35
Breast Shear Wave Elastography
Azra Alizad
Department of Radiology, Mayo Clinic, Rochester, MN, USA
35.1 Introduction
Emerging ultrasound elasticity imaging techniques have been of great interest as an adjunct to breast sonography to improve its sensitivity and specificity for diagnosis of breast cancer. Among elasticity imaging methods, shear wave elastography has gained much attention in recent years as a new imaging modality for differentiation of breast masses. This chapter focuses on applications of shear wave elastography techniques, more specifically comb‐push ultrasound shear elastography (CUSE) in breast imaging. In addition, we will present the results of in vivo results of breast CUSE. Future developments and potential impact of CUSE in breast imaging are also discussed.
35.2 Background
Today, breast cancer remains a major health problem among women around the world. Breast cancer is the leading cause of cancer death in women aged 20 to 59 years and second to lung cancer in women 60 and older. According to the American Cancer Society in 2015, over 231 000 new cases of invasive breast cancer are expected to occur in one year alone, accounting for 29% of all new cancer cases among American women. There is a probability that 1 in 8 women are at risk of developing invasive breast cancer in their lifetime [1]. This data is shown in Table 35.1.
Table 35.1 Estimated new cases and deaths in American women.
| Estimated new cases in 2015 | 231 670 |
| % of all new cancers among American women | 29% |
| Estimated deaths in 2015 | 40 290 |
| % of all cancer deaths among American women | 15% |
Manual breast examination is the oldest, simplest, yet effective, method for detection of breast masses, because the stiffness of breast tumors is significantly different from surrounding tissue. However, it has limited value in detecting deep and small masses.
Current imaging methods, e.g., mammography and ultrasound, carry low specificity rates that lead to a great number of unnecessary (i.e. benign) biopsies. According to the American College of Radiology Imaging Network (ACRIN), in women with dense breasts, using combined whole‐breast screening, ultrasound (US), and mammography yielded a sensitivity of 77.5% versus 50% for mammography or ultrasound alone. However, the increased sensitivity in combined use of US and mammography is associated with a substantial increase in the number of false‐positive cases [2]. Thus, due to low specificity of current imaging modalities, women encounter a high number of unnecessary follow‐up examinations and procedures. Only 20% to 40% of breast biopsies are cancerous [3, 4]. Thus, the rate of false‐positive cases seems to be common with current imaging tools. Consequently, the number of unnecessary benign breast biopsies that are performed annually in the US approaches 1 million cases, with an associated cost of an excision breast biopsy of $2400 per case [5]. Thus, the cost of potentially unwarranted biopsies is around $2 billion annually, which contributes substantially to US healthcare spending [5, 6]. Furthermore, women who undergo biopsy for benign disease experience great emotional distress and morbidity [7].
The efficacy of mammography is also greatly reduced in dense breasts [8, 9]. This is particularly important in the case of high‐ and moderate‐risk women who need to be screened at a younger age [8, 9]. Even though magnetic resonance imaging (MRI) has high sensitivity, the poor specificity results in unnecessary follow‐ups and biopsies that are associated with high extra cost and great emotional distress. Moreover, the limited availability and substantial cost of MRI limit the use of MRI in breast cancer detection [10–12].
To overcome the limitations of current breast imaging modalities, new noninvasive tools for breast imaging with high sensitivity and specificity are being developed. In the last 25 years or so, various ultrasound‐based techniques [13–19] or MRI‐based methodologies [20–25] have been developed to characterize the mechanical response of tissues under external stress.
More research is in progress in the development of ultrasound‐ or MRI‐based elasticity technologies, especially those that provide palpation‐like information, for example, information about tissue stiffness. In palpation, breast masses differ from surrounding tissue [26]. In fact, breast tumors are often stiffer than normal tissue [27] and malignant breast lesions are harder than benign lesions [28, 29]. Hence, new techniques are being developed to noninvasively evaluate the pathology of a tissue based on its mechanical properties [30].
35.3 Breast Elastography Techniques
Elasticity imaging is an emerging field that can noninvasively evaluate the tissue elasticity. From this group, magnetic resonance elastography (MRE) was introduced to differentiate pathological breast tissues from normal and increase diagnostic specificity [23, 31–34]. However, MRE, a MRI‐based technology, is expensive and less likely to be available for a wide range of the patient population.
