Polybenzimidazole (PBI) high temperature polymers and blends
B.G. Dawkins, F. Qin, M. Gruender and G.S. Copeland, PBI Performance Products, Inc., USA
Abstract:
This chapter discusses polybenzimidazole (PBI), PBI blends, and some of their high temperature applications. The chapter first reviews the PBI polymer, including its history, structure, properties, synthesis, advantages and limitations. It then focuses on various PBI blends and their properties. The chapter also summarizes current commercial PBI and PBI blend products and provides examples of PBI and PBI blends in high temperature applications.
7.1 Introduction
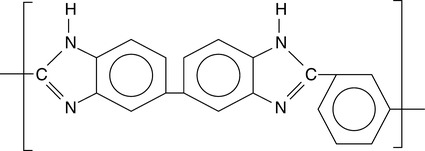
Development work on aromatic polybenzimidazole (PBI) polymer was done in the early 1960s.1,2 Later, NASA and the Air Force Materials Lab sponsored work on PBIs as a non-flammable and thermally stable textile fiber for aerospace and defense applications. In the 1970s NASA used PBI as part of the astronaut’s clothing on Apollo and other space shuttle flights. In 1983 the Celanese Corporation commercialized PBI fibers, spun from solutions of the polymer, and subsequently started development of other PBI forms, including films, fibrids, papers, microporous resins, sizings, coatings, molding resins, as well as reinforced composites. PBI was introduced to the Fire Service as an outer shell protective fabric, typically 40% PBI/60% para-aramid. In the 1990s shortcut PBI fiber was introduced for use in automotive braking systems as a friction formulation material. Also, staple fiber was introduced into the aircraft market as a seat fire blocking layer material, and lightweight fabrics were developed for electric utility and petrochemical applications. During the early 2000s enhanced and next-generation fibers were continuously commercialized and introduced in firefighter turnout gear. In 2005, Celanese sold its PBI business to PBI Performance Products Inc, an affiliate of the InterTech Group of North Charleston, SC, and the world's only commercial producer of PBI polymer (Fig. 7.1) and fiber.
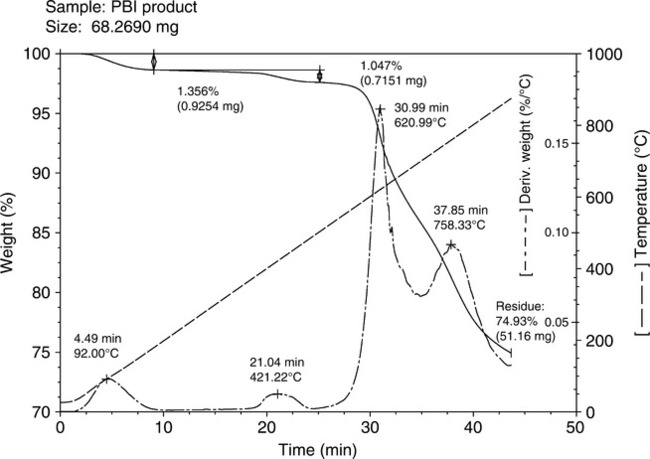
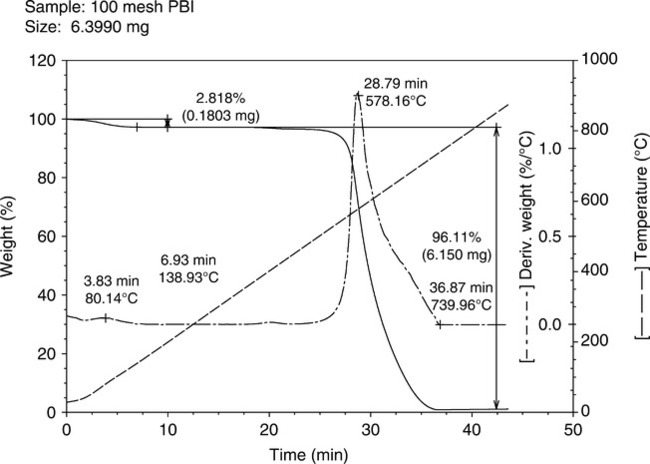
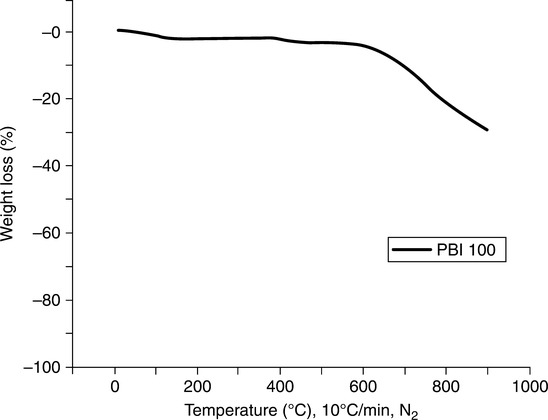
Thermal gravimetric analysis (TGA) of PBI polymer shows that decomposition in air occurs at around 580°C and, for short times at temperature, the polymer can withstand high temperatures without severe loss of mechanical properties. PBI does not burn in air, has high heat resistance (does not melt), possesses the highest compressive strength and mechanical property retention over 205°C of any unfilled resin, and is hydrolytically stable to high pressure steam or boiling water. In an inert atmosphere, such as nitrogen, the high temperature weight loss occurs at 600°C. For example, for a ~10-min exposure time, the suggested maximum temperature is ~ 650°C. Long-term temperature aging can lead to rapid loss of mechanical properties, due to oxidative degradation, and thus ~ 320°C is the maximum temperature capability for exposures exceeding 200 h. Under cryogenic conditions, it has been reported3,4 that PBI maintains its properties in temperatures as low as minus 196°C. PBI is typically resistant to organic acids, chlorinated solvents, alcohols, and weak organic bases, but can be affected by polar aprotic solvents and strong acids and bases. PBI is also known to be hydroscopic with an equilibrium moisture content of ~ 15% by weight, the uptake primarily due to water forming hydrogen bonds with the hydrogen on the imidazole ring of the polymer chain. Thus, sample materials are generally dried at ~ 180°C for several hours in a vacuum oven prior to testing and usage. The polymer also absorbs acid (pKa ~ 5.5), a vital characteristic for fuel cell membranes and other proton-conducting applications. Figures 7.2 and 7.3 show the TGAs in nitrogen and air, respectively, at a rate of 20°C/min.
Due to the several attractive properties of PBI such as commercial availability, high glass transition temperature (Tg, 425–435°C), chemical resistance in hostile environments, and retention of good mechanical properties at both high and cryogenic temperatures, it has been and continues to be examined in high performance polymer blends.5 Thus, blending with other high temperature-resistant polymers targets the high Tg provided by PBI and the improved processability afforded by the component polymers. High temperature-performance polymer needs often emerge where blend technology is required or expedites performance optimization of available materials. Ideally, the resulting blend is a miscible combination where phase separation does not occur during thermoplastic processing.
This chapter covers PBI-based polymer blends, their preparation, their unique properties relative to the companion polymer, and their applications or potential applications. Typically, these blends would consist of more than one polymer, specifically having high performance components or resulting in high performance materials, as opposed to new chemistry to produce new polymer materials. Blending allows for new and unique property tailoring and with almost infinite possibilities, and within targeted performance and price specifications. Such blends take advantage of the high Tg of PBI (425–435°C) relative to the component polymers, the blends being melt processable at high temperatures, miscible or partially miscible, compatible, and applicable to high temperature end uses. With a resulting single Tg polymer blend, specific modifications of processing temperatures may be made, and with adherence to ‘Rule of Mixtures’ type predictable behavior and property ranges.
The chapter is intended to be a general overview, not totally comprehensive, but with coverage of specific and significant polymer blends systems that address specific deficiencies and needs based on current applications and trends in the field of high temperature polymer blends, particularly with PBI. PBI blends, synthesis, structures, modified structures properties, development activities, and applications were reviewed and covered previously.6–11 Much of the previous work7 emphasized the use of polymer materials in application areas traditionally dominated by metals and alloys, such developments including new polymers, control of morphology, reinforcements, and blending, and with blending receiving less attention for applications in the 300–400°C range. Subsequently, much effort has been focused on the preparation and characterization of high performance polymer blends toward developing a better fundamental understanding of the phase behavior of such blends, and end-use potential.
7.2 Processing of polybenzimidazole (PBI)
As a class of polymers, polybenzimidazoles exhibit high thermal and chemical stability. PBI is unique as a commercially available over 400°C Tg resin, but lacks the thermoplastic processability and has relatively poor long-term thermo-oxidative stability, as well as relatively high moisture regain (at 10–15%) vs. polyimides, polycarbonates, and polyarylketones (up to 2%); the regain is apparently due to intermolecular hydrogen bonding between water and N-H groups in the PBI structure. PBIs can be further processed into various product and end-use forms such as fiber, solutions, coatings, molded shapes, film, membranes, foams, and composites. Additionally, blends with other polymer resins and materials can be processed to take advantage of the unique high performance properties, modified for desired thermal, chemical, processability, and mechanical targets, and end-use applications. The thermal stability of polybenzimidazoles is inherent in the wholly aromatic, ladder-like structure (Fig. 7.1), with the presence of three benzene rings in the repeating unit contributing to the polymer’s superior thermal stability properties, stiffness, and toughness. Blending helps to improve processability since the polymer typically does not melt, and variant polymer properties can be achieved, as opposed to varying/tuning the polymer structure and subsequent properties.
