[1] Valiev R.Z, Langdon T.G. Principles of equal channel angular pressing as a processing tool for grain refinement. Progress in Materials Science. 2006;51:881–981.
[2] Zhilyaev A.P, Langdon T.G. Using high-pressure torsion for metal processing: fundamentals and applications. Progress in Materials Science. 2008;53:893–979.
[3] Gubicza J, Dobatkin S, Bakai Z, Chinh N.Q, Langdon T.G. Microstructure and mechanical behavior of severely deformed F.C.C. Metals, Materials Science Forum. 2007;567–568:181–184.
[4] Gubicza J, Chinh N.Q, Lábár J.L, Dobatkin S, Hegedűs Z, Langdon T.G. Correlation between microstructure and mechanical properties of severely deformed metals. Journal of Alloys and Compounds. 2009;483:271–274.
[5] Dalla Torre F, Lapovok R, Sandlin J, Thomson P.F, Davies C.H.J, Pereloma E.V. Microstructures and properties of copper processed by equal channel angular extrusion for 1–16 passes. Acta Materialia. 2004;52:4819–4832.
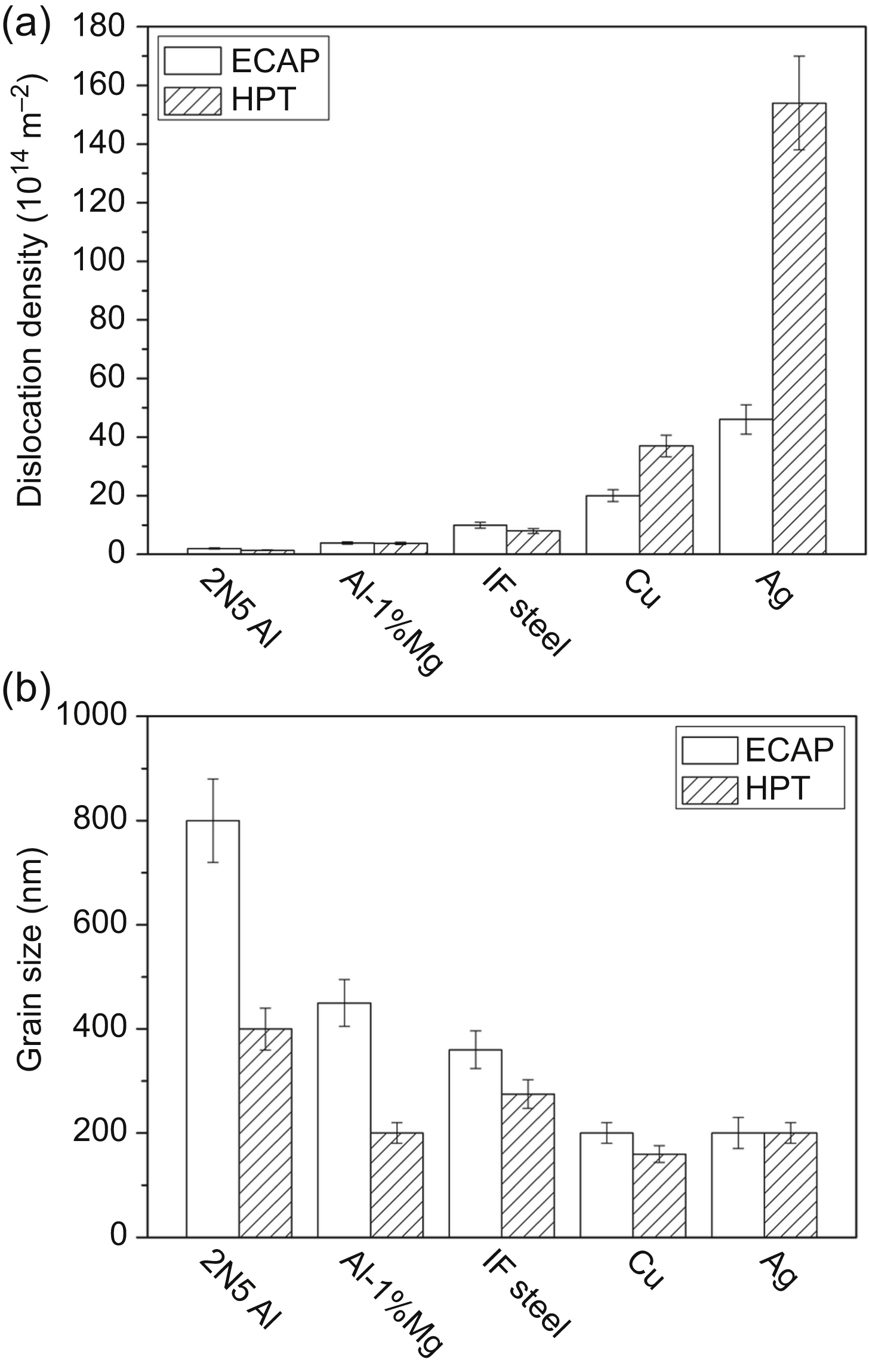
[6] Gubicza J, Chinh N.Q, Horita Z, Langdon T.G. Effect of Mg addition on microstructure and mechanical properties of aluminum. Materials Science and Engineering A. 2004;387–389:55–59.
