13
ORGANOMETALLIC COMPOUNDS
13.1 INTRODUCTION
Organometallic chemistry is the study of chemical compounds containing at least one metal–carbon (M-C) bond, where carbon is part of an organic group. It is also known as organo-inorganics, metallo-organics and metalorganics. Some examples are organolithium, organo magnesium (Grignard reagent) compounds, and metal carbonyls. Living systems contain a variety of organometallic compounds such as haemoglobin, myoglobin and chlorophyll. The specialised field focused on the study of such compounds is known as bioinorganic chemistry.
13.1.1 Organometallic Chemistry Timeline
The timeline of organometallic chemistry is outlined in Table 13.1.
Table 13.1 Timeline of organometallic chemistry

13.2 ORGANOLITHIUM COMPOUNDS
The organolithium compounds characterised by C—Li bond are important in organic synthesis. Organolithium compounds show similar reactivity as Grignard reagent. They are more reactive than Grignard reagents. Lithium is less electronegative than carbon and the C—Li bond is polarised Cδ−—Liδ+. Organolithium compounds behave as a nucleophile and a base.
13.2.1 Preparation of Organolithium Compounds
From Alkyl or Aryl Halides
The reaction of lithium metal at low temperature with an alkyl or aryl halide in a hydrocarbon solvent in inert atmosphere gives alkyl lithium.
![]()
The yield of alkyl lithium is very low, if very pure lithium is used. It is believed that lithium must contain at least 0.02% of sodium for the reaction to proceed smoothly. In the aforementioned reaction, the reactivity of the halides is RI > RBr > RCl.
By Halogen–Metal Exchange Method
This method is useful for the preparation of organolithium reagent that cannot be directly obtained from alkyl halide and metal. In this method, an organic halide is treated with alkyl lithium.
![]()
This method is best suited for the preparation of aryl-lithium derivatives. Thus, phenyl lithium is prepared by treating bromobenzene with n-butyl lithium.

Vinyl lithium is obtained as follows:

By Metalation
Compounds having acidic hydrogen can be easily converted into organolithium compounds by treatment with suitable organolithium compounds.

13.2.2 Properties of Organolithium Compounds
Organolithium reagents are very reactive, powerful nucleophiles and strong bases. They find numerous applications in organic synthesis and are better than the Grignard reagents.
Reaction with Compounds Containing Active Hydrogen
Organolithium reagents react with substrate having active hydrogen such as water, alcohols, amines and carboxylic acid to give the corresponding hydrocarbons.
![]()
Reaction with Carbonyl Compounds
Organolithium reagent reacts with aldehyde to form secondary alcohol and reacts with ketones to form tertiary alcohols. Primary alcohols are obtained by treatment with formaldehyde.

Grignard reagent cannot react with hindered ketones. However, organolithium reagents are less susceptible to steric effects and react with hindered ketones, giving the corresponding 3° alcohols.

Reaction with Alkyl Cyanide
Organolithium reagent reacts with alkyl cyanide to give imine slat; when formed imine salt undergoes hydrolysis first gives the imine, which on acid hydrolysis gives ketone.

Reaction with Epoxide
Epoxide reacts with organolithium reagents to give primary alcohols.

Unsaturated organolithium reagent gives unsaturated alcohol.

Reaction with Carbon Dioxide
Organolithium reagent, the formed carboxylate anion, reacts with a second molecule of reagent to give a ketone.

This method finds application in the conversion of carboxylic acid into ketones. A special feature of this reaction is that the original stereo chemistry of the carboxylic acid is maintained in the formed ketones.
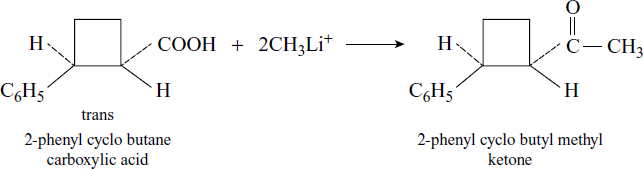
Reaction with Alkenes
Organolithium reagent reacts with alkenes to give alkyl lithium, which reacts with a second molecule of alkene to give the corresponding alkyl lithium. This process continues depending on the relative amount on the alkene.

Electrophilic Displacement
We have already seen the synthesis of vinyl lithium from vinyl bromide in the preparation of vinyl lithium reagent.
![]()
Nucleophilic Displacement
The halogen of the alkyl halide can be substituted with the alkyl group of the organolithium reagent to give hydrocarbon.
![]()
This reaction takes placed by the SN2 mechanism as in the case of Wurtz reaction.
Reaction with α, β-unsaturated Carbonyl Compounds

13.3 ORGANOMAGNESIUM COMPOUNDS
The organomagnesium halides are known as Grignard reagents. These were discovered by Vector Grignard, and were hence named Grignard reagent. Grignard reagents are represented as R—Mg—X, where R = alkyl, aryl, alkenyl, alkynyl and X = Cl, Br, (or) I.
13.3.1 Preparation of Organomagnesium Compounds
Grignard reagents are prepared by the action of magnesium on alkyl halide with anhydrous ether.