PBI polymer is manufactured exclusively in commercial quantities by PBI Performance Products, Inc. The fiber processed from PBI polymer solutions is recognized as high performance for its exceptional thermal stability and chemical resistance. The commercial process utilizes two commercially available monomers, tetraaminobiphenyl (TAB) and diphenylisophthalate (DPIP), in a two-stage melt condensation and solid-state polymerization (SSP) process. The monomers are heated in an inert atmosphere. As the reaction proceeds in the first stage, PBI prepolymer is formed, and water and phenol foaming by-products evolve. The reactor is cooled and the prepolymer crushed. In the second stage reaction, the prepolymer is heated additionally at higher temperature to complete the polymerization and advance the molecular weight to the target value for further processing.
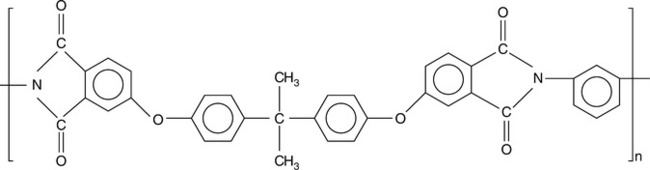
The reactor is cooled and poly (2,2′-(m-phenylene)-5,5′-bibenzimidazole), the only commercially available PBI polymer (Fig. 7.1), is discharged. The polymer is tan/golden brown in color. This particular commercial form of PBI is available in polymer resin form, under the trade name Celazole®, and is also available in solution form (~ 26%, 10% and other levels of polymer in N,N′-dimethyl acetamide (DMAc), or other solvents). PBI polymer of 100-mesh particle size is typically used in the manufacture of dry blends for extruded and injection molded stock shapes. Polymerization can be carried out either in solution or under molten states with various monomers, i.e., an aromatic tetraamine and a diphenyl ester or an anhydride of an aromatic or heterocyclic dicarboxylic acid in a one- or two-stage process. The commercial PBI product is meta-PBI where the phenylene ring is meta-coordinated. The para-PBI polymer can provide superior tensile strength and stiffness, but is not as soluble in useful solvents for downstream processing. Figure 7.4 shows a typical PBI polymerization process reaction.
A great deal of literature on PBI has been developed since the first reported synthesis.7,12–14 Many aromatic PBI variants have been synthesized and studied, but the commercial PBI product is the one cited most often and receiving most attention in developmental applications; it is particularly used as a polymeric matrix material capable of maintaining load bearing properties for short periods of time at temperatures up to ~ 650°C, and with good mechanical properties at elevated temperatures, good chemical resistance, and potentially useful in a variety of aerospace applications.15 Aromatic PBIs are characterized by a high degree of thermal stability. They may be shaped to form fibers, films, and other articles of wide utility which show resistance to degradation by heat, hydrolytic media, and oxidizing media. However, these PBIs are not readily melt processable at desirable low temperatures and pressures, and so shaped articles are not readily fabricated from them. Molded parts, for example, require a two-stage process involving cold compaction for the PBI powders, followed by heating at pressures of 1–3 Kpsi at 425–500°C for a few hours.16
PBI polymer solutions are prepared by dissolving the polymer resin in dimethyl acetamide (DMAc)/LiCl (lithium chloride) under pressure and temperature in an inert atmosphere. The LiCl is added to stabilize the solution, increasing the shelf-life from days to several months. Prior to further processing, the PBI dope solution is filtered to remove insolubles that could interfere with down-stream processing, such as spinning into fiber. Solutions may also be prepared without the added stabilizer, particularly at lower solids levels and for applications requiring the absence of metal or interfering additives.
7.3 PBI blends
Polymer blending allows the opportunity to lower costs without sacrificing the desired properties, the ability to tailor properties without creating completely new polymers, and presents options for high performance blends from synergistically interacting polymer systems. Miscible polymer blends and co-polymerization can offer complementary routes to polymer systems of tailored properties, although co-polymerization can often heighten miscibility and lead to better routes to control cost-performance. The optimum blend system is often derived after evaluation of process ease and performance profiles available, which could involve blending, co-polymerization, or a combination.6 Compared to polymerizations or co-polymerizations to form new polymers or variants, polymer resin blending is often the preferred route17 due to simplicity, reproducibility, processability, and reduced development cost. Such blending work by Chung and others showed that incorporation of small amounts of PBI (3–17%) into Matrimid ® 5218 polyimide increased the thermal stability and selectivity of the blend film for pervaporation dehydration of t-butanol. Chung and Xu18 described solution spinning of hollow fibers based on a miscible blend of PBI and Ultem ® 1010 polyetherimide (PEI). Furthermore, blending can synergistically combine the advantages of different material properties and overcome the deficiencies of the individual components, and miscible blends are desirable for producing final products with uniform performance and stable thermal and mechanical properties. Property targets can thus be tuned to deliver end-use attributes associated with Tg, melt processability, properties tailored to the required application as opposed to over-performance, solubility controls and with alternate solvents, cost controls, i.e., for a confluence of properties and conditions to enter into a variety of different applications. Sudhir and Kratzer19 also have reported fluid separation membranes made from blends of polyimides and polyimidazoles; such membranes are reported to exhibit resistance to interaction with the fluid stream material which can result in plasticizing of the membrane, have superior mechanical properties for performance in high membrane differential pressure and high process temperature environments, can be configured in hollow fiber membranes, and have good performance in long-term contact with aggressive process composition, pressure, and temperature conditions.
Polymer blends may be miscible, immiscible or partial (a combination of the two).16 Miscible blends offer additional desirable and, often needed, end-use properties intermediate to the individual components across the miscible composition range. Typically, these have uniform physical properties. As stated by Flory:20 ‘It is well known that, regarding the mixing of thermoplastic polymers, incompatibility is the rule and miscibility or even partial miscibility is the exception. Since most thermoplastic polymers are immiscible with other thermoplastic polymers, the discovery of a homogeneous mixture or partially miscible mixture of two or more thermoplastic polymers is, indeed, usually unpredictable with any degree of certainty’. And, it is generally known or deduced that most pairs of polymers tend to be totally immiscible, even some of the same type.21 Thus, miscible polymer blends are not common, but can be induced with temperature and relative composition considerations.
A miscible blend of two polymers will generally have properties somewhere between those of the two polymer components. The component with the higher property value, for example Tg, will increase that value of the blend as the relative amount of that component is increased, generally linearly, but deviations may occur if the two polymer components bind more strongly to each other than to themselves, if intermolecular bonding is involved, etc., since stronger binding leads to decreased mobility. However, in most cases, two polymers would be expected to bind less strongly with each other than with themselves. Other properties, such as mechanical properties, resistance to chemicals/radiation/heat, etc., would generally trend the same as Tg with respect to relative amounts of each polymer in the blend. Immiscible mixture or phase-separated blends will typically display a Tg corresponding to each phase, or a Tg for each component if totally incompatible.
There are several technical and manufacturing reasons to blend polymers in various forms, including to facilitate processing, to improve or modify properties (mechanical, physical, chemical, absorbency, thermal and thermal oxidative stability, hydrolytic stability, chemical resistance), to reduce/control costs, etc. The resulting blends and targeted properties are typically performed in concert with product applications or new applications identification (Celanese internal communications). The latter is usually more long-term and encompasses more advanced planning, recognition of market needs, more testing, and eventually insight into specific performance needs at some reasonable price. Applications and end-use testing are usually already available or adaptable, often in the form of standard protocols, whereas this may or may not be the case for new applications or new specialty forms of existing base polymers and materials to meet a needed/required product-market situation. Besides blending, other options to tune polymer properties include sieving to isolate specific particle sizes or molecular weights, cross-linking polymer structures, cross-linking blends, modifying the polymer chain, as well as co-polymerization and post-polymerization treatments. PBI is potentially a good candidate for nucleophilic substitution reactions due to the N-H bonds in the structure.
Typical blend polymers for PBI include poly (aryl ether ketone) (PAEK), polyetherimide (PEI), poly (aryl ether sulfone), poly (phenylene sulfide), and mixtures thereof. Different types of PAEK resins (Fig. 7.5) include PEK, PEEK, PEKEKK, PEKK, PEEKK, etc., several of which have melting points over 300°C. PAEK resins with relatively low Tg values can be further improved by the addition of reinforcement fillers, such as fiberglass, carbon fibers, and ceramic or mineral fillers. Such additions, however, can compromise or be neutral in terms of material weight, creep resistance and relaxation under stress. The predominant factor for high performance applications is Tg, and the use of blending to increase or synergize. PBI resin can be utilized to increase the Tg of the blend, or the other blend component used to provide a lower or intermediate Tg for intermediate applications. PBI is not expected to be detrimental to the other blend component as the other polymer usually becomes the limiting factor, although the other polymer can enhance melt processability of the blend.

