Chapter 8
Nanomaterial Applications in Chemical Sensors
8.1 Generals
We are currently witnessing a remarkable revolution in various fields of science and technology as a result of the development of nanomaterials [1, 2]. This line of development has a considerable impact on the state-of-the-art of chemical sensors, and considerable progress in this area has already been achieved by the application of nanomaterials [3–6].
A nano-object is characterized by the fact that at least one of its dimension is within the nanometer scale, that is, < 10−6 m. From the standpoint of the macroscopic dimension, one can distinguish nanoparticles (zero-dimensional), nanowires (monodimensional) and nanosheets (two-dimensional) nanomaterials. Accordingly, none, one or two of these dimensions, respectively, are above the 10−6 m limit, the remaining dimensions falling within the nanometer range. Numerous biological entities, such as proteins, nucleic acids and viruses are within the nanosize scale and are often designated as biological nanomaterials. Synthetic nanomaterials of various chemical composition are currently available. Such nanomaterials may consist of pure chemical elements (metals, carbon or silicon), inorganic compounds (metal chalcogenides or metal oxides) or organic compounds (polymers).
What is characteristic of nanomaterials is that they normally display specific properties that are fundamentally different from the properties of the bulk material [7, 8]. In the case of metal and semiconductor nanoparticles such differences arise first of all from the characteristic small size that has a major effect on the distribution of electronic levels in semiconductor nanoparticles. As a consequence, these materials exhibit specific and characteristic properties as far as light absorption and emission are concerned. In metal nanoparticles, for example, electron behavior in an electromagnetic field imparts characteristic light-scattering properties. Another significant characteristic is the area/volume ratio. Due to the very small size, an important fraction of the component atoms are not completely involved in chemical bonding with vicinal atoms and this results in increased chemical reactivity and catalytic activity. Last but not least, the size of synthetic nanomaterials matches that of biopolymers such as antibodies, enzymes and nucleic acid. Combined with surface reactivity, the size compatibility allows to produce nanomaterials-biomolecule hybrids with wide applications in biomedicine for therapy or imaging purposes [9–11]. The size compatibility combined with the high specificity of the biocompounds mentioned led to a broad spectrum of applications of nanomaterials in the biosensors area [4, 12–14].
As far as the production of synthetic nanomaterials is concerned, two main fabrication strategies can be distinguished, namely top-down and bottom-up techonologies.
The top-down approach is actually an extension of lithography [1]. In such a method, one starts with a macroscopic block of material that is processed by spatially controlled etching or layering in order to produce microscopic-sized patterns. Such technologies are widely used in the semiconductor industry and are characterized by a high throughput and flexibility. However, physical limitations do not allow the obtaining of structures smaller than 10 nm. In addition, conventional top-down methods may induce severe and uncontrollable structure defects during the etching steps. Nevertheless, such methods are very suitable for the fabrication of organized networks of nano-objects on solid surfaces.
The bottom-up approach is based on the assembly of atoms, ions or molecules driven by specific physical or chemical interactions that determine the crystallographic structure of the product. Typical examples of this kind are crystal growth and the synthesis of large polymers. A critical issue is the control of the nano-object size. This can be achieved by adjusting specific physicochemical parameters. If this is not possible, a monodisperse nano-object product can be obtained by subsequent size-selective separation.
Bottom-up methods are based either on gas-phase processes (chemical vapor deposition) or solution-phase processes [8, 15]. Chemical vapor deposition is a process where gaseous species react yielding a solid product on a solid support. Thus, carbon nanomaterials can be obtained by chemical decomposition of simple organic compounds in the presence of suitable catalyst.
A broad spectrum of solution-phase methods are based on colloid chemistry. Metallic, semiconductor and magnetic nanoparticles can be obtained in this way. Colloidal particles are produced by chemical reactions of suitable precursors, such as inorganic salts or organometallic compounds. The production of these colloidal particles involves initially a nucleation step that is followed by the subsequent growth of the preformed nuclei. The balance between the nucleation and growth velocities determines the size of the resulting particles. Thus, if the nucleation is much faster than the growth, relatively small particles result, whereas fast growth favors the growth of larger particles. These processes are conducted in the presence of organic surfactant molecules that adhere to the surface of the growing crystal. The surfactant layer prevents aggregation of particles and determines to a large extent both the size and size uniformity of the resulting particles.
Another class of solution-phase synthetic methods relies on the sol-gel technique that is well suited for obtaining oxide nanoparticles [15, 16]. In this technique, a metal alkoxide (R-OM, where R is an alkane residue and M is a metal or semimetal) or metal chloride undergoes hydrolysis and polycondensation reactions to form a gel that consists of oxide- or alcohol-bridged networks. Subsequent aging, dehydration, drying and thermal treatment lead to solid particles.
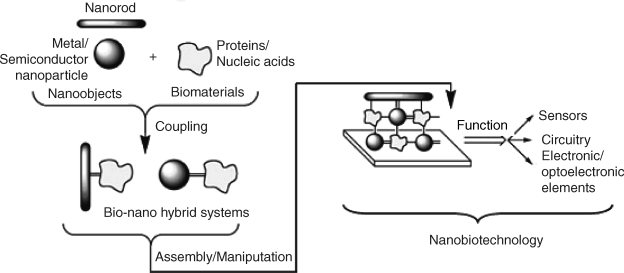
A typical approach to using nanomaterials in biosensors is illustrated in Figure 8.1. In the first step, coupling of synthetic nanomaterials with biocompounds produces bionanohybrid systems. The biocomponent imparts specificity whereas the nanomaterial acts as a label, catalyst or signal-amplifying component [17]. In order to obtain a sensor, the hybrids are assembled on a solid support, which could be a transduction device. Nanomaterials in the resulting assembly can perform also as electron conductors or building blocks of an electronic device adapted for biosensing. Nanoparticle–biocompound hybrids can be obtained by various procedures based on either covalent or noncovalent interactions [18].
Figure 8.1 Integration of nanomaterials and biomolecules to yield functional devices. Adapted with permission from [14]. Copyright 2004 Wiley-VCH Verlag GmBH & Co. KGaA.
The following sections review the main types of nanomaterials as far as synthesis and main physicochemical properties as well as modification and bioconjugation are concerned. Possible applications in conjunction with various transduction methods are discussed in later chapters.
8.2 Metallic Nanomaterials
Metal nanoparticles arouse considerable interest mainly because of their particular electronic properties [19]. As is well known, valence electrons in metals are not bound but can move freely. This imparts good electrical conductivity to metals. However, size confinement in metal nanoparticles imparts to electrons the property of interacting in a specific way with an electromagnetic field. This makes metal nanoparticles valuable for imaging and optical sensing. Among various metallic materials, gold is particularly preferred because of its chemical inertness, which prevents surface oxidation [20].
8.2.1 Synthesis of Metal Nanoparticles
Gold nanoparticles with the size between 1 to 8 nm can be prepared by the reduction of the ![]() anion with various reducing agents such as sodium borohydride (
anion with various reducing agents such as sodium borohydride (![]() ) or citric acid [21]. Stable colloidal gold systems are obtained if the reduction is performed in the presence of a protecting ligand that coats each particle with an adsorbed layer. Citric acid, for example, forms a negatively charged protecting layer that prevents coagulation of the nanoparticles by electrostatic repulsion. As is also well known, thiols form very stable surface layers by sulfur chemisorption on gold. Thiol-capped nanoparticles result from
) or citric acid [21]. Stable colloidal gold systems are obtained if the reduction is performed in the presence of a protecting ligand that coats each particle with an adsorbed layer. Citric acid, for example, forms a negatively charged protecting layer that prevents coagulation of the nanoparticles by electrostatic repulsion. As is also well known, thiols form very stable surface layers by sulfur chemisorption on gold. Thiol-capped nanoparticles result from ![]() -reduction by
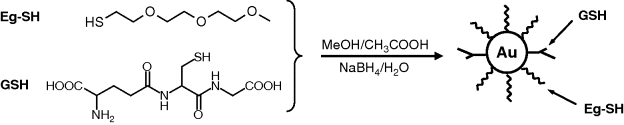
-reduction by ![]() in the presence of a thiol that contains amine or carboxyl groups as the second terminal functionality. Such terminal groups lead to water-soluble nanoparticles and also provide linking sites for subsequent conjugation with biocompounds. Mixed layers on nanoparticles are particularly suited for biosensing. As shown in Figure 8.2, nanoparticles capped with a mixed layer can be obtained by the reduction of
in the presence of a thiol that contains amine or carboxyl groups as the second terminal functionality. Such terminal groups lead to water-soluble nanoparticles and also provide linking sites for subsequent conjugation with biocompounds. Mixed layers on nanoparticles are particularly suited for biosensing. As shown in Figure 8.2, nanoparticles capped with a mixed layer can be obtained by the reduction of ![]() in the presence of two thiol derivatives: the glutathione (GSH) tripeptide and a thiolated (Eg-SH) triethylene glycol. The last component acts as a shielding component to minimize nonspecific interaction of the gold surface with biomolecules in the sample. At the same time, GSH can interact by hydrogen bonding or electrostatic attraction with the target compound. Moreover, GSH provides linking sites (such as –
in the presence of two thiol derivatives: the glutathione (GSH) tripeptide and a thiolated (Eg-SH) triethylene glycol. The last component acts as a shielding component to minimize nonspecific interaction of the gold surface with biomolecules in the sample. At the same time, GSH can interact by hydrogen bonding or electrostatic attraction with the target compound. Moreover, GSH provides linking sites (such as –![]() and –COOH) that are useful for nanoparticle conjugation with biocompounds.
and –COOH) that are useful for nanoparticle conjugation with biocompounds.
Figure 8.2 Synthesis of gold nanoparticles covered by a mixed layer of glutathione (GSH) and thiolated ethylene glycol (Eg-SH). Redrawn with permission from [22]. Copyright 2005 Wiley-VCH GmbH.
Metal nanoparticles can be produced not only as spherical granules but also with various geometries such as rods or cubes. Thus, metal nanorods can be prepared by template electrodeposition in a nanoporous host material such as alumina (![]() ) or by chemical reduction within the nanopores of a polymer membrane. Such approaches are useful for preparing regular networks of nanosized disk electrodes. Metal nanorods can also be obtained as an aqueous suspension by the reduction of the metal ion in the presence of a surfactant that favors growth of an asymmetric shape. Either chemical or electrochemical reduction can be employed to this end [23]. An important feature of a nanorod is the aspect ratio, which represents the ratio between its length and diameter. Light interaction with metal nanorods depends to a large extent on this parameter.
) or by chemical reduction within the nanopores of a polymer membrane. Such approaches are useful for preparing regular networks of nanosized disk electrodes. Metal nanorods can also be obtained as an aqueous suspension by the reduction of the metal ion in the presence of a surfactant that favors growth of an asymmetric shape. Either chemical or electrochemical reduction can be employed to this end [23]. An important feature of a nanorod is the aspect ratio, which represents the ratio between its length and diameter. Light interaction with metal nanorods depends to a large extent on this parameter.
Bimetallic nanoparticles can be prepared starting with silver or copper particles that are plated by more noble metal such as Au, Pt or Pd. Plating occurs by oxidation–reduction reactions of a compound of the plating metal. In platinum-metal core–shell nanoparticle, the platinum metal layer imparts specific catalytic activity.
8.2.2 Functionalization of Gold Nanoparticles
Various methods of functionalizing gold nanoparticles have been developed [10, 14, 22, 24]. Some of them are summarized in Figure 8.3. In one method, nanoparticles capped with negatively charged carboxylic acids or phospholipids are modified by the adsorption of positively charged proteins by electrostatic attraction (Figure 8.3A). This approach is also suited for assembling multiple layers of oppositely charged components, such as proteins and polyelectrolytes. Multiple enzyme layers can be assembled in this way.
Figure 8.3 Methods for functionalization of gold nanoparticles with biological compounds. Adapted with permission from [14]. Copyright 2004 Wiley-VCH Verlag GmBH & Co. KGaA.