Ultrasound‐based elasticity imaging is an emerging field and has been of great interest as an adjunct to breast sonography to improve its sensitivity and specificity for the diagnosis of breast cancer. Various elastography methods are available on many ultrasound systems, with most of them based on some form of tissue deformation (i.e. strain). This can be done either by manual pressure and release, or by taking advantage of normal cardiac or respiratory motion. Conventional quasi‐static elastography is one such technique, which calculates the relative deformation of the tissue or strain. Studies have showed it can increase the specificity of conventional B‐mode US in the detection of breast cancer [30]. Quasi‐static elastography utilizes compression and US‐based strain imaging to obtain maps of relative stiffness [35–39]. However, in most cases, it is a qualitative imaging technology so the results differ with users' experience in data acquisition and interpretation, and their operator dependency may hinder their clinical effectiveness [40]. The newly emerging shear wave elastography (SWE) techniques are the alternative, which use acoustic radiation force to induce shear waves and estimate tissue elasticity from measured shear wave speed. Shear waves travel faster in stiffer tissues and slower in softer tissues; therefore, shear wave elastography could be an optimal technique to be used for differentiation of breast masses [41].
35.3.1 Shear Wave Elasticity Imaging (SWEI)
Shear wave elasticity imaging (SWEI) is the first shear wave elastography method, and was introduced by Sarvazyan et al. [42]. The 1D or 2D transient elastography technique was first performed by using an external vibrator. This technique uses an ultrafast ultrasound acquisition imaging system (5000 frames/s) that allows a real‐time in vivo imaging of transient shear waves propagating in human tissues. The transient elastography technique has been tested to differentiate breast masses [19]. The use of large external vibrators has limited the clinical application of transient elastography. To overcome this limitation, efforts have been made to advance shear wave elastography techniques.
35.3.2 Supersonic Shear Imaging (SSI)
The ultrafast SWE system from Supersonic Imagine performs supersonic shear imaging (SSI), uses an acoustic radiation force, and through a focused ultrasound beam, remotely induces mechanical vibrations in breast tissue. SSI uses a conventional transducer and produces a supersonic regime of moving sources to generate shear waves, and by using ultrafast plane wave imaging captures the propagation of resulting shear waves and quantifies tissue elasticity from measured shear wave speed [41, 43, 44]. Promising results in studies with SSI for differentiation of breast masses have been reported [45–52]. Here, we present a SSI shear wave map and US image of a woman in her 80s who had abnormal screening mammography. Targeted ultrasound of the right breast demonstrates a 0.9 × 0.9 × 0.6 cm hypoechoic mass, which corresponds to the mammographic abnormality as shown in Figure 35.1. A shear wave map using SSI showed a high value of mean elasticity, E mean of 111.8 kPa. The result of pathology confirmed the malignant breast lesion as an invasive mammary carcinoma with mixed ductal and lobular features, grade I.
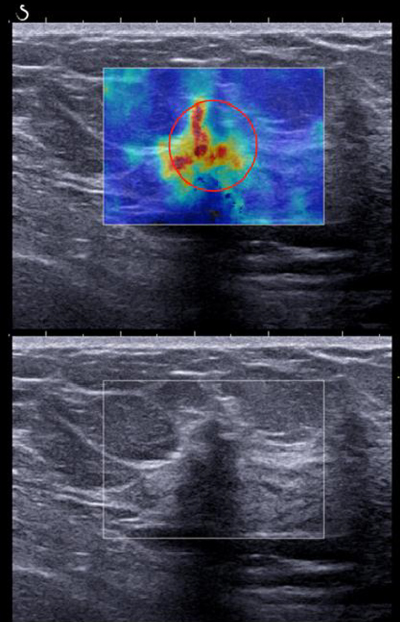
Figure 35.1 SSI and ultrasound images of a patient with invasive mammary carcinoma. The shear wave speed is significantly higher in the lesion.