For the given halogen, the reactivity order of an alkyl group is CH3 > C2H5 > C3H7; hence an increase in
carbon atoms the formation of Grignard reagent becomes difficult.
Reactivity order of alkyl halides is RI > RBr > RCl.
Aryl halide reacts with magnesium to form aryl magnesium halide. For example, phenyl magnesium bromide.
Vinyl halides react with magnesium to form vinyl magnesium halide. In aryl and vinyl halides, the C—X bond is stronger than the C—X bond of alkyl halide. Therefore, a stronger Lewis base is required for the formation of aryl or vinyl Grignard reagent requires more basic tetrahydrofuran (THF).
Mechanism
The reaction occurs via free radical mechanism.

In addition, the Grignard reagent prepared from subtracts have acidic hydrogen.
Example: alkynes, cyclopentadienes, etc.

The substrate for the preparation of Grignard reagent should not have groups like —COOH, —OH, —NH2 and —C≡CH.
Structure
Grignard reagent exists as a coordination complex with ether.

13.3.2 Properties of Organomagnesium Compounds
Grignard reagents may be regarded as polar compounds, and are a source of nucleophile carbon ions.
![]()
Reaction with Compound Containing Active Hydrogen
The Grignard reagent reacts with active hydrogen containing molecules such as H2O, alcohol, NH3 and R—NH2 and forms hydrocarbons.

Reaction with Carbonyl Compounds (Nucleophilic Addition Reaction)
The C—Mg bond in the Grignard reagent is polar and generates a nucleophile carbon (Cδ−—Mgδ+—Br). Being nucleophile in nature, the Grignard reagent attacks the carbonyl group of aldehydes, ketones, esters, anhydrides, acid chlorides and amides. In this reaction, the alkyl group having carbon ionic character becomes attached to the carbonyl carbon and Mgx to the oxygen of carbonyl group to give complex. These addition Products in decomposition with water give the hydroxyl compounds.
Reaction with Ketones

Reaction with Aldehydes

Reaction with Formaldehyde

Reaction with Halides

Reaction with Esters

Reaction with Acid Chloride
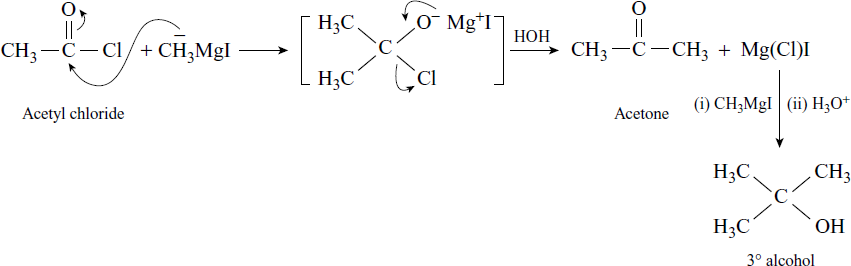
Reaction with Acid Anhydrides

Reaction with Amines

Reaction with Epoxide
Grignard reagent reacts with epoxide to give primary alcohol with the lengthening of carbon chain by two carbon atoms.

Reaction with Carbon Dioxide
Grignard reagent is treated with solid CO2 followed by hydrolysis yield carboxylic acid.

Reaction with Nitriles
When Grignard reagent reacts with nitriles, ketones are formed. If HCN is used instead of alkyl cyanide, an aldehyde is obtained.

Reaction with Oxygen
Oxygen reacts with Grignard reagent of a low temperature to give an adduct, which on acidification gives the corresponding hydroperoxides.

Reaction with Sulphur
Grignard reagent reacts with sulphur to give the corresponding thiols.

Reaction with Carbon Disulphide
Carbon disulphide reacts with Grignard reagent to give an adduct, which on hydrolysis, gives the corresponding di thionic acid.

Reaction with Sulphur Dioxide
SO2 adds on to Grignard reagent to give an adduct, which on hydrolysis gives sulfonic acid.

Reaction with Sulphur Trioxide

Reaction with Iodine
Grignard reagent on reaction with iodine in acetone gives the corresponding alkyl halide.

Reaction with Inorganic Halides
Grignard reagent reacts with inorganic halides to gives organometallic compounds.
![]()
Reaction with Alkyl Halides
![]()
13.4 METAL CARBONYLS
Carbonyls are complex compounds in which carbon monoxide is attached to metals by coordinate linkage. Carbonyls are mainly divided into three types.
- Mononuclear carbonyls: Here, only one metal atom is present.

