15
STRUCTURE AND REACTIVITY OF ORGANIC AND INORGANIC MOLECULES
15.1 INTRODUCTION
By transferring or sharing electrons, a bond is formed between the atoms. The covalent bond plays a vital role in the formation of many organic molecules. A covalent bond is formed with the sharing of electrons between two atoms; it is mainly σ-bond and π- bond.
σ-bond
A sigma bond is formed with end-to-end overlap of orbitals.
π-bond
A π bond is formed with a partial or a side-wise overlap of orbitals.
15.2 HYBRIDISATION
Hybridisation is defined as the “hypothetical intermixing of nearly the same energy [of] atomic orbitals to give [an] entirely new identical equal energy orbitals”.
It is also defined as,
“Combining of atomic orbitals have different energies or nearly the same energy and the formation of a new set of orbitals with equivalent energy and shape”. They are known as hybrid orbital and the phenomenon is known as hybridisation.
15.2.1 Salient Features of Hybridisation
In hybridisation, only orbitals combine; electrons do not combine. The number of hybrid orbitals formed is exactly equal to the number of atomic orbitals that participated in hybridisation. Only similar energy orbitals can combine and form a hybrid orbital. The formed hybrid orbitals are equivalent in energy and shape. Hybrid orbitals can form strong bonds that lead to the formation of more stable molecules. They are directed in space so as to have minimum repulsion between electron pairs.
15.2.2 Important Conditions for Hybridisation
The orbitals undergoing hybridisation should have comparable/nearly the same level of energy. The promotion of an electron is not an essential condition prior to hybridisation. Half-filled or completely filled orbitals can participate in the process.
15.2.3 Types of Hybridisation
There are various types of hybridisation that occur between s, p and d orbitals. Depending on the type of atomic orbitals involved in the process, there are sp, sp2, sp3, sp3d and sp3d2 hybridisation. The shape and bond angles of the molecule depend on the type of hybridisation.
sp Hybridisation
Combining one s and one p orbital and the formation of two equivalent sp hybrid orbitals is known as sp hybridisation. Each sp hybrid orbital has 50% s character and 50% p character. With sp hybridisation, the molecule possesses linear geometry.
sp hybridisation is also known as diagonal hybridisation. The two sp hybrid orbitals point in the opposite direction along the axis with projecting positive lobes and very small negative lobes which provide more effective overlapping, resulting in the formation of stronger bonds.
Example: Formation of beryllium chloride (BeCl2)
Beryllium: ground state electronic configuration – 1s2 2s2
Excited state electronic configuration – 1s2 2s1 2p1
One 2s and one 2p orbitals of beryllium combine to form two equal sp hybrid orbitals. These two sp hybrid orbitals are oriented in the opposite direction, forming an angle of 180°. Each sp hybrid orbital overlaps with the 3p orbital of chlorine axially and forms two Be — Cl sigma bonds. The shape of this molecule is linear, and the bond angle is 180°.

sp2 Hybridisation
Combining one s and two p orbitals and the formation of three equivalent sp2 hybrid orbitals is known as sp2 hybridisation. Each sp2 hybrid orbitals has 33.3% s character and 66.6% p character. With sp2 hybridisation, the molecule possesses trigonal planar geometry. The bond angle between each sp2 hybrid orbital is 120°C.
Example: Formation of boron chloride (BCl3)
Boron – ground state electronic configuration – 1s2 2s2 2p1
Excited state electronic configuration – 1s2 2s1 2px1 2py1 2pz0
One 2s and two 2p orbitals of boron combine to form three equal sp2 hybrid orbitals.

sp3 Hybridisation
Combining one s and three p orbitals and the formation of four equivalent sp3 hybrid orbitals is known as sp3 hybridisation. Each sp3 hybrid orbital has 25% s character and 75% p character. With sp3 hybridisation, the molecule possesses tetrahedron geometry. The bond angle between each sp3 hybrid orbital is 109°281.
Example: CH4, NH3, H2O.
Formation of Methane (CH4)
Carbon – ground state electronic configuration – 1s2 2s2 2px1 2py1 2pz0
Excited state electronic configuration – 1s2 2s1 2px1 2py1 2pz1

In NH3 and H2O, the molecules are involved in sp3 hybridisation. However, they cannot exhibit tetrahedral structure and the bond angle also varies.
Formation of Ammonia (NH3)
Nitrogen – ground state electronic configuration – 1s2 2s2 2px1 2py1 2pz1
In ground state, nitrogen undergoes sp3 hybridisation with one 2s orbital containing two electrons and three 2p orbitals containing one electron each. In these four sp3 hybrid orbitals, three hybrid orbitals have unpaired electrons and one hybrid orbital has a lone pair of electrons on the central metal atom. By these lone pair electrons, repulsion occurs between the lone pair and the bond pair. Due to this repulsion, the geometry of the molecule changes from tetrahedral to pyramidal structure, and the bond angle also varies from 109° 281 to 107° 51.

Formation of Water (H2O)
Oxygen–ground state electronic configuration – 1s2 2s2 2px2 2py1 2pz1
In ground state, oxygen undergoes sp3 hybridisation with one 2s orbital containing two electrons and three 2p orbitals; among three p orbitals, one contains a lone pair and another two contain one electron each. In these four sp3 hybrid orbitals, two hybrid orbitals have unpaired electrons and two hybrid orbital have a lone pair of electrons on the central atom. In these lone pair of electrons, repulsion occurs between the lone pair and the bond pair. Due to this repulsion, the geometry of the molecule changes from tetrahedral to a v-shaped structure, and the bond angle also varies from 109° 281 to 104°.

