2.6 Perception is Personal
According to the inductive method, scientists seek – and find – the truth without being guided by personal opinions or preferences. Inductive science begins with objective observation. The resulting theories always enter the picture at a later stage.
One of the criticisms against this method that we have mentioned is that theories cannot be proved on the basis of observations. This problem remains unresolved in the hypothetico-deductive method. If we define truth in strictly logical terms, we have to live with a certain degree of uncertainty about the truth of our theories, regardless of which method we use. To prove something logically, we need access to all the facts relevant to our problem. This is possible, for example, in mathematics, where theories are derived from axioms laid down by people. Axioms can be said to define the researcher's private universe, because if we make the rules ourselves we have access to a complete set of facts. In the natural sciences, Mother Nature makes the rules and the researcher's job is to find them. For this reason, empirical scientists must play down the role of logical proofs and find ways to compensate for the naturally incomplete information they have to work with – and they have to do this whatever scientific approach they use. One way is to search for many independent types of support for a theory. Although we may not have information sufficient for valid logical proofs, the collected support for a theory may be convincing enough to reduce our doubts in it to a mere minimum. Electromagnetic field theory, for example, has been supported by countless experiments and observations since it was developed in the 1800s and has been employed in widespread technical applications like mobile phones and radar. You would need very compelling reasons to doubt that it is a valid theory.
Another criticism is raised against the inductivist claim that science is based on objective observations. According to the critics, observations cannot be objective as they are colored by theory. This non-objectivity is less problematic in the hypothetico-deductive approach, as it is based on investigating one hypothesis at a time. When testing an hypothesis it is of course natural to be temporarily biased towards one idea. But if we understand the talk of theory-dependent observations as meaning “observations can never be trusted as a source of information”, then science in general is in trouble. Without empirical data there can be no empirical science. The theory-dependence of observation is a main theme in many books about scientific method. Chalmers uses a whole chapter to discuss why it means that the inductive method must be rejected [3]. It is worthwhile to use a little space here to discuss this aspect of observation from a practical point of view. Firstly, I would like to discuss what a sensible definition of objectivity might be. Thereafter, I would like to ask in what sense all observations are theory-dependent.
Imagine that you are a detective arriving at the scene of a murder. A lifeless body lies on the floor of a Victorian library and you have been called there to investigate the crime. Where do you start looking? Presumably, you do not start by investigating the wood paneling on the walls to see if it is made from oak or teak. Nor do you try to determine the optical quality of the glass in the crystal chandelier. No, you probably start by looking at the body. How is it positioned? Are there any indications of violence? It is likely that you continue with particular aspects of the rest of the scene. Did the murderer leave any traces? Is something missing that could provide a clue about the motive? There is an immense wealth of facts that could be observed in the library and to solve the case you obviously have to choose what to observe. Strictly speaking, this means that you are not objective. Still, most people would not accuse you of being biased if you conducted your investigation this way, because they think that objectivity has to do with having an open mind and not jumping to conclusions before looking at the relevant facts. This is the common sense definition of objectivity. Figure 2.4 illustrates how it differs from the strict definition.
Figure 2.4 The observer on the left is strictly objective and pays equal attention to all facts. The observer on the right selectively pays attention to facts relevant to the problem. The larger the number of facts, the less successful the left observer is at solving the problem.
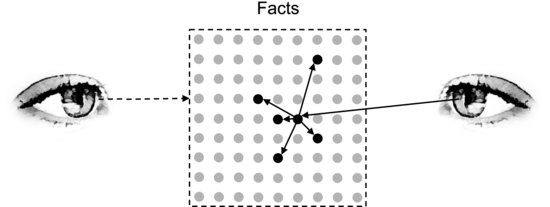
Since our knowledge is incomplete (otherwise we would not need to investigate the murder) it is difficult to determine beforehand which facts are the most relevant, but the examples above show that many facts can be discarded as irrelevant at an early stage. If later findings should suggest that the murderer was looking for diamonds and that these may have been hidden among the crystals of the chandelier, a closer look at the chandelier will be justified. But before we know anything about the motive it would be foolish to pay the chandelier special attention. If objectivity means giving the same weight to every single fact, an objective investigation is probably impossible and, at any rate, it could never solve the case. This strict definition of objectivity is impractical. Regardless of if we are solving a scientific problem or if we are making a cheese sandwich we must prioritize between observations, concentrate on the important ones and disregard the unimportant ones. It is not sensible to say that an observer who does not pay equal attention to all facts is not objective.
