Pollutants and contaminants
7.1 Guidance
7.1.1 What are pollutants and contaminants?
Over the last 20 years environmental matters have become an area of widespread public concern particularly those concerning the issues of pollution and contaminants. Pollutants and contaminants come in many forms and can have an effect on the air, land or water courses and as pollutants move from one medium to another they may be deposited on equipment and equipment housing. They can cause extensive damage.
Pollution of the air can occur in both the troposphere and stratosphere. In the troposphere pollutants from chimneys (for example) are carried by the air and can be deposited over time and distance, thus having a limited life span before they are washed out or deposited on the ground, as shown in Figure 7.1. If pollutants are injected straight into the stratosphere (as with a volcanic eruption) they will remain there for some time and result in noticeable effects over the whole region.
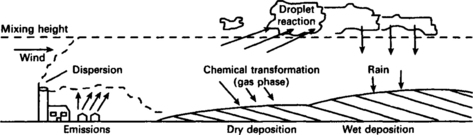
Fig. 7.1 Process involved between the emission of air pollutants and their being deposited on the ground
The roughness of the ground produces turbulence in the air promoting the mixing of pollutants. In general, low wind speeds result in high pollutant concentrations.
Chemical pollution is the introduction of substances into the environment (by man) that are liable to harm human health, living resources and ecological systems, damage structures or generally interfere with the legitimate use of the environment.
Sources of natural pollutants include:
• sulphur – emitted by volcanoes and from biological processes;
• nitrogen – from biological processes in the soil, lightning and biomass burning;
• hydrocarbons – methane from the fermentation of rice paddies, or from fermentation of the digestive tract of ruminants (e.g. cows). Hydrocarbons are also released by insects, coal mining and gas extraction.
Sources of man-made pollutants include:
• carbon dioxide and carbon monoxide produced during the burning of fossil fuels;
• soot formation accompanied by carbon monoxide, which is generally due to inadequate or poor air supply;
• hydrocarbons. Most boilers and central heating units burning fossil fuels result in very low emissions of gaseous hydrocarbons or oxygenated hydrocarbons such as aldehydes.
7.1.2 Introduction
Satisfactory performance during the desired lifetime of equipment depends on many parameters: some are determined by their design, others by the environment in which they have to function. Whilst the design of the equipment can be moderated and/or controlled, special attention has to be paid to polluting and contaminating substances within the atmosphere.
