14
COORDINATION CHEMISTRY
14.1 INTRODUCTION
Alfred Werner, a Swiss chemist and the founder of coordination chemistry, won the Nobel Prize for chemistry in 1913 for his research into the structure of coordination compounds. Prior to him, the concepts of valence bonding and geometry in metal complexes were confusing. He revolutionised the fields of inorganic chemistry and stereochemistry and found applications in many fields such as organic chemistry, analytical chemistry, biochemistry, geochemistry and mineralogy. He proved that stereochemistry is not limited to organic chemistry but is a general phenomenon.
Coordination chemistry is the study of a class of compounds formed by metals. Coordination compound can be explained by the following example:
K4[Fe(CN)6]—Potassium ferrocyanide complex can prepared by an excess of aqueous potassium cyanide being added to aqueous ferrous sulphate, formed as a yellow colour solution. The complex K4[Fe(CN) 6] is called “coordinate compound”. The formation of coordination compound from a metal is called “complexation”. The complex solution contains [Fe(CN) 6]4− and is called “metal complex ion”. It can be isolated as the part [Fe(CN) 6]4− in K4 [Fe(CN) 6] and is called “complex species (or) complex entity”. The formula of the complex species should be represented within brackets—[ ].
14.2 BASIC REQUIREMENTS TO FORMATION OF COORDINATION COMPOUND
- The coordination compound formed by metal and certain number of species is called a ligand. Ligand means binds or that which is attached, having an electron pair for binding with the positive metal. It is the prime requirement for complex formation. Ligands may be anions (CN−, Cl−, SCN−, etc.), neutral molecules (H2O, CO, NH3, etc.) but not positive ions.
- Complex species may be cationic, neutral and anionic.
Examples:
- Cationic complex: [Pt(NH3)4]2+ Tetraammineplatinum(II)
- Neutral complex: [Pt(NH3)2Cl2] Diamminedichloroplatinum
- Anionic complex: [Pt(Cl4)]2− Tetrachloroplatinate(II)
- The stability of the metallic species increases with the complexation of metal ion; hence, complex of metal is more stable than metal ion itself.
- Complex formation is often accompanied by striking changes in colour.
- Depending on the number of ligand bonded to the central metal, the molecule of the complex attains its characteristic structure such as tetrahedral, square planar, octahedral, etc.
- Depending on the type of bonding and unpaired electrons present on the metal, the complex may be diamagnetic or paramagnetic.
- A metal complex differs from an ordinary salt in some respects.
A simple salt is dissolved in water and produce its constituent simple ions.
Example: FeSO4→Fe+2 + SO42−
However, metal complex is dissolved in water and produces complex ion.
Example: K4[Fe(CN)6] → [Fe(CN)6]4− + 4K+
- Some metal complexes are soluble in water, but some are insoluble.
Example for water soluble complex: [Ag(NH3)2]+(diamino silver(I)
Example for water insoluble complex: [Ni((CH3)2—C=N—OH)] Nickel dimethylglyoxime
Some complexes are soluble in organic solvent.
Example: Bis(acetylacetonate)Cu(II)

Double Salt and Coordination Compounds
Double salt is the compound formed by combining two stable compounds.
Example: Ferric alum Fe2(SO4)3·(NH4)2SO4·24H2O
When double salts are dissolved in water, they will completely dissociate into simple ions. For example, when crystals of ferric alum are dissolved in water, the solution shows properties of NH4+, SO42− and Fe2+ ions.
Coordination compound is formed with metal and ligands by dative bonds. When it dissolves in water, it does not break down to simple ion; only the ionic bond breaks and gives metal ion and complex ion.
Example: K4[Fe(CN)6] When dissolved in water the solution, it consists of K+ and [Fe(CN)6]4−; the complex ion does not break down to Fe+2 and CN−2 ions.
Coordination Number
The total number of monodentate ligands attached to the central metal complex is called coordination number; this is equal to number of sigma bonds between the metal and ligand. Coordination number from two to nine is known in complex; four and six are common coordination numbers but three is a rare coordination number.
Oxidation Number
The number denotes the charge on the central metal atom if all the ligands in the complex were removed along with their electron pairs; it is represented by Roman numerals.
Eg.:
[NiCl4]2−
In the above complex ion if all four chlorine ligands are removed from complex ion then the central atom Ni attain a charge of +2. The oxidation number of this metal in this complex is written as II.
[Cr(NH3)6]3+
In the above complex ion if all six ammonia ligands are removed from complex ion then the central atom Cr attain a charge of +3. The oxidation number of this metal in this complex is written as III.
Types of Ligands
A ligand is an ion or a molecule capable of donating an electron pair by participating in the formation of a coordinate bond. Depending on the bonding formation, ligands are divided into unidentate, polydentate, bridging, ambident, flexident ligands, etc.
Unidentate ligand is bound by only one atom at a time as a donor and fills only one coordination position of a given cation.
Example: Cl−, Br−, I−, CN−, SCN−, NO3−, NO2−, R3N, pyridine, CO, H2O, etc.
Poly dentate ligand is bonded by more than one atom at a time as a donor and fills more coordination positions of a given cation. Ligands and their bonding capacity given in Table 14.1.
Table 14.1 Ligands and their bonding capacity

Bridging ligand binds simultaneously with two metals by forming a bridge between the two metals and acting as a ligand to both metals.