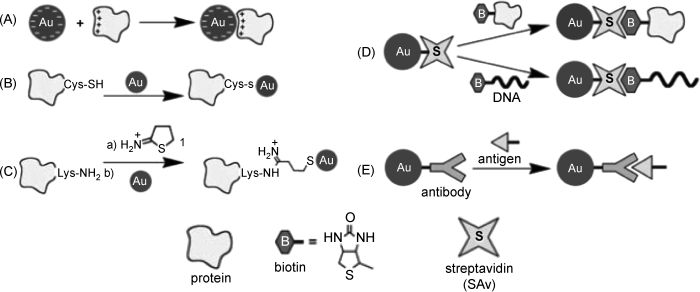
A common functionalization strategy relies on chemisorption of multifunctional thiol derivatives (see for example, Figure 8.2) that can function as bridges for tethering the nanoparticle to a biomolecule. Proteins including cysteine residues are suitable for direct chemisorption by thiol linkage to gold nanoparticles (Figure 8.3B). If no thiol residues are present in the native protein, thiol groups can be incorporated by chemical reaction of the amine groups with 2-iminothiolane (Traut's reagent) or by genetic engineering. A covalent method for the conjugation of proteins and gold nanoparticles is shown in Fig. 8.3C.
Affinity reactions are also widely used in order to link avidin (or streptavidin) tethered particles to biotin-derivatized proteins or nucleic acids (Figure 8.3D). Affinity reactions of carbohydrates with proteins are also useful in this respect.
Of great importance are the immunochemical reactions that make use of antibody-antigen reactions in order to attach proteins or living cells to nanoparticles (Figure 8.3E).
Gold nanoparticles can also be functionalized with metal-ion receptors such as macrocyclic ligands that allow specific detection of the ion of interest [25].
8.2.3 Applications of Metal Nanoparticles in Chemical Sensors
Assemblies of metal nanoparticles can be used for setting up electrical contact at a very small size scale. Moreover, including or growing metal nanoparticles within the sensing part can enhance dramatically the local electrical conductivity, which can be exploited for transduction. Metal nanoparticles can act as catalysts in chemical reactions involved with recognition or transduction processes. In mass-sensitive sensors (such as the quartz crystal microbalance) postrecognition attachment of metal nanoparticles to the receptor-bound analyte brings about a large mass increase with consequent enhancement of the response signal. Signal amplification can also be obtained by means of metal nanoparticles functionalized with multiple active components (for example, an enzyme).
A large number of applications are encountered in the field of optical sensors. Under the effect of the electromagnetic field, electrons at the surface of a metal particle oscillate collectively (localized surface plasmon resonance). This process can be noticed as a specific color, determined by the dispersion of light with the wavelength different from that of the resonance wavelength. At the same time electron oscillation results in a local enhancement of the electromagnetic field that enhances the intensity of Raman lines. On the other hand, metal nanoparticles can accept energy from excited fluorescent molecules and, in this way, reduce the fluorescence intensity (fluorescence quenching). The mentioned effects are useful for transduction in optical sensors and are discussed in more detail in Chapter 20.
Important applications rely on site-specific biocatalytic growth of metal nanoparticles in order to set up electrical contacts in nanostructured sensors [26].
8.3 Carbon Nanomaterials
Natural carbon is found in two allotropic forms: diamond and graphite. Whereas the diamond structure consist of sp3-hybridized carbons, graphite exhibits a honeycomb structure in which each atom is bound to three vicinal carbons in the sp2-hybridization state. Such monoatomic layers can be obtained in the form of graphene (Figure 8.4A) [27, 28]. Macroscopic graphite forms consist of assemblies of stacked layers of this type bound together by van der Waals forces.
Figure 8.4 (A) Graphene structure. (B) Structure of ![]() fullerene. In order to support the curving, both hexagon and pentagon carbon cycles are included in the structure. (A) Reproduced with permission from [32] Copyright 2007 Macmillan Publishers Ltd: Nature Materials. (B) Reproduced with permission from [30]. Copyright 2005 Wiley-VCH Verlag GmbH & Co. KGaA.
fullerene. In order to support the curving, both hexagon and pentagon carbon cycles are included in the structure. (A) Reproduced with permission from [32] Copyright 2007 Macmillan Publishers Ltd: Nature Materials. (B) Reproduced with permission from [30]. Copyright 2005 Wiley-VCH Verlag GmbH & Co. KGaA.
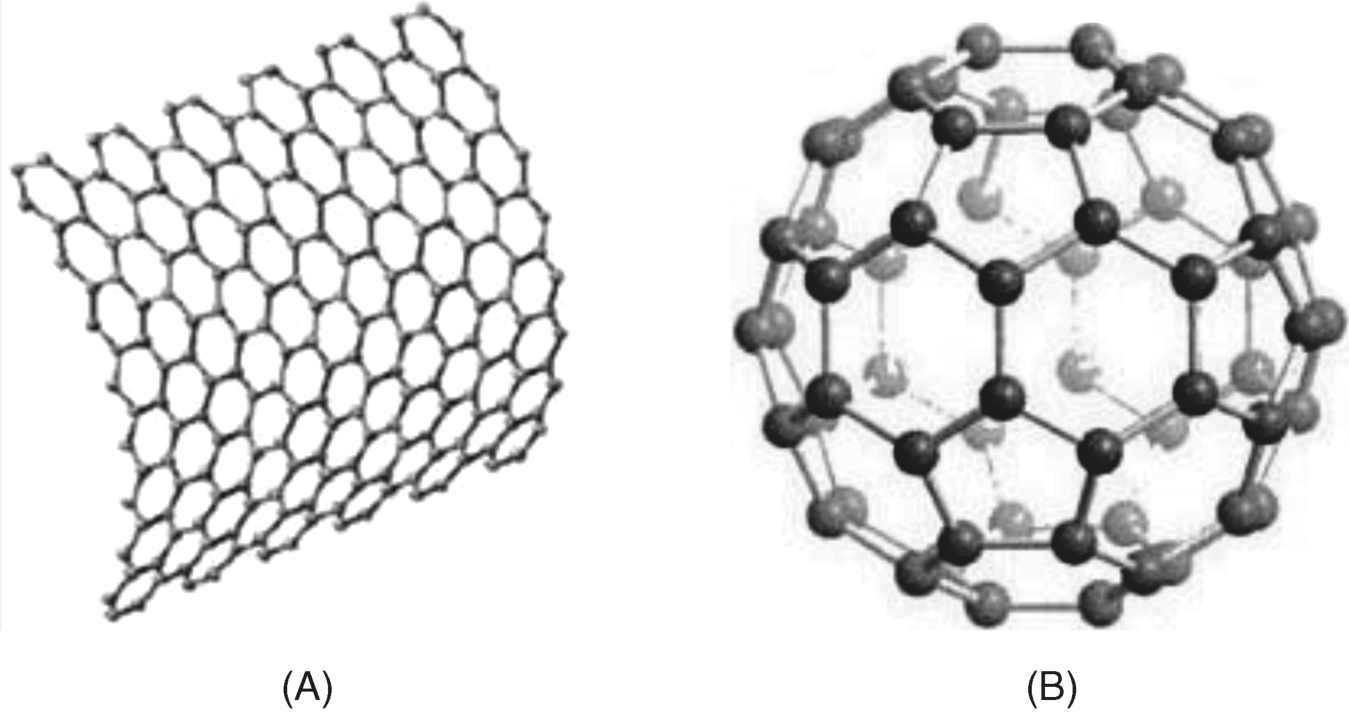
Other carbon allotropic forms such as fullerenes have been obtained by synthetic procedures. A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube (Figure 8.4B) [29] [30]. Fullerenes proved to be versatile materials for chemical sensors, for example being used as mediators in amperometric enzyme sensors [31].
Of particular importance in sensor applications are carbon nanotubes (CNTs) [32–36]. This section reviews the structure, synthesis and chemical properties of such materials. Various applications of CNTs as components of chemical sensors are emphasized in the following chapters.
Carbon nanotubes (CNTs) are hollow cylinders made of graphite structured sheets (graphenes) of graphitic carbon. Therefore, chemical bonds between carbons are of the ![]() -type. Single-walled carbon nanotubes (SWCNTs) consist of a single carbon layer (Figure 8.5) whereas multiwalled species (MWCNTs) consist of a series of concentric nanotubes or a scrolled graphene (“papier mâchè” structure). A perfect SWCNT like that in Figure 8.5 is actually an extended fullerene. However, for practical applications, caps are removed so as to obtain a true tube.
-type. Single-walled carbon nanotubes (SWCNTs) consist of a single carbon layer (Figure 8.5) whereas multiwalled species (MWCNTs) consist of a series of concentric nanotubes or a scrolled graphene (“papier mâchè” structure). A perfect SWCNT like that in Figure 8.5 is actually an extended fullerene. However, for practical applications, caps are removed so as to obtain a true tube.
Figure 8.5 Structure of a single-walled carbon nanotube (SWCNT). Reproduced with permission from [37]. Copyright 2005 Wiley-VCH Verlag GmbH & Co. KGaA.
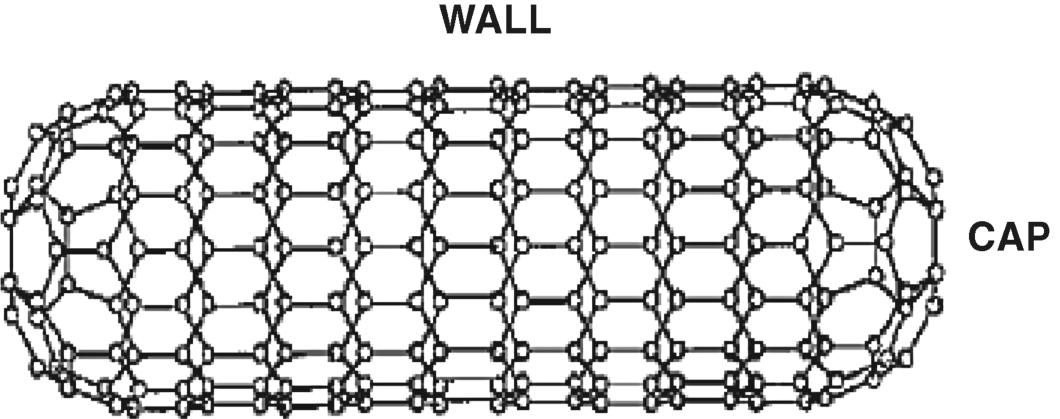
CNTs are single-dimensional materials, with diameters of 1–2 nm and lengths ranging from 50 nm to about 1 cm. They exhibit outstanding electrical, mechanical and photophysical properties that are expected to revolutionize a series of scientific and technological fields including electronics, solid-state materials and biomedicine.
8.3.1 Structure of CNTs
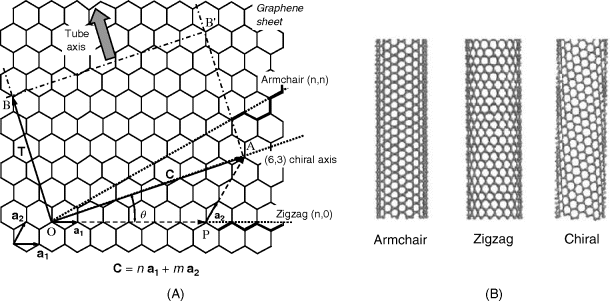
Formally, an SWCNT can be imagined as graphene rolled at a certain chiral angle with a plane perpendicular to the tube long axis. Consequently, a SWCNT can be defined by its diameter and chiral angle, which can range from 0 to 30°.
However, it is more convenient to describe the tube geometry by means of a pair of integer indices (n, m). These indices refer to two equally long unit vectors at 60° angle to each other across a single 6-member carbon ring, as shown in the left-down corner in Figure 8.6. To build up a nanotube, draw a ![]()
![]() vector across the graphene sheet (OP), then draw a
vector across the graphene sheet (OP), then draw a ![]()
![]() vector at 60° to the first one (PA) and add the two vectors. The length of the resultant vector
vector at 60° to the first one (PA) and add the two vectors. The length of the resultant vector ![]() defines the circumference of the nanotube along a plane perpendicular to its axis. Perpendicular to
defines the circumference of the nanotube along a plane perpendicular to its axis. Perpendicular to ![]() , the vector
, the vector ![]() points to the long axis of the cylinder; its length is the shortest repeat distance along this axis.
points to the long axis of the cylinder; its length is the shortest repeat distance along this axis. ![]() and
and ![]() are referred to as chiral vectors and translational vectors, respectively. Together,
are referred to as chiral vectors and translational vectors, respectively. Together, ![]() and
and ![]() define the unit cell of the nanotube as the rectangle OAB'B. The nanotube is obtained by rolling the graphene along the
define the unit cell of the nanotube as the rectangle OAB'B. The nanotube is obtained by rolling the graphene along the ![]() direction making AB' and OB to coincide. Depending on the chiral angle
direction making AB' and OB to coincide. Depending on the chiral angle ![]() , two particular nanotube types can be distinguished: the zigzag (
, two particular nanotube types can be distinguished: the zigzag (![]() , that is,
, that is, ![]() ) and the armchair (
) and the armchair (![]() ,
, ![]() ) forms. For any intermediate
) forms. For any intermediate ![]() value (that is,
value (that is, ![]() , the tube is in the chiral configuration. Figure 8.6 displays the construction of a chiral (6,3) nanotube as well as the
, the tube is in the chiral configuration. Figure 8.6 displays the construction of a chiral (6,3) nanotube as well as the ![]() -axes for the zigzag and armchair particular forms. As this figure demonstrates, such designations derive from the path of the carbon atoms along the particular chiral axis.
