Chapter 9. Polyolefins and Acrylics
9.1. Background
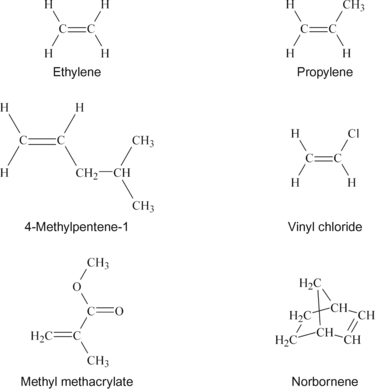
In organic chemistry, an alkene, also called an olefin, is a chemical compound containing at least one carbon-to-carbon double bond. The simplest alkenes, with only one double bond and no other functional groups, form a homologous series of hydrocarbons with the general formula CnH2n. The two simplest alkenes of this series are ethylene and propylene. When these are polymerized, they form polyethylene (PE) and polypropylene (PP), which are two of the plastics discussed in this chapter. A slightly more complex alkene is 4-methylpentene-1, the basis of poly(methyl pentene), known under the trade name of TPX™. If one of the hydrogens on the ethylene molecule is changed to chlorine, the molecule is called vinyl chloride, the basis of polyvinyl chloride, commonly called PVC. Acrylic polymers are also polymerized through the carbon–carbon double bond. Methyl methacrylate is the monomer used to make poly(methyl methacrylate).
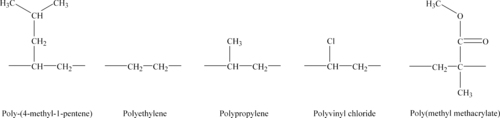
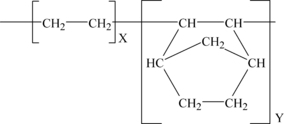
The structures of these monomers are shown in Figure 9.1, with polymer structures shown in Figure 9.2. The copolymer structure using the norbornene monomer is shown later in Figure 9.5.
9.1.1. Polyethylene
PE can be made in a number of ways. The way it is produced can affect its physical properties. It can also have very small amounts of comonomers, which will alter its structure and properties.
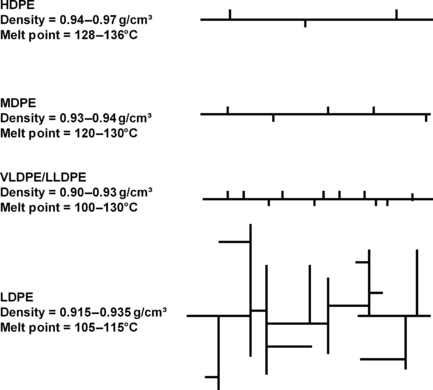
The basic types or classifications of PE, according to the ASTM 1248, are:
• Ultralow-density PE (ULDPE), polymers with densities ranging from 0.890 to 0.905g/cm3, contains comonomer
• Linear low-density PE (LLDPE), polymers with densities ranging from 0.915 to 0.935g/cm3, contains comonomer
• Low-density PE (LDPE), polymers with densities ranging from about 0.915 to 0.935g/cm3
• Medium-density PE (MDPE), polymers with densities ranging from 0.926 to 0.940g/cm3, may or may not contain comonomer
• High-density PE (HDPE), polymers with densities ranging from 0.940 to 0.970g/cm3, may or may not contain comonomer
Figure 9.3 shows the differences graphically. The differences in the branches in terms of number and length affect the density and melting points of some of the types.
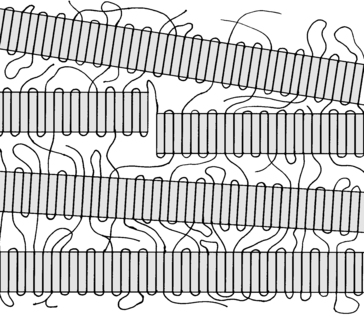
Branching affects the crystallinity. A diagram of a representation of the crystal structure of PE is shown in Figure 9.4. One can imagine how branching in the polymer chain can disrupt the crystalline regions. The crystalline regions are the highly ordered areas in the shaded rectangles of Figure 9.4. A high degree of branching would reduce the size of the crystalline regions, which leads to lower crystallinity.
9.1.2. Cross-linked PE
A modification of HDPE is called cross-linked PE (PEX). It is a form of PE with cross-links and it is commonly abbreviated as PEX or XLPE. The HDPE has undergone a chemical or physical reaction that causes the molecular structure of the PE chains to link together as described in Section 3.3 and Figure 3.11. This reaction creates a three-dimensional structure which has superior resistance to high temperature and pressure. PEX is primarily used in tubing. There are three primary commercial methods for producing PEX tubing:
• Peroxide (or Engel) method (PEX-a)—Cross-links during extrusion while the polymer is molten
• Silane method (PEX-b)—A chemical cross-linking method in which reactive silane groups are grafted onto the polymer backbone
• Radiation or electron-beam method (PEX-c)—The formed tubing passes through a radiation chamber that generates the cross-links
The details are beyond the scope of this book.