Hybridisation Involving d Orbitals
In addition to s and p orbitals, the elements present in the third period contain the d orbital. The energy of 3d orbitals is comparable to the energy of the 3s, 3p and 4s, 4p orbital. Therefore, hybridisation may involve 3s, 3p and 3d or 4s, 3d and 4p. Due to energy differences, there is no possibility of hybridisation involving 4s, 3d and 3p.
sp3d Hybridisation
Combining one s, three p and one d orbital and the formation of five equivalent sp3d hybrid orbitals is known as sp3d hybridisation. With sp3d hybridisation, the molecule possesses trigonal bipyramidal geometry. The bond angles are 90° and 120°.
Example: PF5, PCl5.
Formation of Phosphorous Pentachloride
Phosphorous – ground state electronic configuration – 1s2 2s2 2p6 3s2 3px1 3py1 3pz1 3d0
Excited state outer shell electronic configuration – 3s1 3px1 3py1 3pz1 3d1

sp3d2 Hybridisation
Combining one s, three p and two d orbital and the formation of six equivalent sp3d2 hybrid orbitals is known as sp3d2 hybridisation. With sp3d2 hybridisation, the molecule possesses a square pyramidal (BF6) and octahedral (SF6) geometry.
Formation of Sulphur Hexafluoride
Sulphur – ground state electronic configuration – 1s2 2s2 2p6 3s2 3px2 3py1 3pz1 3d0
Excited state outer shell electronic configuration – 3s1 3px1 3py1 3pz1 3d 2

Its geometry is octahedral and the bond angle is 90°.
dsp2 Hybridisation
Combining one d, one s and two p orbitals and the formation of four equivalent dsp2 hybrid orbitals is known as dsp2 hybridisation. With dsp2 hybridisation, the molecule possesses a square planar geometry.
Example: [Ni(CN)4]−2, [Pt(Cl)4]−2

15.3 BOND POLARISATION
The reaction of organic compounds essentially involves changes in the existing covalent bond present in their molecules. These changes may involve “electronic displacement in the bonds, breaking of bonds, energy changes accompanying the cleavage and formation of new bonds”.
To clearly understand the mechanism of various reactions, it is essential to know electron displacement in covalent bonds, cleavage of covalent bonds and the nature of attacking reagents.
15.3.1 Electron Displacement in Covalent Bonds
The electronic displacement in covalent bonds may occur due to the atoms, functional groups or under the influence of attacking reagents. Due to these electron displacements, the center of different electron densities are created and there is a chance of attack by the reagent.
The factors which create the center of different electron densities in the substrate is mainly due to the following:
- Inductive effect
- Electromeric effect
- Resonance and mesomeric effect
- Hyperconjugation
Inductive Effect
In a covalent bond between two dissimilar atoms, the electron pair does not remain in the centre but is attracted towards the more electronegative atoms. Due to unequal sharing of the electron pair, the bond becomes polar.
It is defined as follows: “The polarity produced in the molecule as a result of higher electronegativity of one atom compared to other is termed as inductive effect”.
It is important to note that the electron pair, although permanently displaced or simply shifted, remains in the same valence shell. The inductive effect is always transmitted along a chain of carbon atoms.
Example: Whenever electron-withdrawing groups are attached to the end of carbon chain, the sigma electrons of the C—X bond are slightly displaced towards the more electronegative atom. However, the intensity decreases as distance from the source atoms increases.
![]()
It is a permanent effect in the molecule and can be observed practically in the form of dipole moment. This type of electron displacement along a carbon chain due to the presence of a source is called “inductive effect or transmission effect”. This effect is represented by an arrow head in the middle of a covalent bond pointing to the direction of electron displacement.
There are two types of inductive effects. They are –I effect and +I effect. The carbon and the hydrogen bond are used as a standard for comparing the tendency of electron attraction and repulsion.
–I Effect
The atom or group which has more power to attract the electron in comparison to hydrogen is said to be –I effect.
![]()
The atoms or groups in the decreasing order are as follows:
![]()
+I Effect
The atom or group which has less power to attract electrons than hydrogen are said to have +I effect. The σ electrons are displaced away from the substituents ‘I’.
![]()
The first group is referred to as attracting or electron withdrawing, while the second group is referred to as the electron repelling or the electron releasing group.
Groups in the decreasing order are as follows:
![]()
Salient Features of Inductive Effect
The salient features of inductive effect are as follows:
- It is a permanent effect in the molecule or ion. Although the shared pair of electrons are permanently shifted towards the more electronegative atom, they remain in the same valence.
- As a result of the electron pair shifting towards more electronegative atom it acquires a partial negative charge and other part acquires positive charge.
- The inductive effect is not confined to the polarisation of one bond but is transmitted along a chain of carbon atom through σ bonds. However, the effect is insignificant beyond the second carbon in the chain.
- Inductive effect brings changes in the physical properties such as dipole moment, solubility, etc. It affects the rate of reaction.
- To explain inductive effect, the carbon and hydrogen bond is taken as a standard, and zero effect is assumed for the bond. Atoms or groups which have great electron withdrawing capacity than hydrogen show –I effect, whereas atoms or groups which have greater electron releasing power show +I effect.
- The phenomenon of inductive effect is very important in organic chemistry as it is helpful in explaining several facts such as reactivity of alkyl halides, dipole moment, reactivity and strength of the acids and strength of bases.
Electromeric Effect
The effect involving the complete transfer of a shared pair of electron to one atom of a compound which is joined by a multiple bond (double (or) triple) at the requirement of attacking reagent is known as electrometric effect. It is indicated by E and is represented by a curved arrow (![]() ) showing an electron pair. It is a temporary effect.
) showing an electron pair. It is a temporary effect.
Direction of the Shift of Electron Pair
When the groups linked to multiple bonds are similar, the shift can occur to either direction. For example, in ethylene, the shift can occur in any one of the carbon atom.

When dissimilar groups are linked on two ends of a double bond, the shift is decided by the direction of the inductive effect.
Example:

Due to the electron repelling nature of methyl group, the electron shift occurs according to equation (1) and not equation (2) path.

The +I effect of —CH2CH3 is larger than that of —CH3 due to transfer of π-electron from C3 to C2.
In case of the carbonyl group, the shift is always towards oxygen, that is, more electronegative atom.
![]()
This effect is common during the addition of polar reagents on >C==C< and >C==O bonds.
There are two types of electrometric effects—+E effect and –E effect
- The transfer of electron towards the attacking reagent is called +E effect.
Example: Addition of acids to alkene.