35.3.3 Virtual Touch Tissue Quantification using Acoustic Radiation Force Impulse
Shear wave elastography using acoustic radiation force impulse (ARFI) has also been used for differentiation of breast masses [53–55]. The ARFI shear wave elastography technique is based on generating an impulse‐focused radiation force to induce shear waves in tissue and then use pulse‐echo ultrasound to detect such waves. Then, the speed of shear waves estimated at each location is used to calculate the stiffness of the tissue [56].
35.3.4 Comb‐push Ultrasound Shear Elastography (CUSE)
Recently, Song et al. [57, 58] have developed an US shear wave elastography technique called Comb‐push ultrasound shear elastography (CUSE) that uses multiple simultaneous acoustic radiation force (ARF) beams. CUSE is a fast quantitative US shear wave elasticity imaging methodology that provides a two‐dimensional map of shear wave speed. CUSE uses acoustic radiation force beams that are laterally distributed and simultaneously excite the tissue and generate shear waves [58]. CUSE uses four focused push beams that are spaced laterally for shear wave production and shear waves traveling in both lateral directions fill the entire field of view (FOV). A directional filter is used to separate the shear waves traveling in opposing directions and estimates the elasticity map of the FOV in one single comb‐push acquisition [37, 59].
35.4 Application of CUSE for Breast Cancer Detection
CUSE was conducted on patients with suspicious masses using a fully programmable ultrasound platform, Verasonics V‐1 system (Verasonics Inc., Redmond, WA), using a linear array transducer L7‐4 (Philips Healthcare, Andover, MA).
In the CUSE technique using Verasonics, after excitation of the tissue, the system instantly shifts to plane wave imaging mode in order to track the propagated shear wave. A graphical user interface was developed using Matlab (MathWorks Inc., MA) to process the obtained CUSE data and then reconstruct the shear wave speed map. As a quality control factor, a normalized cross‐correlation coefficient of the shear wave speed map was used [60]. The performance of CUSE using a research ultrasound scanner has been reported with promising results [61, 62].
An example of breast CUSE, as such is presented in Figure 35.2. This case is a patient in her 70s. The targeted ultrasound of her right breast demonstrates an irregular mass measuring 1.4 cm in the longest dimension. The measured mean shear wave speed within the ROI was 2.63 ± 1.59 m/s with a Young's modulus of 20.75 kPa. Breast biopsy results were a benign fibroadenoma.
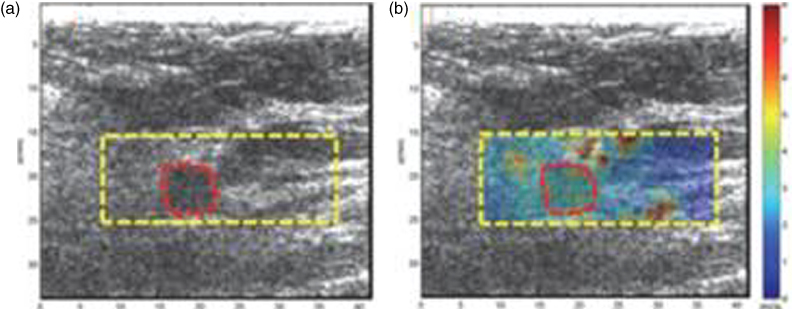
Figure 35.2 US image and CUSE shear wave speed map of breast tissue (dashed rectangle) including the breast mass ROI (dashed circular contour). (a) B‐mode US image, (b) CUSE shear wave speed map. Source: adapted from [61].
CUSE imaging using a Verasonics research US system was conducted for a patient in her 70s with a concerned lump in her right breast. Targeted ultrasound of her right breast shows a hypoechoic mass measuring 2.3 cm in the longest dimension, as shown in Figure 35.3. The measured mean shear wave speed of the ROI was 7.07 ± 1.18 m/s, with a Young's modulus 149.9 kPa. The result of a breast biopsy was malignant grade II invasive ductal carcinoma with ductal carcinoma and calcifications in situ adjacent to it.

Figure 35.3 B‐mode US and CUSE shear wave speed map of a patient's breast (dashed rectangular contour) including the breast mass ROI (red circular contour). (a) B‐mode US image, (b) CUSE shear wave speed map. Source: adapted from [61].