9.1.3. Polypropylene
The three main types of PP generally available:
1. Homopolymers are made in a single reactor with propylene and catalyst. It is the stiffest of the three propylene types and has the highest tensile strength at yield. In the natural state (no colorant added), it is translucent and has excellent see-through or contact clarity with liquids. In comparison to the other two types, it has less impact resistance, especially below 0°C.
2. Random copolymers (homophasic copolymers) are made in a single reactor with a small amount of ethylene (<5%) added which disrupts the crystallinity of the polymer allowing this type to be the clearest. It is also the most flexible with the lowest tensile strength of the three. It has better room temperature impact than homopolymer but shares the same relatively poor impact resistance at low temperatures.
3. Impact copolymers (heterophasic copolymers), also known as block copolymers, are made in a two reactor system where the homopolymer matrix is made in the first reactor and then transferred to the second reactor where ethylene and propylene are polymerized to create ethylene propylene rubber (EPR) in the form of microscopic nodules dispersed in the homopolymer matrix phase. These nodules impart impact resistance both at ambient and cold temperatures to the compound. This type has intermediate stiffness and tensile strength and is quite cloudy. In general, the more ethylene monomer added, the greater the impact resistance with correspondingly lower stiffness and tensile strength.
9.1.4. Polymethyl Pentene
4-Methylpentene-1-based polyolefin is manufactured and marketed solely by Mitsui Chemicals, Inc under the trade name TPX™. This lightweight, functional polymer displays a unique combination of physical properties and characteristics due to its distinctive molecular structure, which includes a bulky side chain as shown in Figure 9.2. Polymethyl pentene (PMP) possesses many characteristics inherent in traditional polyolefins such as excellent electrical insulating properties and strong hydrolysis resistance. Moreover, it features low dielectric, superb clarity, transparency, gas permeability, heat and chemical resistance and release qualities.
It can be used for extruded and film products, injection molded, and blow molded application items, including:
9.1.5. Ultrahigh Molecular Weight PE
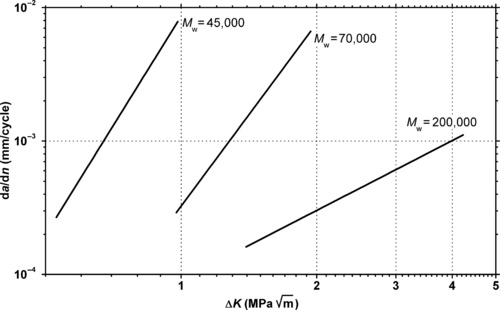
Thermoplastic ultrahigh molecular weight PE (UHMWPE) is also known as high-modulus PE (HMPE) or high-performance PE (HPPE). It has extremely long chains, with molecular weight numbering in the millions (usually between 3.1 and 5.67 million). The high molecular weight leads to very good packing of the chains into the crystal structure. This makes UHMWPE a very tough material, with the highest impact strength of any thermoplastic presently made. It is highly resistant to corrosive chemicals, with the exception of oxidizing acids. It has extremely low moisture absorption and is highly resistant to abrasion. Its coefficient of friction is significantly lower than that of nylon and acetal.
9.1.6. Rigid Polyvinyl Chloride
PVC is a flexible or rigid material that is chemically nonreactive. Rigid PVC is easily machined, heat formed, welded, and even solvent cemented. PVC can also be machined using standard metal working tools and finished to close tolerances and finishes without great difficulty. PVC resins are normally mixed with other additives such as impact modifiers and stabilizers, providing hundreds of PVC-based materials with a variety of engineering properties.
There are three broad classifications for rigid PVC compounds: Type II, CPVC, and Type I. Type II differs from Type I due to greater impact values, but lower chemical resistance. CPVC has greater high-temperature resistance. These materials are considered “unplasticized,” because they are less flexible than the plasticized formulations. PVC has a broad range of applications, from high-volume construction related products to simple electric wire insulation and coatings.
