5
Carbon Nanomaterials for Deep‐Tissue Imaging in the NIR Spectral Window
Stefania Lettieri 1 and Silvia Giordani 2,3
Istituto Italiano di Tecnologia (IIT), Nano Carbon Materials, Turin, Italy
Department of Chemistry, University of Turin, Italy
School of Chemical Sciences, Dublin City University, Ireland
5.1 Introduction
Optical imaging for diagnostic and therapeutic applications is widely diffused, as a noninvasive technique, which involves the use of visible and infrared (IR) light decreasing the exposure of the patient to harmful radiation [1]. Optical imaging can be applied for a live study event at a molecular level and as diagnostic tool for the prevention and treatment of cancer and other diseases [2]. Above all, near infrared fluorescent imaging has gained increasing attention due to the importance of noninvasive whole animal and deep‐tissue imaging. Neuroscientists are also interested in imaging deep inside the brain. Near‐infrared (NIR) light (700–2500 nm) is a transparent light, which can deeply penetrate biological tissues such as skin and blood efficiently compared to visible light. In this spectrum region, the tissue exhibits minimal absorbance and deeper penetration and the scattering is reduced, producing high‐quality images deep in the tissue. Moreover, the damage to the soft tissue is reduced due to the highly transparency of light. In this chapter we will focus on the use of carbon nanomaterials (CNMs) for NIR imaging, highlighting the peculiar characteristics of these nanometer‐size probes that make them good candidate for this application compared to other materials or organic fluorophores.
5.1.1 Transparent Optical Windows in Biological Tissue
Although the NIR spectrum is between 700 and 2500 nm, at wavelengths longer than 950 nm the absorbance from water and lipids start increasing and becomes important at wavelengths longer than 1400 nm, diminishing the effect of the NIR light. In the same way, at wavelengths shorter than 650 nm, the absorbance of other tissue biological elements starts (Figure 5.1). The two “biological transparent windows” shown in Figure 5.1, where minimum tissue auto‐fluorescence is present, are NIR‐I (750–1000 nm) and NIR‐II (1000–1700 nm) windows. At shorter wavelengths (<750 nm) and longer wavelengths (>1700 nm) we incur in elevate tissue background due to the absorbance of biological elementary constituents. Absorbance by water, proteins, and hemoglobin (Hb), for instance, is between 200 and 600 nm [6], making it difficult to work in the visible range as the background will be too high to have clear optical images. The scattering and absorption properties of human skin, subcutaneous and mucous tissues [3], and primary biological elements may influence the wavelength selection and, to some extent, the depth of penetration. Most organic molecules and water absorb in the ultraviolet range, and proteins, which are highly present in cells, also absorb in the UV spectral region (peak around 280 nm).
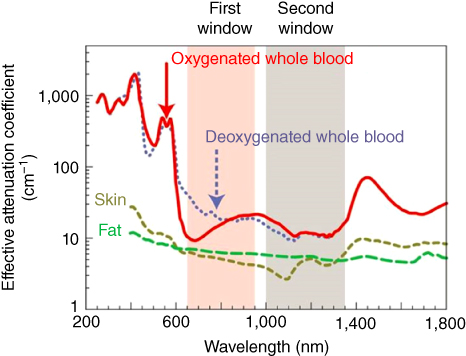
Figure 5.1 Optical windows in biological tissues. Plots of effective attenuation coefficient (on a log scale) versus wavelength show that absorption and scattering from oxygenated blood, deoxygenated blood, skin, and fatty tissue is lowest in either the first or second near‐infrared window [3, 4].
Source: Reprinted by permission from Macmillan Publishers Ltd.: ref. [5]. Copyright (2009).
For instance, oxyhemoglobin (HbO2 ), predominant in vascularized tissue, absorbs in the green and yellow spectrum, but exhibits a cut‐off at about 600 nm, similarly to melanin and human red blood cells [4, 6]. Infrared radiation, on the other hand, is essentially absorbed by collagen [7] and water, with increasingly stronger bands toward longer wavelengths. For this reason, the spectral “therapeutic window” [8] between 650 and 1400 nm allows for a deeper tissue penetration, reaching deeper targets due to the minimal scattering and absorption of biological absorbents.
5.1.2 Near‐Infrared Imaging Materials
The NIR‐I and in particular the NIR‐II windows, offers the possibility to obtain sensitive in vivo fluorescence imaging. In the past years a development in this field producing new long‐wavelength fluorophores and nanocarriers as optical materials have been made. For instance, the absorbance of gold nanoparticles (Au‐NPs) can be tuned toward a longer wavelength region by changing the size of Au‐NPs spherical clusters or changing their morphologies [9]. The fluorescence in the NIR wavelength region is also known for rare‐earth ion‐doped ceramic nanophosphors (RED‐CNPs) under NIR excitation to be used for in vivo imaging [10]. Moreover, several biocompatible NIR fluorophores such as zwitterionic cyanine dyes [11, 12], phosphonated cyanine dyes [13], and quaternary ammonium cyanine dyes [14], have been synthetized to date with emitting properties in the NIR window [15]. In particular, 4,4‐difluoro‐4‐bora‐3a,4a‐diaza‐s‐indacene (BODIPY) derivatives are a class of fluorophores with emerging biological and medicinal applications emitting in the NIR windows [16–18]. These dyes strongly absorb UV light, emitting sharp fluorescence with high quantum yield. Moreover, they are stable organic molecules with low photobleaching effect under continuous excitation, making them promising options for optical imaging. Although the aforementioned materials are promising nano‐tools for NIR deep‐tissue imaging, CNMs can bring important advantages to this field. For instance, CNMs are largely available, their scale‐up production is possible, and they are not expensive. Instead bioimaging materials such as RED‐CNPs can be more challenging and expensive to produce due to the presence of earth ions such as erbium and ytterbium, which are rare and not available as pure elements. Moreover, the extraction and purification of these ions is difficult and therefore expensive. The use of a NIR‐emitting dye alone can be also a problem, as it can be localized randomly in the body. Instead CNMs can be surface chemically functionalized with a ligand, which can selectively recognize cell proteins overexpressed on cancer cells, which can be useful for targeted drug delivery applications. Another advantage of CNMs is their low tendency to photobleach, allowing a prolonged excitation of the CNMs without signal decay, obtaining live and bright images without losing their emission signal. The biocompatibility of CNMs is also a pro of carbon‐based fluorescent probes, which was demonstrated both in vitro and in vivo by several groups in the past years. Other imaging probes, instead, can exhibit toxicity, such as quantum dots due to heavy metals contained in their structure, and hence are not useful for biological applications. Moreover, some CNMs, as carbon nanotubes CNTs, exhibit an intrinsic photoluminescence (PL); this allow the use of the CNMs alone as bioimaging agent, overcoming the issue related to the detachment of an emitting molecule attached to it as the fluorescent tag, which can happen once the nano‐probe is in physiological condition due to enzymatic cleavage. Later in this chapter we will explain in detail some pros and cons of different CNMs as NIR deep‐tissue imaging agents, underscoring which are potentially the most promising.
5.2 Carbon Nanomaterials for NIR Imaging
Since the discovery of fullerene by Kroto, Smalley, and Curl in 1985 [19], CNMs have gained increased interest due to their peculiar optical, electronic, and physical characteristics. In addition to the natural carbon allotropes, diamond, and graphite, which consist of extended networks of sp3 ‐ and sp2 ‐hybridized carbon atoms, respectively, several nanoforms have been discovered [20]. These are fullerene (C60) [19], CNTs [21, 22], carbon nanohorns (CNHs) [23], carbon nano‐onions (CNOs) [24], graphene [25], nanodiamonds (NDs) [26], and carbon dots (CDs) [27] (Figure 5.2). They are promising materials for biomedical applications due to their small size (1–100 nm) and biocompatibility. In addition, they are easily surface chemically functionalized with a therapeutic agent or a targeted ligand, for instance, using different reaction to synthetize specialized nanoparticles, as carbon has the ability to bind to itself and to nearly all elements in almost limitless variety. Furthermore, CNMs are promising tools for high resolution and live imaging, as some of them exhibit intrinsic fluorescence and low photobleaching properties, even when excited with high energy light.
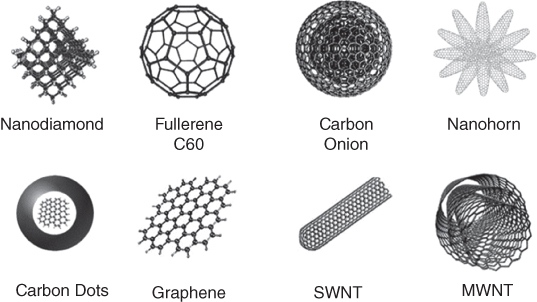
Figure 5.2 Members of the carbon nanomaterial family.
Source: Reproduced from Ref. [28] with permission from The Royal Society of Chemistry. Copyright (2015).
5.2.1 Biocompatibility of CNMs
Among the required characteristics of fluorescent probes and bioimaging agents is their biocompatibility and biosafety. In the past decade, biomedical research on CNMs has demonstrated their suitability as nanoprobes for bio applications [29, 30]. Their small size, optical properties, and large surface area, together with their bio safety, make them unique and promising as nano‐carriers. Several reports have shown their in vitro and in vivo biocompatibility, demonstrating that CNMs are safe for use on living beings.
The toxicity of purified single‐walled carbon nanotubes (SWCNTs), both pristine and oxidized, was tested in a three‐dimensional (3D) cellular model using THP‐1 cell line [31]. No toxic effect was observed after 24 hours of exposure. The tests demonstrated the relevance of purification and surface functionalization of SWNTs in the development of NIR probes to reduced toxicological impact on human health [32], or in the synthesis of carbon‐based nanoparticles for biological application [33]. For instance, both CNTs and carbon nano‐onions, upon surface functionalization, induce very limited if none inflammation both in vitro and in vivo [33]. Recent reports show that fluorescently labeled CNOs exhibit weak inflammatory potential and a low cytotoxicity [33], and they are readily internalized by cancer cells and accumulate in the lysosomes [34, 35]. In vivo studies performed on zebrafish (Danio rerio) during development [36] and on hydra vulgaris as model organisms for nanoecotoxicology have demonstrated their biocompatibility and ecosafety [37]. Similarly, NDs have been used as nano‐carriers for drug delivery systems and other biological applications due to their good biocompatibility [38, 39] and feasible surface modification [40]. For example, NDs have been used as a vehicle for the delivery of cisplatin anchored to the nanoparticle surface through covalent bonds [41].
5.2.2 Fluorescence of CNMs Probes
The NIR‐emitting properties of CNMs can be both intrinsic to the CNM or due to its surface functionalization with a fluorophore molecule. Interesting CNMs as CNTs can absorb and emit light in the far‐red/NIR region, reducing the autofluorescence of the tissue. This property escapes the need to use organic fluorophore to create an imaging platform in the NIR region, avoiding any potential toxicity of the dye or the chemical effort. In the coming section, the origin of the intrinsic fluorescence of CNMs and its advantages will be examined in detail.