35.5 CUSE on a Clinical Ultrasound Scanner
Recently, CUSE has been implemented in a GE LOGIQ E9 (LE9) (General Electric Healthcare, Wauwatosa, WI) ultrasound scanner [63]. We have studied more than 100 patients with breast masses detectable on ultrasound. Here, we present examples of breast CUSE images using a clinical ultrasound scanner. Figure 35.4 presents US and CUSE images of a patient using a clinical ultrasound scanner. The patient is a woman in her 60s with abnormal mammography screening. Targeted ultrasound of her right breast demonstrated a 1.0 × 0.7 × 0.8 cm hypoechoic mass with a hyperechoic rim and angular margins in the right breast. SWE results showed a high elasticity value, E mean = 114.3 kPa. Pathology results demonstrated invasive lobular carcinoma with alveolar variant features (grade II).
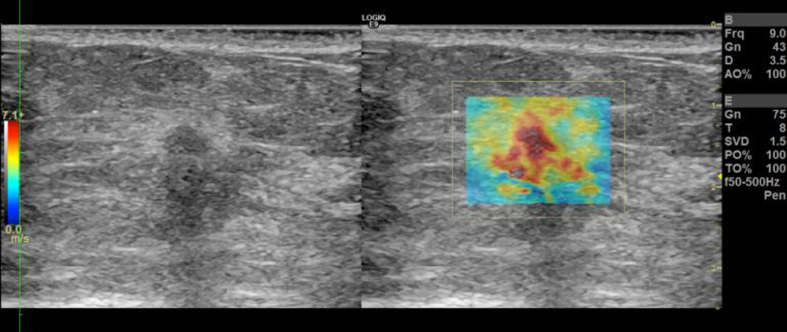
Figure 35.4 B‐mode US and CUSE shear wave speed map of a patient's breast; invasive lobular carcinoma.
CUSE using a clinical US scanner has been performed on a patient in her 40s with an abnormal mammography screening. Targeted US of her right breast showed a hypoechoic complex cystic mass measuring 1.5 × 0.4 × 1.1 cm, as shown in Figure 35.5. SWE showed low elasticity value, E mean = 14.5 kPa. Pathology results of the mass showed clustered apocrine cysts.

Figure 35.5 (a) B‐mode US and (b) CUSE shear wave speed map of a patient's breast; benign clustered apocrine cysts.
An example of breast carcinoma examined by CUSE using a clinical US scanner is shown in Figure 35.6. The patient is a woman in her 70s with a palpable abnormality in her left breast. Targeted US confirmed a 12 × 9 mm oval circumscribed mass, as shown in Figure 35.6. SWE results showed a high elasticity value, with E mean = 132.7 kPa. This mass was later diagnosed by pathology as mucinous carcinoma (grade I).

Figure 35.6 (a) B‐mode US and (b) CUSE shear wave speed map of a patient's breast with a malignant breast mass; mucinous carcinoma (grade I).
Another example of breast cancer evaluated by CUSE imaging using a clinical US scanner is shown in Figure 35.7. The patient is a woman in her 70s with a 1.3 cm asymmetry seen in her screening mammography. Targeted US demonstrated a 1.3 cm irregular region with posterior shadowing, as shown in Figure 35.7. SWE showed a high elasticity value, with E mean = 97.7 kPa. Pathology results classified the mass as invasive mammary carcinoma with mixed ductal and lobular features (grade I), and calcifications present in benign ducts and invasive carcinoma.

Figure 35.7 (a) B‐mode US and (b) CUSE shear wave speed map of a patient's breast with a malignant breast mass; invasive mammary carcinoma.
Figure 35.8 presents ultrasound and CUSE imaging of a patient in her 50s with an asymmetry presented as a 0.7 cm round mass during mammography screening. Targeted ultrasound showed a 0.7 cm round circumscribed mostly anechoic mass. SWE map is E mean = 6.8 kPa. The mass was revealed as a cyst after biopsy.

Figure 35.8 (a) B‐mode US and (b) CUSE shear wave speed map of a patient's breast; benign cyst.
