2
Using Polymers to Enhance the Carbon Nanomaterial Biointerface
Goutam Pramanik1 Jitka Neburkova1,2 Vaclav Vanek1 Mona Jani1 Marek Kindermann1,3,4 and Petr Cigler1
1 Institute of Organic Chemistry and Biochemistry of the CAS, Czech Republic
2 Charles University, First Faculty of Medicine, Prague, Czech Republic
3 Institute of Microbiology of the CAS, Czech Republic
4 University of Chemistry and Technology, Czech Republic
2.1 Introduction
The unique ability of carbon atoms to participate in robust covalent bonds with each other and also with other elements in diverse hybridization states (sp, sp2 , sp3 ) allows them to form a wide range of structures, from small molecules to nanomaterials [1]. Carbon‐based nanomaterials have garnered a great deal of interest over the past three decades, starting with the debut of fullerene in 1985 [2], followed by carbon nanotubes [3] (CNTs) and graphene [4] in 1991 and 2004, respectively. Fullerene, CNTs, and graphene are primarily composed of sp2 carbon atoms with a network of conjugated π electrons. In recent years, carbon dots (CDs) with mixed sp2 and sp3 carbon atoms plus defects and heteroatoms, as well as nanodiamonds consisting of sp3 carbon atoms, have also received a great deal of attention [5, 6]. Due to the quantum confinement effect, these carbon nanomaterials (CNMs) possess many interesting physicochemical properties that are not attainable in bulk carbon materials like diamond and graphite [7, 8]. Over the past decade, there has been a flurry of development of biomedical research applications for graphene, CNTs, nanodiamonds, and CDs, owing to their small size, unique optical properties, and large surface area. In this chapter, we will focus on functionalization of CNMs (CNTs, graphene, nanodiamonds, and CDs) with polymers for biomedical applications. For these applications, a stable dispersion of CNMs in biological medium is a fundamental prerequisite that can be achieved by proper surface functionalization of the nanomaterial [9, 10]. Nonfunctionalized CNMs tend to form stable aggregates due to strong intermolecular interactions, such as van der Waals (vdW) forces and dipole–dipole interactions. Aggregation of nanomaterials results in unwanted changes to their physicochemical properties and makes homogeneous cell labeling very difficult. Polymer coating is an effective, convenient, and widely used approach for transferring CNMs into aqueous solution. Polymer coatings provide a number of distinct advantages over small‐molecule coatings, including ease of synthesis and processing, structural diversity, tunable surface functionality, and flexibility. Multiple contact points between the CNM surface and polymers provide a more robust surface coating than coating with small molecules, which typically have only one binding group. The strong binding also prevents desorption of ligands from the surface, which has been one of the sources of toxicity in practical applications. Most importantly, polymers can be designed to include reactive functional groups that can be further functionalized with biomolecules. The design and structure of polymers for functionalization of CNMs depend on the chemistry of the CNM and its potential applications in a biological environment. Polymer coating of CNMs not only increases the colloidal stability of the particles but also selectively changes or influences the CNM properties, such as reducing nonspecific adsorption of biomolecules or tuning the charge (for gene therapy applications). The polymer shell on the surface of CNMs, rather than the core, determines the major final properties of the coated CNMs. These properties are crucial for potential interactions of particles with a biological environment.
2.2 Colloidal Stability of CNMs
To successfully develop nanomaterials for biomedical applications, a detailed understanding of the factors driving the colloidal stability of nanoparticles (NPs) and their aggregation in biological medium is crucial. A classical theoretical approach based on Derjaguin–Landau–Verwey–Overbeek (DLVO) theory is commonly used to predict the stability of a colloidal system. This approach balances attractive vdW forces and repulsive forces caused by the electrostatic double layer (EDL) (Figure 2.1a,c) [12]. The attractive vdW forces are competed by the EDL which stabilizes the colloidal dispersion. In addition to electrostatic stabilization, other repulsive forces (e.g. hydration forces) and specific attractive forces (e.g. depletion and hydrophobic or magnetic forces) also contribute to the stabilization or destabilization of the colloidal system [13]. The DLVO theory has been extended (X‐DLVO) (Figure 2.1d) by adding these individual forces, assuming that each interaction is completely independent.

Figure 2.1 Colloidal interactions of NPs. (a) At the most basic level, NP aggregation is governed by van der Waals interactions. Permanent or induced dipoles within the NP can result in net attractive forces between NPs and subsequent aggregation. (b) Macromolecules can physically stabilize NPs. Hydrophilic macromolecules can shield the NPs from interactions, and steric stabilization can be combined with charge stabilization for electro‐steric stabilization. (c) The inherent surface charge of NPs, caused by surface ions or functional groups, results in formation of the stern layer, oppositely charged ions adsorbed to the NP surface. Ions with an opposite charge from the stern layer form the diffuse layer. Together, this is referred to as the electric double layer (EDL). The EDL forms a net charge, and when two like particles are in proximity, the EDL repels the two. (d) Illustration of potential energy (ψ) distance (d) curves. They can be used to predict the contribution of the different stabilization approaches and model the stability of NPs in solution.
Source: Reproduced from [11].
The colloidal stability of nanomaterials increases with increasing surface charge. The surface charge of NPs is commonly determined in terms of zeta potential. Zeta potential is the electrostatic potential of the particles measured at the so‐called shear plane, the distance from the surface at which ions are not bound to the particle. A zeta potential of ±30 mV is widely regarded as the borderline between stable and unstable colloids in aqueous solution. The net charge of a nanosystem is balanced by the counterions around the particle. Counterions adsorbed on the charged NP surface comprise the “Stern layer,” and a cloud of ions of opposite net charge surrounding the nanoparticles makes up the “diffuse layer” (Figure 2.1c). The ionic strength of the solution determines the radial size of the diffuse layer. Low ionic strength allows the diffuse layer to extend far from the particle surface, which repels particle–particle interactions. High ionic strength, as in electrolyte‐rich cell culture medium, induces the collapse of colloidal systems by compressing the EDL [14]. During endocytotic uptake by cells, NPs are exposed to varying conditions, such as pH changes from 7.4 (in the extracellular medium) to 5.5 (in late endosomes) to 4.5 (in lysosomes). Nanomaterials with amphoteric surface groups tend to have a neutral charge at pH values near the isoelectric point, which promotes aggregation due to decreased repulsion and enhanced attractive vdW forces [15]. In addition to their acidic pH, lysosomes also contain high levels of hydrolytic enzymes that can degrade the nanomaterial coating [16]. Thus, electrostatically stabilized NPs generally have poor stability in cellular medium and are not well‐suited for biomedical applications.
The influence of NPs on cellular interactions can be assessed by in vitro systems in which NPs interact with the components of the cell culture medium prior to any cellular contact. These culture media contain a complex mixture of proteins, such as serum albumin and globulins, and numerous biomolecules, such as amino acids and salts. The nonspecific adsorption of protein on the surface of NPs increases the hydrodynamic diameter and greatly reduces the mobility of the particles. Changes in the dispersion state of NPs have been shown to influence the cellular response and the fate of NPs in biological environments [17–19].
For successful design of the CNM biointerface, the following must be considered:
- After functionalization, CNMs must be soluble in water and should not aggregate over a broad pH range, in the presence of high concentrations of electrolytes, nor in biological media.
- The surface functionalization of the CNMs must promote biocompatibility and reduce nonspecific interactions.
- Surface functionalization needs to be effective and controllable to permit adjustment of the number and nature of biomolecules attached to CNMs, thus facilitating their applications in targeting, biosensing, and bioimaging.
2.3 Functionalization of CNMs with Polymers
Surface polymers stabilize dispersion of CNMs by steric hindrance. Steric stabilization is a powerful tool to enhance the dispersion stability of CNMs under harsh conditions by preventing two particles from forming attractive vdW interactions due to osmotic pressure and elastic recoil effects (Figure 2.1b) [20]. Unlike pure electrostatic stabilization, steric stabilization also stabilizes NPs in the presence of high salt concentrations, which is important for biomedical applications. Using charged polymers, the stabilization of colloidal systems can be further improved due to combined electro‐steric repulsion [21, 22].
CNMs can be functionalized with polymers via two types of approaches: (i) noncovalent coating through electrostatic or hydrophobic interactions and (ii) covalent modification, which can be divided into “grafting to” and “grafting from” approaches.
2.3.1 Noncovalent Approaches
Polymers can adsorb onto CNMs spontaneously from solution if the interaction of CNMs with the polymer is more favorable than with solvent. Polymer connections to CNMs occur through various mechanisms, depending on the internal structure of the polymer. CNMs can be encapsulated inside amphiphilic polymers via hydrophobic interactions between the hydrophobic portion of the polymer and the hydrophobic CNM surface at multiple coordination points, leaving the hydrophilic portion of the polymer exposed to biological medium. CNMs also can be noncovalently polymer‐coated by electrostatic interactions − for example, between the amine groups of biomolecules and carboxylic acid groups of the CNMs.
2.3.2 Covalent Approaches
The “grafting to” approach is based on the attachment of synthesized or commercial polymers to the CNM surface by various chemical reactions, such as amidation, esterification, or radical coupling. Polymers with suitable reactive functional end/side groups (e.g. ─OH, ─NH2, ─COOH, and ─COCl) can be grafted onto CNMs. This technique is easy to carry out with both linear and dendritic polymers, but the grafting efficiency is always low due to steric hindrance from the pregrafted polymer chains. Polymer chains have to diffuse through an existing layer of polymer to reach complementary groups on the surface.
Introduction of a suitable functional group onto the CNM surface is the first step in the “grafting from” approach. This is followed by growth of polymer chains from the NP surface in a solution containing a mixture of monomers. Carboxylic acid groups, which can be generated on graphene, CNTs, nanodiamonds, and CDs by oxidization procedures, can be used to attach an initiator via esterification or amidation [23, 24]. Many polymerization methods, including atom transfer radical polymerization (ATRP), reversible addition fragmentation chain transfer polymerization (RAFT), nitroxide‐mediated radical polymerization (NMRP), anionic polymerization, and ring‐opening polymerization (ROP) techniques, have been used to functionalize CNMs. ATRP is among the most effective and successfully used approaches to functionalize CNMs with a wide range of polymers while controlling the molecular weight of the grafted polymers [24, 25]. “Grafting from” methods usually yield more dense protective coatings than “grafting to” methods.
2.4 Influence of Polymers on the Spectral Properties of CNMs
The physicochemical properties of CNMs are determined by their intrinsic nanostructure. The surface functionalization of CNMs with polymers can introduce defects into the nanostructures or alter the nano‐environment around the CNMs, which can lead to changes in their intrinsic properties. In this section, we will focus on the effect of polymer functionalization on the spectral properties of various CNMs.
