Chapter 22
Introduction to Fire Protection
Abstract
Students are provided with an overview of fire, what sustains a fire, and the four major sources of ignition. Heat transfer and heat sources are also topics of discussion in this chapter. Finally, the subjects of extinguishing fires and the various systems used to douse a fire are covered.
Keywords
Carbon dioxide; Combustible; Conduction; Convection; Deluge system; Dry chemical; Dry pipe; Flammable; Flammability range; Flash point; Foam; Halon; Ignition point; Radiation; Standpipe; Wet-PipeFire protection in the United States has developed only as a result of the loss of human life and property. Unfortunately, development continues only because loss of life and property continues.
On October 9, 1871, the most famous fire in the United States began when, as legend has it, Mrs O’Leary’s cow kicked over a kerosene lamp. The fire destroyed much of Chicago. Fire Prevention Week is now during the week of October 9th each year in memory of this disaster.
As a result of another fire on December 30, 1909, at the Iroquois theater in Chicago, which killed 602 people, improvements were mandated in the construction of fire protection for theaters. On May 4, 1908, in Collinwood, Ohio, 175 persons (mostly schoolchildren) were killed in a fire at the Lakeview Grammar School. Because of this fire, school fire drills were established.
Four hundred ninety-two persons, mostly serviceman, were killed when a fire swept through the Coconut Grove nightclub in Boston, Massachusetts, on November 28, 1942. As a result of the Coconut Grove fire, regulations improving exits and installation of emergency lighting equipment were made law. In 1958, a total of 95 people died in Chicago’s Our Lady of Angels elementary school. Because of this fire, schools are now inspected.
In May 1976, more than 175 people died at the Beverly Hills Supper Club near Covington, Kentucky, when fire engulfed the nightclub. In January 1981, more than 75 people died at the MGM Grand Hotel in Las Vegas, Nevada, as a result of a fire. Unfortunately, progress in the development of fire protection in the United States seems to occur only after people are killed in fires.
Fire estimates
Additional information on the U.S. fire problem is available from the National Fire Protection Association (NFPA)’s website.
NFPA definition of structure
A structure is an assembly of materials forming a construction for occupancy or use in such a manner as to serve a specific purpose. A building is a form of structure. Open platforms, bridges, roof assemblies over open storage or process areas, tents, air supported, and grandstands are other forms of structures.
| Total Fire Loss (2012) | |||
| All Fires | Deaths | Injuries | Direct Dollar Loss in Millions |
| 1,375,000 | 2,855 | 16,500 | $12,427 |
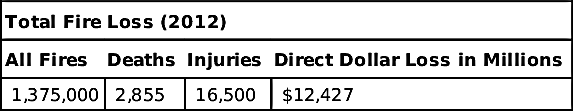
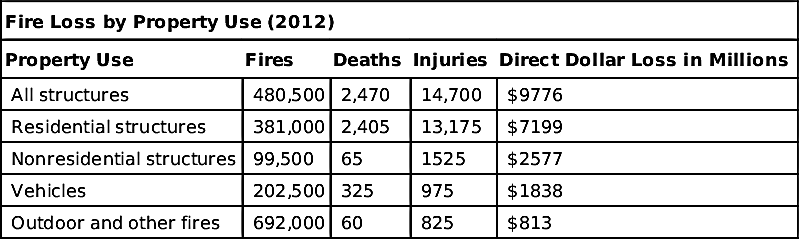
| Fire Loss by Property Use (2012) | ||||
| Property Use | Fires | Deaths | Injuries | Direct Dollar Loss in Millions |
| All structures | 480,500 | 2,470 | 14,700 | $9776 |
| Residential structures | 381,000 | 2,405 | 13,175 | $7199 |
| Nonresidential structures | 99,500 | 65 | 1525 | $2577 |
| Vehicles | 202,500 | 325 | 975 | $1838 |
| Outdoor and other fires | 692,000 | 60 | 825 | $813 |
Fire departments are more advanced today in attempting to save lives and property from fire. The objectives of most fire departments in the U.S. are to:
1. Prevent fires from starting.
2. Prevent loss of life and property when fire starts.
3. Confine fire to the place where it started.
4. Extinguish fires.
More than 4,000 persons die each in year in the United States as a result of fire. Most deaths are the result of breathing smoke or toxic gases. Usually, the victim is a child or elderly person who may become confused and panic in a fire. Teenagers make up the lowest percentage of deaths. The United States has more direct property loss than any other country as a result of fires (Figure 22.1).
