Liquid crystal polymers (LCPs) as a reinforcement in high temperature polymer blends
Abstract:
Liquid crystal polymers (LCPs) are a relatively new class of high temperature polymers that have attracted a great deal of interest, both academically and industrially. This interest has arisen because of the very high mechanical properties that LCPs provide, as well as the relatively low melt viscosity. There have also been attempts to take advantage of these unique properties in mixtures with other polymers. This chapter will summarize those studies and highlight the advantages offered by the use of LCPs in blends, including an effect referred to as a self-reinforcing composite. It is also possible to use LCPs as rheology modifying agents. Future directions in the area are recommended.
5.1 Introduction
Liquid crystal polymers (LCPs) are a relatively new class of high temperature polymers. Blends of these materials have been produced that take advantage of the unique features of the LCPs, including the very high physical properties they offer. This chapter will summarize the activities, both academic and industrial, that have been carried out in this area. The idea of creating a reinforcement species in situ while conducting processing operations of a material which is unreinforced prior to processing is an attractive and elegant way to sidestep problems encountered with conventional filled materials, such as increased viscosity, degradation of the reinforcing species, contamination of the final product and wear on the tooling. This concept is not limited to polymer blends, but occurs in metallurgy as well.
In the field of polymer engineering, several approaches have been considered. One is to exploit the fact that extended-chain crystals of polymers are both more stable (higher melting point) and stronger (not weakened by the presence of chain folding). We will not discuss the extensive literature on solution-grown extended-chain crystals nor that of solid-state extrusion, but confine ourselves to thermoplastic processing. An early example is by Janssen et al.1 in which the possibility of forming a self-reinforcing composite of a single material (polypropylene) by extrusion under conditions such that extended-chain reinforcing fibers are formed within a matrix of chain-folded material. Another approach to this problem is that of in situ polymerization in which a monomer species is created within a matrix such that a post-processing polymerization step can occur, leading to the eventual polymeric reinforcing species. Examples of this are Collyer2 and Kiss et al.3
The focus of this chapter is more specifically blends in which the reinforcing species is a thermotropic LCP which is blended in a rather conventional way with isotropic thermoplastics (TP). This exploits the inherent strength and low viscosity of the LCP in a way which leads to maximum benefit at minimum processing complexity. This has been an actively studied area of polymer engineering for 40 years and continues to be of interest. We note that though much work has been done on the science of LCP blends, there is also a need to review the exploitation aspects in terms of cost considerations, patents and commercial products.
5.2 Researching liquid crystal polymers (LCPs)
Before the advent of the internet and easily accessible online search tools, there was great value in a work which purported to be a complete and exhaustive overview of a specific aspect of a field. Presumably the author would have read essentially every relevant article from the major journals in the field, as well as a selection of obscure journals, from bound volumes and current issues in a library. This was done as a service to others with an interest in the field, to spare fellow researchers the effort of having to hunt down all of the primary materials themselves. The production of review and survey articles was part of the process of digesting new science and incorporating it into the body of established science. The unspoken assertion of the author of such a work was that ‘if it is not in my survey/review article, it is not significant’.
Exhaustiveness as a virtue in its own right has been made obsolete and trivial by online search and research tools, which allow any given article to be the entrée to both an infinite regress and an infinite progress of citations. This chapter can be seen as something of a sampler, in which many references are included as a starting point for locating further related work, rather than the final reference. It is truly an awe-inspiring experience for one who was an active researcher in the pre-internet days, when ‘literature search’ was synonymous with many days in the library, many hours at the copy machine, and many pages of handwritten notes, to experience contemporary online search, such as Web of Science.4 (One of us [GK] would like to express heartfelt appreciation to Lisa Klein of Rutgers for guest access to Web of Science.) In real time one can start with a search such as ‘in situ composite’ and find 175 hits, and see them immediately or export them to a convenient list of abstracts for perusal. Each hit is characterized by a very useful piece of metadata: the number of times the work has been cited in more recent work. This allows one to see immediately which papers have been heavily cited and are ‘required reading’ in the field.
Alternatively, one can start with the 175 ‘in situ composite’ hits, then add additional terms such as ‘polymer’ (26 hits), ‘LCP’ (12 hits) and ‘rheology’ (5 hits) and focus rapidly and efficiently onto the niche of interest, and have the papers (and their reference lists) in hand immediately as PDFs on a USB stick. Thus many days of pre-internet literature search can be accomplished in a single working session. The term continues to be used to describe LCP/thermoplastic polymer blends, as does the term self-reinforcing composite (though the latter is a misnomer, since the matrix does not in fact reinforce itself; there is a distinct reinforcing species).5,6
The references are divided into three categories. First are the traditional references to scientific and trade journals. Second, we have chosen to segregate the patents which are a component of the technical literature to be sure, but not motivated primarily by a desire to disseminate information and instruct, but by a desire to exploit the technology commercially. Many of the questions which naturally occur to the reader are left deliberately obscure in patent writing. Third, we include a category for ‘links and personal communication’ which expands on the ephemeral conversations and letters which had traditionally been referenced to include online resources in the form of links which may or may not be viable for future readers. Online search makes it simple to track down persons who might be willing to confirm or discuss a ‘personal communication’. Also, the internet archive (aka the ‘Wayback Machine’) makes even expired links potentially viable.
