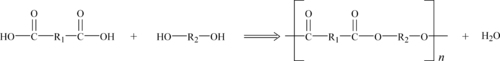Chapter 3. Introduction to Plastics and Polymers
The most basic component of plastic and elastomer materials is polymers. The word polymer is derived from the Greek term for “many parts.” Polymers are large molecules comprised of many repeat units, called monomers that have been chemically bonded into long chains. Since World War II, the chemical industry has developed a large quantity of synthetic polymers to satisfy the materials needs for a diverse range of products, including paints, coatings, fibers, films, elastomers, and structural plastics. Literally thousands of materials can be called “plastics,” although the term today is typically reserved for polymeric materials, excluding fibers, which can be molded or formed into solid or semisolid objects. The subject of this chapter includes polymerization chemistry and the different types of polymers and how they can differ from each other. Since plastics are rarely “neat”, reinforcement, fillers, and additives are reviewed. A basic understanding of plastic and polymer chemistry will make the discussion of fatigue and tribology of specific plastics easier to understand and it also provides a basis for the introductions of the plastic families in later chapters. This chapter is taken from The Effect of Temperature and Other Factors on Plastics book, but it has been refocused on fatigue properties.
3.1. Polymerization
Polymerization is the process of chemically bonding monomer building blocks to form large molecules. Commercial polymer molecules are usually thousands of repeat units long. Polymerization can proceed by one of several methods. The two most common methods are called addition and condensation polymerization.
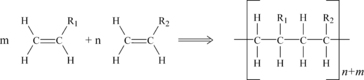
In addition polymerization, a chain reaction adds new monomer units to the growing polymer molecule one at a time through double or triple bonds in the monomer. Each new monomer unit creates an active site for the next attachment. The net result is shown in Figure 3.1. Many of the plastics discussed in later chapters of this book are formed in this manner. Some of the plastics made by addition polymerization include polyethylene, polyvinyl chloride (PVC), acrylics, polystyrene, and polyoxymethylene (acetal).
The other common method is condensation polymerization in which the reaction between monomer units and the growing polymer chain end group releases a small molecule, often water as shown in Figure 3.2. This reversible reaction will reach equilibrium and halt unless this small molecular by product is removed. Polyesters and polyamides are among the plastics made by this process.
Understanding the polymerization process used to make a particular plastic gives insight into the nature of the plastic. For example, plastics made via condensation polymerization, in which water is released, can degrade when exposed to water at high temperature. Polyesters such as polyethylene terephthalate (PET) can degrade by a process called hydrolysis when exposed to acidic, basic, or even some neutral environments severing the polymer chains. As a result the polymer’s properties are degraded.
3.2. Copolymers
A copolymer is a polymer formed when two (or more) different types of monomer are linked in the same polymer chain, as opposed to a homopolymer where only one monomer is used. If exactly three monomers are used, it is called a terpolymer.
Monomers are only occasionally symmetric; the molecular arrangement is the same no matter which end of the monomer molecule you are looking at. The arrangement of the monomers in a copolymer can be head-to-tail, head-to-head, or tail-to-tail. Since a copolymer consists of at least two types of repeating units, copolymers can be classified based on how these units are arranged along the chain. These classifications include:
• Alternating copolymer
• Random copolymer (statistical copolymer)
• Block copolymer
• Graft copolymer.
When the two monomers are arranged in an alternating fashion, the polymer is called, of course, an alternating copolymer:
In the following examples A and B are different monomers. Keep in mind the A and B do not have to be present in a one-to-one ratio. In a random copolymer, the two monomers may follow in any order:
In a block copolymer, all of one type of monomer is grouped together, and all of the other are grouped together. A block copolymer can be thought of as two homopolymers joined together at the ends:
A polymer that consists of large grouped blocks of each of the monomers is also considered a block copolymer:
When chains of a polymer made of monomer B are grafted onto a polymer chain of monomer A we have a graft copolymer:
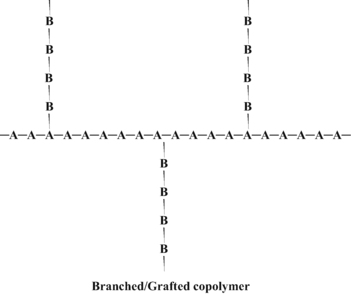 |
High-impact polystyrene, or HIPS, is a graft copolymer. It is a polystyrene backbone with chains of polybutadiene grafted onto the backbone. The polystyrene gives the material strength, but the rubbery polybutadiene chains give it resilience to make it less brittle.