-axes for the zigzag and armchair particular forms. As this figure demonstrates, such designations derive from the path of the carbon atoms along the particular chiral axis.
Figure 8.6 (A) Construction of the unit cell for a (6,3) SWCNT. Bold chemical bonds show the armchair and zigzag disposition of carbons along the cylinder circumference. (B) Armchair, zigzag, and chiral SWCNTs. Adapted with permission from [39]. Copyright 2006 Springer-Verlag.
The n and m indices determine whether the nanotube is a metal-type conductor or a semiconductor. The first case occurs when ![]() (k is an integer), whereas the second one corresponds to
(k is an integer), whereas the second one corresponds to ![]() . The nanotube diameter
. The nanotube diameter ![]() is related to
is related to ![]() and
and ![]() as [38]:
as [38]:
(8.1) ![]()
where a = 0.246 nm is the magnitude of unit vectors. The chiral angle is also unambiguously determined by the ![]() and
and ![]() indices. Therefore, the
indices. Therefore, the ![]() and
and ![]() indices decide both crystallographic and geometrical characteristics as well as the metallic or semiconducting properties of a CNT.
indices decide both crystallographic and geometrical characteristics as well as the metallic or semiconducting properties of a CNT.
As already mentioned, SWCNTs can be obtained as either semiconductor or metal-type conducting materials. In contrast, MWCNTs are always metal-type electric conductors.
8.3.2 Synthesis of CNTs
CNTs can be grown by sublimation of carbon vapors under arc discharge or laser ablation conditions [33, 40, 41]. However, the most promising method for CNT fabrication is based on chemical vapor deposition (CVD) [42]. In this process, CNTs form on metal catalyst particles (Co, Ni or Fe) supported on a refractory material (MgO or ![]() ) heated to about 700 °C, in the presence of a gas mixture that generates carbon vapors. This mixture consists of a carbon feedstock component (acetylene, ethylene or methane) and a process gas (nitrogen, hydrogen or ammonia). Finally, the metal catalyst is removed by acid treatment. As the product contains CNTs of various types and size and also other carbonaceous materials, CNTs of the expected type should be separated by a suitable method such as ultracentrifugation, electrophoresis or size-exclusion chromatography.
) heated to about 700 °C, in the presence of a gas mixture that generates carbon vapors. This mixture consists of a carbon feedstock component (acetylene, ethylene or methane) and a process gas (nitrogen, hydrogen or ammonia). Finally, the metal catalyst is removed by acid treatment. As the product contains CNTs of various types and size and also other carbonaceous materials, CNTs of the expected type should be separated by a suitable method such as ultracentrifugation, electrophoresis or size-exclusion chromatography.
SWCNTs can be grown with a high yield by the HiPCO version of CVD. In this method, carbon monoxide disproportionation to carbon at high temperature and high pressures is catalyzed by iron particles that form by decomposition of gaseous iron pentacarbonyl, purposely added to the CO stream.
CNTs can be grown vertically aligned with respect to the support if the process is conducted in a plasma generated by an electric field, which dictates the growth direction. This method (known as plasma-enhanced CVD) allows regular networks of vertically aligned CNTs to be obtained by using support surfaces with a preformed array of catalyst nanoparticles [42]. Similarly, CNTs can be grown in the form of a nanobrush on a cylindrical support.
CNT assemblies with controlled geometry are very important in sensor development as they allow controlled tailoring of the sensing part. However, the growth of carbon nanotubes with a predefined microscopic structure still remains a major challenge.
CNT processing and application often relies on forming liquid suspensions. As CNTs are hydrophobic, water suspensions can be obtained by ultrasonic agitation in the presence of a surfactant such as sodium dodecylsulfate or sodium colate, followed by centrifugation to remove bundles, ropes and residual catalyst. CNT wrapping with synthetic or biopolymers is equally successful.
8.3.3 Chemical Reactivity and Functionalization
Practical applications of CNTs requires some preliminary processing such as tube opening, changing of tube-wetting properties, tube filling, adsorption at the tube surface, charge transfer to or from the tube or tube doping [43].
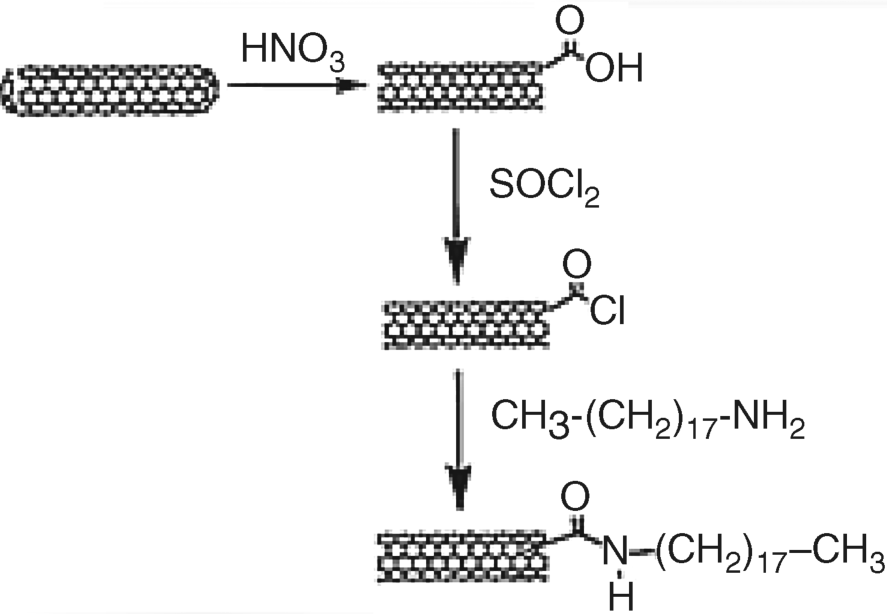
Raw CNTs are closed by hemispherical fragments including carbon pentagons or metal catalyst inclusions. Opening is required for many applications; it can be accomplished by various methods including vapor-phase oxidation, plasma etching, or chemical reaction with strong oxidants (e.g., nitric acid, Figure 8.7). The opened end is terminated with different oxygen groups such as carboxyl, hydroxyl and quinones. Such groups are useful for covalently linking CNTs to a support or to various biocompounds. Thus, the carboxylate group allows further nanotube modification via an ester or amide linkage (Figure 8.7). Oxidative treatment results as a rule in tube shortening and carboxylate formation on the side wall as well.
Figure 8.7 Cap opening and CNT functionalization by a peptide linkage. Reproduced with permission from [48]. Copyright 2005 Wiley-VCH.
Covalent functionalization at the CNT side wall can be effected by various reactions producing reactive functionalities as linking sites [44–47]. However, it is important to note that covalent functionalization at the side wall disrupts the ![]() orbitals system and affects the electronic properties of the nanotube.
orbitals system and affects the electronic properties of the nanotube.
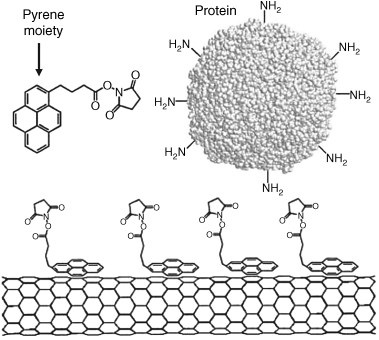
Noncovalent functionalization can be achieved by adsorption on the side wall and has the advantage that the physical properties of the CNT are essentially preserved. Thus, polyaromatic molecules (such as pyrene) adsorb at the graphitic surface of CNTs via ![]() -
-![]() - stacking. As shown in Figure 8.8, reactive groups can thus be attached to the nanotube surface to serve for further conjugation with proteins. Pyrene-derivatized receptors (e.g., cyclodextrin) or nanoparticles can be attached to CNTs in a similar way [49]. DNA can be attached to a pyrene methylammonium-covered CNT. DNA binds to the surface modifier by electrostatic interaction of the anionic phosphate backbone with the positive ammonium group in the modifier.
- stacking. As shown in Figure 8.8, reactive groups can thus be attached to the nanotube surface to serve for further conjugation with proteins. Pyrene-derivatized receptors (e.g., cyclodextrin) or nanoparticles can be attached to CNTs in a similar way [49]. DNA can be attached to a pyrene methylammonium-covered CNT. DNA binds to the surface modifier by electrostatic interaction of the anionic phosphate backbone with the positive ammonium group in the modifier. ![]() -
-![]() - stacking allows single-strand DNA molecules to wrap around a CNT.
- stacking allows single-strand DNA molecules to wrap around a CNT.
Figure 8.8 Protein immobilization on a CNT via N-succinimidyl-1-pyrenebutanoate - π-π stacked at the nanotube surface. Adapted with permission from [50]. Copyright 2009 American Chemical Society.
Various amphiphilic compounds are currently used to stabilize CNT dispersions by adsorption. This approach can also be used in sensor development provided the amphiphile is biocompatible, nontoxic and sufficiently strongly adsorbed so as to resist desorption in biological media with high salt and protein content. Polyethylene glycol-derivatized phospholipids have proved particularly practical in this respect. The two hydrocarbon chains of the lipid strongly adsorb at the nanotube surface with the hydrophilic polyethylene glycol chain extending into the aqueous phase. Conjugation of the biological molecule can be achieved using a functional group (e.g., amine or carboxylate) purposely added at the PEG terminal.
CNTs can be covered by the adsorption of various biocompounds such as proteins, nucleic acids and polysaccharides (e.g., chitosan) that can act as anchors for attaching other compounds of interest.
Encapsulation into the nanotube cavity is another method for noncovalent modification. Various inorganic species (including pure elements, metallocenes, inorganic salts, metal oxides, and fullerenes) can be inserted into CNTs and bring about important changes in electronic properties. Metal nanowires can also be grown inside CNTs. Biomolecules (such as small proteins or single-stranded nucleic acids can be entrapped in CNTs by hydrophobic interactions due to the favorable tube diameter (2–10 nm).
Semiconducting CNTs are very promising materials for nanosized semiconductor devices including chemical sensors. Fabrication of CNTs with predefined semiconducting properties is therefore a matter of great interest [43]. Pristine CNTs are naturally p-doped, that is, display only hole conduction. Doping of CNTs shifts the Fermi level, while the band structure remains intact. This changes the population of electronic states near the Fermi level, which reduces the ohmic losses and facilitates carrier injection from contacts. Substitutional doping can be effected by the replacement of some carbon atoms with boron or nitrogen atoms. Boron induces holes and converts the tube into a p-semiconductor, whereas nitrogen, which imparts electrons, renders the tube an n-type semiconductor. Doping can also be achieved by encapsulating atoms, molecules or clusters exhibiting either electron-acceptor or electron-donor properties. Adsorption of some gases with electron-donor/acceptor properties (such as oxygen or ammonia) converts, in a reversible way, intrinsically semiconducting nanotubes into apparent metallic ones. Adsorption of an organic electron acceptor (tetracyanoquinodimethane, TCNQ) or donors (amines) also affects the electrical properties of SWCNTs.
8.3.4 CNT Applications in Chemical Sensors
The advent of CNTs has prompted a spectacular development in the research activity involving applications of such materials in analytical chemistry and, particularly, in chemical sensors and biosensors [51–54]. Integration of CNTs with sensor systems is facilitated by the large variety of available tube derivatization methods that allow the tuning of CNT properties and their conjugation with various biocompounds.
By physical adsorption, covalent binding or incorporation in a polymer, CNTs enable the structuring of chemical sensors at the nanometer scale. On the other hand, a CNT may serve as a support for multiple tags (e.g., fluorophores or enzymes). Once attached to a compound of interest, such a multiple label provides an appreciable enhancement of the response signal.