Due to its very unique and high thermal stability characteristics, PBI blends were studied and developed early on for aerospace and composite materials.22 The discovery of new polymeric alloys by blending commercially available polymers was the trend in materials development. A key reason for growth in this area was the ability to tailor existing materials to a new/unique set of property-performance-price specifications through combinations of materials. For example, a miscible blend based on PBI and polyimide (PI) was discovered and, after thorough investigations, PBI was found not only to have superior thermal/chemical properties, but to also exhibit unique reactivity to enable it to interact with other functionalized polar molecules to form compatible binary systems.23 PBIs reactivity also led to the discovery of interactions between PBI and polyarylate (PA) polymers at Hoechst Celanese, which resulted in the preparation and characterization of PBI/PA film and fiber blends. At a fixed concentration (e.g. 0.5%), it was observed that the inherent viscosity (IV) of the solution blends in N-methyl-2-pyrrolidone (NMP) exceeded the Rule of Mixtures, suggesting PBI and PA exhibited interaction in a dilute solution such that the resulting hydrodynamic sizes of the blends was greater than the calculated averages based on each component. This interaction was further demonstrated by FTIR; the carbonyl stretching shifted downfield, indicating the existence of intermolecular H-bonding between PBI and PA in a film blend. Thus, a new family of high performance, processable, and compatible blends based on PBI and PA were discovered, which exhibited synergistic effects on properties and offered new opportunities for polymer alloys for engineering and aerospace applications.
High temperature polymer blends are typically prepared to take advantage of the high Tg, such as PBI with polyaryletherketones (PAEKs), typically polyetheretherketones (PEEKs) and polyetherketoneketones (PEKKs). PAEKs render PBI more melt processable and provide synergism for varying the end-use thermal and mechanical properties. The miscibility of such blends may be confirmed by the presence of a single glass transition temperature lying between those of the blend components.
7.4 PBI–polyetherketoneketone (PEKK) blends
Celazole® PBI is the low to medium molecular weight form of PBI molding resin, 23 000–37 000 grams per mole, inherent viscosity of 0.55–0.8 dL/g.5 PBI and polyetherketoneketone (PEKK) can be solution blended with blend miscibility over a range of concentration ratios. Targeted blends may lower the moisture absorption vs. PBI, improve tractability vs. PBI, improve strength vs. PEKK, and with thermal and mechanical properties exceeding those predicted by the Rule of Mixtures. Polyarylketones (PAK) have good chemical resistance and moderate compressive strength, but exhibit poorer mechanical properties at elevated temperatures as compared to PBI. There are processing limitations to molding PBI articles, whereas PAK molded articles are limited in thermal and pressure resistance.
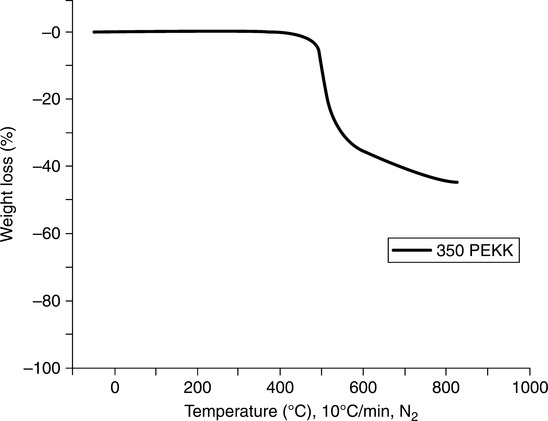
Blend patent work24 describes a process for producing a solution blend of PBI and an amorphous PEKK. The objective was to prepare solution-derived blends of 100-mesh PBI polymer with PEKK, and to evaluate the thermal properties (Figs 7.6 and 7.7 show the TGA curves for PBI and PEKK, respectively). The PBI is mixed with sulfuric acid at a temperature between 40°C and 80°C for 30 min to 2 h to produce a PBI solution, then cooled to room temperature. PEKK is then added to the PBI solution to form a mixture, the mixture is stirred to homogenize, and subsequently poured into an excess of water, methanol, or water/methanol, being stirred swiftly. The aqueous mixture is filtered to produce a blend, and the polymer blend is washed with water and dried. Thus, the common sulfuric acid solvent can be used to form blends in varying ratios, then added to a non-solvent to isolate and recover the macroscopically homogeneous mixture.
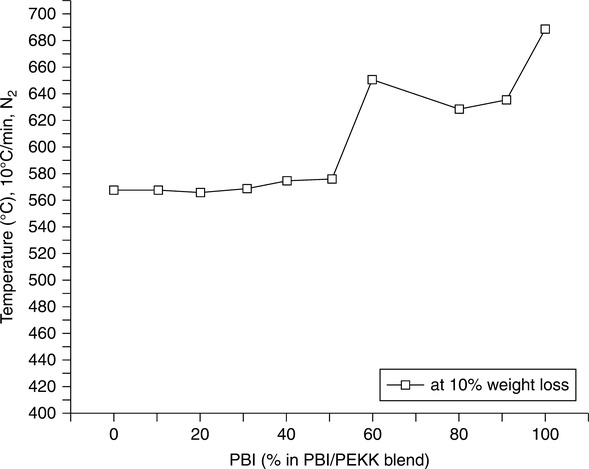
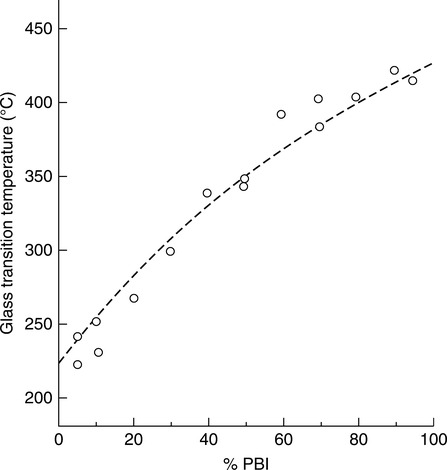
Miscible blends of PBI and PEKK having a single or predominant Tg were found to be feasible from 50/50 to 10/90 (PBI/PEKK) with intimate and uniform mixing. While blends 40/60 to 90/10 may also be miscible, they tend to behave thermally more like PEKK (Tg ~ 150°C, amorphous phase; Tm ~ 335°C, melting point of the crystalline phase) vs. PBI (Tg ~ 430°C). There appeared to be a tendency to lose Tg, and thus potentially heat deflection temperature (HDT), as the PEKK ratio exceeded 40% (Fig. 7.8). The most compatible blends appeared to be 90/10 and 80/20. The amorphous PEKK with greater than 50% PEKK appeared to degrade as the pure PEKK component, whereas the blend with less than 50% PEKK appeared to degrade similarly to pure Celazole® PBI. As expected, the degradation temperature increased toward that of PBI as its content was increased (Fig. 7.9). The semi-crystalline blend containing greater than 30% PEKK appeared to degrade as the homopolymer PEKK, presumably due to the phase-separated domains of the crystalline segments.
This referenced patent also includes a process for producing a melt blend of PBI and PEKK. In this alternative process, the PBI and PEKK are pre-mixed dry to obtain a dry mix. Then, in an extruder with a plurality of heating zones (240–410°C), the dry mix is fed and melted as it passes through the extruder, either single- or twin-screw, and is subjected to the mixing and the temperature generated; the resulting product is a melt blend of PBI–PEKK feasible in varying proportions from 1/99 to 80/20 PBI–PEKK.
PBI powder resin (Celazole® 100-mesh polymer) is typically chard-like in shape. When melt blended, SEM indicates that particle morphology is retained after compounding. Thus, PBI is not dissolving in the PAEK; it is apparently behaving as an encapsulating filler. It appears that the PBI polymer resin immobilizes the lower-melting-point amorphous regions of the PAEK matrix resin, thus imparting enhanced thermo-mechanical properties.
Unfilled grades of partially crystalline polymers such as PEEK are not very useful in molded part forms at temperatures much above their Tg since the softening that occurs leads to a sharp loss in polymer mechanical properties, especially stiffness/modulus. This lowered modulus is reflected in reduced dimensional stability under stress at temperatures above Tg. Polymer mixtures with PBI benefit from PBI’s ability to immobilize the amorphous regions of the PAEK matrix resin, thereby imparting enhanced thermo-mechanical properties not obtainable with other fillers of PAEK, but which fall short of that obtained in a miscible blend of polymers.
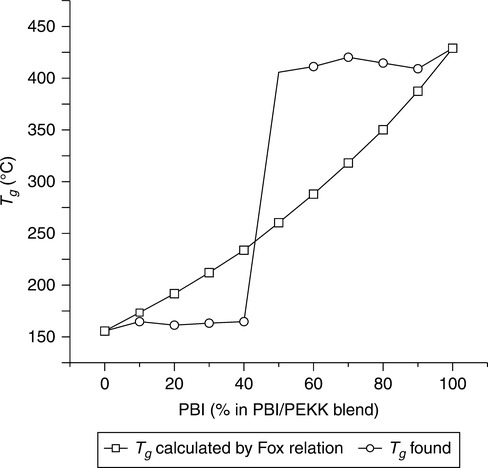
The Fox equation relationship (Fig. 7.10) defines or describes the composition dependence of the Tg of ideal co-polymer or ideal polymer blends (or miscible blends of two polymers) in which no strong interactions are involved. The Fox equation typically is expected to lead to a lower value of Tg than would be given by a simple linear Rule of Mixtures (Blend Inherent Viscosity vs. % Component in the Solution Blends) and reflects the effective higher free volume or randomness due to the presence of two components in the mixture:
where M1 and M2 are the mass fractions, Tg1, and Tg2 are the glass transition temperatures (in Kelvin) of polymers 1 and 2.
One would predict that a blend of PBI resin with PEKK would exhibit thermal properties roughly following the Fox relationship, but unexpectedly it was determined that a PBI–PEKK blend in all proportions from 50/50 to 90/10 PBI–PEKK made by a solution process has a Tg of greater than 400°C, which is greater than would be predicted by the Fox relationship. Note also the TGA curves and thus thermal properties of the PBI and PEKK individual components, as well as for the various PBI-PEKK blends (Fig. 7.8).
