Recycling lithium batteries
B. Scrosati1; J. Garche2; Y.-K. Sun3 1 Italian Institute of Technology, Genova, Italy
2 FCBAT Ulm, Ulm, Germany
3 Hanyang University, Seoul, South Korea
Abstract
The first part of this chapter describes the expected evolution of the electric vehicles in the automobile market and the related production of the battery (mainly lithium) needed to power their electric engine. The content will be then addressed to the description and the evaluation of the processes presently in progress to recycle these batteries with particular attention to the economical, safe, and legislative aspects. These lithium battery recycling technologies are examined in terms of their operational steps, including sorting, separation, leaching, and thermal treatment. Also the historical aspect is here considered by describing a lithium battery recycling process proposed back in the year 2000 but still of actual relevance. Finally, the most recent developments are reported by discussing a recent European project aimed to the recycling of lithium and nickel metal hydride batteries with the recovery of their valuable parts.
Acknowledgment
Y.-K.S. wishes to thank the human resources development program (No. 20124010203310) of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government Ministry of Trade, Industry and Energy.
20.1 Introduction
The interest in sustainable vehicles, namely hybrid and/or electric (HEV, BEV), is increasing worldwide due to the growing concern about global warming and air pollution in large urban cities (Tarascon and Armand, 2001). Predictions suggest that hybrid and/or electric vehicles in the year 2035 will have a 35% share of the automobile market, with an associated, considerable reduction of CO2 emissions.
To be successfully achieved, this important goal requires an efficient power source for the electric engine and, in view of its high energy density, long life, and rate capability, the lithium-ion battery is an ideal candidate for this purpose (Scrosati and Garche, 2010). Indeed, many companies are now engaged in the development of electric vehicles powered by lithium batteries, see Table 20.1.
Table 20.1
Car companies involved in the production of lithium battery-powered BEVs
| Car manufacturer | Model | Cell maker | Pack maker | Weight (kg) |
| Nissan | Leaf | AESC | Nissan | 294 |
| Renault | Kankoo | AESC | Renault | 260 |
| BMW | i3 | Samsung | BMW | 230 |
| Daimler | Smart | Li-Tec | Deutsche Accum | 170 |
| Mitsubishi | i-MiEV | LEJ | LEJ | 175 |
| Fiat | 500 | Samsung | Bosh | 270 |
| TESLA | S | Samsung | TESLA | 540 |

AESC, Automotive Energy Supply Corporation; LEJ, Lithium Energy Japan.
Lithium-ion batteries are the power sources of choice in the consumer electronics market including popular devices, such as mobile phones, notebook, and are presently produced at an annual rate of several billion units, mostly in Asian countries.
For consumer electronics, e.g., for powering mobile phones, only a single cell is sufficient, whereas car driving battery packs require the assemblage of many cells and the inclusion of a safety battery management system (BMS), see Figure 20.1.

The worldwide reserves of lithium carbonate (i.e., the lithium main natural source) are still large. Considering that the yearly production amounts to about 0.16 M tons and that ~ 0.5 kg of Li2CO3 are needed per kWh battery, we can estimate from 80 to 100 years of reserves. Nevertheless, almost 70% of the global lithium deposits are concentrated in South America’s ABC (Argentina, Bolivia, and Chile) salars (Scrosati, 2011), and this poses an inherent risk for the accessibility of the raw material since unexpected events may condition the supply with a resulting impact on the battery price and consequently on the vehicle cost (Fletcher, 2011).
Furthermore, one has to consider that lithium is also the material of choice for applications other than batteries, including pharmaceuticals, ceramics, and glasses. Actually, the present consumption rate of lithium by OEMs is limited to a minor fraction, accounting for only about one quarter of the current lithium production. However, in the prospect of large road diffusion of BEVs (1 million expected in 2020), the amount of lithium needed to meet the market demand is expected to increase considerably. The prices of lithium constantly increased over the last 10 years; at time of publication, prices were $5500–6000 per ton of lithium carbonate, depending on applications. Accordingly, considerable increases are expected if the demand rises. To limit the risks, many battery material manufactures underwent investment in partnerships with the South American ABC countries to secure the lithium supply and hence, to control prices fluctuations.
The above considerations clearly outline the need for recycling lithium car batteries once they have exhausted their operational life, with the final goal of reusing them back to the car manufacturers. The idea is well represented by the general scheme reported below, as proposed by the Japanese Sumitomo company, see Figure 20.2.