Although we have said that review and survey articles are less critical now than in the pre-internet days, nevertheless they represent the best resource for information about discoveries and progress as this field emerged in the 1960s, 1970s and 1980s. It continued to generate substantial interest in the 1990s, by which time online resources were becoming available. In the 2000s and to the present day, LCP blends continue to generate interest both in journal articles and patents, and in conferences.
An indispensable reference which reviewed the state of the art in 1990 is Dutta, Fruitwala, Kohli, and Weiss.7 The rheology of LCPs was reviewed by Wissbrun.8 A more recent review is from 1996,9 particularly Chapter 4 by Cogswell and Wissbrunn10 and Chapter 6 by Collyer.11 Three early papers published in a single issue of Polymer Engineering and Science V27 (1987) have each been cited hundreds of times as of August 2011.12–14 These papers popularized the term in situ composite to indicate the notion that the reinforcing species does not exist per se in the unformed material, but rather comes into existence during processing.
5.3 Liquid crystals
Much of the material in this section can be seen in an online webinar, courtesy of Malvern Instruments.15 Liquid crystals, also known as ordered fluids, are an intermediate state of matter between ordinary liquids and crystalline solids. Like ordinary liquids they flow in response to stress, although some exhibit yield stress. Like crystalline solids they exhibit some degree of long range order, in some cases forming elaborate patterns which extend for distances many orders of magnitude greater than the molecular length. The subject of liquid crystals is vast and has been studied intensely for many decades, the earliest citation being from 1888. The most familiar liquid crystal phenomena are optical effects exploited for displays, and involve low molecular weight compounds.16,17
Liquid crystals can be formed by melting of crystalline solids, in which case they are called thermotropic, or by dissolving certain solids in a solvent, in which case they are called lyotropic. Both of these routes to liquid crystals can give rise to a panoply of structures (called ‘mesophases’), of which the best known are nematic, cholesteric, and smectic.
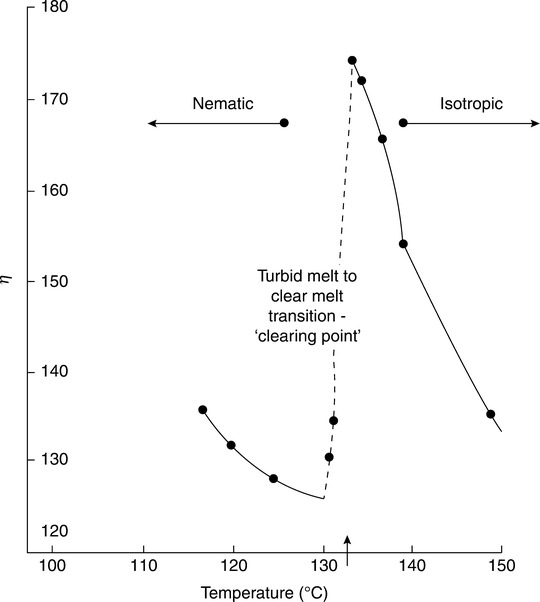
The simplest of the structures is the nematic, which is formed by molecules with high aspect ratio (‘rod-like’) and characterized by a degree of long range order in the orientation of the long axis of the molecules but not in any other geometric feature. A schematic view of a small-molecule nematic such as the extensively studied para-azoxyanisole (p-azoxyanisole), is shown in Fig. 5.1a and 5.1b. Most small-molecule nematics can be heated sufficiently that they lose their orientational order before decomposing, forming a conventional isotropic liquid as shown in Fig. 5.1c. This temperature is often called the ‘clearing point’ because there is usually a loss of turbidity.
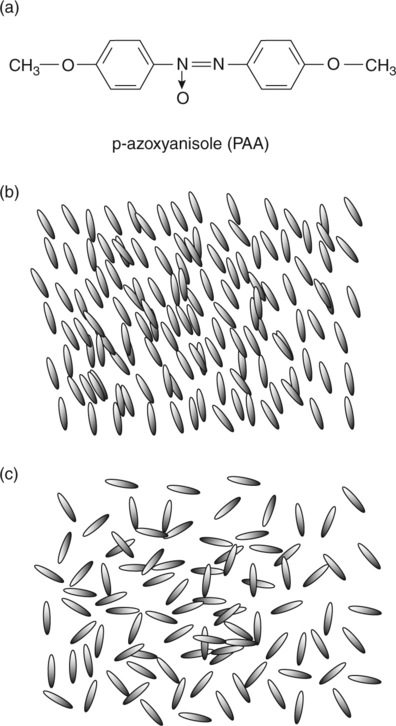
The nematic-to-isotropic transition also occurs for lyotropic small-molecule liquid crystals. Looking at Fig. 5.1b and 5.1c, one can easily imagine that the oriented molecules would slide past one another with relative ease in the nematic. In isotropic melts the molecules at rest have no preferred orientation and would interfere with each other to a greater extent when sheared. Also, they would be expected to display shear-thinning as shear-induced orientation is created. Thus one would expect a lower zero-shear viscosity in the nematic melt, although it exists at a lower temperature than the isotropic melt. This is indeed the case, and a sudden increase in viscosity at the clearing temperature is seen in many nematics as the orientational order at rest disappears (see Fig. 5.2). The rheology of small-molecule liquid crystals was reviewed in 1962.18
5.4 Polymer liquid crystals
Flexible polymers generally do not form mesophases; however, polymers which have a high aspect ratio are sometimes able to. There are two broad categories of rod-like polymers:
• Those which have a rigid backbone, composed entirely of rigid bonds, which happen to be arranged so that the overall molecule is elongated.