The fluorescence of CNTs, which appears mainly in the second near‐infrared window (NIR‐II window, 1000–1700 nm), is distinct from that of other CNMs. CNTs such as single‐walled carbon nanotubes (SWCNTs) absorb a photon with the band gap of the E22 transition (Figure 2.2a), which excites the electron from the second valence band to the second conduction band, leaving a hole behind. The excited electron and the hole form a bound pair called an exciton, which recombines to emit a photon in the NIR‐II region (Figure 2.2b). The excitonic nature of the photoluminescence properties of CNTs makes these nanomaterials highly sensitive to the environment, as well as to the length of the nanotube. Defects in the conjugated system of nanotubes act as discontinuities along their length. The defect sites in a SWCNTs can cause nonradiative recombination of diffusing excitons and lower the quantum yield. Noncovalent coating of SWCNTs with polymers avoids disruption of the π‐network of pristine CNTs and increases the luminescence quantum yield by one order of magnitude [29]. The photoluminescence peak of polymer‐coated nanotubes can be red‐shifted by creating a more polarizable environment around the CNTs due to π–π stacking [30]. Single‐stranded DNA can disperse CNTs in water by helically wrapping around the CNT surface through π‐stacking [31]. Kurnosov et al. found that cysteine doping (from 10−8 to 10−3 M) into an aqueous suspension of nanotubes wrapped with DNA leads to an increase in photoluminescence intensity [32]. The photoluminescence intensity was enhanced by 27% in the presence of 10−3 M cysteine, and the researchers attributed this increase to the passivation of p‐defects on the nanotube by the reactive thiol group of cysteine [32]. On the other hand, the fluorescence intensity of SWCNTs can be quenched upon noncovalent coating with a pyrene‐functionalized poly(3‐hexylthiophene) derivative, due to a strong photo‐induced electron transfer resulting from the noncovalent linkage of SWCNTs to the poly(3‐hexylthiophene) backbone via the pyrene bridge [33]. SWCNTs can be covalently functionalized with polymers via the carboxylic groups at the open ends of the SWCNTs. However, the damage to the conjugated π network during covalent functionalization of SWCNTs is a major concern. The defects created in the conjugated π network during covalent modification decrease the fluorescence quantum efficiency of NIR‐II emission, due to the increased probability of nonradiative recombination of excitons at the defect site [26].
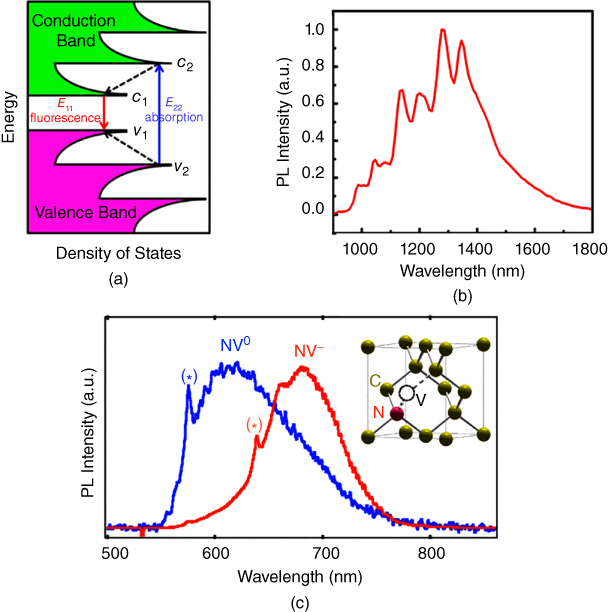
Figure 2.2 (a) Band diagram of a semiconducting SWCNT, showing fluorescence emission after excitation. (b) A NIR photoluminescence spectrum of a SWCNT. (c) Photoluminescence spectra of single NV− and NV0 color centers in nanodiamonds. The zero‐phonon lines of NV− and NV0 are indicated with ⋆ symbols; the emissions are located at 637 and 575 nm, respectively. The inset shows the atomic structure of the NV defect, consisting of a substitutional nitrogen atom (N) associated to a vacancy (V) in an adjacent lattice site of the diamond crystalline matrix.
Source: Adapted with permission from [28]. Copyright (2010) by the American Physical Society.
Source: Adapted with permission from [27]. Copyright (2008) American Chemical Society.
Source: Adapted with permission from [26]. Copyright (2015) American Chemical Society.
A large, pristine graphene sheet is a zero band gap semiconductor with the conduction band and valence band touching at the K points. Graphene sheets typically are expected to be nonfluorescent. However, with decreasing size of the graphene sheet and introduction of functional groups at its edges, band gap opening is predicted due to quantum confinement and edge effects [34, 35]. The origin of fluorescence from graphene oxide (GO) remains poorly understood. Various mechanisms have been proposed, including nanometer‐sized aromatic domains in a GO sheet [36], strong electronic coupling between the carboxylic groups and the neighboring sp2 graphitic carbon π‐electrons [37], and electronic transitions between the non‐oxidized pristine sp2 graphitic carbon regions and oxidized carbon regions at the boundaries [38].
Exceptionally photostable, high‐quantum‐yield NIR fluorescence in nanodiamonds arises from color centers, such as nitrogen vacancy (NV) centers (Figure 2.2c) and silicon vacancy (SiV) centers, present in the diamond core. The emission state of NV centers in fluorescent nanodiamonds can be manipulated by controlling the nanodiamond's size or surface moieties or by changing the surface chemistry at the atomic level. The emission state is also sensitive to the action of the surrounding medium [39–41]. Bradac et al. demonstrated that it is possible to control the intermittency in the luminescence (blinking) of 5‐nm diamonds by embedding them in a poly(vinyl alcohol) film. The electronic discontinuity at the interface was altered, and the luminescence temporal behavior of individual NV centers was restored to a continuous luminescence signal [40]. Petrakova et al. reported modulating nanodiamond fluorescence through noncovalent interactions with charged polymers, such as poly(allylamine) and poly(diallyldimethylammonium chloride), by switching between NV¯ and NV0 energy states [42]. An analogous mechanism has been used to image DNA transfection in cells [43]. In this case, nanodiamonds were coated with cationic poly(ethyleneimine) and modified with DNA. After transfection into cells, the DNA detached from the particles, which resulted in switching between the NV¯ and NV0 energy states [43].
CDs have been widely studied as a green substitute for quantum dots due to their excellent photoluminescence and high biocompatibility. The mode of formation or the mechanism of photoluminescence origin (due to quantum effect, bandgap transition of conjugated π‐domains, different emissive traps on the CD surface, or molecular state) is a matter of debate. Polymers are increasingly becoming an important component for both synthesis and modification of CDs [44]. Surface passivation and functionalization with polymers lead to higher quantum confinement of emissive energy trapped on the CD surface, resulting in higher photoluminescence quantum yield [44]. Quantum‐sized CDs with a surface passivated with poly(propionylethylenimine‐co‐ethylenimine) (PPEI‐EI, with EI fraction ∼20%) showed strong luminescence with two‐photon excitation in the NIR spectral region [45]. Enhancement of emission by chemical crosslinking or physical immobilization of polymer chains on CDs is known as the crosslink‐enhanced emission (CEE) effect [46]. Tao et al. demonstrated the CEE effect by hydrothermally crosslinking polyacrylic acid (PAA) and ethylenediamine chains [47]. CDs developed from poly(thiophene phenylpropionic acid) demonstrated broad absorption (400–750 nm), red‐emission, a strong photoacoustic response, and high photothermal conversion efficiency (η ~ 38.5%) upon NIR laser irradiation, enabling them to act as multifunctional fluorescent, photoacoustic, and thermal theranostics for simultaneous diagnosis and therapy of cancer [48].
Raman spectroscopy provides useful information about the structure, electronic properties, composition, and homogeneity of CNMs, as well as information about the chemical environment around the nanomaterials. Changes in D‐band and G′‐band (D‐band overtone) Raman spectra can be used to probe and monitor structural modifications of the nanotube sidewalls that stem from the introduction of defects and the attachment of different chemical species [49]. Raman shift in the D* band (the second‐order overtone of the D band) is often reported for CNTs embedded in polymers, and can be used to measure the strain or stress applied to the CNTs from the surrounding media [50]. The D‐band in the Raman spectrum of GO arises from the stretching of sp3 carbons of graphene sheets, which occurs at 1366 cm−1 . The G‐band arises from the stretching of sp2 carbons and occurs at 1582 cm−1 . The interaction between polymer and GO shifts the G‐band, due to the change in the electronic structure of graphene [51].
2.5 Functionalizing CNMs with Antifouling Polymers for Bioapplications
When nanomaterials are introduced into cell culture media, the constituent proteins coat the NP surface to form a new bio‐nano interface, the so‐called “protein corona” (Figure 2.3a) [52, 53]. The protein corona is composed of an inner layer of proteins with a lifetime of several hours in slow exchange with the environment (the hard corona) and an outer layer of weakly bound proteins characterized by a faster exchange rate with the free proteins (the soft corona) [53, 54]. It is the hard corona, rather than the pristine NP surface, that interacts with cellular receptors. To avoid nonspecific adsorption of proteins, dense polymeric shells with anti‐fouling properties are needed. Anti‐fouling polymers decrease the nonspecific interactions of nanoparticles with proteins and cells (Figure 2.3b), prolong the time the nanoparticle spends in the circulatory system, improve biodistribution, and can be further modified with a targeting moiety exposed to the surroundings. Coating polymers need to be hydrophilic and ideally neutral or negatively charged. They can be synthetic or natural (such as proteins and polysaccharides). For example, use of SWCNTs for biological research was pioneered by Hongjie Dai and colleagues, who introduced poly(ethyleneglycol) (PEG) on their surfaces [26]. Although PEG is most often used to coat the surfaces of various nanoparticles, other polymers also have excellent properties [55, 56].

Figure 2.3 (a) In serum‐containing media, nanoparticles are covered with proteins (protein corona), which leads to interaction of the nanoparticles with cells and their subsequent internalization. (b) A dense polymeric shell makes nanoparticles resistant to protein adsorption and cell interaction.
Surface functionalization of nanoparticles can be covalent or noncovalent. As described in Section 2.4, covalent functionalization radically decreases the intrinsic optical properties of CNTs. Instead, amphiphilic polymers with a hydrophilic part exposed to solution and a hydrophobic part that interacts noncovalently with the nanotubes are typically used [7]. Phospholipids, the major components of cell membranes, are often used as an optimal hydrophobic component that strongly anchors onto CNTs and creates stable particles with low nonspecific adsorption to proteins [57–59]. Similar properties (i.e. reduction of nonspecific adsorption of proteins) have been observed for pluronic copolymers or PEG with the surfactant Tween, which shielded CNTs more efficiently than other coatings [60, 61]. Robinson et al. [62] introduced a large 90‐kDa amphiphilic poly(maleic anhydride‐alt‐1‐octadecene)‐methoxy‐block‐poly(ethylene glycol) coating to prepare CNTs with a longer blood circulation time than those with a phospholipid‐PEG (PL‐PEG) coating. Nevertheless, Liu et al. pointed out that a long circulation time leads to accumulation in the skin, underscoring the need to find an optimal balance between high uptake in the tumor and accumulation in skin [58].
The length of the PEG chain is an important parameter: longer PEG (5 kDa compared to 2 kDa) provides higher stability, lower nonspecific interaction with proteins, and lower reticuloendothelial system (RES) uptake, as well as longer circulation time and therefore higher tumor uptake upon modification with Arg‐Gly‐Asp (RGD)‐targeting peptide [59]. In addition to length, the branching structure of PEG chains can prolong circulation time. According to one study [57], increasing the PEG size to more than 7 kDa did not significantly improve the particle properties, but branching provided the particles with desirable characteristics for in vivo applications [57].