35.6 Limitations of Breast Shear Wave Elastography
There are some limitations in shear wave elastography. Technical factors, such as the amount of precompression used, can negatively alter the shear wave elastography results. However, that can be controlled by minimizing the compression [67].
Despite the promising results on breast shear wave elastography, significant overlap in elasticity between benign and malignant breast masses has been reported [50, 65, 67–72], which limits the discriminating power of elasticity imaging regardless of the accuracy of the measurement method. Usually false‐positive rates are higher than false‐negative rates. Smaller lesion size and increased breast thickness and depth are associated with false‐negative results. Conversely, large lesion size is associated with false‐positive results [73]. Also, the presence of calcifications in benign lesions can induce the apparent high stiffness regions when they are evaluated by SWE methods, and that may lead to false‐positive results [74, 75].
Future developments in shear wave elastography techniques would help to overcome the current limitations.
35.7 Conclusion
Shear wave elastography is a quantitative elasticity imaging technique that has the potential to help in the better differentiation of breast lesions and can be used complementary to conventional ultrasound to improve our certainty in the final assessment of solid breast masses. Studies on using SSI, the most well‐known shear wave elastography, have shown that the addition of SWE to B‐mode US increased the specificity of breast US mass assessment without the cost of decreasing the sensitivity [45, 64]. It has been shown that adding the elasticity ratio (E ratio) improves the diagnostic performance of shear wave in differentiating benign and malignant breast lesions [49, 65].
Gray scale ultrasound may underestimate the size of cancerous tumor when compared to actual size at histology. Shear wave elastography of such tumors shows peritumoural stiffness 3 mm beyond the tumor border seen in an ultrasound image; as such, tumor size estimation is closer to the actual histological size. Therefore, shear wave elastography may have the potential to accurately estimate cancerous tumor size and help with breast‐conserving surgery planning [66]. However, further studies are required to assess the relationship of the extent of SWE stiffness to grayscale US and final histological size of breast cancer.
Acknowledgments
The work described in this chapter was supported by the National Institutes of Health (NIH) grants R01CA148994, R01CA148994‐S1, R01CA168575, R01EB017213, and R01CA174723 from the NIH‐National Cancer Institute. The content is solely the responsibility of the author and does not necessarily represent the official views of NIH. The author is very grateful to the following individuals for their valuable work during the course of the study that presented in as this chapter: Dr. Max Denis, Dr. Mahdi Bayat, Dr. Mohammad Mehrmohammadi, Ms. Adriana Gregory, and Ms. Cynthia Andrist.
The author, AA, discloses that Mayo Clinic holds patents on the CUSE technology, and Mayo Clinic has also received royalties from companies that have licensed the CUSE technology. The author does not have any financial interest in the technology described in this chapter.
References
- 1 Siegel, R.L., Miller, K.D., and Jemal, A. (2015). Cancer statistics, 2015. CA Cancer J. Clin. 65 (1): 5–29.
- 2 Berg, W.A., et al. (2008). Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. J. Am. Med. Assoc. 299 (18): 2151–2163.
- 3 Saslow, D., et al. (2007). American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J. Clin. 57 (2): 75–89.
- 4 Leach, M.O., et al. (2005). Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet 365 (9473): 1769–1778.
- 5 Burkhardt, J.H. and Sunshine, J.H. (1999). Core‐needle and surgical breast biopsy: comparison of three methods of assessing cost 1. Radiology 212 (1): 181–188.
- 6 Chubak, J., et al. (2010). Cost of breast‐related care in the year following false positive screening mammograms. Med. Care 48 (9): 815.
- 7 Rahbar, G., et al. (1999). Benign versus malignant solid breast masses: US differentiation 1. Radiology 213 (3): 889–894.
- 8 Corsetti, V., et al. (2011). Evidence of the effect of adjunct ultrasound screening in women with mammography‐negative dense breasts: Interval breast cancers at 1 year follow‐up. Eur. J. Cancer 47 (7): 1021–1026.
- 9 Zonderland, H.M., et al. (1999). Diagnosis of breast cancer: contribution of US as an adjunct to mammography 1. Radiology 213 (2): 413–422.
