22
Novel Hydrocolloids for Future Progress in Nanotechnology
Sara Naji‐Tabasi
Department of Food Nanotechnology, Research Institute of Food Science and Technology (RIFST) PO Box, 91895‐157.356, Mashhad, Iran
22.1 Introduction
The food industry is the largest manufacturing sector in the world [1]. The major challenge in this industry is the preservation of the activity and bioavailability of bioactive compounds during food processing, storage, passage through the gastrointestinal tract, and efficient absorption through cells for the development of functional food [2–4]. Nanotechnology is one of the world's fastest‐growing industries, which controls material dimensions on the scale of approximately 1–100 nm [3,5]. Nanosystems can provide a polymeric barrier for core materials against the destructive conditions in the food industry and gastrointestinal tract, improving the stability and direct uptake [6]. Food‐grade biopolymers such as proteins or polysaccharides can be used to produce nanometer‐sized particles.
Hydrocolloids are a heterogeneous group of long‐chain polymers (polysaccharides or proteins) and are used in technical and regulated applications to thicken and/or stabilize formulations [7]. Polysaccharide‐based hydrocolloids are composed of monosaccharides joined by glycosidic bonds, which are suitable carriers for the targeted and controlled release of drugs or nutraceuticals along the human gastrointestinal tract, due to their structural versatility and site‐specific digestion properties [8].
The use of polysaccharides as building blocks in the development of nano‐sized functional food delivery systems is rapidly growing due to their unique multi‐functional groups in addition to their physicochemical properties, including biocompatible, low toxicity, low cost, and stable structure [9]. Natural polysaccharides can be obtained from several resources, including plant (e.g., pectin, cellulose, and starch), animal (chitosan, chitin, and glycosaminoglycan), microbial (e.g., dextran, pullulan, xanthan gum, and gellan gum), and algal origin (e.g., agar, alginate, and carrageenan) [7]. Polysaccharides can be easily modified chemically and biochemically, and new features can be obtained to improve the bioavailability of bioactive compounds included in delivery systems [10,11]. Furthermore, the presence of hydrophilic groups in their structure is a useful strategy to improve the bioavailability of bioactive compounds [11]. Therefore, finding a new source of biodegradable polymers, especially plant‐derived polymers with appropriate properties, is an active area of investigation. Recently, various non‐commercial sources of hydrocolloids have been identified, whose variety of physicochemical properties enables the preparation of a wide array of nanoparticles like Qodume Shirazi (Alyssum homolocarpum) [12], cress (Lepidium sativum) seed gum [13,14], basil (Ocimum bacilicum L.) seed gum [15,16], sage (Salvia macrosiphon) seed gum [17], Balangu (Lallemantia royleana) seed gum [18], and Qodume Shahri (Lepidium perfoliatum) seed gum [19]. Various studies have been conducted to evaluate the potential of the new source of hydrocolloids in nanotechnology. Therefore, in this chapter, various types of novel hydrocolloids used as nanostructural systems in food and pharmacy industry and appropriate techniques for their fabrication have been discussed.
22.2 Importance of Finding New Material Sources in Nanotechnology
Scientists are beginning to construct all sorts of different types of structures with varying functionalities using nanomaterials as their building blocks. Nanotechnology can be applied in the food industry as new tools for the delivery of bioactive compounds to target sites and for the improvement of safety and the nutritional value of food products [20,21]. Also, nanotechnology can be used to build new types of food packages; food quality detection tools; sensors which can detect trace contaminants, gasses, or microbes in food; and other measurement tools [22].
The future of functional food is related to the development of novel nanocarriers for oral delivery systems. Various delivery systems are currently under development to minimize bioactive compound degradation and loss, to prevent harmful side effects, and to increase bioavailability. The choice of a polysaccharide as wall material depends on several factors such as their self‐assembly capability, cost of the raw materials, ease of fabrication of the delivery system, regulatory status, and full attention must be paid to safety and toxicological issues [11]. As a result, polymers derived from plant origin have evoked tremendous interest because they comply more easily with the requisites of biocompatibility, biodegradability, and the absence of toxicity [10]. Researchers are trying to develop better and more efficient nanocarriers with increased bioavailability without compromising the appearance and taste of the food products in which novel carriers are incorporated. Therefore, access to a new source of hydrocolloid with different physicochemical properties can provide nanoparticles with unique properties. On the other hand, the novel delivery system is a new approach to delivering nutraceutical compounds that addresses the limitations of the common delivery systems. The demand for these substances is increasing, and new sources are being developed [23].
22.3 Nanomaterials
Different types of functional nanostructures can be used as building blocks to create novel structures and introduce new functionalities into foods [5]. Nanostructure materials are broadly classified into three: (1) nanofilm, (2) nanofiber, and nanoparticles. Researchers have explored the use of novel sources of hydrocolloids in nanosystems, specifically nanofibers, and nanoparticles, as delivery systems, which are discussed here.
22.3.1 Nanofiber
Nanofibers are defined as fibers with a diameter of less than 100 nm. They can be prepared by interfacial polymerization, electrospinning, and phase separation techniques [24]. Nanofibers possess various advantages such as high porosity with very small pore size, large surface area‐to‐volume ratio, high gas permeability, and superior mechanical properties, for example, stiffness and tensile strength [25,26]. Nanofibers can be employed for various applications such as delivery systems, membrane technology, protective clothing, tissue engineering scaffolds, and wound healing [27,28]. But in the food industry, they are mostly used for food texturizing, enzyme immobilization, filtration, enhancement of film properties [ 26,29], as edible carriers for encapsulation of food additives such as encapsulation of vitamins [30], as phenolic compounds [31], and in bioactive packaging technologies [32].
Electrospinning (ES) is one of the best and simplest techniques for fabrication of ultrathin fibers. There is a wide range of polysaccharides that can be electrospun into fine nanofibers, such as chitosan‐hyaluronic acid [33], cellulose [34], methylcellulose [35], hydroxypropylmethylcellulose [35], chitosan [36], and so on. As advances continue in the area of nanofiber production from food‐grade materials, the use new of natural sources such as basil seed gum (BSG) [37], almond gum [38], cress seed gum (CSG), Alyssum lepidium gum [39], and Ficus‐indica mucilage [40] will likely increase.
22.3.1.1 Basil (Ocimum bacilicum L.) Seed Gum
The outer epidermis of basil seeds (Ocimum bacilicum L.), when soaked in water, swells into a gelatinous mass which consists of considerable amounts of unesterified galacturonic acid [41]. BSG is an anionic gum with a high molecular weight (2320 kDa) and two major fractions [42] composed of glucose, rhamnose, galacturonic acid, arabinose, mannose, glucuronic acid, and galactose [43,44]. It has a random coil conformation in the dilute regime, which can form an ordered conformation under favored conditions such as a high enough concentration, the presence of binding agents, or changes in temperatures and pHs [45]. BSG exhibits shear‐thinning properties and high consistency coefficient and yield stress, which is dependent on concentration and temperature [46,47].
BSG can be used as a natural polymer for the preparation of nanofiber, but it cannot be spun alone and needs to be used with an electrospinning aid agent. Polyvinyl alcohol (PVA) is a good choice as an aid agent due to the presence of a hydroxyl group in its structure [37]. Kurd et al. [37] investigated the electrospinning of different blending ratios of BSG/PVA solution (80:20, 60:40, 40:60, and 20:80) at high‐voltage power (18 and 23 kV) and 25 °C. The optimum condition for preparation of BSG nanofiber is a volume ratio of 60:40 under a voltage of 18 kV, and the distance between needle tip and collector should be kept constant at 14 cm (Figure 22.1) [37]. The BSG fiber diameter (179–390 nm) increases with increasing voltage from 18 to 28 mV. Higher voltage, and consequently a higher electrical field, leads to higher solution feeding rates, causing larger fiber diameters. By increasing the BSG proportion in BSG/PVA blend solution, thinner nanofibers are obtained, which can be attributed to the higher solution viscosity and electrical conductivity of BSG, which reflected a higher charge density over the ejected jet and thus higher elongation. On the other hand, increasing the PVA volume ratio results in uniform BSG nanofibers without bead defects, which is related to the reduction in repulsive forces within the charged polymeric solution. Also, the thermal properties of nanofiber improve with PVA addition.

Figure 22.1 SEM image of nanofibers produced under voltages 18 kV and BSG to PVA volume ratios = 20:80, nozzle‐collector distance = 14 cm.