- The transfer of electron away from the attacking reagent is called –E effect.
Example:

- The transfer of electron away from the attacking reagent is called –E effect.
Resonance or Mesomeric Effect
If a molecule has alternate double bonds, it is said to be a conjugated molecule. In such compounds, the π electrons are delocalised and polarity will develop in the molecule. The same happens when an atom or a group with a lone pair of electron is in conjugation with a conjugated double bond.
“The development of polarity in a molecule as a result of interaction between two π-bonds or a π-bond and atom or a group with a lone pair of electrons is known as mesomeric effect or resonance”.
In a carbonyl group, the oxygen atom is more electronegative than the carbon atom. The π- electrons of the double bond get displaced towards the oxygen atom. The shifting of the electron is shown by a curved arrow ![]() . This will give an ionic structure. The actual structure seems to lie in between structures (i) and (ii) which can be best represented as structure (iii) which is π-electrons drawn preferentially towards oxygen.
. This will give an ionic structure. The actual structure seems to lie in between structures (i) and (ii) which can be best represented as structure (iii) which is π-electrons drawn preferentially towards oxygen.

Like inductive effect mesomeric effect also +M and –M effect.
+M Effect
Electrons transfer away from the atoms or a group. A group which has the capacity to increase the electron density of the rest of the molecules is said to be +M effect or groups which donate the electron to the double or triple bond of the conjugate system are said to be +M effect.
+M effect groups are —Cl, —Br, —I, —NH2, —NHR, —NR2, —OH, —OR, —SR, —SH, —OCH3, etc. The +M effect of a halogen atom in vinyl and allyl halides explains their low reactivity. The +M effect activates benzene nucleus for electrophilic substitution reaction that occurs at ortho and para position.
–M Effect
The transfer of electrons is directed towards atoms or groups; the group which decreases the electron density of the rest of the molecules is said to have –M effect or the group which can withdraw electrons from the double bond or from a conjugated system towards itself due to resonance is said to have –M effect.

With –M effect, electrophile attacks at the M-position (e.g., benzene). –M effect deactivates for electrophilic substitution reactions but activates nucleophilic substitution reactions.
Salient Features of Mesomeric Effect
The salient features of mesomeric effect are as follows:
- π-electrons and a lone pair of electrons are involved in this effect through conjugative mechanism.
- It is a permanent effect in the molecule in the ground state.
- It affects the physical properties such as dipole moment, solubility and rate of reaction of the substance.
Hyperconjugation
The kind of delocalisation involving sigma bond orbital is called hyperconjugation.
There should at least one H-atom at α-carbon with respect to sp2 hybrid carbon; greater the number of C—H bonds at α-carbon to the unsaturated system, greater will be the electron release and thus, greater the hyperconjugation effect.
15.4 REACTION INTERMEDIATES
Short-lived and highly reactive fragments are called reaction intermediate; with the result of reaction intermediate, either homolytic or heterolytic bond fission occurs. The important reactive intermediates are free radicals, carbonians, carbanions, carbenes, nitrenes and benzynes.
15.4.1 Free Radicals
A free radical may be defined as an atom or a group of atoms having odd or unpaired electrons. These results occur due to homolytic fission of a bond and are denoted by putting a dot (.), against the symbol of atom or a group of atom.
Example: Cl•(chlorine free radical), H•(hydrogen free radical) and CH3•(methyl free radical).
Formation of Free Radical
The formation of free radical is initiated by heat, light or catalyst; the chemical reaction which takes place through free radical is known as free radical reaction. Free radicals are highly unstable, generally neutral and paramagnetic in nature. They are short-lived and highly reactive due to the presence of odd electron.
![]()
The three types of free radical reactions that are observed are as follows.
- Mutual combination of free radical

- Reaction between free radical and neutral molecule gives a neutral molecule and new free radical.

- A free radical can lose a neutral molecule to form a new free radical.

Some Important Reactions via Free Radical Formation
- Wurtz reaction giving alkanes
- Substitution reactions of alkanes
- Kolbe electrolytic reaction giving alkanes, alkenes and alkynes
- Anti-Markovnikov’s addition or peroxide effect or Kharasch effect
Relative Stability of Free Radicals
- Tertiary (3°) > Benzyl > allyl > Secondary (2°) > Primary (1°) > Methyl > Vinyl


Structure of Free Radical
The carbon atom of alkyl-free radicals is sp2 hybridised and bonded to only three atoms or groups of atoms. Hence, free radicals have a planar structure with odd electrons situated in the unused p-orbital at right angle to the plane of hybrid orbital.

15.4.2 Carbocations or Carbonium Ions
In a covalent bond, carbon is linked to a more electronegative atom or a group, breaks up by a heterolytic fission. The more an electronegative atom takes the electron pair from carbon; hence carbon loses its electron and thus acquires a positive charge; known as carbonium ion.
![]()
Organic substance carrying a positive charge on carbon atom are known as “carbocation” or “carbonium ion”,
![]()
Carbocation only differs from free radicals which have an odd electron. Carbocation is very reactive due to a carbon atom having a vacant p-orbital. A positively charged carbon atom tries to complete its octet and hence, these ions react readily with those species which can release two electrons for the formation of fourth bond. They react with the nucleophilic reagent.
Carbocations are named by adding the words carbocation to the parent alkyl group. These are also termed primary, secondary and tertiary, depending upon the nature of the carbon atom bearing a positive charge.

Formation of Carbocation
- By heterolysis of halogen compound:

- By protonation of alkene or alcohol:

- By decomposition of diazo compounds:

Some Important Reactions via Carbocation Formation
Some important reactions that take place as a result of carbocation are as follows:
- Elimination reaction to form alkenes from alkyl halides and alcohols
- Electrophilic addition reaction of alkenes, alkynes and alkalines
- SN1 reaction of alkyl halides and diazonium salts
- Pinacol–pinacolone molecular rearrangement
Structure of Carbocation
A carbon atom carrying positive charge has six electrons in its valance shell, i.e., two electrons less than an octet. The positively charged carbon atom in the carbocation is in sp2 hybridisation and has a planar structure.