[7] Gubicza J, Balogh L, Hellmig R.J, Estrin Y, Ungár T. Dislocation structure and crystallite size in severely deformed copper by X-ray peak profile analysis. Materials Science and Engineering A. 2005;400–401:334–338.
[8] Schafler E, Steiner G, Korznikova E, Kerber M, Zehetbauer M.J. Lattice defect investigation of ECAP-Cu by means of X-ray line profile analysis, calorimetry and electrical resistometry. Materials Science and Engineering A. 2005;410–411:169–173. .
[9] Gubicza J, Chinh N.Q, Krállics Gy, Schiller I, Ungár T. Microstructure of ultrafine-grained fcc metals produced by severe plastic deformation. Current Applied Physics. 2006;6:194–199.
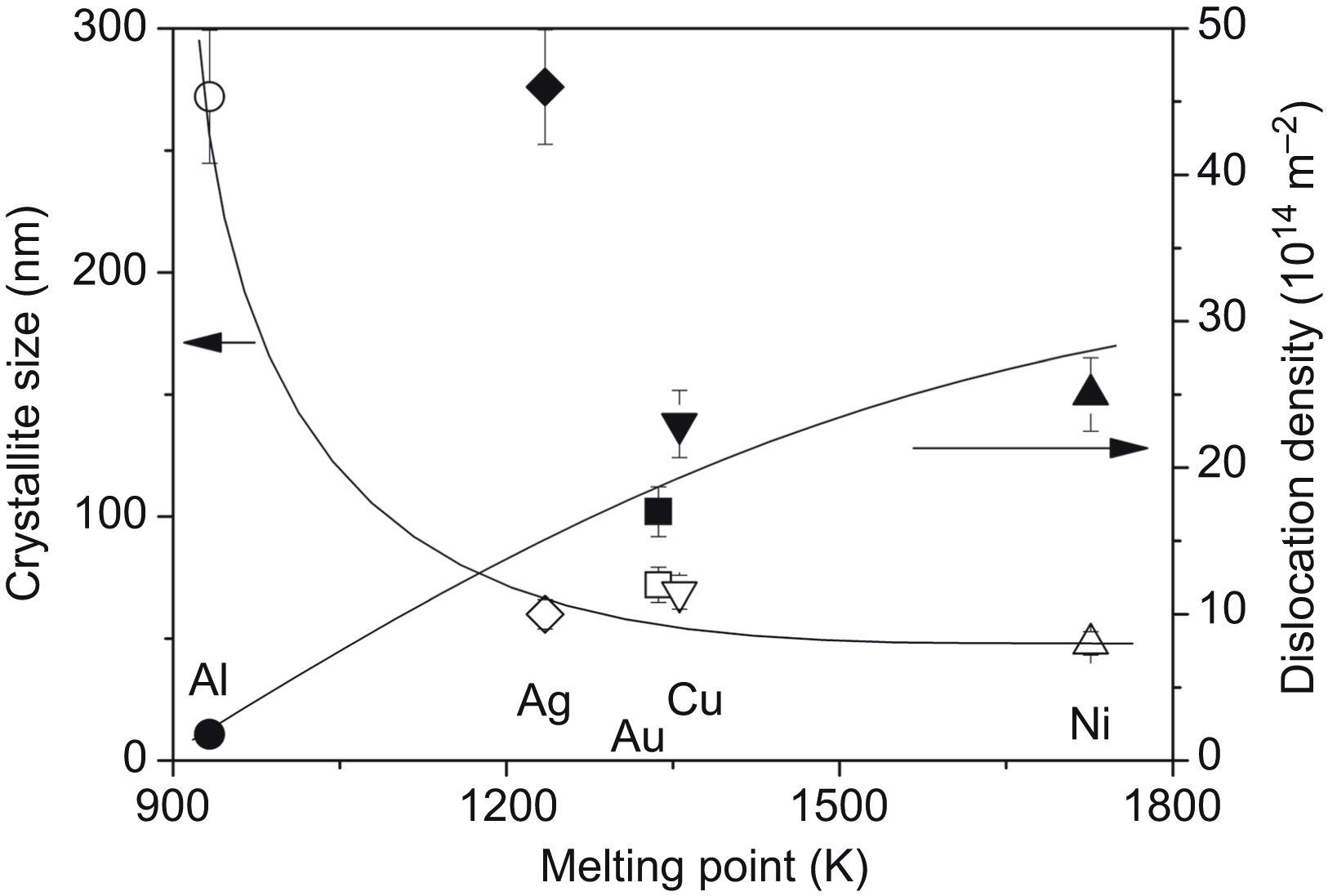
[10] Gubicza J, Chinh N.Q, Lábár J.L, Hegedűs Z, Langdon T.G. Principles of self-annealing in silver processed by equal-channel angular pressing: the significance of a very low stacking fault energy. Materials Science and Engineering A. 2010;527:752–760.
[11] Máthis K, Krajnák T, Kuzel R, Gubicza J. Structure and mechanical behaviour of interstitial-free steel processed by equal-channel angular pressing. Journal of Alloys and Compounds. 2011;509:3522–3525.
[12] Dobatkin S.V, Szpunar J.A, Zhilyaev A.P, Cho J.-Y, Kuznetsov A.A. Effect of the route and strain of equal-channel angular pressing on structure and properties of oxygen-free copper. Materials Science and Engineering A. 2007;462:132–138.
[13] Ungár T, Tichy G, Gubicza J, Hellmig R.J. Correlation between subgrains and coherently scattering domains. Powder Diffraction. 2005;20:366–375.
[14] Huang J.Y, Zhu Y.T, Jiang H, Lowe T.C. Microstructures and dislocation configurations in nanostructured Cu processed by repetitive corrugation and straightening. Acta Materialia. 2001;49:1497–1505.