Source: Adapted from Kurd et al. [37] with permission from Elsevier.
22.3.1.2 Almond (Amygdalus communis L.) Tree Exudate Gum
Almond gum/PVA nanofibers were fabricated by electrospinning as a vanillin carrier. Almond gum is a high‐molecular‐weight (1180 kDa) natural polymer, which exudates from branches, trunks, and fruits of almond gum trees (Amygdalus communis L., a species of genus Prunus belonging to the Rosacea family). The exudation usually starts as a result of a disease (gummosis) or a mechanical injury followed by a microbial attack [38]. The gum exudates are composed, on a dry weight basis, by 2.45% of proteins, 0.85% of fats, and 92.36% of carbohydrates. The latter consist of arabinose, xylitol, galactose, and uronic acid (46.8:10.9:35.5:6.0 mass ratio) with traces of rhamnose, mannose, and glucose [48]. The glycosyl linkage positions were analyzed using gas chromatography–mass spectrometry and showed a main chain composed of galactose units [→3)‐Gal‐(1→] branched mainly with arabinose residues [Ara‐(1→] [49]. Moreover, gum exudates are rich in minerals such as sodium, potassium, magnesium, calcium, and iron [48].
The best conditions for electrospinning of almond gum are as follows: proportion of almond gum to PVA = 80:20 (w/w) and total concentration of 7% (w/w), voltage = 18 kV, needle to collector distance = 15 cm, and flow rate = 0.125 ml h−1. Electrospinning of almond gum–PVA solution (80:20) at 7% (w/w) concentration produces beaded fibers. But adding vanillin (3% (w/w)) to almond gum–PVA solution (in the proportion of 80:20 and 7% (w/w) concentration) increases the viscosity and conductivity of the solution and reduces its surface tension, which therefore resulted in successful electrospinning of uniform and bead‐free nanofibers with a diameter of 77 ± 18 nm.
The release kinetics of vanillin from almond nanofiber in different media (distilled water, 10% ethanol, 50% ethanol, and simulated saliva) follows a pseudo‐Fickian diffusion mechanism: the burst release of vanillin within the first 5 min, followed by sustained release for 180 min. Increasing the vanillin concentration from 1% to 3% (w/w) increases the loading efficiency from 68% to 75%. But loading more vanillin (4%, w/w) increases the bead in fibers. BSG nanofibers have relatively good stability under the dry ambient condition and maintained around 58% of the incorporated vanillin for 90 days [38].
22.3.1.3 Cress (Lepidium sativum) Seed Gum
Cress (Lepidium sativum) seeds are a source of natural gum, which can be used for nanofiber production by electrospinning [50]. Cress seed has been found to contain a high amount of protein and iron, and a significant amount of calcium. As its bran is a source of pentosans (11.0%) with a swelling index of 18–19 ml, it has the potential to be used as a dietary fiber. Cress seed mucilage is an anionic gum with low molecular weight (540 kDa) [13] and intrinsic viscosity (3.92 gr dl−1) [51], which has excellent potential in the fabrication of the nanostructure [52]. CSG has the potential to confer stability to the products undergoing high temperatures, refrigeration, or freezing treatments and has high resistance to pH tolerance, salts, and the synergic effect of sugar. Consequently, the ranges of application of CSG will expand because of its desirable stability in different conditions [ 14, 51].
CSG–PVA nanofiber, produced via electrospinning, has an amorphous structure. The main factors affecting the amorphous structure of CSG–PVA nanofibers are the fast solvent evaporation and the presence of CSG. The morphology and size of CSG fiber are related to the solution viscosity. Under low viscosity, beads are formed rather than uniform fibers due to the domination of surface tension. The better uniformity and smoothness of CSG nanofiber are achieved by increasing the PVA volume ratio, while larger fiber diameters are produced. Also, increasing the CSG–PVA volume ratio increases the electrical conductivity, which is related to the higher electrical conductivity of CSG solution (1175 µS cm−1) due to its weak ionic nature compared to PVA (467 µS cm−1). In the electrospinning process, the ejected jet carries the charges of the CSG solution, and as the charges increase, elongation of the jet occurs by the electrical field, and therefore a reduction in the diameter of the electrospun nanofiber takes place. The increasing electrical conductivity of solutions causes a significant decrease in the nanofiber diameters [50].
Fahami and Fathi [50] have reported that the optimum electrospun CSG/PVA nanofibers (CSG‐to‐PVA ratio 60:40; tip‐to‐collector distance 17 cm, and feed rate 0.2 ml h−1) have an average diameter size range of 95–278 nm with a smooth and uniform surface. But Golkar et al. [39] have found that an aqueous solution of CSG–PVA = 80:20, voltage = 18 kV, polymer concentration = 50%, tip‐to‐collector distance = 10 cm, and feed rate = 0.125 ml h−1 can be successfully used to obtain uniform nanofibers with diameters as low as 139.9 nm. The presence of the CSG in nanofiber has been proved by X‐ray diffraction (XRD) and Fourier transform infrared spectroscopy (FT‐IR). The electrospun nanofibers have higher thermal stability in comparison with CSG [50].
22.3.1.4 Ficus‐indica Mucilage
Thomas et al. [40] has investigated Ficus‐indica (Ofi) cactus mucilage, as a novel polymer for nanofiber preparation, and used it in membrane filtration for water systems. Ofi mucilage is a clear colorless substance comprised of proteins and polysaccharides. It is composed of varying sugars and is considered a linear chain containing galacturonic acid, rhamnose, and galactose, with xylose and arabinose residues attached as side chains. It also contains organic species, which give it the capacity to interact with metals, cations, and biological substances. Like the other high‐molecular‐weight biopolymers, electrospinning nanofibers of Ofi mucilage alone is difficult because of the long polysaccharide chains. PVA and polystyrene are needed to form long polymer chains for electrospinning. The Ficus‐indica mucilage has been electrospun with different volume ratios of mucilage:PVA, mucilage:polystyrene‐D‐limonene, and mucilage:polystyrene–toluene from 30:70 to 70:30. D‐limonene should be used to break down the polystyrene into a solution form. The Ofi cactus mucilage nanofiber membranes have been used as filtration devices for 50 ppb arsenic solutions. PVA:mucilage nanofiber membranes dissolve upon repeated cycling of water solutions, which is attributed to the hydrophilic nature of the PVA and mucilage. On the other hand, 70:30 polystyrene:mucilage nanofiber membranes can remove 9.72% of arsenic from the water. The 50:50 polystyrene:mucilage nanofiber membrane has the ability to remove 18.93% arsenic, which is comparable to a traditional sand columnar filtration (18.33%). The mucilage nanofiber membranes have the potential to serve as the basis for the next generation of economically sustainable filtration devices that make use of a natural non‐toxic material for sustainable water systems. Also, the cactus mucilage nanofiber can be used in tissue scaffolding, enzyme carrier, tissue engineering, air filtration, cell culturing, gas filtration, drug delivery, textiles, sensors, and for many other uses [40].
22.3.2 Nanoparticles
Nanoparticles (NPs) are solid colloidal particles ranging in size from 1 to 100 nm, which are made up of macromolecular materials [11]. But particles >200 nm are not heavily pursued, and nanomedicine often refers to particles <200 nm [53]. The nanoparticle system provides a polymeric barrier for the core materials against the oxidative conditions, resulting in improvement of stability. Hence, the nanoparticles composed of biocompatible and biodegradable polymers like protein and polysaccharide can be used in functional food delivery systems. Different hydrocolloids are used as a nanocarrier in the food industry, such as alginate, chitosan, gum arabic, mesquite gum, starch, and its derivatives (Table 22.1), and are still at the stage of being developed as efficient nanocomponents to find applications in the food industry, which is discussed in this chapter.