The order of reactivity of carbocation is:
Primary (1°) > Secondary (2°) > Tertiary (3°)
Relative Stability of Carbocation
The stability of alkyl carbocation is influenced by resonance, hyperconjugation and inductive effects. The stability decreases as +I effect decreases and molecular mass decreases.
![]()
If α-hydrogen with respect to carbocation, that is, carbon has one or more than one lone pair of electrons, then the lone pair of electrons strongly stabilise carbocation due to delocalisation.
15.4.3 Carbanions
When a covalent bond in which carbon is attached to a lesser electronegative atom breaks up by heterolysis, the atom leaves without taking away the bonding pair of electrons and thus the carbon atom acquires negative charge due to an extra electron.
Organic ions which contain a negatively charged carbon atom are called carbanions.
![]()
Structure and Characteristics of Carbanions
- In carbanion, the carbon carrying the negative charge contains eight electrons in the valance shell; it has octet configuration and is diamagnetic.
- They are highly reactive because the carbon carrying the negative charge is electron-rich and can donate its non-bonding pair of electrons to some other group for sharing. Hence, carbanion behaves as nucleophiles and is ready to attack electrophiles.
- The negatively charged carbon is in a state of sp3 hybridisation and has a pyramidal structure.
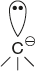
Some Important Reactions via Carbanion Formation
Some important reactions via carbanion formation are as follows:
- Aldol condensation of aldehyde having α-H atom
- Cannizzaro’s reaction of aldehyde without α-hydrogen atom
- Perkin reaction
- Knoevenagel reaction
Relative Stability of Carbanions
- Aromatic > benzyl > allyl > acetyl > vinyl > alkyl (primary) > secondary > tertiary


Stability increases with increase of S-character:

Groups such as —NO2, —CN, —COOC2H5, > C==O, halogen and C6H5 increase the stability of carbanions.
15.4.4 Carbenes
Carbenes are highly reactive, short-lived, diagonal in geometry and neutral species in which a carbon atom has six electrons in the outer shell, out of which two constitute a lone pair and two are shared. Therefore, they are divalent carbon species of carbon containing two unpaired electrons and possess no charge.
Carbenes are sp2 as well as sp hybridised, neutral, transitory reaction intermediates containing a carbon atom with two bonds and two electrons.
- By the decomposition or pyrolysis of aliphatic diazo compounds or ketones.

- By action of a base on a suitable polyhalogen.
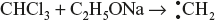
sp2 hybrid carbene are two types singlet and triplet.
Some Important Reactions via Carbene Formation
Carbenes undergo several important reactions as follows:
- Addition reaction (Carbylamine reaction)
- Insertion reaction (Reimer–Tiemann reaction)
- Ring expansion reaction
15.4.5 Nitrenes or Imidogens
Nitrenes are electron-deficient species in which ![]() has a sextet of electron. These are similar to carbenes and the neutral univalent nitrogen intermediate can exist in singlet and triplet forms.
has a sextet of electron. These are similar to carbenes and the neutral univalent nitrogen intermediate can exist in singlet and triplet forms.
Formation of Nitrenes
Nitrenes are formed in the following manner:
- The simplest nitrene is formed when hydrozoic acid is irradiated with UV light in aromatic solvent and forms a small amount of primary aromatic amines.

- Photolysis of alkyl azide:

Some Important Reactions Via Nitrenes Formation
Some important reactions in nitrene formation are as follows:
- Hofmann rearrangement
- Lossen rearrangement
- Schmidt rearrangement
Reactions
The reactions are discussed here.
Addition Alkenes
Alkenes react with nitrenes to form the corresponding cyclic amine.

Addition to Alkanes
Acetyl nitrene reacts with isobutane to form acetyl tertiary butyl amine.

Photolysis of phenyl nitrene forms azobenzene.
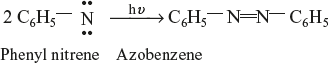
15.4.6 Benzynes
Benzyne is neutral, highly reactive intermediate; it has a formal C≡C bond and can be represented as follows:
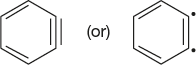
Benzynes are also known as didehydro benzenes.
Stereochemistry
Except for the two hydrogen atoms which are linked a through a ≡ bond, the remaining C-atoms are sp2–hybridised. The C-atoms linked through the ≡ bond are sp hybridised. Due to these two sp hybridised c-atoms, the intermediate is highly strained and is highly reactive. However, the presence of sp hybridisation does not change its aromatic character. Hence, the intermediate still consists of aromatic sextet.

There are three possible ground sates of benzyne.
Example:
- A triplet state:

- A symmetric singlet state, where the two half-filled sp orbitals are symmetric with respect to C2 axis:

- Anti-symmetric singlet:

Formation of Benzynes
By Aryl Halides
By heating Aryl halides with amines benzynes are formed.

By Ortho Amino Benzoic Acid
Ortho amino benzoic acid is treated with dilute nitrous acid by losing nitrogen and carbon dioxide gives benzyne.
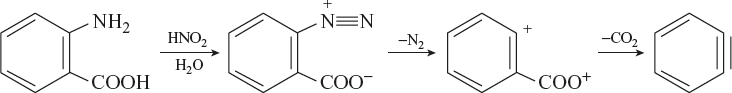
Stability of Benzynes
The structure of benzyne can be represented as follows:

Benzyne is stabilised by resonance between structures (i) and (ii); hence, benzyne can be represented by structure (iii). The extra π bond (iv) is localised and orthogonal to the other π bond by making up the aromatic ring. Benzyne can also be drawn as diradical; here, the π bond (v) splits homolytically, leaving one electron on each of the two atoms that are formally part of that bond. It can be extremely reactive species due to the nature of its (≡) triple bond. In benzyne, the p-orbitals are distorted to accommodate the ‘≡’ bond with the ring system, reducing their effective overlapping. A suitable chemical trap for benzyne is a cyclopentadiene.
There are three possible di radical di de hydro benzynes as 1,2 di de hydro benzyne, 1,3 di de hydro benzyne and 1,4 di de hydro benzyne.

Reaction with Benzynes
Cyclo Addition
[2 + 2] cyclo addition

[2 + 3] cyclo addition
Dimerisation and Trimerisation
Dimerisation of benzyne is a 2+2 cyclo addition reaction, it occurs via a concerted mechanism and the product is biphenylene. Trimerisation of benzyne is a 2+2 cyclo addition reaction that occurs via concerted reaction mechanism and the product is triphenylene.