In addition, PEG‐coating methods need to be carefully optimized to ensure that CNTs possess the desired properties. Long periods of sonication create defects at the SWCNT sidewall, which leads to cutting at the defect site. The commonly used one‐hour sonication period shortens the CNTs [63] and degrades PEG into shorter fragments [64]. Welsher et al. proposed sonication of nanotubes in an excess of sodium cholate, followed by surfactant exchange for PL‐PEG, which protects the CNT fluorescence and targeting properties [63]. Zeineldin et al. showed that 2 kDa PL‐PEG is sufficient to decrease nonspecific binding and block the uptake into cells of nanotubes not fragmented by sonication for one hour. After subsequent modification with folic acid, nanotubes were specifically taken up only by cells expressing folic acid receptor [64].
GO needs to be modified with polymers primarily to reduce its cytotoxicity and increase biocompatibility and physiological stability. GO can be modified covalently by amidic coupling with six‐armed PEG, which leads to particles that are highly stable in medium or sera [65]. If GO is reduced to increase its fluorescence, it becomes water‐insoluble and has to be modified with an amphiphilic polymer. Similar to CNTs, reduced GO can be noncovalently modified with 5 kDa PEG with poly(maleic anhydride‐alt‐1‐octadecene), which ensures stability and prolongs circulation time [66]. Tan et al. focused on the behavior of nanoGO coated with 10 kDa amino‐terminated six‐arm‐branched PEG in serum and on reduction of protein adsorption onto the surface upon polymer modification. The polymer layer altered protein binding selectivity. Some of the proteins (immune‐related factors) adsorbed onto the surface at a higher rate, which could serve for eliminating immune factors evoked by other types of nanomaterials [67].
CDs are often covalently modified with PEG using amidic coupling [68]. Particles coated with PEG have lower in vitro and in vivo toxicity than uncoated particles [69]. In addition to increasing stability and biocompatibility, 5 kDa PEG prevents the creation of a protein corona, prevents interaction with opsonins, prolongs circulation time, and changes the biodistribution [70]. Such particles can be efficiently targeted by RGD peptide to tumor cells [71]. CDs can be created directly from the polymer by partial carbonization. CDs created from PEG do not need to be modified with a surface polymer layer, saving an extra functionalization step. These particles show good stability and prolonged circulation in blood, and after modification with a nuclear localization signal peptide, they can serve as nucleus‐imaging probes [72].
Nanodiamonds are considered the most biocompatible CNM. The purpose of polymer coating is therefore not to reduce their toxicity but to increase stability and reduce nonspecific interactions with proteins and cells to prolong circulation time and enhance the targeting effect. For example, detonation nanodiamonds (DNDs) can be covalently modified with Zonyl polymer (a combination of perfluoroalkyl chains and a PEG block). The Zonyl layer reduced adsorption of the protein bovine serum albumin (BSA) from 80% (of non‐modified particles) to 20% [73]. Covalent modification of high‐pressure high‐temperature (HPHT) nanodiamonds with 5 kDa PEG led to nanoparticles that were stable for at least two weeks, even in 1 M NaCl. These nanodiamonds were taken up by cells after a 24‐hour incubation. In contrast, nonmodified nanodiamonds aggregated during pre‐incubation in phosphate buffer saline (PBS) and stayed on the membrane. Nanodiamonds dispersed in serum with media before addition to cells were partially stabilized by proteins such as serum albumin and entered cells [74]. A PEG coating was used also as interface for nanodiamonds embedded in gold shells [75]. Wu et al. showed that coating HPHT nanodiamonds with PEG‐modified human serum albumin resulted in particles that were stable in 1 M NaCl with no change in size and that were internalized by cells [76].
However, many biomedical applications call for particles with proper anti‐fouling properties that do not enter cells at all. PEG‐coated nanoparticles bind various proteins, depending on the length and conformation of the PEG chain [67, 77, 78]. Moreover, PEG is an immunogenic polymer. PEG on GO stimulates potent cytokine responses [79] and induces deleterious effects and cell death [80]. Other possible surface modifications, such as proteins, polysaccharides, and various synthetic polymers, are becoming more popular. Proteins have turned out to be good stabilization agents, increasing the biocompatibility and stability of nanoparticles, but they usually do not reduce nonspecific interactions with other proteins nor internalization by cells. Engineered M13 bacteriophage modification of CNTs stabilized particles in both PBS and media with serum and enabled their further modification with an antibody against prostate‐specific membrane antigen [81]. Covering GO with complement factor H, an abundant protein in plasma, almost completely protects the particle from complement activation. Coating particles with serum albumin, which has a lesser shielding effect, is less protective, and coating with immunoglobulin G worsens complement activation [82]. Stabilization and enhanced intracellular uptake of serum albumin‐coated HPHT nanodiamonds has been demonstrated in vitro [83] and in vivo [84]. CNTs modified with polysaccharide dextran sulfate proved to be resistant to opsonins and bacterial cells. These nanotubes were modified by a site‐specific conjugation strategy (by oxime ligation) with antibodies that selectively target pathogenic bacteria [78]. GO with a cross‐linked chitosan/dextran coating proved to be stable in physiological conditions with low nonspecific interactions with human serum albumin [85]. Doxorubicin (DOX)‐loaded GO with noncovalently attached chitosan modified with cyclic RGD peptide specifically targets cancer cells [86]. Covalent amidic modification of GO with the polysaccharide hyaluronic acid leads to improved cytotoxicity and negligible hemolytic activity. A key advantage of hyaluronic acid as a polymer coating is its possibility to target cancer cells (with CD 44 determinants overexpressed on their surface). With this combined approach, there is no need to modify nanoparticles with other targeting moieties [87]. An even more straightforward approach is to use hyaluronic acid to prepare CDs, which are not fully carbonized and therefore are modified with hyaluronic acid as a shielding and targeting polymer on the surface [88]. Fructose‐based branched glycopolymer improved the stability of amino‐silica coated DNDs, targeted the particles to five glucose transporters [GLUT] that are expressed on breast cancer cells, and delivered DOX to selectively kill the cancer cells [89].
For exceptional results in targeted delivery and construction of selective biosensors, a perfect stealth coating is needed. All of the coatings described above were prepared by the “grafting to” method, in which polymers are prepared in advance in the solution and then noncovalently or covalently attached to the nanoparticle surface. Various synthetic polymers can polymerize in thicker and denser brushes directly on the surface of particles (“grafting from” approach) [90]. The “grafting from” approach cannot be recommended for CNTs because of their limitation in covalent modification. There are a few reports of using “grafting from” approaches to coat graphene and CDs, but the best‐studied material for preparation of dense brushes is nanodiamonds. GO modified with poly(acrylic acid) grown from graphene was found to have slightly better biocompatibility in vivo, less toxicity, reduced protein adsorption (especially the content of immunoglobulin G in the protein corona), and reduced membrane disruption, as well as a longer circulation time, than GO with 5 kDa PEG grafted to the surface [91]. CDs coated with polycation‐block‐polyzwitterion polymer by surface‐initiated ATRP have an excellent ability to suppress serum albumin adsorption. This type of polymer protects a DNA vector against nonspecific interactions with serum components and results in better transfection efficiency [92].
Hydrophilic, biocompatible hyperbranched polyglycerol grafted from the surface by anionic ROP is commonly used to prepare dense polymeric surfaces. CDs with such surfaces are water‐dispersible particles with low toxicity [93]. On nanodiamond surfaces, polyglycerol performs better than PEG, with lower nonspecific adsorption of lysozyme [94, 95]. These particles have excellent stability [96] and can be further modified with cyclic RGD peptide by azide‐alkyne cycloaddition, giving the particles reasonably high targeting efficiency to cancer cells [97, 98]. Exceptional properties, including no nonspecific interaction with proteins and very high particle stability (even in 1 M NaCl for at least two weeks), were achieved by preparing a dense poly[N‐(2‐hydroxypropyl)methacrylamide] layer on HPHT nanodiamonds. The shell was grown from silica with terminal methacrylate groups by radical polymerization (Figure 2.4a). Addition of azide‐ or alkyne‐ functionalized methacrylate monomer results in the possibility to further modify the nanodiamonds by azide‐alkyne cycloaddition catalyzed by Cu(I) ions [99, 100]. Such nanodiamonds with a dense polymer layer modified with cyclic RGD showed remarkably high specific interaction with integrin‐overexpressing cancer cells, thanks to the complete suppression of nonspecific interactions with the polymer shell (Figure 2.4b) [99]. A similar polymer shell was used to construct programmable quantum diamond nanosensors bearing densely loaded gadolinium(III) complexes [101].
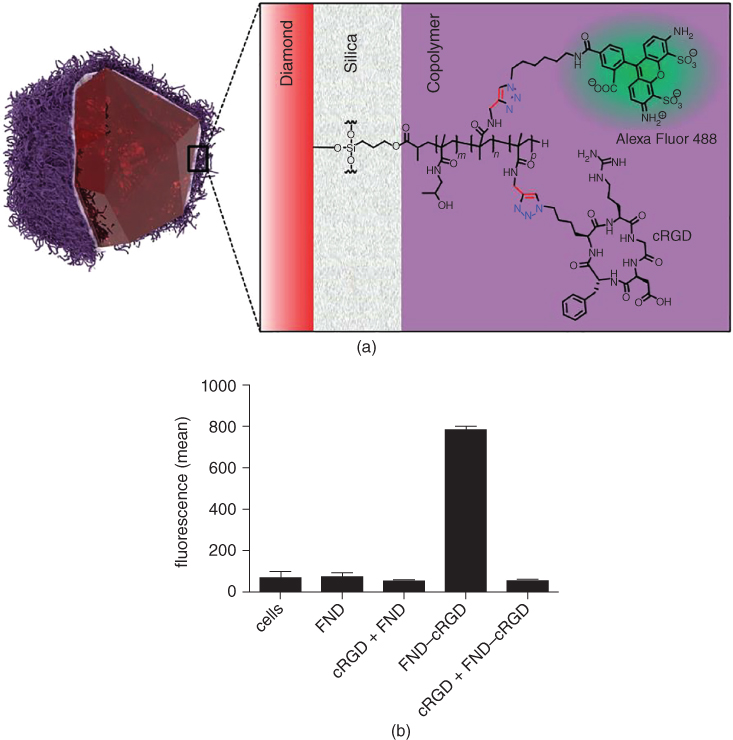
Figure 2.4 (a) Schematic structure of HPHT nanodiamonds with NV centers coated with a thin layer of silica and poly[N‐(2‐hydroxypropyl)methacrylamide] layer modified with cyclic RGD and the secondary fluorescent label Alexa fluor 488. (b) Fluorescence intensity measurement of U87‐MG glioblastoma cells incubated with: nanodiamonds bearing cyclic RGD (FND‐cRGD) with (cRGD+) or without preincubation with free cyclic RGD; nanodiamonds with polymeric shell without RGD modification (FND) with (cRGD+) or without preincubation.
Source: Reproduced from [99].
