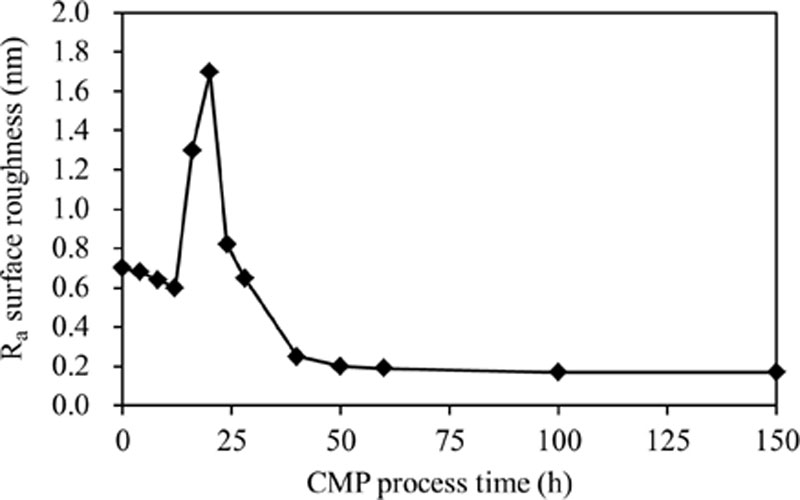
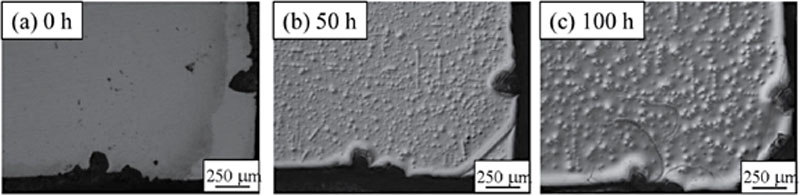
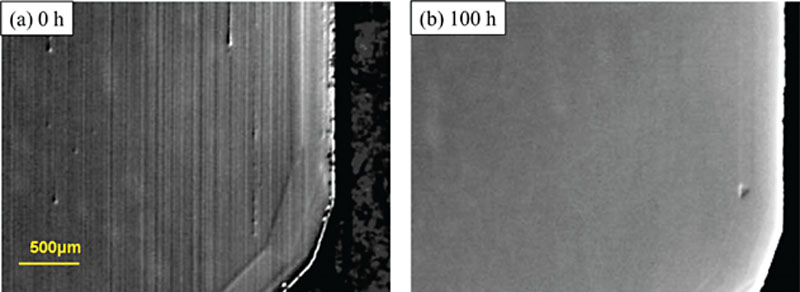
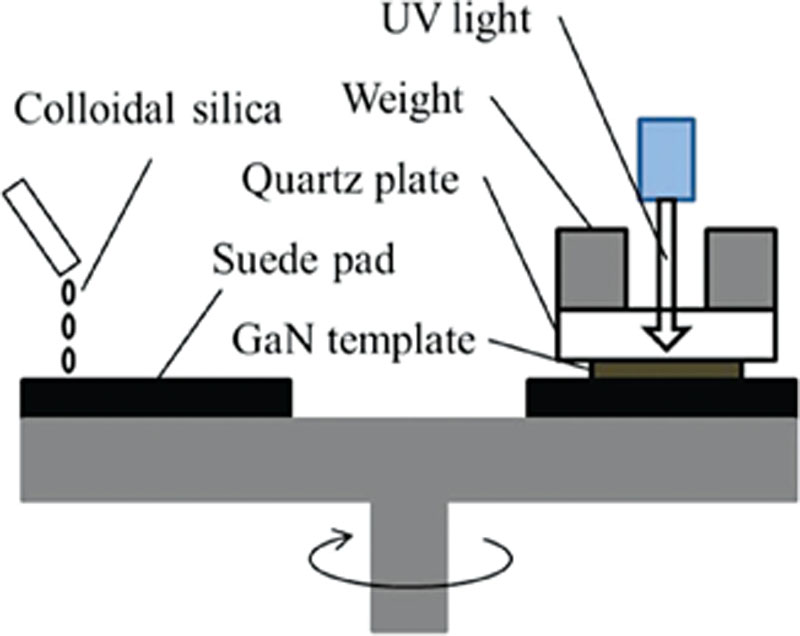
References
[1] Doy TK. Colloidal silica polishing based on micromechanical removal action and its applications. Sens Mater. 1998;3:153.
[2] Gutsche HW, Moody JW. Polishing of sapphire with colloidal silica. J Electrochem Soc. 1978;125:136.