Not incidentally, polymers with extended backbones have high modulus, since elongating the bulk polymer requires bonds to be stretched, rather than bent.
• Those which have a flexible backbone which happens to take on a conformational structure such that the overall molecule is elongated. The best-known example of this is helical molecules such as DNA and PBLG, but in fact much early work was done using a living rod-like polymer, namely tobacco mosaic virus.19,20
Examples exist of both types of rod-like polymers forming both lyotropic and thermotropic mesophases. An exploration of the correspondence between the two types is given in reference 21.
5.4.1 Lyotropic polymer solutions
Rigid backbone polymers are typically difficult to dissolve. A significant application of lyotropic polymer solutions is the nematic spinning dope which is obtained when dissolving para-aromatic polyamides (‘aramids’) in powerful solvents such as concentrated sulfuric acid. Note that the polymer itself is not liquid crystalline, as commonly stated, but rather the spinning dope is a nematic. The best-known example is DuPont’s Kevlar, and the commercial significance of Kevlar inspired a great deal of activity around both lyotropic and thermotropic polymer liquid crystals, starting in the 1960s.22–24
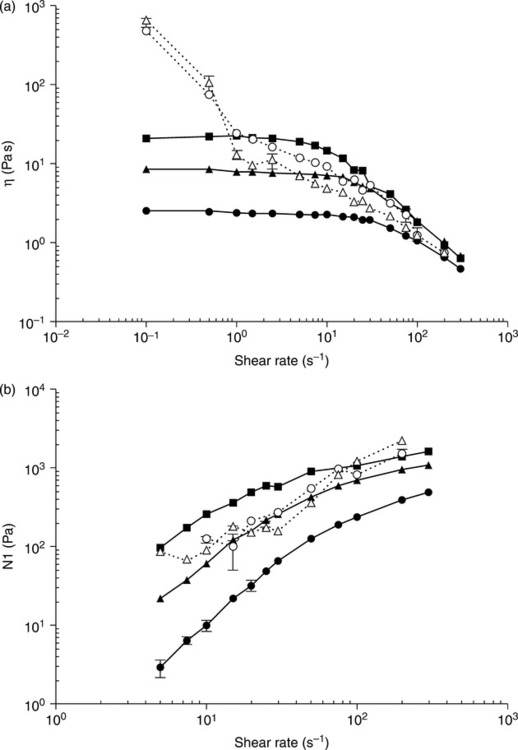
The viscosity behavior seen in small-molecule nematics is echoed in lyotropic polymer solutions. Concentration plays a role analogous to that of temperature. Just as increasing temperature causes the loss of zero-shear orientation in small-molecule nematics, so too does decreasing concentration. When concentration falls below a critical value, which is related to aspect ratio, polymer solute molecules are able to assume random orientation due to the availability of sufficient volume per molecule25, and a phenomenon analogous to the viscosity peak at the clearing point is seen. That is, a peak in zero-shear viscosity vs. concentration is observed with the analogous surprising observation that the more concentrated (anisotropic) solutions have lower viscosity than the less concentrated (isotropic) solutions of the popular model system poly-gamma-benzyl-glutamate in m-cresol.26,27 Furthermore, the effect of the volume-filling orientation is seen to be most pronounced when extrapolated to zero-shear, and it decreases in magnitude while shifting to lower concentration as shear rate is increased (see Fig. 5.3), and eventually disappears entirely when the shear-induced orientation overwhelms the volume-filling effect.