9.1.7. Cyclic Olefin Copolymer
Cyclic olefin copolymer (COC) is an amorphous polyolefin made by reaction of ethylene and norbornene in varying ratios. Its structure is given in Figure 9.5. The properties can be customized by changing the ratio of the monomers found in the polymer. Being amorphous it is transparent. Other performance benefits include:
• low density
• extremely low water absorption
• excellent water vapor barrier properties
• high rigidity, strength, and hardness
• variable heat deflection temperature up to 170°C
• very good resistance to acids and alkalis
9.1.8. Polyacrylics
While a large number of acrylic polymers are manufactured, polymethyl methacrylate PMMA is by far the most common. Nearly everyone has heard of Plexiglas®. PMMA has two very distinct properties that set the products apart from others. First it is optically clear and colorless. It has a light transmission of 92%. The 4% reflection loss at each surface is unavoidable. Second its surface is extremely hard. They are also highly weather resistant.
9.1.9. Other Olefin Acrylic Polymers
There are a large number of acrylic/olefin copolymers manufactured. One of the best known is the copolymer of ethylene and methacrylic acid forming a polymer known as EMAA. This is more commonly known by its trade name Surlyn® made by DuPont. Generally, there is little multipoint data publicly available for these polymers – so they are not included in this book.
9.2. Polyethylene
9.2.1. Fatigue Data
9.3. Polypropylene
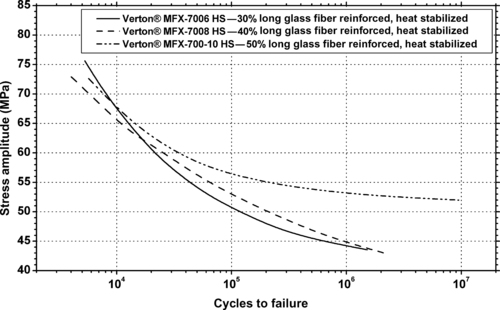
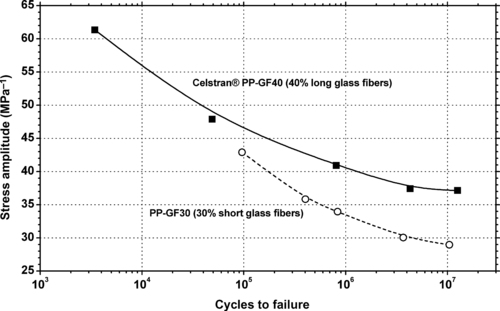
9.3.1. Fatigue Data
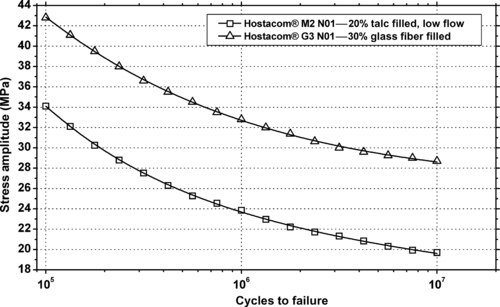 |
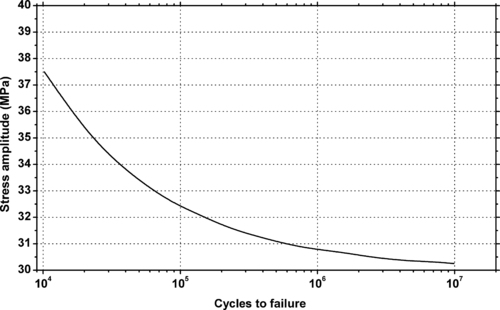
