Chapter 5
Materials and Methods in Chemical-Sensor Manufacturing
5.1 Introduction
Fabrication of a chemical sensor consists of integrating a transducer with the sensing element, which includes receptor sites. Often, this is largely a matter of immobilizing the receptor at the transducer surfaces. However, many sensors require additional active components. At the same time, the sensing element should be designed so as to be able to allow the access of the analyte to the receptor. In addition, implantable sensors should be compatible with living tissue. That is why, in general terms, building up the sensing part of the sensor is not simply a problem of receptor immobilization, although this is a key issue.
Commercialization of chemical sensors brings about additional requirements as far as the fabrication method is concerned. The ideal commercial sensor should be as simple as possible and suitable for mass production. The sensor characteristics should be stable for a sufficiently long period of time under operational and storage conditions. As far as stability under operation is concerned, the requirements are less stringent in the case of disposable sensors, but in this case an inexpensive fabrication technology is sought.
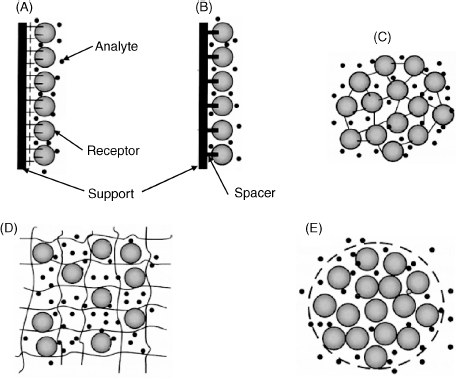
A broad variety of methods for assembling the sensing element has been developed [1]. For example, confinement of an enzyme solution between a perm-selective membrane and the transducer surface is a straightforward method that was widely used at the beginning of the development of enzymatic sensors. However, the stability of this system is rather poor and this method is clearly not suitable for mass production. More advanced approaches to sensor fabrication are summarized in Figure 5.1. In one of these methods the sensing part is assembled as a monolayer on the support surface by physical adsorption (A). More stable systems are obtained by forming strong linkages between the receptor compound and the support, either by covalent bonding or affinity interactions (B). Covalent bonding can also be applied for crosslinking protein molecules (C). Entrapment within a polymer network is another widely used approach (D). Finally, a solution of a biocompound can be encapsulated within vesicles (E). A broad spectrum of immobilization protocols has been developed in the framework of biotechnology, and biosensor science can greatly benefit from this readily available know-how [2].
Figure 5.1 Several methods for building up the sensing layer. (A) Physical adsorption at a solid support; (B) Covalent bonding to the support surface; (C) Support-free crosslinking; (D) Entrapment in a polymer network; (E) encapsulation. The large circles represent biomacromolecules, the smaller circles represent free-diffusing small molecules or ions. Adapted with permission from [3]. Copyright 2011 Wiley-VCH Verlag GmBH & Co. KGaA.
The next sections refer mostly to the use of proteins as bioactive components. Although some of the methods described here are also convenient for nucleic acid immobilization, some particular features relating to the immobilization of nucleic acids are addressed in Chapter 7.
5.2 Noncovalent Immobilization at Solid Surfaces
Immobilization of biocompounds by adsorption involves noncovalent bonding (such as hydrophobic interactions, hydrogen bonding or electrostatic attraction). Such methods are gentle and uncomplicated. Generally, adsorption is achieved by prolonged contact of the support with a solution of the adsorbate. As monolayers are usually formed by such interactions, the analyte can access the sensing part with no restrictions. However, an adsorbed film is not particularly stable and can be damaged by desorption caused by changes in temperature, pH or ionic strength. Nevertheless, an adsorbed layer can be stabilized by crosslinking of adsorbed proteins.
Adsorption of protein molecules conform as a rule to the Langmuir isotherm that relates the surface concentration, ![]() (in mole m−2) with the concentration of the adsorbate in solution c:
(in mole m−2) with the concentration of the adsorbate in solution c:
(5.1) ![]()
K is the equilibrium constant of the adsorption process, ![]() is the maximum achievable surface concentration, and the
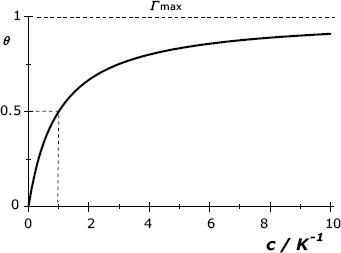
is the maximum achievable surface concentration, and the ![]() ratio is termed the surface coverage. This equation was derived under the assumption that adsorption occurs by equilibrium interaction between the adsorbate and corresponding binding sites at the support surface. It is also assumed that the adsorbed molecules do not interact with each other and that only a single molecular layer forms. As shown in Figure 5.2, the coverage increases with increasing adsorbate concentration and tends asymptotically to a value close to 1 at high concentrations.
ratio is termed the surface coverage. This equation was derived under the assumption that adsorption occurs by equilibrium interaction between the adsorbate and corresponding binding sites at the support surface. It is also assumed that the adsorbed molecules do not interact with each other and that only a single molecular layer forms. As shown in Figure 5.2, the coverage increases with increasing adsorbate concentration and tends asymptotically to a value close to 1 at high concentrations.
Figure 5.2 Graphical representation of the Langmuir isotherm. The concentration is indicated in ![]() units. Half-coverage (
units. Half-coverage (![]() ) is obtained when
) is obtained when ![]() .
.
Despite its intrinsic limitations, adsorption is a method of choice in preliminary investigations, and also for fabricating disposable sensors that are exposed to sample solutions for only short periods of time. Various support materials have been used for adsorption, the most popular being silica, cellulose acetate, activated carbon, and synthetic polymers such as poly(vinyl chloride) (PVC) and polystyrene.
Ion exchangers represent another alternative for noncovalent immobilization. Such materials are porous polymers bearing charged groups that can capture protein molecules bearing the opposite charge (see Figure 5.1A). Electrostatic interactions are affected by the ionic strength of the solution, a quantity that depends on both ion concentrations and ion charges. At a high ionic strength, charged groups can be screened by counterions leading to the attenuation of the electrostatic forces.
5.3 Covalent Conjugation
Outstanding stability of the sensing layer is obtained by chemical reactions resulting in covalent bonds [4, 5]. However, this method can be time-consuming and may need expensive reagents.
In this section, several typical conjugation methods are described by assuming that two species, A and B have to be bound together by a covalent link. Both A and B can be biomacromolecules; alternatively, one of them is a macromolecule and the second is a small molecule or a reactive solid surface. Common reactive groups involved in bioconjugation are hydroxyl, primary amine, carboxyl and sulfhydryl groups that are all ubiquitous in proteins. Bioconjugation can be applied for various purpose such as immobilization on solid supports [5], attaching small molecular labels or linking enzyme or nanoparticle labels to biomacromolecules. The covalent bond should not involve groups located in the vicinity of the active site of the bioreceptor in order to preserve its biological activity.
If the conjugation partners cannot react directly (which is often the case), crosslinking is performed in two stages. In the first stage (activation), a conjugation reagent interacts with the species of interest to append a reactive group to it. This group then reacts with the second species.
A wealth of protocols for covalent crosslinking has been developed within the framework of various biological disciplines [3, 6]. Chemical-sensor technology can benefit advantageously from this vast pool of expertise.
5.3.1 Zero-Length Crosslinkers
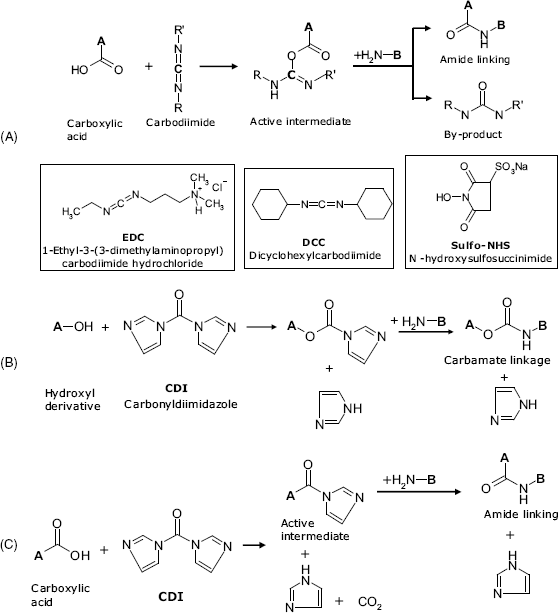
In this approach, one atom of a molecule is covalently attached to an atom of a second molecule in a condensation reaction that results in a two-atom linkage. For example, conjugation of carboxyl and amine derivatives can be performed by compounds of the carbodiimide class. Typical reagents of this type are EDC and DDC that are shown in Figure 5.3A. Such reagents react first with a carboxyl group to yield a reactive intermediate that reacts next with a primary amine group giving an amide linkage. EDC coupling occurs best in the presence of sulfo-NHS (Figure 5.3A) that increases the solubility of the intermediate product and thus enhances the effectiveness of the amine attack. Amide linking can also be obtained by activation with CDI (Figure 5.3B and C).
Figure 5.3 Crosslinking by means of zero-length crosslinkers. (A) Crosslinking of carboxyl and amino groups by means of carbodiimides; (B) crosslinking of alcohols and amines by means of N,N.-carbonyldiimidazole (CDI); (C) crosslinking of carboxyl and amino groups by means of CDI.
5.3.2 Bifunctional Crosslinkers
Bifunctional crosslinkers have a reactive group at each end of a short molecule. By conjugation, such reagents link two species through a spacer arm. When immobilizing a biocompound on a solid support, the presence of a spacer arm has particular advantages. Thus, the immobilized molecule has preserves sufficient mobility that results in enhanced reaction velocity of the recognition process due to less steric hindrance. In addition, the spacer secures some distance from the support that prevents disturbing effects of the interface microenvironment on the immobilized species.
Homobifunctional crosslinkers contain the same reactive group at each end and are used to conjugate similar functionalities. Thus, glutaraldehyde reacts with the amino group of lysine in proteins, and is widely used to perform crosslinking of protein molecules (Figure 5.4A). In this way, a crosslinked protein network forms. The reaction proceeds via a poorly stable Schiff base intermediate that, by reduction with ![]() or NaCNBH3, gives a hydrazone linkage. Enzyme immobilization by crosslinking with glutaraldehyde is a very popular method as it produces a very stable preparation. Crosslinking with glutaraldehyde is also a convenient method to append proteins to amine-functionalized solid supports.
or NaCNBH3, gives a hydrazone linkage. Enzyme immobilization by crosslinking with glutaraldehyde is a very popular method as it produces a very stable preparation. Crosslinking with glutaraldehyde is also a convenient method to append proteins to amine-functionalized solid supports.
Figure 5.4 Crosslinking with homobifunctional crosslinkers. (A) Glutaraldehyde linking of two amine derivatives; (B) Crosslinking of aldehyde derivatives with adipic acid dihydrazide (ADH); (C) Crosslinking of hydroxyl and amine derivatives by means of CDI.

Homobifunctional hydrazides (such as adipic acid dihydrazide, ADH) can be used to conjugate molecules that contain carbonyl or carboxyl groups. Such reagents also react spontaneously with aldehydes to form hydrazone linkages (Figure 5.4B). This reaction is used to attach amine groups to polysaccharides (for example, dextran) after oxidation of the saccharide with periodate, which creates aldehyde groups.
CDI acts as a bifunctional reagent for crosslinking of hydroxyl and amine derivatives via a carbamate spacer, as shown in Figure 5.4C.
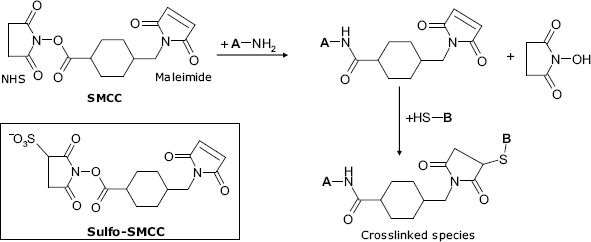
Coupling of amine and thiol derivatives can be achieved by means of a heterobifunctional reagent such as SMCC (succinimidyl-4-(N-maleimidomethyl) cyclohexane-1-carboxylate), as shown in Figure 5.5. This molecule contains two different reactive groups: an N-hydroxysuccinimide (NHS) ester at one end and a maleimide residue at the opposite end. The first group reacts with a primary amine group to yield a reactive intermediate that reacts further via the maleimide fragment with a thiol forming a spacer-linked conjugated species. As antibodies contain a large number of thiol groups, this method is widely used to perform antibody conjugation with an enzymes label.
Figure 5.5 Conjugation by a heterobifunctional reagent. Coupling of an amine and a thiol by means of SMCC (succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate).
5.3.3 Immobilization by Protein Crosslinking
As is apparent from the above discussion, crosslinking reactions can be used to graft biomolecules to solid supports, and to link different molecules in the absence of a support. The second alternative is particularly suited for preparing enzyme gel layers. In order to conserve valuable enzyme, crosslinking is carried out in the presence of an inexpensive protein (such as bovine serum albumin). Glutaraldehyde is a commonly used reagent in this respect. In this case, a critical issue is preserving enzyme activity in the resulting preparation. An advanced version of support-free crosslinking is based on preliminary formation of enzyme aggregates by the addition of salts (salting out effect), organic solvents or polymers. This promotes the precipitation of the enzyme in the form of molecular aggregates under mild conditions that safeguard enzyme activity. Finally, enzyme aggregates are stabilized by crosslinking.
5.4 Supports and Support Modification
5.4.1 General Aspects
Protein immobilization on solid supports (carriers) is a topic of great interest in biotechnology [2, 7], and methods developed in this field are also practical in biosensor research and technology. Many standard bioconjugation reactions can be adapted for grafting biocompounds to functional groups at the support surface either directly, when the support bears reactive groups, or after support activation [4, 5]. This section reviews several suitable support materials for biocompound immobilization and presents typical reactions for support activation and biomolecule grafting to solid supports.
Supports can be made of nonporous or porous materials. Due to the high specific area, porous supports allow the achievement of a high density of immobilized compound, which is beneficial in various applications. Also, pores can act as a diffusion barrier that is favorable in an enzyme sensor as it contributes to expanding the linear response range. Among porous supports, hydrogels occupy a special position as they allow for immobilization by entrapment, and also by covalent grafting.
An important property of solid supports is their wettability, which represents the ability of a liquid to maintain contact with the surface. Wettability is assessed by the contact angle ![]() , which is the angle at which a liquid/gas interface meets a solid surface (Figure 5.6). A contact angle less than 90° indicates that wetting of the surface is favoured, and the fluid will spread over a large area of the surface. Contact angles greater than 90° indicate that wetting of the surface is not favoured and the liquid tends to minimize the contact area with the surface.
, which is the angle at which a liquid/gas interface meets a solid surface (Figure 5.6). A contact angle less than 90° indicates that wetting of the surface is favoured, and the fluid will spread over a large area of the surface. Contact angles greater than 90° indicate that wetting of the surface is not favoured and the liquid tends to minimize the contact area with the surface.
Figure 5.6 The contact angle of a water drop with a hydrophobic surface (A), an intermediate surface (B) and a hydrophilic surface (C).
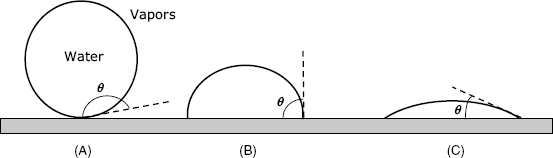
In the case of water, a wettable surface is also termed hydrophilic and a nonwettable surface is termed hydrophobic. Polar functional groups at a surface make it hydrophilic, whereas the absence of such groups renders the surface hydrophobic.
5.4.2 Natural Polymers
Two kinds of natural polymer are particularly suited to enzyme immobilization, namely, polysaccharides and proteins, that provide a hydrophilic, biocompatible environment to the immobilized species. Such materials are prepared in the form of gels and are useful for immobilization by entrapment, but can also be conveniently employed as supports for covalent grafting. Common polysaccharide materials are considered next.
Cellulose is a practical support for protein immobilization but it is susceptible to microbial degradation and is currently used to a lesser extent.
Dextran is a basically linear, water-soluble polysaccharide. It turns into an insoluble porous gel by crosslinking and in this form it exhibits size-exclusion perm-selectivity. Dextran materials are commercially available under the trade name Sephadex. Ionic derivatives of dextran are useful for protein coupling by electrostatic interactions.
Chitosan (Figure 5.7A) is a polysaccharide containing amino groups. It is obtained by processing chitin, which is a byproduct of fishing (crabs, shrimps) and fermentation industries. Chitosan in a soluble form can be mixed with a protein solution and a gel can be formed by glutaraldehyde crosslinking.
