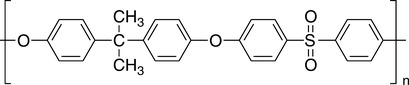
Polysulfones as a reinforcement in high temperature polymer blends
Abstract:
Polysulfones are a class of high temperature polymers that have applications in emerging fields such as membranes. The high temperature nature of the polysulfones allows them to be used in demanding applications that other polymeric materials cannot satisfy. However, as new applications continue to emerge, the properties of the polysulfone need to be modified appropriately. One approach to achieving that goal is through blending with other polymers, both thermoplastics and thermosets. This chapter provides a summary of those blending efforts and suggests additional areas for further research and development work. The use of polysulfones in both membrane applications and as impact-modifiers for thermosetting materials is discussed.
6.1 Introduction
The general term ‘polysulfone’ describes an entire family of thermoplastic polymers which are melt processable. The polymers are particularly known for their toughness and stability at high temperatures. They contain the sub-unit aryl-SO2-aryl, the defining feature of which is the sulfone group. This chapter will discuss blends of polysulfone polymers with other polymers, both thermoplastics and thermosets, and the results obtained from the production of such mixtures.
6.2 Structure and properties of polysulfone
The general chemical structure of polysulfone is shown in Fig. 6.1. It is an amorphous polymer that has a glass transition temperature or Tg of about 185°C. Due to the aromatic backbones present in the chemical structure, polysulfones typically possess high stiffness and strength. Articles made from polysulfone have excellent transparency features.
Polysulfone is highly resistant to mineral acids, alkali and electrolyte materials. It is resistant to oxidizing agents, surfactants and hydrocarbon oils. It is not resistant to low polarity organic solvents such as ketones, chlorinated hydrocarbons and aromatic hydrocarbons. It is also stable in aqueous acids and bases and many non-polar solvents. However, it is soluble in both dichloromethane and methylpyrrolidone.
Polysulfone has one of the highest service temperatures of all melt-processable thermoplastics. Its resistance to high temperatures gives it a role as a flame retardant material, without compromising the strength in the way that usually results from the addition of flame retardants. Its very high hydrolytic stability allows for its use in numerous medical applications that require autoclave and steam sterilization. However, it also has low resistance to some solvents, as already noted, and does undergo weathering effects.
The use of polysulfone allows for the easy manufacturing of membranes with controllable size of pores down to 40 nanometers. Such membranes are used in applications such as hemodialysis, waste water recovery, food and beverage processing, and gas separation. Also, polysulfone can be reinforced with glass fibers to form composite materials. Further, it is used as a dielectric material in capacitor applications.
One of the primary rationales for producing blends of polysulfone with other polymers is to use the polysulfone to impart separation and membrane capabilities to the material and for the second polymer to provide higher temperature performance than is possible with the use of the polysulfone alone. For example, in the next section of this chapter, blends of polysulfone with other high temperature polymers such as polyimides (PIs) and poly-benzimidazole (PBI) will be discussed. Much of that effort is focused on the production of miscible blends that can be fabricated into both symmetric and asymmetric membranes. Later sections of this chapter will focus on the use of polysulfone in mixtures to modify other properties of polymers, particularly the fracture and impact behaviors.
6.3 Issues in blending polysulfone with other high temperature polymers
Following up on the comments from the last section, the first blends to be discussed will be blends of PBI with polysulfone. In an initial investigation of these blends, Chung et al.1 determined that the two polymers do not form miscible mixtures. However, when fibers were spun from the blends, the tensile modulus and strength of the blend fibers were comparable to or better than that of PBI, depending on the processing conditions that were used.
It was later shown2 that the introduction of functional groups, such as sulfonate groups, into the polysulfone polymer entity resulted in the formation of miscible blends with PBI. Further, the degree of sulfonation as well as the overall blend composition controls the observed miscibility behavior. Fourier Transform (FT-IR) Analysis showed that the observed miscibility is due to specific interactions between the N-H group on the PBI and the sulfonate groups on the polysulfone.
Similar observations have been made of blends of polyamide 11 (PA 11) and sulfonated polysulfone (SPSF) prepared by solution casting from dimethyl formamide (DMF).3 In that work, differential scanning calorimetry (DSC) showed a melting point depression of the equilibrium melting point of the PA 11, indicative of an interaction between the two polymers. With lower degrees of sulfonation of the polysulfone, less interaction between the two polymers was observed. FT-IR and Raman spectroscopic techniques were used to confirm the nature of the specific interactions involved.
In EP 0778077A3, gas separation polymer membranes were prepared from mixtures of polysulfone, Udel P-1700, and an aromatic polyimide, Matrimid 5218.4 The two polymers were proven to be completely miscible as confirmed by optical microscopy, glass transition temperature values and spectroscopy analysis of the prepared mixtures. This complete miscibility allowed for the preparation of both symmetric and asymmetric blend membranes in any proportion from 1 to 99 wt. % of polysulfone and polyimide. The blend membranes showed significant permeability improvements, compared to pure polyimides, with a minor change in their selectivity. Blend membranes were also more resistant to the plasticization phenomenon compared with pure polyimides. This work showed the use of polysulfone-polyimide blends for the preparation of gas separation membranes for application in the separation of industrial gases.