[3] Aida H, Takeda H, Koyama K, Katakura H, Sunakawa K, Doi T. Chemical mechanical polishing of GaN with colloidal silica. J Electrochem Soc. 2011;158:H1206.
[4] Farber BY, Yoon SY, Lagerlöf KPD, Heuer AH. Microplasticity during high temperature indentation and the peierls potential in sapphire (α-Al2O3) single crystals. Phys Status Solidi A. 1993;137:485.
[5] Fujikane M, Inoue A, Yokogawa T, Nagao S, Nowak R. Mechanical properties characterization of c -plane (0001) and m -plane (10–10) GaN by nanoindentation examination. Phys Status Solidi C. 2010;7:1798.
[6] Yin L, Vancoille EYJ, Ramesh K, Huang H. Surface characterization of 6H-SiC (0001) substrates in indentation and abrasive machining. Int J Mach Tools Manuf. 2004;44:607.
[7] Nowak R, Pessa M, Suganuma M, Leszczynski M, Grzegory I, Porowski S. Elastic and plastic properties of GaN determined by nano-indentation of bulk crystal. . Appl Phys Lett. 1999;75:2070.
[8] Tapily K, Moutanabbir O, Gu D, Baumgart H, Elmustafa AA. Thermal behavior of the mechanical properties of GaN throughout Hydrogen-induced thin layer transfer. ECS Trans. 2010;33:241.
[9] Drory MD, Ager III JW, Suski T, Grzegory I, Porowski S. Hardness and fracture toughness of bulk single crystal Gallium nitride. Appl Phys Lett. 1996;69:4044.
[10] Hong MH, Pirouz P, Tavernier PM, Clarke DR. Dislocations produced by indentation deformation of HPVE GaN films. Mater Res Soc Symp Proc. 2000;622:1:.
[11] Yonenaga I. Mechanical stability of power device materials, high temperature hardness of SiC, AlN and GaN. Chem Sustainable Dev. 2001;9:19.
[12] Yonenaga I, Hoshi T, Usui A. Hardness of bulk single-crystal Gallium nitride at high temperatures. Jpn J Appl Phys. 2000;39:L200.
[13] Yonenaga I. Thermo-mechanical stability of wide-bandgap semiconductors: high temperature hardness of SiC, AlN, GaN, ZnO and ZnSe. Phys B Condens Matter. 2001;308–310.
[14] Preston FW. The theory and design of plate glass polishing machines. J Soc Glass Tech. 1927;11:214.
[15] Nakamura S, Senoh M, Iwasa N, Nagahama S. High-brightness InGaN blue, green and yellow light-emitting diodes with quantum well structures. Jpn J Appl Phys. 1995;34:L797.
[16] Asif Khan M, Shur MS, Kuznia JN, Chin Q, Burm JW, Schaff WJ. Temperature activated conductance in GaN/AlGaN heterostructure field effect transistors operating at temperatures up to 300˚C. Appl Phys Lett. 1995;66:1083.
[17] Nakamura S, Mukai T, Senoh M. Candela - class high - brightness InGaN/AlGaN double - heterostructure blue - light - emitting diodes. Appl Phys Lett. 1994;64:1687.
[18] Wu XH, Fini P, Keller S, Tarsa EJ, Heying B, Mishra UK, et al. Morphological and structural transitions in GaN films grown on sapphire by metal-organic chemical vapor deposition. Jpn J Appl Phys. 1996;35:L1648.
[19] Belyaev LM. Ruby and sapphire. Moscow: Nauka; 1974.
[20] Shiratsuchi Y, Yamamoto M, Kamada Y. Surface structure of self-organized sapphire (0001) substrates with various inclined angles. Jpn J Appl Phys. 2002;41:5719.
[21] Aida H, Kim S-W, Sunakawa K, Aota N, Koyama K, Takeuchi M, et al. III–nitride epitaxy on atomically controlled surface of sapphire substrate with slight misorientation. Jpn J Appl Phys. 2012;51:025502.
[22] Yuasa T, Ueta Y, Tsuda Y, Ogawa A, Taneya M, Takao K. Effect of slight misorientation of sapphire substrate on metalorganic chemical vapor deposition growth of GaN. Jpn J Appl Phys. 1999;38:L703.
[23] Lu D, Florescu DI, Lee DS, Merai V, Parekh A, Ramer JC, et al. Advanced characterization studies of sapphire substrate misorientation effects on GaN-based LED device development. Phys Status Solidi A. 2003;200:71.