A study was conducted of 20,000 industrial fires. Of these 20,000 fires, 16,000 occurred while the plant or facility was operating. Nearly one-half (50%) of the facilities that had caught fire and were heavily damaged were never rebuilt. Out of the nearly 50% which were rebuilt (10,000), nearly one-third of these (some 3300) were bankrupt in just three years. Therefore, by looking at the results of this study, you can see why it is so important to prevent fires from starting. The chances are about 50–50 that if the plant or building where you currently work burns to the ground, you and all of your co-workers will be out of a job.
| Estimated U.S. Building Fire Causes | ||
| Percent of Fires | Percent of Dollar Loss | |
| Heating and cooking | 16 | 8 |
| Smoking and matches | 12 | 4 |
| Electrical | 16 | 12 |
| Rubbish, ignition source unknown | 3 | 1 |
| Flammable liquid fires and explosion | 7 | 3 |
| Open flames and spark | 7 | 4 |
| Lighting | 2 | 2 |
| Children and matches | 7 | 3 |
| Exposures | 2 | 2 |
| Incendiary, suspicious | 7 | 10 |
| Spontaneous ignition | 2 | 1 |
| Miscellaneous known | 2 | 6 |
| Unknown | 17 | 44 |

Bugbee P. Principles of fire protection. Boston: NFPA; 1978. p. 25.
What is fire?
Fire is unpredictable. It is a rapid, self-sustaining oxidation process accompanied by the evolution of heat and light of varying intensities. In other words, fire spreads quickly and, as long as there is enough air filled with oxygen (O2), fire will continue to burn. During the time it burns, the fire will give off heat and light while the temperature of the fire increases and decreases. Four things are needed for a fire to continue to burn. These four things create the fire triangle (Figure 22.2):
1. Fuel
2. Heat or source of ignition
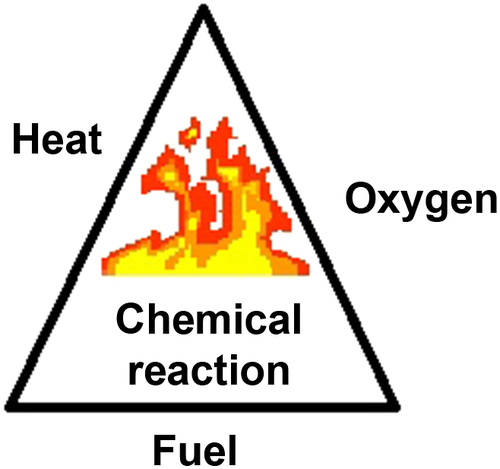
3. Oxygen (21% of air is oxygen)
4. Reaction time of oxidation or the chemical chain reaction of fire.
A fire will continue to burn until:
1. The combustible material (fuel) is either totally consumed or removed.
2. The level of oxygen in the air is reduced enough to stop the fire from burning.
3. The combustible material is cooled below its ignition point.
4. The flames are chemically inhibited.
Let us discuss those points in greater detail.
1. Fire will continue to burn until the material that is burning is either completely burned up or until it is removed from the fire. A log burning in a fireplace is a good example: Once a fire has started in a fireplace, it will continue to burn as long as there is wood to burn. If the wood is used up or taken from the fireplace, the fire will stop.
2. Fire will continue to burn until the oxygen (O2) is lowered to a level where the fire cannot burn. A good example of this is when a pan with grease on the top of a stove catches fire. When you put the lid on top of the pan, the fire is extinguished because there is no air with oxygen for the fire. Fire needs oxygen in the same way a person needs oxygen to breath. If there is no air, no oxygen, a person will suffocate. If a fire is denied oxygen, it will also suffocate and no longer burn.
3. Fire will continue to burn until the material on fire (fuel) is cooled so much that the fire stops. Can you think of an example of how a material is cooled below its ignition temperature? You are right if you thought of water! Water can be placed on many fires, which will cool the material to the point where the fire will stop. (Note: water is not used on all fires.)
4. Fire will continue to burn until the flames are chemically inhibited. This means that the fire will stop when the chain reaction that occurs between the heat, fuel, and oxygen is stopped. Remember that! It will be discussed in greater detail later.
Heat transfer
When an object is on fire, heat is transferred from that object to other objects that are not on fire. Any type of heat transfer occurs in one of three ways:
1. Conduction
2. Radiation
3. Convection
Conduction occurs when two objects are physically touching one another. If two objects are touching and one is burning, the second will become hotter and hotter, and many times will eventually begin to burn. A good example of conduction heat transfer is when a piece of paper is laid on top of a hot pipe or stove. If the pipe or stove are hot enough (usually about 350 °F), the paper will begin to burn.