5.2.3 Covalent and Noncovalent Functionalization
CNMs can be functionalized on their surface both covalently [42–44] and not covalently [44–48] by adsorption of a molecule interacting through π–π stacking with the graphitic layer of the CNMs. The use of surface functionalization is essential to transform these nanoparticles into specialized probe for biological applications − for example, by attaching a fluorescent tag, a ligand, or a drug. Moreover, surface functionalization is key to enhance their solubility: CNMs display poor solubility in aqueous solvents where they are prone to aggregation due to hydrophobic interactions. They show substantial van der Waals interactions and a hydrophobic nature, causing their precipitation in water and most organic solvents.
5.2.4 CNMs as Bioimaging Platforms
In this section we will cover CNMs used for bioimaging in therapeutic windows, underlying their optical and structure characteristics.
5.2.4.1 Fullerene
Fullerene or C60 is a zero‐dimensional (0D) CNM. It consists of sp2 ‐hybridized carbon atoms and it was discovered in 1985 by Kroto, Smalley, and Curl [19] while understanding the mechanisms by which long‐chain carbon molecules are formed in interstellar space and circumstellar shells. In 1990, C60 was successfully prepared in macroscopic quantities by Krätschmer and Huffman [49]. Since then, C60 has been used in different applications spanning from biomedical research to sensing [50]. The nanometer size, biocompatibility [51, 52], intrinsic photoluminescence [53], and hollow cavity of C60, make this nanomaterial suitable for drug delivery and imaging. For example, C60 sphere has been used to load metal ions such as Gd3+ [54] or 99m Tc [55], as a magnetic resonance imaging (MRI) contrast agent, using endohedral functionalization. C60 exhibits a characteristic reddish orange photoluminescence signature in the solid state with a peak at 735 nm [53]. This photoluminescence change in different solvents. C60 in methanol retained this key property that is dependent on the interstitial spacing between C60 molecules in the crystalline structure with a broad peak around 750 nm. This spectroscopic characteristic has been exploited to investigate the interaction of C60 with cells and their uptake by cancer cells. It has been demonstrated that the intracellular C60 retains its PL signature. Bong Hyun Chung and co‐workers reported on hybrid fullerene‐silica nanoparticles (FSNPs) exhibiting a bright fluorescence, high photostability, and low cytotoxicity important for their use in biological applications [56]. In their work, FSNPs were prepared by a reverse microemulsion method with a nonionic surfactant. The average diameter size of these nanoparticles is 61.5 ± 6.0 nm. To assess the prospects of FSNPs as a bioimaging material, their photostability, cell permeability, and cytotoxicity have been tested. The authors observed the PL image of the FSNPs in macrophage (RAW 264.7) cells, human epithelial carcinoma cells (HeLa), estrogen receptor negative cells (SKBr3), and human umbilical endothelial cells (HUVECs) by treating them with 40–160 μg ml−1 FSNP solutions. In particular they observed at the confocal microscopy the FSNPs incorporated in macrophages (RAW 264.7) under 492 nm excitation and >617 nm detection during the initial stage of irradiation. Importantly, continuous excitation by light during cell imaging revealed a high photostability of the FSNPs in the cytoplasmic region of the cells. The author linked the red PL from the nanoparticles with the defects formed in the silica network by fullerenic carbon atoms. These reported particles are promising for bioimaging application for their photostability and high luminescence, besides being easily modified on their surface with biomolecules. A few years later, the same group reported on color‐tunable photoluminescence nanoparticles [57]. Their challenge was to overcome the limit of fullerene nanomaterials due to their low fluorescence efficiencies, which render them unavailable for bioimaging, besides exhibiting limited water solubility. Their strategy was to conjugate tetraethylene glycol (TEG) with fullerene using lithium hydroxide (LiOH) as a catalyst. TEG‐conjugated fullerene nanoparticles (C60‐TEGs) were synthesized by adding LiOH to mixtures of C60 fullerene dissolved in toluene with various concentrations and TEG at a volume ratio of 1 : 1 at room temperature. The color of C60‐TEGs nanoparticles was tunable by varying the C60 concentration in the reaction solution. The emission of C60‐TEGs was linearly red‐shifted from 503 to 550 nm when the C60 concentrations in the reaction solution increased. These nanoparticles were tested on HeLa cells to asset their potentiality as an imaging platform, as they have a good water solubility and tunable fluorescence. The changes of electronic transition state of C60 dependent on the extent of their oxidation and the number of TEG in C60‐TEGs nanoparticles are the reason of the color tunability.
The weak visible absorbance and zero near‐infrared absorbance of fullerenes are their drawbacks. An excellent candidate as live‐cell imaging agents are fullerene‐oligothiophene chromophores with enhanced light‐absorbing capability, shown in Figure 5.3 [58]. The purpose of this work was focused on their application in organic electronic and optics. By incorporating an oligothiophene into the fullerene π‐system by an open‐cage strategy (Figure 5.3, right), the absorbance of this new fullerene material at the visible region was enhanced, besides being extended to the NIR region.

Figure 5.3 Fullerene‐oligothiophene chromophores structure (left); Open‐cage strategy for improving fullerene absorbance (right).
Source: Reproduced from Ref. [58] with permission from Wiley‐VCH Verlag GmbH & Co. KGaA. Copyright © 2012.
Overall, fullerene exhibits an intrinsic photoluminescence that is maintained upon cell internalization, making this material promising as bioimaging probes. The pros of this material are: large availability, possible surface modification with molecules of biological interest, high photostability, and biocompatibility. However, the limitation of fullerene is associated with its PL which doesn't belong to the NIR‐II spectrum window. The aforementioned fullerene‐oligothiophene chromophores are perhaps the most promising fullerene‐based probes, as their emission was shifted to the NIR, but not yet at wavelengths greater than 1000 nm.
5.2.4.2 Carbon Nanotubes
Since their discovery in the late twentieth century [21, 22, 59], CNTs have raised an increasing interest from different fields for their unique chemical, optical [60], electrical, mechanical, and thermal [61] properties. CNT is one‐dimensional (1D) CNM comprised of sp2 carbon atoms organized in single or multiple coaxial tubes of graphitic sheets resulting in SWCNTs and multiple‐walled carbon nanotubes (MWCNTs) respectively. Of particular interest is the use of CNTs for imaging of living object due to their peculiar optical characteristics and biocompatibility [31, 32]. Although several CNM are able to emit light upon photo‐excitation, above all semiconducting SWCNTs play an important role, due to their fluorescence in the NIR‐II window.
As shown in Figure 5.4, light absorption at photon energy E22, which creates an electron–hole pair, is followed by fluorescence emission near E11 in semiconducting SWCNTs. The values of E11 and E22 will vary with tube structure [62]. The purity and chirality of the utilized SWCNTs thereby play an important role. Hence, SWCNTs usually absorb photons in the visible (400–750 nm) and NIR‐I (750–1000 nm) windows, followed by fluorescence emission in the NIR‐II window (1000–1700 nm), which belongs to the biological transparent window. The nonradiative relaxation of the absorbed photon energy is instead emitted in the form of heat [63]. For this reason, SWCNTs have also been used as photosensitizers for photothermal and photodynamic therapy [64, 65]. The unique optical properties of SWCNTs have made them promising candidates as NIR fluorophores for deep‐tissue fluorescence imaging in living objects. SWCNTs have been used as fluorescence tags for in vitro cell imaging [66], ex vivo imaging of tissues and organs [67], and in vivo imaging of organs. Although SWCNTs have been functionalized covalently with fluorophores exhibiting a shorter emission wavelength to be used as imaging probe [68, 69], the use of SWCNTs alone in optical applications using their intrinsic fluorescence is an advantage. In fact, an organic dye can be detached from the CNMs by the enzymatic cleavage of the chemical link, besides affecting their cytotoxicity.
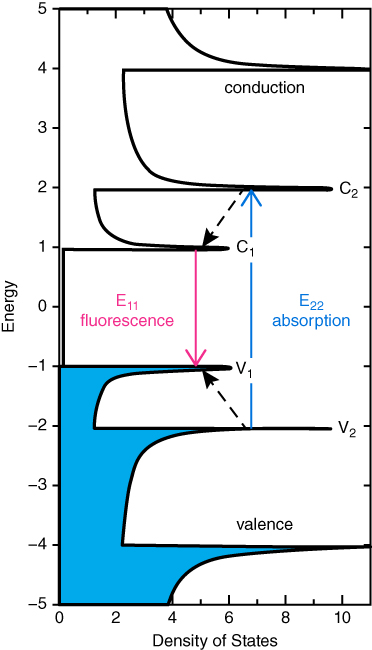
Figure 5.4 Schematic density of electronic states for a single nanotube structure illustrating the transitions leading to SWCNT fluorescence. Solid arrows depict the optical excitation and emission transitions of interest.
A drawback of SWCNTs as fluorophores for bioimaging is its type of fluorescence, which is excitonic, meaning that it is sensitive to its chemical environment as well as the length of the nanotube. For this reason, the fluorescence can be quenched for many factors, meaning that SWCNTs generally exhibit a low quantum yield on the order of 0.1–1% [70, 71]. Moreover, their fluorescence can be affected when covalently functionalized − for example, when oxidized SWCNTs were linked to poly(ethyleneglycol) (PEG), e.g. PEGylation through 1,3‐dipolar cycloaddition [72]. Major efforts have been made to make SWCNTs suitable for fluorescence imaging with sufficient NIR‐II photoluminescence emission. The efforts were focused on enhancing their intrinsic fluorescence and by removing possible quencher from the environment [73–75]. Therefore, if properly functionalized, SWCNTs are promising biomarkers for cell imaging due to their low cytotoxicity, high photostability, absence of quenching, and photobleaching in cells. For example, oxygen doping of SWCNTs can be modified in their NIR band gaps, changing the emitting properties of SWCNTs without suppressing their NIR fluorescence, which can happen using covalent sidewall functionalization [76]. Weisman et al. reported covalently oxygen‐doped nanotubes prepared by exposure to low doses of ozone and then light [77] with stronger and shifted NIR emission compare to pristine one. They used semiconducting species obtained by nonlinear density gradient ultracentrifugation [78] as pristine starting material. The authors explained that the dopant sites harvest light energy by trapping mobile excitons, resulting in an NIR emission at 10–15% longer wavelengths than pristine semiconducting SWCNTs. Moreover, they tested these oxygen‐doped SWCNTs coated with Pluronic F127 surfactant on human uterine adenocarcinoma cells. They were able to obtain images of single nanotubes with enhanced image contrast. Most recently, the effect of polyunsaturated fatty acids (PUFAs) on the near‐infrared photoluminescence of SWCNTs was investigated [79]. PUFAs are used for SWCNTs coating to make them less toxic and with enhanced water solubility; however, their effect on the PL of this nanomaterials needs to be investigated. The authors observed a decrease in the SWCNT bandgap emission (E11) and a new red‐shifted emission (E11 − ) in the presence of PUFAs. Overall the PL changes were attributed to the formation on SWCNTs of oxygen‐containing defects by lipid hydroperoxides through photooxidation.