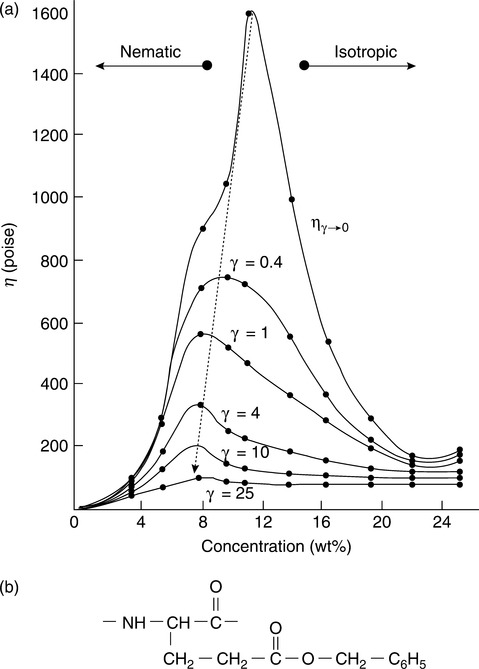
The viscosity behavior is thus simple, straightforward and unsurprising. Not so the normal stress behavior. The same model system gives bizarre and unexpected normal stress behavior in that steady-state values of normal stress are observed at well-defined shear rates intermediate between a low shear rate positive negative transition and a high shear rate negative positive transition, as seen in Fig. 5.4.23 This behavior is well documented, and a satisfactory theoretical model has been developed.28
5.4.2 Thermotropic liquid crystal polymers
Thermotropic liquid crystal polymers are very analogous to small-molecule nematics. They are (partially) crystalline solids which melt to form anisotropic melts. As stated earlier, polymers which are rigid and rod like typically have melting points higher than their decomposition temperatures, so must be processed from solution. This obviously restricts the shapes available to fibers and films. Much effort has been expended to create rod-like polymers which can melt sufficiently to be molded and yet retain the desired mechanical properties. Various approaches to slightly disrupting the rod packing have been tried. (Griffin and Cox21 referred to this process using the vivid phrase ‘frustrated chain packing’.) One approach is to introduce a kink into the rod by using naphthalene moieties in the backbone. Another approach is to introduce non-para linkages so that the molecule rod is less straight. Two other approaches are to add bulky substituents to the aromatic rings (another example of frustrated chain packing), and to incorporate flexible linkages into the backbone.
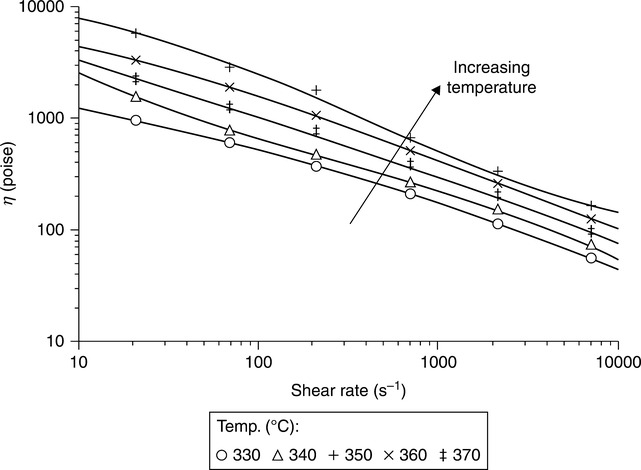
The rheology of liquid crystal polymers was reviewed by Wissbrun.8 A more recent review is from 1996,10 particularly Chapter 4 by Cogswell and Wissbrunn, which makes the point that LCP ‘changes the rules which govern the balance between ease of processing and service performance’ compared to conventional thermoplastics reinforced by the addition of discrete fiber fillers. These reviews describe the essential features of the usual response of thermotropic polymers to shear, namely shear-thinning, lower viscosity than isotropic polymers, and conventional (negative) thermal coefficient of viscosity. However, thermotropic polymers also reveal some rheological surprises. There are no examples of steady-state negative normal stress, but there are observations of transient negative normal stress.8 There is also a report of unconventional (positive) thermal coefficient. Viscosity was observed to increase with temperature over a broad range of temperatures and shear rates for a copolymer of hydroxybenzoic acid (HBA), isophthalic acid (IA), and hydroquinone (HQ) called HIQ29 as seen in Fig. 5.5 for HIQ 35. This unusual phenomenon was confirmed28 and has application in LCP blends, as we will see later in Section 5.6.2.
5.5 Blends of isotropic and anisotropic thermotropic polymers
The concept of blending of isotropic polymers with LCP’s goes even further in the direction of ‘changing the rules’, as quoted in Section 5.4.2, as a strategy for engineering materials which strike exactly the right balance of properties and processability. Since the major component (the matrix) is the isotropic polymer, the overall blend exhibits many of its properties (not the least of which is cost) or at least is heavily biased in that direction. Furthermore, the degree of freedom traversed in optimizing the material is the concentration of the LCP phase, which is easily controlled in compounding or even at the last minute by dry-blending in the hopper, which is certainly easier than varying chemistry or molecular weight. An indispensable reference which reviewed the state of the art in 1990 is Dutta, Fruitwala, Kohli, and Weiss.7 This paper also considered small-molecule liquid crystals as additives to conventional thermoplastics.
5.5.1 Miscibility and compatibility
When blending two or more polymers, the question of miscibility comes up. And if the two components are immiscible, then the next questions to be asked are those of adhesion and compatibilization. Polymers in most cases are not miscible because of low enthalpy of mixing, but examples of miscible blends containing an isotropic and a thermotropic polymer are known. One recent example is a study elucidating the phase diagram of polycarbonate with HBA-HNA copolyester30 which used optical microscopy and transmitted light intensity analysis to determine two separate phase separation temperatures at which ‘slight’ and ‘pronounced’ phase separation occurred. They also used NMR and FTIR to establish that no transesterification was occurring. Their Figure 13 shows a phase diagram with temperature from 220°C to 300°C and LCP content between 20 wt % and 60 wt % exhibiting three well-defined regions.