Figure 5.7 Natural polysaccharides used for enzyme immobilization.
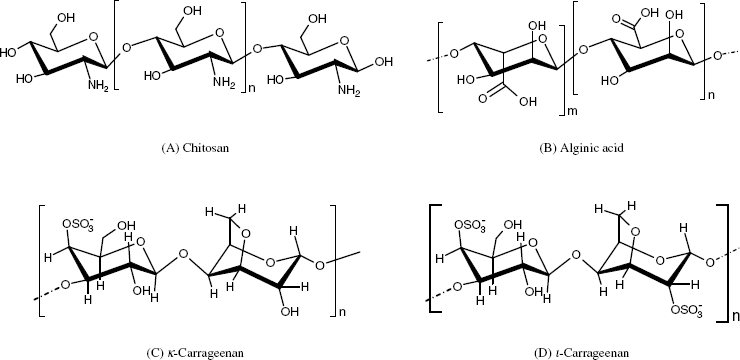
Algal polysaccharides include materials such as agarose and alginates (Figure 5.7B), both of them being able to form gels. Crosslinked agarose (Sepharose) is more satisfactory than crude agarose.
Alginate gels can be formed in the presence of calcium ions or other multivalent counterions. It is very stable between pH 5 and 10 and is mechanically stable in the presence of high concentrations of ions. Enzyme entrapment in alginate gels can be impaired by leakage, but it is a mild and versatile technique.
Carrageenans (Figure 5.7C and D) are extracted from red algae and consist of galactose units that are partially esterified with sulfuric acid. Carragenan gels are very stable at pH > 4.5 and tolerate heat-sterilization conditions. Gelation is induced by calcium and potassium ions but such gels are not stable in the presence of sodium ions.
A common feature of algal polysaccharides and chitosan is the presence of ionic groups such as ![]() ,
, ![]() and
and ![]() . Gelation of such materials can be induced by multivalent ions that interact electrostatically with the charged groups in polysaccharides (ionotropic gelation [8]). A protein can be entrapped in the gel if it is present in the mother liquor.
. Gelation of such materials can be induced by multivalent ions that interact electrostatically with the charged groups in polysaccharides (ionotropic gelation [8]). A protein can be entrapped in the gel if it is present in the mother liquor.
Among protein materials, collagen and gelatin deserve particular mention.
Collagen is abundant in higher vertebrates as a constituent of flesh and connective tissues. It is hydrophilic, water insoluble and displays a high concentration of binding sites. In addition, its fibrous structure and high swellability in water are convenient for enzyme immobilization, which can be performed by adsorption, entrapment and covalent coupling.
Gelatin is a mixture of peptides and proteins produced by partial hydrolysis of collagen on boiling with water. It forms a gel by cooling down the solution to about 40 °C, a temperature that is compatible with many enzymes. However, the gel strength is rather low and crosslinking of the entrapped enzyme is required.
5.4.3 Synthetic Polymers
Synthetic polymers present a large variety of immobilization opportunities due to the possibility of tailoring the chemical structure, morphology and physical properties for diverse applications. In addition, such materials are inert to microbial attack. Within the broad class of synthetic polymers it is possible to distinguish active polymers and inactive polymers. Compounds in the first class bear active groups that can react directly with functional groups in biocompounds in order to perform covalent coupling. Inactive polymers need activation with suitable reagents prior to coupling. Various polymers purposely designed for enzyme immobilization are commercially available.
Polystyrene includes benzene as a pending group that can be easily converted into an amino derivative by nitration and reduction. This can be further activated by diazotization. If the hydrophobicity of polystyrene raises compatibility problems, it is practical to use materials obtained by copolymerization of styrene with hydrophilic monomers such as acrylic acid. Polystyrene is inexpensive, readily available and, after functionalization, displays a high density of binding groups.
Acrylic polymers are available in different forms, each of them including specific reactive groups such as carboxyl, anhydride, amide, hydroxyl, nitrile or epoxide. Thus, polyacrylates and polymethacrylates contain carboxyls and form a negatively charged matrix. Polyacrylamide is widely used both as a matrix for gel entrapment and as a support for covalent coupling. As linear polyacrylamide is water soluble, gelation is induced by crosslinking.
Polyamides, known as nylons are a category of copolymers formed by condensation of ![]() -dicarboxylic acids and
-dicarboxylic acids and ![]() -diamines. Polyamides are available in a variety of physical forms, such as fibers, membranes, powders and tubes. These materials have a good mechanical strength, biological resistance and hydrophilicity. Terminal carboxyl and amino groups are possible reactive groups. In order to increase the density of binding groups, polyamides are subject to mild acid depolymerization that generates carboxyl and amino groups on the surface. The abundant amide groups can be converted into another group that is susceptible to subsequent conjugation with proteins.
-diamines. Polyamides are available in a variety of physical forms, such as fibers, membranes, powders and tubes. These materials have a good mechanical strength, biological resistance and hydrophilicity. Terminal carboxyl and amino groups are possible reactive groups. In order to increase the density of binding groups, polyamides are subject to mild acid depolymerization that generates carboxyl and amino groups on the surface. The abundant amide groups can be converted into another group that is susceptible to subsequent conjugation with proteins.
5.4.4 Coupling to Active Polymers
Certain polymers contain active groups that can be conjugated with biocompounds without preliminary activation. This class includes polymers containing anhydride or epoxide groups or halogens.
The epoxide group (also known as oxirane or ethylene oxide group) is a three-member cycle formed of one oxygen and two carbon atoms (Figure 5.8A). Acid-catalyzed hydrolysis of an epoxide generates a glycol. The epoxide group reacts readily with nucleophile compounds (primary amine, hydroxyl and thiol) in a ring-opening process.
Figure 5.8 Coupling to synthetic polymers containing epoxide (A) carbonate (B) and isocyanate (C) groups.
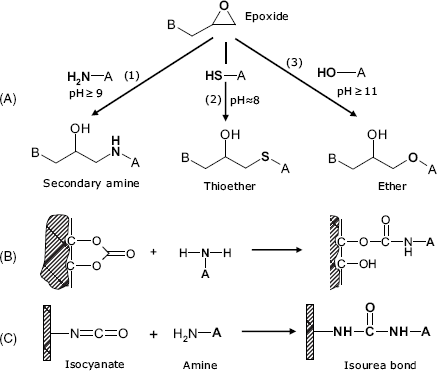
Polycarbonates contain –O–(C=O)–O– groups and can be prepared in either a soluble or an insoluble form suitable for covalent grafting of proteins by reaction with the amino group (Figure 5.8B)
The isocyanate group (–N=C=O) reacts easily with the amino group to produce a urethane linkage (–NH–(C=O)–O–, also called an isourea linkage) that functions as a linker to biomolecules (Figure 5.8C). The isothyocyanate group (–N=C=S) behaves similarly.
Certain halogenated copolymers are suitable for immobilization via reaction of the halogen with primary amines giving secondary amine bonds.
5.4.5 Coupling to Inactive Polymers
Polymers containing amino, hydroxyl and carboxyl groups are widely employed as immobilization supports, but coupling to such materials can only be carried out after prior activation.
Amine groups can be present in raw polymers or can be created by chemical conversion of other functional groups. It can be activated by diazotization, as shown in Figure 5.9A or by reaction with crosslinkers such as glutaraldehyde or trichlorotriazine (cyanuric acid).
Figure 5.9 Common coupling reactions for inactive polymer supports. (A) Amine activation by diazotization; (B) activation of vicinal hydroxyls by cyanogen bromide; (C) trialkoxysilane functionalization; (D) activation of separate hydroxyl by trichlorotriazine (cyanuric chloride); (E) activation of carboxyl group by thionyl chloride; (F) coupling to amide group via acyl azide; (G) Conversion of nitrile group into amine.
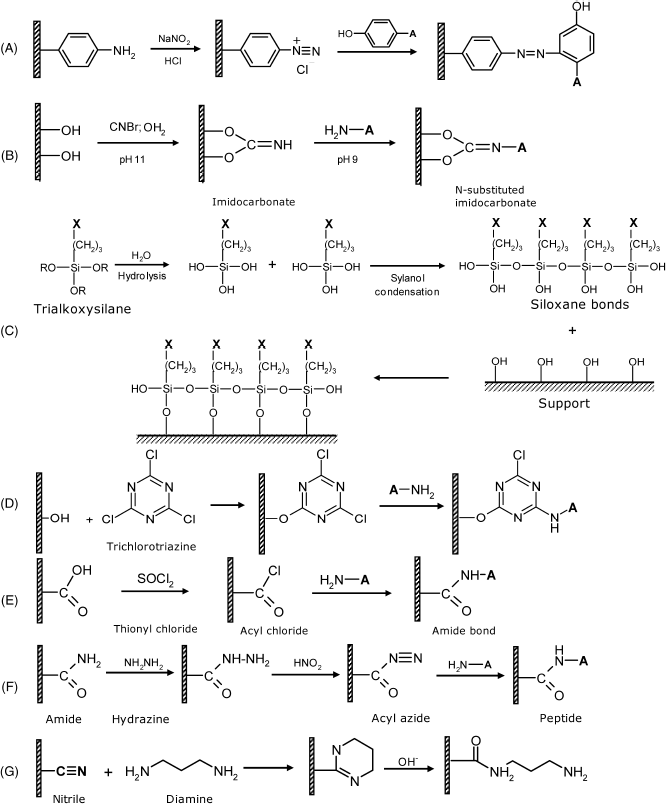
Vicinal hydroxyl groups are found in polysaccharides as well as in polyvinyl alcohol. Oxidation of polysaccharides with periodate produces aldehyde groups that can be crosslinked according to Figure 5.4B. Activation with cyanogen bromide (CNBr) yields reactive imidocarbamates that react with amine groups in proteins to form a peptide bond (Figure 5.9B).
Reactions with trialkoxysilanes provide a wealth of methods for attaching various functionalities (X) for subsequent coupling. (Figure 5.9C). Various groups can be attached in this way (including amino, carboxyl, thiol or epoxide) using suitable trialkoxysilanes. When affinity binding is sought as an immobilization method, the surface can be functionalized by a biotin-derivatized precursor. As thiols bind spontaneously to gold, gold surfaces can be covered with a silica layer by sol-gel chemistry using a thiol-derivatized trialkoxysilane.
Separate hydroxyl groups can be found in synthetic polyols, such as polyethylene glycol and related polymers. Such groups can be activated by reaction with CDI (Figure 5.4C) or with trichloro-s-triazine (Figure 5.9D). Functionalized epoxide derivatives react with hydroxyl as shown in Figure 5.8A.
Carboxyl groups can be activated via the reaction of carbodiimide in the presence of sulfo-NHS (Figure 5.3A). Also, reactions with CDI (Figure 5.3B), thionil chloride (Figure 5.9E) and the Woodward's reagent are commonly employed to this end.
The amide group is present in polyacrylamide and also in Nylon-type polyamides. This group reacts with hydrazine to form an acyl azide derivative that couples with amino groups in biomolecules (Figure 5.9F).
The nitrile group is present in poly(acrylonitrile) and related polymers. This group can be converted to carboxyl by acid or base catalysis. Nitrile activation can also be carried out by reduction to amine or by aminolysis leading to an amine side chain (Figure 5.9G). Further amine activation (e.g., by glutaraldehyde) allows various biocompounds to be grafted to the polymer. Remarkably, the conversion of nitrile groups into amino groups results in the formation of a polymeric gel with a high swelling factor.
5.4.6 Inorganic Supports
Inorganic materials such as controlled-pore glass, silica and metal oxides (![]() ,
, ![]() ) are characterized by high chemical stability that imparts resilience to chemical and thermal treatments. Selection of a support from among such materials is mostly determined by their resilience at extreme pH values. Thus, raw controlled-pore glass is not stable at pH > 8 but its durability is increased by coating it with zirconium oxide.
) are characterized by high chemical stability that imparts resilience to chemical and thermal treatments. Selection of a support from among such materials is mostly determined by their resilience at extreme pH values. Thus, raw controlled-pore glass is not stable at pH > 8 but its durability is increased by coating it with zirconium oxide. ![]() and
and ![]() resist alkaline media well, whereas silica is better suited to acidic solutions. As hydroxyl groups are present at the surface of these materials, their functionalization can be achieved by reaction with alkoxysilanes (Figure 5.9C).
resist alkaline media well, whereas silica is better suited to acidic solutions. As hydroxyl groups are present at the surface of these materials, their functionalization can be achieved by reaction with alkoxysilanes (Figure 5.9C).
5.4.7 Carbon Material Supports
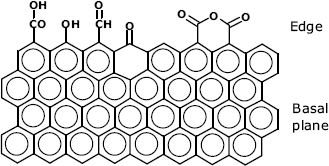
Various forms of graphite and other carbon materials are commonly employed as electrode materials in electrochemical sensors [9]. Graphite consists of parallel sheets of ![]() -bonded carbon atoms held together by van der Waals interactions. The properties of the sheet edge differ essentialy from those of the basal plane (Figure 5.10). Thus, the basal plane is hydrophobic and resistant to chemical attack. Conversely, the unsaturated covalent bond at the edge imparts chemical reactivity and normally various oxygen functionalities are appended to marginal carbons. A high density of carboxyl groups at the edge can be obtained by chemical or electrochemical oxidation. Various forms of graphite materials are available and the immobilization method is selected in accordance with the surface constitution. Surfaces displaying preponderantly basal planes are suitable for adsorptive immobilization of hydrophobic compounds. Conversely, surfaces consisting mostly of edges are hydrophilic. Moreover, in neutral and alkaline solution, dissociation of carboxyl groups imparts to the surface a negative charge. Covalent bonding to such surfaces is achievable via suitable activation of the carboxyl group.
-bonded carbon atoms held together by van der Waals interactions. The properties of the sheet edge differ essentialy from those of the basal plane (Figure 5.10). Thus, the basal plane is hydrophobic and resistant to chemical attack. Conversely, the unsaturated covalent bond at the edge imparts chemical reactivity and normally various oxygen functionalities are appended to marginal carbons. A high density of carboxyl groups at the edge can be obtained by chemical or electrochemical oxidation. Various forms of graphite materials are available and the immobilization method is selected in accordance with the surface constitution. Surfaces displaying preponderantly basal planes are suitable for adsorptive immobilization of hydrophobic compounds. Conversely, surfaces consisting mostly of edges are hydrophilic. Moreover, in neutral and alkaline solution, dissociation of carboxyl groups imparts to the surface a negative charge. Covalent bonding to such surfaces is achievable via suitable activation of the carboxyl group.
Figure 5.10 Graphite structure.
A very common method for integrating biocompounds with carbon materials is based on carbon paste materials [10]. The carbon paste consists of graphite powder mixed with an oil binder and a suitable modifier such as a synthetic or biological receptor. By proper selection of the binder, the modifier nature and the component percentage, a huge variety of carbon paste compositions can be prepared for applications in sensors for inorganic, organic and biological species. Mass production of sensor including similar mixture as planar films can be achieved by screen-printing technology (Section 5.13.2).
Contemporary research in the field of carbon materials focuses on carbon nanomaterials such as graphene, fullerene and carbon nanotubes. This topic, which is of a high interest in chemical-sensor technology, is addressed in detail in Chapter 8.
5.4.8 Metal Supports
Due to their chemical inertness, noble metals such as gold and, to a lesser extent, silver, platinum and palladium are commonly used in various types of chemical sensor.
Molecular monolayers can be assembled at a metal surface by chemisorption of molecules containing a reactive head-group. A widely used method of this kind is based on self-assembly of thiols on gold or other metals.
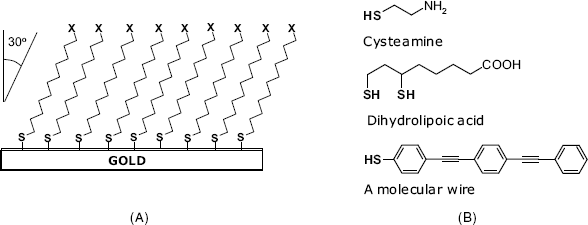
Thiols (R–SH, also referred to as mercaptans) react spontaneously with a gold surface to form strong chemical bonds via the sulfur head group [11]. Under normal conditions, this process is irreversible, which results in high stability of the self-assembled monolayer. In general, the adsorbed alkylthiols molecules form close-packed monolayers with the alkane chain tilted at about ![]() from the vertical (Figure 5.11A). van der Waals interactions between alkyl tails result in a regular pattern that is characteristic of a self-assembled monolayer (SAM). Thiol monolayers can also be formed on silver or platinum supports. Other sulfur derivatives, such as thiones (
from the vertical (Figure 5.11A). van der Waals interactions between alkyl tails result in a regular pattern that is characteristic of a self-assembled monolayer (SAM). Thiol monolayers can also be formed on silver or platinum supports. Other sulfur derivatives, such as thiones (![]() [12]) or thiocarboxylic acids (RC(=O)SH) behave similarly. Selenols (R–SeH) also form self-assembled monolayers on gold [13].
