12
Carbon Nanomaterials for Photothermal Therapies
Jiantao Yu1, Lingyan Yang2, Junyan Yan3, Wen‐Cheng Wang4, Yi‐Chun Chen5, Hung‐Hsiang Chen5, and Chia‐Hua Lin5
1 School of Advanced Materials, Peking University Shenzen Graduate School, Peking University, Shenzhen, China
2 CAS Key Laboratory of Nano‐Bio Interface, Suzhou Institute of Nano‐Tech and Nano‐Bionics, Chinese Academy of Sciences, Suzhou, China
3 Jiangsu Provincial Key Laboratory of Parasite Molecular Biology, Jiangsu Institute of Parasitic Diseases, Wuxi, China
4 Research Center for Environmental Changes, Academia Sinica, Taipei, Taiwan
5 Department of Biotechnology, National Formosa University, Yunlin, Taiwan
12.1 Introduction
Photothermal cancer therapy, also called photothermal therapy (PTT), uses light‐induced heat to treat cancer cells. PTT has attracted attention owing to its advantages over traditional cancer therapies such as chemotherapy, radiation therapy (RT), and surgery. Thermal therapy was discovered during the nineteenth century when cancer patients were administered living bacteria to cause inflammation and subsequent fever. The use of circulating heated water to treat uterine cervical cancer was also reported during that era. Owing to the rudimentary technology of that age, neither clinical applications nor further studies of thermal therapy were explored. Interest in PTT was revived in the 1980s, and owing to the fast development of optical and nanomaterial technology during recent decades, thermal therapy, particularly PTT has become a fast‐growing field of study [1–5].
Unlike traditional thermal therapies, PTT is a relatively mild cancer therapy that primarily uses a hyperthermia treatment administered at temperatures of 41 °C–48 °C [6]. Several cellular reactions take place within this temperature range, including (i) protein denaturation and aggregation, which destroy cellular homeostasis and lower cell survival rates; (ii) cell inactivation for several hours or more at temperatures above 42 °C but below 45 °C, which can increase oxidative stress and subsequently damage proteins, lipids, and nucleic acids, after which cells undergo apoptosis or secondary necrosis processes; and (iii) rapid cellular necrosis at temperatures above 45 °C. Although, as noted by Fuente [7], direct necrosis initiated by hyperthermia can cause bodily inflammation, which should be avoided in the clinical treatment of cancer; useful applications of hyperthermia therapy could induce cell death via cellular apoptosis and secondary necrosis, which do not cause an inflammatory response.
Another factor that limits the clinical application of PTT is the lack of effective light for thermal triggering owing to the intrinsic absorbance by water and hemoglobin and the light‐scattering effect of body tissue [6]. These tissue properties result in the poor penetration of visible light at the tumor site, thereby limiting heat triggering. This effect can be illustrated with the spectrum shown in Figure 12.1.
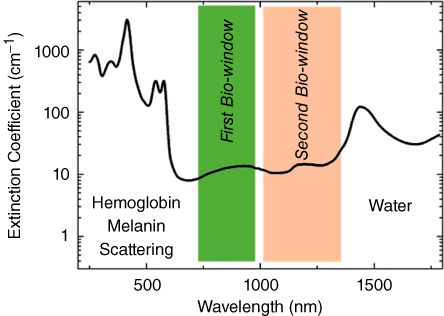
Figure 12.1 Extinction coefficient of a representative tissue [6].
Because of light‐wave extinction by tissue, visible light is unsuitable for PTT applications. However, intrinsic tissue absorption and scattering of light is relatively lower between the wavelengths of 700 and 1400 nm. Light in this region can be divided into two parts: (i) a first bio‐window of 700–980 nm, in which the absorbance by water significantly vanishes but residual scattering persists; and (ii) a second bio‐window of 1000–1400 nm, in which absorbance does not completely vanish but scattering is minimized. Most light‐triggered therapies use lasers within the bio‐windows to achieve deeper tissue penetration and avoid the side effects of normal tissue heating.
To achieve precise heating within the tumor region and avoid the heating of noncancerous tissue, PTT must deliver an efficient thermal reagent to the tumor site that can be triggered by a bio‐window laser to produce heat. Rapid developments in nanotechnology have allowed the study of dozens of nanomaterials, including metallic and semiconducting materials [4, 8], organic polymers [9], carbon nanomaterials [10], and quantum dots (QDs) [11] for both in vitro and in vivo PTT. Carbon nanomaterials are good choices for photothermal reagents because they have (i) relatively higher absorbance in the two bio‐windows described above, (ii) good biocompatibility, (iii) easily modified and functionalized surfaces, and (iv) good dispersibility in bio‐liquids, particularly blood.
Carbon nanomaterials include carbon nanodiamonds and graphite structural nanomaterials. Carbon nanodiamonds have not yet proved efficient for photothermal conversion. However, graphite structural nanomaterials, including graphene oxide (GO) [12], carbon nanotubes (CNTs) [13], carbon nanohorns (CNHs) [14], carbon dots (CDs) [15], graphene dots (GDs) [16], and fullerenes [17] have been widely studied for photothermal applications. In graphite structural nanomaterials, a π‐plasmon absorbance background at the bio‐windows and relatively lower luminance are the mechanisms for good light‐to‐heat conversion efficiency. Therefore, these materials are efficient photothermal reagents. Oxygen‐containing functional groups (particularly carboxyl) produced during synthesis impart hydrophilic and other easily modified properties to graphite structural nanomaterials. Moreover, the stability of their carbon structures and good solubility in bio‐fluids, which aid their ultimate elimination from the body, give graphite structural nanomaterials good biocompatibility.
Liu studied nano‐sized GO (nGO) for in vivo PTT in animals and observed that 24 hours after tail vein injection, nGO accumulated at tumor sites owing to enhanced permeability and retention (EPR), whereas little accumulation occurred in other organs with the exception of the kidneys [12]. In addition to GO, other graphite structural nanomaterials, particularly CNTs, have been studied for applications in PTT [18].
Combinations of carbon nanomaterials with reagents and other nanomaterials for magnetic resonance imaging [19], computed tomography [20], or photoacoustic or Raman imaging [21, 22] have also been studied for imaging‐guided PTT, which is also called theranostics. Because the surfaces of carbon nanomaterials can be easily modified, derived drug loading and biomolecular functionalization are avenues for further combination therapy with chemotherapy, photodynamic therapy, RT, gene therapy, immune therapy, and other modalities.
Given the diversity of carbon nanomaterials, the compatibility of these materials for combination therapy with PTT, and the large number of studies aimed at developing effective photothermal applications for carbon nanomaterials, the aim of this chapter is to provide an overview of the carbon nanomaterials useful for PTT and their current PTT applications. The following topics are covered:
- GO for PTT
- CNTs and CNHs for PTT
- CDs and GDs for PTT
- Fullerenes for PTT
- Carbon nanomaterial‐based nanocomposites for PTT
- Carbon nanomaterial‐based combined therapy with PTT
12.2 GO for PTT
GO, a two‐dimensional carbon nanomaterial, has been studied as a nanomedicine for biomedical applications. As mentioned in the introduction, extinction at the near‐infrared (NIR) windows limits the photothermal applications of nanomaterials. However, GO is an effective PTT reagent owing to its intrinsic absorption in the NIR region, efficient cellular uptake, and easy preparation. Because GO is easily modified, a variety of GO‐based nanomaterials have been developed for cancer and theranostic therapies.
12.2.1 PTT‐Related Physical and Chemical Properties of GO
The aromatic structure of GO suggests that strong optical absorbance in the NIR region will result in NIR light‐triggered photothermal conversion. However, because carbon atoms in GO nanosheets are partially oxidized to sp3 carbon atoms during synthesis, its absorbance is likely to be weaker than that of grapheme [23, 24]. According to Yang et al., the reduction of GO increases NIR region absorbance three‐ to fourfold and significantly improves photothermal conversion (Figure 12.2) [25].

Figure 12.2 (a) Structural illustration of GO, which is related to the photothermal properties (oxygen‐containing groups, especially carboxyl group, are related to the dispersibility; aromatic structure is related to the intrinsic absorbance in the NIR region) and (b) NIR absorption of GO (B) [25].
Furthermore, the carboxyl groups generated on GO during synthesis impart hydrophilic properties, which are vital in biomedical applications. However, the reduction process substantially consumes carboxyl groups, which reduces the dispersibility of reduced GO (rGO). Therefore, additional surface modifications have been investigated to improve the dispersibility of rGO. For example, polyethylene glycol (PEG)‐grafted poly(maleic anhydride‐alt‐1‐octadecene) is directly grafted to the GO surface to improve the dispersibility of rGO, and dopamine is used to reduce GO via coating on the rGO surface to prevent rGO aggregation [9, 26].
12.2.2 GO for in vitro PTT
To assess the in vitro effects of various photothermal reagents, researchers have constructed temperature increase curves by directly measuring the temperatures generated under continuous laser irradiation. Photothermal conversion efficiency is primarily calculated according to the method reported by Roper et al. [27].
Because it has excellent photothermal conversion efficiency, GO and functionalized GO have been widely investigated for in vitro PTT applications. PEG is usually used to improve the dispersibility and stability of GO in the physiological environment [25, 28]. GO easily enters cells and locates in the cytoplasm; however, cellular uptake efficiency can be improved through the use of a positively charged polymer such as polyethyleneimine (PEI) [29–31]. PEI reverses the surface charge of GO, which improves interactions with cell membranes. Fluorescent dyes such as rhodamine B [32] are used to modify the GO surface so that it can be traced for intracellular behavior studies.
Initially, researchers tried to reduce GO to improve its photothermal conversion efficiency. The partially rGO partly removed the oxygen‐containing groups, thereby significantly improving NIR absorbance and photothermal conversion efficiency. Another way to improve photothermal conversion efficiency is to combine GO with other photothermal reagents. For example, an NIR cyanine dye was used to enhance the absorbance of GO and thereby increase photothermal conversion efficiency [33]. Because the interaction between GO and the cyanine dye is sensitive to ambient pH, the absorbance enhancement is dependent on pH; therefore, the GO–cyanine dye system has been designed for pH‐triggered photothermal enhancement, which is accompanied by a pH detection function (Figure 12.3) [22].
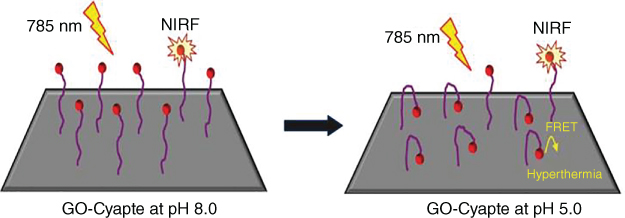
Figure 12.3 pH‐mediated cypate‐enhanced photothermal effect of GO [22].
As illustrated above, significant efforts have been made to improve the photothermal performance of GO. We believe that a good photothermal reagent should have the following properties:
- High NIR absorbance
- High photothermal conversion efficiency
- Good stability in the biotic environment
- Efficient cellular uptake
- Good biocompatibility
In most PTT‐related studies, the 3‐(4, 5‐dimethylthiazol‐2‐yl) 2, 5‐diphenyl tetrazolium bromide (MTT) assay is used to assess the cytotoxicity of photothermal reagents and evaluate biocompatibility [22, 32–34]. Some of these studies have evaluated the toxicity of photothermal reagents, including carbon nanomaterials [35–37]. PEG, chitosan, bovine serum albumin, poly(acrylic acid), and polydopamine (PDA) have been used to modify GO to reduce its cytotoxicity [25, 26, 38–40]. According to a study by Cheng, biopolymers including PDA‐, bovine serum albumin‐, and heparin‐grafted GO show ultralow cytotoxicity even at concentrations of 100 μg ml−1 .