Table 22.1 Summary of nanoencapsulation of bioactive compounds in common polysaccharide wall materials.
| Polysaccharide | Core | Method | Reference |
| ß‐cyclodextrin | Cinnamon and thyme essential oil components | Inclusion complexes | [54] |
| Sodium caseinate–gellan | Lactobacillus casei | Emulsion | [55] |
| Octenyl succinic anhydride modified starch | ß‐Carotene | Nanoemulsion | [56] |
| Pectin–caseinate | Omega‐3 fatty acids | Polyelectrolyte complexation | [57] |
| Alginate | Lactoferrin | Ion gelation (Calcium, thermal, and non‐thermal treatment) |
[58] |
| Pectin–WPC | Saffron extract | Double‐layered multiple Emulsification |
[59] |
| Chitosan–sodium caseinate | Fluorescein, rhodamine B, and riboflavin | Coacervation | [60] |
| Gum arabic–sodium caseinate | Fish Oil | Complexation | [61] |
| Pectin–WPC | Olive leaf phenolic compounds | Complexation | [62] |
| Gum arabic–chitosan | Curcumin | Polyelectrolyte complexation | [63] |
| Dextran–whey protein | B‐carotene | Nanoemulsion | [64] |
| Caseinate–zein–pectin | Curcumin | Complex | [65] |
22.3.2.1 Cress Seed Gum
CSG nanoparticles were fabricated by Taheri and Razavi [66] via the desolvation method (acetone) in three steps: (1) production of an aqueous solution of CSG at pH 9; (2) addition of a desolvating agent of to an aqueous solution of the polysaccharide comprising coacervates, and (3) drying of nanoparticles. They used the central composite design to optimize the process. The particle size of CSG nanoparticles is significantly influenced by the gum concentration, amount of acetone as non‐solvent, and rate of agitation, whereas the relative viscosity of CSG nanoparticles is only affected by the gum concentration. Under the optimum desolvation condition (0.28% w/v gum concentration, 5.12 ml acetone, and rate of agitation 500 rpm), the CSG nanoparticles have a spherical shape with an average size of 20.10 nm and a zeta potential of −32.38 mV (Figure 22.2 and Table 22.2) [66].
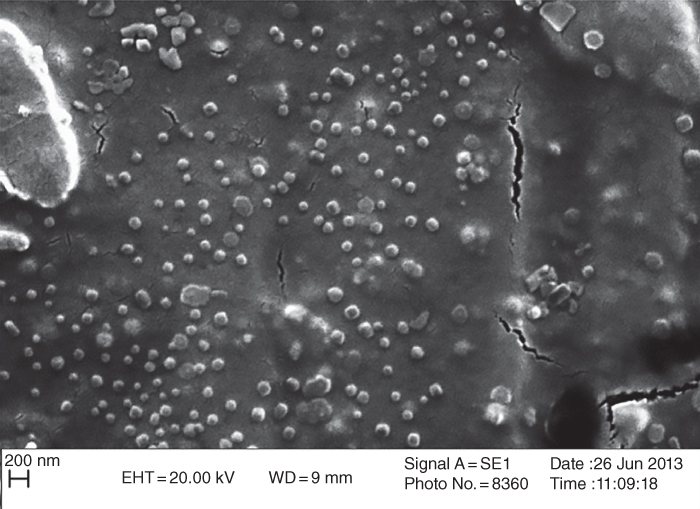
Figure 22.2 SEM images of CSG nanoparticles prepared by intermittent acetone addition at different gum concentrations.
Source: Adapted from Taheri and Razavi [66] with permission from Springer Nature.
Table 22.2 Summary of novel polysaccharide nanoparticles as a delivery system.
| Polysaccharide | Concentration/ratio (% w/v) | Core | Core concentration | Method | Size (nm) | Reference |
| CSG | 0.15, 0.25, 0.35 | — | — | Nanoprecipitation (desolvation) | 28–376 | [66] |
| BSG | 0.1, 0.05, 0.025 | — | — | Ion gelation | 298–596 | [67] |
| BSG | 0.1, 0.05, 0.025 | Glutathione | 0.05 w/v | Ion gelation | 361–401 | [67] |
| BSG | BSG:water (1:200 ratio) | — | — | Supercritical CO2 phase inversion | 60 | [68] |
| Chi–ChG | Chi (0.1, 0.5, 1)‐ChG (4) | Lippia sidoides essential oil | — | Spray dried | 17–429 | [69] |
| Chi–AG | AG (5)‐Chi (4) | Lippia sidoides essential oil | — | Spray dried | 19–472 | [69] |
| Chi–CG | CG (4, 10, 20)‐ChG (4) | Lippia sidoides essential oil | — | Spray dried | 181–483 | [69] |
| CG | 2 |
Larvicide from Moringa
oleifera seeds |
CG–MO volume ratios: 3:1, 2:1, and 1:1 | Spray dried | 288–357 | [70] |
| AHSG | 0.5 | D‐limonene | 10, 20 & 30 w/v | Electrospray | 35–90 | [71] |
| CSM | 0.1 | Chia oil | 0.5, 0.75, and 1.25 mg ml−1 | Nanoemulsion | 163–212 | [72] |
| KG–Chi | ChG (0.04)‐KG (0.06) | Cefixime | 0.05 w/v | Coacervation method | 80–230 | [73] |
| ASG | cASP/pTGF‐β1 weight ratios of 5:1, 10:1, and 20:1 | Gen (transforming growth factor‐beta 1 (TGF‐β1)) | 100 µg ml−1 | Electrostatic complex | 20–159 | [74] |
CSG, Cress seed gum; BSG, Basil seed gum; Chi–ChG, Chitosan gum–Chichá gum; Chi–AG, Chitosan–angico gum; Chi–CG, Chitosan gum–cashew gum; CG, Cashew gum; AHSG, Alyssum homolocarpum seed gum; CSM, Chia (Salvia hispanica L.) seed mucilage; KG–Chi, Kondagogu gum–chitosan gum; ASG, Angelica sinensis gum
The intrinsic viscosity of the CSG nanoparticles lies within the range 0.22–0.43 gr dl−1. Non‐solvent (acetone) and CSG concentrations have significant effects on the intrinsic viscosity of nanoparticles. CSG nanoparticles exhibit an extended random coil conformation when exposed to low amounts of acetone, while a high amount of acetone causes the polyelectrolyte to take on a completely globular conformation. As a result, CSG goes on to exhibit a coil–globule transition above a certain threshold of acetone. The structural properties of the CSG polymer and the changing polarity of the surroundings are the main parameters which control structural changes and guide the different rheological behaviors of CSG nanoparticles [75].
22.3.2.2 Basil Seed Gum
Basil seed mucilage has a wide range of applications in the food and pharmaceutical industries such as thickener disintegrant [76], biodegradable edible film [77], binder [78], pharmaceutical excipient [79], anti‐diabetic agent [80,81], suspending agent [82] and also as matrices for sustained and controlled drug delivery [83,84]. BSG has good bioadhesive qualities, and many drug actives can be released through such bioadhesives, such as steroids, anti‐inflammatory agents, pH‐sensitive peptides, and small proteins such as insulin, as well as local treatments to alleviate pain in the buccal cavity [68]. BSG nanoparticle size, when preparation is with the ion gelling technique, is between 361 and 401 nm (Table 22.2), and the zeta potential is negative (∼−31 mv) with a spherical shape. The particle size distribution of BSG nanoparticles (BSG NPS) increases as the BSG concentration increases (1, 0.5, and 0.25 mg ml−1) with CaCl2 (concentrations 0.7, 0.5, and 0.3 mg ml−1) as a cross‐linking agent. The highest percentages of gum (1 mg ml−1) and cross‐linking agent (0.7 mg ml−1) lead to the largest particle size (595 nm). The smallest medium nanoparticle size (298 nm) can be obtained by the combination of the lowest BSG and CaCl2 concentrations (Figure 22.3). A decrease in nanoparticles size at lower BSG concentrations is attributed to a reduction in solution viscosity, which improves solubility and the gelation process. The intrinsic viscosity of the BSG NPS lies in the range 1.85–3.53 dl gr−1. Formation of BSG nanoparticles considerably reduces the intrinsic viscosity of basil seed polysaccharide (39.17 dl gr−1) [67].
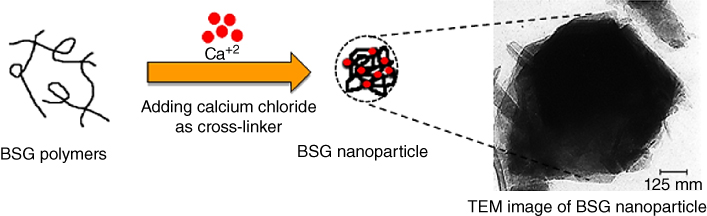
Figure 22.3 Schematic of basil seed gum nanoparticle (BSG NP) formation.
Source: Adapted from Naji‐Tabasi et al. [67] with permission from Elsevier.