Blends of polycarbonate with the HIQ polymer described in Section 5.4.2 were found to be miscible.30 The authors observed by thermal and thermomechanical analysis that the Tg of the blend was greater than that of either pure component, and indeed the Tg of the HIQ component was absent in a blend containing as much as 75% HIQ. They consider the combination of miscibility of LCP with a thermoplastic and a non-intermediate Tg to constitute a novel polymer blend pair.
The minimum performance which is hoped for in a polymer blend is to follow the Rule of Mixtures, which is simply a volume averaged result based on the composition. If both components are polymers, then volume average and weight average are about the same. In many cases the performance of the blend falls below the Rule of Mixtures, due to poor adhesion, degradation or other chemistry during processing, details of orientation and morphology, and a host of other unsurprising problems which can lead to disappointing performance. The surprising and hoped-for result is performance which exceeds that predicted by the Rule of Mixtures, which would represent a synergy. Better yet is performance which exceeds that of either pure component.
5.5.2 Mechanical properties of LCP/polymer blends
The motivation behind much of the work on LCP blends is the desire to achieve improved mechanical properties in a useful regime of cost, performance, and processability. The early work in this area took place shortly after the advent of thermotropic polymers and was an attempt to capitalize on the desirable points of thermotropic polymers while avoiding some of the drawbacks (cost, poor transverse properties, weld lines, etc.)
Synergies in mechanical properties were reported by Isayev and Modic.31 Dynamic mechanical measurements showed local maxima above the Rule of Mixtures in storage modulus for two LCPs blended with polycarbonate. Young’s modulus of extruded strands showed local maxima above the Rule of Mixtures, especially for ‘unsupported’ strands, leading to the possibility that this was a drawing effect. However, local maxima in Young’s modulus were also observed for compression molded films and injection-molded bars. The most striking observation was that of transverse strength in injection-molded bars being higher for blends than for either base polymer (p. 166 and Figure 11 of reference 31). Synergy in viscosity behavior would be the observation of viscosity lower than that predicted by the Rule of Mixtures, or even lower than either neat polymer.
5.6 Processability of LCP/thermoplastic blends
As referred to earlier, LCP/thermoplastic polymer blends represent a route to novel materials that ‘change the rules’ when it comes to the balance and tradeoffs between processability and properties. In addition to the ability of these low-viscosity blends to fill long flow paths (giving greater flexibility in gate location) and thin-wall sections (conserving material) there are other considerations such as energy consumption during processing, abrasion of tools, and surface quality. Abrasion of screws, runners, and molds is particularly important in the case of medical devices, which is an application area that could tolerate the relatively high cost. Metal contamination is clearly unacceptable for medical devices, and surface quality is also critical.
5.6.1 Viscosity ratio
A thorough study of the effect of viscosity ratio (LCP to TP) was carried out by Fekete et al.32 Although some authors have asserted that a viscosity ratio of less than one (LCP less viscous than TP at the processing condition) would be optimal, this was not borne out in this study. Of course ‘processing conditions’ is a broad term, which includes temperature, pressure, shear rate and possibly elongation rate as well. This paper compared flow-direction and transverse strength for a variety of TPs and LCP contents, and concluded with this rule of thumb: ‘The results of the experiments revealed that the reinforcing effect is directly related to the viscosity decrease of the blends compared to the neat thermoplastics.’
A particularly interesting test of the above rule of thumb would be blends containing the HIQ polymer described in Section 5.4.2 in which the unusual positive thermal coefficient of viscosity was attributed to the coexistence of isotropic and anisotropic domains of the coploymer, as determined by the distribution of copolymer ratios and therefore local chain stiffness. As temperature increased, the fraction of the high viscosity isotropic phase increases at the expense of the low-viscosity anisotropic phase. This polymer by itself as the LCP component would open a degree of freedom in varying the viscosity ratio. Furthermore, blending this HIQ LCP with more conventional LCPs with which it is miscible33 would expand the window of control of the LCP component.
