1
ESTIMATION OF MAGNESIUM BY EDTA METHOD
AIM
To estimate the amount of magnesium present in 100 ml of the given solution using approximately 0.01 M ethylenediamine tetraacetic acid (EDTA) solution.
PRINCIPLE
The estimation of magnesium is based on complexometric titration. Magnesium ions in ammonia solution form a wine red colour complex with Eriochrome Black T (EBT) indicator. When the EDTA solution is added, it reacts with the magnesium ions and releases the free indicator. The colour of the solution changes from wine red to blue, which indicates the end point of titration.

EDTA metal ion complex is stable at pH = 10.
PROCEDURE
Part A: Preparation of Standard Magnesium Sulphate Solution
Take approximately 0.25 g of magnesium sulphate crystals in a clean and dry weighing bottle. Weigh the bottle along with the magnesium sulphate crystals accurately up to the fourth decimal place using the rider in an analytical balance. Then, carefully transfer the chemical substance into a clean 100 ml standard flask with the help of a glass funnel. After the transfer, weigh the empty bottle accurately. The difference between these two values gives the weight of magnesium sulphate. Dissolve the magnesium sulphate crystals in a minimum quantity of distilled water and make it up to the mark. Shake the standard flask well to obtain uniform concentration and keep aside the solution for further use.

Weight of magnesium sulphate + weighing bottle (W1) = _____ g
Weight of empty weighing bottle (W2) = _____ g
Weight of magnesium sulphate (W) = (W1 − W2) = _____ g
Gram molecular weight (GMW) of MgSO4 · 7H2O = 246.47

Part B: Standardisation of EDTA Solution
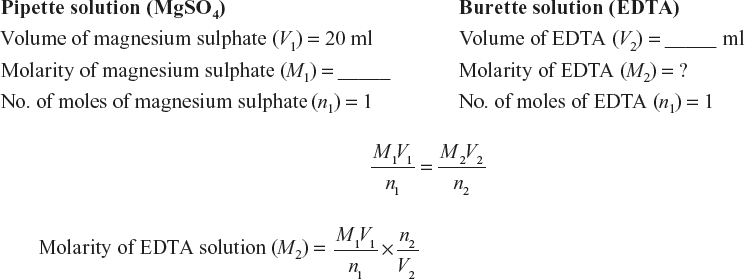
Clean a burette with tap water and then rinse it with distilled water as well as the EDTA solution. Then, fill it with the EDTA solution and note down the initial reading. Pipette out 20 ml of the standard magnesium sulphate solution into a clean conical flask. Add 8 to 10 ml of ammonical buffer solution and two to three drops of the EBT indicator. The solution turns to wine red colour. Titrate this solution mixture against the EDTA solution taken in the burette until the colour changes from wine red to blue, which indicates the end point of titration. Note down the volume of EDTA run down. Repeat the titration until two consecutive concordant values are obtained. Note down the values in a tabular column. From the titre values, calculate the strength of EDTA.

Part C: Estimation of Magnesium
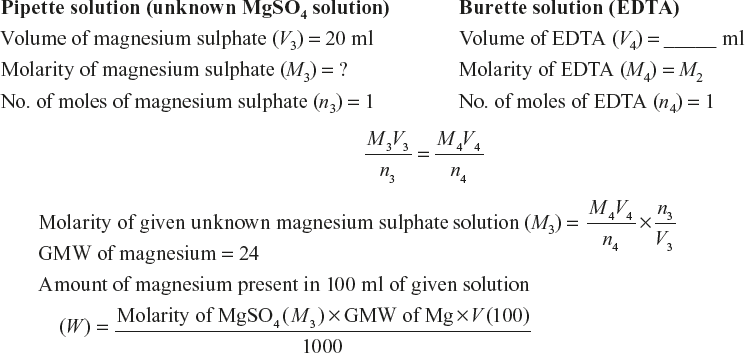
Make up to the mark the given unknown magnesium ion solution by adding distilled water and shake the resulting solution well to get uniform concentration. Fill the burette with the EDTA solution and note down the initial reading. Pipette out 20 ml of the unknown magnesium sulphate solution into a clean conical flask. Then, add 8 to 10 ml of ammonical buffer solution and two to three drops of the EBT indicator. The solution turns to wine red colour. Titrate the solution against the EDTA solution until the colour changes from wine red to blue, which indicates the end point of titration. Repeat the titration until two consecutive concordant values are obtained. Calculate the molarity of the magnesium ion solution and the amount of magnesium present in the whole of the given 100 ml solution from the titre values.

RESULT
Molarity of the magnesium sulphate solution (M1) = _____ M
Molarity of the EDTA solution (M2) = _____ M
Molarity of the unknown magnesium sulphate solution (M3) = _____ M
Amount of magnesium present in 100 ml of the given solution (W) = _____ g
VIVA QUESTIONS
- What is complexometric titration?
- What is the role of the EBT indicator?
- Expand EDTA.
- Give the structure of EDTA.
- What is the end point of titration in this experiment?
- What is the basic principle involved in this experiment?
- Define molarity.
- What is the GMW of MgSO4 ·7H2O?
