11
Multifunctional Graphene‐Based Nanocomposites for Cancer Diagnosis and Therapy
Ayuob Aghanejad1 Parinaz Abdollahiyan1 Jaleh Barar1,2 and Yadollah Omidi1,2
1 Research Center for Pharmaceutical Nanotechnology, Biomedicine Institute, Tabriz University of Medical Sciences, Iran
2 Department of Pharmaceutics, Faculty of Pharmacy, Tabriz University of Medical Sciences, Iran
11.1 Introduction
Cancer has become a major cause of human mortality during the last few decades worldwide. Considering regional and global reports, increasing mortality rates of cancer appears as a primary stimulus to develop advanced diagnostic/therapeutic modalities to treat the disease much more effectively [1, 2]. Currently, the most common methods of cancer diagnosis include different imaging modalities such as single‐photon emission computed tomography (SPECT), positron emission tomography (PET), computed tomography (CT), magnetic resonance imaging (MRI), and mammography. They are used to provide effective detection of malignant sites in tissue/organs for improving the cancer prognosis [3, 4]. The commonly used therapeutic modalities (i.e. chemotherapy and radiotherapy) are often invasive and may inevitably elicit some undesired side effects in other healthy tissues/organs, which must be reduced/controlled by specific/selective targeting of the diseased cells/tissue with an advanced treatment modality. For enhancing therapies specifically/selectively on the target cells/tissue, the current trends are literally focused on targeted diagnosis and treatment of cancer simultaneously. In this line, various advanced multifunctional nanoparticles (NPs)/nanosystems (NSs) have been developed and successfully used for the concurrent detection and treatment of different tumors [5, 6]. Of these, NP‐based molecular probes armed with various imaging and targeting agents in combination with pH‐sensitive and photo/thermo‐responsive structure carrying therapeutic cargos have been developed as carriers to improve the efficacy of targeted diagnosis and therapy [7, 8].
Among deferent types of NPs, the carbon‐based NPs are considered as one of the most attractive nanomaterials that have been engineered in different forms, including single‐walled carbon nanotubes/multiple‐walled carbon nanotubes (SWCNTs/MWCNTs), nanohorns, fullerenes, nanodiamonds, carbon NPs, nanofibers, and graphene derivatives (Figure 11.1). So far, significant progress has been made for the synthesis, functionalization, and applications of the graphene‐based NSs in biomedicine, in large part because of the low‐cost of graphene and simplicity in terms of functionalization and surface modification. As carbon‐based advanced nanomaterial, the graphene‐based NSs have been used for the formulation of multifunctional nanomedicines and theranostics to serve as targeted drug delivery and imaging NSs. Graphene possesses hexagonally regular sp2 hybridized carbon sheets structure with unique physicochemical properties, including high surface area, premier near infrared (NIR) absorption, and high electrical and heat conductivity. As a result, a large number of investigations have been conducted to be used in different biomedical applications such as carriers for drug delivery and targeting, scaffolds for cell/tissue engineering, biosensors, and NIR light absorbance in photothermal therapy/photodynamic therapy (PTT/PDT) [9, 10]. Depending on the biological applications of graphene, different types of functional groups can be grafted onto the graphene sheets to produce functionalized graphene. Owing to the large surface area and amphipathic nature of the graphene oxide (GO) developed by the oxidation of graphite sheets, it is considered as a promising flexible and biocompatible carbon‐based carrier for targeted detection, imaging, and therapy of cancer with extremely minimal cytotoxicity (Figure 11.1).

Figure 11.1 Schematic illustration of different types of carbon nanomaterials.
Source: The image was adapted with permission from [11].
Graphene‐based materials can be fabricated by several approaches, among which the Hummer method is one of the most popular preparation approaches because it is very cost‐effective and facile scalable. GO can be reduced (e.g. by hydrazine hydrate) resulting in reduced graphene oxide (rGO). The characterization of GO can be accomplished by several techniques, including Furrier transform infrared (FT‐IR) spectroscopy, ultraviolet‐visible (UV‐Vis) spectroscopy, X‐ray diffraction (XRD), Raman scattering, scanning electron microscopy (SEM), field emission scanning electron microscope (FESEM), and other electrical measurements.
11.2 Multifunctional Graphene‐Based Composites for the Diagnosis/Therapy of Cancer
Graphene, GO, rGO, and its derivatives are considered as promising materials for cancer monitoring and biosensing due to their various features such as potent UV absorption, fluorescence quenching capability and functionalizable surface (see Figure 11.2) [13]. Further, these materials can be functionalized with various functional groups (e.g. carboxyl, hydroxyl, epoxy, etc.), which makes them as a hydrophilic substrate with trivial intrinsic toxicity and good dispersibility in the physiologic fluids [14]. By far, nanocomposites (NCs) of graphene‐based materials have been developed through modification with deferent types of biocompatible agents and nanoparticles (e.g. magnetic nanoparticles, gold nanoparticles, polymers, proteins, and etc.), resulting in the production of multifunctional NSs for in vitro and in vivo applications in drug delivery, tissue engineering, and multimodal diagnosis/therapy [15]. Furthermore, to maximize the impacts of chemotherapies, the PTT and PDT potential of graphene‐based materials have been combined with the cytotoxic effects of different chemotherapies, which can lead to substantially enhanced detrimental impacts on the malignant cells. The multifunctional GO‐based nanocomposites appear to be very promising for simultaneous imaging and therapy of cancer. For instance, the upconversion nanoparticles (UCNPs) coated with GO and quantum dot (GOQD) have been developed and used as image‐guided probes. In a study, to develop GO‐based multimodal NPs, core‐shell NPs composing of UCNP as a core and a GOQD as a shell were developed. For PDT, hypocrellin A (HA), as a chemotherapy and photo‐sensitizing agent, was conjugated onto the GOQD through a π−π interaction and loaded on the PEGylated UCNP. The engineered HA‐loaded GOQD‐UCNPs were proposed as a multifunctional NS for the simultaneous imaging and therapy of cancer [16]. In a similar study, a hybrid NS of GOQD was developed as a pH‐responsive fluorescent probe to serve as an improved cancer detection agent. The resulting biocompatible composites exhibited upconversion photoluminescence (UCPL) as well as fluorescence, at which the NS was proposed as a fluorescence/photoluminescence‐guided probe for the detection of cancer [17]. The pH‐responsive curcumin‐containing composites of GO/QD have also recently been developed and successfully used in vitro and in vivo for concurrent imaging and chemotherapy of cancer [18].
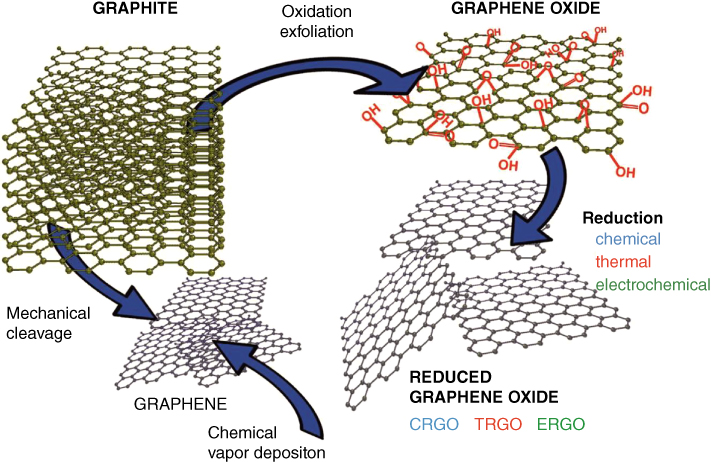
Figure 11.2 The possible ways for the synthesis of graphene derivatives. CRGO: Chemically reduced graphene oxide. TRGO: Thermally reduced graphene oxide. ERGO: Electrochemically reduced graphene oxide.
Source: The image was adapted with permission from [12].