Excellent electrical conduction of metallic CNTs makes them suitable as molecular wires in electrochemical sensors [37, 55, 56]. Thus, in amperometric enzyme sensors A CNT can promote direct electron transfer between the active center of a redox enzyme and a macroscopic electrode. At the same time, catalytic properties, imparted by tube-end oxygen functionalities is useful in applications based on the electrochemical oxidation of the analyte. On the other hand, semiconducting CNTs integrated in field effect transistor structures allow chemical sensors to be developed for inorganic or biological species [49, 55].
SWCNTs exhibit remarkable photophysical properties such as near-infrared fluorescence and very characteristic Raman scattering. Such properties render CNTs suitable for applications as optical labels in chemical sensors, as discussed in detail in Chapter 20.
8.3.5 Carbon Nanofibers (CNFs)
Carbon nanofibers (CNFs), also known as vapor-grown carbon nanofibers (VGCNFs), are cylindrical nanostructures with graphene layers arranged as stacked cones, cups or plates around the fiber axis [57].
CNFs are fabricated by chemical vapor deposition from hydrocarbon precursors using metal-particle catalysts [58]. The main steps of the synthesis are outlined in Figure 8.9. First, adsorption and decomposition of the hydrocarbon molecule at the catalyst particle takes place. Next, the carbon atoms thus formed are dissolved in the catalyst particle and undergo diffusion through it. Carbon reaching the opposite side of the particle precipitates to form a carbon network, thus promoting the growth of the fiber.
Figure 8.9 Grown of CNFs by chemical vapor deposition on a catalyst particle. (a) Decomposition of the precursor and carbon dissolution into the catalyst particle; (b) carbon diffusion through the catalyst particle; (c) nanofiber growth at the particle surface.
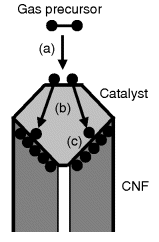
The diameter of CNFs can range between 5 to 500 nm; the diameter is determined by the size of the catalyst particle. The length of CNFs is in the micrometer region.
The structure of the nanofiber depends on the nature of the catalyst metal. Thus, Figure 8.10A(a) shows the so-called herringbone structure, obtained with nickel as catalyst. This structure is composed of stacked nanocones. Under similar conditions, but using iron as catalyst, one obtains a bamboo-type CNF (Figure 8.10A(b)).
Figure 8.10 (A) CNF structure. (a) Herringbone structure formed by stacking of carbon nanocones grown on Ni catalyst particles by chemical vapor deposition; (b) bamboo-type CNF grown under the same conditions with an Fe catalyst. (B) Freestanding vertically aligned CNFs and forests of CNFs. Reproduced with permission from [59]. Copyright 2005 American Institute of Physics.
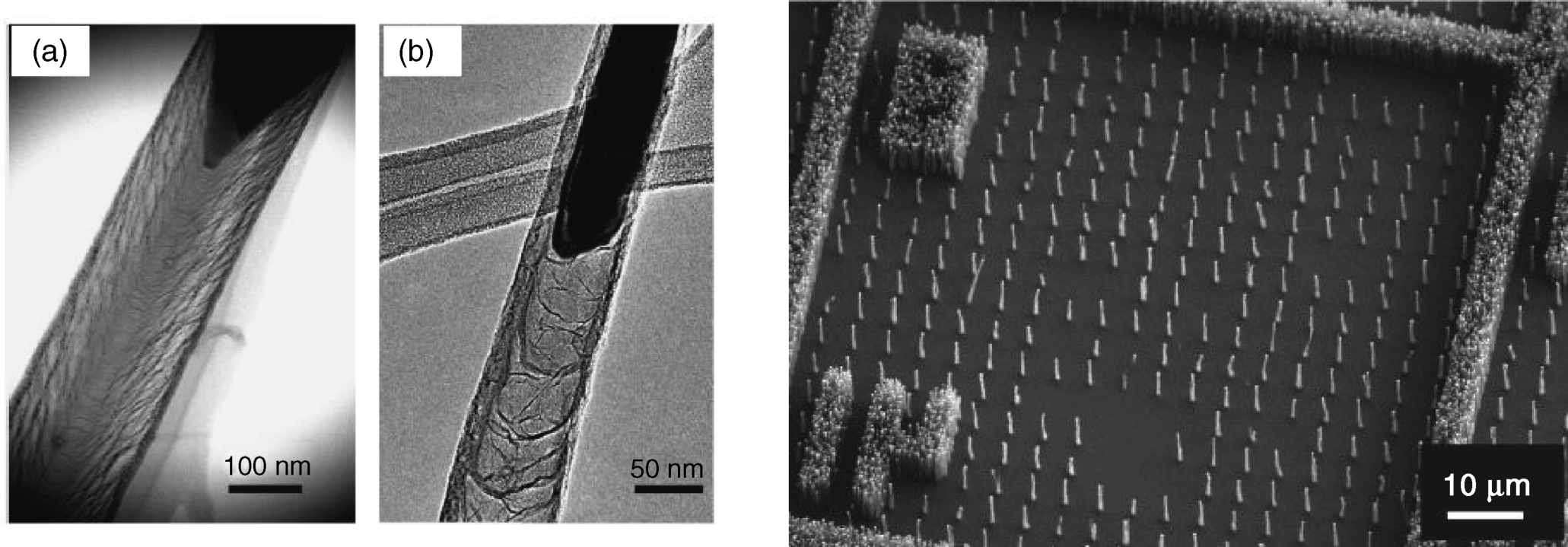
CNFs can be obtained as bundles or as networks of randomly entangled fibers. For applications that require CNFs as individual elements, such as nanoelectronics or chemical sensing, ordered assemblies, such as vertically aligned CNFs, are more suitable. Vertically aligned CNFs can be grown in a nanoporous template (for example, alumina) or on catalyst particles immobilized by photolithography on a flat support in a predetermined pattern [59]. Figure 8.10B shows both individually freestanding structures of CNFs as well as dense mats (“forests”) of fibers.
As far as the application of CNFs in sensor design is concerned, it is important to point out that the structure of CNFs contrasts strongly with that of CNTs. CNTs are well-defined three-dimensional macromolecular structures built up of hexagonal carbon cycles units while CNFs are assemblies of graphenes held together by van der Waals forces. While the functionalization of a CNT should be performed only at the tube end in order to prevent alteration of the conductive properties, CNFs display a high number of reactive sites at the edges of included graphenes. Oxidation of the fiber endows it with a high surface density of hydroxyl and carboxyl group that can interact physically or chemically with the surrounding environment. In addition, oxygen groups can function as binding sites for covalent attachment of biomolecules and other compounds of interest. Good electrical conductivity, combined with multiple possibilities of functionalization, render CNFs very attractive for applications in electrochemical sensors, particularly because CNFs allow direct electron transfer from the catalytic sites of redox enzymes to the electrode [60, 61].
8.4 Polymer and Inorganic Nanofibers
Nanofibers can be obtained by various methods such as template synthesis, phase separation or self-assembly. A versatile method for nanofiber fabrication is electrospinning that allows nanofibers to be formed from solutions or melts [62]. As shown in Figure 8.11A, in this process the liquid is pumped through a nozzle connected to a high-voltage supply. When a sufficiently high voltage is applied to a liquid droplet, the body of the liquid becomes charged, and electrostatic repulsion counteracts the surface tension. As a result, the droplet is stretched and at a critical point a stream of liquid erupts from the surface. This point of eruption is known as the Taylor cone. As the jet dries in flight, the charge migrates to the surface of the fiber. The jet is then elongated by a whipping process caused by electrostatic repulsion initiated at small bends in the fiber. Electrostatic repulsion also produces thinning of the fiber, which is finally deposited on a grounded support that collects the charge.
Figure 8.11 (A) Principle of nanofiber fabrication by electrospinning; (B) Scanning electron micrograph of polystyrene–polyaniline blend fibers. (A) Adapted from http://commons.wikimedia.org/wiki/File:Electrospinning_Diagram.jpg Last accessed 17/05/2012. (B) Reproduced with permission from [66] Copyright 2009 The Conference of Photopolymer Science and Technology.
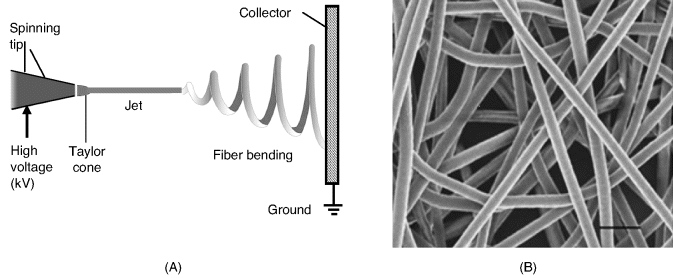
Electrospinning is typically employed to produce polymer fibers [63, 64]. Inorganic fibers can be obtained in the same way, using a solution of precursors that yields by chemical reaction the expected material. Semiconductor metal oxide nanofibers produced by electrospinning followed by annealing, have proved to function as excellent sensing materials in certain gas sensors [65].
Polymer fibers can be obtained either from a single polymer or from polymer blends. In the second case, two different polymers are dissolved in the same solvent and the fiber is spun from this mixed solution. If the two solutions are incompatible, two syringes are used, each of them delivering one of the components to the spinning tip. The morphology of a nanospun polymer mesh of a polystyrene–polyaniline blend is shown in Figure 8.11B.
Owing to the very large specific area, electrospun polymer meshes are suitable as immobilization supports for enzymes since they allow high enzyme loading and facile mass transfer of the substrate. Enzyme immobilization can be performed by well-established methods such as entrapment, adsorption, crosslinking or covalent bonding [67]. In addition, a nanospun mesh can function as a size-selective separation membrane in enzyme sensors to discriminate between small substrate molecules and proteins so as to prevent fouling or interference by the proteins.
Polymer blends that include conducting polymers provide an additional function, namely electrical conductivity, which makes them attractive for application in amperometric enzyme sensors. Nanofibers of polystyrene–polyaniline blends allow glucose oxidase to be immobilized by electrostatic adsorption. The conducting polymer component imparts electrical conductivity and provides direct electron transfer from the immobilized enzyme [66].
The two-syringe electrospinning method is also a handy technique for producing polymer–inorganic composite nanofibers such as carbon nanotube–polymer composites [68].
Metal nanofibers with excellent electrical conductivity can be obtained by electrospinning from a polymer solution containing a metal precursor, which provides nucleation seeds for subsequent growth of a metal coating over the polymer fiber. Thus, gold nanofibers have been fabricated by electrospinning of a polyacrylonitrile solution containing HAuCl4. Nucleation seeds are formed by reduction of occluded HAuCl4 to Au using an NaBH4 solution. Finally, gold deposition has been produced from a solution containing HAuCl4 with hydroxylamine as reducing agent [69]. Enzyme immobilization can be achieved by crosslinking to a layer of cysteamine chemisorbed at the gold surface. This design has been utilized in an amperometric fructose sensor based on fructose dehydrogenase, which performed well in the determination of fructose in blood and beverages.
8.5 Magnetic Micro- and Nanoparticles
Magnetic particles commonly consist of magnetic elements such as iron, nickel and cobalt or some of their chemical compounds. As will be shown later the magnetic behavior depends to a large extent on the particle size. That is why it is useful to distinguish two categories: magnetic beads (round particles of 100–400 μm size) and magnetic nanoparticles that are between 10 and 100 nm diameter. Both categories have proved extremely useful in applications in biosciences owing to the possibility of remote manipulation of the magnetically tagged entities by means of magnetic fields. Remarkable achievements have been made using magnetic particles in diagnostic, therapy and bioimaging applications [70–72]. Also, magnetic particles have proved to be excellent for separation, purification and identification of molecules, cells and micro-organisms tagged with such particles. As far as biosensors are concerned, magnetic particles are useful for remote manipulation of sample components and as transduction labels in magnetic sensors.
8.5.1 Magnetism and Magnetic Materials
The term magnetism denotes the property of some materials, molecules or elementary particles to be subject to forces under the effect of a magnetic field. Under the effect of a magnetic field, any magnetic entity experiences a force at each pole. These forces are equal to each other but are oriented in opposite directions and tend to rotate the magnet until it aligns with the magnetic field as a compass needle. Rigorously speaking, the effect of a magnetic field on a magnet results in a torque, a vector oriented perpendicularly to both forces and the pole axis and with the initial point at the mid-distance between poles. Its magnitude ![]() is:
is:
(8.2) ![]()
where ![]() is the distance between poles,
is the distance between poles, ![]() is the force applied to each pole and
is the force applied to each pole and ![]() is the angle between the force and the pole axis.
is the angle between the force and the pole axis.