7.1.2.1 Pollutants
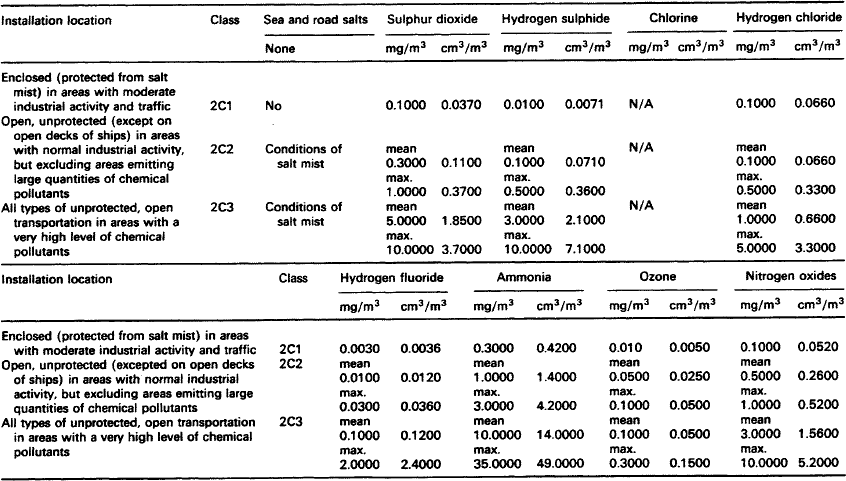
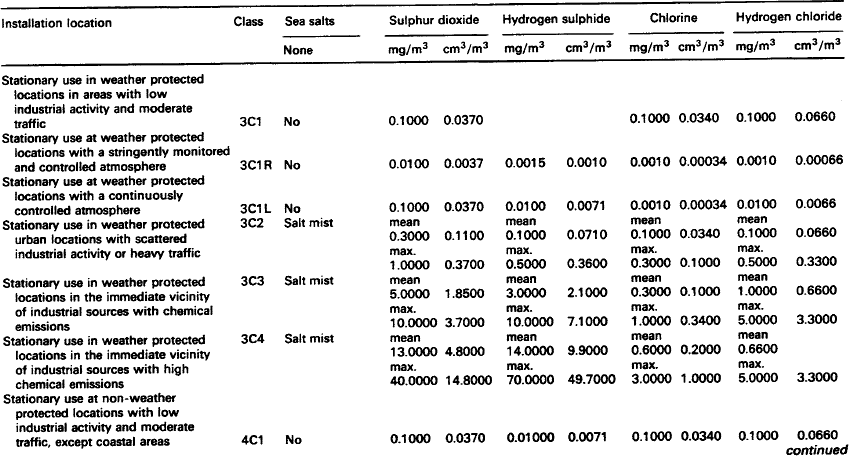
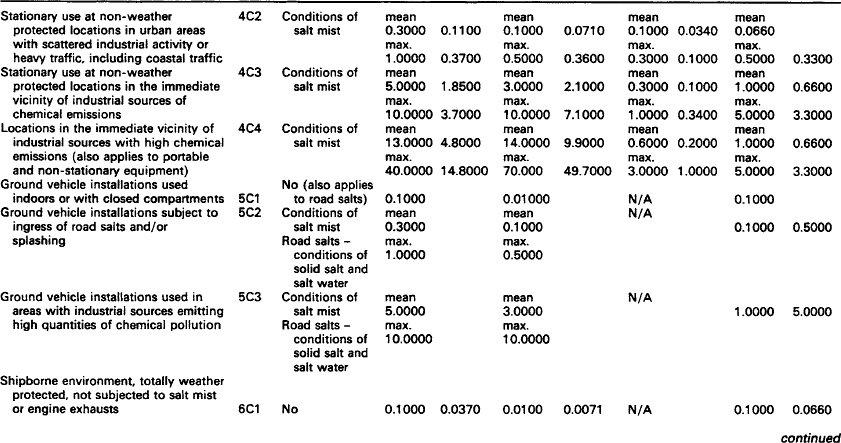
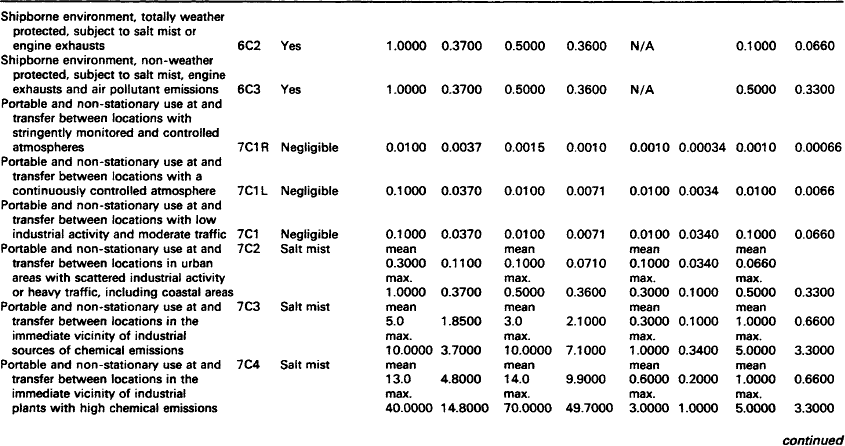
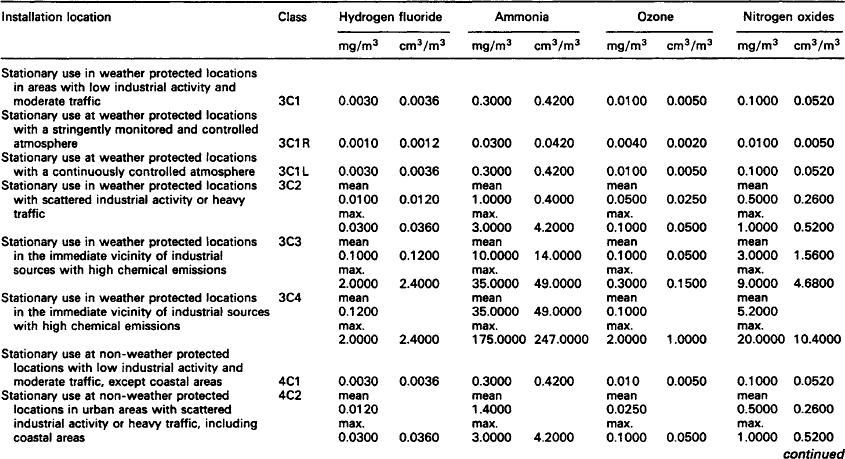
Although pollutant gases are normally only present in low concentrations, they can cause significant corrosion and a marked deterioration in performance of contacts and connectors. The gases in operating environments which cause corrosion are oxygen, water vapour and the so-called pollutant gases which include sulphur dioxide, hydrogen sulphide, nitrogen oxides and chlorine compounds.
Silver and some of its alloys are particularly susceptible to tarnishing by the minute quantities of hydrogen sulphide that occur in many environments. The tarnished product is dark in colour and consists largely of b-silver sulphide. Separable electrical connections employing these materials may, therefore, suffer from increased resistance and contact noise as a result.
The amount of tarnishing (of a metal) is dependent upon the amount of humidity present. Less corrosion occurs below 70% relative humidity (RH) but above 80% RH the rate of tarnishing increases rapidly. Temperature also has an effect on the amount of tarnishing as the nature of the corrosion mechanism has a tendency to change at temperatures above 30°C.
7.1.2.1.1: Hydrogen sulphide Hydrogen sulphide is caused by the bacterial reduction of sulphates in vegetation, soil, stagnant water and animal waste on a worldwide basis. In the atmosphere, hydrogen sulphide is oxidised to sulphur dioxide which, in turn, is brought to the ground by rain. In an aerobic soil, bacteria turns the sulphur dioxide to sulphates. Sulphate reducing bacteria complete the cycle and turn the sulphates to hydrogen sulphide which is the principal natural sulphur input in the atmosphere and is, therefore, a widespread pollutant of air.
7.1.2.1.2: Sulphur dioxide Sulphur dioxide is the pollutant gas most commonly found in the atmosphere and is usually present in high concentrations in urban and industrial locations. In combination with other pollutants and moisture (e.g. humidity) sulphur dioxide is responsible for the formation of high resistance, visible corrosion layers on all but the most noble metals (e.g. silver and gold) and alloys.
Note: Sulphur dioxide has little effect on silver unless both the concentration of the gas and humidity are high.