In another development that was focused on membranes, miscibility in blends of polysulfone with poly (1-vinyl pyrrolidone-co-styrene) (P (VP-S)) copolymers containing various amounts of 1-vinyl pyrrolidone (VP) was examined.5 Copolymers that contained VP from 68 to 88 wt. % were used in that work and were found to be miscible with polysulfone. On the other hand, polysulfone blends with P(VP-S) copolymers containing 65 wt. % VP and those with the P (VP-S) copolymers containing 90 wt. % VP showed two Tg values, indicating that phase separation had occurred. In terms of membrane performance, the solute rejection examined with membranes fabricated from miscible blends was similar to that of a polysulfone membrane, while the solute rejection examined with the membranes fabricated from immiscible blends was lower than that of polysulfone membranes.
6.4 Physical properties of polysulfone blends
The previous section addressed miscibility issues encountered in polysulfone blends and how those relate to membrane performance. However, the functioning as a membrane is just one aspect of parts fabricated from polysulfone blends. This section of the chapter will deal with other properties, primarily mechanical properties.
Kohlman and Petrie6 produced blends of 5%, 10% and 20% by weight of polysulfone in polycarbonate by melt blending. The resulting blends were injection molded into plaques. Mechanical analysis was performed on samples machined from the plaques and consisted of tensile, pendulum impact and ballistic impact testing. The average impact strength and the percentage of ductile failures decreased with increasing amounts of polysulfone. Tensile test results showed that a relationship exists between the percent composition and the yield strength for the molded blends, with the blends showing an improvement in tensile strength. The ballistic testing results showed that a linear relationship exists between the percent polysulfone in the blend and the critical velocity for complete penetration.
In another study,7 Garcia et al. produced blends of polysulfone with a liquid crystal copolyester, Rodrun 5000, by processing methods of direct injection molding and extrusion followed by subsequent injection molding. The blends were immiscible and showed two pure polymer phases. A generally linear relationship of the Young’s modulus of the directly molded blends was observed and was attributed to the counteracting effects of the large orientation of the molded bar skin and its low thickness. There is an improvement in the notched impact strength of the polysulfone with the addition of small amounts of the LCP that indicates a reduction in its notch sensitivity. The behavior of the tensile strength was close to linear with respect to the blend composition, except for a 20/80 blend which showed synergistic behavior. This behavior is reminiscent of what is sometimes observed in rubber-toughened blends.
Ramiro et al.8 studied the mechanical properties of directly injection molded specimens based on blends of poly (etherimide) (PEI) and polysulfone. The molded samples were composed of an almost pure PEI phase and a pure polysulfone phase. The measured modulus of elasticity was near to an additive relationship of the two component polymer values. The impact strength was higher than that of the poly (etherimide). The tensile strength and elongation at break were almost additive, suggesting that there is good interfacial adhesion between the two phases. The reported behavior of the physical properties is again somewhat reminiscent of rubber-modified blends.
Polysulfone/PET blends were obtained by direct injection molding across the entire composition range.9 A slight synergistic behavior was observed in the Young’s modulus, mainly in the 90/10 blend. This is probably due to orientation effects. The measured ductility values were approximately linear with composition. This was generally the case with the polysulfone-rich blends and was attributed to some polysulfone being present in the PET-rich phase. The presence of some broken particles suggested some interfacial adhesion being present.
Summarizing the physical property results, then, on polysulfone blends, it seems that the strength and modulus values can be given by a linear relationship if the proper blend morphology is established. At the same time, it appears to be possible to observe synergistic behaviors in the impact properties for certain blend formulations. These results suggest that a toughening mechanism effect may be occurring in some of these polysulfone blends. That effect will be explored further in the next section of this chapter, when a discussion of polysulfone/thermoset mixtures is provided.
6.5 Polysulfone/thermoset mixtures
It is well recognized that phase separation is a necessary condition for improving the fracture toughness in thermoplastic-modified thermosetting matrices.10,11 For a certain thermoplastic-modified thermosetting matrix, two primary factors control the final morphology and, hence, the ultimate properties of the mixtures: thermodynamics and the kinetics of phase separation during the curing process itself. Both these factors can be modified by changing the thermoplastic content in the mixture and/or varying the curing conditions.
Martinez et al.12 performed such a study of polysulfone-modified diamin-odiphenyl methane-cured diglycidyl ether of bisphenol-A epoxy mixtures. The immiscibility for the mixtures was proven for various polysulfone contents and as a function of the curing conditions used. It was found that the control of the general morphologies can be performed only by varying the processing temperature. For a particular thermoplastic amount, it was discovered that immiscibility lowers the rigidity and the strength but increases the fracture toughness of the mixtures. Those properties, including the fracture toughness, are also dependent upon the percentage of thermoplastic and the subsequent morphologies. The higher values of fracture toughness are achieved for a bicontinuous morphology.