Ambidentate ligand is capable of bonding through one type of donor atom in one complex but through a different donor atom in another complex. Such a ligand has potentially two ligating atoms. Some ambidentate ligands and their names are given in Table 14.2.
Table 14.2 Ambidentate ligands and their names

A flexidentate ligand can bind to a metal with different numbers of coordinate sites.
Example: EDTA is normally a hexadentate ligand but can act as a pentadentate or tetradentate ligand depends on ligand present at that particular situation; hence, it is called a flexidentate ligand.
14.3 NOMENCLATURE OF METAL COMPLEXES
Metal complexes are named systematically based on certain rules recommended by the International Union of Pure Applied chemistry (IUPAC).
14.3.1 Cationic Complex
The cationic complex names begin with the number of ligands, followed by the name of the ligand bonded to the central metal atom (or) ion. The oxidation number of metal is indicated by Roman numerical parenthesis.
![]()
The complex name will be written without space between ligand and metal names and the metal name and the parenthesis in a single entity.
![]()
In normal salts like BaCl2, the salt is named barium chloride and not barium dichloride because the bivalency of barium is implied. Similarly, [Cr(NH3)6]Cl3 is not named tri chloride; but simply chloride. The first part of the name of the complex Hexaamminechromium(III) automatically indicates that it is of three chlorides.
14.3.2 Anionic Complex
The name of the anionic complex will be mentioned by the adding of -ate at the end of the metal ion; however, complex positive ions or neural molecules do not have such an ending.
Example: [Fe(CN) 6]-4 hexacyanoferrate—here, the suffix “ate” is attached to the name of the metal.
Anionic complexes having simple cations like Na+, K+, etc., where the complex begins with the name of a cation.
Example: K4[Fe (CN)6] potassium hexacyanoferrate(II)
The complex ion can put in a bracket [ ]. This indicates that the ions are expected to be formed in a solution when the complex is dissolved in solvent. Some common complex anion shown in Table 14.3.
Table 14.3 Names of complex anions containing metal atoms

Naming Ligands
Negative Ligands
The name of a negative ligand ends in O.
Example:

Neutral Ligand
Neutral ligands are named as such without any special name ending.
Example:

Positive Ligand
Positive ligands end with “ium”.
Example: NH2-NH3+ - hydrazinium
Order of Ligands
- When a complex contains different charged ligands, the order is negative ligand, neutral ligand, positive ligand.
Example: [PtCl2(H2O)(NH2NH3)+] Dichloroaquahydrozoniumplatinum(II)ion
- When a complex contains more than one kind of similar charged ligand, the ligands are listed in the alphabetical order.
[CrCl3(NO2)3]3− Trichlorotrinitrochromate(III)ion
- The number of the same ligands present in the complex uses prefixes such as di, tri, tetra, penta and hexa.
[Ag(NH3)2]+ - Diamminesilver(I)ion.
However, when the name of the ligand itself has a prefix such as di, tri, tetra, etc., to avoid confusion, prefixes such as (di) = bis, (tri) = tris, (tetra) = tetrakis are used to indicate the number of such ligands present in complex.
[Cu(en)2]SO4 -bis(ethyldiamine)copper(II)sulphate
Naming Isomeric Ligands
In isomeric complexes, the ligand is attached through different donor atoms to the metal.
![]()
Designating the Ligand Atom in a Polydentate Ligand
A polydentate ligand has more than one donor site, some (or) all of which may be bonded to the metal.
Example:

14.3.3 Nonionic Complexes
Nonionic complexes are represented with a single entity.
Example: [Fe(NH3)3(CO)3] - Triamminetricarbonyliron(0).
14.3.4 Polynuclear Complex
A complex formed with two or more metal ions per molecule is called a polynuclear complex. The two metal ions in it are bridged by ligands; such bridging ligands are identified by the prefix µ - if there are two or more bridging ligands of the same type, this is indicated by tri -µ, di -µ. If there are two or more bridging ligands of different types, then the prefix µ - is added for each such bridging ligand in the alphabetical order.
Example:
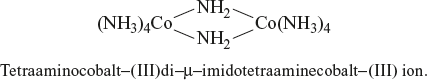
14.3.5 Complex with Metal-Metal Bond
In a complex having a metal-metal bond, the prefix bis or - di is used before the name of metal which involves metal-metal bond. Abbreviations for Polydentate ligands their formulas shown in Table 14.4.

[Br4ReReBr4]-2 bis(tetrabromorhenate)(ReRe) (2−)
Table 14.4 Abbreviation for ligand names

Some important complexes by Scientist names shown in Table 14.5.
Table 14.5 Scientist names for complexes

14.4 THEORIES OF COORDINATION CHEMISTRY
The various theories like Werner’s theory, Sidgwick’s electronic concept theory, valence bond theory (VBT), crystal field theory (CFT), molecule orbital theory (MOT), etc., have successfully explained the properties of complexes and the bonding between the metal and ligand.
14.4.1 Werner’s Theory
In 1893, Alfred Werner proposed this theory at the age of 26; it is now referred to as Werner’s coordination theory. This theory explains the “primary-secondary valence” of metal complexes.
Postulates of Werner’s Theory
- Complexes exhibit primary and secondary valance; primary valance is ionisable and secondary valance is non-ionisable. Primary valance can be satisfied by anions and the secondary valance by anions and neutral molecule.
- The primary valance represents the oxidation number and the secondary valance represents the coordination number.
- All elements tend to satisfy both primary and secondary valance; but in every case, the fulfilment of the secondary valance should be essential.
- Secondary valance is directed towards some fixed positions in structure.
Example: Four coordinated complexes the four valances are arranged in either square planar or a tetrahedral structure and in six coordinated complexes, six valances are directed towards six corners of the octahedron.
- Werner extensively studied “chloroaminecobalt(III)complexes” with silver salt which is shown in Table 14.6.
Table 14.6 Werner studied chloroaminecobalt(III)complexes