The strength of a magnet is given by its magnetic dipole moment, which is the ratio of the maximum torque (experienced at ![]() ) to the strength of the magnetic field. A specific magnetic property of a specimen is the magnetization,
) to the strength of the magnetic field. A specific magnetic property of a specimen is the magnetization, ![]() , which is defined as the specimen magnetic moment divided by its volume. Magnetization depends on the strength of the magnetic field and a characteristic property of the specimen called magnetic susceptibility.
, which is defined as the specimen magnetic moment divided by its volume. Magnetization depends on the strength of the magnetic field and a characteristic property of the specimen called magnetic susceptibility.
The source of magnetic properties is the electron orbital motion around the nucleus (orbital magnetic dipole moment) and the electron's intrinsic magnetic moment (spin). Vector addition of spin and orbital moments results in the total magnetic dipole moment that is responsible for the behavior of the electron in a magnetic field. Paired electrons are required by the Pauli exclusion principle to have their spin magnetic moments pointing to opposite directions, causing their magnetic fields to cancel out. However, an unpaired electron is free to align its magnetic moment with any direction and imparts to the atom a magnetic dipole moment. In the absence of a magnetic field, the atomic magnetic moments are oriented randomly and the overall magnetic moment of the specimen is zero. When an external magnetic field is applied, the atomic magnetic moments will tend to align themselves with the direction of the applied field. Materials displaying such a property are termed paramagnetic materials if the atoms return to random orientation after removal of the magnetic field. The oxygen molecule in the ground state displays typical paramagnetic behavior because it includes two unpaired valence electrons.
A ferromagnetic material is similar to a paramagnetic one in that it consists of atoms with a nonzero magnetic dipole moment. In addition, ferromagnetic materials are able to maintain the alignment of the atomic dipoles after removing the external magnetic field, which imparts to them permanent magnetism. This property is the result of the existence of magnetic domains in ferromagnetic materials. In each magnetic domain, atomic magnetic dipoles adopt, by mutual interaction, a parallel orientation. However, each domain adopts a random orientation and the overall magnetic moment of the specimen is zero. If a magnetic field is applied, domains tend to line up along the direction of the magnetic field. However, due to restricted movement in the solid-state phase, after removal of the magnetic field the domains cannot return to perfectly random orientation and the specimen preserves residual magnetization.
8.5.2 Magnetic Nanoparticles
Colloidal particles of magnetic materials with sizes ranging from nanometer to micrometer sizes can be prepared by high-temperature liquid-phase reactions of suitable precursors in the presence of a surfactant [73–75]. As particle properties are size dependent, it is important to use monodisperse systems, which are characterized by a standard deviation of the diameter of less than 10%. Nanometer-sized magnetic particles are particularly suitable for biological applications because such particles consist of a single magnetic domain. Consequently, magnetic nanoparticles do not maintain residual magnetization after being exposed to a magnetic field, a property termed superparamagnetism. If residual magnetization persisted, particles would tend to form clusters by mutual attraction that would be detrimental to biological applications.
The most common materials for magnetic nanoparticles are ![]() (hematite),
(hematite), ![]() (maghemite) and mixed oxides with the general formula
(maghemite) and mixed oxides with the general formula ![]() , where M is a divalent ion, such as Fe, Co, Mg or Zn. Among these materials, magnetite (
, where M is a divalent ion, such as Fe, Co, Mg or Zn. Among these materials, magnetite (![]() ) is the most widely used.
) is the most widely used.
Colloidal magnetic nanoparticles are covered by a hydrophobic layer (oleic acid or oleamine) that prevents self-aggregation. For biological applications it is essential to convert them into a hydrophilic form and attach functionalities or molecules that are able to interact specifically with the target compound or cell [76]. Conversion of magnetic particles into hydrophilic form can be effected either by surfactant addition or by surfactant exchange.
The surfactant-addition approach relies on forming an additional layer of an amphiphilic compound. The hydrophobic end of the amphiphilic compound interacts with the initial layer to form a double layer that displays a hydrophilic terminus in contact with the aqueous phase. As the surfactant layers are held together by hydrophobic interaction, this structure is not particularly stable. Alternatively, hydrophobic particles can be encapsulated in a polymer layer that contains hydrophilic groups oriented to the aqueous phase.
Surfactant exchange is achieved by direct replacement of the original layer by a compound that fulfills two conditions: it forms strong bonds with the particle surfaces and displays hydrophilic terminal groups at the opposite end. Small bifunctional molecules (such as cystamine or 2,3-dimercaptosuccinic acid) are practical for surfactant exchange either by forming strong metal–sulfur bonds or by chelate binding of carboxylate groups to the metal. Magnetic nanoparticles can also be coated with a silica shell by means of sol-gel chemistry methods [77].
A convenient alternative to the above strategies is based on particle synthesis in the presence of a hydrophilic multidentate ligand (such as citric acid) that covers, in situ, the particle with a hydrophilic layer.
Biological as well as sensor applications of magnetic nanoparticles require them to be able to bind target biocompounds or cells [76]. To this end, the surface layer should be engineered so as to include suitable anchoring groups to which receptors are attached by standard bioconjugation reactions. Thus, covalent linking can be effected if the particle envelope contains groups such as carboxyl, amine, thiol, or carbonyl. Antibodies, nucleic acids and affinity reagents (for example, streptavidin) can be conjugated thereafter with modified magnetic nanoparticles. Abiotic receptors such as macrocyclic ligands, cryptands or calixarenes can be appended in the same way.
Magnetic crystals can be extracted from certain bacteria and algae. Such particles are found as assemblies called magnetosomes that act together like a compass needle to orient the organism in the geomagnetic field. Each magnetite crystal within a magnetosome is surrounded by a lipid bilayer, and specific soluble proteins. Magnetosome crystals have high chemical purity, narrow size ranges and elicit species-specific crystal morphologies.
8.5.3 Magnetic Biosensors and Biochips
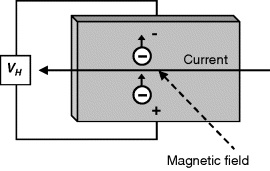
Magnetic biosensors rest on the detection of the stray magnetic field induced by magnetic nanoparticles accumulated near the transducer as a result of a suitable recognition process [78–82]. Various physical effects such as the Hall effect and the giant magnetoresistance effect form the basis of such transducers [78, 80]. The Hall effect is the consequence of the electron drift under the influence of a magnetic field (Figure 8.12). In a thin metal strip, this causes a potential difference to develop between the edges as a function of the strength of the magnetic field. The anisotropic magnetoresisitive effect manifests as a change in material resistance when the magnetization changes from parallel to transverse with respect to the direction of current flow. The giant magnetoresistance effect is the basis of spin-valve transducers. Magnetoresistive transducers have been developed primarily for applications in magnetic random access memory devices but have also proved useful for magnetic biosensing purposes.
Figure 8.12 The Hall effect. A Hall potential difference (![]() ) develops in a thin metal strip crossed by a current and subject to a magnetic field perpendicular to the surface.
) develops in a thin metal strip crossed by a current and subject to a magnetic field perpendicular to the surface.
In order to detect magnetic nanoparticles, a conveniently oriented magnetic field is applied to the sensor. This magnetic field induces a magnetic dipole to each nanoparticle that results in an alteration of the local strength of the magnetic field.
Typical configuration of a magnetic affinity biosensor is shown in Figure 8.13. In the direct detection scheme (Figure 8.13A), the receptor interacts with the target analyte which is attached to magnetic nanoparticles. The unbound analyte molecules will then be washed away. The presence of the target in the sensing layer is indicated by the stray magnetic field induced by the nanoparticles. The sandwich assay format can also be implemented using magnetic nanoparticles. In this case, the sensing layer and nanoparticle tags consist of different ligands, each of them binding to a specific site of the analyte molecules.
Figure 8.13 (A) Configuration of a magnetic sensor based on affinity recognition. (B) Sandwich DNA assay using magnetic nanoparticles as labels. Adapted with permission from [77]. Copyright 2004 Elsevier.
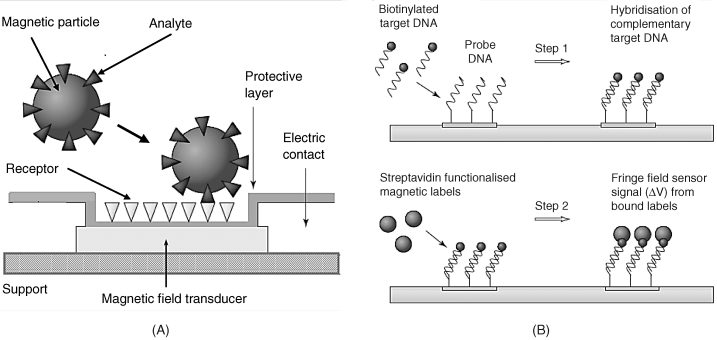
A two-step DNA assay method is shown in Figure 8.13B. In the first step, the biotin-tagged DNA analyte binds by hybridization to the complementary probe attached to the transducer surface. Next, streptavidin-functionalized magnetic nanoparticles are added to the biotin tags of the hybridized probe. Any magnetic particles not bound to the receptor will then be driven away by the action of a magnetic field. Finally, the effect of the stray magnetic field within the sensing layers is recorded.
Current applications of magnetic biosensing are concerned chiefly with biochip development. Magnetic biochips consist of assemblies of minute magnetic sensors with a typical size in the micrometer region. This technology is suitable for developing hand-held biochip instrumentation [81, 83, 84].
Magnetic biosensors exhibit a series of notable advantages. Magnetic labels are very stable and not affected by chemical reactions involved in the sensor operation. Even more important is the absence of interferences from biological media that exert almost no influence on magnetic nanoparticles. Consequently, magnetic transduction is practically free of interference from the sample matrix. It allows the development of low-cost components that provide compact, user-friendly and very sensitive devices. Remote manipulation by a magnetic field can be implemented in order to perform analyte separation or to remove unwanted magnetic particles. However, present magnetic biochips have a fairly low throughput owing to the relatively large area of each sensing site. Much progress is therefore anticipated from the reduction of transducer size.
8.5.4 Magnetic Nanoparticles as Auxiliary Components in Biosensors
Application of magnetic particles in biosensor design brings about a series of advantages as far as sensitivity, reliability, selectivity and fabrication methods are concerned [85–87].
First, magnetically tagged entities (proteins, DNA, living cells) can be easily separated from a crude sample in order to conduct the analysis under interference-free conditions. When necessary, the separated analyte can be subject to additional processing (such as DNA amplification) after separation. This approach works equally well with protein immunosensors and DNA hybridization sensors. It has also proved also useful in the detection of living cells (such as pathogenic micro-organisms and tumor cells) by immunorecognition.
In standard affinity biosensors, the receptor is immobilized at the transducer surface in order to construct the sensing part. Under such circumstances, the recognition reaction is relatively sluggish owing to slow diffusion and the steric restriction imparted to the immobilized receptor. Magnetic particles allow the development of an alternative strategy based on receptor immobilization at the surface of magnetic particles thus letting the transducer interface free of additional components. Once mixed with the sample, the tagged receptor interacts with the analyte to give a tagged analyte–receptor–magnetic particle hybrid. This hybrid can be selectively accumulated at the transducer surface by means of a magnetic field in order to assess the response signal. As the recognition processes are much faster in the solution phase, this procedure results in a substantial reduction in the analysis time.
A series of promising applications of magnetic particles has been demonstrated in the field of enzyme sensors. Thus, in order to avoid the need for prior enzyme immobilization, the enzyme is tagged with magnetic particles and then accumulated at the transducer surface by means of an incorporated magnet. Owing to the size compatibility, enzyme tagging with magnetic nanoparticles does not affect enzyme activity to the same extent as the immobilization on macro-objects does. As a result, magnetic immobilization of the enzyme may result in improved sensitivity.
Signal enhancement in enzyme sensors can also be obtained by using a magnetically tagged cofactor. A rotating magnetic field drives the magnetic particles into motion, thus enhancing the diffusion rate by local convection. The simplest use of the magnetic-field effect consists of switching the enzyme reaction on/off alternately by the action of a magnet on magnetically tagged cofactor molecules that are present in the solution. The sensor turns on when a magnet is placed just under the sensor but it turns off if the magnet is moved to above the solution.