[24] Grudowski PA, Holmes AL, Eiting CJ, Dupuis RD. The effect of substrate misorientation on the photoluminescence properties of GaN grown on sapphire by metalorganic chemical vapor deposition. Appl Phys Lett. 1996;69:3626.
[25] Kim S-W, Aida H, Suzuki T. The effect of a slight mis-orientation angle of c-plane sapphire substrate on surface and crystal quality of MOCVD grown GaN thin films. Phys Status Solidi C. 2004;1:2483.
[26] Kim S-W, Aida H, Suzuki T. Effect of substrate mis-orientation on GaN thin films grown by MOCVD under different carrier gas condition. Phys Status Solidi C. 2005;2:2170.
[27] Kim S-W, Aida H, Suzuki T. Influence of Mis-Orientation of C-plane sapphire substrate on the early stages of MOCVD growth of GaN thin films. Mater Res Soc Symp Proc. 2005;831:E3.40.1.
[28] Aida H, Doi T, Takeda H, Katakura H, Kim S-W, Koyama K, et al. Ultraprecision CMP for sapphire, GaN, and SiC for advanced optoelectronics materials. Curr Appl Phys. 2012;12:S41.
[29] Yoshimoto M, Maeda T, Ohnishi T, Koinuma H, Ishiyama O, Shinohara M, et al. Atomic - scale formation of ultrasmooth surfaces on sapphire substrates for high - quality thin - film fabrication. Appl Phys Lett. 1995;67:2615.
[30] Krukowski S, Grzegory I, Bockowski M, Lucznik B, Suski T, Nowak G, et al. Growth of AlN, GaN and InN from the solution. Int J Mater Product Technol. 2005;22:226.
[31] Motoki K, Okahisa T, Hirota R, Nakahata S, Uematsu K, Matsumoto N. Dislocation reduction in GaN crystal by advanced-DEEP. J Cryst Growth. 2007;305:377.
[32] Yoshida T, Oshima Y, Eri T, Ideda K, Yamamoto S, Watanabe K, et al. Fabrication of 3-in GaN substrates by hydride vapor phase epitaxy using void-assisted separation method. J Cryst Growth. 2008;310:5.
[33] Hashimoto T, Wu F, Speck JS, Nakamura S. A GaN bulk crystal with improved structural quality grown by the ammonothermal method. Nat Mater. 2007;6:568.
[34] Kawamura F, Iwahashi T, Omae K, Morishita M, Yoshimura M, Mori Y, et al. Growth of a large GaN single crystal using the liquid phase epitaxy (LPE) technique. Jpn J Appl Phys. 2003;42:L4.
[35] Taverier PR, Margalith T, Coldren LA, Denbaars SP, Clarke DR. Chemical mechanical polishing of gallium nitride. Electrochem Solid-State Lett. 2002;5:G61.
[36] Pearton SJ, Zolper JC, Shul RJ, Ren F. GaN: Processing, defects, and devices. J Appl Phys. 1999;86:1.
[37] Hanser D, Tutor M, Preble E, Williams M, Xu X, Tsvetkov D, et al. Surface preparation of substrates from bulk GaN crystals. J Cryst Growth. 2007;305:372.
[38] Hayashi S, Koga T, Goorsky MS. Chemical mechanical polishing of GaN. J Electrochem Soc. 2008;155:H113.
[39] Sadakuni S, Murata J, Yagi K, Sano Y, Arima K, Hattori AN, et al. Influence of the UV light intensity on the photoelectrochemical planarization technique for Gallium nitride. Mater Sci Forum. 2010;645–648.
[40] Doi T, Philipossian A, Ichikawa K. Design and performance of a controlled atmosphere polisher for Silicon crystal polishing. Electrochem Solid-State Lett. 2004;7:G158.
[41] Doi TK, Watanabe S, Doy H, Sakurai S, Ichikawa D. Imapact of novel Bell-jar type CMP machine on CMP characteristic of optoelectronics materials. Int J Manuf Sci Technol. 2007;9:5.
[42] Doi TK, Yamazaki T, Kurokawa S, Umezaki Y, Ohnishi O, Akagami Y, et al. Study on the development of resource-saving high performance slurry —Polishing/CMP for glass substrate in a radical polishing environment, using manganese oxide slurry as an alternative for ceria slurry—. Adv Sci Technol. 2011;64:65.
[43] Kitamura K, Doi TK, Kurokawa S, Umezaki Y, Matsukawa Y, Ooki Y, et al. Trial manufacturing of a simultaneous double-side CMP machine housed in a sealed, pressure-resistant container and its polishing characteristics. Key Eng Mater. 2010;447–448.