Some studies have shown an excellent NIR‐absorption ability and payload capacity for rGO. As a result, it might be selected as a delivery system to combine the PTT/PDT with chemotherapy for improved treatment against different types of cancers. For example, in a study, rGO was loaded with doxorubicin (DOX) and chlorin e6 as a photosensitizer, which was then used against cancer in vitro and in vivo. The therapeutic efficacies of graphene‐based NSs were studied in combination with PDT, PTT, and chemotherapy on 2D/3D cultured tumor cells (spheroid/tumoroid) and animal models with results showing the efficacy of nanomedicine‐based PTT compared to chemotherapy and PDT modalities in cancer treatment [19]. Further, in an interesting research, GO was reduced by glucose to develop the glucose‐functionalized biocompatible rGO for PTT of the LNCaP prostate cancer cells. Results suggested that the biocompatible GO/glucose NS could be efficiently used for PTT of cancer [20]. A large number of investigations have been conducted using different types of nucleic acids for targeted gene therapy of cancers. However, the main issue in the gene therapy is the lack of suitable gene delivery system, in large part due to immunogenicity of the viral vectors and genotoxicity of nonviral vectors [21–26]. GO‐based NSs can provide a suitable platform for the delivery genes (see Figure 11.3). [28, 29]. For instance, the GO functionalized with hyaluronic acid (HA), to serve as a fluorescence‐switchable theranostic nanoplatform, was fabricated for simultaneous sensing of miR‐21 and inhibiting its tumorigenicity in vitro and in vivo. The Cy3‐labeled antisense miR‐21 peptide nucleic acid (PNA) probes loaded onto the HA‐GO (HGP21) were shown to specifically target the CD44‐positive MBA‐MB231 cells, in which a fluorescence recovery was observed through an interaction with the cytoplasmic miR‐21. In the miR‐21 knockdown mice induced by HGP21, decreased proliferation and migration of cancer cells with induction of apoptosis and enhanced phosphatase and tensin homolog located on chromosome ten (PTEN) levels were reported. Furthermore, in vivo imaging in the tumor‐bearing mice showed fluorescence signals three hours after the IV injection of the NS, upon which the researchers proposed it as a multifunctional theranostic for the miRNA‐targeted diagnosis/therapy [30]
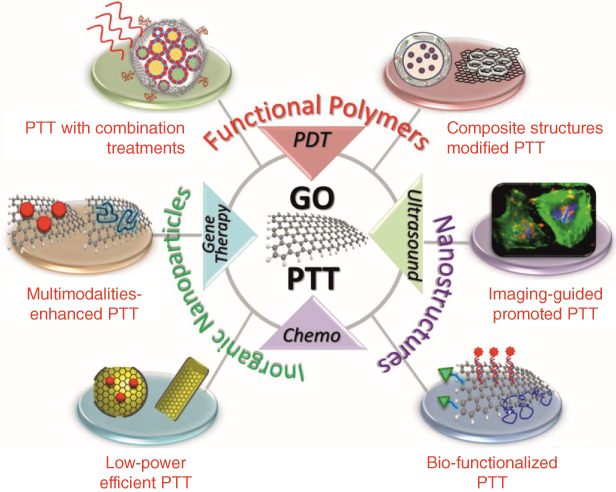
Figure 11.3 Functionalization of graphene‐based nanomaterials for enhanced PTT applications. GO: Graphene oxide. PTT: Photothermal therapy. PDT: Photodynamic therapy.
Source: The image was adapted with permission from [27].
11.2.1 Metal‐Graphene Nanocomposites
11.2.1.1 Gold‐Graphene Composites
Metal‐based NSs, in particular gold NPs (GNPs) and magnetic NPs (MNPs), offer significant prospective tools for the targeted imaging and therapy of cancer. Functionalized NPs with various targeting, imaging, diagnostic and/or therapeutic agents (e.g. pharmaceuticals, antibodies, radionuclides, and optical imaging substances) have so far been developed and showed great capacities as advanced NSs [31]. Of these, the image‐guided therapy modalities offer great possibilities for the simultaneous diagnosis and therapy of cancers. Likewise, the graphene'gold NCs offer unique light‐to‐temperature conversion and optical features, upon which they have been proposed for the cancer biosensing and theranostic applications. For example, in a study NCs of gold nanostars and GO were employed to intensify the NIR photothermal efficacy using an image‐guided therapeutic approach in the epithelial breast cancer cells. The nanocomposites were reported to improve the photothermal effects and exhibit Raman scattering, indicating their potentials as image‐guided therapy probes [32]. Similarly, the hybrid of transferrin functionalized GNP/GO was developed as a turn‐on NIR fluorescent probe for bioimaging of transferrin receptor in cancer cells/tissues. The basis of this NC was on the fluorescence quenching property of GO. This biocompatible NIR fluorescence probe was shown to provide an excellent specificity to target the cognate receptor, exhibiting markedly low cytotoxicity, high stability, and water solubility for cancer imaging [33].
As reported previously, rGO/GNPs composites have been engineered for the delivery of mitoxantrone (MTX) to breast cancer cells. Results demonstrated that graphene‐based drug delivery NSs could improve the delivery and impacts of MTX in cancer cells [34]. In a study, for image‐guided targeted therapy of tumors, photoacoustic/fluorescent theranostic GO/GNPs nanocomposites were developed by conjugating Cy5.5 labeled metalloproteinase‐14 (MMP‐14) onto the graphene nanohybrid. The in vivo studies on SCC7‐bearing mice demonstrated that this theranostic probe could provide an enhanced PTT, and hence might be used for simultaneous diagnosis and therapy of cancer [35].
Recently, the NCs of gold nanorods (GNRs) core and GO shell were developed to serve as a chemophotothermal cancer therapy agent. The nanoscaled GNR/GO composites were further conjugated with HA and loaded with DOX. The engineered stimuli‐responsive NC was shown to exhibit pH‐responsiveness with NIR‐triggered drug‐release properties, providing significantly greater targeting cell death rates than the single chemotherapy or photothermal therapy (1.5‐fold and fourfold, respectively). While showing no/trivial toxicity toward nontargeted cells, the engineered NS displayed simultaneous imaging and therapy impacts. Furthermore, such strategy could be extended to the construction of other nano‐graphene oxide (NGO)‐encapsulated functional nanomaterial‐based carriers [36] Such NIR‐ and pH‐responsive nanocomposites as a multimodal chemophotothermal therapy agent might be used for imaging and therapy of various solid tumors. Similarly, in another investigation, the photothermal properties of the GNRs‐linked PEGylated GO (GNR‐PEG‐GO) nanocomposites were examined in the epidermoid carcinoma A431 cells in the presence and absence of irradiation of Xenon‐lamp light (60 W cm−2 ). The results highlighted a significant decrease in the cell viability (40%) with the light irradiation. In addition, in vivo tumor studies indicated that the engineered NCs could reduce the tumor volumes through PTT [37]. In a study, NCs of GNPs/GO were fabricated as chemophotothermal agents to image and influence the cancer cells via both NIR two‐photon luminescence and Raman scattering. These NCs were proposed to be employed as a nanoscaled pH‐responsive nanocarrier with surface‐enhanced‐Raman‐scattering properties for the delivery of anticancer drugs [38].