| Figure 9.10. |
| Flexural stress amplitude vs. cycles to failure at 23°C and 10Hz of LyondellBasell Industries Polyolefins Hostacom® PP plastics. |
 |
| Figure 9.11. |
| Flexural stress amplitude vs. cycles to failure at 23°C of SABIC Innovative Plastics Thermocomp® MF-1006—30% glass fiber reinforced PP. |
9.4. Ultrahigh-Molecular-Weight PE
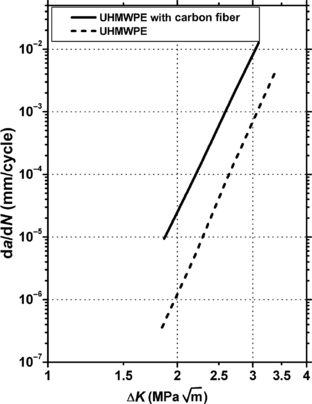
9.4.1. Fatigue Data
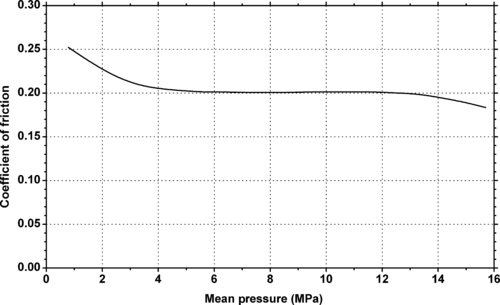
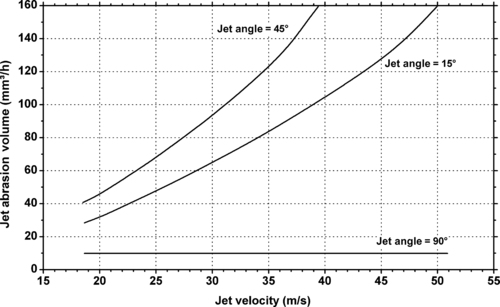
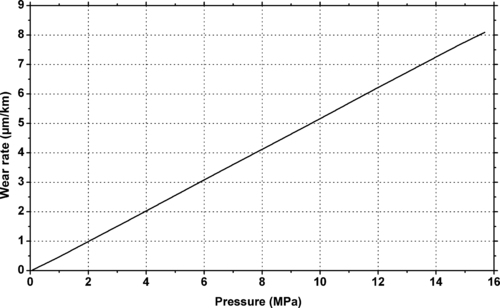
9.4.2. Tribology Data
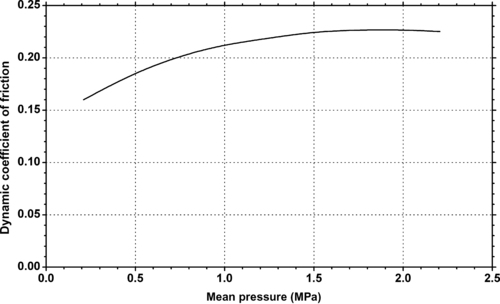 |
| Figure 9.15. |
| Dynamic coefficient of friction vs. pressure at a velocity of 10m/min of Ticona Engineering Polymers GUR® UHMWPE. |
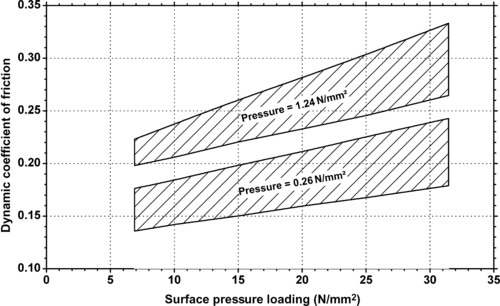 |
| Figure 9.16. |
| Dynamic coefficient of friction vs. sliding speed and pressure of Ticona Engineering Polymers GUR® UHMWPE. |
9.5. Polyvinyl Chloride
9.6. Acrylics
9.6.1. Fatigue Data
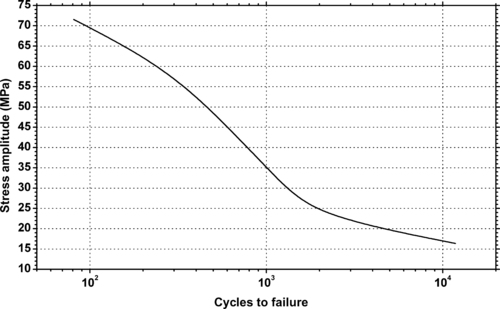 |
| Figure 9.22. |
| Flexural stress amplitude vs. cycles to failure at 23°C and 65% relative humidity of Lucite International Inc Diakon™ CMG302—general-purpose, high-heat-resistant acrylic. |
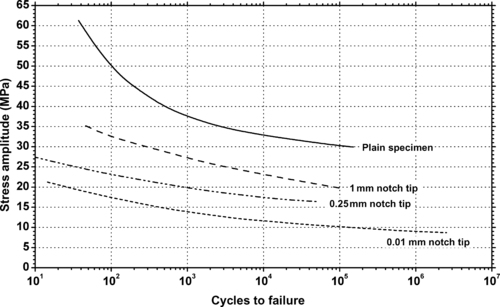 |
| Figure 9.23. |
| Tension/compression stress amplitude vs. cycles to failure at 20°C and 5Hz at different notch sizes of generic acrylic. |
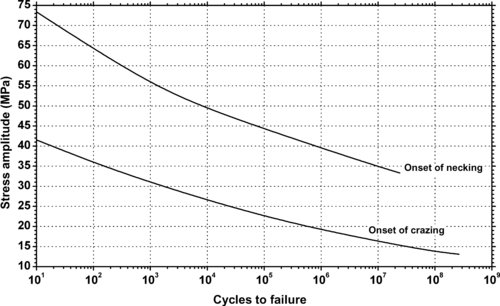 |
| Figure 9.24. |
| Stress amplitude vs. cycles to failure at 23°C and 65% RH by type of failure of Lucite International Inc Diakon™ CMG302—general-purpose, high-heat-resistant acrylic. |
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.