Radiation is when heat travels in space. The degree of heat transfer depends on the size of the objects involved and their distance from one another. Heat from the sun is an excellent example of radiation heat transfer.
Convection is when heat is transferred by circulation of gas. Hot gases, vapors, and liquids rise and the temperature of the material is gradually increased. Think of a “force fan” gas furnace. Gas vapors are ignited and air is heated in the chamber of a furnace until a fan “forces” the heated air from the chamber out into a room.
Heat sources
There are four major sources of ignition or heat energy:
1. Chemical
2. Electrical
3. Mechanical
4. Nuclear
Chemical
Fire is basically a form of chemical reaction—a chemical reaction process known as oxidation. Oxidation is a process that usually produces heat. Because air is the primary source of oxygen, the amount or flow of air will directly affect the rate of burn. Occasionally, an organic substance such as hay may ignite spontaneously. This occurs because the hay will give off heat due to the natural process of oxidation. Usually, when heat is given off from organic matter, the rate at which the heat is released is so slow and the area around the matter so large, the heat does not build up. Should a build-up of heat occur, such as in wet hay in a barn loft, spontaneous ignition may result. When this occurs, it is an example of a chemical form of ignition.
Electrical
Energy that is required to move electrical current through a substance will form heat. When electrical current flows through a wire or another conductor, such as copper or silver, the resistance is low and not much heat will be produced [1]. Potential fire hazards can exist because of electrical heat energy, such as:
1. Resistance
2. Arcing
3. Sparking
4. Static
5. Lightning
Resistance is caused by overloading electrical conductors. A common cause of fires is overloading electrical circuits by plugging in too many lights and appliances.
Arcing occurs when a good electrical connection is not made in a switch or fuse blank. The electrical energy will jump or arc across the space.
Sparking is different from arcing in that it is continuous.
Static occurs when an electrical charge exists on the surfaces of two materials that have been brought together and then separated. If the materials are not grounded, the two surfaces will eventually emit a static spark.
Lightning is another form of electrical energy. A properly installed lightning rod will provide proper protection from lightning.
Mechanical
Energy that is generated when two objects are rubbed together is called mechanical. The friction transforms the energy into heat. Friction is the cause of many fires in industry.
Nuclear
Heat energy that is released from the nucleus of an atom is nuclear. Nuclear energy is used primarily in the generation of electricity.
Flammable and combustible liquids
Material burns because vapors are heated to the point where burning begins and continues. The lowest temperature at which enough vapor will be given off to form a flammable mixture with air is known as the material’s flash point. These vapors form near the surface of the material; with the proper mixture of air, ignition will occur provided there is an ignition source. Consider lighter fluid. If a match is placed near a charcoal briquette that has been soaked in lighter fluid, ignition will normally occur, even if the match never actually touches the charcoal. If the match is not close enough to the briquette to ignite the vapors, ignition will not occur.
Another important term to be familiar with is ignition point. The lowest temperature at which a material must be heated in order to start self-sustained combustion or burning is known as ignition point or ignition temperature. The ignition point of most wood is approximately 350 °F. This means that if a small amount of wood were placed near a hot pipe, once the temperature of the wood reaches 350 °F, it would ignite automatically without another source of flame.
Flammability limit is another term that is important to know when discussing fire. Flammability limit describes the minimal level of mixture of air to vapors below which a flame will not burn. A mixture of air and vapors below the flammability limit means that the mixture is too lean and therefore will not burn. Consider how a carburetor works in a car. The flammability limits of gasoline are 1.4–7.5%. These percentages mean that between 1.4% of gasoline vapors mixed with air and 7.5% of vapors mixed with air, gasoline will ignite. For a carburetor, the normal mixture of gasoline vapors with air is 5%. If too much gas is in the carburetor (above 5%), the gasoline will not ignite and the carburetor is considered “flooded”. If a person will wait until some of the gasoline evaporates, the carburetor will eventually rid itself of the excess vapors and will normally start. Examples of flash point and ignition temperatures of gasoline and kerosene are as follows:
| Flash Point | Ignition Temperature | |
| Gasoline | −45 °F | 536 °F |
| Kerosene | 100 °F | 410 °F |
In the above chart, flash points are more critical. For gasoline, provided the right mixture of air to gasoline vapors exist and provided the temperature of the air directly above the gasoline liquid is above –45 °F, the gasoline will ignite. In most of the United States during most of the year, the temperature will normally be higher than –45 °F. That is why gasoline is so versatile in vehicles. If, however, you happen to be in northern Canada in the middle of February, you may find the temperature of the air is colder than –45 °F. If that is the case, sufficient gas vapors will not be given off and the vehicle will not start. Kerosene, on the other hand, would not give off enough vapors to ignite from a flame unless the temperature of the air immediately above the surface of the liquid is at least 100 °F. That is why kerosene is safer for use in homes than gasoline. With kerosene, a flame source must be placed immediately above the kerosene until the temperature of the kerosene at the source of the flame is 100 °F. Gasoline, on the other hand, would not have to be heated to such a high temperature and therefore ignites and explodes much easier than kerosene.