A 90 kDa amphiphilic poly(maleic anhydride‐alt‐1‐octadecene)‐methoxy poly(ethylene glycol) [C18‐PMH‐mPEG] used for coating noncovalently SWCNTs, which preserved their intrinsic NIR photoluminescence [80], was also reported. The coating also allowed an in vivo long blood circulation of these functionalized CNTs to achieve ultrahigh accumulation into the tumor, and to perform a live imaging of the tumor in the second near‐infrared (NIR‐II, 1.1–1.4 μm) window and ex vivo quantification of the pharmacokinetics. This work was promising to study vessel imaging to distinguish tumors from health tissue, using the abnormal blood vessel's fenestration nearby and inside the tumor area [81]. In another example, the use of highly debundled SWCNTs led to the develop of high contrast NIR agents easily detectable due to their intense and preserved fluorescence [67, 82]. SWCNTs coated with pharmaceutical‐grade Pluronic F108 as a nonionic surfactant with enhanced dispersibility in aqueous media were prepared. The exfoliation process of SWCNTs by ultrasonication in a solution of the artificial surfactant Pluronic F108, destroys the nanotubes aggregate, which are created by strong van der Waals forces. These Pluronic‐suspended SWCNTs were used for imaging of phagocytic cells [82]. The NIR emission allows a strong discrimination between the CNMs emission and the endogenous fluorescence. They demonstrated that the ingested nanotubes maintain their fluorescence, besides been biocompatible and readily taken up by macrophage cells. These SWCNTs are therefore promising tools for studying the bio‐distribution of nanoparticles in organisms and the interaction of SWCNTs with cells and tissue. The same group reported on the study of the pharmacokinetic of CNTs using their intrinsic NIR fluorescence [67]. Importantly, they were able to map the distribution of intravenously injected SWCNTs inside ex vivo rabbit liver tissues. In the latest examples the type of binding between the nanomaterial and the tissue or cells were nonspecific. However, to be able to use SWCNTs as a specific tag for imaging and selectively probing and imaging cells, nanotubes must be functionalized with specific ligands blocking the nonspecific interaction between a biological target and the nanomaterial. To this end, it has been reported on a surface noncovalent functionalization of SWCNTs with PL−PEG(5400 Da)‐NH2, where the PEG chain blocks the nonspecific interaction and enhances the water solubility of the nanotubes, while the amino group can react with different targeted ligands such as Rituxan and Herceptin, antibodies that specifically target the CD20 and HER2 receptors, respectively [66]. They demonstrated a selective SWCNT−antibody binding to cells by detecting the intrinsic band gap NIR fluorescence of nanotubes demonstrating the strong dependence of the interaction between CNTs and living cells from their surface functionalization. Welsher et al. reported another example of targeted imaging using CNTs as probes, describing the conjugation of SWCNTs with an arginine‐glycine‐aspartic acid (RGD) peptide, which interacts specifically with the αvβ3 integrin on the human brain glioblastoma U87‐MG cells [83]. The fluorescent SWCNTs‐RGD conjugates were prepared via the surfactant‐exchange method. A bright NIR‐II signal was observed from the αvβ3 integrin positive U87‐MG cells treated with SWCNT−RGD conjugates compared to the low fluorescence exhibited from cells with a low expression level of this protein. Importantly, in this paper they prepared the SWCNTs, sonicating them with sodium cholate, followed by surfactant exchange to form phospholipid‐polyethylene glycol coated nanotubes. In this way, they obtained bright and biocompatible imaging agents.
Another example of targeted imaging, where SWCNT−RGD conjugate were used to obtain both selective and enhanced fluorescence, was reported and a good selectivity toward αvβ3 integrin positive U87‐MG cells was observed [84]. In this case, they applied a plasmonic gold film, which enhances the NIR‐II fluorescence signals of the SWCNTs bonded to the cell receptors by about ninefold compared to the quartz substrate. The presence of the gold film allowed acquiring high‐quality images under a short exposure time (300 ms with gold enhancement versus one to three seconds without gold). Also, the detection limit was reduced up to 48 pM, decreasing the CNTs concentration. The use of SWCNTs as fluorescent probes is not only limited to the selective detection of a particular cell − for example, as a diagnostic tool to selectively imaging a tumor − but also as a delivery tool to transport proteins [85, 86], DNA [86], and drug molecules [87] inside a cell and monitor at the same time the internalization process of each individual nanotube, using the stable NIR‐II fluorescence of SWCNTs that barely photobleaches under prolonged excitation. The capability of SWCNTs to obtain images in a small time frame, and their spatial and temporal resolution, allowed biologists to study intracellular events, in addition to directly tracking the endocytosis and exocytosis of CNTs [88]. An interesting example was the noninvasive tracking of intracellular fluctuations using SWCNTs as unique fluorescent labels [89]. The authors studied the intracellular dynamics of a motor protein, a kinesin‐1, in fibroblast‐like COS‐7 cells. The SWCNT‐labeled kinesins move along the microtubule tracks, and the high‐resolution imaging allowed tracking the motions of the kinesin‐1 motor proteins inside the cytoplasm with a temporal resolution of 5 ms per frame. The low photobleaching nature of the nanotubes permitted an increase in the excitation power, allowing the acquisition of ultra‐short time dynamics.
Recently, the intrinsic NIR emission of SWCNTs was also used to study the interaction of nanoparticles with a 3D in vitro cell culture model [90]. In particular, SWCNTs were localized and their cell internalization was studied on MCF‐7 breast cancer cell‐derived spheroids, and on SK‐136 cells. They concluded that SWCNTs were readily internalized in the MCF‐7 cell line, while little penetration was observed on the SK‐136 cells.
The in vivo imaging of live animals is also possible, taking advantage of the exquisite optical characteristics of SWCNTs. In fact, the limitation of in vivo imaging are the penetration depth and imaging clarity due to three main factors: photon scattering, photon absorption, and tissue auto‐fluorescence. NIR‐II imaging of SWCNTs allowed deep‐tissue penetration and high‐resolution images. The main challenge in live imaging is to reach a penetration depth greater than 150 μm, keeping the resolving power [91]. NIR‐II fluorescence photons have much less scattering than traditional fluorescence in both visible and NIR‐I windows, allowing deep‐tissue penetration, which is key for imaging on live animals.
For example, in one study, imaging using NIR‐II, NIR‐I, and visible windows was compared [92]. In this study, a deep, noninvasive imaging for surgical guidance of tumors was achieved. Moreover, the SWCNTs were functionalized with the M13 virus to recognize tumor‐targeting peptides. The comparison between other imaging systems was significant. From Figure 5.5 is clear how the SWCNTs display a higher signal‐to‐noise performance compared to visible and NIR‐I window dyes. This is one example of the possible application of CNTs as diagnostic tools for an early and noninvasive detection of tumors to guide their surgical removal.
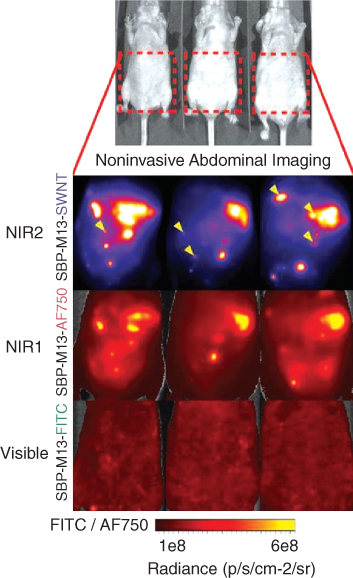
Figure 5.5 Noninvasive imaging of ovarian tumors using SBP–M13 conjugated to SWNTs (NIR‐II), AlexaFluor750 (NIR‐I), and FITC (Visible) (top to bottom). Arrows in the SWNT panel denote nodules visible only by SWNTs (n = 3 animals). (See color plate section for the color representation of this figure.)
Source: Reprinted with permission from ref. [92]. Copyright 2014 National Academy of Sciences.
The successful live imaging of mouse cerebral vasculature using SWCNTs was also performed without the necessity of craniotomy, cranial windows, and skull thinning techniques, which are usually required for brain imaging (Figure 5.6) [93]. The reduced short‐wavelength photon scattering in the NIR‐IIa window allowed an improved imaging resolution to a depth of >2 mm using 3D in vivo imaging through confocal or two‐photon techniques. Moreover, an imaging rate of ∼5.3 frames per second allowed for real‐time recording of blood perfusion in the cerebral vessels with sufficient temporal resolution. Through this system, the authors were able to study a blood flow anomaly in a mouse stroke model. SWCNTs were also tested by Weisman et al. on Drosophila melanogaster (fruit flies) to explore their use as imaging probe in organisms and biological tissue, and their biocompatibility on a vertebrate model [94]. The larvae were fed food containing water‐solubilized pristine SWNTs. SWNT feeding did not affect survival of Drosophila to either pupal and adulthood stage. Moreover, the nanotube feeding did not affect their overall growth. The authors also used the NIR fluorescence of SWCNTs to image the single nanotubes in tissue specimens and therefore nondestructively image their accumulations inside living organisms.
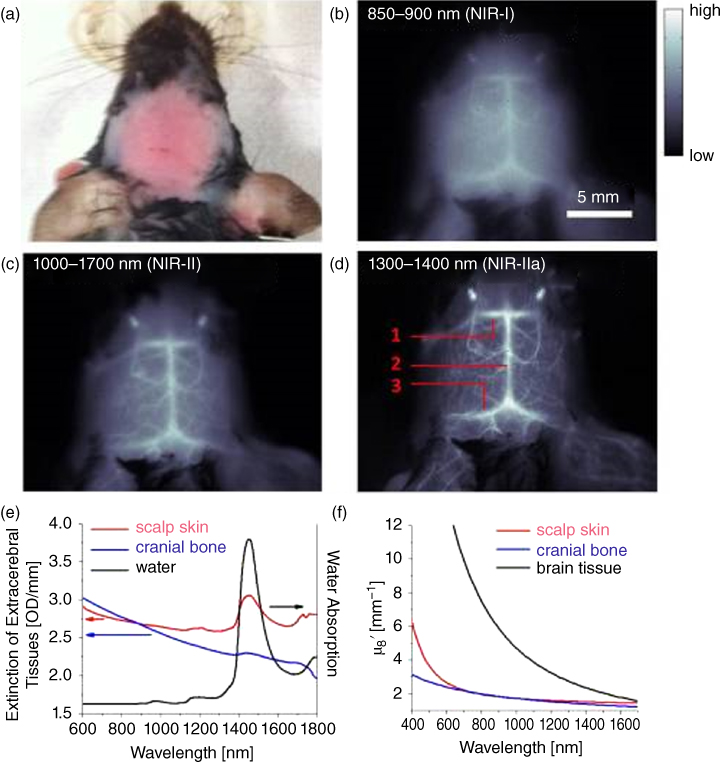
Figure 5.6 (a) A C57Bl/6 mouse head with hair removed. (b–d) Fluorescence images of the same mouse head in the NIR‐I, NIR‐II, and NIR‐IIa regions. In (d), the inferior cerebral vein, superior sagittal sinus and transverse sinus are labeled 1, 2, and 3, respectively. (e) Extinction spectra of scalp (red) and skull (blue) as well as the water absorption spectrum (black). (f) Reduced scattering coefficients μ of scalp skin (red), cranial bone (blue), and brain tissue (black) plotted against wavelength. (See color plate section for the color representation of this figure.)