Reaction with Nucleophile
Benzyne reacts with nucleophilic reagent to form different organic compounds.
Important Organic Reactions
The reactions of organic compounds can be classified into four main types.
- Substitution or displacement reaction
- Addition reaction
- Elimination reaction
- Rearrangement reaction
Substitution Reaction
Which an atom or a group attached to a carbon atom in a substrate molecule is replaced by another atom or a group of atoms, no change occur in the carbon skeleton is observed during the reaction.
Depending on the mechanism, the substitution reactions are further classified into three types as follows.
- Free radical substitution
- Electrophilic substitution
- Nucleophilic substitution
Example:
Addition Reaction
An attacking reagent adds up to the substrate molecule without elimination of any atom or group. Such reactions are given by those compounds which possess double or triple bonds. In this process, the triple bond is converted into a double bond or a single bond and a double bond is converted into a single bond. For each σ bond of the molecule two σ bonds are formed and the hybridisation state of carbon atom changes from sp to sp2 and sp2 to sp.
Addition reactions are also three types.
- Free radical Addition
- Nucleophilic Addition
- Electrophilic Addition
Example:
Elimination Reaction
In these reactions, generally, atoms or groups from two adjacent carbon atoms (α, β) in the substrate molecule are removed and a multiple bond is formed. In the process, two sigma bonds are lost and a new π-bond is formed, that is, a state of hybridisation of the carbon atom changes from sp3 to sp2 and sp2 to sp.
This reaction is also known as β-elimination.
Example:

Some important elimination reactions are as follows:
- Arbylamine
- Reimer-Tiemann reaction
Rearrangement Reaction
The reaction which involves the migration of an atom or a group from one site to another within the molecule, resulting in a new molecular structure is known rearrangement reaction.
Example: Fries rearrangement

Beckmann Rearrangement

15.5 MOLECULAR ORBITAL THEORY
The molecular orbital (MO) theory was proposed by Hund, Jones and Mullikan. This theory treats a molecule in the same way as an atom. The difference is that in an atom, the electrons rotate around only one nucleus, whereas in a molecule, the electrons rotate around more than one nuclei, that is, the molecular orbitals are polycentric.
![]()
The MO theory has the following rules:
- The molecular orbitals are polycentric.
- Electrons are fed into three molecular orbitals one at a time such that each entering electron occupies the lowest possible quantum state, according to the Aufbau principle.
- The electron also obeys Pauli’s exclusion principle.
- Molecular orbitals can be obtained by linear combination of atomic orbitals.
The molecular orbital theory describes electrons as delocalised moieties over adjacent atoms; it is powerful and has extensive approaches that extend beyond the limitations of the valence shell electron pair repulsion (VSEPR) and the valence bond theory (VBT). The VSEPR and the VBT accurately predict bond properties, but they fail to explain structure and magnetic properties of some molecules. The MO theory incorporates the wave character of electrons in developing the MO diagram; these predict physical and chemical properties of a molecule such as structure, bond energy, bond length and bond angle. They also provide information in predicting a molecule’s electronic spectra and para magnetism.
Atomic and Molecular Orbital
Atomic Orbital
The atomic orbital is the region of space around the nucleus of an atom where an electron is likely to be found or the electron density is more.
Molecular Orbital
The molecular Orbital is a combination of atomic orbitals where the region of electron density is most likely to be found in a molecule.
Homo Nuclear Di Atomic Molecules
Molecules consisting of two identical atoms are said to be homo nuclear diatomic.
Example: H2, N2, O2, etc.
Hetero Nuclear Di Atomic Molecules
Molecules consisting of two non-identical atoms are said to be hetero nuclear diatomic.
Example: CO, NO, HF, LiF, etc.
Anti-bonding Molecular Orbitals
Orbitals that are out of phase with each other are called “anti-bonding” orbitals because regions with dense electron probabilities do not merge and may destabilise the molecule.
Bonding Molecular Orbitals
Bonding orbitals are less energetic than anti-bonding atomic orbitals and are in-phase as shown in Figure 15.1.

Figure 15.1 Bonding molecular orbitals
Phases and Nodes
Depends on their wave “up” or “down” displacements, phases are designated as (+) or (-). A node occurs if the phase signs change from (+) to (-) or vice versa. It is important to notice that the phase signs do not symbolise charges. Nodes are regions where the probability of finding an electron is zero.
Sigma (σ) and pi (π) Bond
A sigma-bond is formed as an “end-to-end” overlap of symmetric atomic orbitals.
A pi-bond is formed as a “sideways” overlap of atomic orbitals.
15.5.1 Important Points on Molecular Orbital Diagrams
Some important points on molecular orbital diagrams are as follows:
- The Y-axis of a MO diagram represents the total energy of the orbitals.
- Individual atomic orbitals (AO) are arranged on the far left and far right of the diagram.
- Molecular orbitals formed by overlapping of atomic orbitals are located in the middle of the diagram; they overlap with either a sigma or a pi bond. With respect to their phases, they are designated as bonding, non-bonding or anti-bonding orbitals.
- According to Pauli’s exclusion principle, lower energy MOs are filled first, followed by consecutively higher energy orbitals.
15.5.2 Fundamental Steps for Constructing Molecular Orbitals
The following are common steps to derive simple homo nuclear and hetero nuclear MOs to construct more complicated, polyatomic diagrams.
- Find the electronic configuration of each atom of the molecule and the place on the atomic orbital for that atom.
- Determine if the molecule is homo nuclear or hetero nuclear; if the molecule is homo nuclear, the AOs will be symmetric; hetero nuclear AOs will slightly differ because the more electronegative atom will be placed lower on the diagram due to lone pairs of electrons being more stable on more electronegative elements, thus leading them to be lower in energy.
- Keep in mind the energy of the atomic and molecular orbitals; fill molecular orbitals using energy and bonding properties of the overlapping atomic orbitals. The following factors contribute to the position of one MO with respect to other MOs:
- More nodes = more energetic = higher MOs
- Sigma bond orbitals are stronger than pi bond orbitals
- Anti-bonding MOs are higher in energy than bonding MOs
- Constructive overlap = fewer nodes = more stable (less energetic)
- Destructive overlap = more nodes = less stable (more energetic)
- The number of individual atomic orbitals should be equal to the number of molecular orbitals. Use the MO diagram to predict the properties of the molecule like bond order, bond angle, paramagnetism, etc.
15.5.3 Five Basic Rules of Molecular Orbital Theory
The five basic rules of the MO theory are as follows:
- The number of molecular orbitals = the number of atomic orbitals combined
- Lower energy orbitals are bonding orbitals; higher energy orbitals are anti-bonding.
- Electrons first enter into the lowest available energy orbital.
- According to Pauli’s exclusion principle, the maximum number of electrons in an orbital is two.
- According to Hund’s rule, electrons spread out before pairing up.
Atoms or molecules in which electrons are paired are diamagnetically repelled by both poles of a magnet. Those that have one or more unpaired electrons are paramagnetically attracted to a magnetic field.
15.5.4 Linear Combination of Atomic Orbitals and Type of Atomic Orbitals
Molecular Orbital
According to Hund and Mullikan, a covalent bond is formed when two half-filled atomic orbitals (AO) of two atoms come nearer and then overlap each other to form a new bigger orbital known as a molecular orbital.
The two nuclei may approach each other along a line. When the nuclei come close, the two atomic orbitals of the two atoms combine to form two molecular orbitals. One is bonding and the other is the anti-bonding orbital.
Anti-bonding Orbital
The molecular orbital with higher energy gives rise to a repulsive state called anti-bonding orbital.
Bonding Orbital
The molecular orbital with lower energy gives rise to an attractive state called bonding orbital.
Non-bonding Orbital
These do not participate in a chemical bond. They are inner shell orbitals.
The forming of bonding and anti-bonding orbitals may be explained in terms of wave functions.
Let the wave functions of two atoms A and B be ψA and ψB respectively. These two atomic orbitals may combine in two ways.
- ψb = ψA + ψB (Bonding)
- ψa = ψA – ψB (Anti-bonding)