- 10 Berg, W.A., et al. (2012). Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. J. Am. Med. Assoc. 307 (13): 1394–1404.
- 11 Orel, S.G. (2000). MR imaging of the breast. Radiol. Clin. N. Am. 38 (4): 899–913.
- 12 Heywang‐Köbrunner, S., et al. (2001). International investigation of breast MRI: results of a multicentre study (11 sites) concerning diagnostic parameters for contrast‐enhanced MRI based on 519 histopathologically correlated lesions. Eur. Radiol. 11 (4): 531–546.
- 13 Ophir, J., et al. (1991). Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason. Imaging 13 (2): 111–134.
- 14 Parker, K.J. and Lerner, R. (1992). Sonoelasticity of organs: shear waves ring a bell. J. Ultrasound Med. 11 (8): 387–392.
- 15 Skovoroda, A., Emelianov, S., and O'Donnell, M. (1995). Tissue elasticity reconstruction based on ultrasonic displacement and strain images. IEEE Trans. Ultrason., Ferroelect., Freq. Control 42 (4): 747–765.
- 16 Levinson, S.F., Shinagawa, M., and Sato, T. (1995). Sonoelastic determination of human skeletal muscle elasticity. J. Biomech. 28 (10): 1145–1154.
- 17 Fatemi, M. and Greenleaf, J.F. (1998). Ultrasound‐stimulated vibro‐acoustic spectrography. Science 280 (5360): 82–85.
- 18 Nightingale, K.R., et al. (2001). On the feasibility of remote palpation using acoustic radiation force. J. Acoust. Soc. Am. 110 (1): 625–634.
- 19 Bercoff, J., et al. (2003). In vivo breast tumor detection using transient elastography. Ultrasound Med. Biol. 29 (10): 1387–1396.
- 20 Muthupillai, R., et al. (1995). Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science 269 (5232): 1854–1857.
- 21 Chenevert, T.L., Skovoroda, A.R., and Emelianov, S.Y. (1998). Elasticity reconstructive imaging by means of stimulated echo MRI. Magn. Reson. Med. 39 (3): 482–490.
- 22 Kruse, S., et al. (2000). Tissue characterization using magnetic resonance elastography: preliminary results. Phys. Med. Biol. 45 (6): 1579.
- 23 Sinkus, R., et al. (2000). High‐resolution tensor MR elastography for breast tumour detection. Phys. Med. Biol. 45 (6): 1649.
- 24 Sinkus, R., et al. (2005). Imaging anisotropic and viscous properties of breast tissue by magnetic resonance‐elastography. Magn. Reson. Med. 53 (2): 372–387.
- 25 Plewes, D.B., et al. (2000). Visualization and quantification of breast cancer biomechanical properties with magnetic resonance elastography. Phys. Med. Biol. 45 (6): 1591.
- 26 McDonald, S., Saslow, D., and Alciati, M.H. (2004). Performance and reporting of clinical breast examination: a review of the literature. CA Cancer J. Clin. 54 (6): 345–361.
- 27 Sarvazyan, A. (2001). Elastic properties of soft tissues. In: Handbook of Elastic Properties of Solids, Liquids and Gases (ed. M. Levy, H.E. Bass, R.R. Stern, A.G. Every, and V. Keppens), vol. 3, 107–127. Academic Press.
- 28 Krouskop, T.A., et al. (1998). Elastic moduli of breast and prostate tissues under compression. Ultrason. Imaging 20 (4): 260–274.
- 29 Sewell, C.W. (1995). Pathology of benign and malignant breast disorders. Radiol. Clin. N. Am. 33 (6): 1067–1080.
- 30 Schaefer, F., et al. (2011). Breast ultrasound elastography – results of 193 breast lesions in a prospective study with histopathologic correlation. Eur. J. Radiol. 77 (3): 450–456.
- 31 Mariappan, Y.K., Glaser, K.J., and Ehman, R.L. (2010). Magnetic resonance elastography: a review. Clin. Anat. 23 (5): 497–511.
- 32 McKnight, A.L., et al. (2002). MR elastography of breast cancer: preliminary results. Am. J. Roentgenol. 178 (6): 1411–1417.