BSG can be used as a new carrier for oral delivery of tripeptide compound like glutathione (GSH). BSG–GSH nanoparticle sizes vary between 361 and 401 nm. The entrapment and loading efficiencies of glutathione are in the range 6.5%–43% and 7%–13%, respectively. The entrapment and loading efficiencies of NPs increase with increasing BSG concentration. The release speed of glutathione in pH 1.2 is lower in comparison with pH 6.8, and smaller amounts of glutathione will be destroyed in the stomach during digestion. The glutathione release follows non‐Fickian and Fickian mechanisms in the stomach and intestine environments, respectively.
Akbari et al. [68] have used the supercritical CO2 (SC‐CO2) modified gel drying method to produce drug delivery materials from basil seed mucilage. The supercritical phase inversion technique for hydrogel drying can generate a nanometric structure from BSG. The most homogeneous nanostructure (60 nm mean pore size diameter, 78 m2 g−1 Brunauer–Emmett–Teller (BET) surface area with no agglomeration) is obtained with 2.5% methanol in the non‐solvent stream. A more uniform and porous structure is obtained by SC‐CO2 phase inversion in comparison with the oven‐dried process. The morphology of BSG can be controlled by the composition of the co‐solvent (methanol) in the non‐solvent stream. Furthermore, FTIR analyses have proved that the nature of the final product does not change during the supercritical drying procedure.
The widespread use of SC‐CO2 to generate a porous structure from natural polymers like BSG is limited due to the fact that these polymers are only soluble in aqueous solution (pure water or acidified water), which is a solvent with very little affinity/solubility in SC‐CO2. To overcome this limitation, it is necessary to use a co‐solvent in the supercritical fluid to increase the mutual affinity between solvent and non‐solvent. The biocompatible structure obtained by this method demonstrates a high level of porosity, with good interconnectivity in the pore network system. Furthermore, the obtained product can be loaded with various types of drugs in its structure and used as a controlled bioactive and drug delivery medium [68].
22.3.2.3 Cashew (Anacardium occidentale) Tree Exudate Gum
Cashew gum (CG) is an exudate from cashew (Anacardium occidentale) trees from the Brazilian northeast and is composed of D‐galactose (72%), D‐glucose (14%), and arabinose (4.6%), and also rhamnose (3.2%) and glucuronic acid (4.7%). The main chain is formed by 1–3‐linked β‐D‐galactose units, with the other sugars being present in polysaccharide branches. CG has been used for several applications, including a polyacrylamide hydrogel component, a polyelectrolyte complex with chitosan, and as a polymeric matrix for the delivery of sensitive substances [70].
Paula et al. [70] have also investigated the potential of CG for nanoencapsulation of larvicide from Moringa oleifera (MO) seeds by spray drying, with an operational yield of 70%. The NPs present a unimodal distribution and particle sizes ranging from 288 to 357 nm (Table 22.2). The biopolymer CG entraps the MO‐active principle and has a protective effect, as observed by the positive values of the zeta potential of the NPs and by the decrease in the thermal decomposition temperature of CG present in the NPs. The loading and entrapment efficiencies are proportional to the amount of MO incorporated in the NPs, with values from 2.6% to 4.4% of MO. In vitro release profiles for the CG–MO 1:1 and CG–MO 2:1 samples show prolonged release with a Fickian diffusional profile. The CG–MO 1:1 sample exhibits 78% mortality after 48 h [70].
Paula et al. [69] have prepared nanoparticles of chitosan–cashew gum (Chi–CG) for encapsulation Lippia sidoides essential oil (LSEO). The chitosan‐to‐CG ratio (5:1, 10:1, 2.5:1, 1:1, 1:2.5) play major roles in the nanoencapsulation process and nanoparticle properties. Chi–CG nanoparticle average size varies from 181 to 483 nm (Table 22.2) and exhibits a unimodal distribution, with a polydispersity index in the range 0.280–0.851. A ratio 1:1 sample has the smallest particle size (181 nm), while the ratio 10:1 sample has the largest (483 nm). In general, samples with a higher Chi ratio have larger sizes than the samples with a greater CG ratio. Increasing the Chi ratio increases the amount of NH3+ groups, leading to a greater repulsion between the chains, and consequently the particle size increases. The zeta potential for Chi:CG matrices varies between +30 and +46.4 mV, indicating that the surface of nanoparticles is positively charged by NH3+ sites of chitosan. Increasing the Chi ratio increases the oil loading and encapsulation efficiency. Chi–CG nanoparticles with 5:1 and 10:1 ratios have oil loading and encapsulation efficiencies of around 15% and 60%, respectively. The sample with the highest CG content (1:2.5) has an oil loading of 8.2% and an encapsulation efficiency of 33%. Therefore, Chi plays the major role in oil encapsulation, because when a lower Chi ratio is used for matrix preparation, a lower oil content in the nanoparticle is obtained. On the other hand, increasing CG in the matrix leads to higher and faster oil release, within 60 hours. After 48 h, the sample with the highest CG content releases 100% of LSEO, while a higher Chi proportion results in the release of 70% of the oil present [69].
22.3.2.4 Chichá (Sterculia striata) Gum
Chichá gum (ChG) is extracted from the Chichá tree (Sterculia striata) in the Northeastern region of Brazil, which is a heteropolymer containing partially acetylated chains and a high concentration of uronic acid (42.2%), galactose (23.4%), rhamnose (28.8%), and xylose (5.6%). The polyanionic character of this gum arises from the presence of carboxylic acid groups, through which intermolecular interactions may occur. The technological interest in the Chichá gum, which has been proven to be compatible with many synthetic polymers, comes mainly from its biodegradability and mechanical properties [85]. Paula et al. [69] also have prepared Chi–ChG nanoparticles for encapsulation of LSEO at different ratios (Chi:CG ratios of 5:1, 1:1, 1:5). The sample with the ratio 1:1 has the smallest size (17 nm), and the sample with the highest Chi ratio (5:1) has the largest size (429 nm). For these two ratios, the nanoparticles have a unimodal distribution, with a polydispersity index in the range 0.280–0.851. Increasing the ChG ratio (1:5) leads to a bimodal distribution. The zeta potential of ChG changes from −40 to +26 mV. ChG has a large number of carboxylate groups (around 40%) in comparison with the other gums. Thus, when there is a greater ratio of ChG in a matrix, there is an excess of these groups that have not been complexed with positive sites. The effect of the Chi‐to‐ChG ratio on the oil loading and encapsulation efficiencies has been investigated by maintaining a constant gum concentration during preparation. A higher Chi ratio results in higher oil loading. When using a ratio of 5:1, there is a higher oil encapsulation (15%), while for the ratios 1:1 and 1:5, the loading is around 7%. However, increasing ChG concentrations in the range 0.1%–1.0% increases the oil loading from 3.7% to 14.7% (p < 0.05). The release rate of LSEO varied from 70% (for the sample with a low ChG content) to 100% (for the sample with a high ChG content). The higher ChG content in the matrix leads to a faster release rate [69].
22.3.2.5 Angico (Anadenanthera macrocarpa) Gum
Anadenanthera macrocarpa tree is a plant from Northeast of Brazil, whose exudate gum is employed as an adhesive and alternative medicine in some regions of Brazil. Angico gum (AG) is a polysaccharide composed of arabinose (67%), galactose (24%), rhamnose (2%), and glucuronic acid (7%). AG was found to have a broad molar mass distribution with a molecular weight of 3.7 × 106 g mol−1 and an intrinsic viscosity of 11 ml g−1 in 1 M NaCl at 25 °C [86]. AG has large potential applications since their structure and properties are very similar to those of commercial gums such as gum arabic, karaya, and gellan gums. AG has the main chain of galactose linked by beta 1–3 linkage, differing in the side chains and uronic acid content. AG has the capability of forming micelles and small particles, which leads to small sizes in the solution. The Chi–AG particle sizes ranged (5:1, 1:1, and 1:2.5 ratios) from 19 to 472 nm with the zeta potential ranging from +1 to +30 mV. The sample with the highest Chi ratio (5:1) has a bimodal distribution. The polydispersity index is in the range 0.480–0.781. A greater ratio of AG in relation to Chi results in greater oil loading and encapsulation efficiencies. Loading ranges from 6.2% for the sample with the highest Chi content to 12.6% for the sample with the highest AG content. Also, better oil protection during spray drying is obtained for a low Chi ratio. A slow release rate of LSEO is reported for the sample with a high Chi content and for the Chi–AG 1:1 sample [69].