The dried blend resin can either be injection or compression molded into articles with specifically tailored thermal and mechanical properties that lie between the respective properties of the pure PBI and PEKK from which the blend was produced. These blends can be used to enhance films, composites, or alloys. Further, these blends can be reinforced or filled prior to molding to produce unique blended products such as T-series products marketed by PBI Performance Products Inc.
7.5 PBI–polyetherimide (PEI) blends
PEIs (polyetherimides, Fig. 7.11) are basically combination polymers, consisting of both polyimide and polyether units in the backbone. Ultem PEIs, with Tg values in the 200–280°C range, are made on a commercial scale as engineering thermoplastics. Compared to the stiffer polyimides, PEIs can typically be melted and molded, presumably due to the ether linkages, but maintain good thermal stability, high strength, and high modulus.
PBI–PEI blends can be useful in severe environmental conditions and have good impact resistance, solvent resistance, and price/performance characteristics. PBIs are generally more expensive, have high moisture regain, but high Tg values and good solvent resistance. PEIs generally have lower Tg values, lack resistance to certain solvents, but are tractable and relatively less expensive.
Musto and others25 reported FTIR investigation of the blend system consisting of PBI and poly (etherimide) (PEI, Tg ~ 220°C), poly [2,2′-bis[4-(3,4- dicarboxyphenoxy) phenyl] propane-m-benzenediamine], or commercially available Ultem® 1000, showing evidence of hydrogen bonding between the N-H groups of PBI and the imide carbonyl of Ultem® 1000. Such blends, solution cast from a common solvent, such as DMAc, DMF (N,N′-dimethylformamide), and NMP, were found to have a one composition dependent Tg. As-cast blends formed one-phase structures over the entire composition range;26 however, optical microscopy and DSC (differential scanning calorimetry) revealed that the blends phase-separated upon heating above their Tg and did not remix upon cooling. The blends formed upon room temperature casting were non-equilibrium and remained one-phase because they were below Tg. The phase separation of such 50/50 blends was characterized by small-angle X-ray scattering and solid-state nuclear magnetic resonance,27 showing separation upon annealing at temperatures of 310°C and above, although the DSC-determined Tg was reported at 344°C. Upon heating the blend above the blend Tg, the once-cooled blend was found to exhibit two Tg values, indicating phase separation at elevated temperatures. Phase separation in the blend is expected to be influenced by the vitrification of the PBI-rich phase owing to the much higher Tg of PBI (425–435°C) relative to that of PEI (~ 220°C). Blends of these materials are of interest because of the potential of obtaining materials with unique properties not available with either material alone. Such blends exhibit good resistance to chlorinated solvents,7 lowered moisture uptake vs. PBI alone, and an intermediate Tg. Films of PBI, PEI, and PBI–PEI blends of all stoichiometries are soluble in DMAc.
Various compositions of blended aromatic PBIs and aromatic PEIs can be expected to have approximate linear Tg per weight % of PBI (Fig. 7.12) according to their phase diagrams based on single Tg values from single DSC scans on PBI–PEI blend compositions ranging from ~ 5% to 95% Ultem® 1000.28
The PBI amount in such blends should be at a level to render the composition less sensitive to solvents and to increase the Tg of the PEI. Blend compositions 1 to 99 parts by weight of PEI are feasible, but the 65–95% by weight of PBI blends appear to be the preferred ones. PEIs generally have good resistance to chemicals, with the exception being chlorinated solvents, ethylene glycol antifreeze, brake fluid and DMF, among others. Such blend compositions may be useful for molding, whereas PBI is not as useful.28 By varying the nature of the diamine used to synthesize and the position of substitution in the bis-imides, and using different bis-phenols, a large number of aromatic PEIs with a wide range of physical properties can be prepared,29 but blending with PBI offers non-synthesis routes to obtaining similar or improved properties. Cross-linking and addition of fillers and reinforcements may also be incorporated into such blends to improve and target specific property attributes.
Treatment of the PBI component, via acid protonation of the imidazole ring, should improve the thermo-oxidative stability of the PBI, as well as the blend. Such blends are expected to be useful for matrix use up to ~ 260°C, and with good tensile and mechanical properties. Such blends can be useful as films, coating, or for molding. If the blend is mostly PBI, the PEI renders it more tractable and thermally processable, and less susceptible to moisture. If the blend is mostly PEI, the PBI increases the Tg and renders it more resistant to solvents. The composition is impact resistant and withstands severe environments. This same assessment would apply to PBI–PI blends.
7.6 PBI–polyaryletherketone (PAEK)–PEI blends
The targets for tri-blends consisting of PBI, polyaryletherketone (PAEK), and polyetherimide (PEI) include thermal and mechanical properties exceeding those of PAEK and PEI, lower moisture absorption vs. PBI, improved tractability vs. PBI, and improved strength vs. PAEK or PEI. The PAEK options include PEK, PEEK, PEEKK, PEKEKK, PEKK, etc. Some of the earlier work was performed toward reducing cost, crystallinity, and mold shrinkage, lowering warpage, enhancing PBI–PEEK interfacial interaction, modified blend rheology and improved matrix resin Tg, and a concern related to the thermal oxidative stability of PEI relative to improving that of PBI and the overall matrix.
Blend patent work30 demonstrated tri-blends of PBI (Tg, 425–435°C), PAEK (Tg ~ 145°C), and PEI (Tg ~ 218°C), formed by intimately blending by dissolving in a strong acid or by melt blending. Various tri-blends were prepared, including 45/45/10, 37.5/37.5/25, 25/25/50, and 12/12.5/75 PBI/PEEK/PEI. It appeared that incorporation of 10 to 75 parts of Ultem 1000 PEI into a base mix of 50/50 PBI/PEEK resulted in a decrease in the degradation temperature. The incorporation of 10 to 25 parts PEEK into a base mix of PBI/PEI (50/50) resulted in a considerable decrease in degradation. The PBI/PEI blend prepared in sulfuric acid exhibited a higher thermal stability than that prepared in DMAc. The PBI/PEKK di-blend showed evidence of improved properties compared to the tri-blend.
DSC analyses indicated that the Tg of PEEK in the blend increased due to the formation of a miscible blend with the PEI. The crystallinity of PEEK was also found to decrease after mixing with the amorphous PEI. Thermal degradation of the PEI was found to be insignificant for the PEI-filled PBI–PEEK at the 10–35% level. Mechanical properties of PBI–PEEK (50/50) were found to be minimally affected with the addition of up to about 10% PEI. PBI and PEI have been demonstrated to be miscible and to exhibit strong molecular interactions.
PAEKs have good chemical resistance and moderate compressive strength, but poorer mechanical properties at elevated temperatures when compared to PBI. Blends of PBI and PAEK31 and of PBI and PEI28 have been reported, the former using a dry blend with a high speed mixer, and the latter using a mutual polar solvent such as DMAc, NMP, or DMF. These have good attributes, but further improvements are still desirable for some applications requiring enhanced properties.
PEIs typically have high heat resistance, high strength-to-weight ratio, high modulus, excellent non-flammability characteristics, processability on conventional molding equipment, low smoke evolution, high dielectric strength, a stable dielectric constant and dissipation factor over a wide range of temperatures and frequencies, and good chemical resistance, except to, for example, chlorinated solvents, ethylene glycol, and DMF.
The reported tri-blend composition contains a minimum of 10% by weight of each component and a maximum of 75% of any one component. The components are intimately blended and harvested. Blend solutions can be prepared by the use of a common solvent for each of the three components, such as sulfuric acid, methane sulfonic acid, trifluoromethane sulfonic acid, trifluoroacetic acid, dichloroacetic acid, and combinations thereof. Sulfuric acid was found to be the preferred solvent. With melt blending, the components can be intimately blended in an extruder. Prior to blending in the extruder, the components may be dry blended in, for example, a tumbler or high shear mixer. Harvesting, or blend recovery, can be performed by precipitating out the blend in a non-solvent such as water, methanol, or combinations thereof, and followed by filtering, washing, neutralizing any residual solvent, washing the neutralized tri-blend, and drying. When the blend is made by a melt blend process, the tri-blend may be ground to the appropriate particle size needed for further processing.
Applications of such tri-blends include semiconductor devices, including microchips, flat panel displays, and the like. In the manufacture of such apparatus, such blends can be used as components of the vapor deposition chamber, process housing components, and materials holding fixtures or molds, in order to be inert to the vapor deposition environment and to prevent off-gas contamination. These blends may also be used in components of semiconductor etching, ashing, wafer transportation, and as hard-disc media cassettes where support components may function as a seal, insulator, holding or transportation device which must endure exposure to the harsh processing environments. Further applications for such blends can be found in various industrial, chemical, and petrochemical processes where the temperature resistance in chemical environments extends the life cycle of parts used in such fabrication processes.
7.7 PBI–polyarylate (PA) blends
Polyarylates (PA) have the general structure [-OAOOCA′CO-]n where A is a phenyl radical and A′ is a carboxylic acid radical, wholly aromatic polyesters based on diphenols and aromatic dicarboxylic acids, or their derivatives. They have good processability in terms of injection moldability, high softening temperatures, and good thermal and mechanical properties for a variety of industrial applications, particularly those requiring high Tg values and good HDT. PAs are reasonably soluble in methylene chloride, chloroform, NMP, DMF, DMAc, DMSO (dimethylsulfoxide), and interact with and/or plasticize PBI for improved price/performance characteristics.