Source: Reprinted with permission from ref. 93. Copyright 2014 Nature Publishing Group.
SWCNTs are sensitive to changes on their surface, meaning that their emission wavelength and intensity is modulated by perturbation on their surface, with up to single‐molecule sensitivity. Consequently, Heller's group reported on the possible use of SWCNTs as implantable sensors, due to their emission in the NIR spectral windows and high sensitivity [95]. They were able to observe a real‐time optical quantification of hybridization events of microRNA and other oligonucleotides, which are promising disease biomarkers, on live mice using the nanotubes PL changes caused by hybridization events.
Although SWCNTs exhibit an intrinsic fluorescence, examples of surface functionalized CNTs with fluorescent dyes, taking advantages of the peculiar characteristics of the dye molecule, have been reported. For example, a donor‐acceptor material, consisting of a red/near‐infrared absorbing boron‐chelated tetraarylazadipyrromethenes (AZA‐BODIPY) covalently attached, via amide‐bond formation to a SWCNT, was reported [68]. Efficient electron transfer from the photoexcited donor boron azadipyrromethene to the acceptor SWCNT was demonstrated. Similarly, CNTs were functionalized with an AZA‐BODIPY with on/off switching properties controlled by pH [69]. Pristine SWCNTs were first functionalized on their surface with a benzoic acid. The carboxylic functionalities on the CNTs surface were then treated with thionyl chloride to obtain an acid chloride, which consequently reacted with the amino group present on the fluorophore molecule. The on/off switching mechanism of fluorescent single‐walled carbon nanotubes (f‐SWCNTs) was designed to be controlled by the phenol/phenolate interconversion on the fluorophore. When protonated at pH 5, the f‐SWCNTs exhibited an emission band centered at 725 nm, which disappears immediately upon addition of a base due to the presence of the nonemitting phenol form.
CNTs are the most promising CNMs for deep‐tissue imaging, due to their exquisite characteristics in terms of optical properties and biocompatibility. In particular, semiconducting SWCNTs exhibit a fluorescence in the NIR‐II spectral window (1000–1700 nm), which belongs to the biological transparent window allowing deep‐tissue fluorescence imaging in living objects. Moreover, SWCNTs can be used for imaging without any further modification with a fluorescence tag, as for other CNMs, which is advantageous for overcoming the possible detachment of an organic dye by the enzymatic cleavage of the chemical link, which can eventually affect their cytotoxicity or giving a nonspecific fluorescence background. Other pros of CNMs are their high photostability and absence of quenching and photobleaching under prolonged excitation. The capability of SWCNTs to obtain images in a small time frame, and their spatial and temporal resolution, allowed biologist to study events at a cellular level, as the low photobleaching nature of the nanotubes permits increases in the excitation power, allowing the acquisition of ultra‐short time dynamics. One of the cons of CNTs is related to their generally low quantum yield on the order of 0.1–1%, compared to other CNMs, which is linked to their excitonic nature. However, if properly functionalized, this issue can be overcome, because the quantum yield is mainly affected by the chemical environment as well as the length of the nanotube.
5.2.4.3 Graphene Derivatives
Graphene is a two‐dimensional (2D) CNM [25]. It is an atom‐thin layer of hexagonally arranged carbon atoms with sp2 hybridization in two dimensions. This 2D crystal of carbon can be synthesized by different techniques, including mechanical and chemical exfoliations [96], unzipping of CNTs [97], bottom‐up epitaxial growth [98], and chemical synthesis [99]. Besides the optical, mechanical, and electronic characteristics of graphene, its large surface area, high mechanical flexibility, and capability of chemical functionalization directly on its sp2 hybridized carbons, has raised a great interest for a promising material for biological and medical applications [100]. Graphene oxide (GO), besides the above‐listed characteristics, has a strong intrinsic fluorescence emission due to its highly heterogeneous chemical and electronic structure. GO emits in a broad spectrum spanning from the ultraviolet to the near‐infrared [101], and can be seen as a promising contrast agent for in vivo and in vitro fluorescence bioimaging. GO was first discovered in 1859 via harsh oxidative treatment of graphite [102] and then later modified via the Hummers method [103]. Different mechanisms have been proposed to be the reason for this optical property of GO, which is not present on pristine graphene. One proposed suggestion links the fluorescence of GO to the electronic transition between the nonoxidized pristine sp2 carbon domain and the oxidized boundaries of GO sheet [104]. The NIR‐I fluorescence up to ∼1000 nm from both plain GO and PEGylated GO to be used as fluorescent tag for cellular imaging, have been reported by Dai's group [105]. In particular, they explored the use of nanographene oxide (NGO) in biological application, using the intrinsic photoluminescence of NGO for live cell imaging in the NIR. The NGO were conjugated with anti‐CD20 antibody, Rituxan, in order to selectively image cancer cells, besides being functionalized with a PEG molecule to increase their solubility in aqueous medium. Moreover, anticancer drug doxorubicin was loaded on the NGO PEG Rituxan by physisorption via π‐stacking. Multiphoton‐induced fluorescence imaging using PEGylated graphene oxide (GO‐PEG) nanoparticles have been used for deep‐tissue penetration imaging on a mouse brain [106]. The advantages are that the two‐photon excitation wavelength is usually in the range of 700–900 nm or 1000–1350 nm, and three‐photon excitation wavelength is usually in the range of 1000–1350 nm, which belong to the transparent biological window for tissue. The GO‐PEG nanoparticles reported have been demonstrated to have negligible cytotoxicity by a cell proliferation assay and histological analysis. In addition, it has been reported the use of ultra‐small reduced graphene oxide (nano‐rGO) with high NIR absorbance for photo‐thermal therapy [107]. The single‐layered nano‐rGO sheets were ∼20 nm in average lateral dimension and were stabilized in biological media by adsorption of amphiphilic PEGylated polymer chains. The nano‐rGO exhibited sixfold higher NIR absorption than the nonreduced and covalently PEGylated counterpart. A selective cellular uptake in U87MG cancer cells was also obtained by attaching a targeting peptide with the Arg‐Gly‐Asp (RGD) motif to nano‐rGO. The nano‐rGO exhibited little toxicity without the presence of NIR irradiation.
GO exhibits a low cytotoxicity and an intrinsic fluorescence. However, compare to other CNMs, such as CNTs, their PL doesn't belongs to the NIR‐II window, which is needed for high‐resolution deep‐tissue imaging. Hence, although its promising characteristics such as size, easy surface modification with biological tags for targeted drug delivery or imaging systems, and low toxicity, more research needs to be performed in order to reach a longer wavelength emission. On the other hand, GO exhibits an intrinsic emission up to 1000 nm, which other CNMs don't have, and due to that a deep‐tissue imaging in a mouse brain was achieved, making these nanomaterials still promising for deep‐tissue imaging applications.
5.2.4.4 Carbon Dots
CDs, also known as carbon quantum dot or graphene quantum dot, were discovered by serendipity in 2004, while, during a preparative electrophoresis experiment for purifying arc‐discharged SWCNTs [27], have been observed fluorescent carbonaceous nanoparticles. Even since great interest have been focused on these fluorescent carbon‐based nanoparticles spanning from their synthesis to the physicochemical understanding of the origin of their intrinsic fluorescence. In terms of the chemical structure and physical properties, carbon dots are similar to graphene oxide. They distinguish themselves from GO due to the size, CDs being carbonaceous, graphitic nanoparticles smaller than 10 nm [108]. Carbon dots can be prepared by carbon‐containing precursors using both physical and chemicals means [109] − for example, by laser ablation [110, 111], oxidative acid treatment [112], hydrothermal treatment [113], electrochemical oxidation [114], ultrasound irradiation [115], microwave‐assisted synthesis [116], and electron‐beam lithography [117]. The photoluminescence spectra of carbon dots depends on the excitation wavelengths; emission spans from deep ultraviolet, to visible, to the near‐infrared [118]. Their fluorescence can be tuned from 400 to 750 nm by changing its size, meaning that the quantum confinement of the graphitic domain is linked with the photoluminescence. Moreover, their surface passivation is crucial for obtaining their strong fluorescence [110].
Carbon quantum dots (CQDs) compare to semiconductor quantum dots (QDs), which are limited in their biological applications due to toxicity of the heavy metals contained in their structure. CQDs are biocompatible, besides being water soluble and having high cell permeability [119]. Therefore, due to the intrinsic tunable fluorescence and low toxicity, photostability, and resistance to photobleaching [110], CDs have been widely applied for in vitro and in vivo imaging. Although several studies on bioimaging based on CDs have been reported [120], the majority of carbon dots exhibited a short blue or green fluorescence. Tan et al. reported a recent example of near infrared graphene quantum dots (GQDs) as nano‐probe for bioimaging of endogenous ascorbic acid (AA) in living systems [121]. This is an example of GQDs with excitation and emission wavelength both in the NIR region, allowing a direct detection of AA in cells and at the same time deep‐tissue imaging. Interestingly the GQDs were functionalized on their surface with CoOOH nanoflakes, which are fluorescence quenchers. Once AA was introduced, CoOOH was reduced to Co2+ , which resulted in a “turn‐on” fluorescence signal of GNGs. The proposed nano‐probes can be used both for imaging and for sensing application with high sensitivity toward AA.
Another example of NIR bioimaging using graphene quantum dots produced from Mangifera indica (mango) has been recently reported (mGQDs), where the bright red‐luminescent GQDs were produced with a one‐pot microwave‐assisted green‐synthesis route [122]. The average size of mGQDs range from 2 to 8 nm and exhibit excitation‐independent fluorescence emissions in the NIR region between 650 and 750 nm. These fluorescent tags showed excellent photostability and lifetime, besides exhibiting a good cellular uptake and excellent biocompatibility on L929 cells. Interestingly, the PL intensity of mGQDs changed at excitation range of 300–500 nm; they showed a rise in the emission intensity up to the highest emission at 400 nm and then decreased further. A simple reaction process was reported to synthesize blue, green, yellow, and red graphene nanoparticles (GNPs) from carbon fibers [123]. In particular, the authors were interested in developing a method to obtain carbon dots emitting in the near infrared to use them for optical imaging of deep tissues and organs. Their method consisted on breaking down the planar structure of graphene using a mixture of strong acids (sulfuric acid and nitric acid). The carbon dots exhibited a maximum emission wavelength in the range of 460 to 805 nm, depending of the temperature used during the reaction. In general, the reaction temperature was regulated from 65 to 110 °C, where NIR‐emitting nanoparticles were created by using the lower temperature. These nanoparticles were not toxic on MDA‐MB231 cancer cell line, even at high concentrations. They were tested for noninvasive imaging of nude mice. The fluorescence signal was found to be most intense around the heart, liver, spleen, and kidneys at eight hours post‐injection. Due to the biocompatibility, high‐water solubility, and the luminescence stability, the NIR GNPs are potentially attractive probes for high contrast bioimaging. As mentioned before, great effort needs to be done to fabricate QDs emitting in the NIR spectral window. This issue was also afforded by the group of Yang, which reported a photoluminescent polymer–carbon nanodots (PCNDs) emitting in the NIR [124]. The reported PCNDs exhibit a maximum emission centered at 710 nm with a shoulder peak at 665 nm under 540 nm excitation, with a photoluminescence quantum yield of 26.28%. The carbon dots were prepared from dopamine and o‐phenylenediamine since their molecular structures can generate large conjugated sp2 ‐domains. The reaction mixture was heated for eight hours at 200 °C in an autoclave. The cytocompatibility of the PCNDs was evaluated on KB human oral squamous carcinoma cells, demonstrating their low cytotoxicity. Their luminescence stability was also examined by irradiating them with a UV lamp for eight hours, demonstrating their low tendency to photobleach. Live animal imaging on a nude mouse was performed, subcutaneously injecting PCNDs, and a strong fluorescence signal with good signal‐to‐noise ratio in the far‐red region was observed, proving their potential as in vivo bioimaging tags.