Whilst sulphur dioxide also occurs in volcanic emissions, it originates from both natural and man-made sources. Worldwide, natural sources predominate, but in urban and industrial areas the man-made sources prevail. The principal man-made source of sulphur dioxide is the combustion of fossil fuels which also releases other gases such as sulphur trioxide, nitrogen oxides and chlorine compounds. These other combustion products, although usually only present in low concentrations, will corrode most metals and alloys.
In urban areas, burning fossil fuels emits sulphur dioxide into the atmosphere where, unless it is rinsed with rain, it will accumulate. The content can be 10 times to 1000 times that of hydrogen sulphide and will become the dominant cause of corrosion. It should be noted, however, that in equal concentrations, hydrogen sulphide is far more corrosive than sulphur dioxide particularly on silver and copper.
Although the major input of the sulphur cycle is by hydrogen sulphide through natural processes, industrial processes also play a significant part – particularly oil refineries, chemical plants and gas works.
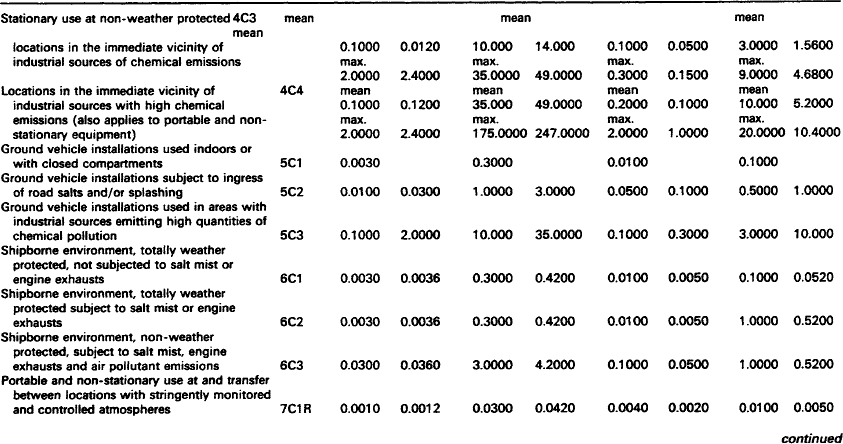
Table 7.1 lists representative concentrations of sulphur dioxide at a range of locations. These levels are sufficient to account for the natural tarnishing of silver. Other sulphurous pollutants are less important.
Table 7.1
Representative concentrations of sulphur dioxide at a range of locations (extracted from a paper (Ref. 1) by kind permission of Herne Consultancy Group)

Some organic sulphur derivatives do tarnish silver, as does elemental sulphur vapour, but these materials probably occur only in small amounts in the atmosphere. The two most common organic sulphurous pollutants are methyl mercaptan and carbon disulphide but these do not tarnish silver.
Although sulphur dioxide alone is less corrosive than other gases (such as sulphur trioxide, nitrogen oxides and chlorine compounds), the most extensive corrosion occurs when combustion products are present together with sulphur dioxide.
7.1.2.2 Contaminants
Contaminants are composed of dust, sand, smoke and other particles that are contained within the air.
Dust, sand and smoke can have an effect on products in various ways, especially:
• ingress of dust into enclosures and encapsulations;
• deterioration of electrical characteristics (e.g. faulty contact, change of contact resistance);
• seizure or disturbance in motion bearings, axles, shafts and other moving parts;
• surface abrasion (erosion and corrosion);
• reduction in thermal conductivity;
• clogging of ventilating openings, bushes, pipes, filters and apertures that are necessary for operation, etc.
The presence of dust and sand in combination with other environmental factors such as water vapour can also cause corrosion and promote mould growth. Damp heat atmospheres cause corrosion in connection with chemically aggressive dust, and similar effects are caused by salt mist. The effects of ion conducting and corrosive dusts (e.g. de-icing salts) also need to be considered.
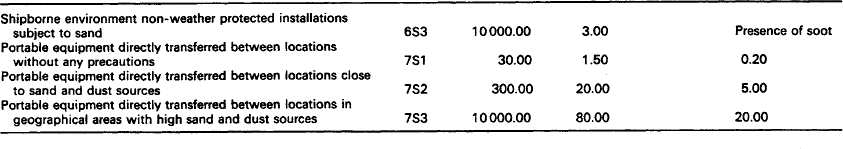
7.1.2.2.1: Dust and sand The concentration of dust and sand in the atmosphere varies widely with geographical locality, local climatic conditions and the type and degree of activity taking place. The amount of dust and sand found in the air is dependent on terrain, wind, temperature, humidity and precipitation. Under certain conditions enormous amounts of dust and sand may be temporarily released and this suspended dust will drift away with the wind (see Table 7.2) depending on the concentration and the size of the particles.
Table 7.2
Concentration of dust and sand (extracted with permission from a paper (Ref. 1) by Herne Consultancy Group)

Particles larger than 150 μm are generally confined to the air layer within the first metre above ground. Within this layer about half of the sand grains move within the first 10 mm above the surface and the remainder within the first 100 mm.
The dust and sand appearing in enclosed and sheltered locations is generated by several sources (e.g. quartz, de-icing salts, fertilisers, etc.) and penetrates into locations via ventilating ducts or badly fitting windows. The dust may also come from cloth or carpets in normal use in the working environment.
7.1.2.2.1.1: Dust The dust found in and around electrotechnical products may be generated by several different sources. The dust may be quartz, coal, de-icing salts, fertilisers, small fibres from cotton or wool (real or artificial) that has been generated from cloth or carpets by normal use in living rooms and offices.