2.6 Functionalization of CNMs with Stimuli‐Responsive Polymers
Stimuli‐responsive polymers respond to their environment by changing their physical and/or chemical properties. They have been designed to be responsive to a variety of stimuli, including pH, temperature, the wavelength or intensity of light, electric or magnetic fields, and the presence of various small molecules and biomolecules. These polymers can respond by various means, such as altering color, transparency, solubility, or shape. Typically, slight changes in the environment are sufficient to induce large changes in the properties of such polymers [102]. Here, we describe conjugates of CNMs with various stimuli‐responsive polymers.
2.6.1 Carbon Nanoparticles with Thermoresponsive Polymers
Among stimuli‐responsive polymers, thermoresponsive polymers have been exploited most abundantly to modify carbon nanoparticles. Some polymers exhibit so‐called lower critical solution temperature (LCST), below which the polymer chains and solvent molecules are homogeneously mixed and above which phase separation occurs. Zydziak et al. employed cyclopentadienyl end‐capped poly(N‐isopropylacrylamide) (PNIPAM‐Cp) that act as dienophiles in a Diels‐Alder reaction to derivatize SWCNTs. The resulting nanoparticles displayed thermoresponsive behavior, precipitating at temperatures greater than 20 °C in aqueous dispersion [103]. Different PNIPAM polymers attached to SWCNTs by pyrene unit π–π stacking have also been used [104]. In another study, thermosensitive nanoparticles with PNIPAM anchored on GO planes were prepared, and these particles exhibited reversible self‐assembly and disassembly around 40 °C [105]. Zhu et al. developed a PNIPAM‐GO thermo‐responsive drug delivery system that releases ibuprofen in a thermo‐controlled way at 22 °C [106]. A similar system for delivery of DOX based on multiwalled CNTs was effective at 37 °C [107]. Dendrimeric polymers with large numbers of terminal functional groups for binding to GO to produce thermosensitive particles with low solution viscosity and excellent solubility relative to linear polymers have also been reported (e.g. based on hyperbranched polyethylenimine partially substituted with N‐isopropylacrylamide) [108].
2.6.2 pH‐Responsive Carbon Nanoparticles
Most pH‐responsive polymers are based on PAA or its analogues. Nanoparticles with a pH‐responsive graphene/PAA assembly formulated through noncovalent interactions exhibit reversible aggregation behavior, depending on the pH of the solution (Figure 2.5) [109].
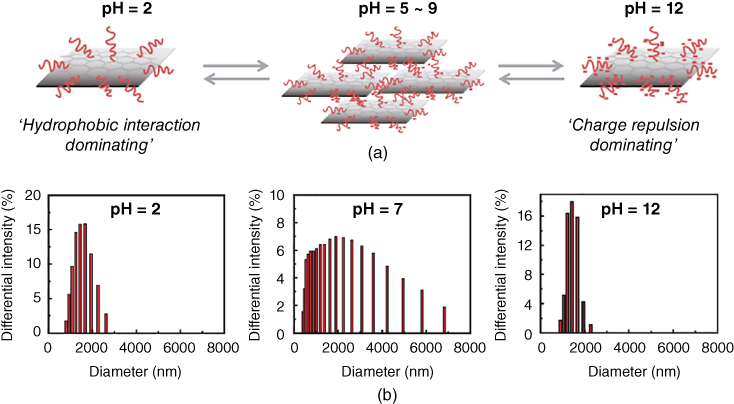
Figure 2.5 (a) Schematic illustration of the pH dependence of the interaction mode for graphene/PAA particles and (b) Dynamic light scattering (DLS) histograms of graphene/PAA solution (1 : 10) at different pH.
Source: Reproduced with permission from [109].
Wang et al. prepared strongly fluorescent CDs with citric acid as a carbon source and hyperbranched polyethyleneimine (PEI) as a surface passivation agent. These particles showed good water solubility and low cell toxicity. PEI, a polycationic polymer, made the CDs exhibit pH‐responsive optical properties, and they were used as pH sensors due to their reversible pH‐responsive fluorescence [110].
Numerous pH‐responsive CNMs have been investigated as potential drug carriers capable of controlled active compound release, primarily for use in cancer therapy. Most of these drug‐delivery strategies take advantage of the difference between normal physiological pH and the much lower extra‐ and intracellular pH of solid tumors. Zhou et al. developed PEI‐treated GO particles coated with a pH‐responsive charge‐reversal polyelectrolyte and integrin monoclonal antibody for targeted delivery and controlled release of DOX into cancer cells [111]. Another strategy for DOX delivery made use of SWCNTs covered with different derivatives of the biopolymer chitosan and folic acid as a targeting moiety. While this system was stable at physiological pH 7.4 and 37 °C, most of the DOX was released at pH 5.5, which is close to the pH of the tumor microenvironment [112]. Similarly, highly fluorescent CDs rich in surface amino groups were coated with PEGylated oxidized alginate polymer and loaded with DOX via acid‐labile Schiff base linkage. The strong fluorescence of the nanoparticles could be utilized for cellular imaging [113]. Another drug delivery system based on CDs, delivering cisplatin(IV) prodrug to tumors, was developed by Feng et al. CDs were converted by dimethylmaleic acid (DMMA) and PEG functionalized poly(allylamine) into pH‐responsive charge‐convertible drug nanocarriers. The particles showed negative charge under normal physiological conditions and could be converted into positively charged species in the tumor extracellular microenvironment [114].
2.6.3 Redox‐Responsive Carbon Nanoparticles
Redox‐responsive systems are mostly based on reducible disulfide bridges. For example, redox‐sensitive, hyaluronic acid‐decorated GO nanosheets were developed for specific, rapid delivery of DOX into tumor cell cytoplasm using NIR irradiation‐controlled endo/lysosome disruption and redox‐triggered cytoplasmic drug release [115].
2.6.4 Multi‐Responsive Carbon Nanoparticles
Recently, many different carbon nanoparticles responsive to multiple stimuli have been developed. Sulfur‐functionalized DNDs conjugated with PNIPAM showed both pH‐ and thermo‐responsive fluorescence; they can be utilized as sensors to detect intracellular pH values and temperature during disease diagnosis and treatment [116]. Wang et al. prepared biocompatible thermo‐ and pH‐responsive PEG‐chitosan@CDs hybrid nanogels by integrating nonlinear PEG, chitosan, and graphitic CDs into a single nanoparticle. These have been used for two‐photon fluorescence bioimaging, pH, and NIR light dual‐responsive drug release, and synergistic therapy [117]. Multi‐walled CNTs have been PEGylated, loaded with DOX by π–π interactions, and covered with the biocompatible polymer polylactide, which is able to form hydrogen bonds with PEG and entrap a drug inside the two types of polymeric chains. The resulting particles were not only temperature‐sensitive, working at temperatures higher than 40 °C, but also pH‐responsive, selectively killing cancer cells by releasing the drug only near cancerous tissues [118]. You et al. described CNT conjugates containing temperature‐responsive PNIPAM chains and disulfide linkages between PNIPAM and CNTs. The disulfide linkages show sensitivity to redox stimuli such as glutathione, thus forming dual‐responsive polymer‐CNTs [119].
CDs have been functionalized with PEI and isobutyric amide groups, which resulted in thermoresponsive species. These particles also responded to other stimuli, including inorganic salts, pH, and loaded organic guests; however, their photoluminescence was negligibly influenced by these stimuli [120].
Sharker et al. prepared multicolor GO nanoparticles that respond to irradiation with UV light and pH changes. The GO was coupled with a polymer conjugated with photochromic spiropyran dye and hydrophobic boron dipyrromethene (BODIPY) dye. The particles can be used for triggered multicolor bioimaging [121].
DOX nanocarriers responsive to pH and the redox environment were prepared via radical polymerization of methacrylic acid from PEG‐modified GO, followed by cross‐linking with cysteamine. While the presence of PEG rendered the drug carrier stealth during blood circulation, the pH‐ and redox‐responsive properties ensured release of DOX in tumor tissues [122].
To deliver cisplatin(IV) prodrug to tumor cells, Feng et al. developed efficient dual pH/redox responsive CDs [123]. CDs covered with a fluorescent thermoresponsive polymer consisting of poly(N‐vinylcaprolactam) showed good biocompatibility and could sense intracellular temperatures. The CDs also demonstrated a turn‐on response to proteins above the LCST, allowing utilization of this material in biosensors [124].
2.7 Functionalization of CNMs with Polymers for Delivery of Nucleic Acids
The development of nonviral, nonimmunogenic, and biocompatible vectors for efficient intracellular transfection of nucleic acids (NAs) is one of the challenges facing future gene therapy approaches [125]. Nonviral nanomaterial‐based gene delivery systems must fulfill the following requirements: (i) cell membrane penetration; (ii) endosomal escape; (iii) release of the NA cargo; (iv) successful gene expression [plasmid DNA (pDNA) delivery] or gene silencing [silencing structure delivery – short interfering RNA (siRNA), micro RNA (miRNA)] related to the effector molecule; and (v) high specificity and no off‐target effects associated with RNA interference (RNAi) [125, 126]. Polymer‐coated CNMs have proven to be one of the most promising nonviral platforms for delivery of genetic materials because of their ability to protect NAs against enzymatic cleavage, the possibility to attach accessory targeting molecules, their high binding capacity, and optical properties suitable for in vitro and in vivo tracking [127]. To construct delivery vectors, CNMs with positively charged surfaces are typically complexed with negatively charged NAs [128–131]. The surface of the CNMs can be functionalized by covalent or noncovalent attachment of macromolecular layer(s) to create reliable nonviral gene delivery vectors. These systems can subsequently be used for covalent or noncovalent bonding of NA molecules.
Liu et al. found that PEG‐coated CNTs are capable of siRNA delivery to afford efficient RNAi of CXCR4 and CD4 receptors on human T cells and peripheral blood mononuclear cells [131]. siRNA was incorporated onto the surface of CNTs via covalent disulfide bonds (Figure 2.6a), which are cleavable by thiol‐reducing enzymes aided by the acidic pH in lysosomes. A shorter PEG chain enhances the cellular uptake of SWCNTs. siRNA attached via a cleavable bond to the CNT exhibits superior transfection efficiency and silencing effects compared to conventional liposome‐based nonviral agents. The synergistic combination of RNAi with NIR photothermal therapy (using siRNA and a highly hydrophilic cationic PEI‐modified CNT complex; Figure 2.6b) greatly enhanced the therapeutic efficacy. siRNA internalization and transfection efficiency were significantly improved upon conjugating tumor‐targeting NGR (Cys‐Asn‐Gly‐Arg‐Cys‐) peptide with SWCNT/PEI/siRNA, which led to stronger suppression in proliferation of PC‐3 cells in vitro [132]. In tumor‐bearing mice, the SWCNT/PEI/siRNA/NGR delivery system exhibited higher antitumor activity due to greater accumulation in tumors without obvious toxicity in main organs. Moreover, there was a significant difference in transfection efficiencies in the order SWCNT/PEI/siRNA/NGR > SWCNT/PEI/siRNA > PEI/siRNA. More details on gene delivery using CNTs as a vehicle can be found in recent reviews [26, 136].