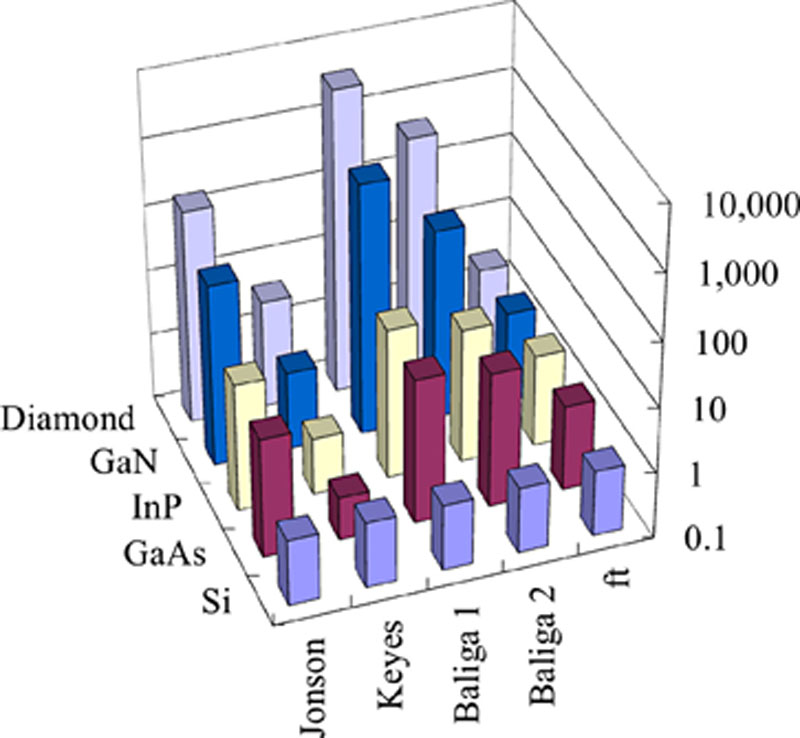
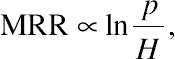 (A.1)
(A.1)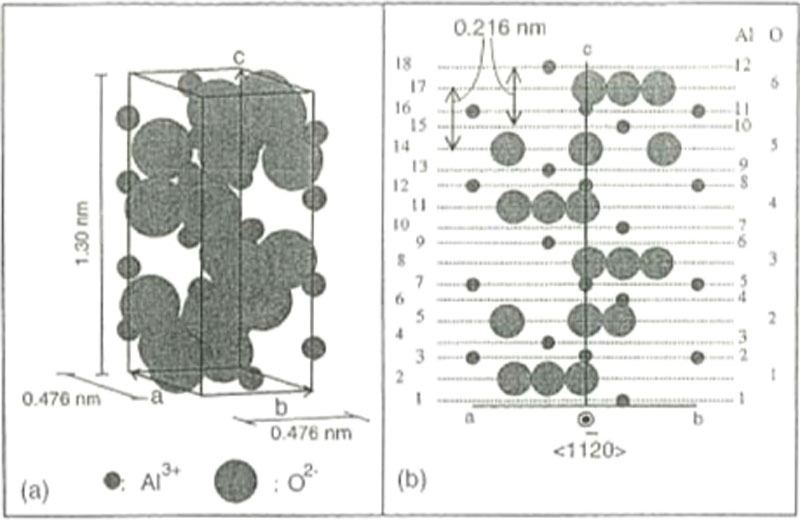

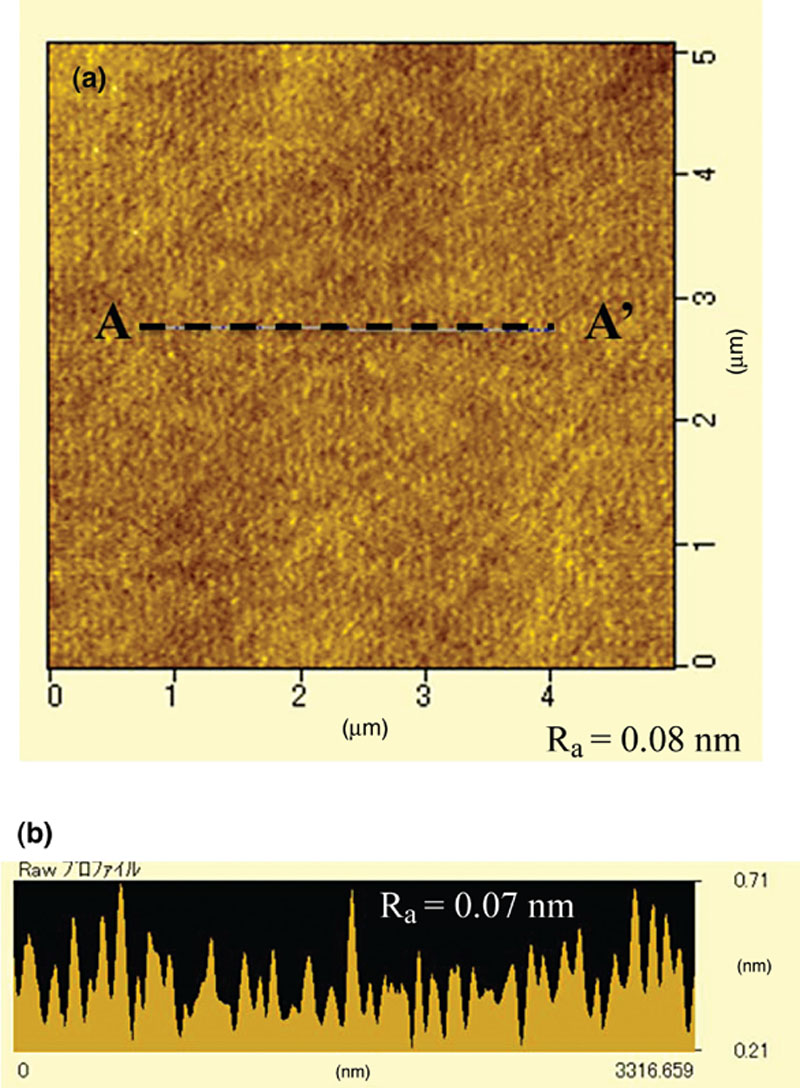
