservation of energy]
- The change in _____________ of a system is equal to the heat added to the system minus the work done by the system.
[Ans. Internal energy]
11.6.2 Multiple-choice Questions
- A cycle consisting of one constant pressure, one constant volume and two isentropic processes is known as
- Carnot cycle
- Stirling cycle
- Otto cycle
- Diesel cycle
[Ans. d]
- Which of the following is an extensive property of a thermodynamic system?
- Pressure
- Volume
- Temperature
- Density
[Ans. b]
- Which of the following is an intensive property of a thermodynamic system?
- Energy
- Volume
- Temperature
- Enthalpy
[Ans. c]
- Closed system is one in which the boundary permits the flow of
- Energy
- Mass
- Energy and mass
- Closed system
[Ans. a]
- The science of measuring temperature is
- Hydrometry
- Gravimetry
- Volumetry
- Thermometry
[Ans. d]
- Water and vapour are in equilibrium at
- Ice point
- Steam point
- Melting point
- Freezing point
[Ans. b]
- Zeroth law of thermodynamics is concerned with
- Internal Energy
- Enthalpy
- Thermal equilibrium
- Entropy
[Ans. c]
- The heat and work are mutually convertible; this statement is known as
- Zeroth law of thermodynamics
- First law of thermodynamics
- Second law of thermodynamics
- Third law of thermodynamics
[Ans. b]
- The first law of Thermodynamics is associated with
- Entropy
- Internal energy
- Reversibility
- Irreversibility
[Ans. b]
- The direction of heat flow is associated with the statement of
- Clausius
- Joules
- Kelvin–Planck
- Charles
[Ans. a]
- The efficiency of engine working on Carnot cycle depends on
- Working medium
- Size of engine
- Temperature range
- None of these
[Ans. c]
- At dead state, the available energy of a system is
- Zero
- Maximum
- Minimum
- None of these
[Ans. a]
- The process of increasing the moisture content in the air is called
- Sensible cooling
- Humidification
- Dehumidification
- Sensible heating
[Ans. b]
11.6.3 Short Answer Questions
- Explain the terms system and surrounding.
Ans.:
System: A definite quantity of matter bounded by some enclosed surface is known as a system. A system is also defined as that part of the universe which is under thermodynamic study.
Examples: For a scientist, the gas enclosed in a cylinder is a system. For a mechanical engineer, the machine is a system.
Surrounding: All the matter which can influence the behaviour of the system is known as surroundings. In other words, except system, rest of the universe is surroundings.
Examples: For an egg placed on the table, the atmosphere around the egg is the surrounding.
Note: The choice of a system and its surrounding is arbitrary.
- Define reversible and irreversible processes and give examples.
Ans.:
Reversible process: The process in which all the changes that occurred in the direct process are repeated in the reverse direction is called reversible process. In a thermodynamic system, the change of the state of absorption of heat energy is the direct process, and the system should release the same amount of heat energy. It changes the state in the reverse direction. This type of process is called reversible process.
For example, very slow process done at constant temperature, Carnot cycle process, etc. A certain amount of heat is given to ice through melting and water is formed. If the same amount of heat is removed from water, then the ice is formed.
Irreversible process: The process in which all the changes that are accepted in the direct process were not repeated in the reverse direction is called irreversible process. All the processes that irreversibly occur are examples of an irreversible process.
Examples: Conduction, radiation, radioactive decay, electrical heating of a wire, etc. If an electric current is passed in the wire, heat is developed in the wire and this cannot be reversed.
- Differentiate between isothermal and adiabatic processes.
Ans.:
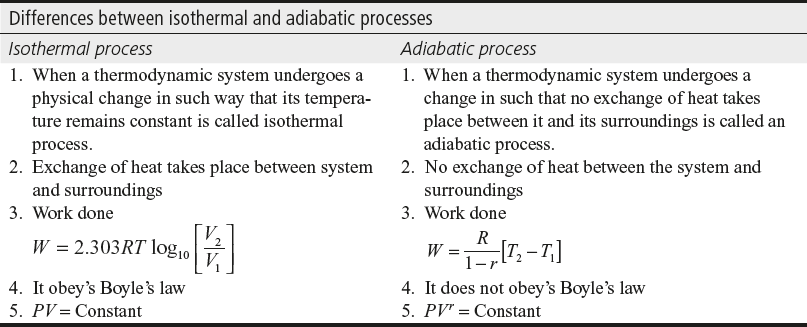
- State first law of thermodynamics.
Ans.: The total energy of an isolated system remains constant though it may change from one state to another. Hence, the absorbed heat of any system is equal to the sum of the increase in internal energy and the work done by the system. According to this law, if a small amount of heat energy (dQ) is supplied to the system, then a part of the energy is used to increase the internal energy (dU) of the system and the remaining energy is used to do external work by the system (dW).
The first law of thermodynamics can be written as dQ = dU + dW.
- What do you mean by indicator diagram? What is its significance?
Ans.: A graph between pressure (P) on the Y-axis and volume (V) on the X-axis is the indicator diagram; this diagram is also called the P–V diagram.
- Define Carnot engine.
Ans.: A Carnot’s engine is a heat engine which can transfer heat energy from a body having higher temperature to lower temperature while transforming the heat energy from one body to another. In Carnot’s engine, the loss of heat energy is very minimum.
- State the importance of second law of thermodynamics.
Ans.: The first law of thermodynamics says that heat and mechanical work is inter conversable, but it doesn’t give any limitation and condition for this conversion. The second law of thermodynamics specifies the direction of the flow of heat engine.
- What is entropy?
Ans.: The thermal property of a body which remains constant during an adiabatic process is called entropy; it remains constant between two adiabatic lines.
11.6.4 Descriptive Questions
Q.1 Explain the concept of internal energy of a system. Formulate first law of thermodynamics and explain its significance.
Q.2 What are reversible and irreversible processes? How does entropy change in each of this process?
Q.3 State and explain the second law of thermodynamics. Describe Kelvin’s scale of temperature and give its importance.
Q.4 State and prove Carnot’s theorem. Indicate how it leads to an absolute scale of temperature.
Q.5 Describe the working of Carnot’s engine and derive an expression for its efficiency.
Q.6 Explain Kelvin–Planck and Clausius statement of the second law of thermodynamics and their importance.
Q.7 Define and explain entropy. Write note on entropy change in reversible and irreversible processes.
Q.8 Draw the entropy diagram for reversible Carnot cycle and show that what is the area of diagram indicates.