From the spectroscopic measurement, the increasing order of energies of molecular orbitals is decided in the following order.
σ1s < σ*1s < σ2s < σ*2s < σ2px < π 2py = π 2pz < π*2py = π*2pz < σ*2px (For homo nuclear atoms; atomic number more than seven and hetero nuclear atoms)
σ1s < σ*1s < σ2s < σ*2s < π 2py = π 2pz < σ2px < π*2py = π*2pz< σ*2px (For homo nuclear atoms; atomic number less than seven)
15.5.5 Molecular Orbital Energy Level Diagrams of Homo Atomic Molecules
Molecular Energy Level Diagrams of Homo Molecules
Hydrogen Molecule
Atomic number = 1
Electronic configuration = 1s1
Total number of electrons = 1 + 1 = 2
Total number of orbitals = 2(σ1sσ*1s)
Molecular electronic configuration = σ1s2
Energy Level Diagram of Hydrogen Molecule

Bond Order
![]()
Number of bonding electrons = 2
Number of anti-bonding electrons = 0
![]()
In hydrogen molecule, single bond exists between two atoms.
Magnetic Property
Here, there are no unpaired electrons. Hence, it shows diamagnetic property.
Helium Molecule
Atomic number = 2
Electronic configuration = 1s2
Total number of electrons = 2 + 2 = 4
Total number of orbitals = 2(σ1s and σ*1s)
Molecular electronic configuration =σ1s2σ*1s2
Energy Level Diagram of Helium Molecule
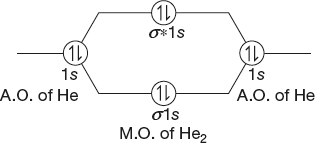
Bond Order
Number of bonding electrons = 2
Number of anti-bonding electrons = 2
![]()
So, Helium molecule does not exist and it is mono atomic.
Magnetic Property
Here, there is no unpaired electron and it is also diamagnetic.
Boron Molecule
Atomic number = 5
Electronic configuration = 1s22s22p1
Total number of electrons = 5 + 5 = 10
Total number of atomic orbitals = 5 + 5 = 10
The MO diagram for diboron requires the explanation of the p orbital overlap; three dumbbell-shaped p-orbitals have equal energy and are oriented mutually perpendicularly or orthogonally. The p-orbitals oriented in the x-direction (px) can overlap end-on end σ bonding (symmetrical) and an anti-bonding σ* molecular orbital. In contrast to the sigma 1s MOs, the σ 2p has some non-bonding electron density at either side of the nuclei and the σ* 2p has some electron density between the nuclei.
The other two p-orbitals, py and pz, can overlap sideways; the resulting bonding orbital has its electron density above and below the plane of the molecule. The orbital is not symmetric around the molecular axis and is therefore a σ orbital. The anti-bonding σ orbital asymmetrical has four lobes pointing away from the nuclei. Both py and pz orbitals form a pair of σ orbitals equal in energy (degenerate) and can have higher or lower energies than that of the sigma orbital.
There are 10 electrons and 10 orbitals; out of 10 electrons, four electrons occupy σ1s and σ*1s. These two are non-bonding orbitals. The next four electrons occupy σ 2s and σ*2s orbitals. Now, the 2p electrons are to be admitted into the molecular orbitals. According to the order of molecular orbital energies, the 2p electrons should occupy σ2px. If it is so, the molecule should be diamagnetic. However, boron is actually paramagnetic. To explain this, it is suggested that σ2py and σ2pz have to be occupied with single electrons. Hence, the molecular electronic configuration of boron is σ1s2 σ*1s2 σ2s2 σ*2s2 π 2![]() π 2
π 2![]()
Energy Level Diagram of Boron

Bond Order
Number of bonding electrons = 6
Number of anti-bonding electrons = 4
![]()
In boron molecule, in between two atoms, a single bond exists.
Magnetic Property
Here, there are two unpaired electrons. Hence, it shows paramagnetic property.
Bond length = 1.59Å, bond dissociation energy = 289 KJ/mole.
Carbon Molecule
Atomic number = 6
Electronic configuration = 1s2 2s2 2p2
Total number of electrons = 12
Total number of orbitals = 10
Molecular orbital electronic configuration
![]()
Energy Level Diagram of Carbon

Bond Order
Number of bonding electrons = 8
Number of anti-bonding electrons = 4
![]()
In a carbon molecule, in between two atoms, a double bond exists.
Magnetic Property
Here, there are no un-paired electrons. Hence, it shows diamagnetism.
Bond length = 1.31Å, Bond dissociation energy = 627.9 KJ/mole
Nitrogen Molecule
Atomic number = 7
Electronic configuration = 1s2 2s2 2p3
Total number of electrons = 14
Total number of orbitals = 10
The molecular electronic configuration is as follows:
![]()
The three σ*2px, σ*2py, σ*2pz anti-bonding orbitals are vacant. Hence, the molecule is highly stable.
Energy Level Diagram of Nitrogen Molecule