5.6.2 Spiral flow
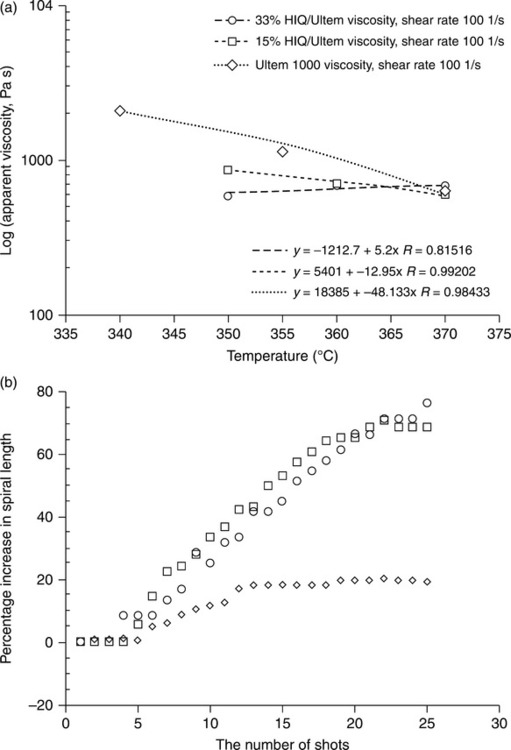
The spiral flow test is commonly used to pragmatically determine the ability of a polymer to tolerate long flow lengths and form thin-walled injection-molded parts. We have mentioned that a particular LCP called HIQ exhibits the unusual property of positive temperature dependence of viscosity, the opposite of all known isotropic thermoplastics. This phenomenon was exploited in LCP/thermoplastic polymer blends to provide self-stabilized viscosity and greater resistance to process drift.34 Figure 5.6 shows viscosity independent of temperature for a 35 wt% HIQ/Ultem blend, and the stabilizing effect on spiral flow length when the mold temperature was abruptly increased by 30°C. This caused a 75% increase in spiral flow length for unblended Ultem after 20 shots but only a 20% increase in length for the blend.
5.6.3 Weld lines
A well-known characteristic of chopped-fiber composites and LCP is that they are extremely sensitive to weld lines in injection molding, so that care must be taken to move the weld lines to non-critical areas within the part. A study of butt-type weld lines compared LCP/TP blends with pure matrix polymer for a number of TPs and determined that there was significant reduction in strength relative to the non-weldline case, but that there were blends in which the weldline strength was nevertheless greater than the pure TP. In every case, the tensile modulus, which is often the more important property for engineering thermoplastics, was greater for the LCP/TP blend weldline than for the maximum observed for the pure TP (in some cases the pure TP had higher modulus for the weldline case than for the non-weldline case). This study did not compare with filled grades, which of course would be the more commercially significant comparison. Weld lines for blends of immiscible polymers in which neither component is an LCP were found to be lower than either pure component.35
5.7 Structure–property relationships of LCP blended materials
Polymers in general have properties which depend strongly on orientation of the chains. LCPs in particular are extremely sensitive to processing conditions which give rise to morphologies that may or may not be favorable for developing favorable mechanical properties. If the LCP is present in spherical or low-aspect ratio domains, then it is used inefficiently for reinforcement, which is particularly problematic given their high cost. Significant effort and sophistication are required to achieve a worthwhile improvement in properties by managing the flow during processing, and the adhesion of the reinforcing domains to the matrix. Another factor to consider is that the highly oriented LCP component can act as a nucleating species to encourage the flexible polymer matrix to crystallize in an oriented rather than a random fashion. The notion is similar to the concept of ‘row-nucleation’, which was studied to such spectacular effect by Keller.
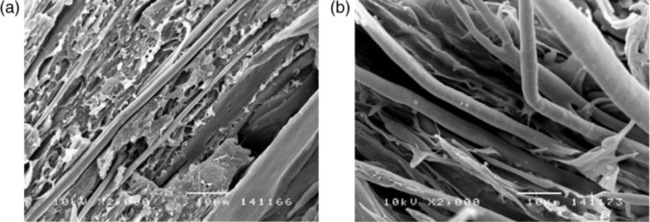
A distinction must be made between extruded and fiber-spun articles, which benefit from uniform shear and elongation flow, both of which are highly efficient at orienting LCP domains, and the complex flows experienced in injection-molded articles. A recent paper on the structure-property relationships of PEN (Polyethylene Naphthalate)/LCP blend fibers used a novel CO2 laser heating method during fiber draw to heat the fiber uniformly rather than outside-in, and achieved synergistic effects.36 The paper included SEM micrographs of well-oriented and well-adhering LCP fibrils, and an example of LCP fibrils is shown in Fig. 5.7. Interestingly, naphthalene dicarboxylate (NDC), a key monomer for PEN, has also been proposed as a lower-cost monomer for LCPs.
Injection-molded articles tend to exhibit skin-core and multi-layer morphologies in which the LCP domain aspect ratio varies considerably with location within the part. The hierarchy of structures found within LCP/TP blends was elucidated by Silverstein et al.,37 who said ‘the microdomains form domains, the domains form microlayers, the microlayers form sublayers, and the sublayers form a layered structure’. The structural hierarchy in PET/CLP blends was characterized by Narh5 who found good agreement of fibril length observed in sublayers with a numerical prediction.
The transition between low-aspect ratio and high-aspect ratio domains can be quite abrupt, as shown in reference 13. In those portions of the part where the flow is favorable to the formation of high-aspect ratio domains, the ‘forest’ of LCP ‘needles’ emerging from the surface can be quite spectacular, especially considering that the domains did not exist prior to the formation of the part, but were formed in situ.