The NIR light used for in vitro studies of carbon nanomaterial‐based PTT varies from less than 0.5 W cm−2 to more than 10 W cm−2 . However, the maximum permissible exposure to lasers in NIR‐1 is 0.33 W cm−2 (808 nm) and that for lasers in NIR‐2 is 1 W cm−2 (1000–1100 nm) (ANSI Z136.1.2007, American National Standard for Safe Use of Lasers). Therefore, researchers have attempted to improve photothermal conversion efficiency to decrease the laser intensity required for PTT. Yang et al. reported that the use of NIR‐808 nm at a laser intensity of 0.15 W cm−2 is effective for in vitro and in vivo photothermal cancer therapy [25].
For evaluating in vitro cancer cell cytotoxicity, the MTT assay is most widely used for quantified assessments, and fluorescence staining is most often used for qualitative evaluations. Surface modification technology is used to increase cell uptake of nanomaterials. For example, cancer cell‐specific aptamers, permeable peptides, folic acid, biotin, and other molecules have been used to modify carbon nanomaterials for this purpose [32, 41–43].
Cytotoxicity studies examine both cell death caused by photothermal effects and cell responses to PTT. Yang et al. showed that for GO, photothermal effects kill cells via apoptosis and the cell response to hypothermal damage is the overexpression of heat shock proteins (HSPs) to maintain homeostasis [32]. Two HSP family members, HSP90 and HSP70, are overexpressed during photothermal treatment (Figure 12.4). However, this HSP overexpression cannot completely mitigate the thermal damage and the decompensatory stage occurs nonetheless. Therefore, cells undergo apoptosis, which may result in secondary necrosis that is expected to be beneficial for clinical PTT applications because it causes the least inflammation [7].

Figure 12.4 HSPs expression profile of MCF‐7 cancer cells after NIR illumination treatment [32].
12.2.3 GO for in vivo PTT
For in vivo applications, GO must be protected from aggregation in the circulatory system. PEGylation is the most widely used GO modification for achieving good stability. Multi‐armed PEG has also been used to enhance stability [25].
Solid tumors under the skin are the most frequently used in vivo models for studies of PTT with GO. Both intratumor and intravenous injections have been used to expose tumors to nanomaterials. NIR illumination is performed after a large amount of nanomaterial aggregates in the tumor via the EPR effect. Although studies have used various nanomaterial concentrations and NIR intensities, most have achieved successful tumor ablation or inhibition. Yang et al. modified a nanographene sheet (NGS) with PEG and conjugated it to an NIR dye to study its in vivo behavior in mice [44]. After intravenous injection, NGS was maintained primarily in the tumor; the amount of NGS stranded in reticuloendothelial systems, including the spleen and liver, was much lower. After subsequent NIR irradiation, the tumors were easily eliminated, and hematoxylin and eosin staining of tissue slides, which is used in many PTT studies, showed no organ damage two days after the disappearance of the tumor (Figure 12.5).
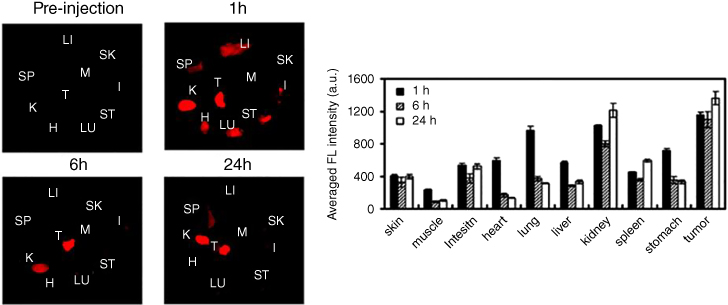
Figure 12.5 Semiquantitative biodistribution analysis of NGS‐PEG‐Cy7 in 4T1 tumor‐bearing mice. (a) Spectrally resolved ex vivo fluorescence images of organs before and after injection of NGS‐PEG‐Cy7. SK: skin, M: muscle, I: intestine, H: heart, LU: lung, LI: liver, K: kidney, SP: spleen, ST: stomach, and T: tumor. (b) Semiquantitative biodistribution of NGS‐PEG‐Cy7 in mice determined by the averaged fluorescence intensity of each organ [44]. (See color plate section for the color representation of this figure.)
12.3 CNTs and CNHs for PTT
CNTs have optical absorbance in the NIR region. Unlike GO, however, they have a long, hollow architecture prepared by rolling graphene. CNTs can be either single‐walled (SWCNTs) or multi‐walled (MWCNTs), and each type has different properties and specific photothermal effects. Owing to their needle‐like structures, CNTs are easily taken up by cells. CNHs are hollow tubules of single‐walled grapheme tubules, which are similar to CNTs and possess short and conically shaped structures [14]. CNHs are capable of absorbing NIR light, leading to significant photothermal effects. This characteristic enhances their biomedical applications, including those in PTT.
12.3.1 Physical and Chemical Properties of CNTs and CNHs Related to PTT
The intrinsic absorbance of CNTs and CNHs in the NIR region are much higher than that of water, which is the main source of heat produced in tissues under NIR illumination (Figure 12.6) [45]. As reported by Torti, MWCNTs allow for greater photothermal conversion and cancer cell killing [46]. CNHs exhibit many advantages over other nanomaterials and serve as promising candidates for PTT. Their size (50–100 nm) is optimal for enhanced penetration and retention effect, leading to excess accumulation at tumor sites [47, 48]. CNHs are more biocompatible than many other nanomaterials requiring metal catalyst [49]. The efficient cellular uptake in tumors and high biocompatibility make CNH a better candidate for PTT than CNT.

Figure 12.6 (a) Absorbance spectra of SWCNT compared with water [45] and (b) photothermal conversion comparation between SWCNT and MWCNT [46].
Although still widely studied, SWCNTs and SWCHNs are not the most efficient carbon nanomaterials for photothermal conversion. According to a study by Trajkovic, the capacity for photothermal cell killing of GO is higher than that of CNTs [50]. There are several reasons for this difference, including the superior photothermal sensitivity and better dispersibility of GO. The poor dispersibility of CNTs and CNHs greatly limits their biomedical application. Therefore, CNTs and CNHs must be modified with hydrophilic polymers to disperse well in physiological environments. Many studies have aimed to improve the performance of CNTs in PTT by introducing hydrophilicity via polymer addition. Among these polymers, the most widely used is PEG, which is nontoxic but interferes with cell processes [51–53].
12.3.2 CNTs and CNHs for in vitro PTT
Similar to assessments of GO‐based PTT, the biocompatibility of CNTs and CNHs have evaluated the toxic responses in vitro to prove their good biocompatibility property [45, 54–56]. Because the testing of the basic PTT performance of CNTs and CNHs is similar to that of GO and other materials, it is not discussed here. As mentioned in the section on GO for PTT, the mechanism of photothermal‐triggered cell death is of great interest to researchers. Therefore, many studies focus on the surface chemistry modification and functionalization of CNTs and CNHs [14, 51, 57–60]. Multi‐armed PEG and PEI have been used to improve dispersibility and cell interaction, whereas peptide [(KFKA7)n] is used to enhance photothermal conversion [8].
In addition to measuring cell death caused directly by heat, studies on cellular apoptosis have been undertaken with the aim of enhancing the efficiency of photothermally induced cell death. Similar to GO, CNTs and CNHs have been studied with respect to the expression of HSPs [61, 62]. A study by Rylander analyzed three HSPs: HSP27, HSP70, and HSP90 [62]. After the administration of PTT adequate enough to cause significant cell death, these three proteins had similar expression profiles. NIR illumination without CNTs causes an increase in HSP expression because the heat generated by water induces a cellular response that protects cells from thermal damage. However, when CNTs are included, significant HSP expression enhancement occurs because a greater HSP response is needed to overcome the hyperthermic damage caused by CNTs under NIR illumination.
Increasing the efficiency of photothermal cancer therapy requires the inhibition of HSP expression. Researchers have directly optimized PTT laser parameters for improved performance via this inhibited expression. The optimized parameters damage cells such that HSPs cannot be overexpressed. However, despite the use of this method, HSP expression has increased. These results may be explained by the intrinsic abundance of HSP90. Therefore, an effective approach may decrease or interfere with the expression of heat shock factor, which takes place upstream of the HSP gene [63].
12.3.3 CNTs and CNHs for in vivo PTT
The in vivo applications of CNTs and CNHs in PPT have also been widely studied, particularly those of functionalized CNTs, which have superior PTT performance [8, 13, 64, 65]. According to the studies by Liu and other researchers, well‐dispersed CNTs efficiently eliminate in vivo solid tumors at very low NIR intensities [66, 67]. Raman scattering analysis has shown that injected CNTs exhibit ultra‐high retention in tumors (Figure 12.7). Choi and coworkers demonstrated exceptional in vivo biocompatibility of PEG‐functionalized SWCNTs and survival longer than six months in a mouse model [68]. In this model, intravenously injected SWCNTs were primarily excreted through the liver and kidneys.

Figure 12.7 Skin accumulation of SWNTs (a and c) Raman images of a skin slice taken from mice injected with SWNT‐100%‐5kPEG‐PMHC18 and (b and d) SWNT‐10%‐5kPEG‐PMHC18. Overlaid Raman images and hematoxylin and eosin‐stained skin slice images [66].
CNHs have been combined along with NIR laser irradiation to reduce the tumor size in vivo, demonstrating their PTT ability [65]. CHN‐induced photothermal tumor ablation can be achieved without triggering any adverse effects on normal tissues [60]. Thermal‐ and CHN‐treated patients showed hair growth at one week after treatment without tumor recurrence (Figure 12.8). These studies provided evidence of the safety of CNTs and CNHs in PTT and other biomedical applications.

Figure 12.8 In vivo photothermal therapy. Representative photographs of 4T1 tumor bearing mice i.v. injected with either saline or SWNHs/C 18 PMH‐PEG and exposed to laser treatment [60].
12.4 CDs and GDs for PTT
The small size and fluorescent properties of CDs and GDs distinguish them from other carbon nanomaterials. Furthermore, the synthetic methods (both top‐down and bottom‐up methods have been developed) and sources for CDs and GDs are also diverse and abundant; therefore, the properties of different CDs or GDs can vary significantly. Benefiting from ultrasmall size and hydrophilic properties, CDs and GDs can be easily dispersed in medium and taken up by cells. Compared with the biocompatibility of semiconductor QDs, that CDs and GDs is superior for biomedical applications, and because they contain aromatic groups, they are also suitable for drug loading and PTT. These properties, combined with their fluorescent properties and ease of surface modification, make CDs and GDs appropriate for theranostic applications.
12.4.1 Physical and Chemical Properties of CDs and GDs Related to PTT
The average size of CDs and GDs is less than 10 nm, with most being approximately 2–5 nm [15, 69, 70]. This small size allows for direct penetration into cells and extensive cellular uptake. Because synthetic routes to CDs and GDs are abundant, a variety of surface properties and modifications are possible [15, 70, 71]. A positively charged surface and residual surface functional groups are beneficial for nanomaterial–cell interactions.