[15] Zhu Y.T, Huang J.Y, Gubicza J, Ungár T, Wang Y.M, Ma E, Valiev R.Z. Nanostructures in Ti processed by severe plastic deformation. Journal of Materials Resources. 2003;18:1908–1917.
[16] Gubicza J, Nam N.H, Balogh L, Hellmig R.J, Stolyarov V.V, Estrin Y, Ungár T. Microstructure of severely deformed metals determined by X-ray peak profile analysis. Journal of Alloys and Compounds. 2004;378:248–252.
[17] Mishin O.V, Juul Jensen D, Hansen N. Microstructures and boundary populations in materials produced by equal channel angular extrusion. Materials Science and Engineering A. 2003;342:320–328.
[18] Zhilyaev A.P, Kim B.-K, Szpunar J.A, Baro M.D, Langdon T.G. The microstructural characteristics of ultrafine-grained nickel. Materials Science and Engineering A. 2005;391:377–389.
[19] Hazra S.S, Gazder A.A, Carman A, Pereloma E.V. Effect of cold rolling on as–ECAP interstitial free steel. Metallurgical and Materials Transactions A. 2011;42:1334–1348.
[20] Chen Y.J, Li Y.J, Walmsley J.C, Dumoulin S, Gireesh S.S, Armada S, Skareta P.C, Roven H.J. Quantitative analysis of grain refinement in titanium during equal channel angular pressing. Scripta Materialia. 2011;64:904–907.
[21] Hallberg H, Wallin M, Ristinmaa M. Modeling of continuous dynamic recrystallization in commercial purity aluminum. Materials Science and Engineering A. 2010;527:1126–1134.
[22] Ungár T, Gubicza J, Ribárik G, Borbély A. Crystallite size distribution and dislocation structure determined by diffraction profile analysis: principles and practical application to cubic and hexagonal crystals. Journal of Applied Crystallography. 2001;34:298–310.
[23] Fátay D, Bastarash E, Nyilas K, Dobatkin S, Gubicza J, Ungár T. X-ray diffraction study on the microstructure of an Al–Mg–Sc–Zr alloy deformed by high-pressure torsion. Zeitschrift fur Metallkunde. 2003;94:842–847.