[12]) or thiocarboxylic acids (RC(=O)SH) behave similarly. Selenols (R–SeH) also form self-assembled monolayers on gold [13].
Figure 5.11 (A) The conformation of an alkanethiol layer at a gold surface. X = H (in alkanethiols) or a functional group (e.g., –COOH, or –![]() ). (B) Common thiol reagents for functionalization of a gold surface by sulfur chemisorption.
). (B) Common thiol reagents for functionalization of a gold surface by sulfur chemisorption.
Mixed layers are made using mixtures of functionalized alkanethiols with unfunctionalized alkanethiols for diluting the functionalities at the modified surface.
Gold electrodes and gold surfaces are widely used in the design of chemical sensors. In such instances, modification by chemisorption of thiols is a method of choice for attaching functional molecules. This can be achieved simply by hydrophobic interaction of a target molecule with a nonfunctionalized thioalkane layer. A clean gold surface is hydrophobic but it turns hydrophilic by chemisorption of –OH terminated thiols. If the end group in the surface layer bears an electric charge (such as in ![]() or
or ![]() ), charged molecules can be attached by electrostatic interactions. A more firm immobilization is achieved by covalent grafting to terminal functionalities in the thiol molecules. Suitable linking groups can be attached to the surface by chemisorption of amino- or carboxyl derivatives such as cysteamine, and dihydrolipoic acid (Figure 5.1B). Remarkably, molecular wires can also be appended to gold surfaces by thiol chemisorption. A molecular wire is a linear molecule containing a system of conjugated
), charged molecules can be attached by electrostatic interactions. A more firm immobilization is achieved by covalent grafting to terminal functionalities in the thiol molecules. Suitable linking groups can be attached to the surface by chemisorption of amino- or carboxyl derivatives such as cysteamine, and dihydrolipoic acid (Figure 5.1B). Remarkably, molecular wires can also be appended to gold surfaces by thiol chemisorption. A molecular wire is a linear molecule containing a system of conjugated ![]() -bonds [14]. Due to delocalization,
-bonds [14]. Due to delocalization, ![]() -electrons can flow along this molecule allowing for electric contact between the metal and redox species within the sensing layer.
-electrons can flow along this molecule allowing for electric contact between the metal and redox species within the sensing layer.
Moreover, thiol-functionalized receptors (such as proteins or oligonucleotides) spontaneously form a chemisorbed surface layer on gold. Many protein molecules contain thiol groups in cysteine residues and react directly. If such groups are absent, one can resort to Traut's reagent (2-iminothiolanel), which reacts with amino groups in proteins and appends a short thiol tail. Other receptors can also be obtained as thiol-derivatives for immobilization by sulfur chemisorption on gold. In order to impart more stability to the surface layer, several thiol groups are appended to the receptor molecule so as to form multiple sulfur–gold linkages.
Chemisorbed close-packed layers only form with long linear thioalkanes. Large receptor molecules form a loose layer leaving a large fraction of the surface unoccupied. In order to prevent nonspecific interactions with this surface, a linear thioalkane assembled in a second step to form a compact layer over the surface left free after receptor immobilization.
Thiol chemisorption is widely used in various kinds of chemical sensor [15–17] such as amperometric sensors, piezoelectric sensors and sensors based on the field-effect transistor. Gold nanoparticles can be crosslinked to various biomolecules by a similar approach. Thiol self-assembly is also an important method in microtechnology [18].
An alternative method for self-assembly on metal surfaces relies on aryl diazonium salts ![]() , where Ar is an aromatic ring and A– is an anion). By electrochemical reduction, such compounds yield a compact film that adheres well to the gold surface. Thus, 4-carboxyphenyl diazonium has been deposited on gold and used for crosslinking polypeptides as metal ion receptors [19]. Compared with thiol chemisorption, diazonium self-assembly produces a more stable and compact layer of short spacers that performs better as linkers to the metal surface.
, where Ar is an aromatic ring and A– is an anion). By electrochemical reduction, such compounds yield a compact film that adheres well to the gold surface. Thus, 4-carboxyphenyl diazonium has been deposited on gold and used for crosslinking polypeptides as metal ion receptors [19]. Compared with thiol chemisorption, diazonium self-assembly produces a more stable and compact layer of short spacers that performs better as linkers to the metal surface.
5.4.9 Semiconductor Supports
Silicon is currently the standard material in microelectronics and is therefore widely available. In addition, well-established micromachining technologies allow for patterning the silicon surface in the form of sensor arrays. In addition, the control of the electronic properties of silicon by coating with bio-organic layers opens up new perspectives in both microelectronics and sensor technology [20, 21].
Modification of the silicon surface begins with activation by a chemical treatment that produces reactive groups at the surface. Thus, hydroxyl groups can be formed by treatment with an oxygen plasma followed by hydrolysis in water. These groups react with trichlorosilanes in the gas phase as shown in Figure 5.12A. The resulting self-assembled monolayer is stabilized by siloxane bonds as well as van der Waals interactions between the alkane chains.
Figure 5.12 (a) Modification of silicon surface by trichlorosilanes. X can be an amino group that is suitable for subsequent crosslinking. (b) Silicon surface modification by self-assembly of diazonium salts.
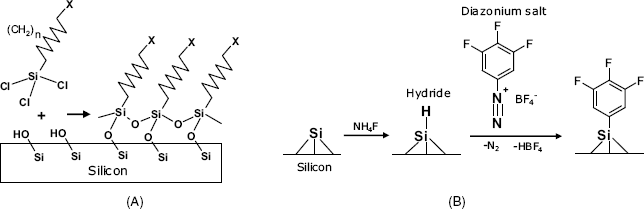
Another approach is based on prior formation of a hydride layer at the silicon surface, either by temperature treatment with ![]() in vacuum or by etching with HF or
in vacuum or by etching with HF or ![]() solutions [22]. Thermally or ultraviolet-driven reactions with a 1-alkene molecule appends it by a Si–C bond. An alkyl layer covalently bound to the silicon surface forms in this way. The terminal methyl in these molecules can be activated by photochemical reactions. Direct linking of functionalized 1-alkenes is hampered by the reactivity of the functional group with the surface hydride. That is why the functional group should be protected prior to grafting and deprotected thereafter. Carboxyl, amino and thiol groups can be appended in this way to the silicon surface. Alternatively, crosslinking reagents can be first attached to silicon in order to perform subsequent grafting of functional groups of interest.
solutions [22]. Thermally or ultraviolet-driven reactions with a 1-alkene molecule appends it by a Si–C bond. An alkyl layer covalently bound to the silicon surface forms in this way. The terminal methyl in these molecules can be activated by photochemical reactions. Direct linking of functionalized 1-alkenes is hampered by the reactivity of the functional group with the surface hydride. That is why the functional group should be protected prior to grafting and deprotected thereafter. Carboxyl, amino and thiol groups can be appended in this way to the silicon surface. Alternatively, crosslinking reagents can be first attached to silicon in order to perform subsequent grafting of functional groups of interest.
An alternative route relies on the reaction of surface hydride with diazonium salts, as shown in Figure 5.12B. Coupling to hydride is initiated by electrochemical oxidation of such salts yielding an aryl radical. Diazonium coupling proceeds spontaneously at room temperature certain compounds (including molecular wires) [23].
A relatively new semiconductor material is doped diamond that is commercially available in the form of thin layers prepared by chemical vapor deposition on various supports. Its main advantages over silicon are the higher chemical stability and the large potential window for electrochemical reactions. Biomolecules can be covalently grafted to a hydrogen-terminated diamond surface [20].
5.5 Affinity Reactions
The high specificity of affinity interactions is advantageously exploited to link various species modified with affinity reagents. The most frequently used system of this kind is the avidin–biotin couple [24–26].
Avidin and streptavidin are proteins with different origin. Avidin is present in egg white, whereas streptavidin is found in the cell membrane of Streptomyces bacteria. These proteins share a common property, namely, both of them combine by affinity interactions with the small molecule biotin (also known as vitamin H or vitamin ![]() , Figure 5.13A). This interaction involves the heterocyclic moiety of biotin and rests on multiple hydrophobic and hydrogen bondings that impart to the complex an outstanding stability (dissociation constant close to
, Figure 5.13A). This interaction involves the heterocyclic moiety of biotin and rests on multiple hydrophobic and hydrogen bondings that impart to the complex an outstanding stability (dissociation constant close to ![]() ). Avidin and streptavidin molecules are homotetramers, each of the four subunit being able to bind a biotin molecule.
). Avidin and streptavidin molecules are homotetramers, each of the four subunit being able to bind a biotin molecule.
Figure 5.13 The biotin–avidin system. (A) the biotin molecule: (B) immobilization of a biotinylated species to a (strept)avidin modified support; (C) conjugation of two biotinylated species by a (strept)avidin bridge.
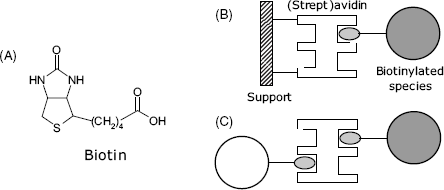
Biotin can be conjugated with proteins by reaction of the carboxyl group with the amino group of lysine. Alternatively, it can be derivatized with reactive groups in order to perform crosslinking with the compound of interest [27, 28]. The second alternative is more convenient because it produces a longer linker and avoids steric hindrance in the interaction with (strept)avidin. Biotin and its derivatives are widely used for conjugation of both proteins [29] and nucleic acids [30] with a (strept)avidin functionalized support (Figure 5.13B). Moreover, due to the multivalent character of (strept)avidin, up to four biotinylated species can be assembled with such a molecule (Figure 5.13C). This property allows the growing of intricate structures such as multilayers or dendrimers.
Avidin is a glycoprotein, whereas streptavidin is a genuine protein that makes it less prone to nonspecific interactions. By removing the saccharides from the avidin molecule, one obtains a product known as NeutrAvidin. As a result of carbohydrate elimination, NeutrAvidin is not prone to nonspecific interactions that can occur in the case of avidin. However, the biotin binding capacity is retained because the carbohydrate is not necessary for this interaction. NeutrAvidin has a near-neutral isoelectric point (pI = 6.3) and is therefore not electrically charged in neutral solutions. Consequently, nonspecific electrostatic interactions are also reduced to a minimal level.
Coupling by affinity proceeds under gentle conditions with a high specificity and yields very stable products. However, the cost of materials can be prohibitive in some applications.
5.6 Thin Molecular Layers
Assembly of one or several molecular layers on a solid support is a practical method for building up the sensing part of the sensor and integrating it with a transducer [31]. This method allows the smart structuring of the sensing layer according to predefined conditions and also provides a biocompatible environment for biological receptors. Such layers can be obtained by self-assembly, a process in which a disordered system of pre-existing components forms an organized structure as a consequence of specific, local interactions among the components. A self-assembly process is spontaneous and is often reversible.
A self-assembled layer can also be obtained by strong interactions (such as chemisorption) with a solid surface. Hydrophobic interactions provide another alternative that is appropriate for assembling surfactants and other amphiphilic compounds. Electrostatic interaction between successive layers allows regular sequences of molecular layers to be assembled by the so-called layer-by-layer method. Organized sequences of molecular layers can also be obtained by affinity interactions.
Such methods belong to the field of Supramolecular Chemistry that addresses large and complex entities formed from distinct molecules assembled by noncovalent bonding [32]. Supramolecular chemistry is essential in living organisms as many biocompounds are built up according to supramolecular chemical principles and provide a rich source of inspiration for synthetic and semisynthetic chemistry. The quaternary structure of some proteins, as well as affinity interactions and formation of enzyme–substrate complexes, are typical examples of supramolecular interactions.
5.6.1 Self-Assembly of Amphiphilic Compounds
Typical amphiphilic compounds are surfactants, which are molecules with a hydrophilic head and a long hydrophobic tail. The hydrophilic head can be either an ionized group (such as ![]() or
or ![]() ) or a polar, nonionic moiety, while the hydrophobic tail is a long hydrocarbon chain. Among various classes of surfactants, phospholipids (such as phosphatidylcholine, (Figure 5.14)) deserve a particular mention. Phospholipids are of interest because they are present in natural membranes and, therefore, are compatible with biocompounds.
) or a polar, nonionic moiety, while the hydrophobic tail is a long hydrocarbon chain. Among various classes of surfactants, phospholipids (such as phosphatidylcholine, (Figure 5.14)) deserve a particular mention. Phospholipids are of interest because they are present in natural membranes and, therefore, are compatible with biocompounds.
Figure 5.14 Structure of phosphatidylcholine, a typical phospholipid (A) and its molecule conformation (B).
When a small amount of surfactant is mixed with water it tends to accumulate at the surface with the hydrophobic tail sticking out from the water. However, if the surfactant concentration is over a certain level (termed the critical micellar concentration), its molecules assemble in small molecular aggregates called micelles in which the hydrophilic heads are oriented toward the aqueous phase (Figure 5.15A). Although this figure shows only a spherical micelle, micelles can also be assembled as rods. Rod-like micelles can further assemble into hexagonal arrangements looking like a submillimeter honeycomb.
Figure 5.15 Structure of a micelle (A) and a liposome (B). (A) Adapted with permission from http://commons.wikimedia.org/wiki/File:Micelle_scheme-en.svg Last accessed 17/05/2012. (B) Adapted with permission from http://commons.wikimedia.org/wiki/File:Liposome_scheme-en.svg Last accessed 17/05/2012.
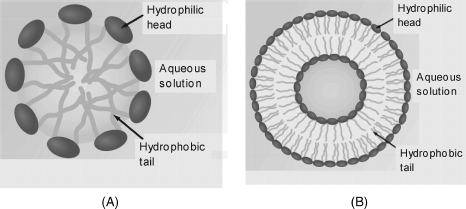
A more advanced degree of organization is achieved in liposomes (Figure 5.15B). Liposomes are artificially prepared vesicles made of surfactant bilayers with a minute amount of aqueous phase occluded within.
Liposomes can be obtained by evaporation of the organic solvent of a phospholipid solution so that a thin film is formed at the bottom of round bottom flask. Then, a liposome suspension in an aqueous buffer solution is formed under stirring or sonication. Controlled-size liposomes form when using surfactant micelles or proteins as templates.
A liposome can consist of a single bilayer (Figure 5.15B) (unilamellar liposome) or of many bilayers stacked upon one another (multilmellar liposomes). Multilamellar liposomes are larger in size but of nonuniform size and undergo fast sedimentation, which may limit their application.
Small molecules, but also proteins, can be entrapped in liposomes when present in the mother liquor. Enzyme entrapment in liposomes stabilizes the enzyme and provides a barrier to inhibitors such as small molecules and metal ions. Selective permeability can be imparted to liposomes by forming size-selective or charge-selective pores in the bilayer.
5.6.2 Bilayer Lipid Membranes
Bilayer lipid membrane are essential structure in living cells and can be easily prepared artificially using natural phospholipids [33–35]. A widely used method for preparing supported lipid bilayers is the Langmuir–Blodgett technique [36].
The Langmuir–Blodgett technique gives rise to molecular layers attached to an inert solid support by adsorption (Figure 5.16). In order to prepare a Langmuir–Blodgett film, a tiny concentration (far less than the critical micellar concentration) of amphiphilic molecules are spread at the air/water interface where they adjust themselves with the hydrophobic tail sticking out from water to form a disordered layer. Lateral compression of this layer produces a close-packed monolayer that is consolidated by mutual molecular interactions. This layer is then transferred to a solid surface by dipping followed by slow retraction. The hydrophilic end will be oriented towards the support surface when the surface is hydrophilic, whereas an opposite orientation will be adopted with hydrophobic supports.
Figure 5.16 Deposition of a Langmuir–Blodgett film on a hydrophobic solid support. (A) random film at the surface of a diluted surfactant solution; (B) close-packed film formed by compression; (C) transfer of the film by dipping the support into the solution; (D) bilayer formation by retracting the support. Adapted with permission from [34]. Copyright 2008 John Wiley & Sons, Ltd.