Primary effects: The dust itself can have one or more of the following harmful effects:
Secondary and combined effects:
Dust may be defined as ‘particulate matter of unspecified origin, composition and size ranging from 1 μm to 150 μm originating from quartz, flour, organic fibres, etc.’. Particles of less than 75 μm, because of their low terminal velocity, can remain suspended in the atmosphere for very long periods through the natural turbulence of the air. In sheltered and enclosed locations, the maximum grain size tends to be smaller (e.g. less than 100 μm) than non-weather protected locations due to the filtering effect of the shelter.
Dust and sand can act either as a physical agent, chemical component, or both, in causing the deterioration of materials or functions of equipment. Dust can also act as an unwanted abrasive on moving components of machinery which may accelerate corrosive action as well as affecting electrical property.
Whilst dust and sand can accelerate the corrosion of metallic surfaces by removing protective coatings or by disturbing semi-protective films of corrosion products, the degree of surface abrasion will depend on the velocity of the particles hitting the surface.
Note: A marked deterioration in the optical quality of aircraft windscreens has been reported after test flights at heights of 60 m and speeds of between 290 m/s and 320 m/s over the North African deserts.
Sand and the majority of dusts which are deposited on insulated surfaces are poor conductors in the absence of moisture. The presence of moisture, however, will result in the dissolving of the soluble particles and the formation of conducting electrolytes. For example, the leakage currents flowing over contaminated power line insulators can be of the order of one million times greater than those which flow through clean, dry insulators.
Dust adhering to the surface of materials may contain organic substances that provide a source of food for micro-organisms.
A reduction in heat transfer rates can be caused by the formation of insulating layers and can lower the efficiency of cooling systems.
7.1.2.2.1.2: Sand Sand is the term applied to ‘segregated unconsolidated accumulation of detrital sediment, consisting mainly of tiny broken chips of crystalline quartz or other mineral, between 100 μm and 1000 μm in size’. Particles greater than 150 μm are unable to remain airborne unless continually subjected to strong winds, induced air flows or turbulence. Sand is generally harder than most fused silica glass compositions and can, consequently, scratch the surface of most glass optical devices. Pressure applied over trapped grains of sand can cause fractures to occur in equipment.
The electrostatic charges produced by friction of the particles in sand storms can interfere with the operation of equipment and sometimes be dangerous to personnel. The breakdown of insulators, transformers and lightning arresters, and the failure of car ignition systems has been known to occur as a result of such charges. The electrostatic voltages produced can be large. Voltages as high as 150 kV have made telephone and telegraph communications inoperable during sand storms.
Quartz, because of its hardness, can result in rapid wear or damage to products, particularly moving parts. However, erosion of material requires that the presence of dust and sand is combined with a high velocity air stream over an extensive period of time.
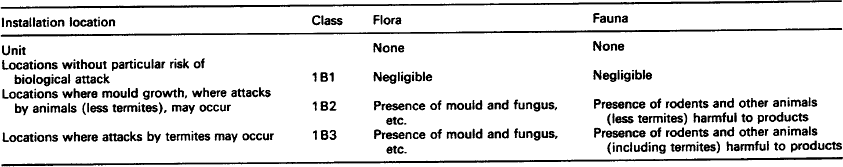
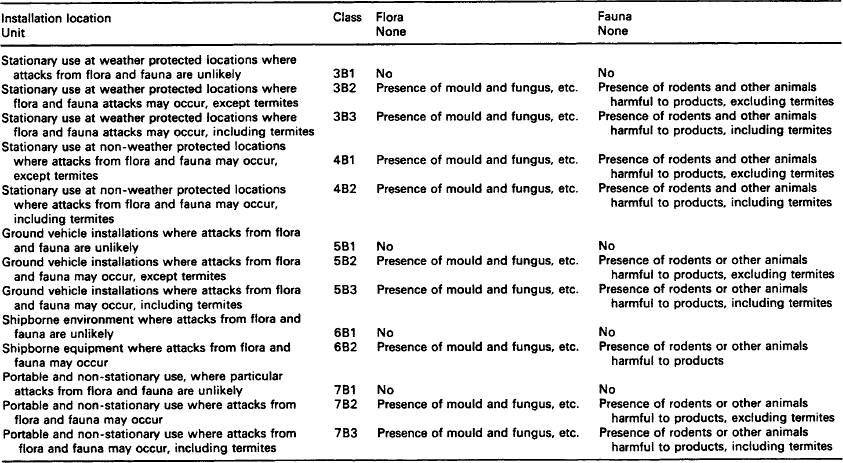
7.1.2.3 Fauna and flora
With a few exceptions, fauna (rodents, insects, termites, birds, etc.) and flora (plants, trees, seeds, fruit, blossom, mould, bacteria and fungi, etc.) may be present at all locations where equipment is stored, transported or used. Whilst fauna may be the cause of damage inside buildings as well as open-air locations, damage by flora will predominantly occur in open-air conditions. Moulds and bacteria can, however, be present both inside buildings and in open-air conditions.
The concentration of this flora and fauna depends on temperature and humidity. In warm, damp climates, fauna and flora, especially insects and micro-organisms such as mould and bacteria, will find favourable conditions for life. Humid or wet rooms in buildings, or rooms in which processes produce humidity, are suitable living spaces for rodents, insects and micro-organisms. The range of temperature in which moulds may grow is from 0°C to 40°C, and the most favourable temperatures for many cultures is between 20°C and 30°C.
If surfaces of the products carry layers of organic substances (e.g. grease, oil, dust), or deposits of animal or vegetable origin, such surfaces are ideal locations for the growth of moulds and bacteria.