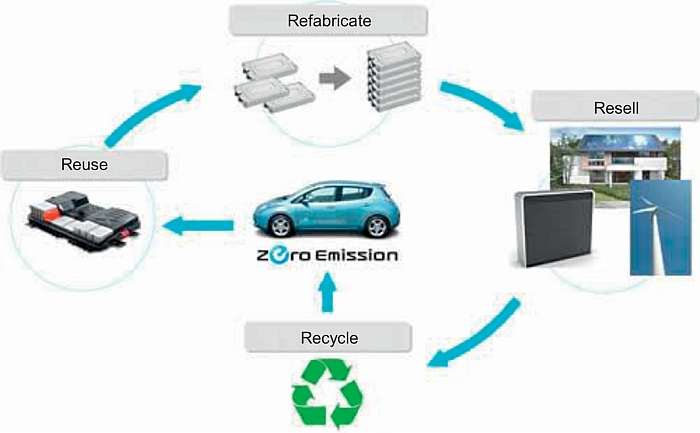
The future of battery recycling, however, is still uncertain; see below.
20.2 Battery recycling
The main goal of recycling is that of separating battery components, as well as of removing waste from the environment. However, the process is affected by a series of issues that make it very challenging. About 100,000 tons of spent batteries are forecast in the prospect of 1 million EV cars on the road and their treatment is not straightforward.
The main problem is in the collection rate, which is still at a very limited extent, even for lithium batteries used in mobile electronic devices. Although the circulating number of these devices, and hence of their batteries, is extremely high, the collection is scarce for a series of reasons. First, there are not many lithium battery collection points in the municipalities. In principle, the shops selling lithium battery-operating devices should serve as collection points for the related exhausted batteries. However, this rarely happens and the customer, out of laziness or forgetfulness, tends to drop the old telephone with the included battery in a drawer. The problem is serious to the point that often the capacity of the plants is not matched by the number of received spent batteries. Obviously, the situation is even more of concern for the car batteries, considering the very limited number of EVs presently in the road.
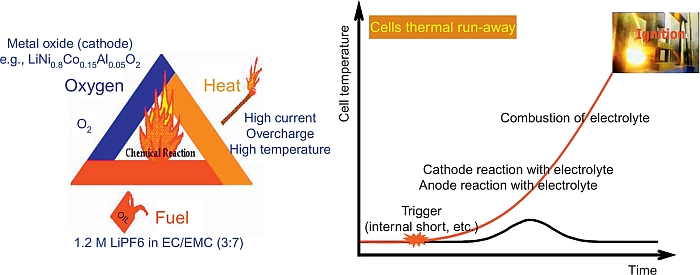
Another serious issue is associated with the intrinsic safety risk owing to the high reactivity of the lithium batteries, especially if they arrive at the recycling plant still with residual charge or if they are damaged. In fact, if overheated or overcharged, as it may happen by shorting when they are stored in masses, the batteries can enter a state of thermal runaway which can eventually lead to fire or even explosion, see Figure 20.3. In addition, metallic lithium can also form on the graphite anode by overcharge and/or by abnormal deposition, whose high reactivity greatly increases the risk of explosion. The energy released by these explosions is powerful enough to melt the metal containers with resulting serious safety hazards. Indeed, battery fire incidents did take place in large lithium battery storage sites, see examples in Figures 20.4 and 20.5 (Green, 2014).


The other serious issue is related to the fact that the lithium battery market is in continuous evolution with the advent of many new chemistries, see Table 20.2. Further, in addition to the rechargeable Li-ion batteries, also primary lithium batteries, using cathodes such as manganese oxide or thionyl and sulfuryl chloride, are still in the market and they may arrive to the plant as well. Finally, also the electrolyte may widely change, passing from a variety of liquid organic solutions to polymer membranes. Clearly, this high diversity makes it difficult to developed an universally valid recycling process, as well as affecting its economics, since the new chemistries may not involve components worth being recovered.