Several investigations have focused on a family of high performance blends of PBI and PA, as well as high modulus aramides (HMA).22,32–34IR spectra, transmission electron spectroscopy (TEM), and thermal mechanical analysis (TMA) showed that the degree of interaction depended on process conditions and LiCl stabilizer addition. Viscometry and FTIR measurements confirmed the existence of intermolecular hydrogen bonding between the two polymers in solution and in films cast from solutions. Solutions of PBI and a PA in 1-methyl-2-pyrrolidinone were found to be homogeneous and increased in stability with increasing PA concentration. The blend films were found to have significantly better solvent resistance than PA films alone. Phase separation can occur with removal of the solvent. The blend properties of PBI–PA and PBI–HMA fibers were found to vary with fiber aging, whereas 80/20 PBI–PS blend fibers showed no aging effects, i.e., no deterioration of mechanical properties.
Polyarylates are high temperature, high performance, thermoplastic polymers with good combinations of thermal and mechanical properties. They also have good processability, which allows them to be molded into a variety of useful articles. They are, however, limited in the aerospace industry, where the chemical resistance is limited in chlorinated solvents and inorganic acids. Blends have been reported with polyesters, 35 as well as PEIs36 and polycarbonates37 and others. The property shortcomings of polyarylates can be substantially improved by incorporating PBI as a miscible blend.38 Beneficial properties are reported to be maintained and solvent, resistance, thermal, and physical properties can be improved at a moderate additional cost.
Chung and others reported38 on novel membranes comprised of a blend of polyarylate and PBI polymers, the blend membranes exhibiting enhanced properties over membranes prepared solely from either polymer alone. The addition of polyarylate allows the composition to be more thermally processable and less susceptible to moisture. The PBI renders the polyarylate less reactive to solvents and increases its thermal stability. Additionally, such blend membranes were found to show high regeneration capacity while retaining good flux ranges. Such membranes demonstrated good separation characteristics and thus may provide an improved membrane with enhanced qualities.
FTIR and other analyses39 confirmed the existence of intermolecular hydrogen bonding between PBI and PA; the carbonyl band shifts from 1741 to 1730 cm− 1 in an 80/20 blend. Also, miscibility and compatibility were indicated further by the clarity (visual and microscopic examination) of films prepared from the 80/20 blend; TGAs showed blends conforming to the Rule of Mixtures, and X-ray diffraction (XRD) analysis confirmed that PBI and PA interact and are compatible on a molecular scale; blends were found to have physical properties adhering to the Rule of Mixtures values; the thermal stability of the blends at 550°C increased proportionately as the relative PBI concentration increased; and after blending, the hydrophilicity of the PBI decreased dramatically. Thus, PAs and aromatic PBIs were found to be miscible in all proportions, and synergistic effects of one polymer over the other’s weak properties can be achieved.
PA is soluble in common organic solvents such as methylene chloride and tetrahydrofuran, whereas PBI is insoluble in these solvents. The two polymers can be simultaneously dissolved in a common solvent (DMF, DMAc, DMSO, NMP, concentrated acid, such as sulfuric), or solution-derived miscible compositions of each polymer may be separately dissolved in separate volumes of a mutual solvent, and desired proportions of the resulting solutions can be mixed together to form a solution of the two polymers. If the blend is mostly PBI, the less expensive polyarylate should be added to an extent to take advantage of rendering the blend more tractable and processable, and less susceptible to moisture. If the blend is mostly polyarylate, then one should take advantage of PBI’s ability to render the blend less sensitive to solvents, particularly chlorinated hydrocarbons. The preferred blend, according to the referenced patent, falls somewhere around 40 to 60 parts by weight of PBI. Articles in the form of films, fibers, fibrets, and molded structures may be prepared from such blends. Molding compositions may also incorporate into the polymer matrix ~ 1 to 50 wt%, preferably 10 to 30 wt% based on the total weight of the molding composition, of a solid filler and/or reinforcing agent, i.e. glass fibers, carbon fibers, synthetic polymer fibers, aluminum fibers, wool fibers, silica, clays, mica, talc, graphite, etc.38,40
7.8 PBI–polysulfone (PS) blends
PBI and polysulfone (PS) do not form miscible blends,41 but the tensile modulus and strength of their blend fibers were found to be comparable to, or better than, that of PBI fibers, depending on the process conditions. Fiber spun from 80/20 PBI–PS solution blends were found to have PBI-like limiting oxygen indices (LOI) and flame shrinkage behavior. The 80/20 blend fibers were found to have comparable or superior mechanical performance to that of miscible PBI–Ultem blend fibers, and with higher modulus but lower elongation than stabilized PBI.
Chung and others also reported42 stable solutions of PBI (70–95 wt%) and PS (5–30 wt%) blends, substantially free of the typical salt stabilizers, such solutions being useful for the production of films, fibers, and molded articles exhibiting enhanced mechanical properties. Polysulfones are useful tractable polymers, but the degree of reduction in thermal and mechanical properties is usually proportional to the wt% of the melt-processable polymer addition. Blends of PBI and PS are generally prepared by solution blending to form a dope solution for extrusion or casting; alternatively, the resin blend may be precipitated, recovered, dried, and molded, as is or after milling. It was determined that small amounts of PS to PBI can decrease moisture takeup (typically at ~ 15%), reduce the processing temperature, increase the tractability, and reduce the cost of resulting blend products.
It has been reported43 that the introduction of functional groups in the polymer chain of PS, such as sulfonate groups, can result in the formation of miscible blends with PBI. Thus, the miscibility behavior of a series of blends of PBI and sulfonated PS at various sulfonation levels has been studied by dynamic mechanic analysis (DMA), FTIR, and FT-Raman spectroscopy. Partially miscible or miscible blends were obtained when the sulfonation level was higher than 10 mol%. FTIR band shifts associated with the N-H and sulfonate groups were shown to account for the induced miscibility in terms of specific interactions. Also, preliminary results were shown to demonstrate the potential usefulness of PBI–PS polymer membranes as electrolytes for fuel cells.
Sulfonation of PS has been found to cause moderate increases in the Tg from 190°C to 210–225°C, and with the PBI–sulfonated PS miscible blend exhibiting excellent miscibility characteristics based on the hydrogen bonding between the N-H group of PBI and the sulfonated groups of the PS. Thermal stabilities at over 200°C were achievable, and the miscibility was found to be dependent on the PBI–sulfonated PS ratio as well as the degree of sulfonation.44,45
7.9 PBI–polyimide (PI) and PBI–polyamide-imide (PAI) blends
PBI may be blended with polyimides (PIs) to improve processability, and thermoplastic PIs can offer improved thermo-oxidative stability. However, PIs often lack the thermal and mechanical attributes required to perform in high use-temperature applications (> 300°C). Thus, blending with PBI can accomplish intermediate thermal and mechanical properties.
7.9.1 PBI–PI blends
Various miscible blends based on aromatic PBI and commercially available PIs have been reported.7,46,48 Blend miscibility was evidenced by infrared (IR) spectra and a single Tg lying between that of the constituent polymers. The intermolecular interactions involving the N-H (from PBI) and carbonyl groups (from PI) were the major driving forces for the miscibility affording the possibility for enhanced melt processing of such blends.49 The IR spectral shifts are eliminated upon phase separation.50,51 Previous efforts52 found PBI to be miscible with synthetic PI blends of poly (amic acids) using DMAc co-solvent, cast film or precipitated into powder. Single composition dependent Tg values between those of the homopolymers were determined, and miscibility was determined to arise solely from hydrogen bonding between the carbonyl from the 5-membered ring and N-H.
Chenevey and others53 reported on homogeneous blends of PBIs and PIs, obtained by mixing in a mutual solvent, for conversion into solvent-resistant films and fibers having good properties. The blends comprised 25–40 wt% PBI (intrinsic viscosity 0.7 to 1.2 in 97% sulfuric acid) and 75 to 60 wt% PI, soluble in DMAc, DMF, and DMSO and, optionally, a stabilizer such as lithium chloride and other agents may be added to the blend solution to promote stability during mixing and storage. Alternatively, such blends may be prepared by melt mixing solid dry mixtures of PBI and PI in devices such as heated screw mixers that provide efficient mixing and heating with limited residence times such as to minimize degradation at high temperature. The blends were reported to be highly solvent resistant, highly resistant to flame embrittlement, and to have single Tg values (at least 270°C). Additionally, the blends were found to retain the desirable features of the PI, including high heat resistance, high strength-to-weight ratio and modulus, and excellent non-flammability characteristics and processability. The blends were found suitable for formation of fibers, films, fibrids, and compression molded articles. Typical fiber properties were reported at or over 3.5 g/denier at 25°C, elongation of at least 25%, and modulus of at least 65 g/denier. Film properties included tensile strength of at least 12 000 psi, elongation of at least 40%, and modulus of at least 300 000 psi.
PBI and PI have been found to be soluble in DMAc, as well as DMF and NMP, and thus blends can be prepared by solution mixing, followed either by precipitation into a non-solvent like methanol or by casting a film. The Tg of a 50/50 blend has been measured at ~ 380°C. The ratio of PI in the blend should be governed by the level of moldability needed and the decreased susceptibility to moisture regain required of the application. Heat-induced cross-linking may lead to further utility of such blends, such as modified softening temperature and solvent resistance under stress-enhanced Tg with aging conditions.54
7.9.2 PBI–PAI blends
Polyamide-imides (PAIs) are thermoplastic amorphous polymers prepared by the condensation of an aromatic diamine, such as methylene diamine, and an anhydride, such as trimellitic acid chloride. PAIs have good mechanical, thermal, chemical resistance, high strength, melt processability, and high heat capacity. They can be processed into a variety of forms, such as injection or compression molded articles, coatings, films, fiber, and adhesives. The typical heat deflection temperature for neat molded PAI is ~ 278°C, but reinforcements are often used to improve mechanical properties. PAIs are generally soluble in strong aprotic solvents such as NMP and DMAc, and thus miscible blends with PBI are feasible.