Overall, CDs are promising carbon‐based fluorescent tags because they are easily synthetized, have an average small diameter, are soluble in aqueous medium, and have a low tendency to photobleach. However, their emission spans from deep ultraviolet, to visible, to the far‐red/near‐infrared, up to 750 nm, which is not enough to performed deep‐tissue imaging.
5.2.4.5 Carbon Nano‐onions
Multi‐shell fullerenes, known as carbon nano‐onions (CNOs) [24, 125], are structured by concentric shells of sp2 carbon atoms. First discovered by Iijima in 1980 [126], Ugarte reported the in situ transformation of amorphous carbon into onionlike graphitic nanoparticles [24]. These materials can be easily produced in high quantities through several methods, including arc discharge [127], pyrolysis [128, 129], chemical vapor deposition (CVD) [130], and thermal annealing of detonation nanodiamonds (d‐NDs) in vacuum [131] or under inert atmosphere [130, 132]. Their surface can be conveniently functionalized by covalent means through chemical reaction directly with the sp2 carbon atoms present on the material surface [133], or by noncovalent interactions through adsorption of organic molecules by π–π stacking between the outer graphitic layer of the CNO and the aromatic moiety of a bioactive molecule [134]. These well‐established surface modifications (e.g. oxidation [135], cycloaddition [136], fluorination [137], and radical addition of diazonium compounds [33, 138]) allow chemists to create specialized nanoparticles. Of particular interest, due to their average size (5 nm) and potential industrial scalable production, are pristine CNOs prepared by thermal annealing. These nanoparticles don't show an intrinsic fluorescence, compared to others carbon nanoforms mentioned previously; however, they can be fluorescently labeled to obtain imaging nanoprobes. As mentioned before, we have shown that fluorescently labeled CNOs exhibit weak inflammatory potential and a low cytotoxicity [33], they are readily internalized by cancer cells and accumulate in the lysosomes [34, 35] and they are biocompatible, as shown by in vivo studies performed on zebrafish (D. rerio) during development [36]. Therefore, CNOs are attractive CNMs for imaging [139], diagnostic, and therapeutic applications.
Our group has been deeply investigating CNOs as imaging probes. For example, CNOs have been functionalized with fluoresceinamine through a coupling reaction between the amine of the dye molecule and the carboxylic groups on the surface of CNOs, to lead to an amide bond formation on the outer layer of the nano‐onion [140]. These fluorescently labeled nanoprobes were readily internalized by HeLa cells, without significant cytotoxicity. These functionalized CNOs showed bright fluorescence upon cell internalization from the confocal microscopy, demonstrating their potentiality as imaging tools for theranostic applications. One of the first reports on the use of onionlike nanoparticles for imaging applications in the NIR spectral window, was published in 2011 [129]. The authors prepared water‐soluble CNOs from wood waste. Fluorescence emission in the visible and NIR windows were caused by their surface passivation and quantum confinement nature. These CNOs were successfully used for imaging the life cycle of D. melanogaster.
In a recent report from our group, boron difluoride azadipyrromethene fluorophores were covalently attached to carbon nano‐onions (azaBODIPY‐CNO) to produced NIR fluorescent carbon‐based material [18]. The peculiarity of this probe was its pH‐dependent switching (on–off) of the fluorescence, characteristic that was preserved upon cell internalization. In this study, pristine CNOs prepared by thermal annealing were first functionalized with a benzoic acid, and then grafted with the dye molecules though a coupling reaction between the ─COOH functionalities of the CNOs surface and the amino groups of the fluorophores. These azaBODIPY‐CNOs do not exhibit toxicity on HeLa cells, allowing the safe use of these nanoparticle in imaging applications. Moreover, in vitro imaging of HeLa cells was acquired and a strong NIR emission intensity was clearly seen in the cells at pH 4.5, compared to a weak trace NIR emission at pH 8.5 (Figure 5.7a).

Figure 5.7 (a) AFM (left), HRTEM (center), and confocal microscopy (right) images of azaBODIPY‐CNO (red color), internalized in HeLa Kyoto cells in the case of the confocal microscopy image. (b) 3D Laser confocal microscopy z‐stacking image of fluorescent CNOs aggregates deposited on polystyrene, which illustrates the intense red fluorescence of the BODIPY‐functionalized CNOs. Excitation at 647 nm; detection of the fluorescence in a range of 700 ± 35 nm. (See color plate section for the color representation of this figure.)
Source: Reproduced from Ref. [17] with permission from Wiley‐VCH Verlag GmbH & Co. KGaA. Copyright © 2015.
Source: Reproduced from Ref. [18] with permission from The Royal Society of Chemistry.
We also reported on the functionalization of CNOs with a π‐extended distyryl‐substituted boron dipyrromethene (BODIPY) derivative with intense far‐red/near‐infrared fluorescence synthetized by our group [17]. The terminal bromo substituent on the BODIPY molecule allows for the subsequent immobilization of the fluorophore on the surface of carbon nano‐onions, which leads to potential imaging agents for biological and biomedical applications. The fluorophores exhibited a maximum emission centered at 669 nm in DMSO, and a high fluorescence quantum yield of 0.82. The maximum emission of crystalline BODIPY was also calculated, which was bathochromically shifted relative to that of the dye in solution and was centered at 703 nm. Moreover, to investigate the fluorescence of the far‐red/NIR BODIPY‐tagged CNOs, laser confocal microscopy imaging was performed on the bulk material, as show in Figure 5.7b.
Overall, CNOs are promising tools for biological applications, due to their low cytotoxicity and biocompatibility. However, these CNMs lack intrinsic fluorescence, making necessary their surface functionalization with a NIR dye for deep imaging applications. The CNOs surface functionalization with a dye molecule is not necessarily a drawback, as it has been shown that the dye molecule maintains its emission properties when attached to the CNOs surface, and upon cell internalization. Another advantage of CNOs is their potentiality to be covalently and noncovalently surface functionalized also with drugs and specific ligand to obtain targeted drug delivery and imaging systems.
5.2.4.6 Nanodiamonds
NDs consist of sp3 carbon atoms only. NDs made from detonation of explosive compounds were first found in the detonation soot, together with graphitic, nondiamond carbon [26]. NDs have unique optical properties promoted by the presence of fluorescent defect centers. Above all the defect centers giving tunable absorption and emission properties to the NDs, the most common is the nitrogen vacancy, which promotes a fluorescence emission at 576 nm (neutral center, N − V0 ) and 638 nm (negative center, N − V− ) [141, 142]. Overall, NDs exhibit fluorescence in the 500–800 nm range. Therefore, NDs for their photostability, low photobleaching properties [143], intrinsic fluorescence, and high fluorescence quantum yield, ranging from 0.7 to 1 [143, 144], are promising nanomaterials for imaging applications. Furthermore, NDs are highly biocompatible and exhibit low cytotoxicity [143]. They can be covalently or noncovalently surface functionalized without affecting the intrinsic optical properties, allowing their surface modification with targeted molecules or a drug [41]. Because of these advantageous characteristics, NDs have been widely used for in vitro [143] and in vivo whole animal imaging [145, 146]. Intracellular imaging has been performed using NDs. For example, a 3D fluorescence‐based optical sectioning of a single 293T human kidney cell has been performed, confirming the intracellular uptake of fluorescent nanodiamonds (FNDs) powders emitting at ∼700 nm [143]. Interestingly, no sign of photobleaching was found for FND even after eight hours of continuous excitation with the Hg lamp, which can be observed on other fluorophores (e.g. F8801, Molecular Probes). Besides exhibiting a bright fluorescence and a low photobleaching, FND showed low cytotoxicity. Chang and Yu reported on the use of FND emitting at 600–800 nm for long‐term in vivo imaging [147]. FNDs exhibit a fluorescence lifetime greater than 15 ns, allowing the use of fluorescence lifetime imaging microscopy (FLIM), which separates the FND emission from the autofluorescence of cells and tissue. By combining the FLIM technique with the spontaneous labeling of primary cells with FNDs by endocytosis, they were able to track transplanted lung stem/progenitor cells over the course of seven days into mice, with single‐cell resolution. A similar imaging technique was used to obtain a background‐free, real‐time in vivo imaging, exploiting the NV− NDs centers exhibiting a fluorescence lifetime of up to 20 ns [148]. The authors were able to acquire fluorescence images of HeLa cells labeled with FNDs in whole blood covered with a chicken breast of ∼0.1 mm thickness at the single‐cell level. The main idea was to find a noninvasive technique to detect transplanted cells, such as stem cells, in vivo. The use of FNDs to track quiescent cancer stem cells (CSCs) which can cause tumors, has also been studied [149]. Moreover, genotoxicity tests of FNDs on human fibroblasts and breast cancer cells indicate that the nanoparticles neither cause DNA damage nor impair cell growth. Most recent research has studied the development of molecular labeling using NDs of 70 nm with streptavidin conjugation and a standard antibody labeling approach for imaging applications [150]. NDs have been also tested for in vivo fluorescence imaging. The first example from Chang et al. was performed in living Caenorhabditis elegans (C. elegans) [145]. In this study, C. elegans worms were fed with FNDs. It was observed that their localization was mainly in the intestinal cells. In another study, FNDs were tested in a live rat [151], by injecting intraperitoneally 100 nm diamonds over a period of five months. No toxicity was observed on the animal after histopathological analysis of various tissues and organs, indicating the potentiality of NIR‐emitting NDs for long‐term in vivo imaging. Real‐time background‐free selective imaging of C. elegans and mice using FNDs has been proposed, exploiting the unique properties of N‐V center in NDs [146].
Although NDs exhibit a bright fluorescence between 500 and 800 nm, besides having a high fluorescence quantum yield, ranging from 0.7 to 1, and resistance to photobleach, their short emission allows them to be mainly used for in vitro imaging, due to their strong scattering associated with these shorter‐wavelength fluorescence photons. On the positive side, NDs are highly biocompatible and exhibit low cytotoxicity. Moreover, they can be covalently or noncovalently surface functionalized without affecting the intrinsic optical properties, allowing their surface modification with targeted molecules or a drug.