Table 20.2
Chemistries of anodes and cathodes for Li batteries
| Anodes | ||
| Graphite, C | C – 10% Si | Lithium titanium oxide, Li4Ti5O12, LTO |
| Cathodes | ||
| Lithium cobalt oxide, LCO | Lithium nickel cobalt aluminum oxide, LiNi0.8Co0.15Al0.05O2, NCA | Lithium nickel manganese oxide, LiNi0.32Mn0.33Co0.33O2, NCM |
| Lithium manganese oxide, LiMn2O4, LMO | LMO + NCA | Lithium iron phosphate, LiFPO4, LFP |
| Lithium nickel manganese oxide, LiNi0.5Mn1.5O4, LMNO | ||

The average composition of a lithium battery is shown in Table 20.3. We see that the amount of cobalt and nickel, namely, the most economically interesting metals, is reduced to a minor fraction, this leading to a net negative value for recycling if lithium is the only recovered material. Therefore, although lithium is 100% recyclable, current economics do not make it worthwhile for automotive purposes. Hence, lithium battery recycling would be at the present only motivated for ecological benefits and for adherence to environmental laws.
Table 20.3
Average composition of lithium batteries (percentage)
| Element | Composition (%) | Element | Composition (%) |
| Co | 1 | C (graphite) | 19 |
| Ni | 3 | Fe | 21 |
| Li | 5 | Others | 16 |
| Cu | 7 | ||
| Mn | 11 | ||
| Al | 17 |

Indeed, the European Commission has mandated a Battery and Accumulator Directive, which imposes to the state members the following targets (Eurostat).
![]() A 45% collection rate for waste-portable batteries to be met by September 2016.
A 45% collection rate for waste-portable batteries to be met by September 2016.
![]() A recycling efficiency to ensure that a high proportion of the weight of waste batteries is recycled, this including 65% of lead-acid, 75% of nickel–cadmium, and 50% of “other waste batteries,” the latter likely referring to lithium batteries.
A recycling efficiency to ensure that a high proportion of the weight of waste batteries is recycled, this including 65% of lead-acid, 75% of nickel–cadmium, and 50% of “other waste batteries,” the latter likely referring to lithium batteries.
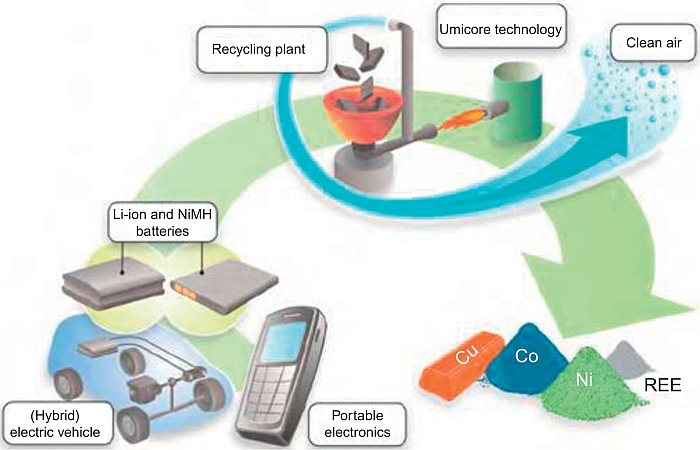
Considering the present low economic value, these targets can be met only if subsidies are provided, usually adding a tax to each manufactured battery, as indeed is the case. Under this scheme, battery recycling plants are now operating in Europe (e.g., Batrec in Switzerland, Umicore in Belgium, and SNAM and Recupyl in France) to honor the mandate (ICBR, 2014). Plants are also in force under different schemes in the United States (e.g., Toxco) and in Japan (e.g., Sony and Sumitomo Metal). Due to the still scarce production of lithium-ion batteries of EV types, the recycling is for the moment limited to the portable ones. However, EV battery recycling is expected to gain quite a significant importance in the years to come, this enhancing the role of the experience obtained with the present small-scale prototypes.
20.3 Recycling technologies
“Some current industrial technologies are based on hydrometallurgical processes” (Xu et al., 2008), according to the schematic flow sheet reported in Figure 20.6. Others are based on a pyrometallurgical process. As an example of a pyrometallurgical process, Figure 20.7 illustrates in a general schematic form the flow sheet of the process in force at the Umicore Plant in Belgium (Batteryrecycling umicore).