For PTT applications, photothermal conversion effects are of primary importance. The optical absorbance of CDs varies in the NIR region. Zhang found only weak prolonged absorbance from π–π* interactions in the NIR region [72]. However, Jing described a CD synthesized from hydrophobic cyanine dye and PEG via the solvothermal process and showed that its photothermal conversion efficiency reached approximately 38.7%, which is higher than that of graphitic pencil nanodots (27%; Figure 12.9) [15]. The increased photothermal conversion efficiency may originate with the residual functional groups on the CD surface. To increase the photothermal effects, researchers usually use other nanomaterials to prepare nanocomposites with CDs and GDs [16, 73–77].

Figure 12.9 UV‐vis absorption spectra of CDs synthesized from hydrophobic cyanine dye (CyCD) (gray line), cyanine (CyOH) and CyOH aggregated particles [15].
12.4.2 CDs and GDs for in vitro PTT
Because CDs have high solubility, their surfaces require no modifications with hydrophilic molecules to improve dispersibility. Furthermore, the fluorescent properties of CDs are advantageous in biomedical applications because they allow for simultaneous tumor imaging and NIR‐triggered thermal therapy [15, 71, 78]. Fluorescence also aids the monitoring of the intracellular behavior of CDs. Both CDs and GDs can also be used for photoacoustic imaging. Therefore, in vitro PTT studies of CDs usually include cellular imaging analyses. Benefiting from easy surface modification, CDs are often functionalized with aptamers and drugs. Furthermore, because the synthesis of CDs and GDs is flexible, a wide range of element‐doped CDs and GDs with various properties have also been developed for photothermal applications. For example, Lee et al. synthesized CDs with different degrees of nitride doping to enhance photothermal conversion efficiency (Figure 12.10) [69]. An N content of 7 M saturated the CDs and maximized photothermal conversion efficiency. Zhang synthesized B‐doped GDs for simultaneous T1‐weighted imaging and PTT applications [79].

Figure 12.10 (a) UV‐vis absorbance spectra and (b) PA amplitude spectrum with different N‐doped CDs [69].
12.4.3 CDs and GDs for in vivo PTT
Because CDs are fluorescent, their in vivo behavior can be studied directly. Wang reported that after intravenous injection, CDs circulate in the blood without aggregation and accumulate in tumors via the EPR effect [71]. The results of a body distribution study showed that most CDs were located in the tumor.
Another concern for in vivo PTT is the distribution of nanomaterials in reticuloendothelial system organs. Although a large portion of CDs is retained in the liver, compared with the accumulation in the tumor, this proportion is negligible and no tissue damage has been observed. The intratumor temperature reaches 57 °C under NIR671 illumination at a power density of 2 W cm−2 (Figure 12.11).

Figure 12.11 Hematoxylin and eosin‐stained slices of the heart, liver, spleen, lung, and kidney in mice after PTT. Scale bar: 50 μm [71].
Recently, Lan et al. used two‐photon‐excited NIR‐emissive CDs for in vivo PTT [80]. Under 880 nm NIR illumination at 0.5 W for six minutes, the intratumor temperature reached 52 °C and tumor growth was effectively inhibited.
12.5 Fullerenes for PTT
Fullerenes have poor solubility, limiting their applications in the biomedical field. To solve this major problem, various functional fullerenes have been developed using chemical modifications on their surface [81, 82]. The potential of functional fullerenes has been demonstrated in cancer treatment, including photodynamic therapy, PTT, and chemotherapy [81] and tumor diagnosis (magnetic resonance and photoacoustic imaging).
12.5.1 Physical and Chemical Properties of Fullerenes Related to PTT
Fullerenes have much weaker optical limiting effects than other carbon nanomaterials [83]. Functionalized fullerenes are inferior to pristine fullerenes as optical limiters, indicating a lower excited‐state absorption cross‐section [84, 85]. Functionalized fullerenes were found to be heated by exposure to laser irradiation, because of the distortion of their symmetrical cage structure [86]. Moreover, because of their chemical structures, fullerenes possess photo‐physical properties and generate reactive oxygen species by exposure to visible light, indicating that fullerenes may be more suited for photodynamic therapy than for PTT in cancer treatment [87].
12.5.2 Fullerenes for in vitro PTT
Because fullerenes have poor solubility, their surfaces require modifications for improved dispersibility. Krishna et al. reported that polyhydroxy fullerenes (PHF) and carboxy fullerenes can be heated under low‐intensity NIR laser irradiation (785 nm). PHF‐coated silica nanoparticles were dosed onto mesothelial lung carcinoma cells. After 10 seconds of NIR laser irradiation, bubbles formed, and floating cellular fragments were immediately observed (Figure 12.12). These results clearly indicate that NIR laser triggered the photothermal effects of PHF, further supporting the notion that these nanomaterials can effectively eliminate cancer cells in vitro [86].
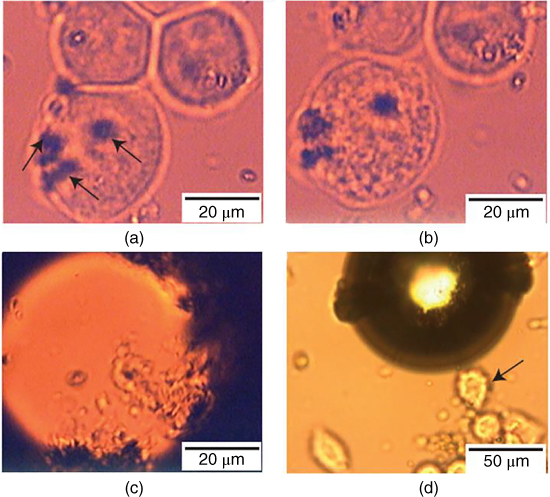
Figure 12.12 Photothermal ablation of cancer cells dosed with PHF‐coated silica nanoparticles. (a) Nanoparticle agglomerates (indicated by arrows) in contact with cells before exposure to a 785‐nm laser. (b) Cells appear granulated after five seconds of irradiation. (c) Cells are destroyed after 10 seconds of irradiation, accompanied by bubble formation. (d) Cells (indicated by arrow) adjacent to the irradiated region remain intact [86].
12.5.3 Fullerenes for in vivo PTT
PHFs are water‐soluble, rapidly excreted and biodegradable carbon nanoparticle, and have antioxidant, and neuroprotective properties in living organisms [17, 86]. These properties make PHF an ideal candidate for cancer PTT. After chitosan‐PHF nanoparticles were injected and exposed to NIR laser, tumors in a cross‐sectional area decreased by an average of 32% within 2 hours of treatment, with only a blister visible 20 hours post‐treatment (Figure 12.13) [17]. Therefore, PHF may enable the safe and effective use of PTT for various cancer types.

Figure 12.13 Treatment of a mice tumor with CP‐0.25 (chitosan‐polyhydroxy fullerenes) nanoparticles. Photographs of mouse tumor (a) two hours and (b) 20 hours after laser irradiation, only a blister was visible after 20 hours (within dotted lines). (c) and (d) Histological sections of remaining tumor stained with hematoxylin and eosin demonstrate areas of necrosis [17]. (See color plate section for the color representation of this figure.)
12.6 Carbon Nanomaterial‐Based Nanocomposites for PTT
Owing to their robust performance in PTT applications, carbon nanomaterials have attracted strong interest for combinations with other nanomaterials or reagents for multifunctional performance. Easy surface functionalization and robust interaction with other reagents allow easy synthesis of carbon nanomaterials into nanocomposites. For example, the carboxyl groups on the surface of GO make it amenable to the amidation reaction and CDs synthesized from various reagents with surface functional groups offer potential for further modification. Nanocomposites are most often used to achieve enhanced PTT effects and develop multifunctional nanomedicines and are synthesized accordingly.
12.6.1 GO‐Based Nanocomposites for PTT
Good dispersibility, presence of surface carboxyl groups, and large surface area allow GO to react and form nanocomposites easily under appropriate conditions. Currently, a wide variety of nanocomposites are based on GO. In addition to the use of chemical reagents such as glucose, dopamine, or citric acid to produce rGO, noble metals such as gold, compound such as CuS, and NIR dyes such as cypate have been used to synthesize GO nanocomposites with enhanced photothermal effects, including greater photothermal conversion [22, 32, 88]. Yun et al. synthesized a nanocomposite of gold nanorods (AuNRs), iron oxide, and GO [89]. AuNRs show enhanced absorbance in the NIR region owing to the longitudinal surface plasmon resonance of free electrons along the elongated axis, and iron oxide has absorbance in the NIR region as well. The NIR absorbance and photothermal effects of this GO nanocomposite are significantly higher than those of GO alone (Figure 12.14).
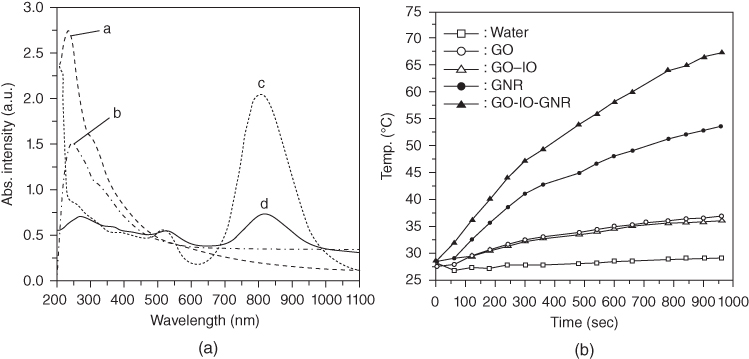
Figure 12.14 (a) UV–Vis absorption spectrum of a. GO, b. GO‐IO, c. GNR, d. GO‐IO‐GNR and (b) temperature variation of five different samples upon laser irradiation [89].
Dembereldorj et al. used an AuNR‐PEG‐GO nanocomposite for PTT and achieved efficient in vitro cancer cell killing and in vivo tumor growth inhibition [90]. Fe3O4 nanoparticles have also been used to synthesize GO composites with enhanced photothermal effects [77]. Arginine and cypate have been combined with rGO to enhance NIR absorbance [22, 91].
Beyond the use of other nanomaterials to enhance the photothermal conversion efficiency, the cytotoxicity of nanocomposites can be improved by increasing the capacity to cause cell death (apoptosis). For example, Yu et al. used a peptide to functionalize PDA‐modified rGO to increase cancer cell death via the bystander effect triggered by PTT [26].
12.6.2 CNT‐Based Nanocomposites for PTT
Because CNTs are hollow nanotubes, they are synthesized into nanocomposites in a manner that differs from that of GO, which is modified by adding other materials to the surface. CNTs can be functionalized with other nanomaterials both on the surface and in the CNT cavity. For example, Kim et al. coated the surface of CNTs with gold to produce gold CNTs with outstanding photothermal conversion efficiency [92], whereas Liu et al. used metal oxide to fill CNTS and enhance photothermal effects [93]. Wang et al. also applied silver and gold to CNTs for theranostic applications with enhanced Raman scattering imaging and PTT capabilities [58]. Folic acid and QDs (CdSe/ZnSe) have been used simultaneously used to modify the surface of CNTs for PTT applications, and blood protein has been used to enhance the biocompatibility of CNTs [94]. CNTs with surfaces modified with glycated chitosan show enhanced PTT efficiency via elevated immune response [64].