- 33 Lorenzen, J., Sinkus, R., and Adam, G. (2003). [Elastography: Quantitative imaging modality of the elastic tissue properties]. RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 175 (5): 623–630.
- 34 Sinkus, R., et al. (2007). MR elastography of breast lesions: understanding the solid/liquid duality can improve the specificity of contrast‐enhanced MR mammography. Magn. Reson. Med. 58 (6): 1135–1144.
- 35 Ginat, D.T., et al. (2009). US elastography of breast and prostate lesions 1. Radiographics 29 (7): 2007–2016.
- 36 Garra, B.S., et al. (1997). Elastography of breast lesions: initial clinical results. Radiology 202 (1): 79–86.
- 37 Yoon, J.H., et al. (2011). Interobserver variability of ultrasound elastography: how it affects the diagnosis of breast lesions. Am. J. Roentgenol. 196 (3): 730–736.
- 38 Barr, R.G. (2010). Real‐time ultrasound elasticity of the breast: initial clinical results. Ultrasound Q. 26 (2): 61–66.
- 39 Cho, N., et al. (2010). Sonoelastographic strain index for differentiation of benign and malignant nonpalpable breast masses. J. Ultrasound Med. 29 (1): 1–7.
- 40 Chang, J.M., et al. (2011). Breast mass evaluation: factors influencing the quality of US elastography. Radiology 259 (1): 59–64.
- 41 Bercoff, J., Tanter, M., and Fink, M. (2004). Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans. Ultrason., Ferroelect., Freq. Control 51 (4): 396–409.
- 42 Sarvazyan, A.P., et al. (1998). Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound Med. Biol. 24 (9): 1419–1435.
- 43 Fink, M. and Tanter, M. (2010). Multiwave imaging and super resolution. Phys. Today 63 (2): 28–33.
- 44 Montaldo, G., et al. (2009). Coherent plane‐wave compounding for very high frame rate ultrasonography and transient elastography. IEEE Trans. Ultrason., Ferroelect., Freq. Control 56 (3): 489–506.
- 45 Chang, J.M., et al. (2011). Clinical application of shear wave elastography (SWE) in the diagnosis of benign and malignant breast diseases. Breast Cancer Res. Treat. 129 (1): 89–97.
- 46 Athanasiou, A., et al. (2010). Breast lesions: quantitative elastography with supersonic shear imaging – preliminary results 1. Radiology 256 (1): 297–303.
- 47 Cosgrove, D.O., et al. (2012). Shear wave elastography for breast masses is highly reproducible. Eur. Radiol. 22 (5): 1023–1032.
- 48 Li, G., et al. (2013). Performance of shear wave elastography for differentiation of benign and malignant solid breast masses. PloS One 8 (10).
- 49 Evans, A., et al. (2012). Differentiating benign from malignant solid breast masses: value of shear wave elastography according to lesion stiffness combined with greyscale ultrasound according to BI‐RADS classification. Br. J. Cancer 107 (2): 224–229.
- 50 Evans, A., et al. (2010). Quantitative shear wave ultrasound elastography: initial experience in solid breast masses. Breast Cancer Res. 12 (6): R104.
- 51 Plecha, D.M., et al. (2014). Addition of shear‐wave elastography during second‐look MR imaging–directed breast US: effect on lesion detection and biopsy targeting. Radiology 272 (3): 657–664.
- 52 Lee, S.H., et al. (2014). Two‐view versus single‐view shear‐wave elastography: comparison of observer performance in differentiating benign from malignant breast masses. Radiology 270 (2): 344–353.
- 53 Yao, M., et al. (2014). Diagnostic value of virtual touch tissue quantification for breast lesions with different size. Biomed. Res. Int. 142504.
- 54 Bai, M., et al. (2012). Virtual touch tissue quantification using acoustic radiation force impulse technology initial clinical experience with solid breast masses. J. Ultrasound Med. 31 (2): 289–294.
- 55 Ianculescu, V., et al. (2014). Added value of Virtual Touch IQ shear wave elastography in the ultrasound assessment of breast lesions. Eur. J. Radiol. 83 (5): 773–777.