7.1.2.3.1: Effects of flora and fauna Fauna and flora can affect equipment (during storage, transportation and/or use) in various ways, the most important being deterioration by mechanical forces and deterioration by deposits.
7.1.2.3.1.1: Deterioration by mechanical forces The functioning of equipment and materials can be affected by physical attacks of fauna. Small animals and insects that feed from, gnaw at, eat into and chew at materials are particular problems as are termites cutting holes into material.
Materials such as wood, paper, leather, textiles, plastics (including elastomers) and even some metals such as tin and lead are all susceptible to attack.
Larger animals can also cause damage by stroke, impact or thrust. These attacks can cause:
7.1.2.3.2: Deterioration by deposits Deposits from fauna (especially insects, rodents, birds, etc.) can be caused by the presence of the animal itself, nest building, deposited feed stocks and metabolic products such as excrement and enzymes, etc.
Deposits from flora may consist of detached parts of plants (leaves, blossom, seeds, fruits, etc.) and the growth layers of cultures of moulds or bacteria. These attacks can lead to:
These in turn can cause an interruption of electrical circuits, malfunctioning of mechanical parts and clouding of optical surfaces (including glass).
7.1.2.4 Mould
Mould can cause unforeseen deterioration of assembled specimens, whether constructed from mould resistant materials or not.
Fungi grows in soil and in, or on, many types of common material. It propagates by producing spores which become detached from the main growth and later germinate to produce further growth.
The spores are very small and easily carried by the wind (or moving air). They also adhere to dust particles carried in the air.
Contamination can also occur due to handling. Spores may be deposited by the hands or in the film of moisture left by the hands.
7.1.2.4.1: Germination and growth Moisture is essential in allowing the spores to germinate and when a layer of dust or other hydrophilic material (i.e. moisture retaining) is present on the surface, sufficient moisture may be abstracted by it from the atmosphere.
In addition to high humidity, spores require (on the surface of the specimen) a layer of material that will absorb the moisture. Mould growth is also encouraged by stagnant air spaces and lack of ventilation.
When the relative humidity is below 65%, no germination or growth will occur. The higher the relative humidity above this value, the more rapid the growth will be. Spores can survive long periods of very low humidity and even though the main growth may have died, they will germinate and start a new growth as soon as the relative humidity becomes favourable again (i.e. in excess of 65%). The optimum temperature of germination for the majority of moulds is between 20°C and 30°C
7.1.2.4.2: Effects of mould growth
7.1.2.4.2.1: Primary effects Moulds can live on most organic materials, but some of these materials are much more susceptible to attack than others. Mould growth normally occurs only on surfaces exposed to the air, and those which absorb or adsorb moisture will generally be more prone to attack.
Even where only a slightly harmful attack on a material occurs, the formation of an electrically conducting path across the surface due to a layer of wet mycelium (i.e. the vegetative part of fungus) can drastically lower the insulation resistance between electrical conductors supported by an insulation material. When the wet mycelium grows in a position where it is within the electromagnetic field of a critically adjusted electronic circuit, it can cause a serious variation in the frequency/impedance characteristics of the circuit.
Among the materials very susceptible to attack are leather, wood, textiles, cellulose, silk and other natural materials. Most plastic materials are less susceptible, but are also attacked. Plastic materials may contain non-polymerised monomers, oligomers and/or additives which may exude to the surface and be a nutrient for fungi. A copious growth may occur on the surface where these secondary materials are exposed. Some plastic materials depend, for a satisfactory life span, on the presence of a plasticiser which, if it is readily digested by fungi, will eventually give rise to failure of the main material.
Mould attack on materials usually results in a decrease of mechanical strength and/or changes in other physical properties.
7.1.2.4.2.2: Secondary effects The growing mould on the surface of a material can yield acid products and other electrolytes which will cause a secondary attack on the material. This attack can lead to electrolytic or ageing effects, and even glass can lose its transparency due to this process. Oxidation or decomposition may be facilitated by the presence of catalysts secreted by the mould.
7.1.2.4.3: Prevention of mould growth All insulating materials used should be chosen to give as great a resistance to mould growth as possible, thus maximising the time taken for mycelium to grow and minimising any damage to the material consequent upon such growth.
The use of lubricants during assembly (e.g. varnishes, finishes, etc.) is frequently necessary in order to obtain the required performance or durability of a product. Such materials should be chosen with regard to their ability to resist mould growth, for even though it can be shown that the lubricants do not support mould growth, they may collect dust which in turn will support mould growth.
Moisture traps which might possibly be formed during the assembly of equipment and in which mould can grow should be avoided. Examples of such less obvious traps are between unsealed mating plugs and sockets, or between printed circuit cards and edge connectors in particular attitudes. Other preventives of mould growth include:
• complete sealing of the equipment in (and with) a dry, clean atmosphere;
• continuous heating within an enclosure can ensure a sufficiently low humidity;
• operation of equipment within a suitable controlled environment;
Where the material and functioning of the equipment allows such treatment, ultraviolet radiation or ozone may be used for sterilisation. Air currents of adequate velocity flowing over the parts can retard the development of mould growth and acaricides (i.e. mites and ticks) can be used to control the action of mites.
7.1.3 Test standards
All proposed equipment, components or other articles should be tested in their production configuration without the use of any additional external devices that have been added expressly for the purpose of passing these tests.
When tested, the sample (components, equipment or other article) shall perform as stipulated in the procuring specification and over the designated temperature range.