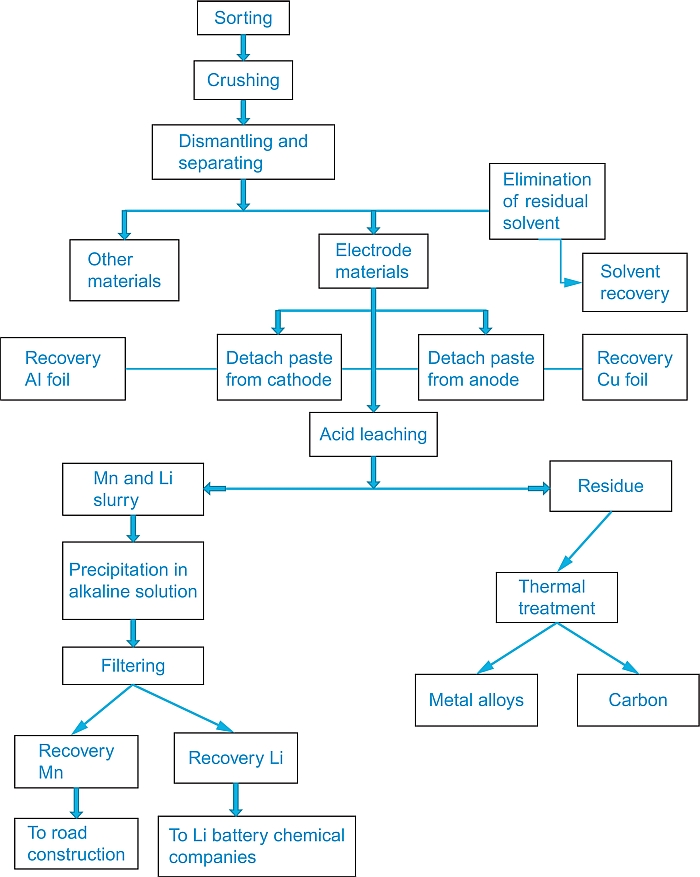
20.3.1 Sorting
Due to the lack of devoted collection, the lithium batteries arrive at the plant in mixture with all other different kinds. Then the process must first involve sorting the batteries according to their chemistries to avoid cross-contamination. For this step all the received batteries are placed in a moving belt and the most common procedure is still of manual type, that is, performed by persons who select the battery types and separate them into designated containers, see Figure 20.8. Obviously, this step greatly contributes to the cost of the process, but allows automatic procedures, generally exploiting X-ray or cameras, to gradually develop. Figure 20.9 shows a system of this type developed at the Refind Technologies, a company in Sweden with the commercial name of Optisort (www.refind.se).


20.3.2 Dismantling and separation
The sorted lithium batteries are dismantled with the removal of the combustible components, such as plastics and insulators, by a burning process, posing attention to clean the combustion gases by scrubbers before releasing them in the atmosphere. The naked cells are then chopped into small pieces and heated to a metal-liquefying temperature. Special safety precautions have to be undertaken for this step. Commonly, liquid nitrogen is used to freeze the batteries before shredding, crushing, and removing the lithium and the other battery components.
The anode is separated by the cathode, and their covering paste of both is detached to recover the aluminum and copper, respectively, foils. The step also involves the elimination of residual electrolyte solution (eventually back-recovered by distillation) and of the separator.
20.3.3 Acid leaching
This step, aimed to leach out dust, iron scraps, and paper residue to finally transfer the metals to an aqueous slurry, is carried out by treating the electrode mass with an acid solution, generally sulfuric, with the addition of hydrogen peroxide as reducing agent. The temperature of operation is around 80 °C. Metals are leached with the following sequence: aluminum > lithium > cobalt > copper. The leaching process is obviously of potential danger to the environment due to emission of corrosive chlorine or sulfur oxide gases. Therefore, special apparatus must be provided to treat these gases, thereby resulting in an additional impact on the overall recycling cost.
20.3.4 Filtration
By this step, approximately 100% of the lithium and 95% of the manganese are usually recovered in a mixture. The latter is separated by the former first by precipitation in hydroxide, followed by the addition of a basic solution and then by filtration.
20.3.5 Thermal treatment
This step is carried out to eliminate carbon and organic compounds from the solid residue coming from the acidic leaching. The process is usually carried out in an electric arc, high-temperature furnace. Occasionally, a second heating treatment is added to complete the process. The residual steel alloy can be directly considered for metallurgical applications.
