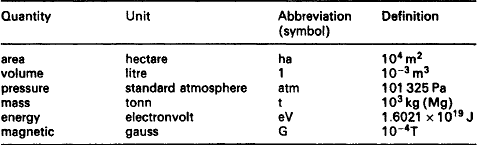
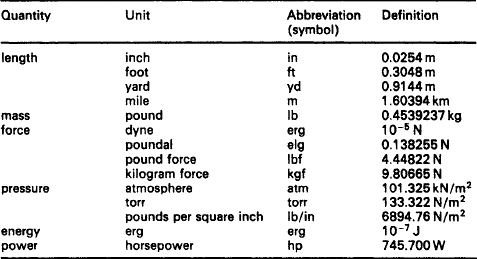
SI units for existing technology
As Gregor M. Grant explained in his article published in the April/May 1997 issue of Electro Technology, the Système International d’Unités (SI) was a child of the 1960s, a creation of the 11th General Conference on Weights and Measures (Conférence Générale des Poids et Mesures (CGPM)). This assembly endorsed the Italian physicist Professor Giovanni Giorgi’s MKS (i.e. metre-kilogram-second) system of 1901 and decided to base the SI system on it. Seven basic units were adopted, as shown in Table A, each of which was harmonised to a standard value.
Table A
| SI nomenclature | Abbreviation | Quantity |
| metre | m | length |
| kilogram | kg | mass |
| second | s | time |
| ampere | A | electrical current |
| kelvin | K | temperatures |
| mole | mol | amount of substance |
| candela | cd | luminous intensity |
Of the seven units, only the kilogram (kg) is represented by a physical object, namely a cylinder of platinum–iridium kept at the International Bureau of Weights and Measures at Sèvres, near Paris, with a duplicate at the US Bureau of Standards.
The metre (m), on the other hand, ‘is the length of the path travelled by light in a vacuum during a time interval of 1/2999 792 458 of a second’.
The second (s) has been defined as ‘the duration of 9192631 770 periods of radiation corresponding to the energy-level change between the two hyperfine levels of the ground state of caesium-133 atom’.
The ampere (A) is ‘that constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circular cross section and placed 1 m apart in vacuum, would produce between these conductors a force equal 2 × 10–7 newtons per metre length’.
The unit of temperature is the kelvin (K), which is a thermodynamic measurement as opposed to one based on the properties of real material. Its origin is at absolute zero and there is a fixed point where the pressure and temperature of water, water vapour and ice are in equilibrium, which is defined as 273.16 K.
The mole (mol) is ‘that quantity of substance of a system which contains as many elementary entities as there are atoms in 0.012 kg of carbon-12’. For definition purposes the entities must be specified (e.g. atoms, electrons, ions or any other particles or groups of such particles).
Finally there is the candela (cd), the unit of light intensity. This is defined as ‘the luminous intensity, in the perpendicular direction, of a surface of 1/6000000 m2 of a black body at the temperature of freezing platinum under a pressure of 101 325 N/m2′.
Two years before the creation of SI units, another international agreement had made the prefixes mega and micro official and introduced some new ones, such as the nano whose name derives from the Greek ‘nanos’ meaning dwarf (see Table B). Its symbol is n, and its mathematical representation is 10–9, indicating the number of digits to the right of the decimal point, in this case 0.000000001.
Table B
| Measurement | Symbol | Equivalent to |
| millimetre | mm | 0.001 m or 10−3m |
| micrometre | μm | 0.000 001 m or 10−8m |
| nanometre | nm | 0.000 000 001 m or 10−9m |
| picometre | pm | 0.000 000 000 001 m or 10−12m |
| femtometre | fm | 0.000 000 000 000 001 m or 10−15m |
| attometre | am | 0.000 000 000 000 000 001 m or 10−18m |
| zeptometre | zm | 0.000 000 000 000 000 000 001 m or 10−21m |
| yoctometre | ym | 0.000 000 000 000 000 000 000 001 m or 10−24m |
Even these minute quantities, however, soon became inadequate and, by 1962, it was decided that a thousandth of a picometre be designated a femtometre and one-thousandth of this new measurement be termed an attometre. Later on, the zeptometre and yoctometre were introduced.
Basic SI units
Many SI units are named after people but when these units are written in full, they do not necessarily require initial capital letters, e.g. amperes, coulombs, newtons, siemens.
All the above examples are expressed in the plural, but note that siemens does not drop the final ‘s’ in the singular as this was derived from a person’s name (i.e. Siemens) thus we have one newton, but one siemens.
Small number SI prefixes
Within the SI units there is a distinction between a quantity and a unit. Length is a quantity, but metres (abbreviated to m) is a unit.
Large number SI prefixes
Table C
| Measurement | Symbol | Equivalent to |
| megametre | Mm | 1 000 000 m or 106 m |
| gigametre | Gm | 1 000 000 000 m or 109 m |
| tarametre | Tm | 1 000 000 000 000 m or 1012 m |
| petametre | Pm | 1 000000000000000m or 1015 m |
| exametre | Em | 1 000 000 000 000 000 000 m or 1018 m |
| zettametre | Zm | 1 000 000 000 000 000 000 000 m or 1021 m |
| yottametre | Ym | 1 000 000 000 000 000 000 000 000 m or 1024 |
Deprecated prefixes
Some non-SI fractions and multiples are occasionally used (see below), but they are not encouraged.
Derived units
Some units, derived from the basic SI units, have been given special names, many of which originate from a person’s name (i.e. Siemens).
Units without special names
Other derived units, without special names, are listed below.
Table F
| Quantity | Unit | Abbreviation |
| area | square metres | m2 |
| volume | cubic metres | m3 |
| density | kilograms per cubic metre | kg/m3 |
| velocity | metres per second | m/s |
| angular velocity (angular frequency) | radians per second | rad/s |
| acceleration | metres per second per second | m/s2 |
| pressure | newtons per square metre | N/m2 |
| electric field strength | volts per metre | Vm |
| magnetic field strength | amperes per metre | A/m |
| luminance | candelas per square metre | cd/m2 |