In complex (A), primary valence is satisfied by three chloride ions and secondary valence is satisfied by 6 NH3 molecules. According to the complex structure, ammonia molecules are tightly bound to cobalt, hence they do not dissociate in solution, but chloride ions are far away from cobalt and are less firmly held by metal. Hence, all the three chloride ions dissociate in the solution, giving 3Cl− ions and [Co (NH3)6]3− with a total of four ions.
- The bond between metal and ligand is a coordinate covalent bond; it is a type of covalent bond.
Defects of Werner’s Theory
Werner’s theory describes the structure of many coordination compounds successfully; however, it does not explain the nature of bonding within the coordination sphere.
- This theory unable to account for the four coordinated and six coordination among complexes.
- This theory is unable to explain some of the four coordination complexes as square planar, whereas some others are tetrahedral complexes.
14.4.2 Sidgwick’s Electronic Concept Theory
According to Sidgwick, the metal and the ligand involve a coordinate bond. Here, the ligand donates the electrons to the central metal atom to form coordination compounds. These ligands are known as donors and the metal ions are the acceptors. The bond between the donor and acceptor is called coordinate, or dative or semi-polar bond. The coordinate bond is generally represented by the “→” arrow, starting from the donor pointing towards the acceptor L→M.
Effective Atomic Number
Effective atomic number (EAN) is also known as 18 electron rule or Nobel gas rule. According to Sidgwick, the EAN of the central metal ion is the total number of electrons around the central metal ion, including those gained through coordination by the ligand. The EAN is equal to the atomic number of the next higher inert gas. Examples of complexes which obey EAN rule and which have exception from EAN rule are shown in Table 14.7 and 14.8 respectively.

Table 14.7 Examples of complexes which obey the EAN rule

Table 14.8 Examples of complexes which are exception to the EAN rule

Defects of Sidgwick’s Theory
- This theory does not explain the stability of metal complexes properly. As the donation of electrons by the ligand to the metal ion would cause accumulation of unfavourble negative charge over the electro positive charge on the central metal, it will reduce the stability of the metal complex.
- The electron pair is donated by H2O, NH3, etc., to the central metal ion by 2s2 pair of electron. The 2s2 pair has no bonding characteristics; in order to make them useful for bonding, the electron should be promoted to a higher energy level. This requires more energy than what is usually available in the bond formation.
- According to Sidgwick’s theory, coordination compounds must be covalent; but their complexes are ionic in nature. Here, the force acting in between the metal and ligand is essentially electrostatic.
Later Structural Theories of Coordination Chemistry
Based on modern principle of bonding and overcome to drawbacks of Sidgwick’s and Werner’s theories, the valence bond theory, crystal field theory and molecule orbital theories are proposed.
14.4.3 Valance Bond Theory
In 1935, Linus Pauling and Slater proposed the valence bond theory (VBT). This theory is primarily concerned with the structure and magnetic behaviour of metal complexes. It is also called Pauling theory.
Postulates of Valence Bond Theory
- The central metal makes available a number of vacant orbitals equal to its coordination number for the formation of the covalent bond with the ligand orbital.
- A covalent bond formed by the end of an overlap of a vacant orbital of metal and filled orbital ligand leads to the formation of M-L σ bond.

- It is also possible to form a π bond by sideways overlapping of the filled metal orbital with a suitable vacant ligand orbital.

- A strong covalent bond is formed when only the orbitals overlap to the maximum extent; this is possible when the metal vacant orbital undergoes hybridisation.
- Numerous combinations of hybridisation are possible with s, p and d orbitals to give different geometries.
Examples:

Spin only Magnetic Moment
A species should have at least one unpaired electron to possess paramagnetic nature. It is attracted by an external magnetic field; theoretically, the paramagnetic moment of an ion can be calculated by its number of unpaired electron using the following spin only formula.
![]()

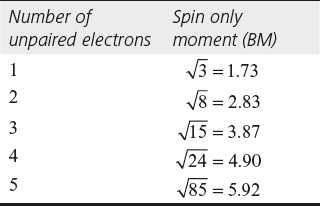
When the species does not contain an unpaired electron, it is diamagnetic.
Depending on the number of d electrons in the metal ion, the VBT can be applied to guess the structure of the complex.
Number of d electrons = Atomic number of central metal atom - Number of electrons lost in ion formation - atomic number of previous inert gas.
Examples:
- [Ni(NH3)4]2+
Ni atomic number = 28
Number of electrons lost in +2 ion formation = 2
Atomic number of previous inert gas = 18
Number of d electrons in Ni = 28 - 2 - 18 = 8
d8 configuration

Sp3 hybridisation - Tetrahedral structure
Two lone pair electrons - Paramagnetic
Observed magnetic moment = 2.83 BM.
- [Ni(CN)4]2−

dsp2 hybridisation: square planar
No lone pair of electrons: dia magnetic
Observed magnetic moment = 0 BM.
- [Co(NH3)6]+3
Co-atomic number = 27
Number of electrons lost in +3 ion formation = 3
Atomic number of previous inert gas = 18
Number of d electrons in Co = 27 - 3 - 18 = 6
d6 configuration
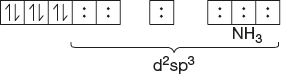
d2sp3 hybridisation: Octahedral
No lone pair of electrons: Dia magnetic
Observed magnetic moment = 0 BM.
Defects of Valance Bond Theory
- The energy level splitting of metals is not explained by the VBT.
- It is unable to account or predict the relative energies of the different alternative structure for a complex.
- The reaction and mechanism of the complexes are not explained by this theory.
- It does not explain why certain four coordinated complexes are tetrahedral, whereas others are square planar.
- It does not indicate why certain ligands involved are in the outer orbital complex, whereas some others form the inner orbital complex.
- This theory does not account for the detailed magnetic properties.