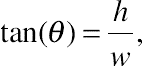 (A.3)
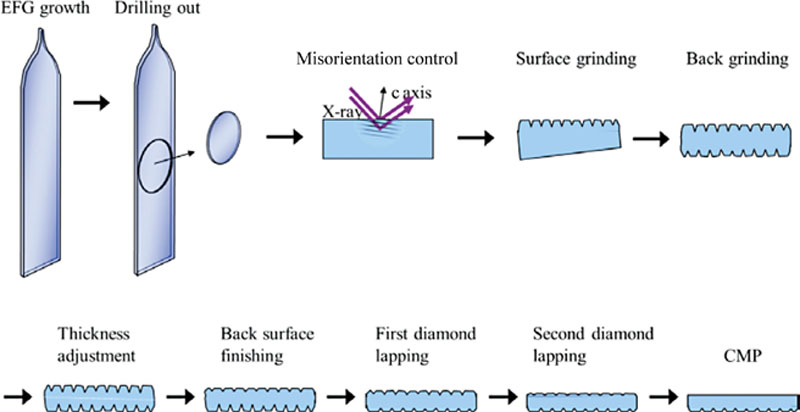
(A.3)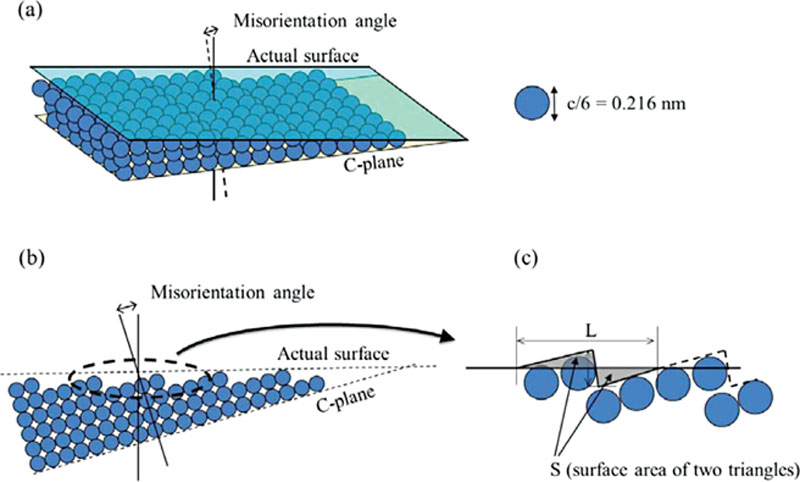
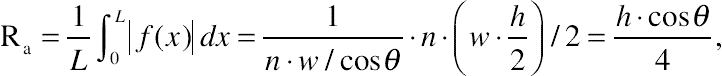 (A.4)
(A.4)