12.6.3 CD‐ and GD‐Based Nanocomposites for PTT
Unlike GO or CNTs, CDs and GDs are usually added to other materials owing to the ultrasmall size of the dots. Similar to CNTs, the fluorescent properties of CDs and GDs are useful for in vitro and in vivo monitoring of nanocomposites [75]. For example, this fluorescence allows monitoring of the intracellular distribution of CD‐tailed CNTs and the distribution of rGO in vivo [73].
CD‐decorated CNTs and GO also have high photothermal efficiency. As illustrated above, CDs and GDs can be doped with inorganic and organic materials to form nanocomposites. For example, chitosan has been combined with CDs to form a nanogel with good dispersibility and biocompatibility for PTT applications [74]. Metal–organic frameworks are newly developed materials that have been used to synthesize nanocomposites with GDs [95]. These composites show enhanced photothermal conversion and cancer‐cell−killing capabilities under NIR illumination.
12.7 Carbon Nanomaterial‐Based Combined Therapy with PTT
12.7.1 Chemotherapy
Chemotherapy is among the most commonly used treatment approaches for cancer owing to its high toxicity to tumor cells. Doxorubicin (DOX) belongs to the anthracycline class of chemotherapeutic agents, which are also toxic to cells in normal tissue and cause severe suppression of hematopoiesis [96]. Liu et al. have developed a method to load DOX onto branched PEG‐functionalized SWCNTs for in vivo cancer therapy [97]. The microenvironment of tumor tissues is acidic and promotes the release of DOX from the SWCNT carrier. Free DOX is less likely to be released in normal tissues because the pH therein is neutral. Because SWCNT–DOX is stable at neutral pH but released in acidic environments, studies have suggested that the SWCNT–DOX combination has a toxicity lower than that of free DOX [98].
As a DOX carrier, GO has a loading capacity as high as 200% [99]. Therefore, to create a drug carrier with photothermal sensitivity, Zhang et al. developed DOX‐loaded PEGylated nGO (nGO‐PEG‐DOX) for combination chemotherapy and PTT in a single system [100]. The authors suggested that nGO‐PEG‐DOX treatment promotes the destruction of tumor cells without patient weight loss or tumor recurrence and concluded that chemo‐PTT using nGO‐PEG‐DOX has advantages over chemotherapy or photothermal treatments alone, which have comparatively poor therapeutic effects.
In addition, DOX molecules can be noncovalently loaded onto nano‐rGO via π‐stacking. Hu et al. have developed a method to load DOX onto rGO/dextran, thereby combining chemotherapy and external NIR PTT [101]. Compared with chemotherapy alone, the rGO/DOX/dextran combination achieved good therapeutic effects with low DOX concentrations.
12.7.2 RT
RT is an effective method for treating cancer [102]. RT kills cancer cells largely through the formation of free radicals, which have high energy that promotes DNA strand breaks. Generally, the radioisotopes used for internal RT can be divided into three categories: α‐particle emitters, β‐particle emitters, and Auger electron emitters. β‐Particle emitters (e.g. 90 γ, 67 Cu, 131 I, 186 Re, and 188 Re) are the most widely used radioisotopes in cancer therapy because they release electrons with low energy and low cytotoxicity [102]. However, RT also has drawbacks such as the fast excretion of injected radionuclides, dose dependence, and nonspecific systemic distribution [103]. For optimal anti‐tumor effects, other therapeutic strategies are combined with RT [104].
Zhao et al. have reported that radionuclide 131 I‐labeled SWCNT@PDA‐PEG enables nuclear imaging and radioisotope cancer therapy. A remarkable synergistic anti‐tumor effect is provided by the NIR photo‐absorption of SWCNT, PTT, and 131 I‐based RT [105].
Several studies have suggested that GO or rGO could be labeled with 125 I, 64 Cu, or 66 Ga to trace the in vivo behavior of these isotopes during cancer treatment [106, 107]. Chen et al. coated rGO with 131 I and PEG to combine RT and PTT. Taking advantage of the 131 I‐RGO‐PEG trace, they found that the nanomaterial remained in the blood circulation for a long period and accumulated in the tumor tissue. Moreover, compared with free 131 I, 131 IRGO‐PEG showed enhanced cellular uptake and thus improved radiotherapeutic efficacy against cancer cells. This study presented a simple approach to the fabrication of graphene‐based multifunctional nanotheranostic applications that use PTT and enhance RT [108].
12.7.3 Photodynamic Therapy (PDT)
Along with PTT, PDT plays a key role in cancer therapeutics. PDT uses photosensitizers stimulated under a suitable wavelength to transform energy into reactive oxygen species that induce cytotoxicity by reacting with other biomolecules [109, 110]. Compared with chemotherapy and radiotherapy, PDT shows fewer side effects and improves tumor‐specific cell death, which suggests that the combination of PTT and PDT has a promising future in cancer treatment [111].
In a study by Marangon et al., MWCNTs and the photosensitizer m‐tetrahydroxyphenylchlorin were combined for PTT/PDT cancer treatment [112] and showed that the route to apoptosis changed depending on the irradiation conditions. Jiang et al. demonstrated that water‐soluble single‐walled CNHs and metal phthalocyanines can be hybridized for PTT and PDT. SWNHs‐tetrasulfonic acid tetrasodium salt copper phthalocyanine (TSCuPc) show good anticancer efficacy by combining noninvasive PTT and PDT [113].
Cao et al. have developed a GO‐PEG‐Ce6 system that can be excited at 660 and 808 nm to kill 4T1 breast cancer cells by combing PTT and PDT [114]. In their study, PDT alone did not effectively inhibit tumor growth, and PTT alone initially inhibited tumor growth but the tumor recurred during the second week after treatment. By comparison, the PTT/PDT combination therapy not only promoted tumor regression but also resulted in tumor‐free status until day 60 [114]. Similarly, Jiang et al. developed a PTT/PDT system by loading water‐soluble phthalocyanine onto graphene. In this system, graphene acts as both a drug carrier and PTT agent and phthalocyanine is the PDT agent. The results showed that the PTT/PDT system exhibits better anticancer efficacy in vitro [115]. Recently, GO and fullerene C60 were used for PTT/PDT combination therapy. The GO‐C60 hybrid showed good solubility and a robust capability to form reactive oxygen species in HeLa cells [116]. Owing to its synergistic effects, the GO‐C60 hybrid performed better than either treatment alone in the inhibition of cancer cells [116].
12.7.4 Gene Therapy
Gene therapy is a novel approach in which nucleic acid polymers are delivered into cells as a drug to treat disease. Since the first successful human genome transfer in 1989 [117], gene therapy has been extensively studied as a method for treating immunodeficiency [118], diabetes [119], cardiovascular disease [120], and cancer [121]. Gene therapy is considered a safe and effective treatment for cancer and uses exogenous nucleic acids, which suppress oncogene expression or restrain the proliferation of intractable tumors [122, 123].
RNA silencing, such as that using small interference RNA (siRNA), is the most broadly used form of gene therapy. It has low toxicity and works in a dose‐dependent manner with high gene knockdown efficiency [124, 125]. The main limitations of RNA use include easy degradation in biological fluids [126] and the low cellular uptake efficiency of siRNA in vivo; therefore, several types of nanomaterials, such as AuNRs, QDs, silica nanoparticles, and metal nanomaterials, have been functionalized and developed into siRNA delivery systems for biomedical applications in vitro and in vivo [127–129].
Owing to its superior absorption and strong NIR conversion properties, functionalized GO has been incorporated into ideal gene delivery systems [130, 131]. The large, specific surface area and exceptional surface‐to‐volume ratio are additional advantages of GO in drug or gene delivery applications in biomedicine [132, 133]. Combinations of PTT and gene delivery therapy in a single platform hold great promise in cancer therapy owing to their enhanced therapeutic effects.
Functionalized GO has been applied extensively for siRNA delivery. In an in vivo anti‐tumor study, Yin used multifunctionalized monolayer GO as a gene delivery system to efficiently co‐deliver HDAC1 and K‐Ras siRNAs to pancreatic cancer cells from the MIA PaCa‐2 cell line. In this study, the synergistic effects of combined gene silencing therapy and NIR light thermotherapy showed significant anticancer efficacy, inhibiting tumor volume growth by more than 80%. One‐third of the tumor‐bearing mice in the study were cured by treatment with GO–siRNA nanoformulations and NIR light treatment [134].
Drug resistance remains a major challenge for anticancer treatment, and the delivery of siRNA combined with a suitable agent for killing drug‐resistant cells is a new approach for silencing the expression of efflux transporters and overcoming drug resistance. siRNA disrupts cellular drug resistance pathways by silencing relevant gene expression, which can re‐sensitize cancer cells that have acquired resistance to anticancer drugs [135–137]. Zeng et al. developed an integrated nGO nanodelivery system for the efficient and targeted co‐delivery of DOX and siRNA against drug efflux transporter expression to treat drug‐resistant tumors at an irradiation of less than 808 nm. The results suggest that composite vesicles may be powerful tools for drug delivery that achieve improved therapeutic efficacy and address drug resistance in combined photo‐chemotherapy [138].
Another study reported the first use of photodynamic therapy to enhance siRNA transfection efficiency by using an nGO‐PEG‐PEI conjugate [131]. In addition to affecting the direct photothermal ablation of cancer cells, NIR laser irradiation under a low‐power density raised cell membrane permeability without damaging cells owing to the strong NIR optical absorbance of the conjugate.
In addition, the unparalleled optical and electrical properties of CNTs have been exploited to introduce DNA and siRNA into cells, including some cells that are difficult to transfect. CNTs can also allow the controlled release of DNA or siRNA for targeted gene therapy. CNTs can further enhance the therapeutic effect of DNA and siRNA by affecting their conformational structure and transient conformational changes. The linear structure of CNTs (the diameter matches that of DNA and siRNA) and their significant flexibility are advantages in these combinations. The synergistic effect of CNTs and DNA or siRNA delivery is a promising lead for the development of powerful multi‐functional nanomedical treatments for cancer [139, 140].
PEI‐grafted three‐dimensional oxidized mesoporous carbon nanospheres (MCNs) have also been developed for combination photothermal gene therapy. MCNs have advantages in gene‐loading capacity, NIR‐mediated PTT, and multiple controlled drug release performance owing to their porous structure and dispersed sp2‐hybridized pore walls. Meng et al. loaded a therapeutic gene (a plasmid DNA encoding ING4 and pING4) onto MCNs to suppress tumor cell proliferation and angiogenesis [141]. This nanomaterial successfully combines gene therapy and PTT into a single platform and not only delivers a therapeutic gene to the tumor for gene therapy but also eliminates the tumor via photothermal ablation. More important, improved gene therapy accompanied by NIR photothermally enhanced gene release was also achieved using this nanoplatform.
12.7.5 Immune Therapy
The emergence of cumulative toxicity and resistance to chemotherapy has emphasized the importance of immunotherapy as an adjunctive anticancer therapy. Anti‐tumor immunotherapy generally has few side effects and good patient tolerance and significantly improves prognosis [142–144]. Some clinical trials of immunotherapy have shown encouraging results in the treatment of malignant tumors such as melanoma, malignant glioma, and renal cell carcinoma, in which chemotherapy is often ineffective [145–148].