Before we leave our detective to go about his/her investigation at the scene of this hideous crime we may stop and ask how he/she knows what to observe and what to ignore. He/she probably has an idea about how murders happen and how such events leave traces on a crime scene. This knowledge comes from a mixture of training, experience and common sense. We can think of such knowledge as a filter placed between the facts of the scene and the mind of the investigator. It filters out irrelevant information and transmits the aspects that should be prioritized. A good filter clearly distinguishes between the relevant and irrelevant facts, while a poor one is less reliable. The quality of the filter could be called the investigator's power of observation and it can be improved with training, experience and reflection. This means that not all observers observe the same things. Even a single observer may observe different things on different occasions if the filter should change over time. Acknowledging that observation is a skill does not necessarily imply that skilled observers are less objective than others – at least not if we use the common sense definition of objectivity. It could simply mean that skilled observers are more efficient at getting an objective view of the problem under study. We should not empty our heads of all knowledge every time we try to solve a problem. At least not if we want to solve it.
Let's turn to the other question. In what sense are all observations theory-dependent? What observers see depends on their knowledge, skill and personality. Perception, in other words, is personal. Chalmers uses several examples to underline this [3]. One of them is how a student and an expert radiologist perceive X-ray images. Where the student may only see shadows of the heart and ribs, with a few spidery blotches between them, the expert sees a wealth of significant features. With training, the student may gradually forget about the ribs and begin to see the lungs as well as a rich panorama of details on them. Another example from Chalmers is an amusing entry from Johannes Kepler's notebook, made after observation through a Galilean telescope. It reads, “Mars is square and intensely coloured”. This statement can be said to have relied on the theory that the telescope gave a true picture of reality, which it clearly did not. Chalmers proceeds to say that this means that “observation statements do not constitute a firm basis on which scientific knowledge can be founded, because they are fallible”.
The point I wish to make is that the fact that observations, and observation statements, are fallible does not undermine their role as fundamental information carriers in science. I think Chalmers would agree with this but I still want to make the point because it is easy to be overwhelmed by the alleged problems with observation when reading statements like this. It is worth mentioning again that if observations could not be trusted there could be no science. We know from a vast body of experience that science is, in fact, a highly successful endeavor. Of course, observers can make mistakes when observing or when explaining what they see, just as philosophers can make mistakes while thinking or expressing their thoughts. That does not undermine the fundamental role of thoughts in philosophy. When discussing the theory-dependence of observation it is easy to indulge in pessimistic statements about how unreliable observations are and forget that the proof of the pudding is, in fact, in the eating. If faulty observations lead to a theory that is incorrect, this will not go unnoticed. Observations must be repeated and elaborated independently by several researchers to have an impact. Scientists do not necessarily make fewer mistakes than other people, neither are they less prone to be led astray by their pet ideas, but the methods of science have elements built into them that make it more difficult for scientists to fool themselves. For observations to have scientific impact we demand a high degree of inter-observer correlation. This means that many observers must be able to see the same thing and that it must be possible for other researchers to successfully repeat our experiments. Before publication, scientific results are examined and criticized by other researchers in anonymous peer review processes. These systems are in place because we are aware of the risk of mistakes, and to increase the degree of objectivity in the results.