Of course the adhesion between the LCP domains and the matrix must be poor for the elongated domains to pull out and appear needle-like, rather than breaking, so this vivid image is not necessarily a favorable one. A better image from the point of view of useful reinforcement is one of broken, but clearly elongated, domains, in which the flow-induced internal structure of the LCP domain may be visible in a brittle fracture surface of the domain in the blend product.
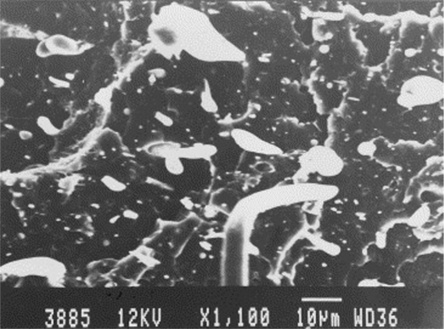
In other cases the adhesion between matrix and LCP reinforcement is good. The next SEM micrograph, Fig. 5.8, shows a rather similar forest of tapered needles, but in this case the tapering was due to drawdown during break (as opposed to brittle fracture) and not to pull out of poorly adhering domains, since the adhesion is seen to be excellent.
Recent work in in situ composites has included the incorporation of single and multi-wall carbon nanotubes (SWCNT and MWCNT). Notably, a low-cost alternative exists in the form of thermal chemical vapor deposition-grown carbon nanofibers (CNF) which are slightly inferior due to greater diameter and shorter length, but are available in kg quantities.38 Carbon nanotubes and LCP seem to be highly complementary because both have a high aspect ratio. LCP is thought to promote good dispersion of CNF, which is necessary to develop good mechanical properties efficiently. In addition, the good dispersion promotes the formation of a conductive network by achieving a percolation threshold at lower CNF loading, leading to improved electrical conductivity at lower cost.
The adhesion between CNF and LCP appears to be favorable, or can be made so using surface treatment of the CNF,39 leading to the observation of ‘bridging’ nanofibers which transfer stress from the matrix to the LCP reinforcement, particularly under conditions where the LCP domains are also undergoing fibrillation.40
5.8 Commercial LCP blends
This section contains a description of commercial activity in LCP blends. In addition, a discussion of patent activity on the topic is provided.
5.8.1 Patents
Much of the early publication in this field was in the form of patents. There was a flurry of patent activity in the early 1980s in the area of LCP/thermoplastic polymer blends, starting with Takayanagi at Asahi Chemical.41,42 Early contributions were also made by Cogswell, Griffin and Rose at ICI.43,44 A number of patents came out of Celanese, including a string by Froix combining LCP with a number of different matrix polymers.45–52 Most of these were relatively straightforward composition of matter patents, though reference 52 was unusual in that in addition to the composition of the blend it specified the size and aspect ratio of the LCP domains within the blend. Isayev at University of Akron also produced at least one patent in the area at this time.53
Celanese remained active in this area and produced some interesting technology in the mid-1990s. Many of these involved blends in which both components were anisotropic (Section 5.4), but Makhija et al.54 describe a technique for LCP/isotropic thermoplastic blends in which components are fed sequentially into a mixing die to give additional control over the morphology. Either the LCP or the isotropic polymer can be first, followed by the other component. In some cases solid polymer is fed into an extruded stream of melt. The patent gives numerous examples of PET first vs. LCP first vs. simultaneous feed, and shows improved mechanical properties.
A relatively recent patent from Foster-Miller55 describes a multiaxially oriented film in which the LCP component of the blend provides barrier properties and controls CTE, while allowing operations such as heat-sealing and microwave heating of food. This was the technology licensed by the Superex spin-off discussed below.
A quite recent Celanese patent is that of Jester,56 which describes blending a ‘stretchable’ LCP into a thermoplastic so that the reinforcement can deform with the matrix and thereby maintain adhesion during post-processing steps. One of the most recent patents we found in the area of LCP blends is actually an LCP/LCP blend rather than an in situ composite.57 It appears that this blend was developed for a specific application (housing for air-conditioner with high-speed fan) which required rigidity, long flow paths, thin walls, and low processing temperature for excellent ‘weldability’. It demonstrates the progress which continues to be made in filling specific niches by engineering materials via blending rather than chemistry. It would be an interesting exercise to determine if this specific housing could have been made equally well but at lower cost with an in situ composite blend.
5.8.2 Commercial products
LCP blends have ‘shown promise’ for many years, and yet there have been relatively few actual commercial applications. A great disadvantage of LCPs is their high cost relative to glass fiber reinforcement, which makes it hard to compete purely on mechanical properties. It therefore becomes necessary to compete with conventional plastics on the basis of other properties which confer high value. Examples are electrical properties, gas barrier properties, and controlled thermal expansion coefficient. Pure LCPs excel in these areas, and blends containing a significant amount of LCP can improve conventional thermoplastics significantly.58 The improvement in processability is a bonus which may tip the balance in favor of an LCP-modified grade, or in some cases make an application possible which otherwise might not be, such as thin-walled objects. CTE control is a major issue with fiberoptic cables, and approaches using LCP have been patented. A blend of LCP with Nylon 12 was reported to show rapid decrease in CTE with LCP content, reduced to 25% of the pure Nylon value as LCP content reached 20%, near-zero CTE at LCP content of 40% and slightly negative CTE with even greater LCP content.