[24] Escaig P.B. Sur le glissement dévié des dislocations dans la structure cubique à faces centrées. Journal de Physique. 1968;29:225–239.
[25] Argon A.S, Moffatt W.C. Climb of extended edge dislocations. Acta Metallurgica. 1981;29:293–299.
[26] Ngan A.H.W, Wen M. Atomistic simulations of Paidar–Pope–Vitek lock formation in Ni3Al. Computational Materials Science. 2002;23:139–145.
[27] Ungár T, Schafler E, Gubicza J. In: Zehetbauer M.J, Zhu Y.T, eds. Microstructure of Bulk Nanomaterials Determined by X-Ray Line Profile Analysis, in the Book Entitled Bulk Nanostructured Materials. Wiley–VCH; 2009: 978-3-527-31524-6:361–386.
[28] Gubicza J. X-ray Line Profile Analysis in Materials Science. Hershey, PA, USA: IGI-Global; 2014.
[29] Gubicza J, Ribárik G, Goren-Muginstein G.R, Rosen A.R, Ungár T. The density and the character of dislocations in cubic and hexagonal polycrystals determined by X-ray diffraction. Materials Science and Engineering A. 2001;309–310:60–63. .
[30] Ungár T, Gubicza J. Dislocation structure and crystallite size distribution in hexagonal nanomaterials from X-ray peak profile analysis. Zeitschrift fur Metallkunde. 2002;93:694–698.
[31] Chatterjee P, Sen Gupta S.P. An X-ray diffraction study of strain localization and anisotropic dislocation contrast in nanocrystalline titanium. Philosophical Magazine A. 2001;81:49–60.
[32] Máthis K, Gubicza J, Nam N.H. Microstructure and mechanical behavior of AZ91 Mg alloy processed by equal channel angular pressing. Journal of Alloys and Compounds. 2005;394:194–199.
[33] Agnew S.R, Duygulu O. A mechanistic understanding of the formability of magnesium: examining the role of temperature on the deformation mechanisms. Materials Science Forum. 2003;419–422:177–188.
[34] Yoo M.H. Slip, twinning, and fracture in hexagonal close-packed metals. Metallurgical and Materials Transactions A. 1981;12:409–418.
[35] Stráská J, Janecek M, Gubicza J, Krajnák T, Yoon E.Y, Kim H.S. Evolution of microstructure and hardness in AZ31 alloy processed by high pressure torsion. Materials Science and Engineering A. 2015;625:98–106.
[36] Caceres C, Lukac P. Strain hardening behaviour and the Taylor factor of pure magnesium Philosophical Magazine A. Philosophical Magazine. 2008;88:977–989.
[37] Janecek M, Cízek J, Gubicza J, Vrátná J. Microstructure and defect structure evolution in MgAlZn alloy processed by severe plastic deformation. Journal of Materials Science. 2012;47:7860–7869.
[38] Lapovok R, Estrin Y, Popov M.V, Langdon T.G. Enhanced superplasticity in a magnesium alloy processed by equal-channel angular pressing with a back-pressure. Advanced Engineering Materials. 2008;10:429–433.
[39] Figueiredo R.B, Langdon T.G. Principles of grain refinement and superplastic flow in magnesium alloys processed by ECAP. Materials Science and Engineering A. 2009;501:105–114.
[40] Figueiredo R.B, Langdon T.G. Principles of grain refinement in magnesium alloys processed by equal channel angular pressing. Journal of Materials Science. 2009;44:4758–4762.
[41] Koike J. New deformation mechanisms in fine-grain Mg alloys. Materials Science Forum. 2003;419–422:189–194.
[42] Galiyev A, Kaibyshev R, Gottstein G. Correlation of plastic deformation and dynamic recrystallization in magnesium alloy ZK60. Acta Materialia. 2001;49:1199–1207.
[43] Gubicza J, Dobatkin S.V, Khosravi E, Kuznetsov A.A, Lábár J.L. Microstructural stability of Cu processed by different routes of severe plastic deformation. Materials Science and Engineering A. 2011;528:1828–1832.
[44] Schafler E. Effects of releasing the hydrostatic pressure on the nanostructure after severe plastic deformation of Cu. Scripta Materialia. 2010;62:423–426.
[45] Zehetbauer M.J, Stuwe H.P, Vorhauer A, Schafler E, Kohout J. The role of hydrostatic pressure in severe plastic deformation. Advanced Engineering Materials. 2003;5:330–337.