3.3. Linear, Branched and Cross-Linked Polymers
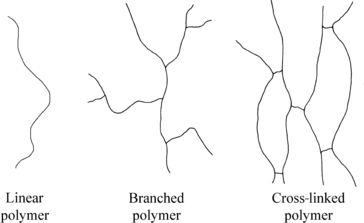
Some polymers are linear, a long chain of connected monomers. Polyethylene, PVC, Nylon 66, and polymethyl methacrylate are some linear commercial examples found in this book. Branched polymers can be visualized as a linear polymer with side chains of the same polymer attached to the main chain. While the branches may in turn be branched, they do not connect to another polymer chain. The ends of the branches are not connected to anything. Cross-linked polymer, sometimes called network polymer, is one in which different chains are connected. Essentially the branches are connected to different polymer chains on the ends. These three polymer structures are shown in Figure 3.3.
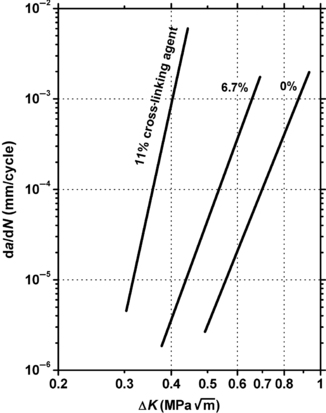
A higher amount of cross-linking in plastics generally leads to higher fatigue crack propagation rates. This is shown in Figure 3.4. This figure shows the fatigue crack propagation rate increases as the amount of cross-linking increases in polymethyl methacrylate. The data to the left side of the plot has the highest amount of cross-linking. The uncross-linked data on the far right has the best performance.
3.4. Molecular Weight
A polymer’s molecular weight is the sum of the atomic weights of individual atoms that comprise a molecule. It indicates the average length of the bulk resin’s polymer chains. All polymer molecules of a particular grade do not all have the exact same molecular weight. There is a range or distribution of molecular weights. The average molecular weight can be determined by several means, but this subject is beyond the scope of this book. Low-molecular-weight polyethylene chains have backbones as small as 1000 carbon atoms long. Ultrahigh molecular weight polyethylene chains can have 500,000 carbon atoms along their length. Many plastics are available in a variety of chain lengths, or different molecular weight grades. These resins can also be classified indirectly by a viscosity value, rather than molecular weight. Within a resin family, such as polycarbonate, higher molecular weight grades have higher melt viscosities. For example, in the viscosity test for polycarbonate, the melt flow rate ranges from approximately 4g/10min for the highest molecular weight, standard grades to more than 60g/10min for lowest molecular weight, high flow, specialty grades.
Selecting the correct molecular weight for your injection molding application generally involves a balance between filling ease and material performance. If your application has thin-walled sections, a lower molecular weight/lower viscosity grade offers better flow. For normal wall thicknesses, these resins also offer faster mold cycle times and fewer molded in stresses. The stiffer flowing, high-molecular-weight resins offer the ultimate material performance, being tougher and more resistant to chemical and environmental attack.