14.4.4 Crystal Field Theory
The crystal field theory was first proposed by J. H Van Vleck and H. Bethe. This theory involves an electrostatic approach to bonding in complexes. It was first applied to ionic type crystalline substance. Therefore, it is called crystal field theory.
- This theory is useful in describing the metal-ligand interaction and is especially helpful in explaining the visible absorption spectra of metal complexes.
- This theory considers the metal ion as being placed in an electrostatic field created by surrounding molecules or ions.
- This electric field changes the energies of the d electrons in the case transition metal ion. Many properties of complexes are related to these energy changes. This theory considers the bonding in complex to be entirely electrostatic.
Assumptions of the Crystal Field Theory
- This theory considers a complex as a combination of a central ion surrounded by other ions or molecules with electrical dipoles called ligands. It regards these ligands as point charges or point poles.
- The bond between the metal and ligand is purely ionic.
- The interaction between the electron of the metal cation and the ligand is entirely repulsive. These repulsive forces are responsible for the splitting of the d orbitals of the metal ion.
- In its simplest form, the crystal field theory does not consider the overlapping between the metal orbital and ligand orbital.
- The d-orbital which degenerates in a free metal ion has its degeneracy destroyed by the approach to the ligands during the formation of the complex.
Crystal Field Splitting of d-orbital in Different Geometrics
The five d-orbitals in isolated gaseous metal ions are degenerated; when a spherically symmetric field of negative charges is placed around the central metal ion, all the five orbitals will be raised in energy as a result of the repulsion between the negative field or negative electrons. Although they still remain degenerated, they will have higher energy than before.
Splitting of d-orbitals in Octahedral Symmetry
- Six ligands participate in forming of octahedral complex. They are positioned symmetrically along the axes of a Cartesian coordinate system with metal ion at the origin.
- In the case of spherically filed, all the d-orbitals will be raised in energy relative to the free ion due to negative charge repulsion; in these cases, not all the orbitals will be affected to the same extent. The orbitals laying along the axes (dz2 and dx2 - y2) will be more strongly repelled than orbital with lobes directed between the axes (dxy, dzx and dyz). The d-orbitals are thus split into two sets with the dz2 and dx2 − y2 at a higher energy than the other three.
- The orbitals are split into two sets as doubly degenerate (eg) and other is triply degenerated (t2g).
t2g - triply degenerated orbitals are dxy, dyz and dzx.
eg - doubly degenerated orbitals are dz2 and dx2 − y2.
- The splitting of the five d-orbitals into eg and t2g energy levels by approaching the ligand is called crystal field splitting. This is the major feature of the crystal field theory. The energy difference between eg and t2g levels is denoted by Δo or 10Dq and is called crystal field splitting energy.
Crystal Field Stabilisation Energy
The difference between t2g and eg orbitals is the crystal field stabilisation energy; it is denoted by Δo or 10Dq. The magnitude of Δo depends on the field strength of the ligand as well as the metal ion. It is assumed that the sum of the energies of the five d-orbitals in the free state must be equal to the sum of the energies of five d-orbitals in the octahedral configuration. Splitting occurs in such a way that there is no net change in energy for the fully occupied orbital. If we consider the energy of the free state as zero, then
![]() (1)
(1)
∴ ![]() (2)
(2)
![]() (3)
(3)
Multiply equation (3) by two and subtract it from equation from (2).
∴ 
Since t2g levels are degenerated,
![]()
On substituting the value of Edxy, namely −0.4Δo in equation (3)
∴ 
Since eg orbitals are degenerate
![]()
This means that presence of an electron in the t2g level will stabilise the complex to the extent ‘0.4 Δo’. This stabilisation energyis called “crystal filed stabilisation energy”. The presence of electron in eg level will destabilise the complex to the extent of 0.6 Δo.
Splitting of d-orbitals in Tetrahedral Symmetry
The metal ion along with its d-orbitals are arranged in the centre of the cube, the ligands occupy alternatively in four corners of the cube. Here, two ligands are in the top face of the cube and other two are in the bottom face but none of the ligand approaches the metal along the Cartesian coordinate axes. Hence, the orbitals along the axes (dz2, dx2 - y2) will be less strongly repelled than those orbitals between the axes (dxz, dxy and dyz). With this, the previously degenerate set of five orbitals is now split into two sets, one at a higher energy and one at lower energy. The higher energy set of orbitals (dxy, dyz and dzx) is labelled as t2 and the lower energy set (dz2 and dx2 - y2) is labelled as e. (The subscript “g” disappears here because the tetrahedron lacks the centre of inversion). The energy separation between the two levels is denoted by Δt (or) 10 Dq.
The crystal field splitting in the tetrahedral field is intrinsically smaller than the octahedral field because there are only four ligands surrounded to the metal, but none of them have a direct effect on the d-orbital. The relationship between the two crystal field separation energies may be represented as Δt = 4/9Δo.
The tetrahedral geometry can occur with the following reasons:
- When ligands are too large, less ligand repulsion occurs.
- When metal ion with zero crystal field stabilisation energy (CFSE), that is, metal atom having the d0, d5 and d10 electronic configuration or small CFSE—d2 and d7 electronic configuration.
Example:

Splitting of d-orbitals in Square Planarl Symmetry
This is the special case of octahedral symmetry; the removal of two ligands from z-direction completely in octahedral symmetry leads to square planar geometry. The geometry is favoured by the metal ion having a d8 configuration in the presence of a strong field. This combination gives low spin complexes where the first four orbitals are occupied and the high energy dx2 − y2 orbitals are unoccupied.