- 56 Nightingale, K., et al. (2002). Acoustic radiation force impulse imaging: ex vivo and in vivo demonstration of transient shear wave propagation. In: Proceedings. 2002 IEEE International Symposium on Biomedical Imaging.
- 57 Song, P., et al. (2013). Comb‐push ultrasound shear elastography (CUSE) with various ultrasound push beams. IEEE Trans. Med. Imaging 32 (8): 1435–1447.
- 58 Song, P., et al. (2012). Comb‐push ultrasound shear elastography (CUSE): a novel method for two‐dimensional shear elasticity imaging of soft tissues. IEEE Trans. Med. Imaging 31 (9): 1821–1832.
- 59 Song, P., et al. (2014). Fast shear compounding using robust 2‐D shear wave speed calculation and multi‐directional filtering. Ultrasound Med. Biol. 40 (6): 1343–1355.
- 60 Cohn, N.A., et al. (1997). An elasticity microscope. Part I: methods. IEEE Trans. Ultrason., Ferroelect., Freq. Control 44 (6): 1304–1319.
- 61 Denis, M., et al. (2015). Comb‐push ultrasound shear elastography of breast masses: initial results show promise. PLoS One 10 (3): e0119398.
- 62 Denis, M., et al. (2015). Update on breast cancer detection using comb‐push ultrasound shear elastography. IEEE Trans. Ultrason., Ferroelect., Freq. Control 62 (9): 1644–1650.
- 63 Song, P., et al. (2015). Two‐dimensional shear‐wave elastography on conventional ultrasound scanners with time‐aligned sequential tracking (TAST) and comb‐push ultrasound shear elastography (CUSE). IEEE Trans. Ultrason., Ferroelect., Freq. Control 62 (2): 290–302.
- 64 Berg, W.A., et al. (2012). Shear‐wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology 262 (2): 435–449.
- 65 Youk, J.H., et al. (2013). Diagnostic value of commercially available shear‐wave elastography for breast cancers: integration into BI‐RADS classification with subcategories of category 4. Eur. Radiol. 23 (10): 2695–2704.
- 66 Mullen, R., et al. (2014). Shear‐wave elastography contributes to accurate tumour size estimation when assessing small breast cancers. Clin. Radiol. 69 (12): 1259–1263.
- 67 Barr, R.G. and Zhang, Z. (2012). Effects of precompression on elasticity imaging of the breast development of a clinically useful semiquantitative method of precompression assessment. J. Ultrasound Med. 31 (6): 895–902.
- 68 Tanter, M., et al. (2008). Quantitative assessment of breast lesion viscoelasticity: initial clinical results using supersonic shear imaging. Ultrasound Med. Biol. 34 (9): 1373–1386.
- 69 Barr, R.G. (2012). Shear wave imaging of the breast still on the learning curve. J. Ultrasound Med. 31 (3): 347–350.
- 70 Tozaki, M. and E. Fukuma (2011). Pattern classification of ShearWaveTM Elastography images for differential diagnosis between benign and malignant solid breast masses. Acta Radiol. 52 (10): 1069–1075.
- 71 Lee, S.H., et al. (2013). Differentiation of benign from malignant solid breast masses: comparison of two‐dimensional and three‐dimensional shear‐wave elastography. Eur. Radiol. 23 (4): 1015–1026.
- 72 Lee, E.J., et al. (2013). Diagnostic performances of shear wave elastography: which parameter to use in differential diagnosis of solid breast masses? Eur. Radiol. 23 (7): 1803–1811.
- 73 Yoon, J.H., et al. (2013). Shear‐wave elastography in the diagnosis of solid breast masses: what leads to false‐negative or false‐positive results? Eur. Radiol. 23 (9): 2432–2440.
- 74 Gregory, A., et al. (2015). Effect of calcifications on breast ultrasound shear wave elastography: an investigational study. PloS One 10 (9): e0137898.
- 75 Gregory, A., et al. (2015). An experimental phantom study on the effect of calcifications on ultrasound shear wave elastography. In: Engineering in Medicine and Biology Society (EMBC), 37th Annual International Conference of the IEEE.