Chen and others55 reported on blends of PBIs and polyamide-imides having fluorine-containing linking groups, 5–95 wt% PBI. The F-containing linking groups were prepared by forming the polycondensation product of one or more aromatic diamines, one or more trifunctional acid anhydride monomers, and one or more tetrafunctional aromatic dianhydrides, at least one of the monomers containing the group, CF3-C(H2)-R, linking two aromatic moieties where R is CF3 or phenyl. PAI typically has a Tg of ~ 275°C; however, it exhibits poor flow properties, rendering difficulties with injection molding and spinning into fibers. The fluorine-containing linking group improves flow characteristics, improves solubility in most organic solvents, such a trichloroethylene as compared to PI, and improves hydrophobic properties as well as thermo-oxidative stability. Thus, applications in wire enamel coatings, laminates, molded products, films, fibers and impregnating varnishes may be significantly improved, including thermal stability, particularly when blended with PBI. Such blends with fillers, reinforcing agents, and post-heat treatment with or without sulfuric acid, can be used in film and fiber forms where shrinkage can be minimized and resistance to solvents and acids is enhanced.
7.10 PBI–poly (bisphenol-A carbonate) (PC) and PBI–polybenzoxazole (PBO) blends
Polycarbonate blends with PBI may be used to improve the blend miscibility by intermolecular interactions (hydrogen bonding) as compared to polyimides, and subsequently improved relative thermal stability. Due to the high temperature performance attributes of PBI and PBO, blending would be expected to enhance further the thermal characteristics and end-uses, and with good solubility in typical solvents for the individual polymer resins.
7.10.1 PBI–PC blends
Musto and others56 pursued PBI blended with poly (bisphenol-A carbonate) (PC), poly-[2,2-propane-bis(4-phenyl carbonate)], as a means to investigate polymers that are comparatively stronger proton acceptors compared to PI. PBI possesses a strong tendency to form hydrogen bonds acting as proton donors, and thus bisphenol A was selected as a candidate toward enhancing the strength of the intermolecular interactions with PBI and thus improving miscibility, in addition to its reactivity, thermal stability, and ability to form miscible blends. Hydrogen bonding interactions occur among the N-H groups of PBI and the carbonyl groups of PC as evidenced from FTIR spectra. The Tg of the blend was found to occur at ~ 240°C as opposed to ~ 220°C calculated from the Fox equation. Thus, PBI was found to have a profound impact on the stability of PC, and with PC degradation in the presence of PBI occurring mainly by dissociation of the carbonate groups via catalyzed hydrolysis. Based on an FTIR quantitative analysis of the carbonyl region of a 50/50 wt% blend, the estimated Tg of the blend was found to be close to the value predicted by the Fox equation.
7.10.2 PBI–PBO blends
Attempts have been made to combine PBI and polybenzoxazole (PBO) to obtain more thermally stable and tractable polymers.57 Both polymers have received attention due to their chemical resistance, thermal stability, and good mechanical properties at high temperature. Co-polymers have also been reported,58,59 with the claim that phase separation would not easily take place compared with solution mixing/blending of the two polymers. It was found that the initial decomposition temperature of the co-polymer was ~ 590°C, with 5% weight loss at 590–600°C, and with ~ 50 wt% of residue remaining at 770°C, demonstrating reasonably good thermal stability. The co-polymer was found to be soluble in hot DMF, DMAc, NMP, and sulfuric acid.
7.11 PBI–poly(4-vinyl pyridine) (PVPy) and other blends
The good miscibility of PBI–PVPy blends can be useful for property modifications in the plastics industry, particularly in the areas of processability, where intermediate Tg values are sufficient and economical, and with retention of good mechanical properties at elevated temperatures. Blending can be achieved by solution mixing, and hydrogen bonding promotes compatibility.
7.11.1 PBI–poly(4-vinyl pyridine) (PVPy) blends
In pursuit of high temperature polymer blends, the miscibility of such polymer systems have been studied previously.7,47,48,60 PVPy was further investigated,61 based on pyridine being a strong base capable of forming hydrogen bonds with the N-H group of PBI. Blends were prepared by solution mixing in DMAc, and PBI was shown to be miscible with PVPy over the entire composition range. Blends of all compositions were shown to have a single Tg, lying close to or above the weight average Tg of the two components. PVPy was found to begin to decompose at temperatures over 375°C. FTIR showed that miscibility was promoted by interaction of H-bonding, and even small amounts of H-bonding may be sufficient to promote miscibility. Such blending may have utility in the pursuit of useful property modifications.
7.11.2 Other blends
Calundann and others62 reported on novel miscible blends comprising from about 5 to 95 wt% of PBI and 95 to 5 wt% of an aromatic polyamide, aromatic polyamide-hydrazide or aromatic polyamide containing heterocyclic linkages, preferably at least 50 to about 95 wt% of the PBI and from 50 to 5 wt% of the polyamide component. Useful blend solvents include NMP, DMF, DMAc, and DMSO; heating may be required to obtain clear viscous solutions. The polyamide-type components were characterized as having high Tg values and HDTs, but with inferior non-flammability characteristics. The non-PBI component was recommended to be present in the blend, mostly PBI, at a sufficient level to result in a blend with improved physical properties (tensile strength and modulus) relative to PBI, more thermally tractable than PBI, and also less susceptible to moisture, and while maintaining the attractive features of PBI. The PBI level in a blend consisting mostly of the polyamide component was recommended at a level such that the blend had increased thermal stability and non-flammability relative to the polyamide component. Such blends were found to have good thermal, flame, and solvent resistance, improved physical properties, good price/performance characteristics, and to be useful in severe environmental conditions. Moldable blends may be prepared by freeze milling the dry blends to 100 microns or less in diameter. Blends in the form of films, fibers, fibrets, or molded articles may be post-treated with heat or sulfonating agents in order to minimize shrinkage and increase solvent and acid resistance.
7.12 PBI commercial products
For engineers and researchers searching for materials for specific high temperature applications, data and information about commercially available PBI and PBI blends are important. PBI commercial products include PBI fibers, PBI polymers, compression molded PBI products, melt-processable PBI/PEEK blends, and PBI solutions. Their typical properties are summarized in this section.
7.12.1 Commercial PBI fibers
The commercially available PBI fibers are 1.5 denier staple and short cut fibers. The typical properties of PBI fibers are shown in Table 7.1. PBI fibers are highly thermally stable and maintain good strength after exposure to flame. They do not burn, melt or drip. The natural color of PBI fiber is golden brown. Black PBI fibers have been developed recently. PBI fibers can be easily blended with other staple fibers during yarn spinning or nonwoven manufacturing. Para-aramid and FR-Rayon fibers are used most to blend with PBI fibers to produce high performance flame resistant fabrics. Since the 1990s, more and more cities have chosen the fabrics made of 40% PBI with 60% para-aramid as the outer shells for fire fighter garments because they outperform other fabrics in fire protection while providing more comfort to wear. Sold under the trade names of PBI Gold® and PBI Matrix®, these PBI fabrics have reduced burn injuries and saved many lives.
Table 7.1
Properties of PBI staple fibers
| Property | Imperial unit | Metric unit |
| Denier per filament (DPF) | 1.5 denier | 1.7 dtex |
| Tenacity | 2.7 g/d | 2.4 dN/tex |
| Breaking elongation | 27% | 27% |
| Initial modulus | 45 g/d | 40 dN/tex |
| Crimp | 9.5/inch | 3.7/cm |
| Specific gravity | 1.4 | 1.4 |
| Moisture regain at 68°F (20°C) 65% RH | 15% | 15% |
| Boiling-water shrinkage | < 1.0% | < 1.0% |
| Hot-air shrinkage at 400°F (205°C) | < 1.0% | < 1.0% |
| Specific heat | 0.3 BTU/lb F | 1.3 kJ/(kg K) |
| Limiting oxygen index | > 41% | > 41% |
| Standard cut length* | 1/8, ¼, 1½, 2, 3, 4 inch | 3, 6, 38, 50, 76, 102 mm |
7.12.3 Compression molded PBI products
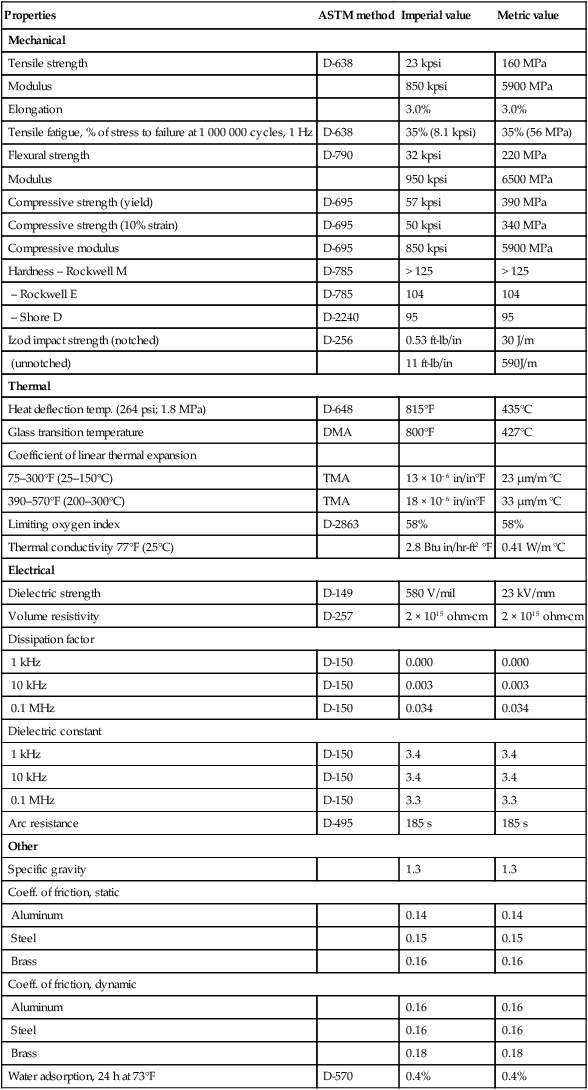
Compression molded PBI stock shapes are commercially available under the trade name Celazole® U-60. High temperature and high pressure are needed to compression mold PBI. PBI stock shapes can be further machined into specific parts. With the highest Tg among thermoplastics at about 427°C (DMA method), Celazole® U-60 also has the highest compressive strength at 57 kpsi. The superior physical properties, high temperature properties and outstanding chemical resistance make Celazole® U-60 the world’s highest performing plastic material. Typical properties data of Celazole® U-60 are summarized in Table 7.2.