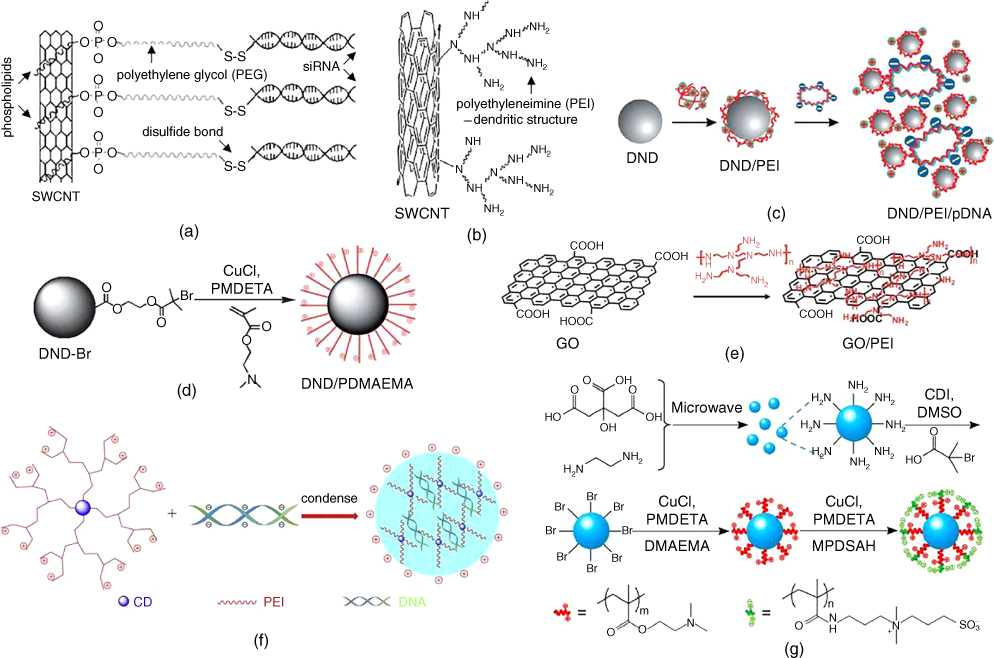
Figure 2.6 Various CNM surface functionalizations for gene delivery. (a) Noncovalent functionalization of SWCNTs with PL‐PEG‐NH2 for conjugation of thiol–siRNA through disulfide linkages. (b) Covalently attached PEI on SWCNTs obtained by cationic polymerization of aziridine. (c) Noncovalent PEI grafting based on electrostatic interaction used for pDNA delivery. (d) “Grafting from” method using ATRP to construct polycation‐functionalized nanodiamonds (DND/PDMAEMA). (e) GO noncovalently functionalized with PEI polymers, forming positively charged GO‐PEI complexes. (f) Branched PEI‐functionalized CDs condensed with DNA by electrostatic interaction. (g) The synthetic route to CDs, CD‐Br, and CD‐polymers.
Source: Reprinted with permission from [92]. Copyright (2014) American Chemical Society.
Source: Reprinted from [135], Copyright (2012), with permission from Elsevier.
Source: Reproduced from Ref. [129] with permission of The Royal Society of Chemistry.
Source: Reproduced from Ref [134] with permission of The Royal Society of Chemistry.
Source: Reprinted with permission from [133]. Copyright (2009) American Chemical Society.
Source: Reprinted from [132], Copyright (2013), with permission from Elsevier.
Source: Reprinted from [131].
Nanodiamonds coated with polycationic polymers such as PEI and poly(allylamine) are a very promising platform as gene delivery vehicles. DNDs coated with low‐molecular‐weight PEI (0.8 kDa) (Figure 2.6c) exhibit higher transfection efficiency compared to amine‐terminated DNDs (DND‐NH2) without PEI modification or PEI itself. The transfection efficiencies of pDNA varied as a function of the DND/pDNA weight ratio and decreased in the following order: DND/PEI (0.8 kDa)/pDNA > PEI/pDNA > DND‐NH2/pDNA > DND/pDNA > pDNA. Even though both DND/PEI/pDNA and DND‐NH2/pDNA complexes were successfully internalized, only DND/PEI/pDNA exhibited high transfection efficiency. This could be because DND‐NH2/pDNA remained imprisoned in the endosomal space [133]. A similar approach was used for HPHT NDs, which were tested in a Ewing sarcoma cell model [137]. In contrast, a recently published study focusing on plasma hydrogenated cationic DNDs (DND‐H) with a primary size of 7 nm showed that cationic DNDs without a macromolecular coating conjugated with siRNA can potently inhibit EWS/FLI‐1 gene expression in a Ewing sarcoma cell line [138]. Zhang et al. demonstrated that a cationic nanodiamond‐polymer brush, synthesized by ATRP of 2‐(dimethylamino)ethyl methacrylate (DMAEMA) (Figure 2.6d), provides higher gene expression than PEI (25 kDa)/pDNA with lower cytotoxicity [134].
The abundance of carboxylic groups on GO enables simple functionalization with common polycationic polymers such as branched PEI (1.2 and 10 kDa) via electrostatic interactions [129, 139, 140]. Positively charged PEI‐coated GO/PEI complexes (Figure 2.6e) can bind and protect pDNA and also provide a higher gene expression level and lower cytotoxicity compared to PEI (10 kDa) [129]. Covalent conjugation of GO with branched PEG (10 kDa) and branched PEI (25 kDa) in a ~1 : 1 : 5 GO:PEG:PEI weight ratio helped avoid aggregation in the presence of sera (tested sera concentrations: 0%, 10%, 20%, 30%). This GO/poly(ethyleneglycol)‐polyethyleneimine (PEG‐PEI) complex was successfully used for pDNA and siRNA delivery. Compared with PEI and GO/PEI, GO/PEG‐PEI exhibits higher transfection efficiency and almost no interference from sera. Moreover, the strong absorption of GO in the NIR region allows synergistic increase in the delivery of siRNA under NIR laser irradiation at low power density, owing to photothermally induced local heating, which facilitates intracellular trafficking [139]. An extensive review of the application of graphene‐based nanosheets to gene delivery was recently published [141].
Fluorescent PEI‐coated CDs (carbon dot‐polyethyleneimine (CD‐PEI)) can be used as vectors for NA delivery. Positively charged CD‐PEI, synthesized via one‐step microwave pyrolysis of glycerol and branched PEI (25 kDa), allows pDNA binding via electrostatic interactions. The pDNA condensation capability and cytotoxicity of CD‐PEI/pDNA is a function of pyrolysis time, possibly due to the destruction of PEI during the formation of CDs. CD‐PEI/pDNA (Figure 2.6f) are able to mediate gene transfection with higher or comparable efficiency, and with lower cytotoxicity, than PEI alone (25 kDa). Furthermore, for cell transfection in serum‐containing media, some CD‐PEI/pDNA particles can achieve a gene expression level superior that obtained with PEI (25 kDa), although it is slightly lower than in the serum‐free case (tested sera concentrations: 0%, 10%). The proper pyrolysis time is the key to balance the transfection efficiency and cytotoxicity of CD‐PEI/pDNA [135]. The block polymer poly‐[DMAEMA]‐block‐poly[N‐(3‐(methacryloylamino)propyl)‐N,N‐dimethyl‐N‐(3‐sulfopropyl)ammonium hydroxide], covalently grafted from CDs by surface‐initiated ATRP, can serve as a condensing agent for pDNA (Figure 2.6g) [92]. Particles modified with this polycation/polyzwitterion copolymer showed less cytotoxicity and enhanced serum stability and cellular internalization compared with particles modified with non‐zwitterionic polymers.
In general, carbon nanomaterial‐polyethyleneimine (CNM‐PEI) complexes have better transfection efficiency than PEI alone (25 kDa) in serum‐containing media. The transfection efficiency of PEI (25 kDa) declines dramatically with increasing serum concentration (tested sera concentrations: 10%, 30%, 50%) [92]. Even though CNM‐based carriers do not offer as high transfection efficiency as viral‐based vectors, they are able to maintain high cellular uptake, reduce adverse effects, and specifically target cells. These results highlight the importance of polymer coating of CNMs for gene delivery applications.
2.8 Outlook
In this chapter, we have provided an overview of how to use polymer coating to control the interface between CNMs and biosystems. Different CNMs require different biointerfacing approaches to make them compatible with biological environments for use in biomedical applications. For example, noncovalent polymer coating is preferable for CNTs because it avoids the risk of losing the NIR‐II emission during covalent modification. A graphene surface can be functionalized with polymers by both covalent and noncovalent approaches; however, noncovalent functionalization preserves the extended π‐conjugation of the graphene sheet, while covalent functionalization creates sp3 defects in the graphene ring. On the other hand, nanodiamonds can be conveniently functionalized by both noncovalent and covalent surface treatments without affecting the intrinsic optical properties of the NV centers buried inside the diamond core. Although CD surfaces are rich in ─OH and ─COOH functional groups, their surface can be further functionalized with PEG or other polymers. The use of polymers as coatings for CNMs is now well‐established, and advances in synthetic and biointerfacing techniques have led to a wealth of literature describing in vitro and in vivo investigations of CNMs.
In addition to polymer coatings, another commonly used biointerfacing approach involves silica coating of CNMs [53, 90, 142]. Silica coating has been studied as a means to enhance colloidal stability of CNMs due to silica's high stability, low cost, chemical inertness, processability, and optical transparency. A silica layer can confer on CNM cores both steric and electrostatic protection. Another popular biointerfacing approach is micellar solubilization through noncovalent wrapping of CNMs with a surfactant [143–145]. Although micelle‐stabilized CNMs can be stable in aqueous solution for several months, the difficulty in purifying these particles and removing excess, usually toxic surfactant molecules raises biocompatibility concerns.
As illustrated in this chapter, intense efforts and interest have been invested by many laboratories to functionalize the CNMs and explore their potential application in medicine. However, it is important to recognize that translation of CNMs from an interesting nanomaterial to an effective biomedical product is still at the nascent stages. Most conclusions on biomedical applications of CNMs have been demonstrated in vitro and in vivo. It remains to be seen whether such strategies will be clinically realistic and efficacious by minimizing adverse reactions. The clinical translation can be impeded by fundamental limitations of human physiology (i.e. vessel pore size, renal and hepatic clearance, RES), potential toxicity, and/or interference with other medical tests [146]. To increase the likelihood of clinical translation of CNMs, at least the following three criteria [146] should be considered. (i) CNMs should composed of nontoxic materials and/or biodegradable to clearable (renal or hepatic) components. (ii) Surface coatings of the CNMs should minimize the nonspecific tissue/organ uptake, and binding to serum proteins. (iii) Hydrodynamic diameter of CNMs or degradation products should be ≤5.5 nm to facilitate complete renal elimination.
Polymer coatings can significantly increase the hydrodynamic diameter of CNMs. The large size of a polymer coating can make CNMs incompatible with biosensor applications, and targeting smaller organelles becomes difficult, as does renal excretion. The size of polymer‐coated CNMs (nanodiamonds, CNT, graphene) typically exceeds the renal filtration cut‐off size (≤5.5 nm) [147], which increases their chance of becoming entrapped inside the liver and spleen and raises long‐term toxicity concerns. In this regard, due to their compact size, single‐digit nanodiamonds, and CDs can afford favorable renal clearance. Therefore, for clinical applications, small‐sized CNMs (nanodiamonds, CNT, graphene) with a biodegradable, thin, compact polymer coating are desirable.