Nanoparticle‐mediated photothermal ablation is currently used for local cancer therapy, but its effectiveness remains low in controlling metastatic cancer, which causes most patient deaths. Ideally, PTT for cancer should not only eliminate the primary tumor but also recognize, pursue, and destroy any remaining tumor cells at sites of distant metastases. Combining PTT with immunotherapy is a promising strategy for treating patients with metastatic tumors [149, 150]. CNTs, which are excellent photothermal agents, can act as potential adjuvants for the activation of natural and adaptive immune responses [151]. Yang et al. reported that oxidized MWCNTs have anti‐tumor effects in Babl/c mice after subcutaneous injection, and these CNTs are expected to be useful for cancer immunotherapy [152].
Moreover, Wang et al. reported the photothermal ablation of primary tumors with SWCNTs in combination with anti‐CTLA‐4 antibodies, which can prevent the development of tumor metastasis in mice [64]. They also found that polymer‐coated SWCNTs were useful not only for photothermal tumor destruction, which releases tumor‐associated antigens, but also as immunological adjuvants to promote the maturation of dendritic cells and production of anti‐tumor cytokines, a unique behavior among PTT agents. These results demonstrate the effectiveness of using adjuvant nanomaterial‐based PTT in combination with immunotherapy to treat cancer metastasis.
Tumor cells do not normally produce effective anti‐tumor immune responses because they are resistant to the primary processing and expression of key molecules [153]. On the contrary, tumor cell death induced by PTT can release tumor antigens into the surrounding environment. At the same time, immune adjuvants promote antigen uptake and expression by specialized antigen‐presenting cells, thus triggering specific anti‐tumor immunity [149]. Therefore, PTT can be combined with immunotherapy to enhance immune response and make tumor residue and metastasis more sensitive to immune‐mediated killing [154]. In immune adjuvants, unmethylated oligonucleotides containing cytosine guanine (CpG) motifs are effective modulators of cancer immunotherapy [155]. The mammalian immune system can recognize CpG oligodeoxynucleotides through Toll‐like receptor 9 and secrete a number of proinflammatory cytokines, including tumor necrosis factor‐alpha and interleukin‐6, which stimulate a cascade of innate and adaptive immune responses [156, 157]. Tao et al. synthesized an nGO‐PEG‐PEI nanocomposite consisting of nGO sheets with covalently conjugated PEI and PEG that is a promising carrier of CpG adjuvant and avoids rapid degradation by nucleases. The GO‐PEG‐PEI‐CpG nanocomplex significantly promotes the production of proinflammatory cytokines and enhances immunostimulatory effects. Tao et al. were also the first to demonstrate that the photothermal heating of GO can effectively enhance the intracellular transport of CpG using NIR irradiation, thereby improving the immune response. Their results showed that combined photothermal immunotherapy is more effective than immunotherapy or PTT alone in in vivo cancer models [158].
12.7.6 Theranostic Applications
The early detection and effective treatment of cancer remains a prominent challenge in the twenty‐first century. Cancer is the leading cause of death worldwide, with an estimated 17 million deaths anticipated in 2030 [159]. Accurate and sensitive diagnosis is the most important means of cancer management. At present, tumor marker detection and cancer cell recognition can be carried out using several techniques, including chemiluminescence, enzyme‐linked immunosorbent assay, radioimmunoassay, and flow cytometry [160]. However, there is growing demand for simpler and more accurate, sensitive, and low‐cost testing methods. Nanomaterials have provided great advancements in diagnostic applications, and among these, graphene‐based biosensors have gained attention for their promise in making accurate and sensitive detection a priority in stopping cancer progression.
Most current methods fail to detect cancers at an early stage. Being photostable and less toxic than conventional fluorescent dyes, graphene‐based composites are suitable for diverse imaging modalities including optical imaging, ultrasonography, magnetic resonance imaging, and computed tomography [161]. In addition, the graphene‐based fluorescence quenching of biosensors reportedly target a wide range of cellular components and processes, such as DNA, RNA, proteins, metal ions, apoptosis, and adenosine‐5'‐triphosphate (ATP) production [162]. Branched PEG‐GO composites exhibit photoluminescence in the NIR range, which is the basis for NIR probes suitable for cancer imaging modalities. At present, graphene‐based composites are a favorable choice for biosensor fabrication.
The development of multimodal imaging and therapy instruments is a widely studied field in which research efforts will boost the clinical benefits and returns of hybrid nanotechnology [163]. In one study, a fluorescent/photoacoustic imaging‐guided photothermal agent was developed by seeding gold nanoparticles onto GO that was covalently functionalized with a NIR dye–labeled matrix metalloproteinase‐14 peptide substrate. Notably, this graphene/gold‐based probe exhibited strong NIR absorbance and high photoconversion efficiency for multimodal fluorescence in photoacoustic image‐guided enhanced PTT of SCC7 tumors [21]. Turcheniuk investigated the potential of PEG‐functionalized rGO‐enrobed AuNRs for the photothermal destruction of human glioblastoma astrocytoma (U87MG) cells in mice. Au NRs@rGO‐PEG composites are ideal multifunctional theranostic nanostructures that can result in effect efficient photothermal destruction of tumors in mice at low doses of NIR light excitation, and they act as fluorescent cellular markers owing to the presence of an NIR dye integrated onto the rGO shell. The results of the study show that novel nanomaterials can effectively destroy solid tumors and be used as excellent multifunctional therapeutic agents in PTT [164].
12.8 Conclusions and Perspectives
From a summary of the literature in this section, different carbon nanomaterials have both tumor detection and cancer‐cell−killing capabilities. These characteristics result in carbon nanomaterials have huge developmental potential in cancer treatment. Specific carbon nanomaterials, such as graphene, have extremely large surface areas that can be loaded with siRNA, DNA, or other biological molecules and chemotherapy drugs, thereby endowing them with capabilities of drug delivery and gene transfection. In relevant in vitro and in vivo studies on cancer photohyperthermia, it can be seen that carbon nanomaterials have good NIR photothermal conversion efficiency. In addition, the drug delivery and test capabilities of carbon nanomaterials can result in synergistic therapeutic effects during cancer treatment and decrease damage due to normal cells during treatment. As carbon nanomaterials have unique optical characteristics, many studies have used it in photodynamic therapy in cancer cells. The above characteristics have enabled carbon nanomaterials to become the next generation of cancer therapy drugs with the greatest developmental potential.
In our opinion, GO, CDs, and CNHs have great potential for PTT development attributable to their superior relevant physical and chemical characteristics. GO is an effective PTT reagent because of its intrinsic NIR absorption capability, efficient cellular uptake, and easy preparation. Reduction of GO can be used to increase the NIR region absorbance and improve photothermal conversion. NIR absorption and photothermal conversion effects are of primary importance for PTT. CDs and GDs are effective in PTT applications because of their high solubility, efficient cellular uptake, and abundant synthetic routes. Moreover, fluorescent properties of CDs are advantageous in biomedical applications because they allow for simultaneous tumor imaging and PTT. CNHs are particularly effective because of their intrinsic NIR absorption, efficient cellular uptake, and high biocompatibility. CHNs are produced by laser ablation of graphite, hence, there is no toxicity associated with the presence of metal catalysts.
It is necessary to carry out further relevant clinical and translational medicine studies before carbon nanomaterials can be used in the treatment of actual cancers. In addition, it is worth noting that progress in animal safety evaluation studies on carbon nanomaterials lags far behind studies on other cancer therapies and this has resulted in a controversy on the biological safety of carbon nanomaterials. The results of some studies have found that carbon nanomaterials have high biocompatibility. However, the studies of some researchers have also proven that exposure to carbon nanomaterials may result in different degrees of biological toxic effects to living organisms. Our research team carried out an evaluation of biological toxicity of GO and rGO and found that these two carbon nanomaterials may affect the normal immune function of biological organisms. The biological toxicity and relevant physical and chemical characteristics of carbon nanomaterials, such as particle size, surface charge, and specific surface modification are intimately connected. Therefore, we must carry more and deeper safety evaluation studies on carbon nanomaterials, including evaluation of biocompatibility and pharmacodynamics (absorption, distribution, metabolism, and elimination, ADME) to further address current safety concerns of carbon nanomaterials in cancer therapy applications.
Undoubtedly, carbon nanomaterials are one of the most researched and most promising drugs in cancer photohyperthermia. During the high development process of carbon nanomaterials, we must pay more attention to potential biological toxicity risk to ensure the biological safety of carbon nanomaterials during cancer photohyperthermia treatment and increase the feasibility of carbon nanomaterials in cancer therapy applications.
References
- 1 Kang, S., Lee, J., Ryu, S. et al. (2017). Gold nanoparticle/graphene oxide hybrid sheets attached on Mesenchymal stem cells for effective Photothermal cancer therapy. Chemistry of Materials 29: 3461–3476.
- 2 Wang, X., Liu, H., Chen, D. et al. (2013). Multifunctional Fe3O4@P(St/MAA)@Chitosan@Au core/shell nanoparticles for dual imaging and photothermal therapy. ACS Applied Materials & Interfaces 5: 4966–4971.
- 3 Abdolahad, M., Janmaleki, M., Mohajerzadeh, S. et al. (2013). Polyphenols attached graphene nanosheets for high‐efficiency NIR mediated photodestruction of cancer cells. Materials Science & Engineering C‐Materials for Biological Applications 33: 1498–1505.
- 4 Tang, S., Chen, M., and Zheng, N. (2014). Sub‐10‐nm Pd nanosheets with renal clearance for efficient near‐infrared photothermal cancer therapy. Small 10: 3139–3144.
- 5 Zhang, L., Xia, K., Bai, Y.‐Y. et al. (2014). Synthesis of gold nanorods and their functionalization with bovine serum albumin for optical hyperthermia. Journal of Biomedical Nanotechnology 10: 1440–1449.
- 6 Jaque, D., Martinez Maestro, L., del Rosal, B. et al. (2014). Nanoparticles for Photothermal therapies. Nanoscale 6: 9494–9530.
- 7 Perez‐Hernandez, M., del Pino, P., Mitchell, S.G. et al. (2015). Dissecting the molecular mechanism of apoptosis during photothermal therapy using gold nanoprisms. ACS Nano 9: 52–61.
- 8 Hashida, Y., Tanaka, H., Zhou, S. et al. (2014). Photothermal ablation of tumor cells using a single‐walled carbon nanotube‐peptide composite. Journal of Controlled Release 173: 59–66.
- 9 Hu, Z., Zhao, F., Wang, Y. et al. (2014). Facile fabrication of a C‐60‐polydopamine‐graphene nanohybrid for single light induced photothermal and photodynamic therapy. Chemical Communications 50: 10815–10818.
- 10 Robinson, J.T., Tabakman, S.M., Liang, Y. et al. (2011). Ultrasmall reduced graphene oxide with high near‐infrared absorbance for photothermal therapy. Journal of the American Chemical Society 133: 6825–6831.
- 11 Ge, J., Jia, Q., Liu, W. et al. (2015). Red‐emissive carbon dots for fluorescent, photoacoustic, and thermal theranostics in living mice. Advanced Materials 27: 4169–4177.
- 12 Yang, K., Hu, L., Ma, X. et al. (2012). Multimodal imaging guided photothermal therapy using functionalized graphene nanosheets anchored with magnetic nanoparticles. Advanced Materials 24: 1868–1872.
- 13 Antaris, A.L., Robinson, J.T., Yaghi, O.K. et al. (2013). Ultra‐low doses of chirality sorted (6,5) carbon nanotubes for simultaneous tumor imaging and photothermal therapy. ACS Nano 7: 3644–3652.