- Di or binuclear carbonyls: In these carbonyls, two metal atoms are present.
Example: Fe2(CO)9, CO2(CO)9
- Polynuclear carbonyls: In these carbonyls, more than two metal atoms are present.
Example: Fe3(CO)12
13.4.1 Ligand
Substances which can donate unpaired or non-bonded or a lone pair of electrons are called ligands. The Lewis dot structure of carbon monoxide is as follows:

Carbon monoxide molecule has a lone pair of electrons of carbon as well as oxygen atoms. In the formation of carbonyls, carbon monoxide donates the lone pair of electrons from carbon to metal atom. As a result, the two atoms are linked by a coordinate bond. Transition elements have a vacant ‘d’ orbital which can easily accommodate the donated electrons.
13.4.2 Effective Atomic Number
Effective atomic number (EAN) is the number of electrons present in the metal atom and the number of electrons gained from the ligand to attain the next inert gas electronic configuration. Some important carbonyls and their EAN number shown in Table 13.2.
EAN = number of electrons present in the metal atom + number of electrons gained from ligand
Table 13.2 Carbonyls and their EAN number

13.4.3 Preparation of Carbonyls
- By passing carbon monoxide over reduced nickel at 30–50°C under atmospheric pressure.

- At high temperature and 200–400 atmospheric pressure, iron reacts with carbon monoxide to form iron pentacarbonyl.

- Metal halides react with carbon monoxide at high temperature and pressure in the presence of sodium metal and give the concerned metal carbonyl.

13.4.4 Properties of Carbonyls
Solubility
In carbonyls, covalent bonds are present. Hence, they dissolve in organic substances.
Reaction with Halogens
Gaseous halogens react with nickel carbonyls and give nickel halides and carbon monoxide.
![]()
Reaction with Acids
Among the hydrohalide acids, carbonyls react with hydroiodic acid and give nickel iodide and release carbon monoxide and hydrogen.

Aqueous halogen acids do not react with carbonyls but aqueous sulphuric acid reacts with nickel carbonyl and gives nickel sulphate.
![]()
13.4.5 Structure of Carbonyls
Nickel Tetracarbonyl
The atomic number of nickel is 28. The outermost electronic configuration is 3d8 4s2 4p0. The energies of 3d and 4s electrons are not very much different; hence, in the presence of carbon monoxide molecule, the two 4s electrons shift to 3d orbitals. Thus, four orbitals (one from 4s, three from 4p) undergo sp3 hybridisation and form 4 sp3 hybrid orbitals to form nickel tetracarbonyl complex with carbon monoxide molecules.
The outermost electronic configuration of nickel atom at ground state is as follows:
![]()
Shifting of electrons from 4s to 3d orbitals in the presence of CO forms four vacant orbitals and undergoes sp3 hybridisation to give four equivalent sp3 hybridised orbitals.

Electronic configuration of nickel at nickel tetracarbonyl

Iron Pentacarbonyl
The atomic number of iron is 26. The outermost electronic configuration is 3d6 4s2 4p0.
![]()
In the presence of ligands, all unpaired d electrons are paired and the 4s electrons shift to the empty d orbital. Now, one d orbital, one s orbital and three p orbitals are empty. They undergo dsp3 hybridisation and have a trigonal bipyramidal structure.
In presence of ligands,

The electronic configuration of iron in iron pentacarbonyl is as follows.

Structure of Chromium Hexacarbonyl
The atomic number of chromium is 24. The electronic configuration is 3d5 4s1 4p0.
![]()
In the presence of ligand, all unpaired electrons are paired to form two d orbitals, one s orbitals, three p empty orbitals and undergo sp3d2 hybridisation and attain octahedral structure.
In presence of ligands,

The electronic configuration of chromium in chromium hexacarbonyl is as follows.

Structure of Fe2 (CO)9
In this molecule, three carbon monoxide molecules are bonded in between two iron atoms. The remaining six ligands; three are bonded with one iron atom and another three bonded with another iron atom.

Structure of Fe3(CO)12
In between three iron atoms each, six carbon monoxide molecules are bonded as two bridges.

13.5 REVIEW QUESTIONS
13.5.1 Fill in the Blanks
- The structure of Grignard reagent is __________.
[Ans.:
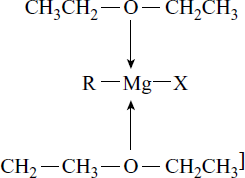
- R—X + Mg
 __________.
__________.
[Ans.: R—Mg—X]
- Reactivity order for halides in organolithium is __________.
[Ans.: RI > RBr > RCl]
- The covalent bond between carbon and metal forms __________.
[Ans.: organometallic compounds]
- The charge carried by carbon in organometallic reagents is __________.
[Ans.: negative]
- The charge of metal atom in organometallic reagent is __________.
[Ans.: positive]
13.5.2 Multiple-choice Questions
- Grignard reagent is
- R—Li
- R—Mg—R
- R—Mg
- R—Mg—X
[Ans.: (d)]
- Alkyl lithium reagent structure is
- R—Li
- R—Li—X
- Both (a) and (b)
- None of these
[Ans.: (a)]
13.5.3 Short Answer Questions
- Write any two methods of preparation of organolithium reagents.
Ans.: From alkyl or aryl halides
The reaction of lithium metal at low temperature with an alkyl or aryl halide in a hydro carbon solvent in inert atmosphere gives alkyl lithium.

By metalation
Compound having acidic hydrogen can be easily converted into organolithium compound by treatment with suitable organolithium compound.

- Write any two properties of organomagnesium reagents.
Ans.: Reaction with CO2

Reaction with ketones

13.5.4 Descriptive Questions
Q.1 Give a detailed note on organolithium reagents.
Q.2 Briefly explain organomagnesium reagent.