11.2.1.2 Magnetic Graphene Nanocomposites
Combination of MNPs with GO/rGO has resulted in the production of unique NCs with magnetic properties. Of various multimodal NSs, MNPs have widely been utilized as an efficient multifunctional imaging and therapy because of their trivial toxicity, magnetic behaviors, surface modification, and drug‐loading potentials. The theranostic ability of MNPs can be combined with graphene, resulting in the development of hybrid composites. In a study, the modified magnetic graphene nanocomposites (MGNCs) were developed for delivery of 5‐fluorouracil (5‐FU) to the liver hepatocellular HepG2 cells. The results of in vitro studies confirmed the biocompatibility of engineered MGNCs, from which the release pattern was found to be pH‐dependent to some extent [39]. In another attempt, to evaluate the MGNCs potentials as a PTT agent in the ablation of drug‐resistant pancreatic cancer, GO was modified with MNPs and used to diagnose/treat regional lymph nodes (RLNs), dissection of which happens to be the only cure in the metastatic pancreatic cancer. To emulate the currently used clinical operations, the NS was injected into the tumor in the animal models, at which the MGNCs were transported to RLNs via lymphatic vessels. Once tested by the MRI, the developed MGNCs showed powerful ability of dual‐modality mapping of the regional lymphatic system, while the dark color of the NS appeared to provide remarkable information important for the surgeon to intraoperatively recognize the RLNs from other surrounding tissue. It was also shown that the diseased tissue can be ablated by the MGNCs through a NIR‐based PTT. Presenting a conceivable low cytotoxicity in the normal tissues, the engineered MGNCs were proposed as safe agents for the in vivo PTT, which might provide great potentials for bioimaging and PTT of the RLNs dissection [40].
In an attempt for the development of dual‐targeted multimodal NS, multifunctional GO/cobalt ferrite (CoFe2O4/GO) NCs were engineered by means of the sonochemical method and loaded with DOX. The developed NC was used as a chemotherapy and MRI nanoplatform. The NCs displayed a pH‐responsive drug release behavior with higher drug release at the acidic pH of 5.5. The NC also exhibited a relaxivity coefficient of about 93 mM−1 s−1 with a dose‐dependent T2‐weighted enhancement in the magnetic field [41]. In another study, GO functionalized bovine serum albumin grafted with folic acid (FA‐BSA) was constructed as a drug carrier NC to deliver DOX to target cancer cells. The in vitro analysis demonstrated that the DOX‐loaded nanohybrid could display a pH‐responsive trend and significant anticancer activity [42]. Likewise, bovine α‐lactalbumin (BLA) functionalized GO nanosheets have been developed and used against breast cancer (MDAMB‐231, MCF‐7, and HaCaT) cells in comparison with the 3T3 normal cells. The engineered nanosheets were compared to the GO NPs. The cytotoxicity and hemolysis analyses demonstrated an excellent dose‐dependent toxicity in the cancer cells tested together with an improved biocompatibility of BLA‐functionalized NPs [43]. Similarly, the nanocrystals of cobalt ferrite/graphitic carbon shell (CoFe/GC) loaded with DOX have been developed as multifunctional biocompatible integrated NS to combine PTT with drug delivery and MR imaging. These nanocrystals demonstrated pH‐dependent release of DOX as well as an enhanced intracellular drug delivery under the NIR laser radiation, leading to an increased cytotoxicity in the breast cancer cells. As a result, this multimodal NS was proposed as theranostic with the combined potentials of MRI, PTT and delivery of anticancer agents [44].
PTT as one of the most advanced methods is used for the treatment of cancer by means of a photosensitizing agent and a certain type of light. In this approach, the accumulated photosensitizers are delivered to the tumor cells and then activated by a laser beam or other light sources, resulting in the production of the active forms of oxygen detrimental to tumor vasculature endothelial and cancer cells. Likewise, GO decorated MNPs were developed as a NIR‐absorbing NSs that were successfully used for the light‐mediated PDT. The NS displayed a great capability in the production of the reactive oxygen species (ROS) under the NIR irradiation. The production of ROS appeared to be due to the electron transfer between the GO and MNPs and the production of hydroxyl radicals because of the reaction with oxygen‐producing superoxide radicals and H2O2. As a result, these NCs were proposed to serve as the NIR‐triggered PDT in cancer therapy [45]. NCs composed of GO/silica‐coated superparamagnetic nanoparticles (SPIONs) functionalized with folic acid (FA) have been developed and used for delivery of DOX molecules as pH‐dependent controlled‐release nanohybrids [46]. In a similar attempt, Wang et al. reported FA‐functionalized nanohybrids based on SPION/GO for the encapsulation of DOX as a pH‐sensitive nanocarrier. The researchers concluded that the NS might be used as a dual‐targeted drug delivery system (DDS) through combined passive (magnetic field) and active (FA) targeting mechanisms [47].
11.2.2 Polymeric Graphene Nanocomposites
Polymeric NCs have been developed using various types of NPs (e.g. magnetic, gold, and graphene NPs), and widely used for the cancer therapy. However, occurrence of systemic cytotoxicity together with the inherent poor‐solubility of these NSs have often limited their biological applications. Polymeric nanohybrids can be harnessed to improve the biocompatibility and multi‐responsiveness of DDSs in the cancer therapy.
In a study, magnetic graphene nanosheets (MGNs) were engineered, coated with Pluronic F127 (PF127) and loaded with DOX. The resultant NS was used for the diagnosis and therapy of cancer simultaneously through the NIR‐mediated PTT in combination with chemotherapy, while displaying MRI potential [48]. Similarly, in another study, the iron oxide‐graphene NCs, stabilized by polyvinylpyrrolidone (PVP) and poly(vinyl alcohol) (PVA) polymers, were loaded with paclitaxel (PTX) and DOX to treat the human cervical cancer HeLa cells. This NC showed a good loading capacity with a pH‐responsive release of DOX molecules and high efficiency. Once under the magnetic field, the NS showed excellent potential for imaging as well as synergistic PTT and chemotherapy effects. It should be noted that arming graphene sheets with PVP was shown to produce a pH‐trigged nanocarrier for delivery of anticancer agents (e.g. DOX). The cytotoxic effects of such NS have been evaluated on various cells lines, including HeLa, NIH‐3T3, HCT‐116, and 9HEK‐293 cells and SCC. The results revealed that the NS can impose profound cytotoxicity in the cancer cells [49].
Surface‐modified GO polymeric NSs were shown to serve as targeted DDS for PDT [50]. In this line, Huang et al. have developed PVP‐coated GO nanocomposites decorated with ACDCRGDCFCG peptide anchoring sites and loaded with chlorin e6 (Ce6) photosensitizer. The resulting targeted NS was found to improve the PDT by increasing the accumulation of Ce6 in tumor cells [51]. In an interesting study, Dou et al. developed NCs composed of rGO decorated bismuth sulfide (Bi2S3). The NCs were further functionalized with PVP and loaded with DOX, and successfully used for the chemophotothermal therapy of cancer. In fact, this dual NIR‐and pH‐responsive NS showed great effectiveness both in vitro and in vivo. Various cancer cells (BEL‐7402, MCF‐7, Hela, and HepG2 cells) and the human hepatoma (BEL‐7402) tumor‐bearing mice were treated with the NS and then irradiated by the NIR ray. The results showed marked synergistic antitumor effects inhibiting the growth of cancer. Besides, due to the X‐ray and NIR absorption capability of the engineered NS, it was proposed to be employed for the image‐guided synergistic chemophotothermal treatment of cancer [52]. Robinson et al. reported on the development of PEGylated rGO functionalized with RGD (Arg‐Gly‐Asp peptide) motif as a homing agent to selectively target the U87MG cancer cells. On the basis of in vitro biological stability and effective photoablation of the nano‐rGO in the presence of NIR irradiation, the researchers suggested it as a promising candidate for the NIR‐mediated PTT of cancer cells [53].
In a study, polymeric GNPs/GO theranostics were developed through a double‐microemulsion of GNPs into poly(lactic acid) microcapsules. The surface was further modified with GO through the electrostatic deposition technique. As shown in Figure 11.4, the engineered micro‐composite was developed to serve as a prognostic contrast agent in the simultaneous ultrasound/CT imaging in combination with PTT for multimodal image‐guided cancer therapy [54].

Figure 11.4 The CT imaging of mice after intramuscular injection of GNPs/GO/PLA theranostics. The white arrow points to the injected site. GNPs: Gold nanoparticles. GO: Graphene oxide. PLA: Poly(lactic acid).