Some descriptions from the trade literature of the 1990s indicated that commercial products were being launched. Although some of the blends described were development projects, a few sound as if they were commercial at the time. Hoechst-Celanese (actually its Polyplastics joint venture with Daicel in Japan) had two Vectra grades X037 and X039 which were PC/LCP blends, neither of which have survived.
A spin-off from Foster-Miller in Waltham MA called Superex Polymer marketed a material in the mid-1990s for $2-$3/lb which was described as a PET/LCP blend called ‘Super PET’ in a Nasa Tech Report link.59 See also reference 55. This material was intended to prevent gas ingress into food, egress from carbonated beverages (especially beer), and water penetration into power cables.60 This material was offered as a licensable technology patent until at least 2000 and possibly beyond. The company was folded back into Foster-Miller in 2000, and Foster-Miller itself was acquired by QinetiQ in 2004.61 Hoechst-Celanese has become Ticona, and they have two grades of PolyPhenylenSulfide (PPS)/LCP, sometimes described as a blend and sometimes as an alloy.62 It is not clear from the product literature, but the matrix component is indeed the PPS.63
5.8.3 LCP/LCP blends
This section of the chapter deals with blends in which both components are LCP. Among the series of patents awarded to Froix in the 1980s was US 4,267,289, issued in May 1981.64 This is a composition of matter patent which does not indicate the benefit but simply claims blends in which one LCP component uses a naphthalene moiety to render the aromatic polyester tractable, whereas the other component uses methyl, chloro, or bromo groups on the aromatic ring.
Another early work in this area is that of DeMeuse and Jaffe from Celanese65 who used several techniques such as rheology, X-ray diffraction, and calorimetry to demonstrate that a model system consisting of blends of two LCP polyesters of differing HBA/HNA ratios were in fact not miscible. This is in contrast to low molecular weight liquid crystals in which two liquid crystals which form the same type are expected to be miscible. This paper also demonstrated that transesterification did not occur at an appreciable rate under the conditions used for melt rheology measurements. They also discuss the concept that for copolymers in which the distribution of copolymer ratio is extremely wide, there could be phase separation within a nominally homogeneous copolymer. This is precisely the mechanism invoked to explain the anomalous temperature dependence of viscosity of HIQ LCP.29
Another study of miscibility of LCP/LCP blends also used the HIQ LCP, blended with Vectra A950 thermotropic polyester.30 This paper confirmed the biphasic nature of HIQ and that HIQ is miscible with Vectra. It determined that the Cox-Merz rule (equivalence of dynamic and steady shear viscosity) was obeyed for the HIQ 45 and blends with Vectra A950 and the anomalous temperature of viscosity dependence was manifested for pure HIQ. They did not, however, indicate what blend composition of HIQ/Vectra manifested viscosity independent of temperature (since Vectra has conventional temperature dependence of viscosity). The same paper also determined that HIQ is miscible with polycarbonate, but the Cox-Merz rule is not followed. They concluded that in both systems the overall behavior is dominated by the less ordered component, whether it be the PC component or the isotropic component of biphasic HIQ.
US Patent 5,393,848, Charbonneau et al., is a Celanese patent from 1995 which discusses in situ polymerization of HIQ to form an LCP/LCP blend in situ.66 The idea here is to create HIQ by polymerizing the monomers within a matrix of molten LCP, for example Vectra. This is a clever way to gain better control over the polymerization process and avoid formation of the problematic biphasic melt. The attention paid to HIQ is because it is produced from three relatively common and low-cost monomers, hydroxybenzoic acid, isophthalic acid, and hydroquinone, avoiding the use of expensive specialty monomers. This method is also proposed as a method to create LCPs which would not be tractable if synthesized conventionally.
A very recent LCP/LCP blend patent57 was already mentioned in Section 5.5.1. A companion patent also issued to Ueno Fine Chemicals is US 7,842,760, issued 20 November 2010, claims essentially the same blend but with emphasis on its dielectric properties.67
5.9 Conclusions and future trends
The rule of thumb (‘… the reinforcing effect is directly related to the viscosity decrease of the blends compared to the neat thermoplastics’) stated by Fekete et al.32 is intriguing but of course too simple and limited. There is a need for a more general theory or predictive model of LCP blends which would allow input of rheological parameters, parameters related to adhesion, orientability, degradation temperature, etc. and of course the cost of the components, and allow one to compare the expected material to commercially available grades of glass-filled thermoplastics, etc.
We feel confident that given the dual benefit of reinforcement and processability improvement, there are high value niches which would be best filled by an LCP blend, particularly a relatively low-cost variant. The potential low cost and unusual rheology of HIQ polymers opens up interesting scientific and commercial possibilities. It would give an opportunity to control viscosity ratio in a novel way. It could be used either alone, or blended with Vectra, since the two seem to be miscible. We would like to see a resurgence of interest in LCP blends and the introduction of commercially successful in situ composite products after all these years.