To say that all observations are theory-dependent is, in practice, misleading. Some observations are indeed heavily theory-dependent, while others are not. For instance, if our detective remarks that the victim lies face-down on the floor, this statement does not presuppose any theoretical knowledge at all. Stating that the victim was beaten with a blunt instrument only presupposes that such instruments leave other types of traces on a body than sharp ones. To call this knowledge a theory empties the word of any meaning relevant in science. If, on the other hand, scientists say that they have been observing a single electron oscillating in the electric field of a laser beam, this observation statement clearly relies on involved, high-level theory. If this theory turns out to be false it could render the observation statement invalid but, as we soon shall see, this is not necessarily so.
For those who think that observation statements are always interpretations in the light of theories, Hacking provides ample examples of the opposite [8]. I am going to mention a few of them briefly here. One of them is the discovery of double refraction in Iceland Spar (calcite) by Erasmus Bartholin in 1689. If you put a piece of this material over a printed page you see the text double. Iceland Spar was the first known producer of polarized light. This phenomenon had to wait well over a century for Fresnel's theoretical explanation, so it is clear that the discovery did not rely on theory. Another optical phenomenon that was discovered long before any theoretical explanation is diffraction. Grimaldi, and later Hooke, discovered that there was illumination in the shadow of an opaque body. Careful observation revealed regularly spaced bands at the edge of the shadow, which come from diffraction. These are examples of phenomena discovered by alert observers. What about deliberate experiments then, do not they always involve theoretical assumptions? If we return for a second to the frustrated rental car customer from the beginning of this chapter, we may recall that walking counter-clockwise around the car clearly was an experiment without theoretical motivation. Similar examples are not uncommon in science. One of Hacking's examples is David Brewster, who experimentally determined the laws of reflection and refraction of polarized light, and also managed to induce birefringence (polarizing properties) in materials under stress. These things were later theoretically explained within Fresnel's wave theory but Brewster himself was advocating the Newtonian view that light consisted of rays of corpuscles. As Hacking puts it, “Brewster was not testing or comparing theories at all. He was trying to find out how light behaves.” This summarizes the important point that our understanding of Nature's machinery often begins with the discovery of an interesting phenomenon, and not necessarily with theoretical considerations. I could go on quoting Hacking, whose book is full of convincing examples like these. To establish that there are plenty of examples also outside it, and thereby strengthen the point, I would like to add a couple of examples of my own choosing. I will first briefly mention Gregor Mendel, who is known to many as a brilliant experimental biologist. The experiments leading to his discovery of the laws of inheritance are described in a separate example in Chapter 6. Here, I will restrict myself to pointing out that this discovery had to wait half a century to be acknowledged by the scientific community, because there was no theory available that gave them meaning. My other example comes from the history of chemistry. In this connection I cannot resist referring back to the observation statement “the gas will not light”, used in the section on inductivism to exemplify a theoretically colored statement. The following example involves similar statements but made in a scientifically more relevant context.
Example 2.2: Joseph Priestley and the discovery of oxygen Before Antoine Lavoisier introduced the oxygen theory, the phlogiston theory was the prevailing theory of combustion. Phlogiston was believed to be a material that escaped from substances during combustion. It can be thought of as a slightly modernized version of Aristotle's fire element, with the difference that its existence had found some support in experiments. Besides combustion, phlogiston was also believed to be involved in calcination of metals. When metals were heated in air they were believed to change because they lost phlogiston. The resulting calxes (oxides) changed back into metals when heated with charcoal, as they regained phlogiston from it. Since the charcoal only left a small amount of ash after the process it was presumed to be rich in phlogiston. Furthermore, combustion could not be sustained in vacuum since air was needed to absorb phlogiston. Correspondingly, combustion in a sealed container ceased when the air inside it was saturated with phlogiston. The theory thus explained a range of phenomena, but it had a weak point: metal oxides lose weight when they are reduced to metals. How could that be the case when they gained phlogiston? [8]
The gas formed when reducing calxes with charcoal was called fixed air. We know it as carbon dioxide. It had been found that fixed air was produced also during fermentation and respiration and it was known not to support life. In August 1774 the English chemist Joseph Priestley collected a gas that had been formed when heating Mercurius Calcinatus (mercury oxide) without charcoal. His investigations showed that the gas had properties opposite to those of fixed air: “what surprised me more than I can well express, was that a candle burned in this air with a remarkably vigorous flame” [9]. Later, he noticed that a mouse survived twice as long in the gas as in ordinary air. Encouraged by this result he decided to breathe the gas himself: “I fancied that my breast felt peculiarly light and easy for some time afterwards. Who can tell that but in time, this pure air may become a fashionable article in luxury. Hitherto only two mice and myself have had the privilege of breathing it” [9]. He was breathing pure oxygen but he called it dephlogisticated air because, in the phlogiston system, a gas was thought to promote combustion if it was deficient in phlogiston. This gave the phlogiston in the burning material somewhere to go [9].