Source: The image was adapted with permission from [54].
Recently, a chitosan (CS)‐based biodegradable hydrogel, consisting of polyethyleneimine (PEI) modified GO/MNP, was developed and loaded with DOX. The hydrogel was used as a minimally invasive system for the treatment of cancer lesions through magnetically induced local hyperthermia. Both in vitro and in vivo results revealed profound antitumor impacts of the hydrogel in the cancer cells and tumor tissue in the presence of magnetic field, at which it was introduced as a system with the synergetic effects of hyperthermia and chemotherapy [55]. Similarly, a versatile drug delivery NS based on functionalized GO with carboxymethyl chitosan (CMCS) was engineered to combine the PTT with chemotherapy using DOX. The modified biocompatible GO composites displayed pH‐responsive release behavior and effectively induced cell death in the liver cancer SMMC‐7721 cells [56]. In another investigation, to produce a pH‐sensitive carrier, surface‐carboxylated mesoporous silica‐coated magnetic GO modified with the CS polymer (i.e. by electrostatic interaction) and loaded with DOX was developed. The nanocarrier showed an improved stability and solubility in biological environments with a pH‐triggered release of DOX. As a result, the engineered NS was proposed as a potential candidate for the cancer therapy [57].
In a recent study, using electrospinning techniques, pH‐responsive GO/CS/polyethylene oxide (PEO) nanofibers loaded with DOX were developed and successfully used for the treatment of lung cancer. These nanofibers showed an excellent drug loading efficacy with a pH‐dependent release of cargo drug [58]. Some reports have clearly highlighted that the routine clinical treatments (chemotherapy, radiotherapy, and surgical resection) of brain cancer are very aggressive approaches with a high mortality rate. To tackle this issue, Su et al. reported on the development of an alternative noninvasive PTT for brain tumors using porphyrin functionalized GO. The in vitro findings revealed marked ablation of the malignant cells with specific targeted PTT [9]. Surface modification of graphene and its derivatives is considered as an effective strategy to improve their solubility that is associated with their cytotoxicity effects. To investigate the impacts of GO nanocomposites in the cancer therapy, Liu et al. developed silica and GO core/shell NSs. To enhance the stability and solubility of the NCs, they were conjugated to the serum proteins and used as an advanced DDS to deliver DOX to the cervical cancer cells. The in vitro analyses confirmed markedly higher cytotoxicity induced by the NSs under irradiation with NIR laser. In addition, the tumor growth in animal models treated with the NS was substantially inhibited in the presence of the NIR irradiation, indicating its impacts as a PTT agent [59].
By far, a variety of graphene‐based magnetic nanocomposites have been engineered to serve as a chemophotothermal therapy agent, which can be used as magnetic‐field‐assisted DDS. In this line, to elicit chemophotothermal synergistic impacts on the HeLa cancer cells using magnetic‐field‐assisted DDS, Chen et al. capitalized on PEGylated MNPs/Au core‐shell nanoparticles modified with rGO and loaded with DOX. These researchers showed that the DOX‐loaded NS could induce synergistic chemotherapeutic impacts on the cells through magnetic‐field‐guided delivery of drug combined with PTT [60]. Likewise, in another study, the Ag/GO nanocomposites loaded with DOX and functionalized with DSPE‐PEG2000‐Asn‐Gly‐Arg (nitrogen‐doped graphene (NGR)) motif were developed. This multimodal NS, as a NIR‐controlled drug delivery nanohybrid, was used for active targeting of the breast cancer. The in vitro experiments confirmed the ability of the engineered NS in terms of NIR‐mediated drug release and chemo‐photothermal therapy. Further, the in vivo biodistribution and the X‐ray imaging studies confirmed the potential of Ag/GO‐DOX‐NGR nanosystems for the simultaneous diagnosis and therapy of cancer [61].
It should be highlighted that the incidences of inevitable unwanted side effects of chemotherapy, which can lead to failure of chemotherapy, have accelerated researches towards the development of multimodal targeted NSs. To mitigate adverse reactions of the conventional chemotherapies, GO hybridized alginate nanogels were fabricated and encapsulated with DOX, which was used to treat the lung cancer A549 cells. The NS was shown to be markedly internalized by the cancer cells, resulting in an enhanced anticancer activity as a multimodal nanomedicine [62]. In another attempt, surface functionalized GO with poly(dopamine) was used for the encapsulation of cytarabine hydrochloride as a chemophotothermal therapy agent. Both in vitro analysis in the HeLa cells and in vivo animal studies of the NS confirmed its biocompatibility, sustained‐release profile, responsiveness to the NIR laser, and the synergistic chemotherapeutic efficiency (under 808 nm NIR laser) and significantly suppressing of tumor growth, indicating great therapeutic efficacy of this versatile nanohybrid [63].
In an attempt to improve the stability and biocompatibility of graphene‐based drug delivery vehicles, FITC‐functionalized poly(N‐isopropylacrylamide) (PNIPAM) grafted GO was developed and encapsulated with indomethacin (IMC) and DOX. The engineered NS showed a pH‐responsive release pattern for both IMC and DOX and a great ability for the fluorescence imaging of cancerous cells [64]. Further, it should be pointed out that the polymeric NSs of GO and thermo‐responsive nanogels (NGs) based on the NIPAM and acrylic acid have previously been developed as nanoscaled stimuli‐responsive (e.g. pH and temperature) DDS [65]. Such nanoporous structures can be used for the encapsulation of anticancer agents (e.g. DOX, PX, MTX) to treat solid tumors such as the cervical cancer. These nanocomposites exhibited sustained‐release pattern at the lower temperatures and the neutral pH. However, drug release was significantly accelerated at the higher temperatures and the acidic pH. Such dual‐mediated release by both temperature and pH appeared to enhance the cytotoxicity associated with the drug‐loaded NS, at which they were proposed as dual‐stimuli responsive NS for delivery of anticancer agents into the target cancer cells [65]. In an interesting experiment, pH‐sensitiveGO‐based nanovehicles were developed through functionalization of GO with 2,3‐dimethylmaleic anhydride and modification with poly(allylamine hydrochloride) and polyethylene glycol (PEG). The NS was loaded with DOX and used to serve as an anticancer agent. The NS showed an improved toxicity in the cancer cells, in large part because of the pH‐mediated release of the drug in the tumor microenvironment. In addition, the photothermal conversion ability of nanohybrid was found to enhance the therapeutic effectivity in the cancer cells [66].
11.2.3 Graphene Biomaterials for MR Imaging
The MRI is considered as an appropriate noninvasive plausible approach for the 3D monitoring of entire tissue in the human body [67]. It should be noted that the relaxivity of any contrast agent used in the MRI corresponds to the relaxation rates of the solution conversion as the function of concentration, MNPs as a contrast agent may affect the two relaxation rates (1/T1 and 1/T2). As a result, two corresponding relaxivities (i.e. r 1 and r 2 ) may define the relaxivity of the MNPs, depending on some factors, including temperature, field strength, and media. In the clinical applications, it is routine to cite r 1 and r 2 values at 1.5T in plasma at 37 °C that corresponds the body temperature. In this imaging technique, spin−spin relaxation of adjoining water molecules can be affected by MNPs, and hence, the T 2‐weighted MRI signals may be altered swiftly [68]. Further, MNPs show higher level of transverse relaxivity (r 2 ), which can result in an improved high‐quality MRI. Thus, the modification of MNPs as the core with various types of shells is a well‐known and appropriate strategy for the enhancement of the T 2 contrast efficacy [69]. In this regard, the functionalized GO/MNP nanohybrids were developed as the T 2‐weighted MRI contrast agent to be used in cellular imaging. The functionalization of the GO/MNPs was accomplished through the alteration with anionic and cationic polyelectrolyte via layer‐by‐layer assembly. The engineered nanohybrids were found to have a low cytotoxicity, while they presented outstanding r 2 values important for the early detection and monitoring of cancers [70]. Similarly, Chen et al. reported on the development of PEGylated GO modified with β‐FeOOH nanorods (GO‐PEG‐β‐FeOOH), which showed an ultra‐high r 2 value of 303.81 mM−1 s−1 . The engineered GO‐PEG‐β‐FeOOH nanocomposites loaded with DOX were reported as safe multimodal theranostic agents that could be used for simultaneous MR imaging and cancer therapy [71]. In another study, the intercalated manganese ions in the dextran functionalized graphene nanoplatelets were developed as MRI contrast agent. The relaxivity, partition coefficient, and thermostability analyses were performed to indicate the physicochemical properties of nanoplatelets. The NS showed high degrees of biocompatibility and thermal stability. In addition, the r 1 value of nanoplatelets was found to be significantly greater than that of the clinical gadolinium‐based MRI contrast agents [72].