22.3.2.6 Ghodome Shirazi (Alyssum homolocarpum) Seed Gum
Alyssum homolocarpum seed gum (AHSG) has a low molecular weight (3.66 × 105 Da), medium intrinsic viscosity (18.34 dl g−1) at 25 °C, zeta potential −25.81 mV (at neutral pH), a relatively flexible chain with a chain flexibility parameter of 618.54, and an activation energy of 0.51 × 107 J kg mol−1. AHSG is likely a galactan‐type polysaccharide with high total sugar content (85.33%) and a few uronic acids (5.63%) [87]. AHSG is a novel encapsulating material because it is a tasteless, odorless, and inexpensive hydrocolloid, with good water solubility. Khoshakhlagh et al. [71] introduced electrosprayed AHSG nanoparticles as a novel and efficient carrier for encapsulation of bioactive ingredients. The anionic nature of AHSG may facilitate the electrospraying process due to the presence of high electric charges on the droplets, which compete with the surface tension of the solution, causing the jet to break up into droplets.
Khoshakhlagh et al. [71] have also investigated the feasibility of developing AHSG nanocapsules containing D‐limonene by electrospraying. For the fabrication of AHSG nanocapsules, D‐limonene emulsions with constant AHSG concentration (0.5% w/w) and various flavor concentrations (10%–30% based on gum weight) with 0.1% Tween 20 should electrospray at a voltage of 20 kV and 0.1 ml h−1 of flow rate. The morphology of AHSG nanocapsules exhibits a strong dependence on solution properties. AHSG solution has aggregated irregular shaped nanoparticles with electrospraying, but by incorporation of 10% and 20% D‐limonene, spherical nanocapsules are obtained (Table 22.2). However, it should be noticed that the higher content of D‐limonene (30%) changes the morphology of nanocapsules to nanofibers (Figure 22.4). The morphology of the materials obtained by electrospraying largely depends on the physical characteristics of the starting polymeric solution. AHSG solution has higher surface tension and electrical conductivity than D‐limonene in water emulsion. The high electrical conductivity of the solution increases the net charge density carried by the electrospraying jet. As a result, AHSG solution destabilizes the jet and electrospraying process, eventually leading to the production of heterogeneous structures. Increasing the D‐limonene content leads to an increase in the mean diameter (35–90) of AHSG nanoparticles, which is attributed to insufficient emulsifier available to completely cover the surface of the oil droplets at high oil concentrations, which leads to droplet aggregation and increases the mean droplet diameter. The emulsion samples containing 10% and 20% D‐limonene have narrower droplet size distribution curves with a single peak and small span. However, 30% limonene leads to a bimodal and wide size distribution with a high span, indicating the high degree of polydispersity, because oil droplet aggregate in the emulsion. The encapsulation efficiency decreases to 87.38 ± 4.76% and 74.93 ± 1.62% when the initial D‐limonene loadings increase to 20% and 30%, respectively. On the other hand, the surface oil content increases significantly as the initial flavor loading increases. The emulsion stability decreases with increasing D‐limonene‐to‐AHSG ratio, due to the lack of enough surfactant for full coverage of the flavor droplets. 20% D‐limonene in water emulsion is sufficient for the formation of AHSG electrosprayed nanocapsules since they have high encapsulation efficiency along with the minimum use of wall material. D‐limonene‐loaded nanocapsules have a fully amorphous structure. The thermal stability of D‐limonene increases after encapsulation, which is a promising result for protection of sensitive bioactive compounds in thermally processed food [71].
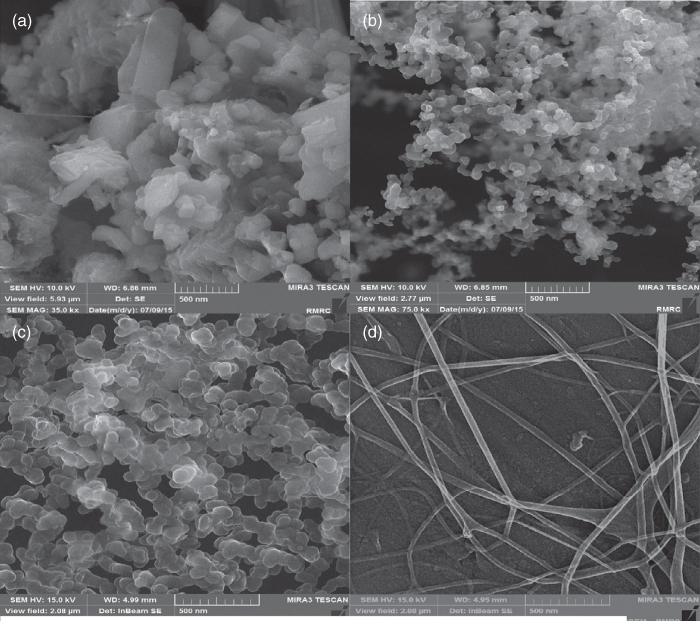
Figure 22.4 FE‐SEM images and size distribution of AHSG nanostructures obtained at a constant voltage (20 kV), flow rate (0.1 ml h−1), and needle‐to‐tip distance (15 cm), with different D‐limonene loadings – a: 0%; b: 10%; c: 20%; d: 30%.
Source: Adapted from Khoshakhlagh et al. [71] with permission from Elsevier.
22.3.2.7 Chia (Salvia hispanica L.) Seed Mucilage
Chia (Salvia hispanica L.) is a herbaceous plant from the Lamiaceae family, originally from southern Mexico, and its use may be as whole seeds, flour, seed oil, and mucilage. Chia seed mucilage (CSM) is an anionic hydrocolloid with excellent water and oil retention capacity, which has high viscosity even at low concentrations [88]. CSM is recognized as a heteropolysaccharide with a high molar mass at approximately 4.9 × 105 Da. CSM was composed on a dry basis of 10.02 ± 1.14% ash, 4.25 ± 0.00% protein, 0.39 ± 0.04% lipids, 74.04 ± 0.13% total fiber, 38.47 ± 4.82% soluble fiber, and 35.57 ± 4.82% insoluble fiber. CSM consists of d‐xylose, α‐D‐glucose, and 4–0‐methyl‐α‐D‐glucuronic acid, as their main simple components, in the ratio 2:1:1, respectively [89]. CSM is a promising polymer for use as wall material, as reported by Timilsena et al. (2016) [88], who utilized this gum in combination with chia protein isolate to microencapsulate chia oil. Also, CSM has been used as a wall material for encapsulation of different concentrations of chia seed oil (CSO) (0.5, 0.75, and 1.25 mg ml−1 of CSO). A low CSM concentration (0.1% w/v) is recommended for nanoparticle fabrication to ensure nanoparticle formation because it has high molar mass. High amounts of oil in the organic phase increase the viscosity of the solution, which also increases the nanoparticle size. Also, the increase in oil concentration may influence the emulsion stability, increasing the mean diameter. The optimum pH for fabrication of CSM nanoparticles is pH 4, which decreases the mean diameter. The decrease in pH solution reduces the electrostatic repulsion between the particles, reducing the number of groups with a similar charge, which decreases the particle size. The formulation with 1.25 mg ml−1 of CSO has been reported as the optimal formulation due to the high amount of oil, albeit satisfactory mean diameter (205 nm) and distribution characteristics. The optimum formulation of CSO‐NP has a spherical shape, an average size of 205 ± 4.24 nm, and a zeta potential of −11.58 ± 1.87 mV with an encapsulation efficiency of 82.8% and loading capacity of 35.38% (Table 22.2). CSO‐NP has high encapsulation efficiency, which can enhance the oil stability against oxidation. In addition, CSM has high viscosity, even at low concentrations, which minimizes the oil circulation and oscillation inside the nanoparticles, thus decreasing oil migration to the surface and improving the encapsulation efficiency even at low concentrations. Furthermore, CSO‐NP is thermally stable at temperatures up 300 °C, which makes it suitable for protecting thermo‐sensitive bioactive compounds. Also, CSO NPs create high stability against oxidation. Therefore, CSM represents a promising alternative to synthetic polymers in nanoencapsulation of bioactive oil [72].
22.3.2.8 Kondagogu (Cochlospermum gossypium) Exudate Gum
Kondagogu gum (KG) is a non‐toxic polysaccharide derived as an exudate from the bark of Cochlospermum gossypium (Bixaceae family), a native tree of India, which is one of the major gum producing centers in India and marketed through Girijan Co‐operative Corporation Ltd., Visakhapatnam, India [90]. The primary structure of this gum is made up of sugars such as arabinose, galactose, glucose, mannose, rhamnose, glucuronic acid, and galacturonic acid with a sugar linkage (1 → 2) ß‐D‐Gal p, (1 → 6) ß‐D‐Gal p, (1 → 4)‐d‐Glc p A, 4‐O‐Me‐α‐D‐Glc p A, (1 → 2) α‐l‐ Rha. It has a molecular weight of 7.2 × 106 Da [91,92]. As KG does not have an independent identity in the world market, it was earlier grouped under gum karaya and exported along with it. KG can be used as suspending agents and matrix formers for controlled drug delivery due to its gelling property [90].