- 14 Whitney, J.R., Sarkar, S., Zhang, J. et al. (2011). Single walled carbon nanohorns as photothermal cancer agents. Lasers in Surgery and Medicine. 43: 43–51.
- 15 Zheng, M., Li, Y., Liu, S. et al. (2016). One‐pot to synthesize multifunctional carbon dots for near infrared fluorescence imaging and photothermal cancer therapy. ACS Applied Materials & Interfaces 8: 23533–22541.
- 16 Wo, F., Xu, R., Shao, Y. et al. (2016). A multimodal system with synergistic effects of magneto‐mechanical, photothermal, photodynamic and chemo therapies of cancer in graphene‐quantum dot‐coated hollow magnetic nanospheres. Theranostics 6: 485–500.
- 17 Krishna, V., Singh, A., Sharma, P. et al. (2010). Polyhydroxy fullerenes for non‐invasive cancer imaging and therapy. Small 6: 2236–2241.
- 18 Robinson, J.T., Welsher, K., Tabakman, S.M. et al. (2010). High performance in vivo near‐IR (> 1 mu m) imaging and photothermal cancer therapy with carbon nanotubes. Nano Research 3: 779–793.
- 19 Khazaei, M., Nasseri, S., Ganjali, M.R. et al. (2016). Response surface modeling of lead (II) removal by graphene oxide‐Fe3O4 nanocomposite using central composite design. Journal of Environmental Health Science and Engineering 14.
- 20 Zhang, Y., Zhang, H., Wang, Y. et al. (2017). Hydrophilic graphene oxide/bismuth selenide nanocomposites for CT imaging, photoacoustic imaging, and photothermal therapy. Journal of Materials Chemistry B 5: 1846–1855.
- 21 Gao, S., Zhang, L., Wang, G. et al. (2016). Hybrid graphene/Au activatable theranostic agent for multimodalities imaging guided enhanced photothermal therapy. Biomaterials 79: 36–45.
- 22 Guo, M., Huang, J., Deng, Y. et al. (2015). pH‐responsive cyanine‐grafted graphene oxide for fluorescence resonance energy transfer‐enhanced photothermal therapy. Advanced Functional Materials 25: 59–67.
- 23 Dikin, D.A., Stankovich, S., Zimney, E.J. et al. (2007). Preparation and characterization of graphene oxide paper. Nature 448: 457–460.
- 24 Casiraghi, C., Hartschuh, A., Qian, H. et al. (2009). Raman spectroscopy of graphene edges. Nano Letters 9: 1433–1441.
- 25 Yang, K., Wan, J., and Zhang, S. (2012). The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra‐low laser power. Biomaterials 33: 2206–2214.
- 26 Yu, J., Lin, Y.‐H., Yang, L. et al. (2017). Improved anticancer photothermal therapy using the bystander effect enhanced by antiarrhythmic peptide conjugated dopamine‐modified reduced graphene oxide nanocomposite. Advanced Healthcare Materials 6.
- 27 Roper, D.K., Ahn, W., and Hoepfner, M. (2007). Microscale heat transfer transduced by surface plasmon resonant gold nanoparticles. Journal of Physical Chemistry C 111: 3636–3641.
- 28 Yan, X., Hu, H., Lin, J. et al. (2015). Optical and photoacoustic dual‐modality imaging guided synergistic photodynamic/photothermal therapies. Nanoscale 7: 2520–2526.
- 29 Liu, C., Liu, H., and Lu, C. (2017). Polyethyleneimine‐modified graphene oxide/PNIPAm thermoresponsive hydrogels with rapid swelling/deswelling and improved mechanical properties. Journal of Materials Science 52: 11715–11724.
- 30 Chen, B., Liu, M., Zhang, L. et al. (2011). Polyethylenimine‐functionalized graphene oxide as an efficient gene delivery vector. Journal of Materials Chemistry 21: 7736–7741.
- 31 Ren, T., Li, L., Cai, X. et al. (2012). Engineered polyethylenimine/graphene oxide nanocomposite for nuclear localized gene delivery. Polymer Chemistry 3: 2561–2569.
- 32 Yang, L., Tseng, Y.‐T., Suo, G. et al. (2015). Photothermal therapeutic response of cancer cells to aptamer‐gold nanoparticle‐hybridized graphene oxide under NIR illumination. ACS Applied Materials & Interfaces 7: 5097–5106.
- 33 Wang, Y.‐W., Fu, Y.‐Y., Peng, Q. et al. (2013). Dye‐enhanced graphene oxide for photothermal therapy and photoacoustic imaging. Journal of Materials Chemistry B 1: 5762–5767.
- 34 Rong, P., Wu, J., Liu, Z. et al. (2016). Fluorescence dye loaded nano‐graphene for multimodal imaging guided photothermal therapy. RSC Advances 6: 1894–1901.
- 35 Yan, J., Chen, L., Huang, C.‐C. et al. (2017). Consecutive evaluation of graphene oxide and reduced graphene oxide nanoplatelets immunotoxicity on monocytes. Colloids and Surfaces B:Biointerfaces 153: 300–309.
- 36 Li, Y., Wu, Q., Zhao, Y. et al. (2014). Response of microRNAs to in vitro treatment with graphene oxide. ACS Nano 8: 2100–2110.
- 37 Chong, Y., Ma, Y., Shen, H. et al. (2014). The in vitro and in vivo toxicity of graphene quantum dots. Biomaterials 35: 5041–5048.
- 38 Mahmoudi, N. and Simchi, A. (2017). On the biological performance of graphene oxide‐modified chitosan/polyvinyl pyrrolidone nanocomposite membranes: in vitro and in vivo effects of graphene oxide. Materials Science & Engineering C‐Materials for Biological Applications 70: 121–131.
- 39 Cheng, C., Nie, S., Li, S. et al. (2013). Biopolymer functionalized reduced graphene oxide with enhanced biocompatibility via mussel inspired coatings/anchors. Journal of Materials Chemistry B 1: 265–275.
- 40 Xu, M., Zhu, J., Wang, F. et al. (2016). Improved in vitro and in vivo biocompatibility of graphene oxide through surface modification: poly(acrylic acid)‐functionalization is superior to PEGylation. ACS Nano 10: 3267–3281.
- 41 Yang, Y., Jiang, Y., Wang, Z. et al. (2012). Skin‐permeable quaternary nanoparticles with layer‐by‐layer structure enabling improved gene delivery. Journal of Materials Chemistry 22: 10029–10034.
- 42 Zhang, Z., Wang, M., Gao, D. et al. (2016). Targeted Raman imaging of cells using graphene oxide‐based hybrids. Langmuir 32: 10253–10258.
- 43 Chen, H.‐M., Lin, C.‐J., Jheng, K.‐R. et al. (2014). Effect of graphene oxide on affinity‐immobilization of purple membranes on solid supports. Colloids and Surfaces B:Biointerfaces 116: 482–488.
- 44 Yang, K., Zhang, S., Zhang, G. et al. (2010). Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Letters 10: 3318–3323.
- 45 Zhou, F., Wu, S., Song, S. et al. (2012). Antitumor immunologically modified carbon nanotubes for photothermal therapy. Biomaterials 33: 3235–3242.
- 46 Burke, A., Ding, X., Singh, R. et al. (2009). Long‐term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near‐infrared radiation. Proceedings of the National Academy of Sciences of the United States of America 106: 12897–12902.
- 47 Iijima, S., Yudasaka, M., Yamada, R. et al. (1999). Nano‐aggregates of single‐walled graphitic carbon nano‐horns. Chemical Physics Letters 309: 165–170.
- 48 Bandow, S., Kokai, F., Takahashi, K. et al. (2000). Interlayer spacing anomaly of single‐wall carbon nanohorn aggregate. Chemical Physics Letters 321: 514–551.
- 49 Warheit, D.B., Laurence, B.R., Reed, K.L. et al. (2004). Comparative pulmonary toxicity assessment of single‐wall carbon nanotubes in rats. Toxicological Sciences: An Official Journal of the Society of Toxicology 77: 117–125.
- 50 Markovic, Z.M., Harhaji‐Trajkovic, L.M., Todorovic‐Markovic, B.M. et al. (2011). In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials 32: 1121–1129.
- 51 Zhao, J. and Schaefer, D.W. (2008). Morphology of PEG‐stabilized carbon nanofibers in water. Journal of Physical Chemistry C 112: 15306–15310.
- 52 Lee, J.U., Huh, J., Kim, K.H. et al. (2007). Aqueous suspension of carbon nanotubes via non‐covalent functionalization with oligothiophene‐terminated poly(ethylene glycol). Carbon 45: 1051–1057.
- 53 Pang, S.N.J. (1993). Final report on the safety assessment of polyethylene glycols (PEGS) ‐6, −8, −32, −75, −150, −14M, −20M. Journal of the American College of Toxicology 12: 429–457.
- 54 Roldo, M., Power, K., Smith, J.R. et al. (2009). N‐Octyl‐O‐sulfate chitosan stabilises single wall carbon nanotubes in aqueous media and bestows biocompatibility. Nanoscale 1: 366–373.
- 55 Murakami, T., Nakatani, M., Kokubo, M. et al. (2013). Mechanism of cell interactions with water‐dispersed carbon nanohorns. Nanoscience and Nanotechnology Letters 5: 402–407.
- 56 Miyawaki, J., Yudasaka, M., Azami, T. et al. (2008). Toxicity of single‐walled carbon nanohorns. ACS Nano 2: 213–226.
- 57 Armentano, I., Marinucci, L., Dottori, M. et al. (2011). Novel poly(L‐lactide) PLLA/SWNTs nanocomposites for biomedical applications: material characterization and biocompatibility evaluation. Journal of Biomaterials Science‐Polymer Edition 22: 541–556.
- 58 Wang, X., Wang, C., Cheng, L. et al. (2012). Noble metal coated single‐walled carbon nanotubes for applications in surface enhanced Raman scattering imaging and photothermal therapy. Journal of the American Chemical Society. 134: 7414–7422.
- 59 Elhissi, A.M.A., Ahmed, W., Hassan, I.U. et al. (2012). Carbon nanotubes in cancer therapy and drug delivery. Journal of Drug Delivery 2012: 837327.
- 60 Chen, D., Wang, C., Nie, X. et al. (2014). Photoacoustic imaging guided near‐infrared Photothermal therapy using highly water‐dispersible single‐walled carbon nanohorns as theranostic agents. Advanced Functional Materials 24: 6621–6628.
- 61 Miyako, E., Deguchi, T., Nakajima, Y. et al. (2012). Photothermic regulation of gene expression triggered by laser‐induced carbon nanohorns. Proceedings of the National Academy of Sciences of the United States of America 109: 7523–7528.
- 62 Fisher, J.W., Sarkar, S., Buchanan, C.F. et al. (2010). Photothermal response of human and murine cancer cells to multiwalled carbon nanotubes after laser irradiation. Cancer Research 70: 9855–9864.
- 63 Egger, M.E., Huang, J.S., Yin, W. et al. (2013). Inhibition of autophagy with chloroquine is effective in melanoma. Journal of Surgical Research 184: 274–281.
- 64 Wang, C., Xu, L., Liang, C. et al. (2014). Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti‐CTLA‐4 therapy to inhibit cancer metastasis. Advanced Materials 26: 8154–8162.