Merits of Crystal Field Theory
- This theory predicts the most favourable geometry of complex.
- It also accounts for the fact certain four coordinated complexes are square planar, whereas the outer ones are tetrahedral.
- It also explains that certain ligands form the outer orbital octahedral complexes, whereas others form the inner orbital octahedral complexes. They, in turn, correspond to high spin and low spin complexes.
- This theory is successful in interpreting the magnetic properties taking into orbital contribution as well.
- This theory represents the colour of transition of metal complexes.
- It also explained the spectral properties of metal complexes.
Limitations of Crystal Field Theory
- In several complexes, the bonding strength and chemical properties cannot be solely explained on the basis of electrostatic attraction as emphasised by this theory.
- This theory fails to explain the relative strength of ligand.
14.4.5 Common Single Atomic Ligands and their Field Strength
According to field strength, the single atomic ligands are arranged in Table 14.9 from weak field to strong field. When weak ligand approaches the metal, outer orbitals of the metal are involved in hybridization, that is, high spin complexes (6 coordinated complexes –sp3d2, 4 coordinated complexes –sp3). When strong ligand approaches the metal, inner orbitals of the metal are involved in hybridization, that is, low spin complexes (6 coordinated complexes –d2sp3, 4 coordinated complexes –dsp2).
Table 14.9 Single atomic ligands and their bonding capacity from weak field to strong field

The entries in the table are sorted by field strength directly binding through the stated atom, that is, as a terminal ligand. The ‘strength’ of the ligand may change when the ligand binds in an alternative binding mode as follows:
- When it bridges between metals;
- When a linear ligand that is forced through steric interactions to bind in a nonlinear fashion, the conformation of the ligand gets distorted.
14.4.6 Molecular Orbital Theory of Coordination Complexes
Molecular orbital theory was proposed by Pauling; it explains mainly magnetic properties, bond strength or bond order, bond length and the covalent character of metal and ligand.
For example, [Co(NH3)6]+3 is a complex in which metal and ligand α-bonding are involved.
This complex contains NH3 ligand which can only participate as a α donor to the metal ion. Now, construct the molecular orbital diagram for octahedral complex.
Let us consider the metal orbitals. The valance orbitals available are 3d, 4s and 4p. A total of nine orbitals are available and these orbitals are suitable to be used in α bonding. This means that the orbitals that we can use should have their lobes pointed along axes. Obviously, 4s orbitals are quite suitable. This called a1g. All 4p orbitals are also suitable. These are labelled as t1u. Now, let us inspect the 3d orbitals; only two of them are suitable. The orbitals dx2 − y2 and dz2 are suitable since they have the lobes along the axes. These are labelled as eg; however, the orbitals dxz, dyz and dxy are not suitable. Since the ligand orbitals cannot overlap with them to give a positive overlap, this cannot be utilised by the metal for α bonding. These remain non-bonding and are labelled as t2g.
Thus, a valance orbital of the metal six is suitable for forming σ-bonding (a1g, t1u, eg); three are non-bonding orbitals (t2g).
Let us assume that by linear combination of the ligand orbitals, we are able to generate six orbitals of symmetry, called ligand group orbital as that of the six metal bonding orbitals. These orbitals on the metal and the ligand combine.
Salient Features of the Molecular Orbital Diagram can be Summarised as Follows:
- The lower energy orbitals a1g, t1u, eg are closer in energy to the ligand orbitals.
- The t2g orbital remains non-bonding. Their energy is not changed after forming the metal orbitals.
- The higher energy orbitals a1g*, t1u*,
 are closer in energy to the metal orbital.
are closer in energy to the metal orbital. - The energy separation between non-bonding and the
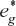 orbitals represent the Δ0 of the crystal field diagram.
orbitals represent the Δ0 of the crystal field diagram. - If this separation is large, low spin complexes are formed; if the separation is small, high spin complexes are formed.
Thus, [CO (NH3)6]3+ [CO has 6 electrons, 6 NH3 ligands have 12 electrons] thus electronic configuration
 . In contrast, [CO F6]3− has the electronic configuration
. In contrast, [CO F6]3− has the electronic configuration 