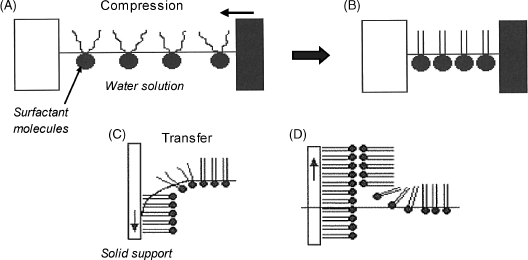
Successive molecular layers can be assembled by the Langmuir–Blodgett technique by repeating the above procedure. In each new layer, molecules are oriented such as to fit the pertinent external moieties in the previous layer. Many applications rely on molecular bilayers. Bilayers formed in this way mimic natural membranes and are thus compatible with many proteins and other biocompounds.
A phospholipid bilayer does not always fulfill the stability requirements imposed by a chemical sensor. Better stability results when using a porous support such as an ultrafiltration membrane. In such a case, microlayers are supported in pores of 1–5 μm diameter. Bilayers formed on a smooth surface can be stabilized by additional treatments such as crosslinking of reactive terminal groups, or postassembly covalent grafting to the solid surface.
A sensing element can be assembled by incorporating a suitable receptor into a bilayer membrane [35, 37]. The receptor can be introduced directly into the surfactant solution prior to the formation of the membrane. Alternatively, the receptor can be codeposited with the surfactant layer at the air/solution interface before the formation of the bilayer. Another method rests on the encapsulation of the receptor molecules in liposomes that are then left to fuse with a preformed bilayer.
Supported bilayers can also be prepared by assembling a phospholipid layer on a preformed alkane thiol self-assembled monolayer. An example is shown in Figure 5.17 that illustrates a method for immobilizing carboxyl-functionalized calixarene. This receptor interacts with cytochrome c by electrostatic interaction between cationic lysine groups in the protein and anionic carboxylate groups in the receptor.
Figure 5.17 Sensing layer for cytochrome c detection using carboxylated calixarene as receptor. The receptor is embedded in a phospholipid layer attached by hydrophobic interaction on an alkanethiol layer assembled on gold. Adapted with permission from [38]. Copyright 2011 Wiley-VCH Verlag GmBH & Co. KGaA.

Firm attachment of a bilayer to a support is achieved in tethered bilayer lipid membranes [39]. In this approach, the first layer is formed by chemisorption of a thiol-tethered lipid to a gold surface, whereas the second layer is obtained by self-assembly of the second layer over the first one.
5.6.3 Alternate Layer-by-Layer Assembly
Molecular multilayers can be assembled on solid supports by consecutive adsorption of alternate monolayers bearing opposite electric charges.
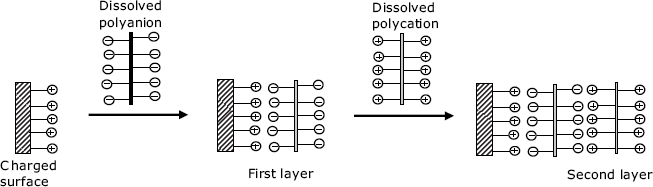
Layer-by-layer assembly does not provide oriented films as the Langmuir–Blodgett technique does, but it can be carried out using a very simple procedure. The solid support should bear ionic groups and the multilayer is assembled using solutions of polyanionic or polycationic compounds. Figure 5.18 illustrates this technique for the case of a positively charged support surface. The first layer is formed by immersing the support in a solution containing a polyanion. After rinsing, the support is immersed in a solution of the polycation to form the second layer. This sequence can be reiterated in order to add more layers. The layer-by-layer technique provides a high degree of control over the width of the layer, owing to the linear growth of the thickness with the number of bilayers.
Figure 5.18 Build up of a multilayer assembly by sequential adsorption of anionic and cationic polyelectrolytes, for example, poly(vinyl sulfate) and poly(allylamine), a polyanion and a polycation, respectively.
Charged protein molecules can be assembled as multilayers simply by substituting the second surfactant by the pertinent protein. As the electric charge is the only restriction, different proteins can be assembled within the same film as consecutive layers (Figure 5.19). In this way, multi-enzyme sensors can be produced.
Figure 5.19 Assembly of different proteins at a solid support using the layer-by-layer method. Mb is myoglobin; Lys is lysozyme; PSS is the poly(styrenesulfonate) polyanion. Adapted with permission from [40]. Copyright 1995 American Chemical Society.
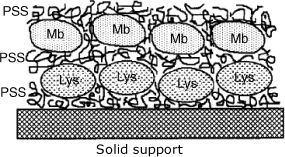
Organized multilayers can also be prepared by exploiting the multivalent character of (strept)avidin in its interaction with biotinylated species. Molecules grafted with multiple biotin units can be assembled sequentially with avidin layers by strong affinity interactions.
The layer-by-layer technique is equally practical for integrating nanoparticles or living cells with the sensing layer. Nanostructured films can be fabricated in this way [41]. This method has proved effective for assembling the sensing layer in sensors based on various transduction methods, such as amperometric, optical and field effect semiconductor devices.
5.7 Sol-Gel Chemistry Methods
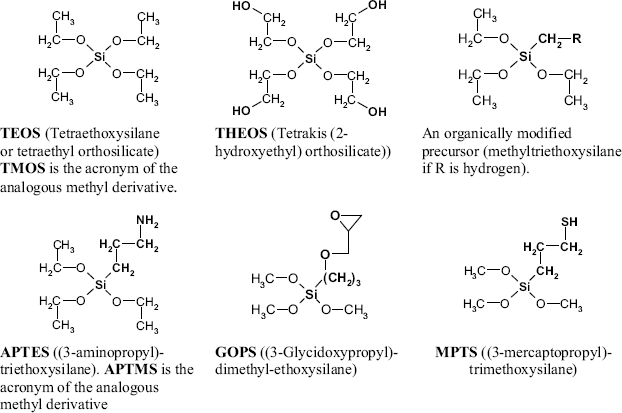
Sol-gel chemistry addresses the preparation of gels by chemical reactions of dissolved precursors [42, 43]. The product is a loose network of polymer chains including an appreciable amount of solvent. Typical materials in this class are the silica gels that form by condensation of orthosilicic acid in acidic solutions of ![]() . They consist of crosslinked polymer chains of –O–Si–O– units. Sol-gel chemistry allows the preparation of similar materials that include organic substituents bound to either silicon or oxygen atoms. The synthesis relies on esters of orthosilicic acids (Figure 5.20) and proceeds by a sequence of hydrolysis (5.2) and condensation (5.3) reactions:
. They consist of crosslinked polymer chains of –O–Si–O– units. Sol-gel chemistry allows the preparation of similar materials that include organic substituents bound to either silicon or oxygen atoms. The synthesis relies on esters of orthosilicic acids (Figure 5.20) and proceeds by a sequence of hydrolysis (5.2) and condensation (5.3) reactions:
Figure 5.20 Typical precursors for the synthesis of silica materials by sol-gel chemistry.
The above sequence produces linear polymer molecules but further hydrolysis and condensation result in a network of crosslinked polymer chains. It is important to note that siloxanes are not stable in an alkaline solutions.
The constitution of the final product depends on the balance of the reaction rates of hydrolysis and condensation, the solution pH being a key parameter. In neutral solutions, hydrolysis is very slow relative to condensation, but this situation is reversed in an alkaline solution. At the same time, if the available amount of water is limited, hydrolysis is much slower than condensation. Fast hydrolysis favors formation of colloidal particles (sols), whereas fast condensation promotes formation of large clusters that, by crosslinking, form a continuous three-dimensional network including water (a gel).
Entrapment of biomolecules in silica gel can be effected in two stages. In the first stage, a sol is produced by reaction of the precursor (for example, TEOS) in an acidic solution. Biopolymers are immobilized after shifting the pH into the neutral region where the structure of the biopolymer is not altered. Under these conditions, the condensation is accelerated yielding a gel that includes biopolymer molecules within its pores (Figure 5.21). However, the alcohol formed in reaction (5.2) can cause protein denaturation. The alcohol should therefore be removed before proceeding to biopolymer entrapment. Some additive, such as poly(ethylene glycol), polysaccharides, polyelectrolytes or ionic surfactants have beneficial effects on the stability of entrapped enzymes as the entrapped molecules are surrounded by additive molecules. In order to prevent precipitation, THEOS should be used as precursor. It is completely miscible with water and, by hydrolysis, it produces ethylene glycol, which is a biocompatible organic solvent. By using THEOS and suitable additives, the immobilization can be effected in a single step consisting of gel formation in the presence of the guest molecules.
Figure 5.21 Entrapment of a phosphatase enzyme in a sol-gel silica network. Adapted with permission from [48]. Copyright 2005 American Chemical Society.
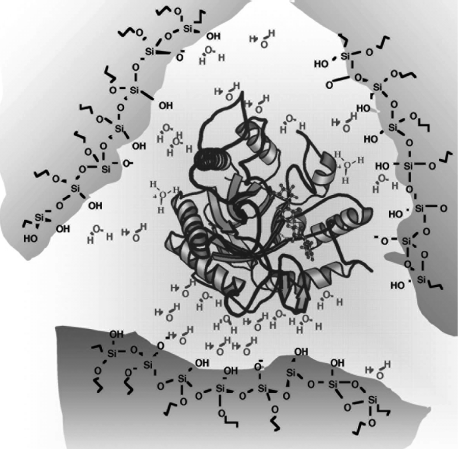
Due to the mild reaction conditions, the sol-gel method is a convenient method for the entrapment of enzymes and other biological materials including living cells and organisms [44, 45]. For example, Figure 5.21 shows an enzyme encapsulated by the sol-gel method in the presence of a surfactant. Remarkably, the enzyme activity is preserved even if the pH in the aqueous phase is very far from the optimum pH of the enzyme. Note that silanol groups can be ionized at a suitable pH imparting negative charges to the gel pore surface. This property brings about some selectivity towards positively charged guest molecules.
The properties of the gel pores can be engineered by using organically modified precursors. Such molecules include an organic group linked directly to a silicon via a carbon–silicon bond, as in methyltrietohysilane and APTES (Figure 5.20). A material obtained by the condensation of organically modified precursors is known as ormosil [46]. One or two C–Si bonded substituents can be introduced in the precursor molecule. Depending on its properties, the substituent imparts to the pore surfaces specific characteristics, such as hydrophobicity (through an alkyl substituent) or an electric charge (APTES with protonated amine groups). By noncovalent interactions, the substituents contribute to the stabilization of the immobilized molecule. The C–Si bonded arm can be designed such as to contain a reactive end group (Figure 5.20), such as amino (in APTES and APTMS), epoxide (in GOPS), or thiol (in MPTS). The reactive group serves as an anchor for covalent conjugation of proteins and another compounds.
Organically modified precursors can be used to activate certain support materials in view of further immobilization of receptor molecules. So, materials with exposed hydroxyls at the surface (glass, cellulose, pretreated carbon) can be modified with reactive groups by reaction with APTES, GOPS or MPTS. A similar treatment can be applied to silicon surfaces after preparing a silicon oxide layer by oxidation and hydrolysis to yield hydroxyl groups. Receptor molecules are then covalently conjugated to the silane derivative linker via the appended reactive group.
Sol-gel chemistry represents a very convenient method for enzyme entrapment due to the mild reaction conditions and the possibility of engineering the surface structure so as to impart specific properties such as hydrophilicity, hydrophobicity or electric charge [45]. Various immobilization protocols based on sol-gel chemistry are available [47].
In summary, sol-gel chemistry is a mild and straightforward method for immobilization of biopolymers and cells in biocompatible matrices. Also, small molecules can be integrated either by inclusion or covalent attachment to the gel network.
Sol-gel entrapment preserves the three-dimensional structure of proteins and hence their biological activity. However, protein denaturation is possible due to the alcohol byproducts. Nanomaterials can be included in the gel matrix in order to bring about additional functionality such as direct electron transfer from redox enzymes to the electrode in amperometric sensors.
This section has focused on silica sol-gel materials, but it is important to note that other materials (such as metal oxides) can be obtained in the gel form by analogous chemistry [49].
5.8 Hydrogels
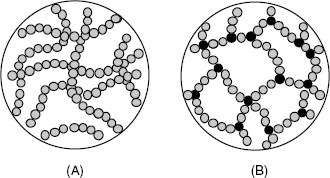
Hydrogels are solid–liquid systems in which a polymeric matrix forms a loose three-dimensional network including a high proportion of water, up to 90% of the total mass [50, 51]. The polymer network results from crosslinking either by physical interactions between polymer molecules or by covalent bonding (Figure 5.22). A xerogel forms from a gel by drying with unhindered shrinkage. Xerogels retain high porosity and a very large specific surface area along with a very small pore size (1–10 nm). When the solvent is removed under supercritical conditions, the network does not shrink and a highly porous, low-density material known as an aerogel is produced.
Figure 5.22 Physically (A) and chemically (B) crosslinked hydrogels. The blank space is filled with water. Adapted from http://commons.wikimedia.org/wiki/File:Structures_of_macromolecules.png?uselang=fr. Last accessed 17/05/2012.
5.8.1 Physically Crosslinked Hydrogels
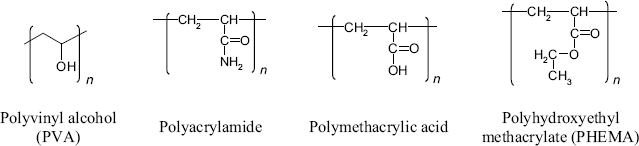
As shown in Figure 5.22A, the stability of a physically crosslinked hydrogel is secured by noncovalent bonding such as hydrogen bonding, van der Waals forces and hydrophobic interactions. Such hydrogels can be obtained from natural polysaccharides (such as dextran or agarose). Certain natural polysaccharides include additional functionalities, such as the amine in chitosan, carboxyl in alginate and carboxyl and sulfonate in some carrageenans (Figure 5.7). Such groups impart particular properties such as an electric charge and can also be practical for conjugation with biocompounds. Protein materials, such as collagen and gelatin are alternative hydrogel materials. Physically crosslinked hydrogels can also be obtained from mixtures of components, such as PVA (Figure 5.23), carboxymethyl cellulose or sodium silicate.
Figure 5.23 Synthetic polymers for hydrogels.
A common procedure for obtaining physically crosslinked hydrogels consists of successive freezing and thawing of the precursor solution in water or a water–organic solvent mixture (freeze-thaw method). The product of this process is often termed a cryogel. During gel formation, growing ice crystals determine the shape and size of the pores that develop after defrosting of the sample. Biocompounds or cells are incorporated in the hydrogel if they present in the system. Alternatively, the species of interest can be adsorbed onto a preformed gel surface or appended to reactive sites purposely included in the gel precursor molecule [52].
5.8.2 Chemically Crosslinked Hydrogels
Such hydrogels consist of networks of branched polymer molecules held together by covalent bonds (Figure 5.22B) and are obtained by polymerization in solution [53]. Typical polymers for such applications are summarized in Figure 5.23. Alternatively, hydrogels can be obtained from prepolymers crosslinked by addition of water (polyurethanes) or by ultraviolet irradiation. Incorporation of a biocomponent into the hydrogel can be effected by adding it to the reaction mixture.
Entrapment in hydrogels is a mild method for immobilization of biomacromolecules or cells. The gel is biocompatible and its matrix is sufficiently loose to allow for penetration of small analyte molecules such as enzyme substrates or oligonucleotides. This method is therefore widely used for the preparation of the sensing element in various types of chemical sensors.
The previous presentation addresses application of hydrogels as a passive matrix for the immobilization of a bioreceptor within the sensing part. However, certain functionalized hydrogels can perform as an active component in the sensing–transduction process. Such functions can be carried out by redox hydrogels, eletroconductive hydrogels or stimuli-responsive hydrogels.
5.8.3 Redox Hydrogels
Redox hydrogels include metal complexes of ruthenium or osmium appended to the main polymer chain [54, 55]. As the metal ion is stable in two oxidation states, electron flow is possible by electron hopping from a reduced metal ion to the oxidized ion under the effect of an applied potential difference. Redox hydrogels are essential components of certain types of amperometric enzyme sensors as they allow direct electron transfer from a redox enzyme molecule to the electrode to be performed. Redox hydrogels have also led to excellent applications in amperometric biosensors based on redox enzyme labels, such as in immunosensors and in nucleic acid sensors. This topic is addressed in detail in Chapters 14 and 16.