Another important term is fire loading. Fire loading describes what occurs when too many combustibles or flammables are put into one area. One 55-gallon drum of kerosene may be proper storage. Six 55-gallon drums of kerosene may be considered excessive, thereby creating an unsafe fire loading problem.
The Factory Mutual Insurance Company gives seven suggestions for properly controlling flammables:
1. Segregate the hazard by distance (do not put too much in the same area).
2. Confine or enclose the hazard by using proper containers.
3. Ventilate to prevent explosive mixtures.
4. Install explosion venting where needed.
5. Eliminate sources of ignition.
6. Educate those involved as to the hazards and proper safeguards.
7. Provide adequate fire protection.
Extinguishing fires
Water is the most commonly used substance in fire protection (Figure 22.3). Water is used in fire hoses and sprinkler systems. At ordinary temperatures, water is relatively stable. A fire will be put out only when water is felt at the source of the fire or combustion. Once the ignition temperature of the material is cooled by the water to a point where flame cannot continue, the fire will be extinguished. Water has a great cooling effect. When water is sprayed onto a fire, steam results, which indicates the fire is cooling. Steam is important in extinguishing a fire. When water is converted into steam, the volume of water increases by approximately 1700 times. Large volumes of steam displace an equal volume of air; in a fire situation, the steam causes oxygen to be reduced. When oxygen is reduced, the fire will also be reduced.
Water is used primarily in sprinkler systems, which were first designed in 1878. Sprinklers are generally considered to be 95% successful at extinguishing fire. The 5% failure rate is primarily the result of human error. A sprinkler system cannot work if a valve that controls the water flow is closed. If a sprinkler system is properly maintained, it will normally work as it was intended. Most sprinkler systems are of the “wet-pipe” type. In a wet-pipe system, water is maintained in the sprinkler system at all times under pressure. Usually, sprinkler heads are closed with a small amount of solder. The solder is designed to melt at a temperature of between 135 and 500 °F, depending on the type of sprinkler head. Most sprinkler heads are designed to open at 165 °F (the temperature at which the solder melts.)

Dry-pipe sprinkler systems
A dry-pipe sprinkler system provides protection from freezing because water is not contained in the sprinkler piping. Instead, a moderate amount of air is pressurized in the sprinkler pipes. When the solder to the sprinkler head melts, the air is released. Water (which is stored behind a valve) rushes through the pipes to the open head, where it then discharges. The dry-pipe system is especially useful in those areas where freezing occurs.
Deluge system
For areas that need extra fire protection, a deluge system is often used. These systems are normally controlled by heat/temperature detectors. When a detector senses heat, it signals an alarm that automatically releases the water. Deluge systems have open sprinkler heads at all times. There is no solder that keeps the head closed. As the name implies, a deluge system is designed to literally deluge or flood an area with a great amount of water in a short period of time. Deluge systems are used for high hazard areas, such as flammable liquid dispensing areas.
Standpipes
In order to provide a readily available means to manually fight a fire, many buildings contain standpipes. Standpipes are classified by the size of their hose connections. A Class I system is a 2½ in. hose, which allows fire departments to provide a great amount of water to a fire. A Class II system is a 1½ in. hose, which is designed to be used by building occupants until the fire department arrives. Those hoses are lightweight, woven jacket, and rubber lined. Class III systems have connections for both 2½ and 1½ in. hoses. Water for the standpipes is usually provided by city water mains, pressure tanks, automatic fire pumps, or gravity tanks.
Foam extinguishing systems
Foam extinguishing systems have been used for many years, particularly in the petroleum-chemical industry. Foam breaks down and vaporizes its water content under attack by heat and flame. When other fire extinguishing agents are used with foam, the foam may become ineffective. This is especially true if water is used in conjunction with foam. One of the most common methods of using foam is by a fire department truck hose line nozzle.