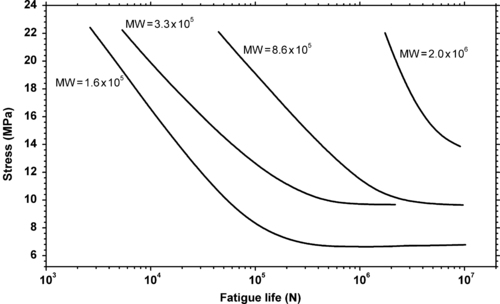
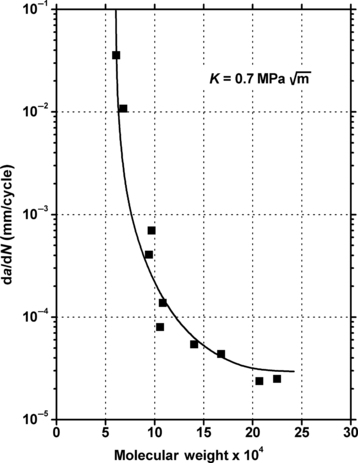
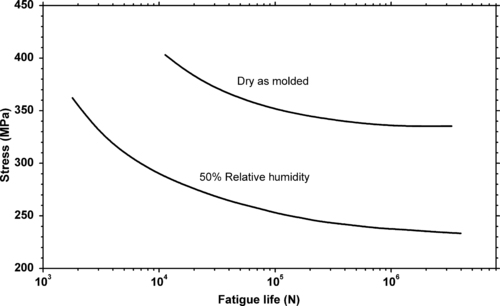
Molecular weight of the polymers that are used in engineering plastics affects fatigue lifetimes. While it is not always known exactly what the molecular weights are, as mentioned above higher flowing plastics of a given series of products generally are lower molecular weight polymers. In general, higher molecular weight provides improved resistance to cyclic fatigue damage. This is demonstrated in Figure 3.5 and Figure 3.6. Figure 3.5 shows the effect of molecular weight of polystyrene on fatigue life. Figure 3.6 shows the effect of molecular weight on fatigue crack propagation rates of the same polymer, polystyrene.
3.5. Thermosets versus Thermoplastics
A plastic falls into one of two broad categories depending on its response to heat: thermoplastics and thermosets. Thermoplastics soften and melt when heated and harden when cooled. Because of this behavior, these resins can be injection molded, extruded or formed via other molding techniques. This behavior also allows production scrap runners and trimmings, to be reground and reused.
Unlike thermoplastics, thermosets react chemically to form cross-links, as described earlier that limit chain movement. This network of polymer chains tends to degrade, rather than soften, when exposed to excessive heat. Until recently, thermosets could not be remelted and reused after initial curing. Recent advances in recycling have provided new methods for remelting and reusing thermoset materials.
3.6. Crystalline versus Amorphous
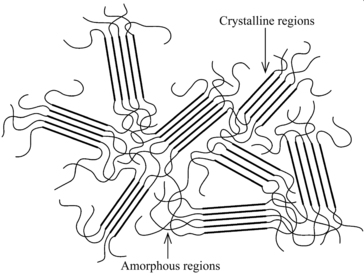
Thermoplastics are further classified by their crystallinity, or the degree of order within the polymer’s overall structure. As a crystalline resin cools from the melt, polymer chains fold or align into highly ordered crystalline structures as shown in Figure 3.7.
Some plastics can be completely amorphous or crystalline. Often plastics specifications will report what percent of it is crystalline as a percent, such as 73% crystallinity. Generally, polymer chains with bulky side groups cannot form crystalline regions. The degree of crystallinity depends upon both the polymer and the processing technique. Some polymers such as polyethylene crystallize quickly and reach high levels of crystallinity. Others, such as PET polyester, require slow cooling to crystallize. If cooled quickly, PET polyester remains amorphous in the final product.