5.3 Conclusions and Outlook
This chapter covered different types of CNMs and their potential in NIR bioimaging application. Broad studies have proven their suitability in biological applications, demonstrating their low toxicity and high biocompatibility both in vitro and in vivo. Most importantly, the peculiar optical characteristics of CNMs (e.g. NIR emission, low photobleaching) make them suitable for real‐time imaging with impressive high resolution, which is hard to achieve using other fluorescently labeled nanomaterials or organic dyes. Above all, semiconducting SWCNTs are the most promising carbon‐based fluorescent agents for live animal imaging. Their emitting properties allow deep‐tissue penetration, permitting live imaging without surgical intervention. Although more studies need to be performed on CNMs to confirm that they are undoubtedly the perfect nanomaterial for theranostic applications, they are promising tools for noninvasive optical imaging, and we envisage a great future in NIR imaging applications.
Acknowledgments
The authors would like to acknowledge the Istituto Italiano di Tecnologia (IIT) for funding and the COST Action CA 15107 (MultiComp).
References
- 1 Balas, C. (2009). Review of biomedical optical imaging‐a powerful, non‐invasive, non‐ionizing technology for improving in vivo diagnosis. Meas. Sci. Technol. 20 (10): 104020.
- 2 Weissleder, R. and Ntziachristos, V. (2003). Shedding light onto live molecular targets. Nat. Med. 9 (1): 123–128.
- 3 Bashkatov, N., Genina, E., Kochubey, V.I., and Tuchin, V.V. (2005). Optical properties of human skin, subcutaneos and mucous tissues in the wavelength range from 400 to 2000 nm. J. Phys. D: Appl. Phys. 38 (15): 2543–2555.
- 4 Friebel, M., Helfmann, J., Netz, U., and Meinke, M. (2009). Influence of oxygen saturation on the optical scattering properties of human red blood cells in the spectral range 250 to 2,000 nm. J. Biomed. Opt. 14 (3): 034001–034006.
- 5 Smith, A.M., Mancini, M.C., and Nie, S. (2009). Bioimaging: second window for in vivo imaging. Nat. Nanotechnol. 4 (11): 710–711.
- 6 Boulnois, J.L. (1986). Photophysical processes in recent medical laser developments: a review. Lasers Med. Sci. 1 (1): 47–66.
- 7 Lazarev, Y.A., Grishkovsky, B.A., and Khromova, T.B. (1985). Amide I band of IR spectrum and structure of collagen and related polypeptides. Biopolymers 24 (8): 1449–1478.
- 8 Parrish, J.A. (1981). New concepts in therapeutic photomedicine; photochemistry, optical targeting and the therapeutic window. J. Invest. Dermatol. 77 (1): 45–50.
- 9 Bardhan, R., Grady, N.K., Cole, J.R. et al. (2009). Fluorescence enhancement by au nanostructures: nanoshells and nanorods. ACS Nano 3 (3): 744–752.
- 10 Kamimura, M., Kanayam,a, N., Tokuzen, K. et al. (2011). Near‐infrared (1550 nm). in vivo bioimaging based on rare‐earth doped ceramic nanophosphors modified with PEG‐b‐poly(4‐vinylbenzylphosphonate). Nanoscale 3 (9): 3705–3713.
- 11 Choi, H.S., Gibbs, S.L., Lee, J.H. et al. (2013). Targeted zwitterionic near‐infrared fluorophores for improved optical imaging. Nat. Biotechnol. 31 (2): 148–153.
- 12 Hyun, H., Henary, M., Gao, T. et al. (2016). 700‐nm Zwitterionic near‐infrared fluorophores for dual‐channel image‐guided surgery. Mol. Imag. Biol. 18 (1): 52–61.
- 13 Hyun, H., Wada, H., Bao, K. et al. (2014). Phosphonated near‐infrared fluorophores for biomedical imaging of bone. Angew. Chem. Int. Ed. 53 (40): 10668–10672.
- 14 Hyun, H., Owens, E.A., Wada, H. et al. (2015). Cartilage‐specific near‐infrared fluorophores for biomedical imaging. Angew. Chem. Int. Ed. 54 (30): 8648–8652.
- 15 Hong, G., Antaris, A.L., and Dai, H. (2017). Near‐infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 1: 0010.
- 16 McDonnell, S.O., Hall, M.J., Allen, L.T. et al. (2015). Supramolecular photonic therapeutic agents. J. Am. Chem. Soc. 127 (47): 16360–16361.
- 17 Bartelmess, J., Baldrighi, M., Nardone, V. et al. (2015). Synthesis and characterization of far‐red/NIR‐fluorescent BODIPY dyes, solid‐state fluorescence, and application as fluorescent tags attached to carbon nano‐onions. Chem. Eur. J. 21: 9727–9732.
- 18 Giordani, S., Bartelmess, J., Frasconi, M. et al. (2014). NIR fluorescence labelled carbon nano‐onions: synthesis, analysis and cellular imaging. J. Mater. Chem. B 2 (42): 7459–7463.
- 19 Kroto, H.W., Heath, J.R., O'Brien, S.C. et al. (1985). C60: buckminsterfullerene. Nature 318: 162.
- 20 Hirsch, A. (2010). The era of carbon allotropes. Nat. Mater. 9 (11): 868–871.
- 21 Iijima, S. (1991). Helical microtubules of graphitic carbon. Nature 354: 56–58.
- 22 Iijima, S. and Ichihashi, T. (1993). Single‐shell carbon nanotubes of 1‐nm diameter. Nature 363: 603–605.
- 23 Karousis, N., Suarez‐Martinez, I., Ewels, C.P., and Tagmatarchis, N. (2016). Structure, properties, functionalization, and applications of carbon nanohorns. Chem. Rev. 116 (8): 4850–4883.
- 24 Ugarte, D. (1992). Curling and closure of graphitic networks under electron‐beam irradiation. Nature 359 (6397): 707–709.
- 25 Novoselov, K.S., Jiang, D., Schedin, F. et al. (2005). Two‐dimensional atomic crystals. Proc. Natl. Acad. Sci. U.S.A 102 (30): 10451–10453.
- 26 Greiner, N.R., Phillips, D.S., Johnson, J.D., and Volk, F. (1988). Diamonds in detonation soot. Nature 333 (6172): 440–442.
- 27 Xu, X., Ray, R., Gu, Y. et al. (2004). Electrophoretic analysis and purification of fluorescent single‐walled carbon nanotube fragments. J. Am. Chem. Soc. 126 (40): 12736–12737.
- 28 Baptista, F.R., Belhout, S.A., Giordani, S., and Quinn, S.J. (2015). Recent developments in carbon nanomaterial sensors. Chem. Soc. Rev. 44 (13): 4433–4453.
- 29 Cellot, G., Cilia, E., Cipollone, S. et al. (2009). Carbon nanotubes might improve neuronal performance by favouring electrical shortcuts. Nat. Nanotechnol. 4 (2): 126–133.
- 30 Bianco, A., Kostarelos, K., and Prato, M. (2011). Making carbon nanotubes biocompatible and biodegradable. Chem. Commun. 47 (37): 10182–10188.
- 31 Movia, D., Prina‐Mello, A., Bazou, D. et al. (2011). Screening the cytotoxicity of single‐walled carbon nanotubes using novel 3D tissue‐mimetic models. ACS Nano 5 (11): 9278–9290.
- 32 Movia, D., Del Canto, E., and Giordani, S. (2010). Purified and oxidized single‐walled carbon nanotubes as robust near‐IR fluorescent probes for molecular imaging. J. Phys. Chem. C 114 (43): 18407–18413.
- 33 Yang, M., Flavin, K., Kopf, I. et al. (2013). Functionalization of carbon nanoparticles modulates inflammatory cell recruitment and NLRP3 inflammasome activation. Small 9 (24): 4194–4206.
- 34 Bartelmess, J., De Luca, E., Signorelli, A. et al. (2014). Boron dipyrromethene (BODIPY). functionalized carbon nano‐onions for high resolution cellular imaging. Nanoscale 6 (22): 13761–13769.
- 35 Frasconi, M., Marotta, R., Markey, L. et al. (2015). Multi‐functionalized carbon nano‐onions as imaging probes for cancer cells. Chem. Eur. J. 21 (52): 19071–19080.
- 36 d'Amora, M., Rodio, M., Bartelmess, J. et al. (2016). Biocompatibility and biodistribution of functionalized carbon nano‐onions ( f‐CNOs ). in a vertebrate model. Sci. Rep. 6 (33923): 1–9.
- 37 Marchesano, V., Ambrosone, A., Bartelmess, J. et al. (2015). Impact of carbon nano‐onions on hydra vulgaris as a model organism for nanoecotoxicology. Nanomaterials 5 (3): 1331–1350.
- 38 Mochalin, V.N., Shenderova, O., Ho, D., and Gogotsi, Y. (2011). The properties and applications of nanodiamonds. Nat. Nanotechnol. 7 (1): 11–23.
- 39 Vaijayanthimala, V. and Chang, H.‐C. (2008). Functionalized fluorescent nanodiamonds for biomedical applications. Nanomedicine 4 (1): 47–55.
- 40 Krueger, A. and Lang, D. (2012). Functionality is key: recent progress in the surface modification of nanodiamond. Adv. Funct. Mater. 22 (5): 890–906.
- 41 Guan, B., Zou, F., and Zhi, J. (2010). Nanodiamond as the pH‐responsive vehicle for an anticancer drug. Small 6 (14): 1514–1519.
- 42 Dyke, C.A. and Tou,r, J.M. (2004). Covalent functionalization of single‐walled carbon nanotubes for materials applications. J. Phys. Chem. A. 108 (51): 11151–11159.
- 43 Georgakilas, V., Kordatos, K., Prato, M. et al. (2002). Organic functionalization of carbon nanotubes. J. Am. Chem. Soc. 124 (5): 760–761.
- 44 Singh, P., Campidelli, S., Giordani, S. et al. (2009). Organic functionalisation and characterisation of single‐walled carbon nanotubes. Chem. Soc. Rev. 38 (8): 2214–2230.
- 45 Hirsch, A. (2002). Functionalization of single‐walled carbon nanotubes. Angew. Chem. Int. Ed. 41 (11): 1853–1859.
- 46 Zhao, Y.‐L. and Stoddart, J.F. (2009). Noncovalent functionalization of single‐walled carbon nanotubes. Acc. Chem. Res. 42 (8): 1161–1171.
- 47 Petrov, P., Stassin, F., Pagnoulle, C., and Jérôme, R. (2003). Noncovalent functionalization of multi‐walled carbon nanotubes by pyrene containing polymers. Chem. Commun. 0 (23): 2904–2905.
- 48 Sapsford, K.E., Algar, W.R., Berti, L. et al. (2013). Functionalizing nanoparticles with biological molecules: developing chemistries that facilitate nanotechnology. Chem. Rev. 113 (3): 1904–2074.
- 49 Krätschmer, W., Lamb, L.D., Fostiropoulos, K., and Huffman, D.R. (1990). Solid C60: a new form of carbon. Nature 347 (6291): 354–358.