Harika et al. [73] have used KG–chitosan gum NPs to develop Cefixime delivery successfully. KG–Chi nanoparticles have been prepared by adding KG (0.04% w/v) to a mixture of chitosan (0.02% w/v) and Cefixime (0.05% w/v) at different polymeric ratios to yield four different formulations, that is, 1:1, 2:1, 1:2, and 2:2. The KG–Chi nanoparticles have average sizes in the range 80.6 ± 3.5 to 230.6 ± 7.3 nm, and the entrapment efficiency varies between 78.0 ± 1.5 and 93.2 ± 2.5% (Table 22.2). The surface charge on the particles could control the particle stability of the nanoparticulate formulation through strong electrostatic repulsion of particles with each other. KG–Chi NPs are spherical or rod‐shaped with a smooth surface morphology. The amount of drug released decreases with increase in polymer concentration due to the increase in the thickness of the polymeric membrane, which decreases the diffusion of drug through it. The release of Cefixime from nanoparticles exhibits a biphasic pattern with an initial burst release followed by sustained drug release up to 32 h [73].
22.3.2.9 Angelica sinensis Polysaccharides
Angelica sinensis polysaccharides (ASP) are isolated from the root of Angelica sinensis (Oliv.) Diels, which is a famous Chinese herbal medicine. ASP has various biological activities, such as gastrointestinal protection, anti‐tumor, immunomodulation, hematopoiesis, and radioprotection. Deng et al. [74] have used ASP after modification with branched low‐molecular‐weight polyethylenimine (1200 Da) for preparation nanoparticles. The cationized Angelica sinensis polysaccharides (cASP) has been applied for delivery of plasmid encoding transforming growth factor‐beta 1 (TGF‐β1). cASP has combined with TGF‐β1 to form a spherical nano‐scaled particle. This nanoparticle has been applied to transfect rat bone marrow mesenchymal stem cells and human umbilical cord mesenchymal stem cells. It has been reported that cASP/pDNA nanoparticles (cASP/pDNA weight ratio 10:1) have the greatest transfection efficiency in both cells, which was significantly higher than those of Lipofectamine 2000 and PEI (25 kDa). The cASP‐pTGF‐β1 nanoparticles (10:1) are well dispersed and have a uniformly distributed size ranging from 20 to 50 nm. This, however, seems to be slightly smaller than the findings from dynamic light scattering, in which the particle size of the cASP‐pTGF‐ β1 complex (10:1) was distributed between 20.7 and 77.4 nm with an average diameter of 62 nm. cASP nanoparticles are less toxic than Lipofectamine 2000 and PEI (25 kDa), which makes cASP the best candidate for a novel non‐viral gene vector (Table 22.2) [74].
22.4 Conclusions and Future Trends
One of the major challenges in nanotechnology is discovering and developing new nanostructures with new features. Hydrocolloids are one of the major polymers in the fabrication of nanomaterials. Therefore, new sources of natural hydrocolloids have evoked great attention in the development of nanotechnology, especially in the food and pharmacy industries. Although various investigations have been conducted on using new source of polysaccharides for preparing nanofibers or nanoparticles, further research is still needed to evaluate the retention and release behavior of encapsulated bioactive materials under various processing conditions (such as temperature, pressure, humidity, light exposure, and storage time) and formulations for use in different industries. As a result, more attention needs to be paid to this area of nanotechnology research for the further development of novel hydrocolloids.
References
- 1 Tozuka, Y., Takeshita, A., Nagae, A. et al. (2006). Specific inclusion mode of guest compounds in the amylose complex analyzed by solid state NMR spectroscopy. Chemical and Pharmaceutical Bulletin 54: 1097–1101.
- 2 Gómez‐Mascaraque, L.G., Lagarón, J.M., and López‐Rubio, A. (2015). Electrosprayed gelatin submicroparticles as edible carriers for the encapsulation of polyphenols of interest in functional foods. Food Hydrocolloids 49: 42–52.
- 3 Eltayeb, M., Bakhshi, P.K., Stride, E., and Edirisinghe, M. (2013). Preparation of solid lipid nanoparticles containing active compound by electrohydrodynamic spraying. Food Research International 53: 88–95.
- 4 Cook, M.T., Tzortzis, G., Charalampopoulos, D., and Khutoryanskiy, V.V. (2012). Microencapsulation of probiotics for gastrointestinal delivery. Journal of Controlled Release 162: 56–67.
- 5 Niosi, J. and Reid, S.E. (2007). Biotechnology and nanotechnology: science‐based enabling technologies as windows of opportunity for LDCs? World Development 35: 426–438.
- 6 Yaktine, A. and Pray, L. (2009). Nanotechnology in Food Products: Workshop Summary. National Academies Press.
- 7 Saha, D. and Bhattacharya, S. (2010). Hydrocolloids as thickening and gelling agents in food: a critical review. Journal of Food Science and Technology 47: 587–597.
- 8 Hu, B. (2013). Biopolymer based nano‐delivery systems for enhancing bioavailability of nutraceuticals. Chinese Journal of Polymer Science 31: 1190–1203.
- 9 Venkatesan, J., Anil, S., Kim, S.‐K., and Shim, M.S. (2016). Seaweed polysaccharide‐based nanoparticles: preparation and applications for drug delivery. Polymers 8: 30.
- 10 Liu, Z., Jiao, Y., Wang, Y. et al. (2008). Polysaccharides‐based nanoparticles as drug delivery systems. Advanced Drug Delivery Reviews 60: 1650–1662.
- 11 Martínez, A., Fernández, A., Pérez, E. et al. (2012). Polysaccharide‐based nanoparticles for controlled release formulations. In: The Delivery of Nanoparticles (ed. A.A. Hashim), 185–222.
- 12 Koocheki, A., Mortazavi, S.A., Shahidi, F. et al. (2009). Rheological properties of mucilage extracted from Alyssum homolocarpum seed as a new source of thickening agent. Journal of Food Engineering 91: 490–496.
- 13 Karazhiyan, H., Razavi, S., Phillips, G.O. et al. (2011). Physicochemical aspects of hydrocolloid extract from the seeds of Lepidium sativum. International Journal of Food Science & Technology 46: 1066–1072.
- 14 Naji, S. and Razavi, S. (2014). Functional and textural characteristics of cress seed (Lepidium sativum) gum and xanthan gum: effect of refrigeration condition. Food Bioscience 5: 1–8.
- 15 Razavi, S., Mortazavi, S.A., Matia‐Merino, L. et al. (2009). Optimisation study of gum extraction from basil seeds (Ocimum basilicum L.). International Journal of Food Science & Technology 44: 1755–1762.
- 16 Naji‐Tabasi, S. and Razavi, S.M.A. (2016). New studies on basil (Ocimum bacilicum L.) seed gum: part II—emulsifying and foaming characterization. Carbohydrate Polymers 149: 140–150.
- 17 Bostan, A., Razavi, S.M., and Farhoosh, R. (2010). Optimization of hydrocolloid extraction from wild sage seed (Salvia macrosiphon) using response surface. International Journal of Food Properties 13: 1380–1392.
- 18 Mohammad Amini, A. and Razavi, S. (2012). Dilute solution properties of Balangu (Lallemantia royleana) seed gum: effect of temperature, salt, and sugar. International Journal of Biological Macromolecules 51: 235–243.
- 19 Koocheki, A., Taherian, A.R., and Bostan, A. (2013). Studies on the steady shear flow behavior and functional properties of Lepidium perfoliatum seed gum. Food Research International 50: 446–456.
- 20 Rashidi, L. and Khosravi‐Darani, K. (2011). The applications of nanotechnology in food industry. Critical Reviews in Food Science and Nutrition 51: 723–730.
- 21 Duncan, T.V. (2011). Applications of nanotechnology in food packaging and food safety: barrier materials, antimicrobials and sensors. Journal of Colloid and Interface Science 363: 1–24.
- 22 Miller, G., van Schaik, D., and Adelaide, R. (2008). Nanotechnology in Food and Agriculture. Radio Adelaide.