MO diagram for octahedral complex
Metal complex ligand

14.5 FACTORS AFFECTING THE STABILITY OF COORDINATION COMPOUNDS
It is not possible to conclude a particular ligand or particular metal complexes of high or low stability. No single factor is expected to account for the relative stability of coordination compounds. They are affected many physical and chemical properties as discussed here.
Charge of the Metal Complex
The complex formation is essential a reaction between cation (metal ion) and ligand. The charge on metal ion is more important to deciding the stability of metal complex. Greater the positive oxidation state of the central metal ion greater will be the attraction for the ligand; hence, greater will be the stability of the complex with same ligand. When metal ion is in a more oxidation state, that complex is more stable when compared to low oxidation metal complex.
Size of the Central Metal Ion
Keeping the charge constant as the size of the metal ion decreases, the specific charge per unit surface area increases. Hence, the metal attraction for the ligand increases. Generally, the size of the metal decreases and the stability of metal complex increases.
Outer Electronic Configuration
We compare the stability of complexes formed by similarly charged and sized metal ions belonging to two subgroups of the same periodic family. This stability is explained by outer electronic configuration.
Exmaple: K+ and Cu+ potassium are non-transition elements, whereas copper is a transition metal. Cations of d-block elements are invariably far more stable complex than non-transition metal complexes.
Potassium has inert gas configuration (n - 1) s2p6 in the outer shell, whereas copper has pseudo-inert gas configuration (n - 1) s2p6d10 in the outermost shell. It is a well-known fact that the latter configuration is much poorer in shielding the excess positive charge is located on the nucleus then the formed configuration. Hence effective nuclear charge on copper is more than potassium, that is copper will behave as a greater nuclear charge. Therefore, Cu+ has greater attraction for electrons offered by the ligands. Consequently, copper form more stable complex than potassium
Nature of the Ligand Atom
The complexes formed by halide ion have been widely studied for metals the order of stabilities as it follows the sequence F−>Cl−>Br−>I−. However, this order is reversed for a few metals like Pt+2, Cu+, Ag+, Hg+2 and Ti+ where back donation occurs from metal to ligand occur in the donation to the transfer of electron from ligand to the metal. For this back donation from metal to ligand to occur, the ligand must possess a vacant orbital capable of receiving electrons.
Basicity of Ligand
Basicity is a measure of electron pair donation; greater the basicity of ligand, greater will be the tendency to donate electron pairs. This means that a more basic ligand will form more stable complex.
Chelating Ability of the Ligand
The stabilities of complexes are increased by the coordination of the ploy dentate ligand. Coordination of such ligands produces a ring structure with the metal atom forming a part of the ring system. Such ring structure complexes are called chelates. This process of chelate formation is called chelation. The ligand that forms a chelate is called a chelating agent. Due to chelation, extra stability is conferred on the complex. This extra stability is termed as the chelating effect.
Number of Chelating Rings
Greater the number of chelate rings, greater will be the stability of complex. Some other factors are as follows:
- Size of chelate ring
- Steric effect
- Stereo chemical requirement of ligands
14.6 DETERMINATION OF COMPLEX ION FORMATION
Many methods are used to confirm the formation of complex compounds in a reaction as discussed here.
Formation of Precipitate
When a neural covalent complex is formed in a polar medium, it gets precipitated. The formation of the precipitate on the addition of a ligand indicates the formation of a neural covalent complex.
Example:

Change in Chemical Behaviour
The altered reactivity of the metal ions in the presence of the ligands is still a useful indication of complex formation.
Example: Fe+3 is not precipitated as Fe(OH)3 by NH4OH in the presence of tartaric acid. This indicates the formation of Fe3+ tartrate complex.
Dissolution of an Insoluble Compound in the Presence of Complexing Agents
Many water insoluble substances dissolve readily in the presence of a reagent that forms a soluble complex.
Example: Dissolution of AgCl in aqueous ammonia indicates the formation of soluble complex [Ag(NH3)2]Cl.
![]()
Colour Change in Solution (Spectral Method)
A colour change of the solution with the addition of another reagent indicates the formation of a new species. In many cases, the colour of the uncomplex metal ion gets altered and in some cases, a totally new colour is produced.

pH Method
The addition of the ligand to metal in solution complex is formed by the releasing of protons.
Example: Cu+2 ion is treated with salicylaldoxime to form the complex [Cu(sal)2] with the release of protons.
![]()
Conductivity Measurement
If the complex formation reaction involves a change in the number of ions, then there would be changes in the conductivity of the solution. The change in conductance can be used to detect the complex formation.
14.7 STABILITY OF COORDINATION COMPOUNDS
The subject of stability of metal complexes is important in understanding the properties of complexes. Many variables associated with the central metal ion and the ligand are greatly complicated. There are two types of stability—thermodynamic stability and kinetic stability.
Thermodynamic Stability
This measures the extent of the complex formed or transformed into another species by metal ligand bond energy stability constant when system reached equilibrium.
Kinetic Stability
This refers to the speed of the reaction with transformation, leading to attainment of equilibrium.
Thermodynamic Stability and Kinetic Stability
The thermodynamic stability of a complex depends upon the difference in energy between the reactants and the products; greater the reaction energy, greater the thermodynamic stability.
The kinetic stability of the complex depends on the difference in energy between the reactants and the activated complex, that is, activation energy. Greater the activation energy, lesser the reaction rate, implying that the complex is inert.
If the term “stability” is used without any modification, then it refers to thermodynamic stability.
In thermodynamic point of view, metal-to-ligand bond energies, stability constant and the thermodynamic variables derivable from them from being stable or unstable have to be considered.
In kinetic stability, the rates and the mechanism of reactions and also with the energies involved in the formation of the activated complex have to be considered. In the kinetic point of view, it will be more proper to speak of complex as being inert or labile rather than stable or unstable. Very often, these two groups of terms are used in correctly. A stable complex may be inert or labile and an unstable complex may be labile or inert.

Example: CN−ion forms a very stable complex with Ni+2
![]()
Ni+2 prefers CN− rather than H2O as a ligand. Thus, [Ni(CN)4]2− is thermodynamically more stable than [Ni(H2O)6]2+. However, when “14C-labelled” CN− (14CN−)is added to the solution, it is almost instantaneously incorporated into complex.
![]()
This means that the complex, [Ni(CN)4]2− is kinetically labile. Thus the stability of complex of this does not ensure inertness.
The Method of Continuous Variation
This method is used for solution where only one complex is formed.
Determination of Composition of Complex by Job’s Method
The sum of the total concentration C of a complexing agent Cx and metal ion CM is held constant and only their ratio is varied.
![]()
A wavelength of light is selected such that where the complex absorbs strongly with metal and ligand but not individually.
A plot of the mole fraction of the ligand in the mixture x versus absorbance gives a triangular curve.