- 65 Zhang, M., Murakami, T., Ajima, K. et al. (2008). Fabrication of ZnPc/protein nanohorns for double photodynamic and hyperthermic cancer phototherapy. Proceedings of the National Academy of Sciences of the United States of America 105: 14773–14778.
- 66 Liu, X., Tao, H., Yang, K. et al. (2011). Optimization of surface chemistry on single‐walled carbon nanotubes for in vivo photothermal ablation of tumors. Biomaterials 32: 144–151.
- 67 Liu, Z., Tabakman, S.M., Chen, Z. et al. (2009). Preparation of carbon nanotube bioconjugates for biomedical applications. Nature Protocols 4: 1372–1382.
- 68 Moon, H.K., Lee, S.H., and Choi, H.C. (2009). In vivo near‐infrared mediated tumor destruction by photothermal effect of carbon nanotubes. ACS Nano 3: 3707–3713.
- 69 Lee, C., Kwon, W., Beack, S. et al. (2016). Biodegradable nitrogen‐doped carbon nanodots for non‐invasive photoacoustic imaging and photothermal therapy. Theranostics 6: 2196–2208.
- 70 Liu, Z., Chen, W., Li, Y. et al. (2016). Integrin alpha(v)beta(3)‐targeted C‐Dot nanocomposites as multifunctional agents for cell targeting and photoacoustic imaging of superficial malignant tumors. Analytical Chemistry 88: 11955–11962.
- 71 Jia, Q., Ge, J., Liu, W. et al. (2017). Self‐assembled carbon dot nanosphere: a robust, near‐infrared light‐responsive, and vein injectable photosensitizer. Advanced Healthcare Materials 6.
- 72 Tu, X., Ma, Y., Cao, Y. et al. (2014). PEGylated carbon nanoparticles for efficient in vitro photothermal cancer therapy. Journal of Materials Chemistry B 2: 2184–2192.
- 73 Hu, S.‐H., Chen, Y.‐W., Hung, W.‐T. et al. (2012). Quantum‐dot‐tagged reduced graphene oxide nanocomposites for bright fluorescence bioimaging and photothermal therapy monitored in situ. Advanced Materials 24: 1748–1754.
- 74 Wang, H., Mukherjee, S., Ji, J. et al. (2017). Biocompatible chitosan‐carbon dot hybrid nanogels for NIR‐imaging‐guided synergistic photothermal‐chemo therapy. ACS Applied Materials & Interfaces 9: 18639–18649.
- 75 Nair, L.V., Nagaoka, Y., Maekawa, T. et al. (2014). Quantum dot tailored to single wall carbon nanotubes: a multifunctional hybrid nanoconstruct for cellular imaging and targeted photothermal therapy. Small 10: 2771–2775.
- 76 Nandi, S., Bhunia, S.K., Zeiri, L. et al. (2017). Bifunctional carbon‐dot‐WS2 nanorods for photothermal therapy and cell imaging. Chemistry‐A European. Journal 23: 963–969.
- 77 Wang, S., Zhang, Q., Luo, X.F. et al. (2014). Magnetic graphene‐based nanotheranostic agent for dual‐modality mapping guided photothermal therapy in regional lymph nodal metastasis of pancreatic cancer. Biomaterials 35: 9473–9483.
- 78 Huang, P., Lin, J., Wang, X. et al. (2012). Light‐triggered theranostics based on photosensitizer‐conjugated carbon dots for simultaneous enhanced‐fluorescence imaging and photodynamic therapy. Advanced Materials 24: 5104–5110.
- 79 Wang, H., Revia, R., Wang, K. et al. (2017). Paramagnetic properties of metal‐free boron‐doped graphene quantum dots and their application for safe magnetic resonance imaging. Advanced Materials (Deerfield Beach, Fla) 29.
- 80 Lan, M., Zhao, S., Zhang, Z. et al. (2017). Two‐photon‐excited near‐infrared emissive carbon dots as multifunctional agents for fluorescence imaging and photothermal therapy. Nano Research 10: 3113–3123.
- 81 Chen, Z., Ma, L., Liu, Y. et al. (2012). Applications of functionalized fullerenes in tumor theranostics. Theranostics 2: 238–250.
- 82 Bosi, S., Da Ros, T., Spalluto, G., and Prato, M. (2003). Fullerene derivatives: an attractive tool for biological applications. European Journal of Medicinal Chemistry 38: 913–923.
- 83 Wang, Q., Qin, Y., Zhu, Y. et al. (2008). Optical limiting performances of multi‐walled carbon nanotubols and [C60]fullerols. Chemical Physics Letters 457: 159–162.
- 84 Cai, X., Jia, H., Liu, Z. et al. (2008). Polyhydroxylated fullerene derivative C(60)(OH)(24) prevents mitochondrial dysfunction and oxidative damage in an MPP(+) ‐induced cellular model of Parkinson's disease. Journal of Neuroscience Research 86: 3622–3624.
- 85 Ryan, J.J., Bateman, H.R., Stover, A. et al. (2007). Fullerene nanomaterials inhibit the allergic response. Journal of Immunology 179: 665–672.
- 86 Krishna, V., Stevens, N., Koopman, B., and Moudgil, B. (2010). Optical heating and rapid transformation of functionalized fullerenes. Nature Nanotechnology 5: 330–334.
- 87 Arbogast, J.W., Darmanyan, A.P., Foote, C.S. et al. (1991). Photophysical properties of C60. Journal of Physical Chemistry A. 95: 11–12.
- 88 Bai, J., Liu, Y., and Jiang, X. (2014). Multifunctional PEG‐GO/CuS nanocomposites for near‐infrared chemo‐photothermal therapy. Biomaterials 35: 5805–5813.
- 89 Yun, K.‐H., Seo, S.‐H., Kim, B.‐M. et al. (2013). Critical enhancement of photothermal effect by integrated nanocomposites of gold nanorods and iron oxide on graphene oxide. Bulletin of the Korean Chemical Society 34: 2795–2799.
- 90 Dembereldorj, U., Choi, S.Y., Ganbold, E.‐O. et al. (2014). Gold nanorod‐assembled PEGylated graphene‐oxide nanocomposites for photothermal cancer therapy. Photochemistry and Photobiology 90: 659–666.
- 91 Hashemi, M., Omidi, M., Muralidharan, B. et al. (2017). Evaluation of the photothermal properties of a reduced graphene oxide/arginine nanostructure for near‐infrared absorption. ACS Applied Materials & Interfaces 9: 32607–32620.
- 92 Kim, J.‐W., Galanzha, E.I., Shashkov, E.V. et al. (2009). Golden carbon nanotubes as multimodal photoacoustic and photothermal high‐contrast molecular agents. Nature Nanotechnology 4: 688–694.
- 93 Liu, X., Marangon, I., Melinte, G. et al. (2014). Design of covalently functionalized carbon nanotubes filled with metal oxide nanoparticles for imaging, therapy, and magnetic manipulation. ACS Nano 8: 11290–11304.
- 94 Ge, C., Du, J., Zhao, L. et al. (2011). Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proceedings of the National Academy of Sciences of the United States of America 108: 16968–16973.
- 95 Yao, X., Niu, X., Ma, K. et al. (2017). Graphene quantum dots‐capped magnetic mesoporous silica nanoparticles as a multifunctional platform for controlled drug delivery, magnetic hyperthermia, and photothermal therapy. Small 13.
- 96 Morelli, D., Menard, S., Colnaghi, M.I., and Balsari, A. (1996). Oral administration of anti‐doxorubicin monoclonal antibody prevents chemotherapy‐induced gastrointestinal toxicity in mice. Cancer Research 56: 2082–2085.
- 97 Liu, Z., Fan, A.C., Rakhra, K. et al. (2009). Supramolecular stacking of doxorubicin on carbon nanotubes for in vivo cancer therapy. Angewandte Chemie International Edition 48: 7668–7672.
- 98 Liu, Z., Sun, X.M., Nakayama‐Ratchford, N. et al. (2007). Supramolecular chemistry on water‐soluble carbon nanotubes for drug loading and delivery. ACS Nano 1: 50–56.
- 99 Yang, X.Y., Zhang, X.Y., Liu, Z.F. et al. (2008). High‐efficiency loading and controlled release of doxorubicin hydrochloride on graphene oxide. Journal of Physical Chemistry C 112: 17554–17558.
- 100 Zhang, W., Guo, Z.Y., Huang, D.Q. et al. (2011). Synergistic effect of chemo‐photothermal therapy using PEGylated graphene oxide. Biomaterials 32: 8555–8561.
- 101 Hu, Y.F., He, L., Ding, J.X. et al. (2016). One‐pot synthesis of dextran decorated reduced graphene oxide nanoparticles for targeted photo‐chemotherapy. Carbohydrate Polymers 144: 223–229.
- 102 Hong, H., Zhang, Y., Sun, J.T., and Cai, W.B. (2009). Molecular imaging and therapy of cancer with radiolabeled nanoparticles. Nano Today 4: 399–413.
- 103 Zhao, W.L. and Robbins, M.E.C. (2009). Inflammation and chronic oxidative stress in radiation‐induced late normal tissue injury: therapeutic implications. Current Medical Chemistry 16: 130–143.
- 104 Ting, G., Chang, C.H., Wang, H.E., and Lee, T.W. (2010). Nanotargeted radionuclides for cancer nuclear imaging and internal radiotherapy. Journal of Biomedicine and Biotechnology .
- 105 Zhao, H., Chao, Y., Liu, J.J. et al. (2016). Polydopamine coated single‐walled carbon nanotubes as a versatile platform with radionuclide labeling for multimodal tumor imaging and therapy. Theranostics 6: 1833–1843.
- 106 Hong, H., Yang, K., Zhang, Y. et al. (2012). In vivo targeting and imaging of tumor vasculature with radiolabeled, antibody‐conjugated nanographene. ACS Nano 6: 2361–2370.
- 107 Hong, H., Zhang, Y., Engle, J.W. et al. (2012). In vivo targeting and positron emission tomography imaging of tumor vasculature with Ga‐66‐labeled nano‐graphene. Biomaterials 33: 4147–4156.
- 108 Chen, L., Zhong, X.Y., Yi, X. et al. (2015). Radionuclide I‐131 labeled reduced graphene oxide for nuclear imaging guided combined radio‐ and photothermal therapy of cancer. Biomaterials 66: 21–28.
- 109 Yang, T., Liu, L., Deng, Y.B. et al. (2017). Ultrastable near‐infrared conjugated‐polymer nanoparticles for dually photoactive tumor inhibition. Advanced Materials 29.
- 110 Xu, J.T., Gulzar, A., Liu, Y.H. et al. (2017). Integration of IR‐808 sensitized upconversion nanostructure and MoS2 nanosheet for 808 nm NIR light triggered phototherapy and bioimaging. Small 13.
- 111 Chen, Y.J., Wu, Y.K., Sun, B.B. et al. (2017). Two‐dimensional nanomaterials for cancer nanotheranostics. Small 13.
- 112 Marangon, I., Menard‐Moyon, C., Silva, A.K.A. et al. (2016). Synergic mechanisms of photothermal and photodynamic therapies mediated by photosensitizer/carbon nanotube complexes. Carbon 97: 110–123.
- 113 Jiang, B.P., Hu, L.F., Shen, X.C. et al. (2014). One‐step preparation of a water‐soluble carbon Nanohorn/phthalocyanine hybrid for dual‐modality photothermal and photodynamic therapy. ACS Applied Materials and Interfaces 6: 18008–18017.