Magnetic switching has also been applied to control the state of a dual-enzyme sensor in which only one of the enzyme cofactors is magnetically tagged. This enzyme will be active only under the effect of a magnet placed under the sensor. Combined with a suitable selection of a specific working parameter (such as the electrode potential in the case of an amperometric sensor), the use of a magnetic field permits sequential determination of two different analytes using a sensing layer composed of a mixture of two enzymes. See ref. [86] for more details.
8.5.5 Outlook
Magnetic nanoparticles can be readily conjugated with biocompounds (such as proteins and nucleic acids) and thus allow remote manipulation by magnetic fields in order to perform separation or concentration of such entities. In addition, combination with magnetic particles is suited for triggering or amplifying certain recognition processes. Such applications can be implemented in conjunction with various biorecognition methods and transduction procedures.
Moreover, in combination with magnetic transducers, functionalized magnetic particles allow the development of magnetic biosensors and biochips characterized by high selectivity and sensitivity.
8.6 Semiconductor Nanomaterials
Although doped silicon is currently the most widely used semiconductor material for applications in electronics, compound semiconductor nanomaterials are particularly applied in the field of chemical sensors and biosensors. Such nanocrystals consist of compounds of group II and VI elements (for example, CdSe and CdTe) or group III and V elements (for example, InP and InAs). Semiconductor nanocrystals are also known as quantum dots.
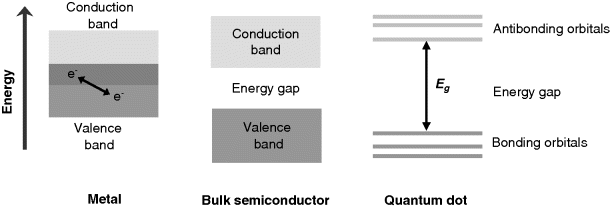
The main difference between bulk semiconductors and quantum dots arises from the distribution of the electronic energy levels. As shown in Figure 8.14, conduction and valence bands in a metal overlap partially allowing electron transition between them. This is the reason for the high electrical conductivity of metallic materials. In a bulk semiconductor, the valence and conduction bands are separated by an energy gap. By contrast, due to electron confinement a quantum dot displays a series of discrete energy levels in both the bonding and antibonding electronic states. These two groups of orbitals are separated by a relatively broad energy gap. The discrete level distribution is similar to that in atoms and imparts quantum dots with particular properties as far as light absorption or emission are concerned. Thus, light absorption occurs only if the photon energy matches the difference between a bonding and an antibonding level.
Figure 8.14 Distribution of electronic energy levels in metals, bulk semiconductors and semiconductor nanocrystals.
8.6.1 Synthesis and Functionalization of Quantum Dots
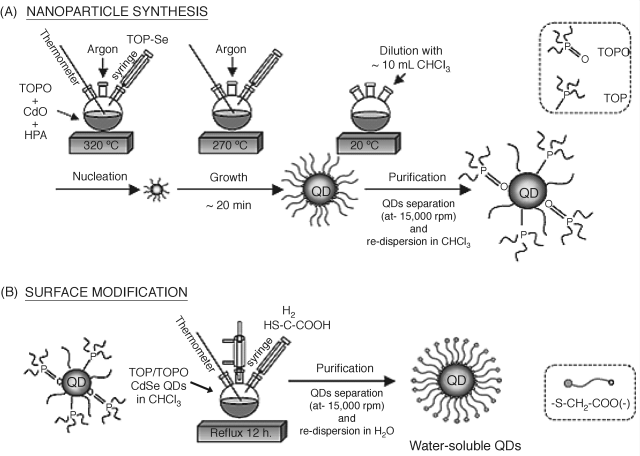
Quantum dots for use in analytical applications can be easily prepared by using one-step colloid chemistry methods [88–91]. In the initial stage, the synthesis is conducted in a high boiling point organic solvent whose molecules form coordination bonds with the particle surface (Figure 8.15A). Such a capping group performs two tasks: it saturates unoccupied metal orbitals at the surface and prevents, by steric hindrance, irreversible aggregation of the particles. Samples with the standard deviation of the particle diameter ≤ 5% are referred to as monodisperse. Quantum dots thus prepared are coated with a hydrophobic layer and are therefore not suitable for analytical applications in aqueous solutions. In order to obtain hydrophilic quantum dots, the surface ligand layer needs to be exchanged with a hydrophilic one by prolonged contact with a ![]() or
or ![]() capped thiol, as shown in Figure 8.15B. The hydrophilic moiety in such ligands is exposed to the solution and renders the quantum dot compatible with water. In addition, this group allows biocompounds to be conjugated with the quantum dot by means of suitable linkers [92].
capped thiol, as shown in Figure 8.15B. The hydrophilic moiety in such ligands is exposed to the solution and renders the quantum dot compatible with water. In addition, this group allows biocompounds to be conjugated with the quantum dot by means of suitable linkers [92].
Figure 8.15 Synthesis of quantum dots as colloidal particles. (A) Synthesis of hydrophobic colloidal quantum dots. (B) Surface modification by cap exchange. TOP: trioctylphosphine; TOPO: tri-![]() -octylphosphine oxide; HPA: hexylphosphonic acid. Alternatively, the step (B) can be effected after chloroform removal by centrifugation followed by redispersion in methanol. Reproduced with permission from [93]. Copyright 2006 Elsevier.
-octylphosphine oxide; HPA: hexylphosphonic acid. Alternatively, the step (B) can be effected after chloroform removal by centrifugation followed by redispersion in methanol. Reproduced with permission from [93]. Copyright 2006 Elsevier.
An alternative to previous the cap-exchange method relies on covering hydrophobic quantum dots with amphiphilic shells such as polymers or phospholipids. In such an arrangement, the hydrophobic moiety of the cap attaches to the original hydrophobic layer by hydrophobic interactions. In order to perform further functionalization of the quantum dot, the amphiphilic layer should include suitable reactive groups.
Another quantum-dot-encasing method involves the growth of a hydrophilic silica shell on the quantum dot by a sol-gel reaction [77]. Reactive functionalities attached to this layer provide convenient binding sites for conjugation with biocompounds.
As already mentioned, covalent bonding to functionalized capping ligands provides various possibilities for quantum dot bioconjugation [93]. In addition, affinity interactions (such as avidin–biotin) represent an alternative method for preparing quantum-dot–biocompound conjugates [94]. Host–guest chemistry provides another recognition method based on functionalized quantum dots. In this alternative, host molecules (cyclodextrin, crown ether or calixarene) are attached to the quantum dot to function analyte-recognizing receptor. Charged biocompounds (antibodies, DNA) can be appended by electrostatic attraction to quantum dots capped with charged ligands.
Hydrophilic quantum dots can also be obtained directly by conducting the synthesis in aqueous solutions in the presence of a thio-derivative (HS-R) as stabilizing and size-regulating agent [95]. For example, the synthesis of hydrophilic CdTe quantum dots proceeds according to reactions (8.3) and (8.4). In the first step, a precursor is formed by the reaction of ![]() with
with ![]() in an alkaline aqueous solution under nitrogen. Quantum dots (QDs) grow subsequently under prolonged heating at 100 °C.
in an alkaline aqueous solution under nitrogen. Quantum dots (QDs) grow subsequently under prolonged heating at 100 °C.
The capping ligand in this case is a short chain thiol containing one or more hydrophilic groups (![]() ,
, ![]() or
or ![]() ) at the end. Such groups prevent aggregation by electrostatic repulsion and, in addition, permit facile linking to a biocompound using typical bioconjugation reactions. ZnSe quantum dots can be prepared in a similar way.
) at the end. Such groups prevent aggregation by electrostatic repulsion and, in addition, permit facile linking to a biocompound using typical bioconjugation reactions. ZnSe quantum dots can be prepared in a similar way.
The previous methods refer to the preparation of colloidal quantum dots. It is also possible to obtain quantum dots embedded in solid materials such as polymers. It is important to note in this respect that various quantum-dot–polymer composites have been devised [96]. Thus, polymer quantum dot blends can be obtained simply by mixing these two materials taking care to avoid phase separation and interruption of polymer-chain entanglement. Nonaggregated nanoparticles can be grown straight in a polymer matrix with the advantage of obtaining controlled localization of nanoparticles within the composite. This technique can produce composites with a gradient of nanocrystals content along a selected direction. For the purpose of further functionalization of the composite, it is convenient to resort to thiol-terminated polymers or dendrimers as encapsulating materials. Dendritic encapsulation is advantageous in that it provides many chain ends for further covalent of suitable modifiers including biocompounds. Finally, polymer encapsulation can be achieved using quantum dots functionalized with molecules capable of initiating polymerization reactions.
8.6.2 Applications of Quantum Dots
Application of quantum dots in biomedicine and chemical sensors relies on their luminescence properties, that is, light emission by excited species [97]. By excitation, electrons can be promoted from the ground bonding orbital to an antibonding one provided the excitation energy overcomes the energy gap. An excited quantum dot emits light at a characteristic wavelength that is determined by the energy gap. As the gap energy depends on the particle size, the emission wavelength can be tuned by adjusting the particle size. The optical properties and applications of quantum dots are discussed in detail in Chapter 20.
8.7 Silica Nanoparticles
8.7.1 Synthesis, Properties, and Applications
Silica (silicon dioxide) nanoparticles can be prepared by hydrolysis and polymerization of tetraethylorthosilicate (TEOS) in ethanol–water mixtures in the presence of ammonia (Ströber method [98]). The particle size is determined by the precursor concentration and the reaction time. Monodisperse spherical silica particles can be formed also by a similar reaction occurring within micelles in a nonpolar solvent (reverse microemulsion method [99]). The chemistry of such processes is typical of sol-gel chemistry, except that particular precautions are needed to prevent large-scale gelation.
Silica is chemically inert under normal conditions and its application rests on its porous structure that imparts a high surface area. Pore diameters can be adjusted between 2 and 10 nm. At the same time, the specific pore volume is very high (> 0.9 ![]() ). In addition, silica particles exhibit good chemical stability and resistance to mechanical stress.
). In addition, silica particles exhibit good chemical stability and resistance to mechanical stress.
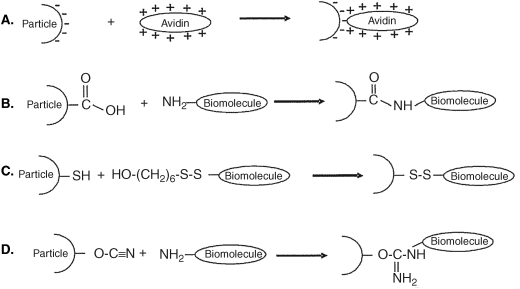
Such characteristics render silica particles well suited for immobilization of various compounds by pore entrapment or covalent linking [77, 100, 101]. Due to ionized Si-OH groups, the pore surface is negatively charged and positively charged compounds are therefore suitable for pore electrostatic immobilization (Figure 8.16A). Surface immobilization can be achieved if the particle is grown in the presence of a second precursor that is derivatized with a reactive bridging group. In a further step, the target compound is linked to the functionalized surface by standard bioconjugation reactions (Figures 8.16C and D).
Figure 8.16 Various bioconjugation methods for silica nanoparticles. (A) avidin–biotin linking; (B) formation of peptide linkages by reaction with carboxyl-derivatized particles; (C) disulfide bonding; (D) formation of peptide linkages by cyanogen bromide modification. Adapted with permission from [101]. Copyright 2004 Wiley-VCH Verlag GmBH & Co. KGaA.
Various functional nanomaterials (such as quantum dots, magnetic nanoparticles or metal nanoparticles) can be integrated with silica to yield nanohybrids. Growing a silica layer on functional nanoparticle prevents self-aggregation, reduces toxicity and brings about chemical groups useful for further functionalization.
8.8 Dendrimers
8.8.1 Properties and Applications
Dendrimers are repeatedly branched, roughly spherical, 2–7 nm diameter large polymer molecules [102]. The name comes from the Greek word dendron, which translates as “tree”. Dendrimers have three major portions: a core, an inner shell, and an outer shell (Figure 8.17A). These compounds are grown as successive spherical layers assembled by covalent bonding, each layer being identified by its generation number.
Figure 8.17 (A) Structure of a dendrimer. Branching points are located on equidistant circles. (B) Structure of a dendron. Adapted from http://commons.wikimedia.org/wiki/File:Graphs.jpg Last accessed 17/05/2012.