The following are the most common tests used for checking the effect of pollutants and contaminants:
| IEC 68.2.10 | Basic environmental testing procedures – Test J and guidance – Mould growth |
| IEC 68.2.42 | Basic environmental testing procedures – Test Kc: Sulphur dioxide test for contacts and connections |
| IEC 68.2.43 | Basic environmental testing procedures – Test Kd: Hydrogen sulphide test for contacts and connections |
| IEC 68.2.45 | Basic environmental testing procedures – Test Xa and guidance – Immersion in cleaning solvents |
| IEC 68.2.68 | Basic environmental testing procedures – Dust and sand |
| ISO 9225 | Corrosion of metals and alloys – Corrosivity of atmospheres – Measurement of pollution |
7.1.4 Other related standards and specifications
| IEC 34.5 | Rotating electrical machines – Classification of degrees of protection provided by enclosures for rotating machines |
| IEC 68.2.1 I | Basic environmental testing procedures – Test Ka: Salt mist |
| IEC 68.2.17 | Basic environmental testing procedures – Test Q: Sealing |
| IEC 68.2.18 | Environmental testing – Test R and guidance: Water |
| IEC 68.2.3 | Basic environmental testing procedures – Test Ca: Damp heat, steady state |
| IEC 68.2.52 | Basic environmental testing procedures-Test Kb: Salt mist, cyclic (sodium chloride solution) |
| IEC 144 | Degrees of protection of enclosures for low voltage switchgear and controlgear |
| IEC 529 | Classification of degrees of protection provided by enclosures |
| IEC 721.1 | Classification of environmental conditions – Environmental parameters and their severities |
| IEC 721.2.3 | Classification of environmental conditions – Environmental conditions appearing in nature – Air pressure |
| IEC 721.2.5 | Classification of environmental conditions – Environmental conditions appearing in nature – Dust, sand, salt mist |
| IEC 721.2.7 | Classification of environmental conditions – Environmental conditions appearing in nature – Fauna and flora |
| IEC 721.3.4 | Classification of environmental conditions – Classification of groups of environmental parameters and their severities – Stationary use at non-weather protected locations |
| ISO 14001 | Environmental management systems – Specification with guidance for use |
7.2 Typical examples of contract requirements – pollutants and contaminants
The requirement for equipment to conform to various environmental specifications is becoming commonplace in today’s contracts. More and more specifications are being used to describe the various conditions that equipment is likely to experience when being used, stored or whilst in transit.
The following are the most common environmental requirements found in modern contracts concerning pollutants and contaminants.
7.2.1 Pollutants
• Although the severity of pollution will depend upon the location of the equipment, the effects of pollution have to be considered in the design of equipment and components.
• Facilities need to be provided to reduce pollution by the effective use of protective devices. In most cases the protection against water and solid objects is normally specified using the protection degree as defined in IEC 529.
• In all cases the requirements of ISO 14001 regarding environmental protection and the prevention of pollution have to be met.
7.2.2 Contaminants
Regarding contaminants, most contracts will require consideration of the following:
• chemically active substances as specified in IEC 721.3.1 to IEC 721.3.7 inclusive to include:
• biologically active substances as specified in IEC 721.3.1 to IEC 721.3.7 inclusive;
• flora and fauna as defined in IEC 721.2.7;
• dust as defined in IEC 721.2.5;
• sand if specified for the application with ranges and values taken from IEC 721.3.1 to IEC 731.3.7 inclusive.
7.2.3 Mould
• In an assembled state, equipment needs to operate when exposed to airborne mould spores and within climates that will be conducive to the growth of moulds.
• Insulating materials have to be chosen to provide as much resistance to mould growth as possible and all materials used shall be chosen with regard to their ability to resist mould growth.
7.2.4 Tests
Test methods for determining the suitability of a specimen shall include:
| IEC 68.2.10 | Environmental testing procedures – Test J and guidance: Mould growth |
| IEC 68.2.42 | Environmental testing procedures – Test Kc: Sulphur dioxide test for contacts and connections |
| IEC 68.2.43 | Environmental testing procedures – Test Kd: Hydrogen sulphide test for contacts and connections |
| IEC 68.2.45 | Environmental testing procedures – Test Xa and guidance: Immersion in cleaning solvents |
| IEC 68.2.68 | Environmental testing procedures – Dust and sand |
| ISO 9225 | Corrosion of metals and alloys – Corrosivity of atmospheres – Measurement of pollution |
7.4 Tests
This section details some of the test standards which may be applied to equipment and contains:
• a list of the most used environmental tests that a purchaser will normally require a manufacturer to adhere to;
Note: Full details of each of these recommended (sometimes mandatory) tests are contained in the relevant ISO, IEC or other standard. A full list of these standards is supplied in the reference section of this book. Copies of all these standards may be obtained from any National Standards Organisation.
7.4.1 Mould growth (IEC 68.2.10 Test J)

7.4.1.1 Introduction
Surface contamination in the form of dust, splashes, condensed volatile nutrients or grease may be deposited on equipment. When that equipment is exposed (in use, storage or transportation) to the atmosphere, and without proper protective covering, mould growth will occur.
7.4.1.2 Purpose of this test
The purpose of this test is to investigate unforeseen causes of deterioration in assembled specimens, whether constructed from mould resistant materials or not.