Recently, rGO nanoplatelets intercalated by manganese ions and functionalized with iodine were developed to serve in the CT and MRI applications. The in vitro cytotoxicity analysis and CT and MR imaging of the human kidney epithelial (A498) and mouse fibroblasts (NIH3T3) cells demonstrated that this nanoplatform can serve as a great carbon‐based multimodal contrast agent [73].
Further, some reports have shown that the deposition of manganese ferrite (MnFe2O4) SPIONs on the GO could generate the GO/MnFe2O4 nanohybrids. The resulting magnetic NCs, which pose imperceptible in vivo cytotoxicity, could be used as an effective contrast agent with improved T 2 ‐weighted signals in both in vitro and in vivo MR imaging. These nanohybrids loaded with DOX were shown to provide synergistic chemophotothermal impacts on the cancer cells [74].
11.3 Multimodal Graphene‐Based Composites for the Radiotherapy of Cancer
The growing interest in effective bioimaging and targeted therapy of tumor cells/tissue is one of the main goals among scientists worldwide. Recently, several studies have been carried out to incorporate diagnostic/therapeutic radioisotopes with NSs that are able to induce combined PTT/PDT effects, and hence to be used as theranostics. Owing to an optimal biocompatibility and low cytotoxicity of GO and its composites, they have a great capacity to serve as multimodal radiolabeled theranostics. Recently, PEGylated nano rGO (nRGO‐PEG) was reported to serve as in vivo PTT nanoprobe in the 4T1 murine breast cancer cells−bearing mice. The biodistribution and tumor accumulation studies of the NS were performed using 125 I‐labeled NSs in the animal models. The developed rGO‐PEG exhibited strong NIR absorption capacity with passive targeting mechanism in solid tumors. Furthermore, rGO‐PEG presented efficient tumor ablation after an intravenous injection in the presence of a low laser power density. Such compelling findings suggested that both the size and the surface chemistry of nanomaterials could impose critical effects on the in vivo behaviors of GO nanocomposites and their potential in PPT of cancer [75]. In a similar study, to develop a nuclear image‐guided probe to combine the PTT with radiotherapy, the PEGlyted rGO was radiolabeled with 131 I (131 I‐rGO‐PEG) and used as a radiopharmaceutical against cancer cells. The in vivo gamma imaging illustrated marked accumulation of 131 I‐rGO‐PEG in the tumor sites after an intravenous injection. Furthermore, this NS was shown to induce an enhanced PTT effect in the presence of NIR light irradiation and an emission of the ionizing high‐energy X‐ray (by 131 I) simultaneously [76]. In another study, to target and diagnose cancer cells, Song et al. developed hybrid GNR/rGO nanocomposites loaded with DOX and radiolabeled with 64 Cu. The NS was successfully used for simultaneous in vivo PET imaging and chemophotothermal therapy in the presence of NIR in the U87MG tumor‐bearing animals. The nanocomposites showed high accumulation in the TME through passive targeting mechanism and strong photoacoustic trait in the tumor site. The use of this NS resulted in a significant inhibition in the tumor growth, in large part because of the synergistic effects of chemophotothermal therapy [77].
Taken together, the GO offers unique characteristics, including (i) physicochemical properties (e.g. biocompatibility, high surface area, thermal conductivity, and photoacoustic trait), (ii) remarkable advancements in biomedical applications, and (iii) in vivo impacts (i.e. GO‐based theranostics) on cancer cells. Thus, it is envisioned that they have great potential for translation into the clinical applications. Among many different attempts for biomedical cancer diagnosis/therapy, in a study, GO was functionalized with monoclonal antibody (mAb) specific to the follicle‐stimulating hormone receptor (FSHR) and radiolabeled with 64 Cu for targeted PET imaging of the angiogenic biomarkers and therapy. This multimodal NS was used to track the angiogenesis during breast cancer progression and metastasis. The in vitro and in vivo studies on the breast cancer cell line and animal model showed great targeting potential and efficacy (Figure 11.5). As a result, the NS was proposed to serve as multimodal theranostic for simultaneous early diagnosis and targeted therapy [78].

Figure 11.5 The in vivo bioluminescence and PET imaging with 64 Cu‐labeled GO. (a) Serial BLI and its signal intensity in the mice after intravenous injection of cbgLuc‐MDA‐MB‐231 cells. (b) PET imaging of cbgLuc‐MDA‐MB‐231 tumor‐bearing mice after injection of 64 Cu‐NOTA‐GO, 64 Cu‐NOTA‐GO‐FSHR‐mAb, and 64 Cu‐NOTA‐GO‐FSHR‐mAb. (c) The cross‐sectional slices of cbgLuc‐MDA‐MB‐231 tumor‐bearing mice at four hours post‐injection of 64 Cu‐NOTA‐GO, 64 Cu‐NOTA‐GO‐FSHR‐mAb, and 64 Cu‐NOTA‐GO‐FSHR‐mAb. GO: Graphene oxide. BLI: Bioluminescence image. PET: Positron emission tomography. FSHR: Follicle‐stimulating hormone receptor. mAb: Monoclonal antibody. (See color plate section for the color representation of this figure.)
Source: The image was adapted with permission from [78].
In another interesting investigation, the GO/Fe3O4@SiO2 nanocomposite was radiolabeled with Rhenium‐188 (188 Re‐ GO/Fe3O4@SiO2) and loaded with gambogic acid (GA). The NS was introduced as a multifunctional theranostic and successfully used for magnetic drug delivery and radionuclide therapy in VX‐2‐bearing nude mice. While the release of the GA was shown to be pH‐responsive, the in vivo biodistribution of the NSs was found to be dependent on their morphology. The peanut‐like nanocomposites were found to be accumulated in the lung, spleen, and liver more than the spherical ones, and could markedly inhibit the tumor cell growth as compared to the nontargeted therapy [79].