- 114 Cao, J.B., An, H.Q., Huang, X.L. et al. (2016). Monitoring of the tumor response to nano‐graphene oxide‐mediated photothermal/photodynamic therapy by diffusion‐weighted and BOLD MRI. Nanoscale 8: 10152–10159.
- 115 Jiang, B.P., Hu, L.F., Wang, D.J. et al. (2014). Graphene loading water‐soluble phthalocyanine for dual‐modality photothermal/photodynamic therapy via a one‐step method. Journal of Materials Chemistry B 2: 7141–7148.
- 116 Li, Q., Hong, L., Li, H.G., and Liu, C.G. (2017). Graphene oxide‐fullerene C‐60 (GO‐C‐60) hybrid for photodynamic and photothermal therapy triggered by near‐infrared light. Biosensors and Bioelectronics 89: 477–482.
- 117 Rosenberg, S.A., Aebersold, P., Cornetta, K. et al. (1990). Gene‐transfer into humans‐immunotherapy of patients with advanced melanoma, using tumor‐infiltrating lymphocytes modified by retroviral gene transduction. New England Journal of Medicine 323: 570–578.
- 118 Hacein‐Bey‐Abina, S., Hauer, J., Lim, A. et al. (2010). Efficacy of gene therapy for X‐linked severe combined immunodeficiency. New England Journal of Medicine 363: 355–364.
- 119 Kojima, H., Fujimiya, M., Matsumura, K. et al. (2003). NeuroD‐betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nature Medicine 9: 596–603.
- 120 Yla‐Herttuala, S. and Martin, J.F. (2000). Cardiovascular gene therapy. Lancet 355: 213–222.
- 121 El‐Aneed, A. (2004). An overview of current delivery systems in cancer gene therapy. Journal of Controlled Release 94: 1–14.
- 122 Cai, H., Chen, M.S., Sun, Z.Y. et al. (2013). Self‐adjuvanting synthetic antitumor vaccines from MUC1 glycopeptides conjugated to T‐cell epitopes from tetanus toxoid. Angewandte Chemie‐International Edition 52: 6106–6110.
- 123 Hatakeyama, H., Akita, H., and Harashima, H. (2011). A multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: a strategy for overcoming the PEG dilemma. Advanced Drug Delivery Reviews 63: 152–160.
- 124 Aagaard, L. and Rossi, J.J. (2007). RNAi therapeutics: principles, prospects and challenges. Advanced Drug Delivery Reviews 59: 75–86.
- 125 Buckingham, S.D., Esmaeili, B., Wood, M. et al. (2004). RNA interference: from model organisms towards therapy for neural and neuromuscular disorders. Human Molecular Genetics 13: R275–R288.
- 126 Whitehead, K.A., Langer, R., and Anderson, D.G. (2009). Knocking down barriers: advances in siRNA delivery. Nature Reviews Drug Discovery 8: 129–138.
- 127 Yin, F., Yang, C.B., Wang, Q.Q. et al. (2015). A light‐driven therapy of pancreatic adenocarcinoma using gold nanorods‐based nanocarriers for co‐delivery of doxorubicin and siRNA. Theranostics 5: 818–833.
- 128 Lee, J.M., Yoon, T.J., and Cho, Y.S. (2013). Recent developments in nanoparticle‐based siRNA delivery for cancer therapy. Biomed Research International .
- 129 Yildirimer, L., Thanh, N.T., Loizidou, M. et al. (2011). Toxicology and clinical potential of nanoparticles. Nano Today 6: 585–607.
- 130 Kim, H., Namgung, R., Singha, K. et al. (2011). Graphene oxide‐polyethylenimine nanoconstruct as a gene delivery vector and bioimaging tool. Bioconjugate Chemistry 22: 2558–2567.
- 131 Feng, L.Z., Yang, X.Z., Shi, X.Z. et al. (2013). Polyethylene glycol and polyethylenimine dual‐functionalized nano‐graphene oxide for photothermally enhanced gene delivery. Small 9: 1989–1997.
- 132 Chung, C., Kim, Y.K., Shin, D. et al. (2013). Biomedical applications of graphene and graphene oxide. Accounts of Chemical Research 46: 2211–2214.
- 133 Yang, K., Feng, L.Z., Shi, X.Z., and Liu, Z. (2013). Nano‐graphene in biomedicine: theranostic applications. Chemical Society Reviews 42: 530–547.
- 134 Yin, F., Hu, K., Chen, Y. et al. (2017). SiRNA delivery with PEGylated graphene oxide nanosheets for combined photothermal and genetherapy for pancreatic cancer. Theranostics 7: 1133–1148.
- 135 He, Y.L., Zhang, L.F., Chen, Z.Z. et al. (2015). Enhanced chemotherapy efficacy by co‐delivery of shABCG2 and doxorubicin with a pH‐responsive charge‐reversible layered graphene oxide nanocomplex. Journal of Materials Chemistry B 3: 6462–6472.
- 136 He, C.B., Liu, D.M., and Lin, W.B. (2015). Self‐assembled nanoscale coordination polymers carrying siRNAs and cisplatin for effective treatment of resistant ovarian cancer. Biomaterials 36: 124–133.
- 137 Zheng, W.J., Cao, C.W., Liu, Y.N. et al. (2015). Multifunctional polyamidoamine‐modified selenium nanoparticles dual‐delivering siRNA and cisplatin to A549/DDP cells for reversal multidrug resistance. Acta Biomaterials 11: 368–380.
- 138 Zeng, Y.P., Yang, Z.Y., Li, H. et al. (2017). Multifunctional nanographene oxide for targeted gene‐mediated thermochemotherapy of drug‐resistant tumour. Scientific Reports 7.
- 139 Xu, H.Y., Meng, J., and Kong, H. (2010). What are carbon nanotubes' roles in anti‐tumor therapies? Science China Chemistry 53: 2250–2256.
- 140 Cheung, W., Pontoriero, F., Taratula, O. et al. (2010). DNA and carbon nanotubes as medicine. Advance Drug Deliver Reviews 62: 633–649.
- 141 Meng, Y., Wang, S.S., Li, C.Y. et al. (2016). Photothermal combined gene therapy achieved by polyethyleneimine‐grafted oxidized mesoporous carbon nanospheres. Biomaterials 100: 134–142.
- 142 Berd, D., Sato, T., Maguire, H.C. et al. (2004). Immunopharmacologic analysis of an autologous, hapten‐modified human melanoma vaccine. Journal of Clinical Oncology 22: 403–415.
- 143 Yu, J.S., Liu, G.T., Ying, H. et al. (2004). Vaccination with tumor lysate‐pulsed dendritic cells elicits antigen‐specific, cytotoxic T‐cells in patients with malignant glioma. Cancer Research 64: 4973–4979.
- 144 Cai, D., Mataraza, J.M., Qin, Z.H. et al. (2005). Highly efficient molecular delivery into mammalian cells using carbon nanotube spearing. Nature Methods 2: 449–454.
- 145 Rosenberg, S.A., Yang, J.C., Topalian, S.L. et al. (1994). Treatment of 283 consecutive patients with metastatic melanoma or renal‐cell cancer using high‐dose bolus interleukin‐2. Journal of American Medical Association 271: 907–913.
- 146 Rosenberg, S.A., Yang, J.C., White, D.E., and Steinberg, S.M. (1998). Durability of complete responses in patients with metastatic cancer treated with high‐dose interleukin‐2 – identification of the antigens mediating response. Annals of Surgery 228: 307–317.
- 147 Chatterjee, M., Draghici, S., and Tainsky, M.A. (2006). Immunotheranostics: breaking tolerance in immunotherapy using tumor autoantigens identified on protein microarrays. Current Opinion in Drug Discovery 9: 380–385.
- 148 Muller, A.J. and Scherle, P.A. (2006). Targeting the mechanisms of tumoral immune tolerance with small‐molecule inhibitors. Nature Reviews Cancer 6: 613–625.
- 149 Chen, W.R., Singhal, A.K., Liu, H., and Nordquist, R.E. (2001). Antitumor immunity induced by laser immunotherapy and its adoptive transfer. Cancer Research 61: 459–461.
- 150 Naylor, M.F., Chen, W.R., Teague, T.K. et al. (2006). In situ photoimmunotherapy: a tumour‐directed treatment for melanoma. The British Journal of Dermatology 155: 1287–1292.
- 151 Meunier, E., Coste, A., Olagnier, D. et al. (2012). Double‐walled carbon nanotubes trigger IL‐1 beta release in human monocytes through Nlrp3 inflammasome activation. Nanomedicine 8: 987–995.
- 152 Yang, M., Meng, J., Cheng, X.L. et al. (2012). Multiwalled carbon nanotubes interact with macrophages and influence tumor progression and metastasis. Theranostics 2: 258–270.
- 153 Hodge, J.W., Guha, C., Neefjes, J., and Gulley, J.L. (2008). Synergizing radiation therapy and immunotherapy for curing incurable cancers – opportunities and challenges. Oncology 22: 1064–1070.
- 154 Guo, L.R., Yan, D.D., Yang, D.F. et al. (2014). Combinatorial photothermal and immuno cancer therapy using chitosan‐coated hollow copper sulfide nanoparticles. ACS Nano 8: 5670–5681.
- 155 Krieg, A.M. (2006). Therapeutic potential of toll‐like receptor 9 activation. Nature Reviews Drug Discovery 5: 471–484.
- 156 Klinman, D.M., Yi, A.K., Beaucage, S.L. et al. (1996). CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proceedings of the National Academy of Sciences of the United States of America 93: 2879–2883.
- 157 Kumagai, Y., Takeuchi, O., and Akira, S. (2008). TLR9 as a key receptor for the recognition of DNA. Advanced Drug Delivery Reviews 60: 795–804.
- 158 Tao, Y., Ju, E.G., Ren, J.S., and Qu, X.G. (2014). Immunostimulatory oligonucleotides‐loaded cationic graphene oxide with photothermally enhanced immunogenicity for photothermal/immune cancer therapy. Biomaterials 35: 9963–9971.
- 159 Yan, M., Sun, G.Q., Liu, F. et al. (2013). An aptasensor for sensitive detection of human breast cancer cells by using porous GO/Au composites and porous PtFe alloy as effective sensing platform and signal amplification labels. Analytica Chimica Acta 798: 33–39.
- 160 Zhang, X.Y., Peng, Y., Bai, J.L. et al. (2014). A novel electrochemical sensor based on electropolymerized molecularly imprinted polymer and gold nanomaterials amplification for estradiol detection. Sensors and Actuators B: Chemical 200: 69–75.
- 161 Orecchioni, M., Cabizza, R., Bianco, A. et al. (2015). Graphene as cancer theranostic tool: progress and future challenges. Theranostics 5: 710–723.
- 162 Feng, L., Dreyfus, R., Sha, R.J. et al. (2013). DNA patchy particles. Advanced Materials 25: 2779–2783.
- 163 Jin, Y.S., Wang, J.R., Ke, H.T. et al. (2013). Graphene oxide modified PLA microcapsules containing gold nanoparticles for ultrasonic/CT bimodal imaging guided photothermal tumor therapy. Biomaterials 34: 4794–4802.
- 164 Turcheniuk, K., Dumych, T., Bilyy, R. et al. (2016). Plasmonic photothermal cancer therapy with gold nanorods/reduced graphene oxide core/shell nanocomposites. RSC Advances 6: 1600–1610.