20.4 Early work
The interest in lithium battery recycling dates back to early 2000, obviously motivated by the economic benefit of recovery cobalt that at the time was still largely used as the main component of the cathode. Although the activity in developing lithium battery recycling processes was meanly academic, the results obtained were of definite importance even in view of scaling up to industrial value. As a relevant example, we quote the laboratory-scale lithium battery recycling process developed in 1999 at the University of Rome (Contestabile et al., 2001). It is significant to quote a sentence reported in this seminal paper. “If we consider the expected overall world market evolution of lithium batteries and that their average life is limited, we can easily understand how the correct disposal of spent lithium batteries may become a serious problem in the years to come” (Figure 20.10).

It is, in fact, to be pointed out how this early process anticipated the concepts that are presently in force in modern plants (compared Figure 20.6) with some obvious differences, such as the absence of the thermal process difficult to carry out at a laboratory scale, however, benefitting by an easy and low-cost procedure conceived with a series of easily performing and low-cost steps. It is then worthwhile to recall the process in this review by describing in detail the related steps since they may be useful to inspire operation in modern plants.
Similarly to the present cases, the process required an initial crushing step, performed manually, opening the battery by cutting the steel cases and removing the inner parts. The hazard associated with this operation was clear also at the time, hence the crushed parts were immersed in liquid nitrogen for inertization (compare with Section 20.3.2). The operation involved a scrubbing step to trap the gases that evolved by the thermal decomposition of the electrolyte due to their possible toxicity.
The next step was directed to the separation of the components, that is, the cathode mass from the metal case. This operation was cleverly achieved by mechanical ridding, exploiting the different weight of the two components. The selective separation of the electrode materials was conducted by treating their mass with N-methylpyrrolidone (NMP) at about 100 °C for 1 h. The choice of the solvent was motivated by the fact that PVDF, that is, the binder used to hold the graphite anode and the lithium cobalt oxide cathode electrode materials on Cu and Al, respectively, current collectors, is highly soluble in NMP (i.e., around 200 g/kg of solvent). The following filtration step allowed to separate and then to recover the substrates. The separation of the graphite and lithium cobalt oxide powders presented some difficulties due to their low particle size, and it was achieved by decanting the solution and then repeatedly washing with water the powders. The recovered NMP was then recycled since, due to its high solubility for PVDF, it could be reused for the following cycles.
The quantitative dissolution of lithium cobalt oxide was achieved by treating the separated residual powders with a small volume of 4 M HCl for 1 h at about 80 °C, using an acid/sample ratio of about 10. Analysis run by inductively coupled plasma emission spectroscopy gave a cobalt to lithium ratio of 7.23/1.30. The side product, that is, the carbon powder settling on the bottom, was separated and recovered by solution decantation.
The cobalt dissolved in the hydrochloric solution was finally recovered as cobalt hydroxide Co(OH)2 by addition of a 4 M NaOH solution. Due to its very low solubility, the precipitation of Co(OH)2 begun at a pH value of 6 and was considered to be completed at pH 8. Alternatively, the precipitation could be obtained using a solution of a weaker base, such as NH4OH, which, however, forms stable complexes with cobalt causing the partial dissolution of the hydroxide and thus, preventing from a quantitative recovery. On a possible industrial scale, this step can be controlled by using an adequate pH sensor. The Co(OH)2 precipitate can be easily separated from the solution by filtration, dried, and directed to chemical companies for battery reusing.
20.5 Recent developments
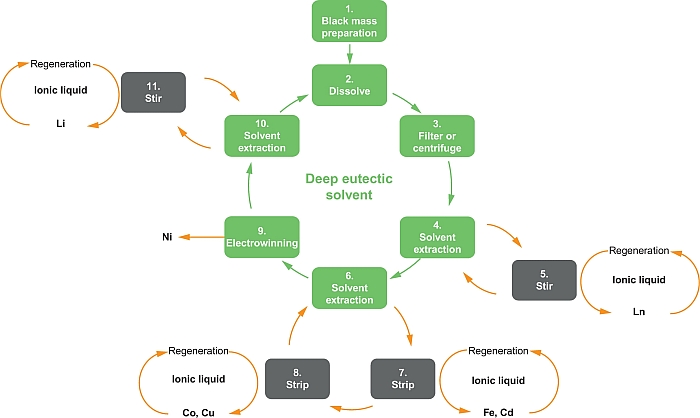
An European project by the acronym CoLaBATS (Colabats) was launched in October 2013 with the aim of developing new industrial processes with the capabilities to retrieve cobalt, lanthanides, nickel, and lithium from spent waste rechargeable batteries, The project, which incorporates 10 collaborative industrial and academic partners, is expected to have a positive impact on recycling efficiencies and the purity of recovered metals in comparison to current routes. In particular, compared to hydro/pyrometallurgical processing, the primary method being developed in the project will have the potential to reduce landfill, critical metal consumption, and environmental impacts.