Table 7.2
PBI Celazole® U-60 typical properties
| Properties | ASTM method | Imperial value | Metric value |
| Mechanical | |||
| Tensile strength | D-638 | 23 kpsi | 160 MPa |
| Modulus | 850 kpsi | 5900 MPa | |
| Elongation | 3.0% | 3.0% | |
| Tensile fatigue, % of stress to failure at 1 000 000 cycles, 1 Hz | D-638 | 35% (8.1 kpsi) | 35% (56 MPa) |
| Flexural strength | D-790 | 32 kpsi | 220 MPa |
| Modulus | 950 kpsi | 6500 MPa | |
| Compressive strength (yield) | D-695 | 57 kpsi | 390 MPa |
| Compressive strength (10% strain) | D-695 | 50 kpsi | 340 MPa |
| Compressive modulus | D-695 | 850 kpsi | 5900 MPa |
| Hardness – Rockwell M | D-785 | > 125 | > 125 |
| – Rockwell E | D-785 | 104 | 104 |
| – Shore D | D-2240 | 95 | 95 |
| Izod impact strength (notched) | D-256 | 0.53 ft-lb/in | 30 J/m |
| (unnotched) | 11 ft-lb/in | 590J/m | |
| Thermal | |||
| Heat deflection temp. (264 psi; 1.8 MPa) | D-648 | 815°F | 435°C |
| Glass transition temperature | DMA | 800°F | 427°C |
| Coefficient of linear thermal expansion | |||
| 75–300°F (25–150°C) | TMA | 13 × 10- 6 in/in°F | 23 μm/m °C |
| 390–570°F (200–300°C) | TMA | 18 × 10- 6 in/in°F | 33 μm/m °C |
| Limiting oxygen index | D-2863 | 58% | 58% |
| Thermal conductivity 77°F (25°C) | 2.8 Btu in/hr-ft2 °F | 0.41 W/m °C | |
| Electrical | |||
| Dielectric strength | D-149 | 580 V/mil | 23 kV/mm |
| Volume resistivity | D-257 | 2 × 1015 ohm-cm | 2 × 1015 ohm-cm |
| Dissipation factor | |||
| 1 kHz | D-150 | 0.000 | 0.000 |
| 10 kHz | D-150 | 0.003 | 0.003 |
| 0.1 MHz | D-150 | 0.034 | 0.034 |
| Dielectric constant | |||
| 1 kHz | D-150 | 3.4 | 3.4 |
| 10 kHz | D-150 | 3.4 | 3.4 |
| 0.1 MHz | D-150 | 3.3 | 3.3 |
| Arc resistance | D-495 | 185 s | 185 s |
| Other | |||
| Specific gravity | 1.3 | 1.3 | |
| Coeff. of friction, static | |||
| Aluminum | 0.14 | 0.14 | |
| Steel | 0.15 | 0.15 | |
| Brass | 0.16 | 0.16 | |
| Coeff. of friction, dynamic | |||
| Aluminum | 0.16 | 0.16 | |
| Steel | 0.16 | 0.16 | |
| Brass | 0.18 | 0.18 | |
| Water adsorption, 24 h at 73°F | D-570 | 0.4% | 0.4% |
Celazole® U-60SD is a special grade of PBI products with extremely low metallic ion contents (< 1 ppm per species) and is generally used in the semiconductor industry due to its high purity. Its mechanical properties are similar to U-60. Other U-60 grades include U-60ESD, an electrostatic dissipative grade used to prevent static buildup in the manufacture of certain electronic parts, and U-60CF, a high strength, high modulus form of U-60. Moisture absorption of PBI is higher than many other thermoplastics. PBI parts should be dried before use in applications in which parts are heated rapidly to above 300°C.
7.12.4 Commercial PBI–polyetheretherketone (PEEK) blends
As discussed in Section 7.2, PBI polymers can be compounded with many other high temperature plastics to make different PBI blends. Currently, four PBI–PEEK(polyetheretherketone) blends are commercially available under the following trade names:
These four blends are sold in pellet form for injection-molding applications. Typical properties of molded Celazole® TU-60, TL-60, TF-60C, and TF-60V products are listed in Table 7.3.
Table 7.3
Typical properties for commercial PBI–PEEK blends
| Properties | ASTM test method | TU-60 | TL-60 | TF-60C | TF-60V | ||||
| Unreinforced Melt processable | Self-lubricating Melt processable | Carbon fiber reinforced Melt processable | Glass fiber reinforced Melt processable | ||||||
| Imperial value | Metric value | Imperial value | Metric value | Imperial value | Metric value | Imperial value | Metric value | ||
| Tensile strength Modulus | D-638 | 14.5 kpsi 730 kpsi |
100 MPa 5000 MPa |
16 kpsi 2600 kpsi |
110 MPa 18,000 MPa |
33 kpsi 3500 kpsi |
230 MPa 24,000 MPa |
22 kpsi 1750 kpsi |
150 MPa 12000 MPa |
| Elongation Flexural strength strength | D-790 | 2.2% 25.4 kpsi 725 kpsi |
2.2% 175 MPa 5000 MPa |
0.9% 23 kpsi 1900 kpsi |
0.9% 158 MPa 13,000 MPa |
1.4% 46.5 kpsi 3050 kpsi |
1.4% 320 MPa 21 000 MPa |
1.4% 32.6 kpsi 1810 kpsi |
1.4% 1810 kpsi 12 500 MPa |
| Compressive strength (yield) Modulus | D-695 | 30 kpsi 430 kpsi |
206 MPa 2900 MPa |
18 kpsi 740 kpsi |
123 MPa 5100 MPa |
32 kpsi 5100 MPa |
220 MPa 3800 MPa |
32 kpsi 530 kpsi |
220 MPa 3600 MPa |
| Hardness – Rockwell A Specific gravity | D-785 | 25 1.3 |
25 1.3 |
24 1.43 |
24 1.43 |
30 1.41 |
30 1.41 |
30 1.52 |
30 1.52 |
| Poisson’s ratio Coefficient of thermal expansion 75–300°F | TMA | 19 × 10− 8 in/in °F | 34 μm/m °C | 0.42 14 × 10− 6 in/in °F |
0.42 26 um/m °C |
0.39 14 × 10− 8in/in °F |
0.39 26 um/m °C |
0.34 9 × 10− 6 in/in °F |
0.34 17 um/m °C |
| Temperature of initial (5%) weight loss in air – in nitrogen | TGA TGA |
930° F 930° F |
499°C 499°C |
1116°F 1123°F |
602°C 606° C |
1110°F 1160°F |
599°C 627°C |
1125°F 1175°F |
607°C 63 5° C |
| Thermal conductivity | F-433 | 5.3 Btu in/h ft2 °F | 0.76 W/m °C | 3.1 Btu in/h ft2 °F | 0.45 W/m °C | 2.5 Btu in/h ft2 °F | 0.36 W/m °C | ||
| Heat capacity at 158°F | DSC | 0.28 Btu/lb °F | 1160 J/kg °C | 0.28 Btu/lb °F | 1180 J/kg °C | 0.27 Btu/lb °F | 1130 J/kg °C | ||
| Heat deflection temperature at 264 psi | D-648 | 482° F | 250°C | 590°F | 310°C | 608°F | 320°C | 590°F | 310°C |

Celazole® TU-60 is an unfilled blend of PBI–PEEK. Independently, PEEK is a high performance thermoplastic with excellent mechanical properties, chemical resistance, and thermal stability. The TU-60 blend has even better thermo-mechanical properties than PEEK, particularly at elevated temperatures. And TU-60 can be injection molded to make parts at lower costs than PBI.
Celazole® TL-60 is a self-lubricating grade of the PBI-PEEK blend. It is an injection-molding grade like TU-60 but with added features of low friction and high wear resistance. The ideal applications are for bearings, bushings, and seals where friction, heat and chemicals can shorten the life of other plastic materials.