7.4.1.3 General conditions
The following is a list of the cultures (or spores) most commonly found:
• Aspergillus niger – grows profusely on many materials and is resistant to copper salts;
• Aspergillus terreus – attacks plastic materials;
• Aureobasidium pullulons – attacks paints and lacquers;
• Poecilomyces variotii – attacks plastics and leather;
• Penicillium funiculosum – attacks many materials especially textiles;
• Penicillium ochrochloron – resistant to copper salts and attacks plastics and textiles;
• Scopulariopsis brevicaulis – attacks rubber;
• Trichoderma viride – attacks cellulose textiles and plastics.
Caution: It is the opinion of mycologists and pathologists that conducting a mould growth test can constitute a health hazard. Inhalation of mould spores and the possibility of them coming in contact with the skin (e.g. around the finger nails) is a potential hazard unless special precautions are taken. Details of these precautions are contained in Appendices attached to the IEC 68.2.10 standard.
7.4.1.4 Test conditions
IEC 68.2.10 describes a method for investigating unforeseen causes of deterioration in assembled specimens, whether constructed from mould resistant materials or not. It may be used to assess the extent to which mould will grow and/or the operational deterioration which may be expected from this source.
There are two basic types of test:
7.4.1.4.1: Variant 1 Variant 1 specifies direct inoculation of the specimen with the mould spores. The extent of mould growth is assessed after 28 days’ incubation (or in some cases 84 days’) and this is probably the most frequently used form of test.
7.4.1.4.2: Variant 2 Variant 2 specifies the pre-conditioning of the test specimen with nutrients which support mould growth. This variant is employed in order to assess the secondary effects of mould growth on materials but can also be used to assess the effectiveness of fungicide treatment on equipment. This test is not suitable for simulating the conditions of a very intensive surface contamination (e.g. due to large quantities of organic dust or dead insects).
Details of possible health hazards (when conducting these tests), safety precautions, decontamination procedures together with a flow chart depicting the various test stages are contained in Appendices to IEC 68.2.10.
7.4.2 General corrosion tests (IEC 68.2.42 and IEC 68.2.43 Tests Kc and Kd)
7.4.2.1 Introduction
These general corrosion tests are particularly suitable for providing information on a comparative basis for atmospheres polluted with sulphur dioxide and/or atmospheres containing hydrogen sulphide.
7.4.2.2 Purpose of these tests
7.4.2.2.1: IEC 68.2.42 (Kc) The purpose of this test is to assess the corrosive effects of atmospheres containing sulphur dioxide on the contact properties of precious metal, or precious metal covered contacts, excluding contacts consisting of silver and some of its alloys.
7.4.2.2.2: IEC 68.2.43 (Kd) The purpose of this test is to:
• provide a method for assessing the effects of atmospheres containing hydrogen sulphide on the contact properties of contacts made of silver, silver alloy, silver protected with another layer and other metals covered with silver or silver alloy;
• check wrapped or crimped connections made of the same materials as mentioned above with particular reference to their tightness or effectiveness.
7.4.2.3 General conditions
These tests are not suitable as general corrosion tests as they may not necessarily predict the behaviour of contacts and connections in industrial atmospheres. They are particularly suitable for providing information on a comparative basis and are intended to provide an accelerated means to assess the effects of tarnishing of silver and silver alloys used for contacts and crimped connections.
7.4.2.4 Test conditions
7.4.2.4.1: IEC 68.2.42 (Kc) Test Kc (Sulphur dioxide test for contacts and connections) provides an accelerated means to assess the corrosive effects on contacts and connections of atmospheres polluted with combustion products. It is particularly suitable for giving information of a comparative basis but it is not suitable as a general purpose corrosion test. The standard provides the reader with detailed instructions on the composition of the atmosphere within the test chamber, schematic drawings of the apparatus required to generate the test conditioning atmosphere (see Figure 7.2) and a schematic flow diagram.

Fig. 7.2 Schematic drawing of apparatus for the generation of a conditioning atmosphere (reproduced from the equivalent standard BS 2011:PT2.1Kc (1991) by kind permission of the BSI)
Temperature does not affect the rate or degree of corrosion occurring in the test to any marked degree. However, as temperature and relative humidity are intimately related and as the latter exerts a marked influence on the degree and nature of corrosion of the test, it is essential that the test temperature is closely controlled to enable the relative humidity to be held within the specified limits and produce the required test severity.
Relative humidity has a greater effect on the test severity than the difference in concentration of sulphur dioxide and temperature. The corrosion rate is relatively low at relative humidities below 70%. Corrosion is markedly accelerated, and the nature and properties of the corrosion products change considerably, at relative humidities above 85% (see Figure 7.2).
7.4.2.4.2: IEC 68.2.43 (Kd) Test Kd (Hydrogen sulphide test for contacts and connections) provides details of tests aimed at assessing the effects of atmospheres containing hydrogen sulphide on the contact properties of contacts (and wrapped or crimped connections) made of silver, silver alloy, silver protected with another layer and other metals covered with silver or silver alloy. The major criteria of this test is to determine the change in contact resistance caused by exposure to the hydrogen sulphide containing atmosphere. The tests have been devised to assess the consequence of tarnishing silver and some of its alloys.
The tests have been validated by laboratory and field tests on silver, though limited tests have also been carried out on components with contacts made of some silver alloys. Gold contacts are largely unaffected by the test.
The standard provides the reader with detailed instructions on how to construct a test chamber and the composition of the atmosphere within the test chamber.
7.4.3 Immersion in cleaning solvents (IEC 68.2.45 Test Xa)

7.4.3.1 Introduction
Many components or parts that are going to be mounted on printed circuit boards will be subjected to a solvent cleaning process. This process can affect the marking, encapsulation and coating of parts as well as influencing component characteristics.