5.8.4 Responsive Hydrogels
Certain hydrogels display the outstanding property of changing their volume dramatically under the effect of some physical or chemical stimulus, such as temperature or pH. Such materials are known as responsive hydrogels or smart hydrogels [56–59]. Thus, a response to pH change is secured by appending ionizable acidic groups to the polymer network. Ionization of this group results in a negative charge appearing within the gel. Within the pH range around the apparent ![]() of the acid the gel volume expands by electrostatic repulsion when the pH increases. The same effect is noted with basic group appended polymers, as the protonation of the base produces positive charges, for example, by conversion of a neutral
of the acid the gel volume expands by electrostatic repulsion when the pH increases. The same effect is noted with basic group appended polymers, as the protonation of the base produces positive charges, for example, by conversion of a neutral ![]() group to
group to ![]() . Rigorously speaking, gel expansion is due to the rise in the osmotic pressure as a consequence of the accumulation of counterions within the ionized gel. As this process is reversible, the hydrogel responds to either an increase or a decrease in pH. This physicochemical effect has been exploited in developing pH sensors [60, 61]. As many enzymatic reactions produce hydrogen ions and, consequently, bring about a local pH change, enzyme-containing responsive hydrogels are suitable for substrate determination [59, 62].
. Rigorously speaking, gel expansion is due to the rise in the osmotic pressure as a consequence of the accumulation of counterions within the ionized gel. As this process is reversible, the hydrogel responds to either an increase or a decrease in pH. This physicochemical effect has been exploited in developing pH sensors [60, 61]. As many enzymatic reactions produce hydrogen ions and, consequently, bring about a local pH change, enzyme-containing responsive hydrogels are suitable for substrate determination [59, 62].
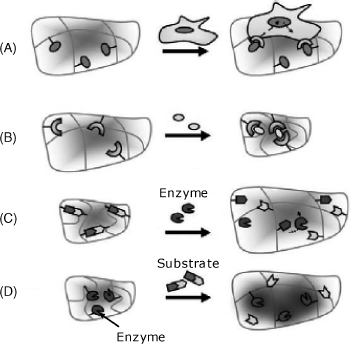
Figure 5.24 summarizes several possible interactions between a responsive hydrogel and a target entity. Thus, living cells can adhere to specific receptors incorporated in the gel (A). Small molecules, ions and also antibodies or other proteins can trigger gel shrinkage by forming bridges between receptor sites within the gel (B). An enzyme that induces the cleavage of chemical bonds within a gel-included substrate causes gel swelling (C). This effect allows the detection of living cells using the effect of a secreted enzyme. A substrate can be sensed if a gel-immobilized enzyme induces reactions with reactive groups appended to the gel network (D).
Figure 5.24 Swelling/collapse of a hydrogel under the effect of chemical stimuli. (A) Interaction of a multitopic target entity with gel-immobilized receptors; (B) bridging of receptors by interaction with the target entity; (C) enzyme detection by cleavage of chemical bonds in the immobilized substrate; (D) Substrate detection by enzymatic reaction with pending groups. Adapted with permission from [59]. Copyright 2007 Elsevier.
The volume change can be reported by various methods [60, 63, 64]. If the gel is confined between a metal support and a metal bending plate, the volume variation causes a variation in the electrical capacity of the capacitor thus assembled. The deformation of a piezoresistive bending plate can be detected by the change in its electrical resistance. Other transduction methods are based on the variation in the electrical resistance of the gel itself in response to the volume change. The change in the gel volume is accompanied by a drastic modification of the refractive index, so, optical methods are particularly suited for transduction. The change in the gel thickness can also be reported by a piezoelectric oscillator. Clearly, an important advantage of smart gel-based sensors arises from the possibility of performing label-free transduction.
In conclusion, hydrogels are outstanding materials for immobilization of both biomacromolecules and living cells. In addition, certain hydrogels can perform as active components of the sensing element, either by providing electrical conductivity or by responding specifically to chemical stimuli. Integration of nanoparticles in hydrogels has led to new materials with very promising properties [65]. Biomolecule-responsive hydrogels appear to be particularly appealing for biosensor development [66].
5.9 Conducting Polymers
Conducting organic polymers are compounds that consist of polyconjugated macromolecules. This particular chemical structure imparts these materials with electrical conductivity, in contrast to common polymers that are insulators. Conducting polymers have backbones of contiguous sp2-hybridized carbon centers. One valence electron on each center resides in a pz orbital, which is orthogonal to the other three sigma bonds. The electrons in these delocalized orbitals have high mobility when the material is doped by oxidation, which removes some of these delocalized electrons. Thus, the conjugated ![]() -orbitals form a one-dimensional electronic band, and the electrons within this band become mobile when the band is partially emptied.
-orbitals form a one-dimensional electronic band, and the electrons within this band become mobile when the band is partially emptied.
Electrochemical deposition is an advantageous method for the synthesis of conducting polymers. This is because it allows for thin films to be prepared at the surface of conducting materials, such as metals or carbon, for application as receptor entrapping matrix in chemical sensors. Alternatively, the synthesis can be conducted so as to obtain a material containing receptor molecules covalently bound to the polymer backbone. In addition, conducting polymers are used as sensing materials in gas sensors and as auxiliary materials in a series of electrochemical sensors. Comprehensive surveys of conducting polymer applications in chemical sensors can be found in refs. [55, 67–69].
Typical conducting polymers are shown in Figure 5.25. Among the conducting polymers, polypyrrole (Figure 5.26A) is the most commonly used [70]; therefore, the subsequent discussion will focus on this compound.
Figure 5.25 Structure of several common conducting polymers.
Figure 5.26 (A) Idealized structure of polypyrrole; (B) electrochemical polymerization of polypyrrole. ![]() is the average fractional charge at the repeating unit of the polymer.
is the average fractional charge at the repeating unit of the polymer.
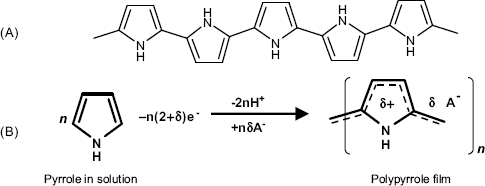
The simplest method of inducing electrochemical polymerization consists of applying a sufficiently positive constant potential to an electrode immersed in an aqueous pyrrole solution. In its first stage, pyrrole oxidation results in radical cations that couple with each other and produce a polymer film. If the electrode potential exceeds a certain limit, the product is an overoxidized polymer.
Clearly, the support should be a conductor material (graphite, platinum) which should be stable under the electropolymerization conditions. Formation of oxides on metal electrodes is detrimental. As the polypyrrole chains bear a positive charge, anions are spontaneously integrated in the network in order to secure electrical neutrality.
According to the idealized structure (Figure 5.26A), the polypyrrole macromolecule features an extended ![]() orbital system. In other words, the
orbital system. In other words, the ![]() electrons are delocalized over the entire macromolecule. Actually, due to the oxidative conditions in the synthesis, many
electrons are delocalized over the entire macromolecule. Actually, due to the oxidative conditions in the synthesis, many ![]() orbitals are not completely filled and such a partially filled orbital includes a positive vacancy. However, vacancies are delocalized as well and each unit in the chain assumes formally a fractional charge,
orbitals are not completely filled and such a partially filled orbital includes a positive vacancy. However, vacancies are delocalized as well and each unit in the chain assumes formally a fractional charge, ![]() (
(![]() ). The electric charge on the polymer backbone is balanced by counterions
). The electric charge on the polymer backbone is balanced by counterions ![]() inserted within the polymer network. The fraction
inserted within the polymer network. The fraction ![]() is called the doping level and the counterion is a dopant. A dopant can be a small inorganic ion or a large polyelectrolyte molecule, that is, a polymer with pendant ionic groups. Due to the charge balance, the doping level also reflects the amount of included counterion. It is worth noting that the type of dopant affects appreciably the properties of the polymer.
is called the doping level and the counterion is a dopant. A dopant can be a small inorganic ion or a large polyelectrolyte molecule, that is, a polymer with pendant ionic groups. Due to the charge balance, the doping level also reflects the amount of included counterion. It is worth noting that the type of dopant affects appreciably the properties of the polymer.
Once prepared, polypyrrole can be subjected to subsequent oxidation or reduction. Such transformations are accompanied by modifications in dopant concentration and consequent modifications of the physical properties. For example, overoxidized polypyrrole is less conductive and more porous.
The most striking property of polypyrrole is its electrical conductivity. From this standpoint, polypyrrole behaves as a semiconductor. Under the effect of an electric field, electrons jump from one molecule to the next one, this process being accompanied by dopant displacement in the opposite direction. The mobility of the dopant has a great effect on the conductivity of the polymer.
Pyrrole can be readily functionalized at the N atom and substituted pyrrole will also undergo electrochemical polymerization, but the arrangement of polymer chains is influenced by the steric effect induced by the substituent.
Taking into account the advantageous properties as well as the easy synthesis of polypyrrole, polypyrrole applications in chemical-sensor design encompasses a very large area [69, 71]. Polypyrrole conductivity is sensitive to gas absorption, which is the basis of the application of polypyrrole in gas sensors [72].
Polypyrrole is widely employed as an entrapment matrix for biological receptors such as proteins and nucleic acids [73]. Physical entrapment is achieved by conducting the synthesis in a solution containing the pertinent receptor. Clearly, this is possible if the receptor molecule bears a negative charge that interacts electrostatically with the positive charge at the polymer backbone. The loose structure of the polymer film permits free diffusion of small analyte molecules and imparts some degree of size-exclusion selectivity.
More control over the structure of the sensing film can be achieved by covalent grafting onto the polymer backbone Thus, pyrrole grafted with a receptor can be copolymerized with crude pyrrole to form a receptor-functionalized film. If the polymerization conditions are not compatible with the receptor, one can resort to another method, namely postpolymerization grafting onto a preformed polymer films. In this approach, the film is prepared by copolymerization of pyrrole and a N-substituted pyrrole monomer. Receptors can then be covalently linked to the polymer through the reactive group at the second monomer unit. Co-polymerization of pyrrole with biotin-functionalized pyrrole allows for subsequent grafting of biotinylated receptors via avidin–biotin linkages. Besides imparting stability to the film, the avidin layer also prevents nonspecific adsorption of sample components.
The stability of a polypyrrole sensing film can be hampered by the hydrophobic character of the backbone that is not compatible with hydrophilic receptor molecules. This problem can be alleviated by synthesizing amphiphilic polymer matrices [74]. Such materials are obtained by the polymerization of monomers consisting of a hydrophilic fragment inserted between two terminal pyrrole residues.
It is also important to point out the use of polypyrrole in fabricating sensor microarrays by electropolymerization. The support in this case is an electrode array. If the electrode at each site is individually connected to the voltage source, particular sensing properties can be imparted to each component of the array by local electrochemical polymerization under specific conditions.
Composites of conducting polymers with various species such as nanoparticles, and organic and bio-organic materials provide various opportunities for chemical-sensor applications [75].
Polypyrrole is extensively employed as the immobilization matrix for various receptors such as enzymes [76–78], nucleic acids [79], and affinity receptors [80, 81].
Other conducting polymers are shown in Figure 5.25. Polyacetylene and poly(para-phenylene) are hydrocarbon compounds, whereas poly(aniline) includes secondary amine bridges that can be protonated to secondary ammonium groups and this makes this material pH-responsive. Polythiophene, like polypyrrole, consists of chains of five-membered heterocycles.
It is worth noting that insulator polymers (such as polyphenol) can also be prepared by electrochemical synthesis [82]. Overoxidized polypyrrole is also a nonconducting polymer. Such polymers are useful as entrapment matrices for enzymes. Due to their insulator character, growing of insulator polymer films is limited to 10–100 nm thickness. Hence, substrate and product diffusion within the film is very fast and allows for a even concentration distribution within it. Electrochemically polymerized dielectric polymers are also employed as insulator layers over transducer surfaces.
Electropolymerization is achievable only at conducting solid surfaces. Plasma polymerization and photochemical polymerization are methods of choice for obtaining polymer coatings on insulator surfaces.
Semiconductor polymers addressed in this section are currently termed simply conducting polymers. This name is assigned also to redox hydrogels. However, the mechanisms of electrical conduction in these two classes of material are fundamentally different.
5.10 Encapsulation
Encapsulation is an immobilization method that consists of enclosing the receptor solution inside a small vesicle (Figure 5.27). The surrounding membrane should be permeable to small molecules (substrate and product) but not to the receptor itself. The capsule size can vary from a few ![]() to a few hundred
to a few hundred ![]() . This method is suitable for the immobilization of sensitive enzymes and whole cells as the native structure of the entrapped species is well preserved. If the membrane remains still permeable to the enzyme one can resort to crosslinking in order to prevent enzyme leakage (Figure 5.27). Crosslinked enzyme aggregates are much larger than individual molecules and are not susceptible to leakage. Crosslinking excludes the necessity to control rigorously the size of membrane pores. Multiple enzyme systems can also be obtained by encapsulation.
. This method is suitable for the immobilization of sensitive enzymes and whole cells as the native structure of the entrapped species is well preserved. If the membrane remains still permeable to the enzyme one can resort to crosslinking in order to prevent enzyme leakage (Figure 5.27). Crosslinked enzyme aggregates are much larger than individual molecules and are not susceptible to leakage. Crosslinking excludes the necessity to control rigorously the size of membrane pores. Multiple enzyme systems can also be obtained by encapsulation.
Figure 5.27 Schematics of enzyme encapsulation and postencapsulation crosslinking. Left: enzyme molecule escape through the pores. Right: bulky enzyme aggregates are tightly confined within the vesicle. Adapted with permission from [2]. Copyright 2005 Wiley-VCH Verlag GmBH & Co. KGaA.
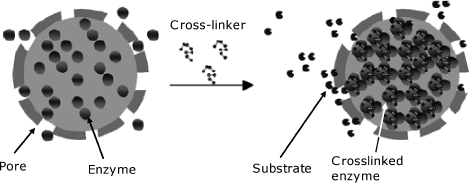
Polymer encapsulation can be achieved by interfacial polymerization. This process is conducted in emulsions formed by mixing an aqueous solution of enzyme with a monomer solution in a nonaqueous solvent. In this way, dispersed aqueous droplets form and the polymerization occurs only at the droplet surface producing vesicles that include enzyme solution. Alternatively, an emulsion can be formed by mixing an aqueous solution of monomer and enzyme with another monomer dissolved in a nonaqueous solvent. In this case, the membrane forms by copolymerization. Encapsulation can also be performed by coating liposome-containing enzymes with a polymer layer.
Other encapsulation methods are based on layer-by-layer technology, sol-gel reactions and hydrogel formation [83].
5.11 Entrapment in Mesoporous Materials
Mesoporous materials are characterized by a regular spatial distribution of pores of similar diameter [84–86]. According to the IUPAC definition, mesoporous materials are porous materials with pore diameters in the range 2–50 nm, which is rather close to the size of proteins. For comparison, microporous materials (such as zeolites) have pore diameters of less than 2 nm, while macroporous materials have pore diameters greater than 50 nm.
Mesoporous silica particles are synthesized by reacting tetraethyl orthosilicate with a template made of micellar rods or another material (Figure 5.28). By reactions similar to that used in the sol-gel method a collection of nanosized spheres or rods is produced that are filled with a regular arrangement of pores. The template can then be removed by a suitable chemical or physical treatment. Periodic mesoporous organosilicates are obtained when organic fragments are included as bridging groups. This modification provides numerous possibilities for tuning chemical and physical properties of the material by varying the structure of the precursors.
Figure 5.28 Schematics of mesoporous silica synthesis. Adapted with permission from [86]. Copyright 2006 Wiley-VCH Verlag GmBH & Co. KGaA.
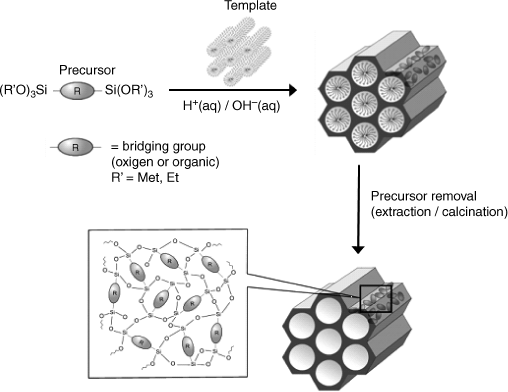
Functionalization of mesoporous materials can be achieving during the synthesis by using precursors substituted with selected functional groups. It is also possible to perform postsynthesis functionalization by grafting other groups onto the material network.
In addition to silica, many other materials can be obtained in the mesoporous form. Thus, nanoporous carbon can be obtained by conversion of sucrose to carbon inside the silica mesopores through mild carbonization. Mesoporous metal oxides and phosphates, as well as mesoporous ceramics, metals and semiconductors have also been obtained.