The mole fraction of the ligand in the mixture x = Cx/C
The mole fraction of the metal in the mixture = CM/C
![]()
The legs of the triangle are extrapolated until they cross. The mole fraction at the point of this intersection gives the formula of complex, since at this point, for the complex Mxn is
![]()
At this point, the ligand and the metal are in the proper relative concentration to give maximum complex formation. Performing the experiment at several different wavelengths at several different values of C would indicate whether there is more than one complex formed in the solution. In such cases, n would not be constant.
The curve may be observed to deviate somewhat from the experiment intersecting lines from the amount of derivation and the stability of constant of the complex can be determined.
Determination of Stability Constant
For 1:1 complex, the ratio of the true absorbance (A) to the extrapolated absorbance (AExt) is the mole fraction of the complex actually formed.
![]()
Where C = Total concentration of the metal (or) ligand, whichever is the limiting concentration at the point considered.

Where K is the stability constant and CM and Cx are the total concentration of metal and ligand respectively.
14.8 APPLICATIONS OF COORDINATION COMPOUNDS
Coordination compounds are used in several areas of chemistry such as analytical, medicinal, industrial chemistry and agriculture.
Metal Complexes in Analytical Chemistry
Inorganic Qualitative Analysis
Coordination chemistry is used in inorganic qualitative analysis. The formation of metal complex is employed in the separation and identification of some metals.
Separation
The principle of masking is used in separationof some metals from each other on qualitative analysis.
Example: Cu+2 and Cd2+ forms insoluble sulphide in the II group. These two metals are separated by complexing them with CN−. Both form complexes but with a difference.