7.4.3.3 General conditions
In many cases, total immersion of printed circuits in the cleaning solvent is required in order to remove fluxes and flux residue. In those cases, components on boards have to withstand a short-term immersion in the relevant cleaning solvent.
7.4.3.4 Test conditions
IEC 68.2.45 is a test procedure whereby specimens to be tested are immersed in a certain solvent at a specified temperature and for a specified time.
Generally speaking the cleaning solvent used depends upon the soldering flux chosen. The three most common solvents used are specified for the purpose of this test and consist of:
• demineralised or distilled water;
• 2-propanol (isopropyl alcohol);
• a mixture of trichlorotrifluorethane and 2-propanol (isopropyl alcohol).
Note: As trichlorotrifluorethane is a potential environmental hazard, it should only be used in exceptional circumstances.
7.4.3.5 Other standards
| IEC 68.2.42 | Environmental testing procedures – Test Kc: Sulphur dioxide test for electrical contacts and connections |
| IEC 68.2.43 | Environmental testing procedures – Test Kd: Hydrogen sulphide test for electrical contacts and connections |
| ISO 9225 | Corrosion of metals and alloys – Corrosivity of atmospheres – Measurement of pollution |
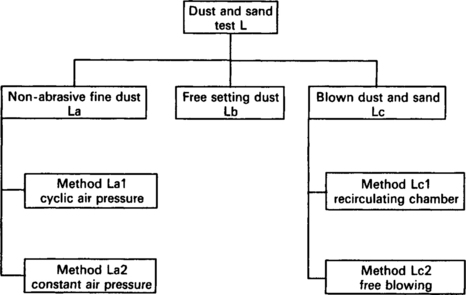
7.4.4 Dust and sand (IEC 68.2.68 Test L)

7.4.4.1 Introduction
This standard specifies test methods to determine the effects of dust and sand suspended in air, on electrotechnical products.
7.4.4.2 Purpose of this test
The test is structured into three groups:
• non-abrasive dust, primarily oriented towards investigation of the seals of the test specimen (Test La);
• free settling dust, oriented towards investigation of the effects which simulate conditions at sheltered locations (Test Lb);
• blown dust and sand, oriented towards investigation of the seals and the effect of erosion when simulating outdoor and vehicle conditions (Test Lc).
The purpose of this test is to determine the effect of:
• ingress of dust into enclosures;
• change of electrical characteristics (for example, faulty contact, change of contact resistance, change of track resistance);
• seizure or disturbance in motion of bearings, axles, shafts and other moving parts;
• contamination of optical surfaces; contamination of lubricants;
• clogging of ventilating openings, bushings, pipes, filters, apertures necessary for operation, etc.
7.4.4.3 General conditions
For non-operational tests the specimen is introduced into the chamber in an unpacked, switched off, ‘ready to use’ state in its normal position unless otherwise specified.
When operational tests are required, the specimen has to be switched on or be electrically loaded.
7.4.5 Corrosivity of atmospheres (ISO 9225)
| Standard No. | ISO 9225 |
| Title | Corrosion of metals and alloys – Corrosivity of atmospheres – Measurement of pollution |
7.4.5.1 Introduction
The ability of an atmosphere to cause corrosion of metals and alloys is controlled by the temperature-humidity complex and the amount of pollution.
When compiling a system specific specification it is useful to know the severity of the corrosive atmosphere into which it will be placed. This will assist in specifying suitable IEC 721 ranges.
This International Standard provides methods for the measurement of deposition rates of sulphur dioxide (SO2) and airborne salinity.
7.4.5.2 Purpose of this test
The purpose of this test is to measure the deposition rates of sulphur dioxide and airborne salinity.
7.4.5.3 General conditions
The test uses three lead dioxide sulphation plates which are exposed to the prevailing atmosphere allowing them to react with airborne sulphur particles.
7.4.5.4 Test conditions
• Determination of sulphur dioxide deposition rate on lead dioxide sulphation plates. Atmospheric sulphur dioxide reacts with the lead dioxide to form lead sulphate. The plates are recovered and the sulphate analysis is performed on the contents to determine the extent of sulphur dioxide capture. The deposition rate of sulphur dioxide is expressed in milligrams per square metre day (mg/(m2·d)). A 30 day ± 2 day exposure period is recommended.
• Determination of sulphur dioxide deposition rate on alkaline surfaces. Sulphur oxides and other sulphur compounds of an acid character are collected on the alkaline surface of porous filter plates saturated by a solution of sodium or potassium carbonate. The deposition rate of sulphur dioxide is expressed in milligrams per square metre day (mg/(m2 d)). A 30 day ± 2 day exposure period is recommended.
• Determination of chloride deposition rate by the wet candle method. A rain protected wet textile surface, with a known area, is exposed to the atmosphere for a specified time. The amount of chloride deposited is determined by chemical analysis. For the results of this analysis the chloride deposition rate is calculated and expressed in milligrams per square metre day (mg/(m2·d)).
7.4.5.5 Other standards
| ISO 7539 | Corrosion of metals and alloys – Stress corrosion testing–General guidance on testing procedures |
| ISO 9223 | Corrosion of metals and alloys – Corrosivity of atmospheres – Classification |
| ISO 9224 | Corrosion of metals and alloys – Corrosivity of atmospheres – Guiding values for the corrosivity categories |
| ISO 9226 | Corrosion of metals and alloys – Corrosivity of atmospheres – Determination of corrosion rate of standard specimens for the evaluation of corrosivity |
| ISO 11845 | Corrosion of metals and alloys – General principles for corrosion testing |