Another important issue is lack of commonly followed standard protocols for synthesis, surface characterization, and toxicity testing of CNMs, which lead to inconsistent results and hinder clinical translation. The broad distribution in diameter, impurities (mainly including amorphous carbon and catalyst particles) also affects the reproducibility. In addition, each new functionality elevates the complexity (e.g. multistep syntheses, purification, and characterization), cost, and regulatory barriers (e.g. owing to multicomponent, heterogeneous formulations) [148]. Therefore, it is recommended that the FDA's regulation be followed regarding nanotechnology products development and characterization and that the FDA be consulted early in the development process to facilitate a mutual understanding of the scientific and regulatory issues for the nanotechnology products (https://www.fda.gov/ScienceResearch/SpecialTopics/Nanotechnology/ucm301114.htm ). Hopefully, a coordinated effort involving all parties, including chemists, pharmacologists, toxicologists, clinicians, pharmaceutical companies, and regulatory authorities, will push the CNM research activity to achieve the ultimate aim of clinical translation to help patients.
Acknowledgments
The work of J.N. was supported by the Grant Agency of the Czech Republic (Project Number 18‐17071S). The work of P.C. was supported by European Regional Development Fund; OP RDE; Project: “Chemical biology for drugging undruggable targets (ChemBioDrug)” (Grant No. CZ.02.1.01/0.0/0.0/16_019/0000729).
References
- 1 Georgakilas, V., Perman, J.A., Tucek, J., and Zboril, R. (2015). Broad family of carbon nanoallotropes: classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev. 115: 4744–4822.
- 2 Kroto, H.W., Heath, J.R., O'Brien, S.C. et al. (1985). C60: Buckminsterfullerene. Nature 318: 162–163.
- 3 Iijima, S. (1991). Helical microtubules of graphitic carbon. Nature 354: 56–58.
- 4 Novoselov, K.S., Geim, A.K., Morozov, S.V. et al. (2004). Electric field effect in atomically thin carbon films. Science 306: 666–669.
- 5 Baker, S.N. and Baker, G.A. (2010). Luminescent carbon nanodots, emergent nanolights. Angew. Chem. Int. Ed. 49: 6726–6744.
- 6 Mochalin, V.N., Shenderova, O., Ho, D., and Gogotsi, Y. (2012). The properties and applications of nanodiamonds. Nat. Nanotechnol. 7: 11–23.
- 7 Liu, Z., Tabakman, S., Welsher, K., and Dai, H. (2009). Carbon nanotubes in biology and medicine, in vitro and in vivo detection, imaging and drug delivery. Nano Res. 2: 85–120.
- 8 Liu, Z., Robinson, J.T., Tabakman, S.M. et al. (2011). Carbon materials for drug delivery & cancer therapy. Mater. Today 14: 316–323.
- 9 Xu, Y., Liu, Z., Zhang, X., and Wang, Y. (2009). A graphene hybrid material covalently functionalized with porphyrin, synthesis and optical limiting property. Adv. Mater. 21: 1275–1279.
- 10 Stankovich, S., Piner, R.D., Nguyen, S.T., and Ruoff, R.S. (2006). Synthesis and exfoliation of isocyanate‐treated graphene oxide nanoplatelets. Carbon 44: 3342–3347.
- 11 Moore, T.L., Rodriguez‐Lorenzo, L., Hirsch, V. et al. (2015). Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 44: 6287–6305.
- 12 Verwey, E.J.W. and Overbeek, J.T.G. (1999). Theory of the Stability of Lyophobic Colloids . Mineola, NY, USA: Courier Dover Publications.
- 13 Kobayashi, M., Juillerat, F., Galletto, P. et al. (2005). Aggregation and charging of colloidal silica particles, effect of particle size. Langmuir 21: 5761–5769.
- 14 Edwards, S.A. and Williams, D.R.M. (2004). Double layers and interparticle forces in colloid science and biology, analytic results for the effect of ionic dispersion forces. Phys. Rev. Lett. 92: 248303.
- 15 Baalousha, M. (2009). Aggregation and disaggregation of iron oxide nanoparticles, influence of particle concentration, pH and natural organic matter. Sci. Total Environ. 407: 2093–2101.
- 16 Soenen, S.J., Parak, W.J., Rejman, J., and Manshian, B. (2015). (Intra)cellular stability of inorganic nanoparticles, effects on cytotoxicity, particle functionality, and biomedical applications. Chem. Rev. 115: 2109–2135.
- 17 Albanese, A. and Chan, W.C.W. (2011). Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano 5: 5478–5489.
- 18 Mahmoudi, M., Lynch, I., Ejtehadi, M.R. et al. (2011). Protein–nanoparticle interactions, opportunities and challenges. Chem. Rev. 111: 5610–5637.
- 19 Murdock, R.C., Braydich‐Stolle, L., Schrand, A.M. et al. (2008). Characterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering technique. Toxicol. Sci. 101: 239–253.
- 20 de Gennes, P.G. (1987). Polymers at an interface; a simplified view. Adv. Colloid Interface Sci. 27: 189–209.
- 21 Domingos, R.F., Tufenkji, N., and Wilkinson, K.J. (2009). Aggregation of titanium dioxide nanoparticles, role of a fulvic acid. Environ. Sci. Technol. 43: 1282–1286.
- 22 McClements, D.J. (2005). Theoretical analysis of factors affecting the formation and stability of multilayered colloidal dispersions. Langmuir 21: 9777–9785.
- 23 Tasis, D., Tagmatarchis, N., Bianco, A., and Prato, M. (2006). Chemistry of carbon nanotubes. Chem. Rev. 106: 1105–1136.
- 24 Li, L., Davidson, J.L., and Lukehart, C.M. (2006). Surface functionalization of nanodiamond particles via atom transfer radical polymerization. Carbon 44: 2308–2315.
- 25 Gonçalves, G., Marques, P.A.A.P., Barros‐Timmons, A. et al. (2010). Graphene oxide modified with PMMA via ATRP as a reinforcement filler. J. Mater. Chem. 20: 9927–9934.
- 26 Hong, G., Diao, S., Antaris, A.L., and Dai, H. (2015). Carbon nanomaterials for biological imaging and nanomedicinal therapy. Chem. Rev. 115: 10816–10906.
- 27 Welsher, K., Liu, Z., Daranciang, D., and Dai, H. (2008). Selective probing and imaging of cells with single walled carbon nanotubes as near‐infrared fluorescent molecules. Nano Lett. 8: 586–590.
- 28 Rondin, L., Dantelle, G., Slablab, A. et al. (2010). Surface‐induced charge state conversion of nitrogen‐vacancy defects in nanodiamonds. Phys. Rev. B 82: 115449.
- 29 Nish, A., Hwang, J.‐Y., Doig, J., and Nicholas, R.J. (2007). Highly selective dispersion of single‐walled carbon nanotubes using aromatic polymers. Nat. Nanotechnol. 2: 640–646.
- 30 Collison, C.J., Pellizzeri, S., and Ambrosio, F. (2009). Spectroscopic evidence for interaction of poly[2‐methoxy‐5‐(2′‐ethylhexyloxy)‐1,4‐phenylenevinylene] conformers and single‐walled carbon nanotubes in solvent dispersions. J. Phys. Chem. B 113: 5809–5815.
- 31 Zheng, M., Jagota, A., Semke, E.D. et al. (2003). DNA‐assisted dispersion and separation of carbon nanotubes. Nat. Mater. 2: 338–342.
- 32 Kurnosov, N.V., Leontiev, V.S., Linnik, A.S., and Karachevtsev, V.A. (2015). Influence of cysteine doping on photoluminescence intensity from semiconducting single‐walled carbon nanotubes. Chem. Phys. Lett. 623: 51–54.
- 33 Li, M., Xu, P., Yang, J. et al. (2011). Synthesis of pyrene‐substituted poly(3‐hexylthiophene). via postpolymerization and its noncovalent interactions with single‐walled carbon nanotubes. J. Phys. Chem. C 115: 4584–4593.
- 34 Son, Y.‐W., Cohen, M.L., and Louie, S.G. (2006). Energy gaps in graphene nanoribbons. Phys. Rev. Lett. 97: 216803.
- 35 Nakada, K., Fujita, M., Dresselhaus, G., and Dresselhaus, M.S. (1996). Edge state in graphene ribbons, nanometer size effect and edge shape dependence. Phys. Rev. B 54: 17954–17961.
- 36 Sun, X., Liu, Z., Welsher, K., and Robinson, J.T. (2008). Nano‐graphene oxide for cellular imaging and drug delivery. Nano Res. 1: 203–212.
- 37 Galande, C., Mohite, A.D., Naumov, A.V. et al. (2011). Quasi‐molecular fluorescence from graphene oxide. Sci. Rep. 1: 85.
- 38 Shang, J., Ma, L., Li, J. et al. (2012). The origin of fluorescence from graphene oxide. Sci. Rep. 2: 792.
- 39 Dolenko, T.A., Burikov, S.A., Rosenholm, J.M. et al. (2012). Diamond–water coupling effects in Raman and photoluminescence spectra of nanodiamond colloidal suspensions. J. Phys. Chem. C 116: 24314–24319.
- 40 Bradac, C., Gaebel, T., Naidoo, N. et al. (2010). Observation and control of blinking nitrogen‐vacancy centres in discrete nanodiamonds. Nat. Nanotechnol. 5: 345–349.
- 41 Bradac, C., Gaebel, T., Pakes, C.I. et al. (2013). Effect of the nanodiamond host on a nitrogen‐vacancy color‐centre emission state. Small 9: 132–139.
- 42 Petrakova, V., Rehor, I., Stursa, J. et al. (2015). Charge‐sensitive fluorescent nanosensors created from nanodiamonds. Nanoscale 7: 12307–12311.
- 43 Petrakova, V., Benson, V., Buncek, M. et al. (2016). Imaging of transfection and intracellular release of intact, non‐labeled DNA using fluorescent nanodiamonds. Nanoscale 8: 12002–12012.
- 44 Zhou, Y., Sharma, S.K., Peng, Z., and Leblanc, R.M. (2017). Polymers in carbon dots, a review. Polymers 9: 67.
- 45 Cao, L., Wang, X., Meziani, M.J. et al. (2007). Carbon dots for multiphoton bioimaging. J. Am. Chem. Soc. 129: 11318–11319.
- 46 Zhu, S., Song, Y., Zhao, X. et al. (2015). The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots), current state and future perspective. Nano Res. 8: 355–381.
- 47 Tao, S., Song, Y., Zhu, S. et al. (2017). A new type of polymer carbon dots with high quantum yield, from synthesis to investigation on fluorescence mechanism. Polymer 116: 472–478.
- 48 Ge, J., Jia, Q., Liu, W. et al. (2015). Red‐emissive carbon dots for fluorescent, photoacoustic, and thermal theranostics in living mice. Adv. Mater. 27: 4169–4177.