- 50 Afreen, S., Muthoosamy, K., Manickam, S., and Hashim, U. (2015). Functionalized fullerene (C60). as a potential nanomediator in the fabrication of highly sensitive biosensors. Biosens. Bioelectron. 63: 354–364.
- 51 Levi, N., Hantgan, R.R., Lively, M.O. et al. (2006). C60‐fullerenes: detection of intracellular photoluminescence and lack of cytotoxic effects. J. Nanobiotechnol. 4 (14): 1–11.
- 52 Aoshima, H., Yamana, S., Nakamura, S., and Mashino, T. (2010). Biological safety of water‐soluble fullerenes evaluated using tests for genotoxicity, phototoxicity, and pro‐oxidant activity. J. Toxicol. Sci. 35 (3): 401–409.
- 53 Capozzi, V., Santoro, M., Celentano, G. et al. (1998). Growth and photoluminescence spectra of C60 single crystals. J. Lumin. 76–77: 395–398.
- 54 Ghiassi, K.B., Olmstead, M.M., and Balch, A.L. (2014). Gadolinium‐containing endohedral fullerenes: structures and function as magnetic resonance imaging (MRI). agents. Dalton Trans. 43 (20): 7346–7358.
- 55 Karam, L.R., Mitch, M.G., and Coursey, B.M. (1997). Encapsulation of 99m Tc within fullerenes: a novel radionuclidic carrier. Appl. Radiat. Isot. 48 (6): 771–776.
- 56 Jeong, J., Cho, M., Lim, Y.T. et al. (2009). Synthesis and characterization of a photoluminescent nanoparticle based on fullerene‐silica hybridization. Angew. Chem. Int. Ed. 48 (29): 5296–5299.
- 57 Jeong, J., Jung, J., Choi, M. et al. (2012). Color‐tunable photoluminescent fullerene nanoparticles. Adv. Mater. 24 (15): 1999–2003.
- 58 Xiao, Z., Ye, G., Liu, Y. et al. (2012). Pushing fullerene absorption into the near‐IR region by conjugately fusing oligothiophenes. Angew. Chem. Int. Ed. 51 (36): 9038–9041.
- 59 Monthioux, M. and Kuznetsov, V.L. (2006). Who should be given the credit for the discovery of carbon nanotubes? Carbon 44 (9): 1621–1623.
- 60 Bachilo, S.M., Strano, M.S., Kittrell, C. et al. (2002). Structure‐assigned optical spectra of single‐walled carbon nanotubes. Science 298 (5602): 2361–2366.
- 61 Ruoff, R.S. and Lorents, D.C. (1995). Mechanical and thermal properties of carbon nanotubes. Carbon 33 (7): 925–930.
- 62 Odom, T.W., Huang, J.‐L., Kim, P., and Lieber, C.M. (2000). Structure and electronic properties of carbon nanotubes. J. Phys. Chem. B 104 (13): 2794–2809.
- 63 O'Connell, M.J., Bachilo, S.M., Huffman, C.B. et al. (2002). Band gap fluorescence from individual single‐walled carbon nanotubes. Science 297 (5581): 593–596.
- 64 Zhou, F., Xing, D., Ou, Z. et al. (2014). Cancer photothermal therapy in the near‐infrared region by using single‐walled carbon nanotubes. J. Biomed. Opt. 14 (2): 21009.
- 65 Moon, H.K., Lee, S.H., and Choi, H.C. (2009). In vivo near‐infrared mediated tumor destruction by photothermal effect of carbon nanotubes. ACS Nano 3 (11): 3707–3713.
- 66 Welsher, K., Liu, Z., Daranciang, D., and Dai, H. (2008). Selective probing and imaging of cells with single walled carbon nanotubes as near‐infrared fluorescent molecules. Nano Lett. 8 (2): 586–590.
- 67 Cherukuri, P., Gannon, C.J., Leeuw, T.K. et al. (2006). Mammalian pharmacokinetics of carbon nanotubes using intrinsic near‐infrared fluorescence. Proc. Natl. Acad. Sci. U.S.A 103 (50): 18882–18886.
- 68 Flavin, K., Lawrence, K., Bartelmess, J. et al. (2011). Synthesis and characterization of boron azadipyrromethene single‐wall carbon nanotube electron donor‐acceptor conjugates. ACS Nano 5 (2): 1198–1206.
- 69 Flavin, K., Kopf, I., Murtagh, J. et al. (2012). Excited state on/off switching of a boron azadipyrromethene single‐wall carbon nanotube conjugate. Supramol. Chem. 24: 23–28.
- 70 Avouris, P., Freitag, M., and Perebeinos, V. (2008). Carbon‐nanotube photonics and optoelectronics. Nat. Photonics 2 (6): 341–350.
- 71 Crochet, J., Clemens, M., and Hertel, T. (2007). Quantum yield heterogeneities of aqueous single‐wall carbon nanotube suspensions. J. Am. Chem. Soc. 129 (26): 8058–8059.
- 72 Liu, Z., Tabakman, S., Welsher, K., and Dai, H. (2009). Carbon nanotubes in biology and medicine: in vitro and in vivo detection, imaging and drug delivery. Nano Res. 2 (2): 85–120.
- 73 Lee, A.J., Wang, X., Carlson, L.J. et al. (2011). Bright fluorescence from individual single‐walled carbon nanotubes. Nano Lett. 11 (4): 1636–1640.
- 74 Kim, S.‐J., Park, J., Jeong, Y. et al. (2014). Metal‐particle‐induced enhancement of the photoluminescence from biomolecule‐functionalized carbon nanotubes. Nanoscale Res. Lett. 9 (1): 85.
- 75 Ju, S., Kopcha, W.P., and Papadimitrakopoulos, F. (2009). Brightly fluorescent single‐walled carbon nanotubes via an oxygen‐excluding surfactant organization. Science 323 (5919): 1319–1323.
- 76 Qin, S., Qin, D., Ford, W.T. et al. (2004). Grafting of poly(4‐vinylpyridine). to single‐walled carbon nanotubes and assembly of multilayer films. Macromolecules 37 (26): 9963–9967.
- 77 Ghosh, S., Bachilo, S.M., Simonette, R.A. et al. (2010). Oxygen doping modifies near‐infrared band gaps in fluorescent single‐walled carbon nanotubes. Science 330 (6011): 1656–1659.
- 78 Ghosh, S., Bachilo, S.M., and Weisman, R.B. (2010). Advanced sorting of single‐walled carbon nanotubes by nonlinear density‐gradient ultracentrifugation. Nat. Nanotechnol. 5 (6): 443–450.
- 79 Chiu, C.F., Saidi, W.A., Kagan, V.E., and Star, A. (2017). Defect‐induced near‐infrared photoluminescence of single‐walled carbon nanotubes treated with polyunsaturated fatty acids. J. Am. Chem. Soc. 139 (13): 4859–4865.
- 80 Robinson, J.T., Hong, G., Liang, Y. et al. (2012). In vivo fluorescence imaging in the second near‐infrared window with long circulating carbon nanotubes capable of ultrahigh tumor uptake. J. Am. Chem. Soc. 134 (25): 10664–10669.
- 81 Maeda, H., Wu, J., Sawa, T. et al. (2000). Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Controlled Release 65: 271–284.
- 82 Cherukuri, P., Bachilo, S.M., Litovsky, S.H., and Weisman, R.B. (2004). Near‐infrared fluorescence microscopy of single‐walled carbon nanotubes in phagocytic cells. J. Am. Chem. Soc. 126 (48): 15638–15639.
- 83 Welsher, K., Liu, Z., Sherlock, S.P. et al. (2009). A route to brightly fluorescent carbon nanotubes for near‐infrared imaging in mice. Nat. Nanotechnol. 4 (11): 773–780.
- 84 Hong, G., Tabakman, S.M., Welsher, K. et al. (2011). Near‐infrared‐fluorescence‐enhanced molecular imaging of live cells on gold substrates. Angew. Chem. Int. Ed. 50 (20): 4644–4648.
- 85 Kam, N.W.S. and Dai, H. (2005). Carbon nanotubes as intracellular protein transporters: generality and biological functionality. J. Am. Chem. Soc. 127 (16): 6021–6026.
- 86 Kam, N.W.S., Liu, Z., and Dai, H. (2006). Carbon nanotubes as intracellular transporters for proteins and DNA: an investigation of the uptake mechanism and pathway. Angew. Chem. Int. Ed. 45 (4): 577–581.
- 87 Feazell, R.P., Nakayama‐Ratchford, N., Dai, H., and Lippard, S.J. (2007). Soluble single‐walled carbon nanotubes as longboat delivery systems for platinum(IV). anticancer drug design. J. Am. Chem. Soc. 129 (27): 8438–8439.
- 88 Jin, H., Heller, D.A., and Strano, M.S. (2008). Single‐particle tracking of endocytosis and exocytosis of single‐walled carbon nanotubes in NIH‐3T3 cells. Nano Lett. 8 (6): 1577–1585.
- 89 Fakhri, N., Wessel, A.D., Willms, C. et al. (2014). High‐resolution mapping of intracellular fluctuations using carbon nanotubes. Science 344 (6187): 1031–1035.
- 90 Jena, P.V., Shamay, Y., Shah, J. et al. (2016). Photoluminescent carbon nanotubes interrogate the permeability of multicellular tumor spheroids. Carbon 97: 99–109.
- 91 Brown, E.B., Campbell, R.B., Tsuzuki, Y. et al. (2001). In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nat. Med. 7 (7): 864–868.
- 92 Ghosh, D., Bagley, A.F., Na, Y.J. et al. (2014). Deep, noninvasive imaging and surgical guidance of submillimeter tumors using targeted M13‐stabilized single‐walled carbon nanotubes. Proc. Natl. Acad. Sci. U.S.A. 111: 13948–13953.
- 93 Hong, G., Diao, S., Chang, J. et al. (2014). Through‐skull fluorescence imaging of the brain in a new near‐infrared window. Nat. Photonics 8 (9): 723–730.
- 94 Leeuw, T.K., Michelle Reith, R., Simonette, R.A. et al. (2007). Single‐walled carbon nanotubes in the intact organism: near‐IR imaging and biocompatibility studies in drosophila. Nano Lett. 7 (9): 2650–2654.
- 95 Harvey, J.D., Jena, P.V., Baker, H.A. et al. (2017). A carbon nanotube reporter of microRNA hybridization events in vivo. Nat. Biomed. Eng. 1: 41.
- 96 Lotya, M., Hernandez, Y., King, P.J. et al. (2009). Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J. Am. Chem. Soc. 131 (10): 3611–3620.
- 97 Kosynkin, D.V., Higginbotham, A.L., Sinitskii, A. et al. (2009). Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 458 (7240): 872–876.
- 98 Kim, K.S., Zhao, Y., Jang, H. et al. (2009). Large‐scale pattern growth of graphene films for stretchable transparent electrodes. Nature 457 (7230): 706–710.
- 99 Cai, J., Ruffieux, P., Jaafar, R. et al. (2010). Atomically precise bottom‐up fabrication of graphene nanoribbons. Nature 466 (7305): 470–473.