The reaction of CN− with CuS is an oxidation reduction reaction coupled with complexation. In both complexes, Cu complex is stable and Cd complex is unstable. The marked difference in the stabilities of copper and cadmium cyano complexes is the basis for separating these two metals.
Reductive Complexation
When a ligand reduces a metal of the complexes, the process is called reductive complexation.
Identification of Metals
Complex formation is used to identify several metals in qualitative analysis.
Complexometric Titration
Certain metal ion reacts with certain ligand stoichiometrically and forms stable metal complexes. This reaction can be used to in volumetric estimation of metal ions.
Example: determination of hardness of water.
Hard water is titrated with EDTA ligand; water containing metals Ca and Mg will form complex with EDTA. From the end point, hardness giving substances present in water can be determine.
Complex in Calorimeter
Some of the metals can be calorimetrically estimated by the formation of coloured complex species in solution.
Coordination Compounds in Gravimetry
The formation of coordination compounds serves as an excellent means for gravimetrically estimating certain metals.
Complexes in Separation of Metals
Some chelating agents are used for separating certain metallic mixtures.
14.9 REVIEW QUESTIONS
14.9.1 Fill in the Blanks
- The formation of coordination compound from metal ion is called __________
[Ans.: Complexation]
- Fe2(SO4)3(NH4)2SO4.24H2O is a __________
[Ans.: Double salt]
- The number of mono dentate ligands attached to metal ion indicates __________
[Ans.: Coordination number]
- Oxidation number of ‘Ni’ in [NiCl4] −2 complex is __________
[Ans.: +2]
- R3N, CO, H2O belongs to __________ ligands.
[Ans.: Unidentate]
 Here, complex Cl belongs to __________ type of ligand.
Here, complex Cl belongs to __________ type of ligand.
[Ans.: Bridging]
- The suffix “ate” is added to __________
[Ans.: Complex anion]
- In Werner’s theory, coordination number represents __________ valence.
[Ans.: Secondary]
- According to Werner’s theory, the bond between ligand and metal __________
[Ans.: Coordinate bond or dative bond]
- The effective atomic number of [V(CO) 6] is __________
[Ans.: 31]
- Does [Mn(CN) 4] complex obey the EAN rule? __________
[Ans.: No]
- __________ theory proposed EAN.
[Ans.: Sidgwick]
- Who proposed valence bond theory? __________
[Ans.: Linus Pauling and Slater]
- On which basis is the shape of complexes explained in the valence bond theory? __________
[Ans.: Hybridisation]
- Square planar complexes exhibiting hybridisation is __________
[Ans.: dsp2]
- Sp hybridisation involving complexes geometry is __________
[Ans.: Linear]
- Spin only moment is explained by _________ theory
[Ans.: Valence bond]
- H. Van Vleck and Bethe proposed __________
[Ans.: Crystal field theory]
- According to crystal field theory, the bond between metal and ligand is __________
[Ans.: Electrostatic attraction]
- Splitting of d-orbitals into __________ and eg is known as crystal field splitting of d-orbitals.
[Ans.: t2g]
- Triply degenerate orbitals are _________ and are designated with __________
[Ans.: dxy, dyz, dzy, t2g]
- __________ orbitals doubly degenerate orbitals and its representation is eg.
[Ans.: dx2−y2 and dz2]
- The energy difference between t2g and eg levels is denoted by __________ and is called __________ energy.
[Ans.: Δ0 (or) 10Dq, crystal field splitting]
- In octahedral complexes, __________ orbitals have lesser energy than eg in excited state.
[Ans.: t2g]
14.9.2 Multiple-choice Questions
- In coordination complexes which of the following donates the electrons?
- Metal
- Ligand
- Both (a) and b
- None of these
[Ans.: b]
- The compound formed by addition of two stable compound and on dilution that forms complete ions is called
- Double salt
- Triple salt
- Coordination compound
- Covalent compounds
[Ans.: a]
- What is the correct name of the given complex [Cu Cl2(NO2)3]−3?
- Trinitrondichlorocopper(III)ion
- Dichlorotrinitrocuprate(III)ion
- Dichlorotrinitrocopper(III)ion
- Trinitrodichlorocuprate(III)ion
[Ans.: b]
- Which of the following is a bidentate ligand?
- Cl−
- EDTA
- Triethyl triamine
- Oxalate
[Ans.: d]
- Which of the following is a neutra ligand?
- Cl−
- NH2—NH3+
- CO
- None of these
[Ans.: c]
- Which ligand behaves as an isomeric ligand?
- M—NO2
- M—Cl
- [M—EDTA]
- [M—(NH3)6]
[Ans.: a]
- In Werner’s theory, primary valence represents
- Oxidation number
- Coordination number
- Both a and b
- None of these
[Ans.: a]
- EAN is explained by
- Werner
- Sidgwick
- Linus Pauling and Slater
- J. H. Van Vleck and Bethe
[Ans.: b]
- Crystal field theory is proposed by
- J.H. Van Vleck and Bethe
- Werner
- Sidgwick
- Linus Pauling and Slater
[Ans.: a]
- According to crystal field theory, d-d splitting in octahedral complexes
- t2g →eg
- eg→ t2g
- T2 → E
- E → T2
[Ans.: a]
- What are the d-orbitals that belong in t2g state?
- dxydyzdzx
- dx2 − y2
- dz2
- dx2 − y2 and dz2
[Ans.: a]
- Which orbitals are double degenerated?
- dxy, dyz
- dxz, dz2
- dx2 − y2, dz2
- dx2 − y2 and dxy
[Ans.: c]
- Spin only formula µ =
[Ans.: a]
- Crystal field stabilisation energy for t2g orbital in octahedral complexes is
- − 0.4 Δ0
- 0.6 Δ0
- − 0.6 Δ0
- 0.4 Δ0
[Ans.: a]
- The presence of electrons in t2g (or) eg level will stabilise the complex is called
- Crystal field stabilisation energy
- Crystal field splitting of d-orbital
- Crystal field theory
- None of these
[Ans.: a]
- The method used to detect the stability of metal complexes is
- PH method
- Job method
- Titration method
- None of these
[Ans.: b]
- In potassium and copper complexes, a form of stable complex is
- Potassium
- Copper
- Both (a) and (b)
- None of these
[Ans.: b]
14.9.3 Short Answer Questions
- Define coordination compound.
Ans.: Compound of metals with certain number of species called ligands bound to the metal is called coordination compound.
- Differentiate double salt and coordination compound.
Ans.: Double salt:
Double salt is formed by the adding of two stable compounds; on dissolution, it breaks down to its simple component ions.
Coordination compounds:
On dissolution, it does not break down into simple ions.
- What is meant by coordination number and oxidation state?
Ans.: The total number of ligands attached to metal complex is called coordination number.
The charge on the complex will be denotes the oxidation state.
- Write the different types of ligands.
Ans.: (i) Monodentate
(ii) Polydentate
(iii) Bridging ligand
(iv) Ambidentate ligand
(v) Flexidentate ligand
- Give the names of the following complexes:
- [Cr(NH3)6]+3
- [PtCl2(H2O)(NH2NH3)]+2
- [CrCl3(NO2)3]3−
- [Cu(en)2]SO4
Ans.: (i) Hexaaminechromium(III)ion.
(ii) Dichloroaquahydrazoniumplatinum(II)ion.
(iii) Trichlorotrinitrochromate(III)ion.
(iv) Biethylenediaminecopper(II)sulphate.
- Write the different theories of coordination chemistry.
Ans.: (i) Werner’s theory
(ii) Sidgwick theory
(iii) Valence bond theory
(iv) Crystal field theory
(v) Molecular orbital theory
- Write the postulates and drawbacks on Werner’s theory.
Ans.: It explains the elements exhibit two types of valence as follows:
- Primary valence
- Secondary valence
Drawbacks:
It is unable to account the 4-coordinated and 6-coordinated complex in coordination chemistry.
It does not explain the nature of bonding between metal and ligand.
- Explain effective atomic number.
Ans.: According to Sidgwick, the total electrons around the metal ion including those gained through the coordination by the ligand is called effective atomic number (EAN).
EAN is equal to the atomic number of the next inert gas atomic number such that metal complexes are stable.
- Explain valence bond theory.
Ans.: In 1935, Pauling and Slater proposed this theory. This theory explains the magnetic properties and structure of the metal complexes.
- Explain crystal field theories and their merits and demerits.
Ans.: This theory was explained by J.H. Van Vleck and Bethe. It was first applied by crystalline substances and on this basis, it was called crystal field theory.
Merits:
- It is successful in interpreting the magnetic properties
- It explains the colour of complexes.
- It gives detailed information about tetrahedral and square planar geometrics of 4 coordinated complexes.
- It is also explains spectral properties of complex.
Demerits:
Bond strength chemical properties cannot be explained.
- Write any two factors affecting the stability of metal complexes?
Ans.: (i) Charge of the metal complex: Greater the charge on metal greater will be the stability of complexes.
(ii) Size of the metal ion: Size decreases the stability of metal complexes increases.
- Write the methods of detection of complexes.
Ans.: (i) Formation of precipitate
(ii) Change in chemical behaviour
(iii) Spectral method
(iv) pH method
(v) Conductivity measurements
14.9.4 Descriptive Questions
Q.1 Write a note on the characteristics of coordination compound.
Q.2 Explain the difference between double salt and coordination compound.
Q.3 Explain the following terms:
- Coordination number
- Complex ion
- Oxidation number
- Ligands
Q.4 Define ligand. Explain the types of ligands with examples.
Q.5 Describe the nomenclature of coordination compounds in detail.
Q.6 Write a detailed note on any two theories of coordination chemistry?
Q.7 Write a short note on crystal field stabilisation energy and splitting of d-orbital in crystal field theory.
Q.8 Explain the factors affecting coordination compounds.
Q.9 Give a brief explanation on the detection of complex ion formation.
Q.10 Explain stability of coordination compounds.
Q.11 Describe Job’s method.
Q.12 Give the application of coordination complexes in analytical chemistry.