Bond Order
Number of bonding orbitals = 10
Number of anti-bonding orbitals = 4
![]()
Hence, triple bond exists in nitrogen molecule.
Magnetic Property
There are no unpaired electrons. Hence, it shows diamagnetism.
Bond length = 1.1Å, bond dissociation energy = 945.6 KJ/mole.
Oxygen Molecule
Atomic number = 8
Electronic configuration = 1s2 2s2 2p4
Total number of electrons = 16
Total number of orbitals = 10
Molecular electronic configuration
![]()
The MO energy level diagram of dioxygen is different from that of the previous diatomic molecules because the p σ MO is lower in energy than the 2π orbitals; this is due to the interaction between the 2s MO and the 2pz MO. Distributing eight electrons over six molecular orbitals leaves the last two electrons as a degenerate pair in the 2pp* anti-bonding orbitals, and the resulting bond order is 2.
Energy Level Diagram of Oxygen Molecule

Bond Order
Number of bonding electrons = 10
Number of anti-bonding electrons = 6
![]()
Hence, the oxygen molecule has double bond.
Magnetic Property
With two unpaired electrons, the oxygen molecule shows para magnetism.
Bond length = 1.21Å, bond dissociation energy = 494.6 KJ/mole
Fluorine Molecule
Atomic number = 9
Electronic configuration is 1s2 2s2 2p5
Total number of electrons = 18
Total number of orbitals = 10
Molecular electronic configuration
![]()
Energy Level Diagram of Fluorine Molecule

Bond Order
Number of bonding electrons = 10
Number of anti-bonding electrons = 8
![]()
Thus, fluorine molecule contains one covalent single bond.
Magnetic Property
As there are no unpaired electrons, the molecule is diamagnetic.
Bond length = 1.42Å, bond dissociation energy = 155 KJ/mole.
15.5.6 Molecular Energy Level Diagrams of Hetero Atomic Molecules
Carbon Monoxide Molecule
Atomic number of carbon = 6 (1s2 2s2 2p2)
Atomic number of oxygen = 8 (1s2 2s2 2p4)
Total number of electrons = 14
Total number of orbitals = 10
Molecular orbital configuration is
![]()
Energy Level Diagram of Carbon Monoxide Molecule

Bond Order
Number of bonding electrons = 10
Number of anti-bonding electrons = 4
![]()
In the CO molecule, triple bond is present.
Magnetic Property
No unpaired electrons are available; hence, it is diamagnetic.
Cyanide Ion (CN−)
Atomic number of carbon = 6 (1s2 2s2 2p2)
Atomic number of nitrogen = 7 (1s2 2s2 2p3)
Total number of electrons = 6 + 7 + 1 = 14 (1 is subtracted when there is a +ve charge; 1 is added when there is a –ve charge.)
Total number of orbitals = 10
Molecular electronic configuration is
![]()
Energy Level Diagram of Cyanide Ion

Bond Order
Number of bonding electrons = 10
Number of anti-bonding electrons = 4
![]()
∴ Triple bond exists in cyanide ion.
Magnetic Property
There are no unpaired electrons; hence, it shows diamagnetism.
Nitric Oxide (NO) Molecule
Atomic number of nitrogen = 7
Electronic configuration = 1s2 2s2 2p3
Atomic number of oxygen = 8
Electronic configuration is 1s2 2s2 2p4
∴ Total number of electrons = 7 + 8 = 15
Total number of orbitals = 10
Molecular electronic configuration is
![]()
Energy Level Diagram of Nitric Oxide Molecule
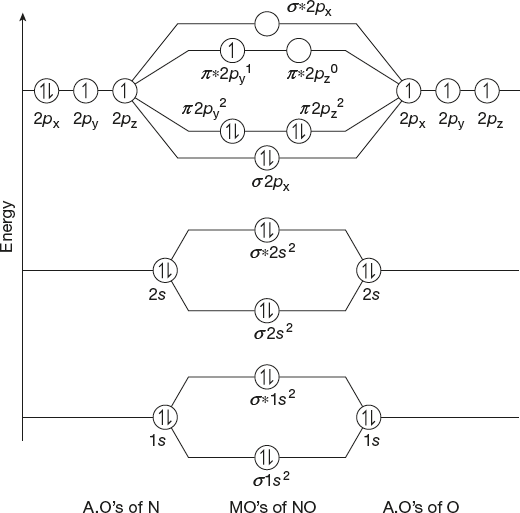
Bond Order
Number of bonding electrons = 10
Number of anti-bonding electrons = 5
![]()
In nitric oxide molecule, two bonds—σ bond and σ bond exist.
Magnetic Property
Here, there are unpaired electrons; hence, it is paramagnetic.
Nitric Oxide Ion (NO−)
Atomic number of nitrogen = 7
Electronic configuration is 1s2 2s2 2p3
Atomic number of oxygen = 8
Electronic configuration is 1s2 2s2 2p4
Total number of electron = 7 + 8 + 1 = 16
Molecular electronic configuration is
![]()

Bond Order
Number of bonding electrons = 10
Number of anti-bonding electrons = 6
![]()
∴ Nitric oxide ion has a double bond.
Magnetic Property
Here, there are two unpaired electrons. Hence, NO− molecules show para magnetism.
15.6 REVIEW QUESTIONS
15.6.1 Fill in the Blanks
- The mixing of 1s orbital 2p orbitals is __________ hybridisation
[Ans.: sp2]
- The bond angle observed in sp hybridisation is __________.
[Ans.: 180°]
- In sp3 hybridisation, the geometry of the molecules is __________
[Ans.: Tetrahedral]
- Structure and hybridisation of NH3 is __________
[Ans.: Pyramidal, sp3]
- SF6 involving __________ hybridisation and possess __________ geometry
[Ans.: sp3d2, octahedral]
- CH4 molecule exhibits __________ hybridisation.
[Ans.: sp3]
- The polarisation of one bond that is caused by the polarisation of an adjacent atom is called __________ effect.
[Ans.: inductive]
- The group or atom which has more power to attract the electron in the comparison of hydrogen is called __________ effect.
[Ans.: positive inductive]
- __________ effect is also known as mesomeric effect.
[Ans.: Resonance]
- Hoffmann reaction involves reaction intermediate __________
[Ans.: nitrene]