As suggestively stated in ref. [85], mesoporous materials are “holes filled with opportunities”. Among these opportunities, application of such materials in biosensors is of interest, particularly for enzyme immobilization by entrapment [87, 88]. Enzyme entrapment can be achieved simply by incubation of the mesoporous material with an enzyme solution. In order to prevent the enzyme from leaking, crosslinking with glutaraldehyde or capping of the pores are recommended. Compatibility of the enzyme with the inner pore surface is a key issue. Thus, depending on the protein characteristics, either a hydrophilic (silica) or hydrophobic (carbon) material should be selected as the support. The electrical conductivity of carbon makes it the material of choice for amperometric enzyme sensors.
As an example, Figure 5.29 shows the configuration of the sensing part of an amperometric glucose sensor based on the detection of hydrogen peroxide. The enzyme is entrapped in ordered mesoporous carbon that displays a hierarchical distribution of pore diameters. Larger mesopores (37 nm diameter) accommodate enzyme molecules, whereas the smaller pores (5.6 nm) secure the diffusion of substrate to the catalytic sites.
Figure 5.29 Immobilization of glucose oxidase in ordered mesoporous carbon for application in an amperometric glucose sensor. Adapted with permission from [89]. Copyright 2009 Wiley-VCH Verlag GmBH & Co. KGaA.
Mesoporous materials are also suitable for entrapping mixed assemblies including enzymes and nanoparticles. It is also interesting to note that certain mesoporous materials allow well-organized assemblies of chemical functionalities that elicit enzyme-like catalytic activity to be obtained. Such biomimetic materials function as artificial enzymes.
5.12 Polymer Membranes
Polymer membranes can perform various functions in chemical sensors [90–92]. Thus, polymer membranes can be employed as matrices for physical entrapment of enzymes. In addition, some membranes feature selective permeability and are therefore useful to prevent interference from certain sample components. A perm-selective membranes can be used simply to contain an enzyme solution as a thin a layer at the transducer surface. Moreover, a polymer membrane can act as a diffusion barriers in enzymatic sensors. This membrane control the rate of substrate diffusion to the immobilized enzyme and allows obtaining a linear response function.
Applications of polymer supports and polymer hydrogels have been discussed before. This section addresses mainly compact polymers that are relevant to chemical sensors. It introduces first several methods for obtaining polymer films and then emphasizes typical applications of polymer membranes in sensor development.
5.12.1 Deposition of Polymers onto Solid Surfaces
Preformed polymers can be obtained in a well-characterized form before they are applied to the underlying surface. Deposition of preformed polymers is carried out using polymer solutions that are cast onto solid surfaces and leave a polymer film after the evaporation of the solvent. The surface should be selected so as to be wetted by the solution. If a hydrophobic receptor is added to the polymer solution, it will be entrapped in the polymer film. This immobilization method is widely used in ion sensors based on the use of macrocyclic compounds or liquid ion exchangers as receptors.
Dip coating is achieved by immersing the support in the polymer solution followed by solvent evaporation. The coverage depends on the immersion time and the polymer concentration. These parameters afford to control of the amount of deposited material.
In the drop coating technique the polymer solutions is applied as several microliter droplets onto the support surface. Spreading the solution out and evaporation results in a thin film. This method is limited to surface areas below 1 cm2 and a uniform coverage cannot be guaranteed. However, it is a very simple technique and permits to control the amount of deposited polymer through the concentration and the volume of the polymer solution.
Spin coating relies on the deposition of polymer solution onto the support while the support is spun at a high speed. Spinning ensures an uniform spreading of the polymer solution that results in an even film thickness. The thickness of the film depends on the rotation speed and the viscosity of the polymersolution. Films as thick as 1 μm can be obtained in this way.
When preparing coated polymer films it is essential to obtain reproducible and compact, pinhole-free layers. This depends on the film thickness and the roughness of the underlying surface. After deposition, the film can be strengthened by crosslinking based on suitable chemical reactions.
A greater degree of control over the film characteristics at the molecular level is achieved by resorting to reactive deposition. Polymer layers covalently grafted onto the support can be obtained using precursors appended through reactive terminal groups that react with the support surface. Thus, triethoxysilanes serves for grafting polymer layers to oxide surfaces.
A very practical method is based on photochemical polymerization that allows the growing of polymer layers at predefined sites. In this approach, photosensitive precursors are first cast onto the surface and then crosslinked by irradiation through a shadow mask. Polymerization only occurs within the irradiated zones. By selective deposition of different polymers at preselected sites, this method allows to produce sensor arrays.
5.12.2 Perm-Selective Membranes
Certain polymer membranes provide selective permeability for some species and are used as selectivity-imparting coatings onto the sensing element in certain sensors. Selectivity can be imparted by the physical size of membrane pores, the solubility of particular compounds in the membrane or specific interactions between the polymer and the diffusing species.
Gas-permeable membranes are permeable to certain gases and are employed to contain an aqueous solution at the sensor surface or as selective coatings onto a solid-state sensing element. The membrane can be either compact or porous. In the case of compact membranes (for example, silicon rubber), gas diffusion through the membrane involves dissolved gas molecules. In the case of porous membranes (for example, Teflon), the gas diffuses through hydrophobic pores that are not permeable to water.
Size-exclusion perm-selective membranes feature micropores that allow the transport of only molecules smaller than the pore size. A typical material of this type is Nucleopore, which is available as about 10-μm thick polycarbonate membranes with uniform pore diameter that can range from 0.01 to 30 μm. Small molecules (for example, an enzyme substrate or a coreagent) can cross this membrane to reach the enzyme layer, whereas large proteins cannot. A typical application of such membranes is in the coating of the sensing element of certain glucose amperometric sensors. The membrane is permeable to glucose and oxygen but prevents the enzyme from leaking away.
A frequently used membrane is based on cellulose acetate (that is, the acetate ester of cellulose) which is available as dialysis membranes and hollow fibers. Cellulose acetate membranes are prepared by solvent casting from a solution of cellulose acetate in acetone onto a flat surface. The membrane is not permeable to anions and large molecules such as proteins. Such a membrane is used as a coating over the electrode surface in amperometric oxygen sensors and also as internal membrane in oxygen-linked enzyme sensors. However, hydrophobic polycarbonate membranes perform better as they have better permeability to oxygen.
Ion-exchanger membranes are made of polymers featuring ionic groups attached to the backbone and display selective permeability to ions bearing an opposite charge. Typical examples in this class are Nafion and poly(vinylpyridine) (Figure 5.30).
Figure 5.30 Chemical structure of two ion exchanger polymers: Nafion (A) and poly(vinylpyridine) (B). ![]() and
and ![]() are countercations and counteranions, respectively.
are countercations and counteranions, respectively.
Nafion is a sulfonated perfluorinated polyether featuring anionic ![]() groups. Nafion can be dissolved and cast to form supported membranes. A particular characteristic of this material is the internal phase segregation. This polymer has a high density of hydrophilic sites (
groups. Nafion can be dissolved and cast to form supported membranes. A particular characteristic of this material is the internal phase segregation. This polymer has a high density of hydrophilic sites (![]() groups), long fragments of hydrophobic chains and is not crosslinked, which imparts to the backbone certain flexibility. In contact with aqueous solutions, the anionic sites separate themselves from the hydrophobic segments forming water-filled pockets and channels. Due to the anionic sites, Nafion behaves as a cation exchanger. Applications of Nafion membranes rest on their selective ionic conductivity. Indeed, under the effect of an electric potential difference, Nafion membrane allow cations to cross it through the water-filled pockets but it is not permeable to anions. This property is exploited in amperometric sensors in which Nafion coating over the sensing part prevents access of certain anionic species that might interfere with sensor functioning.
groups), long fragments of hydrophobic chains and is not crosslinked, which imparts to the backbone certain flexibility. In contact with aqueous solutions, the anionic sites separate themselves from the hydrophobic segments forming water-filled pockets and channels. Due to the anionic sites, Nafion behaves as a cation exchanger. Applications of Nafion membranes rest on their selective ionic conductivity. Indeed, under the effect of an electric potential difference, Nafion membrane allow cations to cross it through the water-filled pockets but it is not permeable to anions. This property is exploited in amperometric sensors in which Nafion coating over the sensing part prevents access of certain anionic species that might interfere with sensor functioning.
Anion perm-selective membranes can be prepared from the cation-exchanger poly(pyridine) (Figure 5.30B). Polypyrrole, with its positively charged backbone is also selectively permeable to anions.
Ion selective membranes can be obtained by incorporating ion exchangers in a poly(vinyl chloride) (PVC) matrix. Thin PVC membranes are prepared by solvent evaporation of PVC solutions on a flat surface. A lipophilic plasticizers, such as bis(2-ethylhexyl)phthalate (also called dioctyl phthalate) or isopropyl myristate, should be incorporated in the PVC solution. The membrane thus obtained occludes the plasticizer in anionic form and is permeable to cations.
Plasticized PVC membranes are widely used as matrices for immobilization of ion receptors in various types of ion sensors, the selectivity arising from the receptor characteristics. Such membranes are obtained by dissolving the receptors along with PVC and a plasticizer in tetrahydrofuran, casting this solution on a flat glass surface and letting the solvent evaporate slowly. Unplasticized PVC membranes that are also lipophilic in nature, elicit selective permeability to certain small organic ions (for example, urate, lactate, ascorbate and oxalate) probably due to residual void interstices in the polymer matrix. Details on ion selective polymer membranes can be found in Chapter 10.
5.13 Microfabrication Methods in Chemical-Sensor Technology
Previous sections pointed to the basic physicochemical methods for the integration of a sensing element with a solid support, which is, in many instances, a component of the transducer. On the laboratory scale, manual fabrication is a common practice. However, mass production of chemical sensors and biosensors needs a high throughput and more reliable fabrication methods. In addition, miniaturization and multiplexing of sensors require methods for accurate deposition of receptors as well-defined patterns at predetermined sites on solid surfaces. Basic methods of this kind are introduced in this section. These methods rely on technologies borrowed from the microelectronics industry, such as the structuring of the surface in the form of thick films (>5 μm) or thin films (<5 μm). Such methods are suitable for creating complex patterns involving one or more receptors and are very suitable for the fabrication of sensor microarray [93,94].
5.13.1 Spot Arraying
Biocompound spot arrays can be formed by the deposition of small solution volumes either manually, using micropipettes or by means of a microdispenser. Commercial printer ink-jet dispensers or commercial instruments used in the fabrication of DNA array can been used to this end. However, the high temperature and shearing stress involved with such devices can be detrimental to proteins. Electrospray devices have also been used to produce enzyme spots of 100–300 μm diameter. However, as this method imparts to the molecule a large electric charge, it may induce enzyme denaturation.
A purposely designed microdispenser for enzyme solutions is shown in Figure 5.31. In this device, the solution is continuously fed in and droplets of about 100 pL are delivered under the impulse from a piezoelectric actuator that vibrates at a high frequency. Enzymes can be attached to the support surface either by sulfur chemisorption, crosslinking or entrapment in a polymer film. In the last two methods, the solution contains immobilization reagents in addition to the enzyme. This general method allows for deposition of mono- or multienzyme structures in the form of mono- or multimolecular layers as about 100-μm-sized patterns [94, 95].
Figure 5.31 Building a biocompound grid microstructure by means of a flow-through microdispenser.
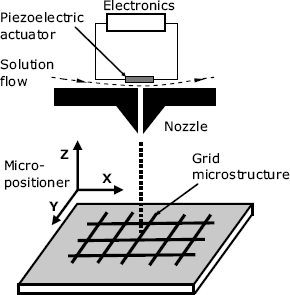
5.13.2 Thick-Film Technology
Thick-film technology is based on the principle of screen printing that is a general method for the deposition of patterned coatings onto flat surfaces [96]. Screen-printing is performed by coating a paste onto a flat support through openings in a screen (Figure 5.32). The paste is pressed onto the screen and the support by a squeegee and the screen determines the pattern of the deposited layer on the support. The success of this operation depends on the viscosity of the paste and also on its thixotropy, a term that defines the capacity of a thick paste or gel to turn into a fluid form under mechanical stress. The paste is a mixture of the sensor materials to be applied, an organic additive (that regulates the thixotropy) and a solvent. After printing, the layer is left to dry and then is subjected to thermal treatment. During this stage, the organic carrier is burned off, and other chemical and physical processes impart adhesion. The paste composition is selected so as to undergo the expected transformation during the heat treatment. A standard mixture consists of silicon, lead, and bismuth oxides that form a dielectric glass at elevated temperatures. A conductive paste can be obtained by including copper oxide that yields copper by reduction. The firing-resistant support is made of ceramic flexible tape. When no high-temperature treatment is required, the support can by a plastic material (such as a polyester).
Figure 5.32 The screen-printing process. Adapted with permission from [97] Copyright 1973 John Wiley & Sons, Ltd.
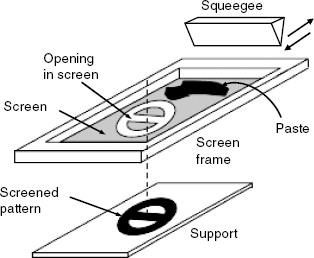
Typically, the screen is formed from a finely woven mesh of stainless steel or synthetic polymer mounted on a metal frame. The mesh is coated with an ultraviolet-sensitive emulsion, for example, polyvinyl acetate sensitized with a dichromate solution, which allows the required patterns to be produced by photolysis.
Screen printing allows the fabrication of 5- to 50-μm thick layers with a resolution of about 100 μm. The width of the sensors thus fabricated can vary from a few mm down to 0.5 mm, which allows the installation of such a sensor in a catheter for in vivo application. Different pastes are commercially available, including, conductive, resistive, dielectric and overglaze pastes. Custom-made pastes are designed for particular applications.
By sequential printing, various patterns with specific properties can be formed on the same support using different masks and different paste compositions. Screen printing can also be used to deposit a polymer film over selected areas of the sensor to function as an immobilization support, as a perm-selective membrane or as an insulator.
This technology is widely used in the fabrication of screen-printed electrodes for use in electrochemical sensors. The paste or ink in this case contains graphite powder and the resulting layer, which is electroconductive, functions as an electrode.
Enzymes can be attached to preformed screen-printed layers by common methods such as adsorption, crosslinking, or entrapment. Alternatively, a second layer can be formed by printing a paste containing an enzyme and, if necessary, a cofactor.
5.13.3 Thin-Film Techniques
Microfabrication is the term used to denote methods for the fabrication of miniature patterns of micrometer size and smaller. This class of technologies is well established in the integrated circuit industry [98] and is also a valuable method in the fabrication of chemical sensors [99, 100]. Microfabrication yields miniature devices and also assemblies of microsensors using readily available equipment. Moreover, electronic circuitry can be integrated with the sensor in order to provide an electronic interface for signal processing. The basic material in microfabrication is silicon; inexpensive silicon wafers (some 250 μm thick) are widely available. A comprehensive survey of microfabrication applications in the field of chemical sensors and biosensors is available in ref. [101].
Microfabrication encompasses a series of unit operations, the most important being patterning, deposition or growth of films, and etching. The thickness of the structure obtained by microfabrication is in the μm range.
Photolithography is a technology for creating micropatterns on flat surfaces (Figure 5.33A). In this technique, light irradiation of a photosensitive coating through a mask creates a pattern of exposed and covered domains that can be further processed so as to create functional units at the microscale. The process begins with spin coating of the support surface with a photosensitive polymer layer (photoresist). This is next irradiated through a photomask with transparent patterns identical to those that are to be transposed onto the support. With a positive photoresist, the irradiated zones are altered and can be dissolved away in the next stage called developing. The pattern on the surface is similar to that on the mask, but about 10 times smaller. In the case of a negative photoresist, photochemical reactions reinforce the exposed material while the shadowed areas remain susceptible to being dissolved away. Clearly, the pattern on the wafer surface is in this case the reverse of that on the mask.
Figure 5.33 Methods of thin-film technology. (A) Photolithography; (B) plasma-enhanced chemical vapor deposition.
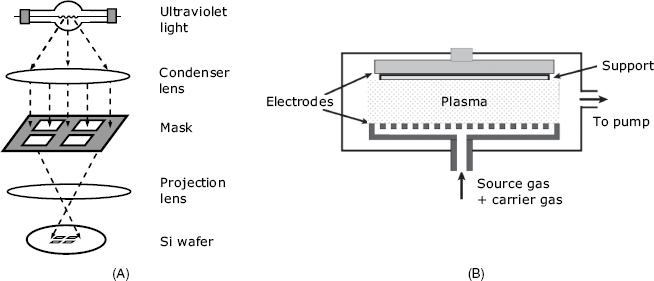
Photomasks are typically transparent fused silica blanks coated with an opaque chromium layer. Chromium is etched by a laser or electron beam under computer control leaving a clear path for the light to travel though.