Antoine Lavoisier, who made more quantitative studies, later discarded the phlogiston theory. He realized that the gas that Priestley had collected was not a variety of air, but a separate component of air with unique properties. It was he who named it oxygen. Since Priestley never stopped supporting the phlogiston theory we know that he interpreted his observations using a false theory. Despite this, his observation statements make perfect sense to us. If his experiments were to be repeated today by someone ignorant of the idea of phlogiston, the results would probably be described very similarly. Clearly, not all observation statements are theoretically colored. Indeed, not all experiments presuppose theory, as Priestley's own reflection on his test with the candle flame shows:
I cannot, at this distance of time, recollect what it was that I had in view in making this experiment; but I know I had no expectation of the real issue of it. If […] I had not happened for some other purpose, to have a lighted candle before me, I should probably never had made the trial; and the whole train of my future experiments relating to this kind of air might have been prevented [10].![]()
There are many examples of scientists who have said that they work from observations to build theories. Charles Darwin, the father of modern biology and one of the most astute intellects in the history of natural science, described himself as “a kind of machine for grinding theories out of huge assemblages of facts” [11]. This sounds like a rather inductivist approach, even though it is probably not a complete description of his way of working. The point is that theories do not pop up from nowhere. They are developed because there is a phenomenon that needs explanation, and the phenomenon must be discovered before a theory can be considered. Once Darwin's great idea had been born, he developed it by testing it against facts collected by him and the many people he corresponded with. His approach probably contained elements from both the inductive and hypothetico-deductive method, and he was not alone in this mode of working. Newton himself said that the best and safest method of research is, “first to inquire diligently into the properties of things, and establishing those properties by experiments and then to proceed more slowly to hypotheses for explanations of them” [4]. I am sure that many with me have experienced, when reviewing data from an experiment, that an unexpected aspect of the data suddenly appears, spurring new ideas that may completely change the course of the investigation. Science does not have to start with a theoretical assumption. Noticing something unexpected that tickles one's curiosity is quite sufficient. There is much wisdom in the famous words attributed to Isaac Asimov, “The most exciting phrase to hear in science, the one that heralds new discoveries, is not Eureka! (I found it!) but rather, ‘hmm … that's funny …’”.
After this discussion of the alleged theory-dependence of observation it might be tempting to ask whether the hypothetico-deductive method really begins with theory? The attentive reader may have noticed that the method assumes that there are already observations to generate hypotheses from. When faced with a seemingly inexplicable phenomenon, we said the scientist should propose speculative theories about its cause. Observation of the phenomenon actually precedes the theories. In the example with the prisms, Newton saw that they disperse white light into several colors before he thought of recombining the colors. Semmelweis noticed the difference in mortality between the two maternity divisions before working out hypotheses. Theory does not necessarily precede observation. More importantly, there are no pure inductivists or falsificationists among working scientists. These are idealized concepts used by philosophers to make it easier to discuss various aspects of research. From a scientist's point of view, the most important thing to keep in mind when comparing the two approaches is that they lead to different types of knowledge. There is nothing wrong with objective observation as a starting point for the advancement of science, but if we want to explain what we see we must – at some point – formulate hypotheses and test them against reality.