[46] Korznikova E, Schafler E, Steiner G, Zehetbauer M. Measurements of vacancy type defects in SPD deformed nickel. In: Proceedings of the 4th International Symposium on Ultrafine Grained Materials 2006 TMS Annual Meeting. Warrendale, PA: TMS; 2006:97–102.
[47] Zehetbauer M, Steiner G, Schafler E, Korznikov A, Korznikova E. Deformation induced vacancies with severe plastic deformation: measurements and modelling. Materials Science Forum. 2006;503–504:57–64.
[48] Setman D, Schafler E, Korznikova E, Zehetbauer M.J. The presence and nature of vacancy type defects in nanometals detained by severe plastic deformation. Materials Science and Engineering A. 2008;493:116–122.
[49] Kilmametov A.R, Vaughan G, Yavari A.R, LeMoulec A, Botta W.J, Valiev R.Z. Microstructure evolution in copper under severe plastic deformation detected by in situ X-ray diffraction using monochromatic synchrotron light. Materials Science and Engineering A. 2009;503:10–13.
[50] Setman D, Kerber M.B, Schafler E, Zehetbauer M.J. Activation enthalpies of deformation-induced lattice defects in severe plastic deformation nanometals measured by differential scanning calorimetry. Metallurgical and Materials Transactions A. 2010;41:810–815.
[51] Hirth J.P, Lothe J. Theory of Dislocations. New York: John Wiley; 1982.
[52] Murr L.E. Interfacial Phenomena in Metals and Alloys. Reading, MA: Addison Wesley; 1975. .
[53] Hegedűs Z, Gubicza J, Kawasaki M, Chinh N.Q, Fogarassy Zs, Langdon T.G. Microstructure of low stacking fault energy silver processed by different routes of severe plastic deformation. Journal of Alloys and Compounds. 2012;536S:S190–S193.
[54] Cízek J, Janecek M, Krajnák T, Stráská J, Hruska P, Gubicza J, Kim H.S. Structural characterization of ultrafine-grained interstitial-free steel prepared by severe plastic deformation. Acta Materialia. 2016;105:258–272.
[55] Gubicza J. Correlation between processing conditions, lattice defect structure and mechanical performance of ultrafine-grained materials. Acta Physica Polonica A. 2015;128:479–486.
[56] Furukawa M, Horita Z, Nemoto M, Langdon T.G. Review: processing of metals by equal-channel angular pressing. Journal of Materials Science. 2001;36:2835–2843.