11.4 Graphene‐Based Nanobiomaterials for Cancer Diagnosis
Given the remarkable photoacoustic and surface functionalization potentials of graphene and its derivatives, they are considered as promising materials for the detection and monitoring of solid tumors. By far, several studies have been carried out to implement different strategies to enhance and improve the photoconversion efficiency of such advanced nanobiomaterials to serve as probes for the early detection of cancer biomarkers in biological fluids of patients. To benefit such potentials, in a study, nanocomposites of GO/GNPs were developed to amplify signals in the surface Plasmon resonance (SPR)‐based detection of micro RNAs involved in the development and progression of cancer. In this study, the thiolated DNAs were immobilized on the gold surface to identify the target miRNAs. The engineered GO/GNPs provided great potential for quantitative early detection of miRNAs (miRNA‐200) with a limit of detection (LOD) as low as 1 fM [80]. In a similar attempt, GO/GNPs nanohybrids were designed for the analysis of miRNA‐141. The fabricated SPR‐based biosensor exhibited exceptional selectivity and sensitivity for the detection of different biomolecules such as miRNA‐141 with a LOD of 0.1 fM [81]. In a study, the nanohybrid of functionalized graphene with polyamidoamine and Ag nanoparticles were developed as a substrate to the sensitive surface‐enhanced Raman spectroscopy for the detection of methimazole (MTZ). This graphene‐based nanohybrid was found to enhance the bands of MTZ with an increased detection sensitivity to a picomolar range [82]. In fact, an exact analysis of biomolecules at a very low level is an important strategy, particularly when their amounts are extremely low. For example, in the blood, the circulating tumor cells (CTCs) and exosomes are very low. Therefore, their selective separation and identification among billions of biological entities in the blood appear to be an enormously complex issue. To tackle such a hurdle, Shi et al. decorated the GO/quantum dots (QDs) with MNPs and conjugated them with anti‐glypican‐3(GPC3) antibody. The NS was proposed to serve as a luminescent agent and successfully used for the two‐photon imaging at 960 nm light in a biological transparency range. The engineered NSs were effectively used for the separation of GPC3‐expressing HepG2 hepatocellular carcinoma tumor CTCs from the blood [83].
11.5 Conclusion
In this chapter, we summarized the recent advancements in engineering and application of the graphene‐based nanomaterials used for the development of multifunctional nanomedicines and theranostics. Owing to the photoacoustic properties of the graphene‐based nanomaterials, they include imaging and PTT/PDT potentials to multifunctional NSs providing great simultaneous imaging and synergistic therapy impacts on the cancer cells. Further, these advanced materials can be used in the development of different types of nanobiosensors to serve as nanoprobes for the detection of extremely low amounts of biological entities such as cancer markers and CTCs. In addition, combination therapy using graphene and its derivatives have widely been extended, resulting in the production of new generation of multimodal pharmaceuticals. In this regard, the ability of graphene nanocomposites as the NIR‐absorbing agents provides great opportunity in terms of variant light‐to‐heat conversion and NIR‐mediated PTT/PDT. These modalities and other image‐guided therapies of cancer through graphene‐based multimodal nanomedicines/theranostics make them outstanding materials that can be functionalized with different types of inorganic and/or organic materials, resulting in hybrid stimuli‐responsive NSs. These seamless NSs offer simultaneous targeting, imaging, and therapy potentials, and many of these NSs are under clinical translation. Despite many advancements, the paramount challenges in the translation of the multifunctional GO graphene‐based structures for imaging and targeted therapy seem to be the development of graphene‐based NSs with high in vivo stability, immune responsibility, and trivial/no toxicity.
Acknowledgment
The authors would like to acknowledge the financial support received from the Research Center for Pharmaceutical Nanotechnology, Biomedicine Institute, Tabriz University of Medical Sciences.
References
- 1 Pineros, M., Znaor, A., Mery, L., and Bray, F. (2017). A global cancer surveillance framework within noncommunicable disease surveillance: making the case for population‐based cancer registries. Epidemiologic Reviews 39 (1): 161–169.
- 2 McGuire, S. (2016). World cancer report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO press, 2015. Advances in Nutrition 7 (2): 418–419.
- 3 Aghanejad, A., Jalilian, A.R., Ardaneh, K. et al. (2015). Preparation and quality control of (68)Ga‐citrate for PET applications. Asia Oceania Journal of Nuclear Medicine and Biology 3 (2): 99–106.
- 4 Aghanejad, A., Jalilian, A.R., Maus, S. et al. (2016). Optimized production and quality control of 68Ga‐DOTATATE. Iranian Journal of Nuclear Medicine 24 (1): 29–36.
- 5 Omidi, Y. (2011). Smart multifunctional Theranostics: simultaneous diagnosis and therapy of cancer. Bioimpacts 1 (3): 145–147.
- 6 Heidari Majd, M., Barar, J., Asgari, D. et al. (2013). Targeted fluoromagnetic nanoparticles for imaging of breast cancer mcf‐7 cells. Advanced Pharmaceutical Bulletin 3 (1): 189–195.
- 7 Fathi, M., Zangabad, P.S., Aghanejad, A. et al. (2017). Folate‐conjugated thermosensitive O‐maleoyl modified chitosan micellar nanoparticles for targeted delivery of erlotinib. Carbohydrate Polymers 172: 130–141.
- 8 Fathi, M., Zangabad, P.S., Barar, J. et al. (2018). Thermo‐sensitive chitosan copolymer‐gold hybrid nanoparticles as a nanocarrier for delivery of erlotinib. International Journal of Biological Macromolecules .
- 9 Su, S., Wang, J., Wei, J. et al. (2015). Efficient photothermal therapy of brain cancer through porphyrin functionalized graphene oxide. New Journal of Chemistry 39 (7): 5743–5749.
- 10 Jeswani, G., Paul, S.D., and Jha, A.K. (2017). Advances in the delivery of cancer therapeutics: a comprehensive review. Current Drug Delivery 15 (1).
- 11 Yan, Q.L., Gozin, M., Zhao, F.Q. et al. (2016). Highly energetic compositions based on functionalized carbon nanomaterials. Nanoscale 8 (9): 4799–4851.
- 12 Filip, J. and Tkac, J. (2014). Is graphene worth using in biofuel cells? Electrochimica Acta 136: 340–354.
- 13 Xu, Y., Sheng, K., Li, C., and Shi, G. (2010). Self‐assembled graphene hydrogel via a one‐step hydrothermal process. ACS Nano 4 (7): 4324–4330.
- 14 Kiew, S.F., Kiew, L.V., Lee, H.B. et al. (2016). Assessing biocompatibility of graphene oxide‐based nanocarriers: a review. Journal of Controlled Release 226: 217–228.
- 15 Rahmanian, N., Eskandani, M., Barar, J., and Omidi, Y. (2017). Recent trends in targeted therapy of cancer using graphene oxide‐modified multifunctional nanomedicines. Journal of Drug Targeting 25 (3): 202–215.
- 16 Choi, S.Y., Baek, S.H., Chang, S.J. et al. (2017). Synthesis of upconversion nanoparticles conjugated with graphene oxide quantum dots and their use against cancer cell imaging and photodynamic therapy. Biosensors and Bioelectronics 93: 267–273.
- 17 Fan, Z., Zhou, S., Garcia, C. et al. (2017). pH‐responsive fluorescent graphene quantum dots for fluorescence‐guided cancer surgery and diagnosis. Nanoscale 9 (15): 4928–4933.
- 18 Some, S., Gwon, A.R., Hwang, E. et al. (2014). Cancer therapy using ultrahigh hydrophobic drug‐loaded graphene derivatives. Scientific Reports 4: 6314–6322.
- 19 Liu, J., Liu, K., Feng, L. et al. (2017). Comparison of nanomedicine‐based chemotherapy, photodynamic therapy and photothermal therapy using reduced graphene oxide for the model system. Biomaterials Science 5 (2): 331–340.
- 20 Akhavan, O., Ghaderi, E., Aghayee, S. et al. (2012). The use of a glucose‐reduced graphene oxide suspension for photothermal cancer therapy. Journal of Materials Chemistry 22 (27): 13773–13781.
- 21 Barar, J. and Omidi, Y. (2013). Intrinsic bio‐signature of gene delivery nanocarriers may impair gene therapy goals. Bioimpacts 3 (3): 105–109.
- 22 Hollins, A.J., Omidi, Y., Benter, I.F., and Akhtar, S. (2007). Toxicogenomics of drug delivery systems: exploiting delivery system‐induced changes in target gene expression to enhance siRNA activity. Journal of Drug Targeting 15 (1): 83–88.
- 23 Omidi, Y., Barar, J., and Akhtar, S. (2005). Toxicogenomics of cationic lipid‐based vectors for gene therapy: impact of microarray technology. Current Drug Delivery 2 (4): 429–441.