Celazole® TF-60C is a carbon-reinforced PBI/PEEK blend. It is an injection-molding grade like TU-60, but with much higher modulus and strength. The improved physical properties are particularly suitable for applications that require superior strength and low deformation at high temperature. It is also static dissipative.
Celazole® TF-60V is a glass-reinforced PBI/PEEK blend. It is an injection-molding grade with properties similar to TF-60C except that TF-60V is a much better thermal and electrical insulator. It is ideally suited for applications that require high strength, lower deformation, and excellent insulation under high temperatures.
7.12.5 PBI solutions
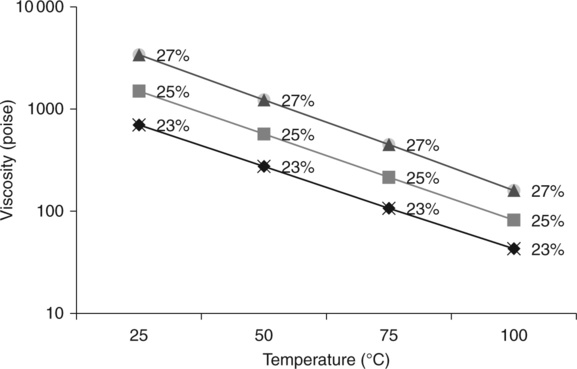
Homogeneous PBI solutions in DMAc (dimethylacetamide) solvent are commercially available at 26% and 10% concentrations. PBI solutions are used for fiber spinning, coating, impregnation, film casting and prepreg applications. The 26% PBI/DMAc solution contains LiCl to keep the solution stable. Solution viscosities at different temperatures are shown in Fig. 7.13. The 26% PBI/DMAc solution can be easily diluted to a lower concentration by mixing with additional DMAc solvent. PBI solution viscosities at different concentrations are shown in Table 7.4. The 10% PBI solution is made without LiCl and is easier to be applied during coating processes.
7.13 PBI in high temperature applications
PBI finds many high temperature applications, not only in protecting fire fighters and military personal, but also in semiconductor, clean energy, oil, gas, chemical, and aerospace industries because of its extremely thermally stable molecular structure and superior physical properties. Examples of some of the applications are briefly discussed in this section.
7.13.1 Molded products of PBI and PBI blends
Compression-molded PBI shapes can be machined into parts of various complexities. As the world’s highest performing thermoplastics, PBI parts are often used in the most demanding environments where other polymer materials fail to meet the temperature and performance requirements. For example, in the dry etch chambers for semiconductor wafer manufacturing, PBI parts are selected because of their high temperature dimensional stability, wear resistance, and plasma resistance. PBI seals, lifting pins, bushing, screws, insulators, and other components have replaced parts made of Quartz, Alumina and polyimide in the plasma etch chambers, providing prolonged life cycle and reduced wafer contamination. PBI parts are also used in flat glass panel manufacturing processes as clamps, guides, and other components. The longer life cycle, superior dimensional stability at high temperature, and the ability to control electrostatic discharge make PBI parts excellent choices.
PBI–PEEK blends TU-60, TL-60, TF-60C and TF-60V are often used for applications where materials with performance superior to PEEK are required. The melt-processable PBI–PEEK blends are cost competitive options to meet the challenges of those applications. Celazole® TL-60, a PBI–PEEK blend with enhanced lubrication properties, works especially well for parts with rotating or sliding surfaces such as bearings and bushings. A study63 examining six high performance plastics, including PBI TL-60, PEEK, polyimide (PI), and polyamide-imide (PAI) through high PV wear tests demonstrated this. The results showed that the TL-60 sample had the lowest coefficients of friction and lowest wear factor. It was the only material in the tests that survived loads at or above 2500 psi at 50 ft/min speed.63
Examples of PBI–PEEK products can be found in bearings, bushings, gears, piston rings, washers, seals, pads, rollers, conveyors, and many other molded parts.
7.13.2 Fabrics made of PBI fiber blends
The fabrics made of 40% PBI with 60% Para-aramid fiber blend are the premier outershell materials for fire fighter suits. PBI fibers improve a fabric–s strength after flame exposure, increase comfort for users, and reduce heat transfer through the fabric. As a result, the PBI–para-aramid fabrics are the first choice for professional firefighters in most large cities in the US, Canada and many cities in Europe. There is an increasing awareness of PBI fabrics in Asia Pacific. Hong Kong, South Korea, Saudi Arabia, and New Zealand are examples of the places in Asia using PBI fabrics to protect their firefighters.
Protection from fire, arc flash, explosions, and other hazards is very important for military personal and for workers in the electrical, oil, gas, and chemical industries. Fabrics made of PBI–para-aramid–FR-Rayon fiber blends perform very well in these applications. Also, PBI–FR-Rayon fiber blends can be knitted to make a soft fabric ideal for protective undergarments or hoods which provide both protection and comfort.
7.13.3 Examples of other applications
PBI short cut fibers have been used to replace asbestos in high temperature applications such as rocket motor insulation. Rubber formulas incorporating PBI short cut fibers showed superior performance in a development of ablative materials for NASA solid fuel rocket motor insulation. As a result, PBI short cut fibers are specified for ablative insulation in the development of new solid fuel rocket motors. Nonwoven fabrics containing PBI are also excellent materials for the fire blocking layers in airplane seats due to their better performance at lighter weight.
PBI membranes have been the critical components in high temperature polymeric electrolyte membrane fuel cells. PBI fuel cells tolerate much more impurities in the H2 fuel compared to low temperature fuel cells. Therefore, they are preferred in fuel cell systems where the H2 supplies are from reformers converting other fuels to H2. PBI fuel cells are also suitable for combined heat and power fuel cell systems. In other applications, PBI membranes have been successfully demonstrated to purify H2 while pumping it against a high pressure. The technology can be applied to recover H2 in metal heat treating ovens as well as many other processes that use H2.
In liquid separation, hollow fiber membranes based on PBI have shown excellent performance for pervaporation dehydration of organic liquids.64–67 For example, a dual layer PEI–PBI hollow fiber membrane with an outer selective layer of PBI showed better performance than most other polymeric membranes in pervaporation dehydration of ethylene glycol.67 Sulfonation modifications of PBI membranes have demonstrated excellent separation efficacies in the dehydration of acetic acid.68 Studies have shown that PBI hollow fiber membranes were effective in separating chromates from solutions.69 Also, PBI nanofiltration hollow fiber membranes are promising candidates as forward osmosis membranes.70 In gas separation, recent studies sponsored by the Department of Energy at Los Alamos National Laboratories and SRI International demonstrated potential applications of PBI membranes in carbon capture and H2 purification from synthesis gas streams at elevated temperatures.71 H2/CO2 selectivity > 40 has been achieved at H2 permeability of 200 GPU at 250°C.72
7.13.4 PBI composites and coatings
With the highest Tg among commercial thermoplastics, PBI can be used as the matrix resin to make composite products with superior thermal properties. The potential exists to increase the temperature range for advanced polymer composites through further development of PBI composite materials.
PBI coatings are highly resistant to heat, chemicals and wear. They are suitable for protecting metal surfaces working under friction at high temperature. US patent 5549946 describes how the surface of a heat-resistant roll for a copying machine is coated with a varnish containing polybenzimidazole as a major resin component.73 US patent 567414 describes how an electrical wire is coated with a polybenzimidazole varnish.74 US Patent Application 12/957601 describes a mixture of polybenzimidazole and polyacrylate.75 US patent 8475925 describes a coating based on polybenzimidazole with superior adhesion characteristics through the inclusion of an epoxy component.76 All of these patents/patent applications advance PBI as a coating by increasing the versatility as a conformal or planar coating in stressful thermal mechanical systems.
7.14 Future trends
PBI’s superior properties, such as high glass transition temperature (Tg), good chemical resistance and excellent mechanical properties at both high and cryogenic temperatures, allow products made of PBI to continue outperforming those made of other commercial high temperature polymers. One trend for PBI application is replacing other materials to improve the performance of an existing high temperature product. In the meantime, technology advancements in defense, aerospace, energy, semiconductor and other industries are demanding material properties that many other performance polymers cannot provide. PBI products are good candidates to meet these new challenges.
Applications of the melt-processable commercial PBI/PEEK blends TU-60, TL-60, TF-60C, and TF-60V are growing. Improvements in processing technologies for these PBI blends are leading to more products with higher quality and efficiency. Knowledge of PBI materials with understanding of specific applications can lead to developments maximizing the benefits of PBI blends for different end uses. Moreover, new and unique properties can be created within targeted performance and price specifications by well-designed PBI blends. Developments of new commercial PBI blends in the future will provide more choices for challenging high temperature applications.
In the protective clothing arena, the trend is continuing for firefighters in more countries to select PBI fabrics for their protective gear. For over two decades, PBI fabrics have been established in North America as the best choice for firefighter turnouts due to their superior overall performance. In recent years, the use of PBI fabrics in the fire services is growing fastest outside North America as countries around the world are seeking better protection for their firefighters. Additionally, there are continuing developments of different PBI fiber blends and fabrics for uses beyond the fire service market. The properties, comfort, and protective performances of these fabrics make them suitable for protective garments for military and police personnel as well as for workers in chemical, oil, gas, and electric industries. An ultra-high performance, unique polymer, PBI will make more and more contributions in high temperature applications as innovations continue in PBI materials, process engineering, and end-use applications.