- 49 Dresselhaus, M.S., Dresselhaus, G., Saito, R., and Jorio, A. (2005). Raman spectroscopy of carbon nanotubes. Phys. Rep. 409: 47–99.
- 50 Fujigaya, T. and Nakashima, N. (2015). Non‐covalent polymer wrapping of carbon nanotubes and the role of wrapped polymers as functional dispersants. Sci. Technol. Adv. Mater. 16: 024802.
- 51 Layek, R.K. and Nandi, A.K. (2013). A review on synthesis and properties of polymer functionalized graphene. Polymer 54: 5087–5103.
- 52 Lynch, I. and Dawson, K.A. (2008). Protein‐nanoparticle interactions. Nano Today 3: 40–47.
- 53 Cigler, P., Neburkova, J., Vavra, J. et al. (2017). Chapter 13, Nanodiamonds embedded in shells. In: Nanodiamonds .
- 54 Mahmoudi, M., Simchi, A., Imani, M. et al. (2010). A new approach for the in vitro identification of the cytotoxicity of superparamagnetic iron oxide nanoparticles. Colloids Surf., B 75: 300–309.
- 55 Lowe, S., O'Brien‐Simpson, N.M., and Connal, L.A. (2015). Antibiofouling polymer interfaces, poly(ethylene glycol). and other promising candidates. Polym. Chem. 6: 198–212.
- 56 Bhattacharya, K., Mukherjee, S.P., Gallud, A. et al. (2016). Biological interactions of carbon‐based nanomaterials, from coronation to degradation. Nanomed. Nanotechnol. Biol. Med. 12: 333–351.
- 57 Liu, Z., Davis, C., Cai, W. et al. (2008). Circulation and long‐term fate of functionalized, biocompatible single‐walled carbon nanotubes in mice probed by Raman spectroscopy. Proc. Natl. Acad. Sci. U.S.A. 105: 1410–1415.
- 58 Liu, X., Tao, H., Yang, K. et al. (2011). Optimization of surface chemistry on single‐walled carbon nanotubes for in vivo photothermal ablation of tumors. Biomaterials 32: 144–151.
- 59 Liu, Z., Cai, W., He, L. et al. (2007). In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat. Nanotechnol. 2: 47–52.
- 60 Chen, R.J., Bangsaruntip, S., Drouvalakis, K.A. et al. (2003). Noncovalent functionalization of carbon nanotubes for highly specific electronic biosensors. Proc. Natl. Acad. Sci. U.S.A. 100: 4984–4989.
- 61 González, M., Tort, N., and Benito, A.M. (2009). Non‐specific adsorption of streptavidin on single walled carbon nanotubes. J. Nanosci. Nanotechnol. 9: 6149–6156.
- 62 Robinson, J.T., Hong, G., Liang, Y. et al. (2012). In vivo fluorescence imaging in the second near‐infrared window with long circulating carbon nanotubes capable of ultrahigh tumor uptake. J. Am. Chem. Soc. 134: 10664–10669.
- 63 Welsher, K., Liu, Z., Sherlock, S.P. et al. (2009). A route to brightly fluorescent carbon nanotubes for near‐infrared imaging in mice. Nat. Nanotechnol. 4: 773–780.
- 64 Zeineldin, R., Al‐Haik, M., and Hudson, L.G. (2009). Role of polyethylene glycol integrity in specific receptor targeting of carbon nanotubes to cancer cells. Nano Lett. 9: 751–757.
- 65 Tian, B., Wang, C., Zhang, S. et al. (2011). Photothermally enhanced photodynamic therapy delivered by nano‐graphene oxide. ACS Nano 5: 7000–7009.
- 66 Yang, K., Wan, J., Zhang, S. et al. (2012). The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra‐low laser power. Biomaterials 33: 2206–2214.
- 67 Tan, X., Feng, L., Zhang, J. et al. (2013). Functionalization of graphene oxide generates a unique interface for selective serum protein interactions. ACS Appl. Mater. Interfaces 5: 1370–1377.
- 68 Anilkumar, P., Cao, L., Yu, J.‐J. et al. (2013). Crosslinked carbon dots as ultra‐bright fluorescence probes. Small 9: 545–551.
- 69 Chen, J., Sun, H., Ruan, S. et al. (2015). In vitro and in vivo toxicology of bare and PEGylated fluorescent carbonaceous nanodots in mice and zebrafish, the potential relationship with autophagy. RSC Adv. 5: 38547–38557.
- 70 Hola, K., Zhang, Y., Wang, Y. et al. (2014). Carbon dots – emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today 9: 590–603.
- 71 Ruan, S., Wan, J., Fu, Y. et al. (2014). PEGylated fluorescent carbon nanoparticles for noninvasive heart imaging. Bioconjugate Chem. 25: 1061–1068.
- 72 Yang, L., Jiang, W., Qiu, L. et al. (2015). One pot synthesis of highly luminescent polyethylene glycol anchored carbon dots functionalized with a nuclear localization signal peptide for cell nucleus imaging. Nanoscale 7: 6104–6113.
- 73 Marcon, L., Kherrouche, Z., Lyskawa, J. et al. (2011). Preparation and characterization of zonyl‐coated nanodiamonds with antifouling properties. Chem. Commun. 47: 5178.
- 74 Rehor, I., Slegerova, J., Kucka, J. et al. (2014). Fluorescent nanodiamonds embedded in biocompatible translucent shells. Small 10: 1106–1115.
- 75 Rehor, I., Lee, K.L., Chen, K. et al. (2014). Plasmonic nanodiamonds, targeted core–shell type nanoparticles for cancer cell thermoablation. Adv. Healthc. Mater. 4: 460–468.
- 76 Wu, Y., Ermakova, A., Liu, W. et al. (2015). Programmable biopolymers for advancing biomedical applications of fluorescent nanodiamonds. Adv. Funct. Mater. 25: 6576–6585.
- 77 Sacchetti, C., Motamedchaboki, K., Magrini, A. et al. (2013). Surface polyethylene glycol conformation influences the protein corona of polyethylene glycol‐modified single‐walled carbon nanotubes, potential implications on biological performance. ACS Nano 7: 1974–1989.
- 78 Kotagiri, N., Lee, J.S., and Kim, J.‐W. (2013). Selective pathogen targeting and macrophage evading carbon nanotubes through dextran sulfate coating and PEGylation for photothermal theranostics. J. Biomed. Nanotechnol. 9: 1008–1016.
- 79 Luo, N., Weber, J.K., Wang, S. et al. (2017). PEGylated graphene oxide elicits strong immunological responses despite surface passivation. Nat. Commun. 8: 14537.
- 80 Mendonça, M.C.P., Soares, E.S., de Jesus, M.B. et al. (2016). PEGylation of reduced graphene oxide induces toxicity in cells of the blood–brain barrier, an in vitro and in vivo study. Mol. Pharmaceutics 13: 3913–3924.
- 81 Yi, H., Ghosh, D., Ham, M.‐H. et al. (2012). M13 phage‐functionalized single‐walled carbon nanotubes as nanoprobes for second near‐infrared window fluorescence imaging of targeted tumors. Nano Lett. 12: 1176–1183.
- 82 Belling, J.N., Jackman, J.A., Yorulmaz, A.S. et al. (2016). Stealth immune properties of graphene oxide enabled by surface‐bound complement factor H. ACS Nano 10: 10161–10172.
- 83 Tzeng, Y.‐K., Faklaris, O., Chang, B.‐M. et al. (2011). Superresolution imaging of albumin‐conjugated fluorescent nanodiamonds in cells by stimulated emission depletion. Angew. Chem. Int. Ed. 50: 2262–2265.
- 84 Mohan, N., Chen, C.‐S., Hsieh, H.‐H. et al. (2010). In vivo imaging and toxicity assessments of fluorescent nanodiamonds in Caenorhabditis elegans. Nano Lett. 10: 3692–3699.
- 85 Xie, M., Lei, H., Zhang, Y. et al. (2016). Non‐covalent modification of graphene oxide nanocomposites with chitosan/dextran and its application in drug delivery. RSC Adv. 6: 9328–9337.
- 86 Wang, C., Chen, B., Zou, M., and Cheng, G. (2014). Cyclic RGD‐modified chitosan/graphene oxide polymers for drug delivery and cellular imaging. Colloids Surf., B 122: 332–340.
- 87 Wu, H., Shi, H., Wang, Y. et al. (2014). Hyaluronic acid conjugated graphene oxide for targeted drug delivery. Carbon 69: 379–389.
- 88 Sharker, S.M., Kim, S.M., Lee, J.E. et al. (2015). In situ synthesis of luminescent carbon nanoparticles toward target bioimaging. Nanoscale 7: 5468–5475.
- 89 Zhao, J., Lai, H., Lu, H. et al. (2016). Fructose‐coated nanodiamonds, promising platforms for treatment of human breast cancer. Biomacromolecules 17: 2946–2955.
- 90 Neburkova, J., Vavra, J., and Cigler, P. (2017). Coating nanodiamonds with biocompatible shells for applications in biology and medicine. Curr. Opin. Solid State Mater. Sci. 21: 43–53.
- 91 Xu, M., Zhu, J., Wang, F. et al. (2016). Improved in vitro and in vivo biocompatibility of graphene oxide through surface modification, poly(acrylic acid)‐functionalization is superior to PEGylation. ACS Nano 10: 3267–3281.
- 92 Cheng, L., Li, Y., Zhai, X. et al. (2014). Polycation‐b‐polyzwitterion copolymer grafted luminescent carbon dots as a multifunctional platform for serum‐resistant gene delivery and bioimaging. ACS Appl. Mater. Interfaces 6: 20487–20497.
- 93 Li, S., Guo, Z., Feng, R. et al. (2017). Hyperbranched polyglycerol conjugated fluorescent carbon dots with improved in vitro toxicity and red blood cell compatibility for bioimaging. RSC Adv. 7: 4975–4982.
- 94 Sotoma, S., Igarashi, R., Iimura, J. et al. (2015). Suppression of nonspecific protein–nanodiamond adsorption enabling specific targeting of nanodiamonds to biomolecules of interest. Chem. Lett. 44: 354–356.
- 95 Zhao, L., Takimoto, T., Ito, M. et al. (2011). Chromatographic separation of highly soluble diamond nanoparticles prepared by polyglycerol grafting. Angew. Chem. Int. Ed. 50: 1388–1392.
- 96 Boudou, J.‐P., David, M.‐O., Joshi, V. et al. (2013). Hyperbranched polyglycerol modified fluorescent nanodiamond for biomedical research. Diamond Relat. Mater. 38: 131–138.
- 97 Zhao, L., Xu, Y.‐H., Akasaka, T. et al. (2014). Polyglycerol‐coated nanodiamond as a macrophage‐evading platform for selective drug delivery in cancer cells. Biomaterials 35: 5393–5406.
- 98 Zhao, L., Xu, Y.‐H., Qin, H. et al. (2014). Platinum on nanodiamond, a promising prodrug conjugated with stealth polyglycerol, targeting peptide and acid‐responsive antitumor drug. Adv. Funct. Mater. 24: 5348–5357.
- 99 Slegerova, J., Hajek, M., and Rehor, I. (2015). Designing the nanobiointerface of fluorescent nanodiamonds, highly selective targeting of glioma cancer cells. Nanoscale 7: 415–420.