After irradiation and development, exposed areas on the surface are processed by etching or film deposition in order to obtain the expected configuration. Finally, the remaining photoresist is removed by attack with a liquid reagent or by ashing with oxygen-containing plasma.
In etching processes, the uppermost layer of the substrate in the areas not protected by photoresist is removed. Plasma or reagent solutions can be used to this end. Plasma etching can be made anisotropic, that is, asymmetric patterns can be etched in this way. Wet etching processes that rely on attack by chemical reagents are generally isotropic in nature, that is, give rise to symmetrical patterns.
Various layers (0.2 to 200 μm thick) can be grown on exposed areas by sputtering, physical vapor deposition or chemical vapor deposition. A metal layer can be formed by sputtering a metal target in vacuum with an argon ion beam. This beam conveys metal atoms to the surface.
In a typical chemical vapor deposition process, the substrate is exposed to one or more volatile precursors that react or decompose at high temperatures on the substrate surface to produce the expected deposit. An advanced version of chemical vapor deposition relies on chemical reactions taking place in a plasma, a highly ionized gas that is produced by electrical discharge or other methods (Figure 5.33B). In plasma, chemical reaction are very fast and this allows the deposition to be performed at lower temperatures, which is often beneficial in the manufacture of semiconductor devices.
After completing the patterning process, a fraction of silicon wafer surface still remains uncoated. The uncoated surface is next passivated by growing films of silicon dioxide or silicon nitride (![]() ) on them. Silicon dioxide is formed by surface reaction in a damp oxygen atmosphere at a high temperature, or by various chemical vapor deposition processes. Better passivation is achieved with silicon nitride layers formed by the gas-phase reaction of dichlorosilane (
) on them. Silicon dioxide is formed by surface reaction in a damp oxygen atmosphere at a high temperature, or by various chemical vapor deposition processes. Better passivation is achieved with silicon nitride layers formed by the gas-phase reaction of dichlorosilane (![]() ) with ammonia.
) with ammonia.
Thin-film methods are used in sensor technology to obtain patterned support surfaces to which the receptor layer is then attached. Gold spot arrays can be prepared by vapor deposition in order to function as support for receptor immobilization by thiol chemisorption. If no site-specific immobilization method is available, one can resort to photolithography to form an array of exposed support spots. Then, the immobilization is performed over the whole area. In this way, the receptor is thus applied on the photoresist-coated domains as well as on the exposed support spots. After removing the photoresist (along with the material deposited on it), only the uncoated spots preserve the sensing layer. This method should be carried out carefully in order to avoid degradation of the receptor by the solvent used to remove the photoresist. This drawback is absent in mask-less photolithography. In this approach, the support is coated with a photolabile layer containing a protein linker, such as biotin. This layer is patterned by irradiation through a mask and then removed from the irradiated zones. Subsequently, the receptor (for example, an avidin-tagged species) will be appended only to the spots that were shaded during the irradiation step.
The resolution of the photolithographic immobilization methods seems to be near 2 μm. Much better resolution can be achieved by soft lithography, which is presented in the next section.
5.13.4 Soft Lithography
In soft lithography a soft stamp (mold) is used to apply a patterned layer to the surface of a solid support [102,103]. As shown in Figure 5.34, a master is first fabricated on a photoresist-covered support by standard photolithography. The polymer stamp is then obtained by pouring a degassed polymer over the master. The stamp materials are elastic polymers, the most common one used being poly(dimethylsiloxane) (PDMS), a silicone-based elastomer. The polymer stamp is cured and then detached from the master. Once the stamp is fabricated, its surface is inked by contact with a thioalkane solution in ethanol. The thioalkane diffuses into the stamp and when the stamp is applied on the surface of a gold support, a thiol self-assembled monolayer (SAM) forms only in the regions of direct contact. The amount of thiol absorbed in the stamp is sufficient for multiple prints. This method is called microcontact printing.
Figure 5.34 Soft lithography: stamp fabrication and microcontact printing. Adapted from http://commons.wikimedia.org/wiki/File:Creating_the_PDMS_Master.JPG (A) and http://commons.wikimedia.org/wiki/File:Inking_and_Contact_Process.JPG (B).
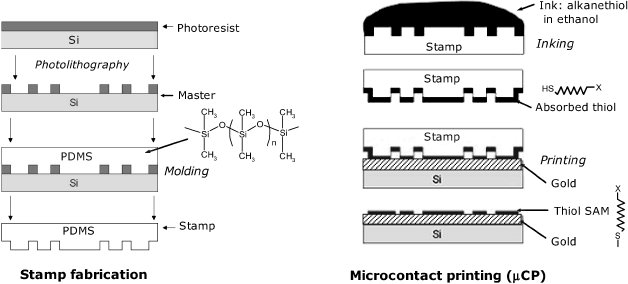
Self-assembly of derivatized thiols impart to the surface various properties, depending on the end group of the thiol. Thus, a plain thioalkane renders the surface hydrophobic and chemically inert. A hydrophilic end group at the thiol end imparts hydrophilicity, whereas reactive end groups allow for crosslinking of compounds of interest. So, either adsorptive or covalent immobilization can be performed on SAMs formed by microcontact printing.
More rugged stamps can be obtained using the PDMS stamp as a master to mold a harder polymer stamp based on oligomers or prepolymers that do not penetrate into PDMS. This technology, which is referred to as replica molding, can produce numerous mold replicas using inexpensive materials that are suitable for mass production.
5.13.5 Microcontact Printing of Biocompounds
Based on the standard soft lithography, various methods for micro- and nanopatterning of surfaces with biocompounds have been developed [104–106].
The SAM microcontact printing technology (Figure 5.34) can be easily transposed to protein printing directly on the support surface by inking the stamp with a protein solution. During the printing process, proteins are transferred to the support surface by adsorption. Thiol-containing biomolecules undergo chemisorption at a gold surface via the sulfur group and protein monolayers can be formed in this way. The main limitation of this method is that the stamp should be inked after each application as the amount of adsorbed protein is limited.
The above problem can be circumvented by using hydrogel stamps swollen with a solution of protein. Such a stamp consists of two parts: a reservoir filled with protein dissolved in a biological buffer and a hydrogel that makes contact with the support and transfers proteins by diffusion.
An advanced method for biopatterning termed affinity-contact printing (α-CP) relies on selective inking with proteins appended to the stamp by affinity interactions (Figure 5.35 left). In this method, the stamp surface is activated by treatment with oxygen plasma. This forms a very thin silica-like layer that includes silanol groups (–Si–OH). Trialkoxysilane-bearing reactive groups are then crosslinked with the silanol groups and finally, capture molecules (e.g., an antibody) is covalently grafted to the surface. The result of this process is a so-called ![]() -stamp. This stamp is then put in contact with a solution containing a mixture of proteins. Affinity interactions occur at the surface leading to the selective capture of target proteins, for example, an antigen. Finally, the antigens are transferred by contact printing to a solid support.
-stamp. This stamp is then put in contact with a solution containing a mixture of proteins. Affinity interactions occur at the surface leading to the selective capture of target proteins, for example, an antigen. Finally, the antigens are transferred by contact printing to a solid support.
Figure 5.35 Affinity contact printing (![]() -CP). Left: main steps: (A) Covalent linking of capturing molecules on a functionalized stamp; (B) Inking of the
-CP). Left: main steps: (A) Covalent linking of capturing molecules on a functionalized stamp; (B) Inking of the ![]() -stamp by affinity bonding of target molecules; (C) Printing on a solid support; (D) stamp removal. Right: Forces involved in the microcontact printing of proteins. Reprinted by permission from Macmillan Publishers Ltd: Nature Biotechnology [107]. Copyright 2001.
-stamp by affinity bonding of target molecules; (C) Printing on a solid support; (D) stamp removal. Right: Forces involved in the microcontact printing of proteins. Reprinted by permission from Macmillan Publishers Ltd: Nature Biotechnology [107]. Copyright 2001.
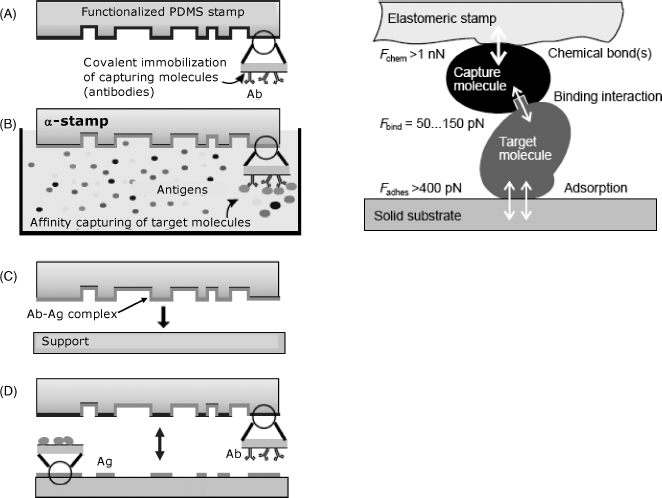
Figure 5.35 (right) shows the strengths of the binding interactions involved in the printing process as determined by atomic force microscopy. It is clear that multiple interactions involved in adsorption provide the driving force for protein transfer from the stamp to the support surface.
The strength of this method arises from the selectivity of the inking process. Only molecules in the ink that fit the capturing molecule are transferred to the stamp. This allows crude protein preparations to be used with no prior separation of the protein of interest.
Various affinity interactions (including avidin–biotin linking) are suitable for inking the stamp. This allows the printing of various proteins (including enzymes) and also nucleic acids and living cells [105, 107].
The ![]() -stamp fabrication method has been further refined in order to enhance the throughput and resolution (<1 μm) of the biomolecule microcontact printing process [108].
-stamp fabrication method has been further refined in order to enhance the throughput and resolution (<1 μm) of the biomolecule microcontact printing process [108].
Microprinting of biocompounds by soft lithography has also been developed so as to allow printing of multiple receptor spots on the same support [109]. This is therefore a valuable technology for fabrication of protein and nucleic acid microarrays.
5.14 Concluding Remarks
A great variety of methods for the fabrication of the sensing element are available. Selection of a particular method depends on a series of factors. First, the fabrication method should be compatible with the receptor and preserve its activity for as long a time as possible. Testing of receptor stability is a key step in developing a chemical sensor, particularly when using sensitive biological materials such as enzymes. Methods for the characterization of immobilized enzymes are well established [110] and most of the standard approaches in this field also apply to enzymatic sensors.
Another important factor is the receptor loading in the sensing layer. Enzyme sensors require, as a rule, a high loading, whereas immunosensors are not particularly demanding as far as this feature is concerned. In general the immobilization should be performed such as to allow the access of the analyte to the binding sites.
The immobilization method should be compatible with the transduction method, which often imposes the restriction of using some particular material as the support for immobilization.
The long-term stability of the sensor depends not only on the stability of the receptor compound but also on the survival of the sensing layer overall. Greater long-time stability is achieved generally at the cost of using more intricate fabrication technology and more expensive reagents. Often, it is preferable to resort to simple and inexpensive methods to fabricate disposable sensors. In this case, the batch reproducibility of the sensor response is the essential issue.
Mass fabrication issue is essential when aiming at the commercialization of sensors. To this end, thick- or thin-film technologies are the methods of choice.
Various immobilization and fabrication protocols are available in the literature [111, 112] and the interested reader can take the advantage of this large pool of expertise.
1. Cass, A.E.G., Cass, T., and Ligler, F.S. (1998) Immobilized Biomolecules in Analysis, Oxford University Press, Oxford.
2. Cao, L. (2005) Carrier-Bound Immobilized Enzymes: Principles, Applications and Design, Wiley-VCH, Weinheim.
3. Bisswanger, H. (2011) Practical Enzymology, Wiley-VCH, Weinheim.
4. Cabral, J.M.S. and Kennedy, J.F. (1991) Covalent and coordination immobilization of proteins, in Protein Immobilization: Fundamentals and Applications (ed. R.F. Taylor), M. Dekker, New York, pp. 73–138.
5. Dugas, V., Elaissari, A., and Chevalier, Y. (2010) Surface sensitization techniques and recognition receptors immobilization on biosensors and microarrays, in Recognition Receptors in Biosensors (ed. M. Zourob), Springer, New York, pp. 47–134.
6. Hermanson, G.T. (2008) Bioconjugate Techniques, Elsevier, Amsterdam.
7. Cao, L.Q. (2005) Immobilised enzymes: science or art? Curr. Opin. Chem. Biol., 9, 217–226.
8. Patil, J.S., Kamalapur, M.V., Marapur, S.C. et al. (2010), Ionotropic gelation and polyelectrolyte complexation: the novel techniques to design hydrogel particulate sustained, modulated drug delivery system: A review. Dig. J. Nanomater. Biostruct., 5, 241–248.
9. Lukaszewicz, J.P. (2006) Carbon materials for chemical sensors: A review. Sens. Lett., 4, 53–98.
10. Švancara, I., Walcarius, A., Kalcher, K. et al. (2009) Carbon paste electrodes in the new millennium. Cent. Eur. J. Chem., 7, 598–656.
11. Wink, T., van Zuilen, S.J., Bult, A. et al. (1997) Self-assembled monolayers for biosensors. Analyst, 122, R43–R50.
12. Ion, A., Partali, V., Sliwka, H.R. et al. (2002) Electrochemistry of a carotenoid self-assembled monolayer. Electrochem. Commun., 4, 674–678.
13. B![]() nic
nic![]() , A., Culetu, A. et al. (2007) Electrochemical and EQCM investigation of L-selenomethionine in adsorbed state at gold electrodes. J. Electroanal. Chem., 599, 100–110.
, A., Culetu, A. et al. (2007) Electrochemical and EQCM investigation of L-selenomethionine in adsorbed state at gold electrodes. J. Electroanal. Chem., 599, 100–110.
14. Tour, J.M. (2003) Molecular Electronics: Commercial Insights, Chemistry, Devices, Architecture And Programming, World Scientific, River Edge.
15. Chaki, N.K. and Vijayamohanan, K. (2002) Self-assembled monolayers as a tunable platform for biosensor applications. Biosens. Bioelectron., 17, 1–12.
16. Gooding, J.J. and Hibbert, D.B. (1999) The application of alkanethiol self-assembled monolayers to enzyme electrodes. TrAC-Trends Anal. Chem., 18, 525–533.
17. Gooding, J.J., Mearns, F., Yang, W.R. et al. (2003) Self-assembled monolayers into the 21(st) century: Recent advances and applications. Electroanalysis, 15, 81–96.
18. Love, J.C., Estroff, L.A., Kriebel, J.K. et al. (2005) Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev., 105, 1103–1169.
19. Liu, G.D. and Lin, Y.H. (2007) Nanomaterial labels in electrochemical immunosensors and immunoassays. Talanta, 74, 308–317.
20. Stutzmann, M., Garrido, J.A., Eickhoff, M. et al. (2006) Direct biofunctionalization of semiconductors: A survey. Phys. Status Solidi A-Appl. Mat., 203, 3424–3437.
21. Ciampi, S., Harper, J.B., and Gooding, J.J. (2010) Wet chemical routes to the assembly of organic monolayers on silicon surfaces via the formation of Si-C bonds: surface preparation, passivation and functionalization. Chem. Soc. Rev., 39, 2158–2183.
22. Boukherroub, R. (2005) Chemical reactivity of hydrogen-terminated crystalline silicon surfaces. Curr. Opin. Solid State Mater. Sci., 9, 66–72.
23. Stewart, M.P., Maya, F., Kosynkin, D.V. et al. (2003) Direct covalent grafting of conjugated molecules onto Si, GaAs, and Pd surfaces from aryldiazonium salts. J. Am. Chem. Soc., 126, 370–378.
24. Wilchek, M. and Bayer, E.A. (1990) Introduction to avidin-biotin technology. Method Enzymol., 184, 5–13.
25. Diamandis, E.P. and Christopoulos, T.K. (1991) The biotin (strept)avidin system - principles and applications in biotechnology. Clin. Chem., 37, 625–636.
26. Anzai, J., Hoshi, T., and Osa, T. (2000) Avidin-biotin mediated biosensors, in Biosensors and Their Application (eds V.C. Yang and T.T. Ngo), Kluwer, New York, pp. 35–46.
27. Hermanson, G.T. (2008) Biotinylation Reagents In Bioconjugate Techniques, Elsevier, Amsterdam, pp. 506–545.
28. Wilchek, M. and Bayer, E.A. (1990) Biotin-containing reagents. Method Enzymol., 184, 123–138.
29. Bayer, E.A. and Wilchek, M. (1990) Protein biotinylation. Method Enzymol., 184, 138–160.
30. Smith, C.L., Milea, J.S., and Nguyen, G.H. (2005) Immobilization of nucleic acids using biotin-strept(avidin) systems, in Immobilisation of DNA on Chips II (ed. C. Wittmann), Springer, Berlin, pp. 63–90.