- 23 Choudhary, P.D. and Pawar, H.A. (2014). Recently investigated natural gums and mucilages as pharmaceutical excipients: an overview. Journal of Pharmaceutics 1‐9.
- 24 Feng, C., Khulbe, K., and Matsuura, T. (2010). Recent progress in the preparation, characterization, and applications of nanofibers and nanofiber membranes via electrospinning/interfacial polymerization. Journal of Applied Polymer Science 115: 756–776.
- 25 Kong, L. and Ziegler, G.R. (2014). Fabrication of pure starch fibers by electrospinning. Food Hydrocolloids 36: 20–25.
- 26 Okutan, N., Terzi, P., and Altay, F. (2014). Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocolloids 39: 19–26.
- 27 Vashisth, P., Pruthi, P.A., Singh, R.P., and Pruthi, V. (2014). Process optimization for fabrication of gellan based electrospun nanofibers. Carbohydrate Polymers 109: 16–21.
- 28 Bhardwaj, N. and Kundu, S.C. (2010). Electrospinning: a fascinating fiber fabrication technique. Biotechnology Advances 28: 325–347.
- 29 Fathi, M.M., Mart, A.A., and McClements, D.J. (2014). Nanoencapsulation of food ingredients using carbohydrate based delivery systems. Trends in Food Science & Technology 39: 18–39.
- 30 Katouzian, I. and Jafari, S.M. (2016). Nano‐encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends in Food Science & Technology 53: 34–48.
- 31 Esfanjani, A.F. and Jafari, S.M. (2016). Biopolymer nano‐particles and natural nano‐carriers for nano‐encapsulation of phenolic compounds. Colloids and Surfaces B: Biointerfaces 146: 532–543.
- 32 Torres‐Giner, S., Gimenez, E., and Lagarón, J.M. (2008). Characterization of the morphology and thermal properties of zein prolamine nanostructures obtained by electrospinning. Food Hydrocolloids 22: 601–614.
- 33 Maeda, N., Miao, J., Simmons, T. et al. (2014). Composite polysaccharide fibers prepared by electrospinning and coating. Carbohydrate Polymers 102: 950–955.
- 34 Kang, Y., Kim, H., Ryu, Y., and Lee, D. (2002). Manufacturing the cellulose web by using electro‐spinning and in‐vitro behaviour of oxidized cellulose web. Journal‐Korean Fiber Society 39: 14–20.
- 35 Frenot, A., Henriksson, M.W., and Walkenström, P. (2007). Electrospinning of cellulose‐based nanofibers. Journal of Applied Polymer Science 103: 1473–1482.
- 36 Matsuda, A., Kagata, G., Kino, R., and Tanaka, J. (2007). Preparation of chitosan nanofiber tube by electrospinning. Journal of Nanoscience and Nanotechnology 7: 852–855.
- 37 Kurd, F., Fathi, M., and Shekarchizadeh, H. (2017). Basil seed mucilage as a new source for electrospinning: production and physicochemical characterization. International Journal of Biological Macromolecules 95: 689–695.
- 38 Rezaei, A., Nasirpour, A., Tavanai, H., and Fathi, M. (2016). A study on the release kinetics and mechanisms of vanillin incorporated in almond gum/polyvinyl alcohol composite nanofibers in different aqueous food simulants and simulated saliva. Flavour and Fragrance Journal 31: 442–447.
- 39 Golkar, P., Allafchian, A., and Afshar, B. (2017). Alyssum lepidium mucilage as a new source for electrospinning: production and physicochemical characterization. IET Nanobiotechnology 12 (3): 259–263.
- 40 Thomas, S. W., Devisetty, M., Katakam, H. C., et al. (2015). Investigation of Novel Opuntia Ficus‐indica Mucilage Nanofiber Membrane Filtration for Water Systems. MRS Online Proceedings Library Archive, 1745.
- 41 Naji‐Tabasi, S. and Razavi, S.M.A. (2017). Functional properties and applications of basil seed gum: an overview. Food Hydrocolloids 73: 313–325.
- 42 Naji‐Tabasi, S., Razavi, S.M.A., Mohebbi, M., and Malaekeh‐Nikouei, B. (2016). New studies on basil (Ocimum bacilicum L.) seed gum: Part I–Fractionation, physicochemical and surface activity characterization. Food Hydrocolloids 52: 350–358.
- 43 Anjaneyalu, Y.V. and Gowda, D.C. (1979). Structural studies of an acidic polysaccharide from Ocimum basilicum seeds. Carbohydrate Research 75: 251–256.
- 44 Tharanathan, R. and Anjaneyalu, Y. (1975). Structure of the acid‐stable core‐polysaccharide derived from the seed mucilage of Ocimum basilicum. Australian Journal of Chemistry 28: 1345–1350.
- 45 Naji‐Tabasi, S., Razavi, S.M.A., Mohebbi, M., and Malaekeh‐Nikouei, B. (2016). New studies on basil (Ocimum bacilicum L.) seed gum: part I‐fractionation, physicochemical and surface activity characterization. Food Hydrocolloids 52: 350–358.
- 46 Naji‐Tabasi, S. and Razavi, S.M.A. (2017). New studies on basil (Ocimum bacilicum L.) seed gum: Part III–Steady and dynamic shear rheology. Food Hydrocolloids 67: 243–250.
- 47 Hosseini‐Parvar, S., Matia‐Merino, L., Goh, K. et al. (2010). Steady shear flow behavior of gum extracted from Ocimum basilicum L. seed: effect of concentration and temperature. Journal of Food Engineering 101: 236–243.
- 48 Mahfoudhi, N., Chouaibi, M., Donsì, F. et al. (2012). Chemical composition and functional properties of gum exudates from the trunk of the almond tree (Prunus dulcis). Revista de Agaroquimica y Tecnologia de Alimentos 18: 241–250.
- 49 Bouaziz, F., Helbert, C.B., Romdhane, M.B. et al. (2015). Structural data and biological properties of almond gum oligosaccharide: application to beef meat preservation. International Journal of Biological Macromolecules 72: 472–479.
- 50 Fahami, A. and Fathi, M. (2018). Fabrication and characterization of novel nanofibers from cress seed mucilage for food applications. Journal of Applied Polymer Science 135.
- 51 Behrouzian, F., Razavi, S.M., and Karazhiyan, H. (2014). Intrinsic viscosity of cress (Lepidium sativum) seed gum: effect of salts and sugars. Food Hydrocolloids 35: 100–105.
- 52 Taherian, A.R., Fustier, P., and Ramaswamy, H.S. (2007). Effects of added weighting agent and xanthan gum on stability and rheological properties of beverage cloud emulsions formulated using modified starch. Journal of Food Process Engineering 30: 204–224.
- 53 Singh, R. and Lillard, J.W. (2009). Nanoparticle‐based targeted drug delivery. Experimental and Molecular Pathology 86: 215–223.
- 54 Cevallos, P.A.P., Buera, M.P., and Elizalde, B.E. (2010). Encapsulation of cinnamon and thyme essential oils components (cinnamaldehyde and thymol) in β‐cyclodextrin: effect of interactions with water on complex stability. Journal of Food Engineering 99: 70–75.
- 55 Nag, A., Han, K.‐S., and Singh, H. (2011). Microencapsulation of probiotic bacteria using pH‐induced gelation of sodium caseinate and gellan gum. International Dairy Journal 21: 247–253.
- 56 Liang, R., Shoemaker, C.F., Yang, X. et al. (2013). Stability and bioaccessibility of β‐carotene in nanoemulsions stabilized by modified starches. Journal of Agricultural and Food Chemistry 61: 1249–1257.
- 57 Bahrani, S., Ghanbarzadeh, B., and Hamishekar, H. (2013). Nanoencapsulation of omega‐3 fatty acids using caseinate‐pectin based complexes: FTIR, DSC, particle size, and encapsulation efficiency. Iranian Journal of Nutrition Sciences and Food Technology 8: 1–15.
- 58 Raei, M., Rajabzadeh, G., Zibaei, S. et al. (2015). Nano‐encapsulation of isolated lactoferrin from camel milk by calcium alginate and evaluation of its release. International Journal of Biological Macromolecules 79: 669–673.
- 59 Esfanjani, A.F., Jafari, S.M., Assadpoor, E., and Mohammadi, A. (2015). Nano‐encapsulation of saffron extract through double‐layered multiple emulsions of pectin and whey protein concentrate. Journal of Food Engineering 165: 149–155.