[Ans.:
 ]
]- Carbanions involving hybridisation are __________
[Ans.: sp3]
- Molecules having __________ involve addition reaction.
[Ans.: double or triple bonds]
15.6.2 Multiple-choice Questions
- The most stable radical is
[Ans.: d]
- The number of unshared electrons in carbine is
- 1
- 2
- 3
- 4
[Ans.: b]
- Nitrene is generated as an intermediate in
- Hoffmann rearrangement
- Curtius rearrangement
- Insertion
- All the above
[Ans.: d]
- Benzyne is generated from
- Chlorobenzene in presence of sodamide in liquid NH3
- Benzene with sodium in liquid ammonia
- Benzene in liquid ammonia
- Addition of heat on benzoic acid
[Ans.: c]
- The nucleophile attack on a carbon-carbon double bond generates
- Carbanion
- carbine
- Carbocation
- free radical
[Ans.: a]
- The peroxide effect occurs
- Free radical mechanism
- Haemolytic fission of double bond
- Heterolytic fission of double bond
- None of these
[Ans.: a]
- Benzyne is a
- 1,2 di hydro benzene
- 1,2 de hydro benzene
- 1,2,3,4 tetra hydro benzene
- Tridozenes
[Ans.: b]




- None of these
[Ans.: b]

[Ans.: a]

- Addition
- substitution
- Elimination
- rearrangement
[Ans.: a]
- The structure of benzene is
[Ans.: c]
15.6.3 Short Answer Questions
- What is hybridisation?
Ans.: The hypothetical intermixing of nearly the same energy atomic orbitals to give an entirely new identical equal energy orbitals is termed hybridisation. The combining of atomic orbitals which have different energy or nearly the same energy and the formation of new set of orbitals with equivalent energy and shape is known as hybrid orbital and this phenomenon is known as hybridisation.
- Write the types of hybridisation and its exhibiting geometrics.
Ans.: 1. SP–linear
2. Sp2 hybridisation–trigonal planar
3. Sp3–tetrahedral
4. Sp3d2–octahedral or square pyramidal
5. dsp2–square planar
- Describe inductive effect.
Ans.: The polarisation of one bond caused by the polarisation of an adjacent atom is called inductive effect. It is two types:
- Negative effect (-I effect)
- Positive effect (+I effect)
- Write note on electrometric effect.
Ans.: The effect involving the complete transfer of a shared pair of electron to one atom of a compound which is joined by a multiple bond (double (or) triple) at the requirement of attacking reagent is known as electrometric effect. It is indicated by E and js represented by a curved arrow
 showing the electron pair. It is a temporary effect.
showing the electron pair. It is a temporary effect.
It is two types:
- 1 +E effect
- -E effect
- Explain resonance or mesomeric effect.
Ans.: The development of polarity in a molecule as a result of interaction between two p-bonds or a p-bond and an atom or a group with a lone pair electrons is known as mesomeric effect or resonance.
- What do you mean by reactive intermediates? What are the types of intermediate?
Ans.: Short-lived and highly reactive fragments are called reactive intermediate. They are of the following types:
- Free radical
- Carbocation or cabonium ion
- Calbanion
- Calbene
- Nitrene
- Benzyne
- Write the reactions involving free radical intermediate.
Ans.: 1. Wurtz reaction
2. Anti-Markovnikov’s addition
3. Kolbe electrolysis
4. Substitution
- Write the formation of carbocation.
Ans.:
By Heterolysis process:

By protonation of alkenes or alcohol:

By decomposition of diazo compounds:

- Give different types of reactions?
Ans.: The types of reactions are as follows:
- Addition reaction
- Elimination
- Substitution
- Rearrangement
- Write a short note on substitute reaction.
Ans.: When an atom or a group attached to a carbon atom in substrate molecule is replaced by another atom or a group of atoms during the reaction and there is no change occur in carbon skeleton, it is called substation. It is of three types:
- Free radical substitution
- Nucleophilic substitution
- Electrophilic substitution
- Describe elimination reaction?
Ans.: In these reactions, generally, atoms or groups from two adjacent carbon atoms (α, β) in the substrate molecule are removed and a multiple bond is formed. This is called elimination reaction.
- Write the types addition reaction.
Ans.: Based on the involving reactive intermediates, the addition reaction is classified into three types.
- Free radical
- Nucleophilic addition
- Electrophilic addition
- Define bonding and anti-bonding molecular orbitals.
Ans.: Anti-bonding orbital: The molecular orbital with higher energy gives rise to a repulsive state called anti-bonding orbital.
Bonding orbital: The molecular orbital with lower energy gives rise to an attractive state called bonding orbital.
- Give the increasing energy order of molecular orbitals.
Ans.: σ1s < σ*1s < σ2s < σ*2s < σ2px < π 2py = π 2pz < π*2py = π *2pz < σ*2px (For homo nuclear atoms; the atomic number is more than seven)
σ1s < σ*1s < σ2s < σ*2s < π 2py = π 2pz < σ2px < π *2py = π *2pz < σ*2px (For homo nuclear atoms; the atomic number is less than seven)
- What is the bond order present in B2, C2 and N2?
Ans.: Boron molecule (B2) - 1
Carbon molecule (C2) -2
Nitrogen molecule (N2) -3
15.6.4 Descriptive Questions
Q.1 Give a detailed note on hybridisation and its salient features, important conditions and types.
Q.2 Explain the inductive effect with suitable examples.
Q.3 Describe electrometric effect and resonance effect with suitable examples.
Q.4 Describe the reactive intermediate in detail.
Q.5 Write a note on the types of reactions. Explain all reactions with examples.
Q.6 Give a brief note on molecular orbital theory and its importance.
Q.7 Explain molecular orbital diagrams of N2 and O2 molecules. What is the difference in their molecular energy levels?
Q.8 Explain the molecular energy diagrams of CO and NO.