31. Davis, F. and Higson, S.P.J. (2005) Structured thin films as functional components within biosensors. Biosens. Bioelectron., 21, 1–20.
32. Ariga, K. and Kunitake, T. (2006) Supramolecular Chemistry: Fundamentals and Applications, Springer, Berlin.
33. Hianik, T. and Passechnik, V.I. (1995) Bilayer Lipid Membranes: Structure and Mechanical Properties, Kluwer, London.
34. Hianik, T. (2008) Biological membranes and membrane mimics, in Bioelectrochemistry: Fundamentals, Experimental Techniques and Applications (ed. P.N. Bartlett), John Wiley & Sons, Chichester, pp. 86–156.
35. Nikolelis, D.P., Krull, U.J., Ottova, A.L. et al. (1996) Bilayer lipid membranes and other lipid-based methods, in Handbook of Chemical and Biological Sensors (eds R.F. Taylor and J.S. Schultz), Institute of Physics Publishing, Bristol, pp. 221–256.
36. Petty, M.C. (1996) Langmuir-Blodgett Films: An Introduction, Cambridge University Press, Cambridge.
37. Trojanowicz, M. (2001) Miniaturized biochemical sensing devices based on planar bilayer lipid membranes. Fresenius J. Anal. Chem., 371, 246–260.
38. Mohsin, M.A., B![]() nic
nic![]() , F.G., Oshima, T. et al. (2011) Electrochemical impedance spectroscopy for assessing the recognition of cytochrome c by immobilized calixarenes. Electroanalysis, 23, 1229–1235.
, F.G., Oshima, T. et al. (2011) Electrochemical impedance spectroscopy for assessing the recognition of cytochrome c by immobilized calixarenes. Electroanalysis, 23, 1229–1235.
39. Knoll, W., Morigaki, K., Naumann, R. et al. (2004) Functional tethered bilayer lipid membranes, in Ultrathin Electrochemical Chemo- and Biosensors: Technology and Performance (ed. V.M. Mirsky), Springer, Berlin, pp. 239–252.
40. Lvov, Y., Ariga, K., Ichinose, I. et al. (1995) Assembly of multicomponent protein films by means of electrostatic layer-by-layer adsorption. J. Am. Chem. Soc., 117, 6117–6123.
41. Siqueira, J.R., Caseli, L., Crespilho, F.N. et al. (2010) Immobilization of biomolecules on nanostructured films for biosensing. Biosens. Bioelectron., 25, 1254–1263.
42. Wright, J.D. and Sommerdijk, N.A.J.M. (2003) Sol-Gel Materials: Chemistry and Applications, Taylor & Francis, London.
43. Lev, O. and Sampath, S. (2010) Sol-gel electrochemistry, in Electroanalytical Chemistry: A Series of Advances, vol. 23 (eds A.J. Bard and C.G. Zoski), CRC Press, Boca Raton, pp. 211–304.
44. Shchipunov, Y. (2008) Entrapment of biopolymers into sol-gel-derived silica nanocomposites, in Bio-Inorganic Hybrid Nanomaterials: Strategies, Syntheses, Characterization and Applications (eds E. Ruiz-Hitzky, K. Ariga, and Y. Lvov), Wiley-VCH, Weinheim, pp. 75–112.
45. Gupta, R. and Chaudhury, N.K. (2007) Entrapment of biomolecules in sol-gel matrix for applications in biosensors: Problems and future prospects. Biosens. Bioelectron., 22, 2387–2399.
46. Tripathi, V.S., Kandimalla, V.B., and Ju, H. (2006) Preparation of ormosil and its applications in the immobilizing biomolecules. Sens. Actuators B, 114, 1071–1082.
47. Dave, B.C., Dunn, B., Valentine, J.S. et al. (1998) Sol-gel matrices for protein entrapment, in Immobilized Biomolecules in Analysis (eds A.E.G. Cass, T. Cass, and F.S. Ligler), Oxford University Press, Oxford, pp. 1–14.
48. Frenkel-Mullerad, H., and Avnir, D. (2005) Sol-gel materials as efficient enzyme protectors: Preserving the activity of phosphatases under extreme pH conditions. J. Am. Chem. Soc., 127, 8077–8081.
49. Niederberger, M. and Antonietti, M. (2007) Nonaqueous sol-gel routes to nanocrystalline metal oxides, in Nanomaterials Chemistry: Recent Developments and New Directions (eds C.N.R. Rao, A. Müller, and A.K. Cheetham), Wiley-VCH, Weinheim, pp. 119–138.
50. Jagur-Grodzinski, J. (2010) Polymeric gels and hydrogels for biomedical and pharmaceutical applications. Polym. Adv. Technol., 21, 27–47.
51. Omidian, H. and Park, K. (2010) Introduction to hydrogels, in Biomedical Applications of Hydrogels Handbook (eds R.M. Ottenbrite, K. Park, and T. Okano), Springer, New York, pp. 1–16.
52. Plieva, F.M., Galaev, I.Y., Noppe, W. et al. (2008) Cryogel applications in microbiology. Trends Microbiol., 16, 543–551.
53. Kuckling, D., Arndt, K.F., and Richter, A. (2009) Synthesis of hydrogels, in Hydrogel Sensors and Actuators: Engineering and Technology (eds G. Gerlach and K.-F. Arndt), Springer, Berlin, pp. 15–67.
54. Heller, A. (2006) Electron-conducting redox hydrogels: design, characteristics and synthesis. Curr. Opin. Chem. Biol., 10, 664–672.
55. Inzelt, G. (2008) Conducting Polymers: A New Era in Electrochemistry, Springer, Berlin.
56. van der Linden, H.J., Herber, S., Olthuis, W., and Bergveld, P. (2003) Stimulus-sensitive hydrogels and their applications in chemical (micro)analysis. Analyst, 128, 325–331.
57. Kuckling, D. (2009) Responsive hydrogel layers – from synthesis to applications. Colloid Polym. Sci., 287, 881–891.
58. Tokarev, I. and Minko, S. (2009) Stimuli-responsive hydrogel thin films. Soft Matter, 5, 511–524.
59. Ulijn, R.V., Bibi, N., Jayawarna, V. et al. (2007) Bioresponsive hydrogels. Mater. Today, 10, 40–48.
60. Richter, A., Paschew, G., Klatt, S. et al. (2008) Review on hydrogel-based pH sensors and microsensors. Sensors, 8, 561–581.
61. Guenther, M. and Gerlach, G. (2009) Hydrogels for chemical sensors, in Hydrogel Sensors and Actuators: Engineering and Technology (eds G. Gerlach and K.-F. Arndt), Springer, Berlin, pp. 165–195.
62. Urban, G.A. and Weiss, T. (2009) Hydrogels for biosensors, in Hydrogel Sensors and Actuators: Engineering and Technology (eds G. Gerlach and K.-F. Arndt), Springer, Berlin, pp. 197–219.
63. Deligkaris, K., Tadele, T.S., Olthuis, W. et al. (2010) Hydrogel-based devices for biomedical applications. Sens. Actuators B-Chem., 147, 765–774.
64. Gawel, K., Barriet, D., Sletmoen, M. et al. (2010) Responsive hydrogels for label-free signal transduction within biosensors. Sensors, 10, 4381–4409.
65. Schexnailder, P. and Schmidt, G. (2009) Nanocomposite polymer hydrogels. Colloid Polym. Sci., 287, 1–11.
66. Miyata, T. (2010) Biomolecule-responsive hydrogels, in Biomedical Applications of Hydrogels Handbook (eds K. Park and T. Okano), Springer Science+Business Media, LLC, New York, NY, pp. 65–86.
67. Cosnier, S. and Holzinger, M. (2011), Electrosynthesized polymers for biosensing. Chem. Soc. Rev., 40, 2146–2156.
68. Chandrasekhar, P. (1999) Conducting Polymers, Fundamentals and Applications: A Practical Approach, Kluwer, Boston.
69. Lange, U., Roznyatouskaya, N.V., and Mirsky, V.M. (2008) Conducting polymers in chemical sensors and arrays. Anal. Chim. Acta, 614, 1–26.
70. Ramanavicius, A., Ramanaviciene, A., and Malinauskas, A. (2006) Electrochemical sensors based on conducting polymer- polypyrrole. Electrochim. Acta, 51, 6025–6037.
71. Giuseppi-Elie, A., Wallace, G.G., and Matsue, T. (1998) Chemical and biological sensors based on electrically conducting polymers, in Handbook of Conducting Polymers (eds T.A. Skotheim, R.L. Elsenbaumer, and J.R. Reynolds), Marcel Dekker, New York, pp. 963–991.
72. Potje-Kamloth, K. (2002) Chemical gas sensors based on organic semiconductor polypyrrole. Crit. Rev. Anal. Chem., 32, 121–140.
73. Cosnier, S. (2003) Biosensors based on electropolymerized films: New trends. Anal. Bioanal. Chem., 377, 507–520.
74. Cosnier, S. (1997) Electropolymerization of amphiphilic monomers for designing amperometric biosensors. Electroanalysis, 9, 894–902.
75. Hatchett, D.W. and Josowicz, M. (2008) Composites of intrinsically conducting polymers as sensing nanomaterials. Chem. Rev., 108, 746–769.
76. Cosnier, S. (1999) Biomolecule immobilization on electrode surfaces by entrapment or attachment to electrochemically polymerized films. A Review. Biosens. Bioelectron., 14, 443–456.
77. Bartlett, P.N. and Cooper, J.M. (1993) A review of the immobilization of enzymes in electropolymerized films. J. Electroanal. Chem., 362, 1–12.
78. Schuhmann, W. (1995) Conducting polymer based amperometric enzyme electrodes. Mikrochim. Acta, 121, 1–29.
79. Bidan, G., Billon, M., Calvo-Munoz, M.L. et al. (2004) Bio-assemblies onto conducting polymer support: Implementation of DNA-chips. Mol. Cryst. Liquid Cryst., 418, 983–998.
80. Cosnier, S. (2005) Affinity biosensors based on electropolymerized films. Electroanalysis, 17, 1701–1715.
81. Sadik, O.A. (1999) Bioaffinity sensors based on conducting polymers: A short review. Electroanalysis, 11, 839–844.
82. Miao, Y.Q., Chen, J.R., and Wu, X.H. (2004) Using electropolymerized non-conducting polymers to develop enzyme amperometric biosensors. Trends Biotechnol., 22, 227–231.
83. Park, B.W., Yoon, D.Y., and Kim, D.S. (2010) Recent progress in bio-sensing techniques with encapsulated enzymes. Biosens. Bioelectron., 26, 1–10.
84. Ozin, G.A., Arsenault, A.C., and Cademartiri, L. (2009) Microporous and mesoporous materials from soft building blocks, in Nanochemistry: A Chemical Approach to Nanomaterials, RSC Publ., Cambridge, pp. 521–592.
85. Bonifacio, L.D., Lotsch, B.V., Ozin, G.A. et al. (2010) Periodic mesoporous materials: Holes filled with opportunities, in Comprehensive Nanoscience and Technology (eds D. Andrews, G. Scholes, and G. Wiederrecht), Academic Press, Amsterdam, pp. 69–125.
86. Hoffmann F., Cornelius, M., Morell, J. et al. (2006) Silica-based mesoporous organic-inorganic hybrid materials. Angew. Chem. Int. Edit., 45, 3216–3251.
87. Vinu, A., Gokulakrishnan, N., Mori, T. et al. (2008) Immobilization of biomolecules on mesoporous structured materials, in Bio-inorganic Hybrid Nanomaterials: Strategies, Syntheses, Characterization and Applications (eds E. Ruiz-Hitzky, K. Ariga, and Y. Lvov), Wiley-VCH, Weinheim, pp. 113–157.
88. Lee, C.H., Lin, T.S., and Mou, C.Y. (2009) Mesoporous materials for encapsulating enzymes. Nano Today, 4, 165–179.
89. Lee, D., Lee, J., Kim, J. et al. (2005) Simple fabrication of a highly sensitive and fast glucose biosensor using enzymes immobilized in mesocellular carbon foam. Adv. Mater., 17, 2828–2833.
90. Tierney, M.J. (1996) Practical examples of polymer-based chemical sensors, in Handbook of Chemical and Biological Sensors (eds R.F. Taylor and J.S. Schultz), Institute of Physics Publishing, Bristol, pp. 349–369.
91. Emr, S.A. and Yacynych, A.M. (1995) Use of polymer-films in amperometric biosensors. Electroanalysis, 7, 913–923.
92. Reddy, S.M. and Vadgama, P.M. (1997) Membranes to improve amperometric sensor characteristics, in Handbook of Biosensors and Electronic Noses: Medicine, Food, and the Environment (ed. E. Kress-Rogers), CRC Press, Boca Raton, Fla, pp. 111–135.
93. Shoji S. (2000) Micromachining for biosensors and biosensing systems, in Biosensors and Their Applications (eds V.C. Yang and T.T. Ngo), Kluwer, New York, pp. 225–241.
94. Gaspar S., Schuhmann, W., Laurell, T. et al. (2002) Design, visualization, and utilization of enzyme microstructures built on solid surfaces. Rev. Anal. Chem., 21, 245–266.
95. Mosbach, M., Zimmermann, H., Laurell, T. et al. (2001) Picodroplet-deposition of enzymes on functionalized self-assembled monolayers as a basis for miniaturized multi-sensor structures. Biosens. Bioelectron., 16, 827–837.
96. Lambrechts, M. and Sansen, W. (1992) Biosensors: Microelectrochemical Devices, Institute of Physics Publishing, Bristol.
97. Rikoski, R.A. (1973) Hybrid Microelectronic Circuits: The Thick Film, Wiley New, York.
98. Licari, J.J. and Enlow, L.R. (1998) Hybrid Microcircuit Technology Handbook: Materials, Processes, Design, Testing, and Production, Noyes Publications, Westwood, N.J.
99. Hierlemann, A. (2005) Integrated Chemical Microsensor Systems in CMOS Technology, Springer-Verlag Berlin Heidelberg, Berlin, Heidelberg.
100. Madou, M. and Kim, H.L. (1996) Integrated circuit manufacturing techniques applied to microfabrication, in Handbook of Chemical and Biological Sensors (eds R.F. Taylor and J.S. Schultz,), Institute of Physics Publishing, Bristol, pp. 45–82.
101. Hierlemann A., Brand, O., Hagleitner, C. et al. (2003) Microfabrication techniques for chemical/biosensors. Proc. IEEE, 91, 839–863.
102. Xia, Y. and Whitesides, G.M. (1998) Soft lithography. Angew. Chem. Int. Ed., 37, 550–575.
103. Gates, B.D., Xu, Q.B., Stewart, M. et al. (2005) New approaches to nanofabrication: Molding, printing, and other techniques. Chem. Rev., 105, 1171–1196.
104. Delamarche, E. (2004) Microcontact printing of proteins, in Nanobiotechnology: Concepts, Applications and Perspectives (eds C.M. Niemeyer and C.A. Mirkin), Wiley-VCH, Weinheim, pp. 31–52.
105. Truskett, V.N. and Watts, M.P.C. (2006) Trends in imprint lithography for biological applications. Trends Biotechnol., 24, 312–317.
106. Mendes, P.M., Yeung, C.L., and Preece, J.A. (2007) Bio-nanopatterning of surfaces. Nanoscale Res. Lett., 2, 373–384.
107. Bernard, A., Fitzli, D., Sonderegger, P. et al. (2001) Affinity capture of proteins from solution and their dissociation by contact printing. Nature Biotechnol., 19, 866–869.
108. Renault, J.P., Bernard, A., Juncker, D. et al. (2002) Fabricating microarrays of functional proteins using affinity contact printing. Angew. Chem. Int. Ed., 41, 2320–2323.
109. Ganesan, R., Kratz, K., and Lendlein, A. (2010) Multicomponent protein patterning of material surfaces. J. Mater. Chem., 20, 7322–7331.
110. Buchholtz, K. and Klein, J. (1987) Characterization of immobilized biocatalysts, in Immobilized Enzymes and Cells (ed. K. Mosbach), Academic Press, Orlando, pp. 3–30.
111. Bickerstaff, G.E. (1997) Immobilization of Enzymes and Cells, Humana, Riverview Drive.
112. Rasooly, A. and Herold, K.E. (2009) Biosensors and Biodetection: Methods and Protocols, Humana Press, New York.