- 100 Mao, H.Y., Laurent, S., Chen, W. et al. (2013). Graphene: promises, facts, opportunities, and challenges in nanomedicine. Chem. Rev. 113: 3407–3424.
- 101 Cushing, S.K., Li, M., Huang, F., and Wu, N. (2014). Origin of strong excitation wavelength dependent fluorescence of graphene oxide. ACS Nano 8 (1): 1002–1013.
- 102 Brodie, B.C. (1859). On the atomic weight of graphite. Philos. Trans. R Soc. London 149 (9): 249–259.
- 103 Hummers, W.S. and Offeman, R.E. (1958). Preparation of graphitic oxide. J. Am. Chem. Soc. 80 (6): 1339–1339.
- 104 Shang, J., Ma, L., Li, J. et al. (2012). The origin of fluorescence from graphene oxide. Sci. Rep. 2: 792.
- 105 Sun, X., Liu, Z., Welsher, K. et al. (2008). Nano‐graphene oxide for cellular imaging and drug delivery. Nano Res. 1 (3): 203–212.
- 106 Qian, J., Wang, D., Cai, F.H. et al. (2012). Observation of multiphoton‐induced fluorescence from graphene oxide nanoparticles and applications in vivo functional bioimaging. Angew. Chem. Int. Ed. 51 (42): 10570–10575.
- 107 Robinson, J.T., Tabakman, S.M., Liang, Y. et al. (2011). Ultrasmall reduced graphene oxide with high near‐infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 133 (17): 6825–6831.
- 108 Baker, S.N. and Baker, G.A. (2010). Luminescent carbon nanodots: emergent nanolights. Angew. Chem. Int. Ed. 49: 6726–6744.
- 109 Wang, Y. and Hu, A. (2014). Carbon quantum dots: synthesis, properties and applications. J. Mater. Chem. C 2 (34): 6921–6939.
- 110 Sun, Y., Zhou, B., Lin, Y. et al. (2006). Quantum‐sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 128 (24): 7756–7757.
- 111 Hu, S.‐L., Niu, K.‐Y., Sun, J. et al. (2009). One‐step synthesis of fluorescent carbon nanoparticles by laser irradiation. J. Mater. Chem. 19 (4): 484.
- 112 Tao, H., Yang, K., Ma, Z. et al. (2012). In vivo NIR fluorescence imaging, biodistribution, and toxicology of photoluminescent carbon dots produced from carbon nanotubes and graphite. Small 8 (2): 281–290.
- 113 Pan, D., Zhang, J., Li, Z., and Wu, M. (2010). Hydrothermal route for cutting graphene sheets into blue‐luminescent graphene quantum dots. Adv. Mater. 22 (6): 734–738.
- 114 Zhao, Q.L., Zhang, Z.L., Huang, B.H. et al. (2008). Facile preparation of low cytotoxicity fluorescent carbon nanocrystals by electrooxidation of graphite. Chem. Commun. 0 (41): 5116–5118.
- 115 Park, S.Y., Lee, H.U., Park, E.S. et al. (2014). Photoluminescent green carbon nanodots from food‐waste‐derived sources: large‐scale synthesis, properties, and biomedical applications. ACS Appl. Mater. Interfaces 6 (5): 3365–3370.
- 116 Liu, C., Zhang, P., Tian, F. et al. (2011). One‐step synthesis of surface passivated carbon nanodots by microwave assisted pyrolysis for enhanced multicolor photoluminescence and bioimaging. J. Mater. Chem. 21 (35): 13163–13167.
- 117 Ponomarenko, L.A., Schedin, F., Katsnelson, M.I. et al. (2008). Chaotic Dirac billiard in graphene quantum dots. Science 320 (5874): 356–358.
- 118 Tang, L., Ji, R., Li, X. et al. (2014). Deep ultraviolet to near‐infrared emission and photoresponse in layered n‐doped graphene quantum dots. ACS Nano 8 (6): 6312–6320.
- 119 Lemenager, G., De Luca, E., Sun, Y.‐P., and Pompa, P.P. (2014). Super‐resolution fluorescence imaging of biocompatible carbon dots. Nanoscale 6 (15): 8617–8623.
- 120 Song, Y., Zhu, S., and Yang, B. (2014). Bioimaging based on fluorescent carbon dots. RSC Adv. 4 (52): 27184–27200.
- 121 Feng, L.L., Wu, Y.X., Zhang, D.L. et al. (2017). Near infrared graphene quantum dots‐based two‐photon nanoprobe for direct bioimaging of endogenous ascorbic acid in living cells. Anal. Chem. 89 (7): 4077–4084.
- 122 Kumawat, M.K., Srivastava, R., Thakur, M., and Gurung, R.B. (2017). Graphene quantum dots from mangifera indica: application in near‐infrared bioimaging and intracellular nanothermometry. ACS Sustainable Chem. Eng. 5 (2): 1382–1391.
- 123 Nurunnabi, M., Khatun, Z., Reeck, G.R. et al. (2013). Near infrared photoluminescent graphene nanoparticles greatly expand their use in noninvasive biomedical imaging. Chem. Commun. 49 (44): 5079–5081.
- 124 Lu, S., Sui, L., Liu, J. et al. (2017). Near‐infrared photoluminescent polymer–carbon nanodots with two‐photon fluorescence. Adv. Mater. 29 (15): 1603443.
- 125 Ugarte, D. (1995). Onion‐like graphitic particles. Carbon 33 (7): 989–993.
- 126 Iijima, S. (1980). Direct observation of the tetrahedral bonding in graphitized carbon black by high resolution electron microscopy. J. Cryst. Growth 50 (3): 675–683.
- 127 Sano, N., Wang, H., Chhowalla, M. et al. (2001). Synthesis of carbon “onions” in water. Nature 414 (6863): 506–507.
- 128 Sonkar, S.K., Roy, M., Babar, D.G., and Sarkar, S. (2012). Water soluble carbon nano‐onions from wood wool as growth promoters for gram plants. Nanoscale 4 (24): 7670.
- 129 Ghosh, M., Sonkar, S.K., Saxena, M., and Sarkar, S. (2011). Carbon nano‐onions for imaging the life cycle of Drosophila melanogaster. Small 7 (22): 3170–3177.
- 130 Chen, X.H., Deng, F.M., Wang, J.X. et al. (2001). New method of carbon onion growth by radio‐frequency plasma‐enhanced chemical vapor deposition. Chem. Phys. Lett. 336 (3–4): 201–204.
- 131 Kuznetsov, V.L., Chuvilin, A.L., Butenko, Y.V. et al. (1994). Onion‐like carbon from ultra‐disperse diamond. Chem. Phys. Lett. 222 (4): 343–348.
- 132 Palkar, A., Melin, F., Cardona, C.M. et al. (2007). Reactivity differences between carbon nano onions (CNOs). prepared by different methods. Chem. Asian J. 2 (5): 625–633.
- 133 Bartelmess, J. and Giordani, S. (2014). Carbon nano‐onions (multi‐layer fullerenes): chemistry and applications. Beilstein J. Nanotechnol. 5: 1980–1998.
- 134 Bartelmess, J., Frasconi, M., Balakrishnan, P.B. et al. (2015). Non‐covalent functionalization of carbon nano‐onions with pyrene–BODIPY dyads for biological imaging. RSC Adv. 5 (62): 50253–50258.
- 135 Flavin, K., Kopf, I., Del Canto, E. et al. (2011). Controlled carboxylic acid introduction: a route to highly purified oxidised single‐walled carbon nanotubes. J. Mater. Chem. 21 (44): 17881–17887.
- 136 Georgakilas, V., Guldi, D.M., Signorini, R. et al. (2003). Organic functionalization and optical properties of carbon onions. J. Am. Chem. Soc. 125 (47): 14268–14269.
- 137 Liu, Y., Vander Wal, R.L., and Khabashesku, V.N. (2007). Functionalization of carbon nano‐onions by direct fluorination. Chem. Mater. 19 (4): 778–786.
- 138 Flavin, K., Chaur, M.N., Echegoyen, L., and Giordani, S. (2010). Functionalization of multilayer fullerenes (carbon nano‐onions). using diazonium compounds and “click” chemistry. Org. Lett. 12 (4): 840–843.
- 139 Bartelmess, J., Quinn, S.J., and Giordani, S. (2014). Carbon nanomaterials : multi‐functional agents for biomedical fluorescence and Raman imaging. Chem. Soc. Rev. 44: 4672–4698.
- 140 Frasconi, M., Maffeis, V., Bartelmess, J. et al. (2015). Highly surface functionalized carbon nano‐onions for bright light bioimaging. Methods Appl. Fluoresc. 3 (4): 44005.
- 141 Fu, C.‐C., Lee, H.‐Y., Chen, K. et al. (2007). Characterization and application of single fluorescent nanodiamonds as cellular biomarkers. Proc. Natl. Acad. Sci. U.S.A 104 (3): 727–732.
- 142 Higbie, J.M., Perreault, J.D., Acosta, V.M. et al. (2017). Multiphoton‐excited fluorescence of silicon‐vacancy color centers in diamond. Phys. Rev. Appl. 7 (5): 054010.
- 143 Yu, S.J., Kang, M.W., Chang, H.C. et al. (2005). Bright fluorescent nanodiamonds: no photobleaching and low cytotoxicity. J. Am. Chem. Soc. 127 (50): 17604–17605.
- 144 Gaebel, T., Popa, I., Gruber, A. et al. (2004). Stable single‐photon source in the near infrared. New J. Phys. 6: 1–7.
- 145 Mohan, N., Chen, C.S., Hsieh, H.H. et al. (2010). In vivo imaging and toxicity assessments of fluorescent nanodiamonds in caenorhabditis elegans. Nano Lett. 10 (9): 3692–3699.
- 146 Igarashi, R., Yoshinari, Y., Yokota, H. et al. (2012). Real‐time background‐free selective imaging of fluorescent nanodiamonds in vivo. Nano Lett. 12 (11): 5726–5732.
- 147 Wu, T.‐J., Tzeng, Y.‐K., Chang, W.‐W. et al. (2013). Tracking the engraftment and regenerative capabilities of transplanted lung stem cells using fluorescent nanodiamonds. Nat. Nanotechnol. 8 (9): 682–689.
- 148 Hui, Y.Y., Su, L.‐J., Chen, O.Y. et al. (2014). Wide‐field imaging and flow cytometric analysis of cancer cells in blood by fluorescent nanodiamond labeling and time gating. Sci. Rep. 4: 5574.
- 149 Lin, H.H., Lee, H.W., Lin, R.J. et al. (2015). Tracking and finding slow‐proliferating/quiescent cancer stem cells with fluorescent nanodiamonds. Small 11 (34): 4394–4402.
- 150 Hemelaar, S.R., de Boer, P., Chipaux, M. et al. (2017). Nanodiamonds as multi‐purpose labels for microscopy. Sci. Rep. 7 (1): 720.
- 151 Vaijayanthimala, V., Cheng, P.Y., Yeh, S.H. et al. (2012). The long‐term stability and biocompatibility of fluorescent nanodiamond as an in vivo contrast agent. Biomaterials 33 (31): 7794–7802.