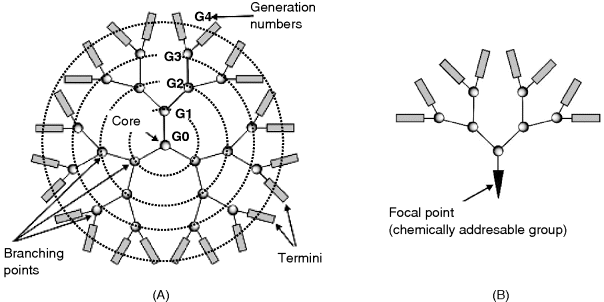
A dendrimer can be synthesized so as to have different functionalities on each of these portions ion order to control characteristic properties such as solubility, thermal stability, and its capability to be conjugated with various compounds. Dendrimer synthesis can be conducted so as to control precisely the size and number of branches of the dendrimer. It is thus possible to assemble a predetermined number of functional groups.
Related compounds are dendrons that contain a chemically addressable group called the focal point with an asymmetric branched structure grown asymmetrically (Figure 8.17B).
Dendrimer applications in chemical sensors rely on their outstanding property of displaying a high density of functional groups at the outer shell. This allows receptors and other molecules to be assembled so as to form multifunctional nanosized assemblies. To this end, one can resort to standard conjugation methods involving functionalized dendrimers [103]. In addition, properly designed dendrimers can encapsulate small molecules at certain sites of appropriate size within the dendritic structure.
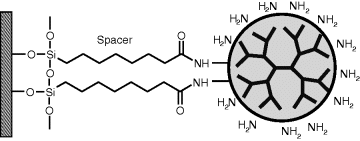
Due to their multivalent nature, dendrimers can be used to add increased functionality to a solid surface. As an example, Figure 8.18 shows a dendrimer attached to a support via siloxane chemistry. Sulfhydryl-focal point dendrons can be assembled on gold surfaces by sulfur chemisorption. Terminal groups in dendrimes and dendrons serve for subsequent conjugation with various compounds of interest.
Figure 8.18 Surface functionalization by dendrimers.
It is not surprising that dendrimers have found various applications in chemical-sensor design [104]. Thus, dendrimers are used as nanosize scaffolds for assembling sensor active components via strong covalent bonds. On the other hand, dendrimers with a suitable structure elicit selective responses to various chemical species including gases, vapors inorganic ions and biomolecules that make them suitable as recognition elements.
8.9 Summary
Various nanomaterials have been applied to chemical-sensor design with extremely promising results. Nanomaterials can be used either as structural components of the sensing part or as functional components that perform a specific task in the recognition or transduction event.
Dendrimers, carbon nanotubes, metal nanoparticles and silica nanoparticles are useful for assembling recognition components such as enzymes or affinity receptors thus allowing to achieve of a high density of active component. This results, as a rule, in enhanced sensor sensitivity. On the other hand, nanostructuring of the sensing part enhances the rate of diffusion of the analyte to the receptor sites.
Conducting carbon nanotubes allow electrical wiring at the nanoscale and can also perform direct electron transfer from the active site of redox enzymes to the electrode in electrochemical sensors.
Nanomaterials are also useful as high-performance labels, particularly in optical sensors. Application of quantum dots, carbon nanotubes, and gold nanoparticles in such sensors have brought about considerable advantages over molecular label-based optical sensors.
In addition, nanomaterials can perform as amplification markers in certain types of sensors. Thus, postrecognition assembly of gold nanoparticles to the sensing element enhances considerably the total mass, which represents a valuable amplification method in mass-sensitive transduction. By postrecognition grafting of dendrimers or liposomes it is possible to enhance the local electric charge, which results in signal enhancement in certain types of electrochemical sensors.
No less interesting is the application of certain nanomaterials (such as semiconductor carbon nanotubes or dendrimers) as recognition materials. By interaction with specific analytes, such materials experience modifications in their physicochemical properties that can be translated into a response signal.
Application of nanomaterials is currently the most active research area in chemical-sensor science and there is no doubt that most of the traditional materials and manufacturing methods will be replaced by nanotechnology-derived ones.
1. Ozin, G.A., Arsenault, A.C., and Cademartiri, L. (2009) Nanochemistry: A Chemical Approach to Nanomaterials, RSC Publ., Cambridge.
2. Cademartiri, L. and Ozin, G.A. (2009) Concepts of Nanochemistry, Wiley-VCH, Weinheim.
3. Asefa, T., Duncan, C.T., and Sharma, K.K. (2009) Recent advances in nanostructured chemosensors and biosensors. Analyst, 134, 1980–1990.
4. Kumar, C.S.S.R. (ed.) (2007) Nanomaterials for Biosensors, Wiley-VCH, Weinheim.
5. Pierce, D.T. and Zhao, J.X. (eds) (2010) Trace Analysis with Nanomaterials, Wiley-VCH, Weinheim.
6. Merkoçi, A. (ed.) (2009) Biosensing Using Nanomaterials, John Wiley & Sons, New York.
7 Vollath, D. (2008) Nanomaterials: An Introduction to Synthesis, Properties and Applications, Wiley-VCH, Weinheim.
8. Cao, G. (2004) Nanostructures and Nanomaterials: Synthesis, Properties and Applications, Imperial College Press, London.
9. Kumar, C.S.S.R. (ed.) (2005) Biofunctionalization of Nanomaterials, Wiley-VCH, Weinheim.
10. de Dios, A.S. and Diaz-Garcia, M.E. (2010) Multifunctional nanoparticles: Analytical prospects. Anal. Chim. Acta, 666, 1–22.
11. Ruiz-Hitzky, E., Ariga, K., and Lvov, Y. (eds) (2008) Bio-Inorganic Hybrid Nanomaterials: Strategies, Syntheses, Characterization and Applications, Wiley-VCH, Weinheim.
12. Kalantar-zadeh, K. and Fry, B. (2008) Nanotechnology-Enabled Sensors, Springer, Boston.
13. Wang, J. and Katz, E. (2005) Biomaterial-nanoparticle hybrid systems for sensing and electronic devices, in Bioelectronics: from Theory to Applications (eds I. Willner and E. Katz), Wiley-VCH, Weinheim, pp. 231–264.
14. Katz, E. and Willner, I. (2004) Integrated nanoparticle-biomolecule hybrid systems: Synthesis, properties, and applications. Angew. Chem. Int. Ed., 43, 6042–6108.
15. Talapin, D.V., Lee, J.S., Kovalenko, M.V. et al. (2010) Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev., 110, 389–458.
16. Shchipunov, Y. (2008) Entrapment of biopolymers into sol-gel-derived silica nanocomposites, in Bio-inorganic Hybrid Nanomaterials: Strategies, Syntheses, Characterization and Applications (eds E. Ruiz-Hitzky, K. Ariga, and Y. Lvov), Wiley-VCH, Weinheim, pp. 75–112.
17. Song, S., Qin, Y., Huang, Q., et al. (2010) Functional nanoprobes for ultrasensitive detection of biomolecules. Chem. Soc. Rev., 39, 4234–4243.
18. Arvizo, R.R., De, M., and Rotello, V.M. (2007) Proteins and nanoparticles: Covalent and noncovalent conjugates, in Nanobiotechnology II: More Concepts and Applications (eds C.A. Mirkin and C.M. Niemeyer), Wiley-VCH, Weinheim, pp. 65–97.
19. Kumar, C.S.S.R. (ed.) (2009) Metallic Nanomaterials, Wiley-VCH, Weinheim.
20. Daniel, M.C. and Astruc, D. (2004) Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev., 104, 293–346.
21. Schmid, G. (2010) Noble metal nanoparticles, in Nanoparticles: From Theory to Application (ed. G. Schmid), Wiley-VCH, Weinheim, pp. 214–239.
22. Zheng, M. and Huang, X. (2005) Biofunctionalization of gold nanoparticles, in Biofunctionalization of Nanomaterials (ed. C.S.S.R. Kumar), Wiley-VCH, Weinheim, pp. 99–124.
23. Feldheim, D.L. and Foss, C.A. (2001) Electrochemical synthesis and optical properties of gold nanorods, in Metal nanoparticles: Synthesis, Characterization, and Applications (eds D.L. Feldheim and C.A. Foss), Marcel Dekker, New York, pp. 163–182.
24. Shad Thaxton, C. and Mirkin, C.A. (2004) DNA-gold nanoparticles conjugates, in Nanobiotechnology: Concepts, Applications and Perspectives (eds C.M. Niemeyer and C.A. Mirkin), Wiley-VCH, Weinheim, pp. 288–307.
25. Drechsler, U., Erdogan, B., and Rotello, V.M. (2004) Nanoparticles: Scaffolds for molecular recognition. Chem.-Eur. J., 10, 5570–5579.
26. Baron, R., Willner, B., and Willner, I. (2007) Biocatalytic growth of nanoparticles for sensors and circuitry, in Nanobiotechnology II: More Concepts and Applications (eds C.A. Mirkin and C.M. Niemeyer), Wiley-VCH, Weinheim, pp. 99–121.
27. Allen, M.J., Tung, V.C., and Kaner, R.B. (2010) Honeycomb Carbon: A review of graphene. Chem. Rev., 110, 132–145.
28. Ratinac, K.R., Yang, W., Gooding, J.J. et al. (2011) Graphene and related materials in electrochemical sensing. Electroanalysis, 23, 803–826.
29. Krueger, A. (2010) Fullerenes-cages made from carbon, in Carbon Materials and Nanotechnology, Wiley-VCH, Weinheim, pp. 33–122.
30. Hirsch, A. and Brettreich, M. (2005) Fullerenes: Chemistry and Reactions, Wiley-VCH, Weinheim.
31. Chaniotakis, N. (2007) Fullerene-based electrochemical detection methods for biosensing, in Nanomaterials for Biosensors (ed. C.S.S.R. Kumar), Wiley-VCH, Weinheim, pp. 101–122.
32. Geim, A.K. and Novoselov, K.S. (2007) The rise of graphene. Nature Mater., 6, 183–191.
33. Krueger, A. (2010) Carbon nanotubes, in Carbon Materials and Nanotechnology, Wiley-VCH, Weinheim, pp. 123–281.
34. Harris, P.J.F. (1999) Carbon Nanotubes and Related Structures: New Materials for the Twenty-First Century, Cambridge University Press, Cambridge.
35. Lukaszewicz, J.P. (2006) Carbon materials for chemical sensors: A review. Sens. Lett., 4, 53–98.
36. Strano, M.S., Boghossian, A.A., Kim, W.J., et al. (2009) The Chemistry of single-walled nanotubes. MRS Bull., 34, 950–961.
37. Wang, J. (2005) Carbon-nanotube based electrochemical biosensors: A review. Electroanalysis., 17, 7–14.
38. Terrones, M. (2004) Carbon nanotubes: Synthesis and properties, electronic devices and other emerging applications. Int. Mater. Rev., 49, 325–377.
39. Merkoci, A. (2006) Carbon nanotubes in analytical sciences. Microchim. Acta, 152, 157–174.
40. Mann, D. (2006) Synthesis of carbon nanotubes, in Carbon Nanotubes: Properties and Applications (ed. M. O'Connell), Taylor & Francis, Boca Raton, pp. 19–49.
41. Joselevich, E., Dai, H., Liu, J. et al. (2008) Carbon nanotube synthesis and organization, in Carbon Nanotubes: Advanced Topics in the Synthesis, Structure, Properties and Applications (eds A. Jorio, G. Dresselhaus, and M.S. Dresselhaus), Springer, Berlin.
42. Vajtai, R., Wei, B., George, T.F. et al. (2007) Chemical vapor deposition of organized architectures of catbon nanotubes for applications, in Molecular Building Blocks for Nanotechnology (eds G.A. Mansoori, T.F. George, L. Assoufid, and G. Zhang), Springer, New York, pp. 188–209.
43. Sgobba, V. and Guldi, D.M. (2009) Carbon nanotubes-electronic/electrochemical properties and application for nanoelectronics and photonics. Chem. Soc. Rev., 38, 165–184.
44. Hermanson, G.T. (2008) Buckyballs, fullerenes, and carbon nanotubes, in Bioconjugate Techniques, Elsevier, Amsterdam, pp. 627–648.