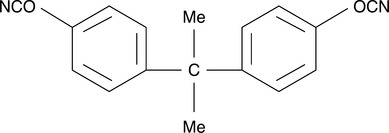
Hwang et al.13 have investigated mixtures of polysulfone with bisphenol A dicyanate, the structure of which is shown in Fig. 6.2. When blends with less than 10 wt. % of bisphenol A dicyanate were cured isothermally, they were phase separated due to a nucleation and growth mechanism. On the other hand, with more than 20 wt. % polysulfone, the blends were phase separated by spinodal decomposition. These different mechanisms for the phase separation are important because in thermoset/thermoplastic blending, the fracture toughness is determined by the morphology formed as a consequence of the phase separation.
Those preliminary studies were later extended to include the addition of organic montmorillonite nanoscale filler to the polysulfone/dicyanate mixtures.14 The major reason for the introduction of the filler is the significant improvement in properties that can be obtained at low clay contents. The exfoliated configuration for the clay is of particular interest because it maximizes the interactions, making the entire surface of the clay layers available for interactions with the polymer matrix. This should lead to dramatic changes in mechanical and physical properties.
In the quoted study, it was found that the polysulfone is initially miscible with the cyanate prepolymer and phase separates in spherical microdomains during the course of the cyanate polymerization process. The flexural modulus was not significantly modified by the thermo-plastic/organoclay addition. On the other hand, the fracture toughness was slightly improved with the addition of polysulfone and clay to the cyanate.
All of these studies highlight the importance of obtaining the correct morphology in the polysulfone/thermoset mixtures in order to be able to maximize the fracture toughness improvement observed. The mixtures often begin as miscible blends and, upon curing of the thermoset component, phase separation occurs. It is control of that phase separation process that ultimately leads to the attainment of a particular physical property profile. Variables that can affect the phase separation process include the overall mixture composition as well as the curing temperature of the thermoset component in the mixture. Among the blend composition variables, the molecular weight of the two components is very significant due to its effect on the overall mixture viscosity, an important factor in controlling the phase separation kinetics.
These conclusions imply that fundamental studies of the phase separation process in polysulfone/thermoset mixtures are needed to better understand the kinetics of the situation. General conclusions about the range of variables that allow for the development of a certain morphology compared to others are needed to be able to take full advantage of the fracture toughness enhancements that have been observed in certain poly-sulfone/thermoset mixtures. It is through such an understanding that the optimum polysulfone/thermoset mixture can be defined and developed.
Variables that should be examined in these type of studies are the molecular weights of the two component materials, both the thermoplastic and thermoset components. It should be determined if both of these affect the viscosity in similar ways, or if it is not the overall viscosity that affects the phase separation kinetics but how the overall mixture viscosity is achieved. Those studies should be done on a single system as a function of the curing temperature, and a complete understanding of the resulting morphologies should be obtained. Having obtained that basic and fundamental understanding on a single system, the goal would be to apply that knowledge to be able to design the required features of other systems. That way, systems with particular and specific property profiles can be designed and fabricated.
6.6 Conclusions
There are two general application areas for which polysulfone blends are currently being utilized. The first is the general development of high temperature separation membranes that use the membrane features of polysulfone in combination with the high temperature performance provided by the other polymer in the mixture. In general, miscibility of the two polymers is desired in this application, and much work has already been devoted to the development of miscible polymer mixtures. As with most polymer blends, one of the key unanswered questions on this topic is what are the key polymer features that lead to miscibility. Much effort has been provided to a trial-and-error approach in this regard to define miscible polymer pairs. It would be quite desirable to establish the general rules of miscibility and better define the relationship between miscible mixtures and membrane performance.
The other general application of polysulfone blends is in the area of impact-modified materials. The general approach adopted here has proven to be useful when the polysulfone is blended with both thermoplastic and thermoset matrix polymers. In this case, immiscibility of the two polymers is the desired phase structure, but control of the phase separation process is needed to be able to optimize the enhancement in fracture and impact properties of the matrix polymer. In other words, there is more to the observed effect than simply blending the two polymers together, but an understanding of the effect of the mixing parameters on the subsequent blend morphology needs to be obtained.
This leads to the one area in which additional research work in polysulfone blends is necessary. That area revolves around the general need to better understand the phase structure of polysulfone blends and how a particular phase structure can be obtained.
Polymer features that lead to miscibility with polysulfone should be further quantified to be able to optimize the membrane separation characteristics of polymer mixtures. On the other hand, in the case of immiscible polysulfone blends, it is desirable to better define the features of the blend components that lead to a particular morphology. Some of those features are perhaps going to be different in the case of thermoplastic and thermoset matrix materials, but viscosity is certainly going to be relevant in both cases. However, in order to best utilize the polysulfone blends that have been discussed in this chapter, more work is required to better comprehend their structure–property–processing relationships.
6.7 Sources of further information and advice
No books solely focused on polysulfone polymers or blends of polysulfones are available. A good general source of information on many of the general features of polysulfones is the Society of Plastics Engineers (SPE) encyclopedia.15 There, information about many of the chemical and physical properties can be found.