The proposed processes will employ task specific ionic liquids (TSILs) to target key metals in lithium-ion and Ni metal hydride (NiMH) batteries, with enhancements made using ultrasonics which can be used to promote breakdown of the battery electrode structures to finally lead to efficient metal recovery. Ionic liquids, that is, organic salts liquid at room temperature (see Figure 20.11) have many positive attributes including no volatility, high thermal stability, low cost, reduced environmental impact, low toxicity, and reusability (minimal processing) (Armand et al., 2009; Gebresilassie Eshetu et al., 2014).

Due to their favorable properties, and, in particular to the liquid state and high thermal stability, the use of TSILs allows for reactions at much lower temperatures than other recycling processes. They are therefore much cheaper to operate, and their utility has already been demonstrated in electroplating processes.
The aim of the process, which is schematically represented by the flow sheet of Figure 20.12, is to develop new industrial processes for the recycling of waste batteries. The focus is to create hydrometallurgical recovery capability suitable for industrial application (and therefore also having regard to the fate of the other components including battery casing materials). Specific objectives are to recover cobalt from spent lithium-ion batteries and nickel, cerium, lanthanum, and other rare earth metals from spent nickel metal hydride batteries, with a purity expected to be greater than 95%.
20.6 Government regulations
Battery recycling has been a task for many years motivated by environmental awareness and waste legislation mandates. With the public’s increase in environmental sensibility, growing attention is paid to sustainable management of natural resources. The public also has an increasing concern with the hazardous properties of metals and substances, a concern that certainly encompasses batteries of all types. European (if not worldwide) regulations have designated all batteries as hazardous waste that require treatment before disposal, with the following tasks to be accomplished, in order of priority:
1. Waste reduction at the origin, by means of cleaner products and processes.
2. Recovery of valuables from wastes, where possible.
3. Treatment of nonrecoverable wastes to make them safe and disposable.
These regulations require large efforts to be devoted to the collection and recycling of batteries of any kind, despite the possibility that they may contain a low content in heavy metals. To cope with these directives, several recycling plants are in operation in Europe, the United States, and Japan (ICBR, 2014). Initially, the activity was mainly restricted to zinc–carbon batteries, namely the common “dry” primary AA or AAA cylindrical cells that are largely present in the low-value electronic market. For these dry cells, regulations have been imposed requiring that they be produced as “mercury-free” systems, which is the case for European and American manufacturers. However, the market globalization has favored circulation from countries where environmental sensitivity is not as acute as in Europe and the United States, with the result that a considerably large amount of mercury is still recovered when recycling these batteries. Interestingly, the majority of the plants are still treating mostly consumer batteries, such as the quoted dry cells, and the rechargeable NiCd and NiMH batteries, while little attention has thus far been devoted to the collection and the recycling of lithium batteries. On the contrary, recycling of conventional automotive batteries, such as starting lighting and ignition (SLI) lead-acid, are in full operation worldwide.
The low recycling rate of lithium batteries is rather surprising since they are products that strongly influence our everyday life. Due to their favorable characteristics, lithium-ion batteries are in fact the power sources of choice in the consumer electronics market and, as such, are sold by several billions per year. Primary lithium batteries are mainly marketed to generic consumer markets for use in cameras, watches, and similar, whereas lithium-ion secondary batteries are marketed for mobile devices of increasing sophistication, such as cellular phones and laptop computers.
The large expansion of these markets (it is assumed that today billions of cellular phones are circulating worldwide) is evidence of the importance of the problem, which will only worsen with the expected advent of a high number of lithium-ion battery-powered electric cars. We need to increase the number of recycling plants for treating these batteries which, despite being rechargeable, will inevitably come to the end of their life at some point. Even though in the last few years protocols and plants have been developed in Europe, the United States, and Japan, much still needs to be done to assure a full collection and an effective recycling program for lithium-ion batteries. We hope that this review will provide the motivation and the stimulus for achieving this important goal in the near future.