45. Niyogi, S., Hamon, M.A., Hu, H., et al. (2002) Chemistry of single-walled carbon nanotubes. Accounts Chem. Res., 35, 1105–1113.
46. Tasis, D., Tagmatarchis, N., Bianco, A. et al. (2006) Chemistry of carbon nanotubes. Chem. Rev., 106, 1105–1136.
47. Yang, W.R., Thordarson, P., Gooding, J.J., et al. (2007) Carbon nanotubes for biological and biomedical applications. Nanotechnology., 18, 12.
48. Bekyarova, E., Haddon, R.C., and Parpura, V. (2005) Biofunctionalization of carbon nanotubes, in Biofunctionalization of Nanomaterials (ed. C.S.S.R. Kumar), Wiley-VCH, Weinheim, pp. 41–71.
49. Zhao, Y.L. and Stoddart, J.F. (2009) Noncovalent functionalization of single-walled carbon nanotubes. Accounts Chem. Res., 42, 1161–1171.
50. Chen, R.J., Zhang, Y.G., Wang, D.W. et al. (2001) Noncovalent sidewall functionalization of single-walled carbon nanotubes for protein immobilization. J. Am. Chem. Soc., 123, 3838–3839.
51. Le Goff, A., Holzinger, M., and Cosnier, S. (2011) Enzymatic biosensors based on SWCNT-conducting polymer electrodes. Analyst, 136, 1279–1287.
52. Wang, J., Liu, G., and Lin, Y. (2007) Nanotubes, nanowires, and nanocantilevers in biosensor development, in Nanomaterials for Biosensors (ed. C.S.S.R. Kumar), Wiley-VCH, Weinheim, pp. 56–100.
53. Ye, J.S. and Sheu, F.S. (2007) Carbon nanotube-based sensor, in Nanomaterials for Biosensors (ed. C.S.S.R. Kumar), Wiley-VCH, Weinheim, pp. 27–55.
54. Balasubramanian, K. and Burghard, M. (2006) Biosensors based on carbon nanotubes. Anal. Bioanal. Chem., 385, 452–468.
55. Jacobs, C.B., Peairs, M.J., and Venton, B.J. (2010) Review: Carbon nanotube based electrochemical sensors for biomolecules. Anal. Chim. Acta, 662, 105–127.
56. Gooding, J.J. (2005) Nanostructuring electrodes with carbon nanotubes: A review on electrochemistry and applications for sensing. Electrochim. Acta, 50, 3049–3060.
57. Tibbetts, G.G., Lake, M.L., Strong, K.L. et al. (2007) A review of the fabrication and properties of vapor-grown carbon nanofiber/polymer composites. Compos. Sci. Technol., 67, 1709–1718.
58. De Jong, K.P. and Geus, J.W. (2000) Carbon nanofibers: Catalytic synthesis and applications. Catal. Rev. -Sci. Eng., 42, 481–510.
59. Melechko, A.V., Merkulov, V.I., McKnight, T.E., et al. (2005) Vertically aligned carbon nanofibers and related structures: Controlled synthesis and directed assembly. J. Appl. Phys., 97, 39.
60. Vamvakaki, V., Fouskaki, M., and Chaniotakis, N. (2007) Electrochemical biosensing systems based on carbon nanotubes and carbon nanofibers. Anal. Lett., 40, 2271–2287.
61. Huang, J.S., Liu, Y., and You, T.Y. (2011) Carbon nanofiber based electrochemical biosensors: A review. Anal. Methods, 2, 202–211.
62. Ramakrishna, S. (2005) An Introduction to Electrospinning and Nanofibers, World Scientific, Hackensack, NJ.
63. Ramakrishna, S., Lala, N.L., Garudadhwaj, H., et al. (2007) Polymer nanofibers for biosensor applications. In Molecular Building Blocks for Nanotechnology (eds G.A. Mansoori, T.F. George, L. Assoufid, and G. Zhang), Springer, New York, pp. 377–392.
64. Potyrailo, R.A. (2006) Polymeric sensor materials: Toward an alliance of combinatiorial and rational design tools? Angew. Chem. Int. Ed., 45, 702–723.
65. Ding, B., Wang, M.R., Yu, J.Y. et al. (2009) Gas sensors based on electrospun nanofibers. Sensors, 9, 1609–1624.
66. Shin, Y.J., Wang, M., and Kameoka, J. (2009) Electrospun nanofiber biosensor for measuring glucose concentration. J. Photopolym. Sci. Technol., 22, 235–237.
67. Ramakrishna, S., Lala, N.L., Garudadhwaj, H., et al. (2007) Polymer nanofibers for biosensor applications, In Molecular Building Blocks for Nanotechnology (eds Mansoori, G.A., George, T.F., Assoufid, L., and Zhang, G.), Springer, Berlin, pp. 377–392.
68. Yeo, L.Y. and Friend, J.R. (2006) Electrospinning carbon nanotube polymer composite nanofibers. J. Exp. Nanosci., 1, 177–209.
69. Marx, S., Jose, M.V., Andersen, J.D. et al. (2011) Electrospun gold nanofiber electrodes for biosensors. Biosens. Bioelectron., 26, 2981–2986.
70. Kumar, C.S.S.R. (ed.) (2009) Magnetic Nanomaterials, Wiley-VCH, Weinheim.
71. Varadan, V.K., Chen, L., and Xie, J. (2008) Nanomedicine: Design and Applications of Magnetic Nanomaterials, Nanosensors and Nanosystems, John Wiley & Sons, Chichester.
72. Tamanaha, C.R., Mulvaney, S.P., Rife, J.C. et al. (2008) Magnetic labeling, detection, and system integration. Biosens. Bioelectron., 24, 1–13.
73. Varadan, V.K., Chen, L., and Xie, J. (2008) Magnetic nanoparticles, in Nanomedicine: Design and Applications of Magnetic Nanomaterials, Nanosensors and Nanosystems, John Wiley & Sons, Chichester, pp. 85–128.
74. Frey, N.A., Peng, S., Cheng, K. et al. (2009) Magnetic nanoparticles: Synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev., 38, 2532–2542.
75. Krylova, G., Bodnarchuk, M.I., Tromsdorf, U., et al. (2010) Synthesis, properties and applications of magnetic nanoparticles, in Nanoparticles: From Theory to Application (ed. G. Schmid), Wiley-VCH, Weinheim, pp. 239–310.
76. Thode, C.J. and Williams, M.E. (2009) Approaches to the biofunctionalization of spherical and anisotropic iron oxide nanomaterials, in Magnetic Nanomaterials (ed. C.S.S.R. Kumar), Wiley-VCH, Weinheim, pp. 507–550.
77. Jin, Y.H., Li, A.Z., Hazelton, S.G., et al. (2009) Amorphous silica nanohybrids: Synthesis, properties and applications. Coordin. Chem. Rev., 253, 2998–3014.
78. Graham, D.L., Ferreira, H.A., and Freitas, P.P. (2004) Magnetoresistive-based biosensors and biochips. Trends Biotechnol., 22, 455–462.
79. Varadan, V.K., Chen, L., and Xie, J. (2008) Magnetic biosensors, in Nanomedicine: Design and Applications of Magnetic Nanomaterials, Nanosensors and Nanosystems (eds V.K. Varadan, L. Chen, and J. Xie), John Wiley & Sons, Chichester, pp. 329–454.
80. Wang, S.X. and Li, G. (2008) Advances in giant magnetoresistance biosensors with magnetic nanoparticle tags: Review and outlook. IEEE Trans. Magn., 44, 1687–1702.
81. Kasatkin, S.I., Vasil'eva, N.P., and Murav'ev, A.M. (2010) Biosensors based on the thin-film magnetoresistive sensors. Autom. Remote Control, 71, 156–166.
82. Llandro, J., Palfreyman, J.J., Ionescu, A. et al. (2010) Magnetic biosensor technologies for medical applications: A review. Med. Biol. Eng. Comput., 48, 977–998.
83. Germano, J., Martins, V.C., Cardoso, F.A., et al. (2009) A portable and autonomous magnetic detection platform for biosensing. Sensors, 9, 4119–4137.
84. Rife, J.C., Miller, M.M., Sheehan, P.E. et al. (2003) Design and performance of GMR sensors for the detection of magnetic microbeads in biosensors. Sens. Actuators A-Phys., 107, 209–218.
85. Sole, S., Merkoci, A., and Alegret, S. (2001) New materials for electrochemical sensing - III. Beads. TrAC-Trends Anal. Chem., 20, 102–110.
86. Willner, I. and Katz, E. (2003) Magnetic control of electrocatalytic and bioelectrocatalytic processes. Angew. Chem. Int. Ed., 42, 4576–4588.
87. Hsing, I.M., Xu, Y., and Zhao, W.T. (2007) Micro- and nano-magnetic particles for applications in biosensing. Electroanalysis, 19, 755–768.
88. Rogach, A.L. (ed.) (2008) Semiconductor Nanocrystal Quantum Dots: Synthesis, Assembly, Spectroscopy and Applications, Springer, Vienna.
89. Murray, C.B., Kagan, C.R., and Bawendi, M.G. (2000) Synthesis and characterization of monodisperse nanocrystals and close-packed nanocrystal assemblies. Annu. Rev. Mater. Sci., 30, 545–610.
90. Trindade, T., O'Brien, P., and Pickett, N.L. (2001) Nanocrystalline semiconductors: Synthesis, properties, and perspectives. Chem. Mater., 13, 3843–3858.
91. Reiss, P. (2008) Synthesis of semiconductor nanocrystals in organic solvents, in Semiconductor Nanocrystal Quantum Dots: Synthesis, Assembly, Spectroscopy and Applications (ed. A.L. Rogach), Springer, Vienna, pp. 35–72.
92. Mazumder, S., Dey, R., Mitra, M.K., et al. (2009) Review: Biofunctionalized quantum dots in biology and medicine. J. Nanomater., Article ID 815734.
93. Costa-Fernandez, J.M., Pereiro, R., and Sanz-Medel, A. (2006) The use of luminescent quantum dots for optical sensing. TrAC-Trend Anal. Chem., 25, 207–218.
94. Goldman, E.R., Balighian, E.D., Mattoussi, H., et al. (2002) Avidin: A natural bridge for quantum dot-antibody conjugates. J. Am. Chem. Soc., 124, 6378–6382.
95. Gaponik, N. and Rogach, A.L. (2008) Aqueous synthesis of semiconductor nanocrystals, in Semiconductor Nanocrystal Quantum Dots: Synthesis, Assembly, Spectroscopy and Applications (ed. A.L. Rogach), Springer, Vienna, pp. 73–99.
96. Skaff, H. and Emrick, T. (2004) Semiconductor nanoparticles, in Nanoparticles: Building Blocks for Nanotechnology (ed. V. Rotello), Kluwer Academic/Plenum Publishers, New York, pp. 29–82.
97. Zrazhevskiy, P., Sena, M., and Gao, X. (2010) Designing multifunctional quantum dots for bioimaging, detection, and drug delivery. Chem. Soc. Rev., 39, 4326–4354.
98. Stöber, W., Fink, A., and Bohn, E. (1968) Controlled growth of monodisperse silica spheres in micron size range. J. Colloid Interface Sci., 26, 62–69.
99. Arriagada, F.J. and Osseoasare, K. (1995) Synthesis of nanosize silica in aerosol OT reverse microemulsions. J. Colloid Interface Sci., 170, 8–17.
100. Wang, L., Zhao, W.J., and Tan, W.H. (2008) Bioconjugated silica nanoparticles: Development and applications. Nano Res., 1, 99–115.
101. Drake, T.J., Zao, J.X., and Tan, W.H. (2004) Bioconjugated silica nanoparticles for bioanalytical applications, in Nanobiotechnology: Concepts, Applications and Perspectives (eds C.M. Niemeyer and C.A. Mirkin), Wiley-VCH, Weinheim, pp. 444–457.
102. Newkome, G.R., Vögtle, F., and Moorefield, C.N. (2001) Dendrimers and Dendrons: Concepts, Syntheses, Applications, Wiley-VCH, Weinheim.
103. Hermanson, G.T. (2008) Dendrimers and dendrons, in Bioconjugate Techniques, Elsevier, Amsterdam, pp. 346–390.
104. Jayaraman, N. (2007) Dendrimers and their use as nanoscale sensors, in Nanomaterials Chemistry: Recent Developments and New Directions (eds C.N.R. Rao, A. Müller, and A.K. Cheetham), Wiley-VCH, Weinheim, pp. 249–297.