- 100 Rehor, I., Mackova, H., Filippov, S.K. et al. (2014). Fluorescent nanodiamonds with bioorthogonally reactive protein‐resistant polymeric coatings. ChemPlusChem 79: 21–24.
- 101 Rendler, T., Neburkova, J., Zemek, O. et al. (2017). Optical imaging of localized chemical events using programmable diamond quantum nanosensors. Nat. Commun. doi: 10.1038/ncomms14701.
- 102 Wei, M., Gao, Y., Li, X., and Serpe, M.J. (2016). Stimuli‐responsive polymers and their applications. Polym. Chem. 8: 127–143.
- 103 Modular ambient temperature functionalization of carbon nanotubes with stimuli‐responsive polymer strands. Polym. Chem. , (RSC Publishing) doi: 10.1039/C2PY20928D.
- 104 Guo, Z., Yin, H., Feng, Y., and He, S. (2016). Functionalization of single‐walled carbon nanotubes with thermo‐responsive poly(N‐isopropylacrylamide), effect of the polymer architecture. RSC Adv. 6: 37953–37964.
- 105 Deng, Y., Zhang, J.Z., Li, Y. et al. (2012). Thermoresponsive graphene oxide‐PNIPAM nanocomposites with controllable grafting polymer chains via moderate in situ SET–LRP. J. Polym. Sci., Part A: Polym. Chem. 50: 4451–4458.
- 106 Zhu, S., Li, J., Chen, Y. et al. (2012). Grafting of graphene oxide with stimuli‐responsive polymers by using ATRP for drug release. J. Nanopart. Res. 14: 1–11.
- 107 Pourjavadi, A., Tehrani, Z.M., Shirvani, T. et al. (2016). Dendritic multi‐walled carbon nanotube with thermoresponsive shells, a good carrier for anticancer drugs. J. Ind. Eng. Chem. 35: 332–340.
- 108 Liu, Y., Li, W., Hou, L. et al. (2014). Thermosensitive hyperbranched polyethylenimine partially substituted with N‐isopropylacrylamide monomer, thermodynamics and use in developing a thermosensitive graphene composite. RSC Adv. 4: 24263.
- 109 Lee, J.Y. and In, I. (2011). pH‐responsive optical modulation of chemically reduced graphene through noncovalent interaction with poly(acrylic acid). Chem. Lett. 41: 127–128.
- 110 Wang, C., Xu, Z., and Zhang, C. (2015). Polyethyleneimine‐functionalized fluorescent carbon dots, water stability, pH sensing, and cellular imaging. ChemNanoMat 1: 122–127.
- 111 Zhou, T., Zhou, X., and Xing, D. (2014). Controlled release of doxorubicin from graphene oxide based charge‐reversal nanocarrier. Biomaterials 35: 4185–4194.
- 112 Ali Mohammadi, Z., Aghamiri, S.F., Zarrabi, A., and Talaie, M.R. (2015). A comparative study on non‐covalent functionalization of carbon nanotubes by chitosan and its derivatives for delivery of doxorubicin. Chem. Phys. Lett. 642: 22–28.
- 113 Jia, X., Pei, M., Zhao, X. et al. (2016). PEGylated oxidized alginate‐DOX prodrug conjugate nanoparticles cross‐linked with fluorescent carbon dots for tumor theranostics. ACS Biomater. Sci. Eng. 2: 1641–1648.
- 114 Feng, T., Ai, X., An, G. et al. (2016). Charge‐convertible carbon dots for imaging‐guided drug delivery with enhanced in vivo cancer therapeutic efficiency. ACS Nano 10: 4410–4420.
- 115 Yin, T., Liu, J., Zhao, Z. et al. (2017). Redox sensitive hyaluronic acid‐decorated graphene oxide for photothermally controlled tumor‐cytoplasm‐selective rapid drug delivery. Adv. Funct. Mater. 27: 1604620.
- 116 Su, S., Wei, J., Zhang, K. et al. (2015). Thermo‐ and pH‐responsive fluorescence behaviors of sulfur‐functionalized detonation nanodiamond‐poly(N‐isopropylacrylamide). Colloid Polym. Sci. 293: 1299–1305.
- 117 Wang, H., Di, J., Sun, Y. et al. (2015). Biocompatible PEG‐chitosan@carbon dots hybrid nanogels for two‐photon fluorescence imaging, near‐infrared light/pH dual‐responsive drug carrier, and synergistic therapy. Adv. Funct. Mater. 25: 5537–5547.
- 118 Pistone, A., Iannazzo, D., and Ansari, S. (2016). Tunable doxorubicin release from polymer‐gated multiwalled carbon nanotubes. Int. J. Pharm. 515: 30–36.
- 119 You, Y.‐Z., Hong, C.‐Y., and Pan, C.‐Y. (2007). Preparation of smart polymer/carbon nanotube conjugates via stimuli‐responsive linkages. Adv. Funct. Mater. 17: 2470–2477.
- 120 Yin, J.‐Y., Liu, H.‐J., and Jiang, S. (2013). Hyperbranched polymer functionalized carbon dots with multistimuli‐responsive property. ACS Macro Lett. 2: 1033–1037.
- 121 Sharker, S.M., Jeong, C.J., Kim, S.M. et al. (2014). Photo‐ and pH‐tunable multicolor fluorescent nanoparticle‐based spiropyran‐ and BODIPY‐conjugated polymer with graphene oxide. Chem. Asian J. 9: 2921–2927.
- 122 Zhao, X., Yang, L., Li, X. et al. (2015). Functionalized graphene oxide nanoparticles for cancer cell‐specific delivery of antitumor drug. Bioconjugate Chem. 26: 128–136.
- 123 Feng, T., Ai, X., Ong, H., and Zhao, Y. (2016). Dual‐responsive carbon dots for tumor extracellular microenvironment triggered targeting and enhanced anticancer drug delivery. ACS Appl. Mater. Interfaces 8: 18732–18740.
- 124 Kavitha, T., Kim, J.‐O., and Jang, S. (2016). Multifaceted thermoresponsive poly(N‐vinylcaprolactam) coupled with carbon dots for biomedical applications. Mater. Sci. Eng., C 61: 492–498.
- 125 Devarajan, P.V. (ed.) Targeted Drug Delivery, Concepts and Design . Springer.
- 126 Jackson, A.L. and Linsley, P.S. (2010). Recognizing and avoiding siRNA off‐target effects for target identification and therapeutic application. Nat. Rev. Drug Discov. 9: 57–67.
- 127 Karimi, M., Ghasemi, A., Sahandi Zangabad, P. et al. (2016). Smart micro/nanoparticles in stimulus‐responsive drug/gene delivery systems. Chem. Soc. Rev. 45: 1457–1501.
- 128 Chen, M., Zhang, X.‐Q., Man, H.B. et al. (2010). Nanodiamond vectors functionalized with polyethylenimine for siRNA delivery. J. Phys. Chem. Lett. 1: 3167–3171.
- 129 Feng, L., Zhang, S., and Liu, Z. (2011). Graphene based gene transfection. Nanoscale 3: 1252–1257.
- 130 Kim, S., Choi, Y., and Park, G. (2017). Highly efficient gene silencing and bioimaging based on fluorescent carbon dots in vitro and in vivo. Nano Res. 10: 503–519.
- 131 Liu, Z., Winters, M., Holodniy, M., and Dai, H. (2007). siRNA delivery into human T cells and primary cells with carbon‐nanotube transporters. Angew. Chem. Int. Ed. 46: 2023–2027.
- 132 Wang, L., Shi, J., Zhang, H. et al. (2013). Synergistic anticancer effect of RNAi and photothermal therapy mediated by functionalized single‐walled carbon nanotubes. Biomaterials 34: 262–274.
- 133 Zhang, X.‐Q., Chen, M., Lam, R. et al. (2009). Polymer‐functionalized nanodiamond platforms as vehicles for gene delivery. ACS Nano 3: 2609–2616.
- 134 Zhang, P., Yang, J., Li, W. et al. (2011). Cationic polymer brush grafted‐nanodiamond via atom transfer radical polymerization for enhanced gene delivery and bioimaging. J. Mater. Chem. 21: 7755.
- 135 Liu, C., Zhang, P., and Zhai, X. (2012). Nano‐carrier for gene delivery and bioimaging based on carbon dots with PEI‐passivation enhanced fluorescence. Biomaterials 33: 3604–3613.
- 136 Karimi, M., Solati, N., Ghasemi, A. et al. (2015). Carbon nanotubes part II, a remarkable carrier for drug and gene delivery. Expert Opin. Drug Deliv. 12: 1089–1105.
- 137 Alhaddad, A., Durieu, C., and Dantelle, G. (2012). Influence of the internalization pathway on the efficacy of siRNA delivery by cationic fluorescent nanodiamonds in the Ewing sarcoma cell model. PLoS One 7: e52207.
- 138 Bertrand, J.‐R., Pioche‐Durieu, C., Ayala, J. et al. (2015). Plasma hydrogenated cationic detonation nanodiamonds efficiently deliver to human cells in culture functional siRNA targeting the Ewing sarcoma junction oncogene. Biomaterials 45: 93–98.
- 139 Feng, L., Yang, X., and Shi, X. (2013). Polyethylene glycol and polyethylenimine dual‐functionalized nano‐graphene oxide for photothermally enhanced gene delivery. Small 9: 1989–1997.
- 140 Kim, H. and Kim, W.J. (2014). Photothermally controlled gene delivery by reduced graphene oxide‐polyethylenimine nanocomposite. Small 10: 117–126.
- 141 Shim, G., Kim, M.‐G., Park, J.Y. et al. (2016). Graphene‐based nanosheets for delivery of chemotherapeutics and biological drugs. Adv. Drug Deliv. Rev. 105 (Part B): 205–227.
- 142 Deng, X., Qin, P., Luo, M. et al. (2013). Mesoporous silica coating on carbon nanotubes, layer‐by‐layer method. Langmuir 29: 6815–6822.
- 143 Vaisman, L., Wagner, H.D., and Marom, G. (2006). The role of surfactants in dispersion of carbon nanotubes. Adv. Colloid Interface Sci. 128–130: 37–46.
- 144 Lotya, M., King, P.J., Khan, U. et al. (2010). High‐concentration, surfactant‐stabilized graphene dispersions. ACS Nano 4: 3155–3162.
- 145 Zhang, X., Wang, S., Liu, M. et al. (2013). Surfactant‐dispersed nanodiamond, biocompatibility evaluation and drug delivery applications. Toxicol. Res. 2: 335–342.
- 146 Choi, H.S. and Frangioni, J.V. (2010). Nanoparticles for biomedical imaging: fundamentals of clinical translation. Mol. Imaging 9: 291–310.
- 147 Liu, J., Yu, M., Zhou, C., and Zheng, J. (2013). Renal clearable inorganic nanoparticles: a new frontier of bionanotechnology. Mater. Today 16: 477–486.
- 148 Wang, J., Choi, H.S., and Wáng, Y. (2015). Exponential growth of publications on carbon nanodots by Chinese authors. J. Thorac. Dis. 7: E201–E205.