- 60 Kurukji, D., Norton, I., and Spyropoulos, F. (2016). Fabrication of sub‐micron protein‐chitosan electrostatic complexes for encapsulation and pH‐modulated delivery of model hydrophilic active compounds. Food Hydrocolloids 53: 249–260.
- 61 da Silva, M.M., Nora, L., Cantillano, R.F.F. et al. (2016). The production, characterization, and the stability of carotenoids loaded in lipid‐core nanocapsules. Food and Bioprocess Technology 1–11.
- 62 Mohammadi, A., Jafari, S.M., Assadpour, E., and Esfanjani, A.F. (2016). Nano‐encapsulation of olive leaf phenolic compounds through WPC–pectin complexes and evaluating their release rate. International Journal of Biological Macromolecules 82: 816–822.
- 63 Tan, C., Xie, J., Zhang, X. et al. (2016). Polysaccharide‐based nanoparticles by chitosan and gum arabic polyelectrolyte complexation as carriers for curcumin. Food Hydrocolloids 57: 236–245.
- 64 Fan, Y., Yi, J., Zhang, Y. et al. (2017). Physicochemical stability and in vitro bioaccessibility of β‐carotene nanoemulsions stabilized with whey protein‐dextran conjugates. Food Hydrocolloids 63: 256–264.
- 65 Chang, C., Wang, T., Hu, Q., and Luo, Y. (2017). Caseinate‐zein‐polysaccharide complex nanoparticles as potential oral delivery vehicles for curcumin: effect of polysaccharide type and chemical cross‐linking. Food Hydrocolloids 72: 254–262.
- 66 Taheri, A. and Razavi, S.M. (2015). Fabrication of cress seed gum nanoparticles, an anionic polysaccharide, using Desolvation technique: an optimization study. Bio Nano Science 5: 104–116.
- 67 Naji‐Tabasi, S., Razavi, S.M.A., and Mehditabar, H. (2017). Fabrication of basil seed gum nanoparticles as a novel oral delivery system of glutathione. Carbohydrate Polymers 157: 1703–1713.
- 68 Akbari, I., Ghoreishi, S.M., and Habibi, N. (2015). Supercritical CO2 generation of nanometric structure from Ocimum basilicum mucilage prepared for pharmaceutical applications. AAPS PharmSciTech 16: 428–434.
- 69 Paula, H.C., Oliveira, E.F., Carneiro, M.J., and de Paula, R.C. (2017). Matrix effect on the spray drying nanoencapsulation of Lippia sidoides essential oil in chitosan‐native gum blends. Planta Medica 83: 392–397.
- 70 Paula, H.C., Rodrigues, M.L., Ribeiro, W.L. et al. (2012). Protective effect of cashew gum nanoparticles on natural larvicide from Moringa oleifera seeds. Journal of Applied Polymer Science 124: 1778–1784.
- 71 Khoshakhlagh, K., Koocheki, A., Mohebbi, M., and Allafchian, A. (2017). Development and characterization of electrosprayed Alyssum homolocarpum seed gum nanoparticles for encapsulation of d‐limonene. Journal of Colloid and Interface Science 490: 562–575.
- 72 de Campo, C., dos Santos, P.P., Costa, T.M.H. et al. (2017). Nanoencapsulation of chia seed oil with chia mucilage (Salvia hispanica L.) as wall material: characterization and stability evaluation. Food Chemistry 234: 1–9.
- 73 Harika, B., Gowthamarajan, K., Chamundeeswari, D. et al. (2016). Fabrication and in‐vitro evaluation of cefixime nanoparticles using gum kondagogu and chitosan as matrix formers. International Journal of Pharmaceutical Sciences and Research 7: 1116.
- 74 Deng, W., Fu, M., Cao, Y. et al. (2013). Angelica sinensis polysaccharide nanoparticles as novel non‐viral carriers for gene delivery to mesenchymal stem cells. Nanomedicine: Nanotechnology, Biology and Medicine 9: 1181–1191.
- 75 Taheri, A. and Razavi, S.M. (2015). The conformational transitions in organic solution on the cress seed gum nanoparticles production. International Journal of Biological Macromolecules 80: 424–430.
- 76 Patel, D., Prajapati, D., and Patel, N. (2007). Seed mucilage from Ocimum americanum Linn. As disintegrant in tablets: separation and evaluation. Indian Journal of Pharmaceutical Sciences 69: 431.
- 77 Khazaei, N., Esmaiili, M., Djomeh, Z.E. et al. (2014). Characterization of new biodegradable edible film made from basil seed (Ocimum basilicum L.) gum. Carbohydrate Polymers 102: 199–206.
- 78 Bhosale, A., Hardikar, S., Pathak, A., and Sable, R. (2009). Investigation of hydrogel isolated from seeds of Ocimum basilicum as binder. Indian Journal of Pharmaceutical Sciences 71: 320.
- 79 Kadam, P.V., Yadav, K.N., Jagdale, S.K. et al. (2012). Evaluation of Ocimum sanctum and Ocimum basillicum mucilage‐as a pharmaceutical excipient. Journal of Chemical and Pharmaceutical Research 4: 1950–1955.
- 80 Imam, H., Lian, S., Kasimu, R. et al. (2012). Extraction of an antidiabetic polysaccharide from seeds of Ocimum basilicum and determination of the monosaccharide composition by precolumn high‐efficiency capillary electrophoresis a. Chemistry of Natural Compounds 1–2.
- 81 Prakash, J. and Gupta, S. (2000). Chemopreventive activity of Ocimum sanctum seed oil. Journal of Ethnopharmacology 72: 29–34.
- 82 Sudam, N., Manish, B., Ritesh, M. et al. (2012). Evaluation of various natural suspending agents for its suspending behaviour using paracetamol as model drug for suspension. Asian Journal of Pharmaceutical & Clinical Research 5.
- 83 Saeedi, M., Morteza‐Semnani, K., Akbari, J. et al. (2015). Evaluation of Ocimum basilicum L. seed mucilage as rate controlling matrix for sustained release of propranolol HCl. Pharmaceutical and Biomedical Research 1: 18–25.
- 84 Akbari, I., Ghoreishi, S., and Habibi, N. (2014). Generation and precipitation of paclitaxel nanoparticles in basil seed mucilage via combination of supercritical gas antisolvent and phase inversion techniques. The Journal of Supercritical Fluids 94: 182–188.
- 85 Zampa, M.F., de Brito, A.C.F., Kitagawa, I.L. et al. (2007). Natural gum‐assisted phthalocyanine immobilization in electroactive nanocomposites: physicochemical characterization and sensing applications. Biomacromolecules 8: 3408–3413.
- 86 Oliveira, M.A., Silva, D.A., Uchoa, D.E. et al. (2007). Synthesis and characterization of carboxymethylated red Angico (Anadenanthera macrocarpa) exudate polysaccharide. Journal of Applied Polymer Science 103: 2985–2991.
- 87 Hesarinejad, M.A., Razavi, S.M., and Koocheki, A. (2015). Alyssum homolocarpum seed gum: dilute solution and some physicochemical properties. International Journal of Biological Macromolecules 81: 418–426.
- 88 Timilsena, Y.P., Wang, B., Adhikari, R., and Adhikari, B. (2016). Preparation and characterization of chia seed protein isolate–chia seed gum complex coacervates. Food Hydrocolloids 52: 554–563.
- 89 de la Paz Salgado‐Cruz, M., Calderón‐Domínguez, G., Chanona‐Pérez, J. et al. (2013). Chia (Salvia hispanica L.) seed mucilage release characterisation. A microstructural and image analysis study. Industrial Crops and Products 51: 453–462.
- 90 Kora, A.J., Sashidhar, R., and Arunachalam, J. (2010). Gum kondagogu (Cochlospermum gossypium): a template for the green synthesis and stabilization of silver nanoparticles with antibacterial application. Carbohydrate Polymers 82: 670–679.
- 91 Vinod, V. and Sashidhar, R. (2009). Solution and conformational properties of gum kondagogu (Cochlospermum gossypium)–a natural product with immense potential as a food additive. Food Chemistry 116: 686–692.
- 92 Vinod, V., Sashidhar, R., Sarma, V., and Vijaya Saradhi, U. (2008). Compositional analysis and rheological properties of gum kondagogu (Cochlospermum gossypium): a tree gum from India. Journal of Agricultural and Food Chemistry 56: 2199–2207.