Carbon dioxide systems
Carbon dioxide (CO2) is a noncombustible gas that for many years was used to extinguish certain types of fires. Carbon dioxide reduces oxygen in a fire to a point where it will no longer support the fire. Carbon dioxide will not conduct electricity. It is heavier than air and, when released from a cylinder, it is about −110 °F, which turns air and water into dry ice. Carbon dioxide can be discharged onto the surface of a burning material by fixed piping or by hand extinguishers. Alternatively, the area can be flooded with the gas until the entire atmosphere in the room is converted to carbon dioxide. Because carbon dioxide removes oxygen in the air, care must be taken when people may be exposed to the gas. A concentration of about 9% carbon dioxide can cause a person to lose consciousness in a short time.
Halon systems
Halon is a material made of hydrogen and carbon. The number of halon, such as 1211 or 1301, was developed by the U.S. Army Corps of Engineers and indicates the chemical composition of the material. Halon 1211 and 1301 are the only two agents recognized by the NFPA as fire extinguishing halons. Both are widely used in the protection of electrical equipment, airline engines, and computer rooms. Because both Halon 1211 and 1301 rapidly vaporize in fire, they leave little residue to clean up. How Halon works is not fully understood, but there is some chemical reaction because the agents are very effective in extinguishing fire. Halon 1211 is used in fire extinguishers and is more toxic than Halon 1301, which is used to protect computer rooms. The low toxicity of Halon 1301 allows it to be discharged safely from total flooding systems where people are located. Contrary to some belief, Halon 1301 does not remove oxygen from the air, as in the case of carbon dioxide. Halon is no longer manufactured in most countries, but can still be found in some electrical switch gear rooms and in computer data centers [2].
Dry chemical extinguishing systems
Dry chemical extinguishing agents consist of fine powders that effectively smother a fire. Dry chemicals have been found to be effective extinguishing agents for fires in flammable liquids and electrical equipment. Dry chemicals are stable both at low and high temperatures. The ingredients in dry chemicals are nontoxic. It is believed that the discharge of dry chemicals into flames breaks up the combustion reaction. Dry chemical systems are not recommended for telephone switchboards or computer protection because of their powdery residue.
Portable fire extinguishers
Portable fire extinguishers are considered to be a first line of defense in extinguishing fires of limited size. Fire extinguishers can be used with little formal or advanced training, but many organizations conduct training exercises for employees so that they are comfortable in using the devices in a stressful situation.
Class A Extinguishers are used on fires in ordinary combustible materials such as water, cloth, paper, etc. They are typically water-based fire extinguishers. Class B Extinguishers are used on flammable liquid fires, such as petroleum or oil-based products. Class C Extinguishers are usually carbon dioxide extinguishers used on electrical equipment fires.
Summary
• Fire protection in the United States has developed only because of the loss of human life and property. National Fire Prevention Week is celebrated each October in the United States to remember the Great Chicago Fire of 1871. Countless other tragedies have occurred in the United States. As a result, fire regulations have been strengthened and fire protection has improved after each disaster.
• Most fire departments attempt to keep fires from starting, prevent loss of life and property when a fire starts, confine fire to the place where it started, and extinguish fire. Nearly 8000 people die each year in the United States from fires. Most of these are deaths of children and elderly persons caused by smoke inhalation.
• Fire is unpredictable, rapid, and self-sustaining. Fire burns because of fuel, heat, oxygen, and a chemical chain reaction that occurs during burning. A fire will continue to burn until one of the four is removed.
• Heat is transferred by conduction, radiation, and convection. The four major sources of ignition are chemical, electrical, mechanical, and nuclear.
• Material burns because vapors are heated to the point where burning begins. The lowest temperature at which enough vapor is given off to form a flammable mixture with air is the material’s flash point. The lowest temperature to which a material must be heated to start burning is ignition point or ignition temperature.
• Water is the most commonly used substance in fire protection. Water is primarily used in sprinkler systems, which can be either wet or dry systems, deluge systems, or standpipes. Foam is used to extinguish fires involving petroleum or chemicals. Carbon dioxide is a noncombustible gas that is used to extinguish certain types of fire. Halon 1211, Halon 1301, and dry chemical powders are also effective fire extinguishing agents. Halon is used to protect computer rooms and aircraft.
Exercises
1. Name the objectives of most fire departments.
2. What four elements are needed for fire to continue to burn?
3. How is heat transferred?
4. What are the major sources of ignition?
5. What are the seven suggestions of the Factory Mutual Insurance Company for controlling flammables?
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.