Crystalline and amorphous plastics have several characteristic differences. Amorphous polymers do not have a sharp melting point, but do have what is called a glass transition temperature, Tg. A glass transition temperature is the temperature at which a polymer changes from hard and brittle to soft and pliable. The force to generate flow in amorphous materials diminishes slowly as the temperature rises above the glass transition temperature. In crystalline resins, the force requirements diminish quickly as the material is heated above its crystalline melt temperature. Because of these easier flow characteristics, crystalline resins have an advantage in filling thin-walled sections of a mold. Crystalline resins generally have superior chemical resistance, greater stability at elevated temperatures, and better creep resistance. Amorphous plastics typically have better impact strength, less mold shrinkage, and less final part warping than crystalline materials. End use requirements usually dictate whether an amorphous or crystalline resin is preferred.
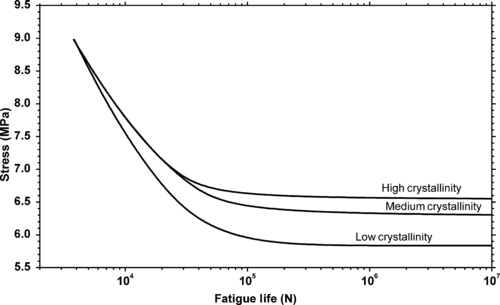
It is generally accepted that crystalline polymers are more fatigue crack propagation resistant than amorphous polymers and so they should also have improved fatigue life. Figure 3.8 shows the effects of crystallinity in PTFE on the fatigue life. In this particular example the thermal history affects the crystallinity of the PTFE, with samples that were cooled more slowly after molding possessing higher levels of crystallinity. This means that not only does it matter which plastic is used to make a part, but the way a part is made may also impact the fatigue performance.
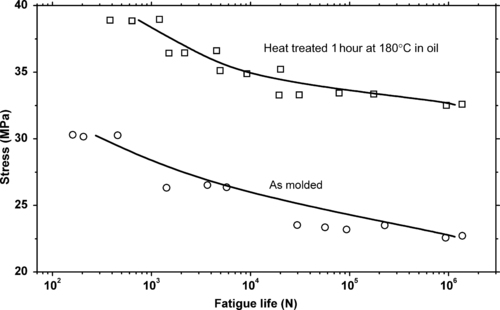
Similarly, thermal history may also affect fatigue life. This is shown in Figure 3.9 which shows the difference heat treatment makes on the fatigue life of polycaproamide. In this case the upper curve has been heat treated for 1 hour at 180°C in oil. The oil keeps oxygen away from the heated polymer. Had the heat treatment been done in air, a different result might be expected.
3.7. Blends
Polymers can often be blended. Occasionally, blended polymers have properties that exceed those of either of the constituents. For instance, blends of polycarbonate resin and PET polyester, originally created to improve the chemical resistance of polycarbonate, actually have fatigue resistance and low-temperature impact resistance superior to either of the individual polymers.
Sometimes a material is needed that has some of the properties of one polymer, and some of the properties of another. Instead of going back into the lab and trying to synthesize a brand new polymer with all the properties wanted, two polymers can be melted together to form a blend, which will hopefully have some properties of both.
Two polymers that do actually mix well are polystyrene and polyphenylene oxide. A few other examples of polymer pairs that will blend are:
• PET with polybutylene terephthalate
• Polymethyl methacrylate with polyvinylidene fluoride
Phase-separated mixtures are obtained when one tries to mix most polymers. But strangely enough, the phase-separated materials often turn out to be rather useful. They are called immiscible blends.
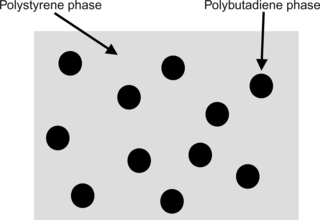
Polystyrene and polybutadiene are immiscible. When polystyrene is mixed with a small amount of polybutadiene, the two polymers do not blend. The polybutadiene separates from the polystyrene into little spherical blobs. If this mixture is viewed under a high-power microscope something that looks like the picture in Figure 3.10 would be seen.
Multiphase polymer blends are of major economic importance in the polymer industry. The most common examples involve the impact modification of a thermoplastic by the microdispersion of a rubber into a brittle polymer matrix. Most commercial blends consist of two polymers combined with small amounts of a third, compatibilizing polymer, typically a block or graft copolymer.