- 24 Omidi, Y., Barar, J., Heidari, H.R. et al. (2008). Microarray analysis of the toxicogenomics and the genotoxic potential of a cationic lipid‐based gene delivery nanosystem in human alveolar epithelial a549 cells. Toxicology Mechanisms and Methods 18 (4): 369–378.
- 25 Omidi, Y., Hollins, A.J., Benboubetra, M. et al. (2003). Toxicogenomics of non‐viral vectors for gene therapy: a microarray study of lipofectin‐ and oligofectamine‐induced gene expression changes in human epithelial cells. Journal of Drug Targeting 11 (6): 311–323.
- 26 Omidi, Y., Hollins, A.J., Drayton, R.M. et al. (2005). Polypropylenimine dendrimer‐induced gene expression changes: the effect of complexation with DNA, dendrimer generation and cell type. Journal of Drug Targeting 13 (7): 431–443.
- 27 Chen, Y.W., Su, Y.L., Hu, S.H., and Chen, S.Y. (2016). Functionalized graphene nanocomposites for enhancing photothermal therapy in tumor treatment. Advanced Drug Delivery Reviews 105 (Pt B): 190–204.
- 28 Paul, A., Hasan, A., Kindi, H.A. et al. (2014). Injectable graphene oxide/hydrogel‐based angiogenic gene delivery system for vasculogenesis and cardiac repair. ACS Nano 8 (8): 8050–8062.
- 29 Zhao, H., Ding, R., Zhao, X. et al. (2017). Graphene‐based nanomaterials for drug and/or gene delivery, bioimaging, and tissue engineering. Drug Discovery Today 22 (9): 1302–1317.
- 30 Hwang, D.W., Kim, H.Y., Li, F. et al. (2017). In vivo visualization of endogenous miR‐21 using hyaluronic acid‐coated graphene oxide for targeted cancer therapy. Biomaterials 121: 144–154.
- 31 Same, S., Aghanejad, A., Akbari Nakhjavani, S. et al. (2016). Radiolabeled theranostics: magnetic and gold nanoparticles. Bioimpacts 6 (3): 169–181.
- 32 Nergiz, S.Z., Gandra, N., Tadepalli, S., and Singamaneni, S. (2014). Multifunctional hybrid nanopatches of graphene oxide and gold nanostars for ultraefficient photothermal cancer therapy. ACS Applied Materials and Interfaces 6 (18): 16395–16402.
- 33 Wang, Y., Chen, J.T., and Yan, X.P. (2013). Fabrication of transferrin functionalized gold nanoclusters/graphene oxide nanocomposite for turn‐on near‐infrared fluorescent bioimaging of cancer cells and small animals. Analytical Chemistry 85 (4): 2529–2535.
- 34 Jafarizad, A., Aghanejad, A., Sevim, M. et al. (2017). Gold nanoparticles and reduced Graphene oxide‐gold nanoparticle composite materials as covalent drug delivery systems for breast cancer treatment. ChemistrySelect 2 (23): 6663–6672.
- 35 Gao, S., Zhang, L., Wang, G. et al. (2016). Hybrid graphene/Au activatable theranostic agent for multimodalities imaging guided enhanced photothermal therapy. Biomaterials 79: 36–45.
- 36 Xu, C., Yang, D., Mei, L. et al. (2013). Targeting chemophotothermal therapy of hepatoma by gold nanorods/graphene oxide core/shell nanocomposites. ACS Applied Materials and Interfaces 5 (24): 12911–12920.
- 37 Dembereldorj, U., Choi, S.Y., Ganbold, E.O. et al. (2014). Gold nanorod‐assembled PEGylated graphene‐oxide nanocomposites for photothermal cancer therapy. Photochemistry and Photobiology 90 (3): 659–666.
- 38 Bian, X., Song, Z.L., Qian, Y. et al. (2014). Fabrication of graphene‐isolated‐Au‐nanocrystal nanostructures for multimodal cell imaging and photothermal‐enhanced chemotherapy. Scientific Reports 4 (6093).
- 39 Fan, X., Jiao, G., Zhao, W. et al. (2013). Magnetic Fe3O4‐graphene composites as targeted drug nanocarriers for pH‐activated release. Nanoscale 5 (3): 1143–1152.
- 40 Wang, S., Zhang, Q., Luo, X.F. et al. (2014). Magnetic graphene‐based nanotheranostic agent for dual‐modality mapping guided photothermal therapy in regional lymph nodal metastasis of pancreatic cancer. Biomaterials 35 (35): 9473–9483.
- 41 Wang, G., Ma, Y., Wei, Z., and Qi, M. (2016). Development of multifunctional cobalt ferrite/graphene oxide nanocomposites for magnetic resonance imaging and controlled drug delivery. Chemical Engineering Journal 289: 150–160.
- 42 Ma, N., Liu, J., He, W. et al. (2017). Folic acid‐grafted bovine serum albumin decorated graphene oxide: an efficient drug carrier for targeted cancer therapy. Journal of Colloid and Interface Science 490: 598–607.
- 43 Mahanta, S. and Paul, S. (2015). Bovine α‐lactalbumin functionalized graphene oxide nano‐sheet exhibits enhanced biocompatibility: a rational strategy for graphene‐based targeted cancer therapy. Colloids and Surfaces B: Biointerfaces 134: 178–187.
- 44 Sherlock, S.P., Tabakman, S.M., Dai, H. et al. (2011). Photothermally enhanced drug delivery by ultrasmall multifunctional FeCo/graphitic shell nanocrystals. ACS Nano 5 (2): 1505–1512.
- 45 He, Y., Del Valle, A., Qian, Y., and Huang, Y.F. (2017). Near infrared light‐mediated enhancement of reactive oxygen species generation through electron transfer from graphene oxide to iron hydroxide/oxide. Nanoscale 9 (4): 1559–1566.
- 46 Yang, X., Wang, Y., Huang, X. et al. (2011). Multi‐functionalized graphene oxide based anticancer drug‐carrier with dual‐targeting function and pH‐sensitivity. Journal of Materials Chemistry 21 (10): 3448–3454.
- 47 Wang, Z., Zhou, C., Xia, J. et al. (2013). Fabrication and characterization of a triple functionalization of graphene oxide with Fe3O4, folic acid and doxorubicin as dual‐targeted drug nanocarrier. Colloids and Surfaces B: Biointerfaces 106: 60–65.
- 48 Li, Y., Liu, J., Dong, H. et al. (2014). Engineering of a Pluronic F127 functionalized magnetite/graphene nanohybrid for chemophototherapy. Nanotechnology 25 (6): 065602.
- 49 Tyagi, N., Attia, N.F., and Geckeler, K.E. (2017). Exfoliated graphene nanosheets: pH‐sensitive drug carrier and anti‐cancer activity. Journal of Colloid and Interface Science 498: 364–377.
- 50 Barar, J. and Omidi, Y. (2014). Surface modified multifunctional nanomedicines for simultaneous imaging and therapy of cancer. Bioimpacts 4 (1): 3–14.
- 51 Huang, P., Wang, S., Wang, X. et al. (2015). Surface functionalization of chemically reduced Graphene oxide for targeted photodynamic therapy. Journal of Biomedical Nanotechnology 11 (1): 117–125.
- 52 Dou, R., Du, Z., Bao, T. et al. (2016). The polyvinylpyrrolidone functionalized rGO/Bi2S3 nanocomposite as a near‐infrared light‐responsive nanovehicle for chemo‐photothermal therapy of cancer. Nanoscale 8 (22): 11531–11542.
- 53 Robinson, J.T., Tabakman, S.M., Liang, Y. et al. (2011). Ultrasmall reduced graphene oxide with high near‐infrared absorbance for photothermal therapy. Journal of the American Chemical Society 133 (17): 6825–6831.