Multiphase polymer blends can be easier to process than a single polymer with similar properties. The possible blends from a given set of polymers offer many more physical properties than do the individual polymers. This approach has shown some success but becomes cumbersome when more than a few components are involved.
Blending two or more polymers offers yet another method of tailoring resins to a specific application. Because blends are only physical mixtures, the resulting polymer usually has physical and mechanical properties that lie somewhere between the values of its constituent materials. For instance, an automotive bumper made from a blend of polycarbonate resin and thermoplastic polyurethane elastomer gains rigidity from the polycarbonate resin and retains most of the flexibility and paintability of the polyurethane elastomer. For business machine housings, a blend of polycarbonate and acrylonitrile butadiene styrene (ABS) resins offers the enhanced performance of polycarbonate flame retardance and UV stability at a lower cost.
3.8. Elastomers
Elastomers are a class of polymeric materials that can be repeatedly stretched to over twice the original length with little or no permanent deformation. Elastomers can be made of either thermoplastic or thermoset materials and generally are tested and categorized differently than rigid materials. They are commonly selected according to their hardness and energy absorption characteristics, properties rarely considered in rigid thermoplastics. Elastomers are found in numerous applications, such as automotive bumpers and industrial hoses.
3.9. Additives
Additives encompass a wide range of substances that aid processing or add value to the final product.4. and 5. Found in virtually all plastics, most additives are incorporated into a resin family by the supplier as part of a proprietary package. For example, you can choose standard polycarbonate resin grades with additives for improved internal mold release, UV stabilization, and flame retardance; or nylon grades with additives to improve impact performance.
Additives often determine the success or failure of a resin or system in a particular application. Many common additives are discussed in the following sections. Except for reinforcement fillers, most additives are added in very small amounts.
3.9.1. Fillers, Reinforcement, Composites
Reinforcing fillers can be added in large amounts. Some plastics may contain as much as 60% reinforcing fillers. Often, fibrous materials, such as glass or carbon fibers, are added to resins to create reinforced grades with enhanced properties. For example, adding 30% short glass fibers by weight to Nylon 6 improves creep resistance and increases stiffness by 300%. These glass reinforced plastics usually suffer some loss of impact strength and ultimate elongation, and are more prone to warping because of the relatively large difference in mold shrinkage between the flow and cross-flow directions.
Plastics with nonfibrous fillers such as glass spheres or mineral powders generally exhibit higher stiffness characteristics than unfilled resins, but not as high as fiber reinforced grades. Resins with particulate fillers are less likely to warp and show a decrease in mold shrinkage. Particulate fillers typically reduce shrinkage by a percentage roughly equal to the volume percentage of filler in the polymer, an advantage in tight tolerance molding.
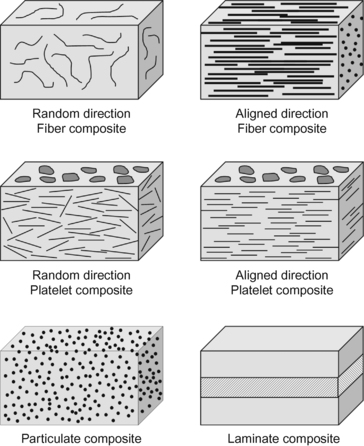
Often reinforced plastics are called composites. Often, the plastic material containing the reinforcement is referred to as the matrix. One can envision a number of ways different reinforcing materials might be arranged in a composite. Many of these arrangements are shown in Figure 3.11.
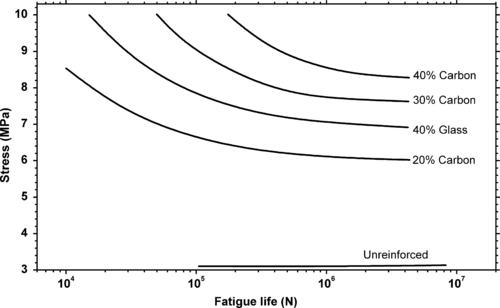
In general fiber reinforcement improves the fatigue strength of a plastic over its unreinforced analog. This is clearly demonstrated in Figure 3.12. It generally follows that carbon fibers enhance the performance over glass fibers at equal loading.
The effect of particulate reinforcement on fatigue properties is less clear and has not been studied as much as fibrous reinforcement.
3.9.2. Combustion Modifiers, Fire, Flame Retardants and Smoke Suppressants
Combustion modifiers are added to polymers to help retard the resulting parts from burning. Generally required for electrical and medical housing applications, combustion modifiers and their amounts vary with the inherent flammability of the base polymer. Polymers designed for these applications often are rated using an Underwriters Laboratories rating system. Use these ratings for comparison purposes only, as they may not accurately represent the hazard present under actual fire conditions.
3.9.3. Release Agents and Antiblocking Agents
External release agents are lubricants, liquids or powders, which coat a mold cavity to facilitate part removal. Internal release agents can accomplish the same purpose. The identity of the release agent is rarely disclosed, but frequently they are fine fluoropolymer powders, called micropowders, silicone resins, or waxes.
3.9.4. Lubricants and Slip Agents, Tribology Additives
As discussion of many additives used to improve slip, dry lubrication and abrasion resistance were discussed in Chapter 2. They are summarized again here.
• PFPE synthetic oil marketed under the trademark Fluoroguard® is an internal lubricant that imparts improved wear and low-friction properties.
• PTFE imparts the lowest coefficient of friction of any internal lubricant.
• Silicone acts as a boundary lubricant because it migrates to the surface of the plastic over time.
• Molybdenum disulfide, commonly called moly is a solid lubricant often used in bearing applications.
• Graphite is a solid lubricant used like molybdenum disulfide.
• Carbon fiber improves mechanical and thermal performance which leads to higher PV limits. Carbon fiber reinforced plastics may have lower coefficient of friction than the base resin. Carbon is softer and less abrasive than glass fiber and some carbon fiber compounds can dissipate static electricity.
• Aramid Fiber, Kevlar® being one, is softer and less abrasive than carbon or glass fiber, this additive is most commonly used for reduction in the wear of the mating surface, especially softer materials.
3.9.5. Catalysts
Catalysts, substances that initiate or change the rate of a chemical reaction, do not undergo a permanent change in composition or become part of the molecular structure of the final product. Occasionally used to describe a setting agent, hardener, curing agent, promoter, etc., they are added in minute quantities, typically less than 1%.
3.9.6. Impact Modifiers and Tougheners
Many plastics do not have sufficient impact resistance for the use for which they are intended. Rather than change to a different type of plastic, they can be impact modified in order to fulfill the performance in use requirements. Addition of modifiers called impact modifiers or tougheners can significantly improve impact resistance. This is one of the most important additives. There are many suppliers and chemical types of these modifiers.
General-purpose impact modification is a very low level of impact modification. It improves room temperature impact strength but does not take into account any requirements for low-temperature (below 0°C) impact strength. For most of these types of applications only low levels of impact modifier will be required (<10%).
Low-temperature impact strength is required for applications that require a certain level of low-temperature flexibility and resistance to break. This is, for example, the case for many applications in the appliance area. For this purpose modifier levels between 5% and 15% of mostly reactive modifiers will be necessary. Reactive modifiers can bond chemically to the base polymer.
Super tough impact strength may be required for applications that should not lead to a failure of the part even if hit at low temperatures (−30°C to −40°C) under high speed. This requirement can only be fulfilled with high levels (20–25%) of reactive impact modifier with low glass transition temperature.
Figure 3.13 shows the effect of one toughener on the Izod performance of a common Nylon 6 plastic. The toughener used in this graph is DuPont’s Fusabond®N MN-493D. The graph shows the improvement in notched Izod performance versus temperature with differing levels of toughener additive. As shown in this figure, the performance can be dramatically improved.