- 54 Jin, Y., Wang, J., Ke, H. et al. (2013). Graphene oxide modified PLA microcapsules containing gold nanoparticles for ultrasonic/CT bimodal imaging guided photothermal tumor therapy. Biomaterials 34 (20): 4794–4802.
- 55 Zhu, X., Zhang, H., Huang, H. et al. (2015). Functionalized graphene oxide‐based thermosensitive hydrogel for magnetic hyperthermia therapy on tumors. Nanotechnology 26 (36): 365103.
- 56 Pan, Q., Lv, Y., Williams, G.R. et al. (2016). Lactobionic acid and carboxymethyl chitosan functionalized graphene oxide nanocomposites as targeted anticancer drug delivery systems. Carbohydrate Polymers 151: 812–820.
- 57 Pourjavadi, A., Mazaheri Tehrani, Z., and Jokar, S. (2015). Chitosan based supramolecular polypseudorotaxane as a pH‐responsive polymer and their hybridization with mesoporous silica‐coated magnetic graphene oxide for triggered anticancer drug delivery. Polymer 76: 52–61.
- 58 Ardeshirzadeh, B., Anaraki, N.A., Irani, M. et al. (2015). Controlled release of doxorubicin from electrospun PEO/chitosan/graphene oxide nanocomposite nanofibrous scaffolds. Materials Science and Engineering. C, Materials for Biological Applications 48: 384–390.
- 59 Liu, Y., Bai, J., Jia, X. et al. (2015). Fabrication of multifunctional SiO2@GN‐serum composites for chemo‐photothermal synergistic therapy. ACS Applied Materials and Interfaces 7 (1): 112–121.
- 60 Chen, H., Liu, F., Lei, Z. et al. (2015). Fe2O3@Au core@shell nanoparticle‐graphene nanocomposites as theranostic agents for bioimaging and chemo‐photothermal synergistic therapy. RSC Advances 5 (103): 84980–84987.
- 61 Shi, J., Wang, L., Zhang, J. et al. (2014). A tumor‐targeting near‐infrared laser‐triggered drug delivery system based on GO@Ag nanoparticles for chemo‐photothermal therapy and X‐ray imaging. Biomaterials 35 (22): 5847–5861.
- 62 Xu, X., Wang, J., Wang, Y. et al. (2017). Formation of graphene oxide‐hybridized nanogels for combinative anticancer therapy. Nanomedicine: Nanotechnology, Biology, and Medicine .
- 63 Zhang, X., Nan, X., Shi, W. et al. (2017). Polydopamine‐functionalized nanographene oxide: a versatile nanocarrier for chemotherapy and photothermal therapy. Nanotechnology 28 (29): 295102.
- 64 Kundu, A., Nandi, S., Das, P., and Nandi, A.K. (2015). Fluorescent graphene oxide via polymer grafting: an efficient nanocarrier for both hydrophilic and hydrophobic drugs. ACS Applied Materials and Interfaces 7 (6): 3512–3523.
- 65 Bardajee, G.R., Hooshyar, Z., Farsi, M. et al. (2017). Synthesis of a novel thermo/pH sensitive nanogel based on salep modified graphene oxide for drug release. Materials Science and Engineering. C, Materials for Biological Applications 72: 558–565.
- 66 Feng, L., Li, K., Shi, X. et al. (2014). Smart pH‐responsive nanocarriers based on nano‐graphene oxide for combined chemo‐ and photothermal therapy overcoming drug resistance. Advanced Healthcare Materials 3 (8): 1261–1271.
- 67 Lin, J., Chen, X., and Huang, P. (2016). Graphene‐based nanomaterials for bioimaging. Advanced Drug Delivery Reviews 105 (Pt B): 242–254.
- 68 Kanakia, S., Toussaint, J., Kukarni, P. et al. (2016). Safety and efficacy of a high performance Graphene‐based magnetic resonance imaging contrast agent for renal abnormalities. Graphene Technology 1 (1): 17–28.
- 69 Zhang, M., Liu, X., Huang, J. et al. (2017). Ultrasmall graphene oxide based T1 MRI contrast agent for in vitro and in vivo labeling of human mesenchymal stem cells. Nanomedicine: Nanotechnology, Biology, and Medicine. .
- 70 Zhou, C., Wu, H., and Wang, M. (2017). Functionalized graphene oxide/Fe3O4 hybrids for cellular magnetic resonance imaging and fluorescence labeling. Materials Science and Engineering C, Materials for Biological Applications 78: 817–825.
- 71 Chen, M.‐L., Shen, L.‐M., Chen, S. et al. (2013). In situ growth of [small beta]‐FeOOH nanorods on graphene oxide with ultra‐high relaxivity for in vivo magnetic resonance imaging and cancer therapy. Journal of Materials Chemistry B 1 (20): 2582–2589.
- 72 Kanakia, S., Toussaint, J.D., Chowdhury, S.M. et al. (2013). Physicochemical characterization of a novel graphene‐based magnetic resonance imaging contrast agent. International Journal of Nanomedicine (8): 2821–2833.
- 73 Lalwani, G., Sundararaj, J.L., Schaefer, K. et al. (2014). Synthesis, characterization, in vitro phantom imaging, and cytotoxicity of a novel Graphene‐based multimodal magnetic resonance imaging ‐ X‐ray computed tomography contrast agent. Journal of Materials Chemistry. B, Materials for Biology and Medicine 2 (22): 3519–3530.
- 74 Yang, Y., Shi, H., Wang, Y. et al. (2016). Graphene oxide/manganese ferrite nanohybrids for magnetic resonance imaging, photothermal therapy and drug delivery. Journal of Biomaterials Applications 30 (6): 810–822.
- 75 Yang, K., Wan, J., Zhang, S. et al. (2012). The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra‐low laser power. Biomaterials 33 (7): 2206–2214.
- 76 Chen, L., Zhong, X., Yi, X. et al. (2015). Radionuclide (131)I labeled reduced graphene oxide for nuclear imaging guided combined radio‐ and photothermal therapy of cancer. Biomaterials 66: 21–28.
- 77 Song, J., Yang, X., and Jacobson, O. (2015). Sequential drug release and enhanced Photothermal and Photoacoustic effect of hybrid reduced Graphene oxide‐loaded Ultrasmall gold Nanorod vesicles for cancer therapy. ACS Nano 9 (9): 9199–9209.
- 78 Yang, D., Feng, L., Dougherty, C.A. et al. (2016). In vivo targeting of metastatic breast cancer via tumor vasculature‐specific nano‐graphene oxide. Biomaterials 1043: 61–371.
- 79 Yang, Y., Liu, Y., Cheng, C. et al. (2017). Rational design of GO‐modified Fe3O4/SiO2 nanoparticles with combined Rhenium‐188 and Gambogic acid for magnetic target therapy. ACS Applied Materials and Interfaces 9 (34): 28195–28208.
- 80 Wang, Q., Li, Q., Yang, X. et al. (2016). Graphene oxide‐gold nanoparticles hybrids‐based surface plasmon resonance for sensitive detection of microRNA. Biosensors and Bioelectronics 77: 1001–1007.
- 81 Li, Q., Wang, Q., Yang, X. et al. (2017). High sensitivity surface plasmon resonance biosensor for detection of microRNA and small molecule based on graphene oxide‐gold nanoparticles composites. Talanta 174: 521–526.
- 82 Saleh, T.A., Al‐Shalalfeh, M.M., and Al‐Saadi, A.A. (2016). Graphene Dendrimer‐stabilized silver nanoparticles for detection of methimazole using surface‐enhanced Raman scattering with computational assignment. Scientific Reports 6 (32185).
- 83 Shi, Y., Pramanik, A., Tchounwou, C. et al. (2015). Multifunctional biocompatible graphene oxide quantum dots decorated magnetic nanoplatform for efficient capture and two‐photon imaging of rare tumor cells. ACS Applied Materials and Interfaces 7 (20): 10935–10943.