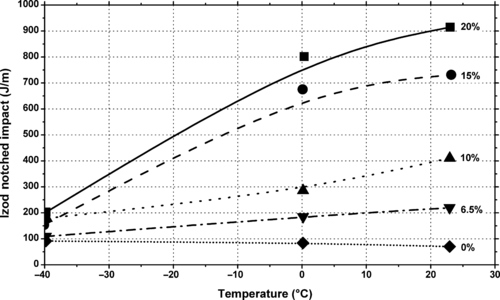 |
| Figure 3.13 |
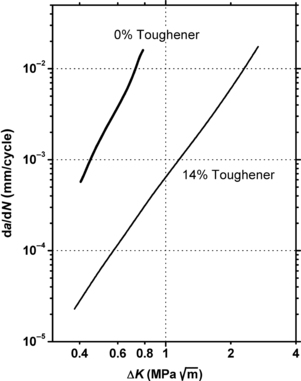
In general toughened plastics are more fatigue crack propagation resistant than the corresponding untoughened analogs. This is graphically demonstrated in Figure 3.14 which compares the fatigue crack propagation rates of toughened and untoughened PVC.
3.9.7. UV Stabilizers
Sunshine and its UV radiation have a deteriorating effect on many polymers. UV stabilizers play an important role in plastics for external uses by counteracting the effects of the sun. UV stabilizers are used in plastic items such as greenhouse film, outdoor furniture, and automotive plastic parts. The amounts added are very small, generally less than 1%.
3.9.8. Antistatic Agents
Antistatic additives are capable of modifying properties of plastics in such a way that they become antistatic, conductive, and/or improve electromagnetic interference shielding (EMI). Carbon fibers, conductive carbon powders, and other electrically conductive materials are used for this purpose.
3.9.9. Plasticizers
Plasticizers are added to help maintain flexibility in a plastic. Various phthalates are commonly used for this purpose. Since they are small molecules they may extract or leach out of the plastic causing a loss of flexibility with time. Just as purposely added small molecules may leach out, small molecules from the environment may be absorbed by the plastic and act like a plasticizer as shown in Figure 3.15.
3.9.10. Pigments, Extenders, Dyes, Mica
Pigments are added to give a plastic color, but they may also affect the physical properties. Extenders are usually cheap materials added to reduce the cost of plastic resins. Dyes are colorants chemically different than pigments. Mica is a special pigment added to impact sparkle or metallic appearance.
3.9.11. Coupling Agents
The purpose of adding fillers is either to lower the cost of the polymer, make it tougher or stiffer or make it flame retardant so that it does not burn when it is ignited. Often the addition of the filler will reduce the elongation at break, the flexibility and in many cases the toughness of the polymer because the fillers are added at very high levels. One reason for the degradation of properties is that the fillers in most cases are not compatible with the polymers. The addition of coupling agents can improve the compatibility of the filler with the polymer. As a result the polymer will like the filler more, the filler will adhere better to the polymer matrix and the properties of the final mixture (e.g., elongation, flexibility) will be enhanced.
3.9.12. Thermal Stabilizers
One of the limiting factors in the use of plastics at high temperatures is their tendency to not only become softer but also thermally degrade. Thermal degradation can present an upper limit to the service temperature of plastics. Thermal degradation can occur at temperatures much lower than those at which mechanical failure is likely to occur. Plastics can be protected from thermal degradation by incorporating stabilizers into them. Stabilizers can work in a variety of ways but discussion of these mechanisms are beyond the purpose of this book.
There are other additives used in plastics, but the ones discussed above are the most common.
3.10. Summary
These first three chapters provide a good basis for analyzing the fatigue and tribology data that follow in the next chapters. If the particular plastic manufacturer’s grade is not found in these chapters, one may find a composition similar from another manufacturer. If the composition is nearly the same, one should expect that data to be representative.
References
1.
2.
3.
4.
5.
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.