[57] Iwahashi Y, Horita Z, Nemoto M, Langdon T.G. The shearing characteristics associated with equal channel angular pressing. Acta Materialia. 1998;46:3317–3331.
[58] Balogh L, Ungár T, Zhao Y, Zhu Y.T, Horita Z, Xu C, Langdon T.G. Influence of stacking-fault energy on microstructural characteristics of ultrafine-grain copper and copper-zinc alloys. Acta Materialia. 2008;56:809–820.
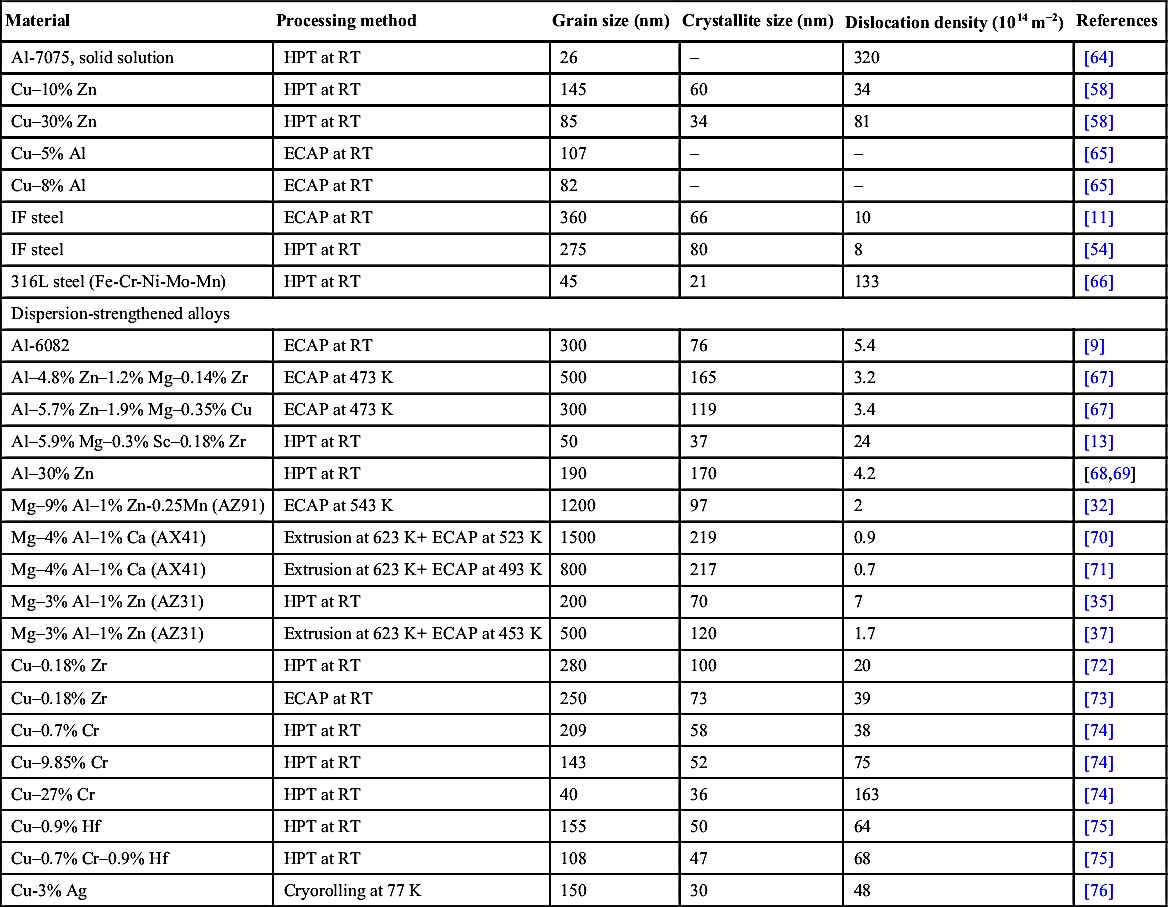
[59] Zhilyaev A.P, Oh-ishi K, Langdon T.G, McNelley T.R. Microstructural evolution in commercial purity aluminum during high-pressure torsion. Materials Science and Engineering A. 2005;410–411:277–280.
[60] Rangaraju N, Raghuram T, Vamsi Krishna B, Prasad Rao K, Venugopal P. Effect of cryo-rolling and annealing on microstructure and properties of commercially pure aluminium. Materials Science and Engineering A. 2005;398:246–251.
[61] Gubicza J, Chinh N.Q, Szommer P, Vinogradov A, Langdon T.G. Microstructural characteristics of pure gold processed by equal-channel angular pressing. Scripta Materialia. 2007;56:947–950.
[62] Zhilyaev A.P, Gubicza J, Nurislamova G, Révész Á, Suriñach S, Baró M.D, Ungár T. Microstructural characterization of ultrafine-grained nickel. Physica Status Solidi A. 2003;198:263–271.
[63] Andreau O, Gubicza J, Zhang N.X, Huang Y, Jenei P, Langdon T.G. Effect of short-term annealing on the microstructures and flow properties of an Al-1% Mg alloy processed by high-pressure torsion. Materials Science and Engineering A. 2014;615:231–239.
[64] Liddicoat P.V, Liao X.-Z, Zhao Y, Zhu Y, Murashkin M.Y, Lavernia E.J, Valiev R.Z, Ringer S.P. Nanostructural hierarchy increases the strength of aluminium alloys. Nature Communications. 2010;1:63 Art. No.
[65] Qu S, An X.H, Yang H.J, Huang C.X, Yang G, Zang Q.S, Wang Z.G, Wua S.D, Zhang Z.F. Microstructural evolution and mechanical properties of Cu–Al alloys subjected to equal channel angular pressing. Acta Materialia. 2009;57:1586–1601.
[66] Gubicza J, El-Tahawy M, Huang Y, Choi H, Choe H, Lábár J.L, Langdon T.G. Microstructure, phase composition and hardness evolution in 316L stainless steel processed by high-pressure torsion. Materials Science and Engineering A. 2016;657:215–223.
[67] Gubicza J, Schiller I, Chinh N.Q, Illy J, Horita Z, Langdon T.G. The effect of severe plastic deformation on precipitation in supersaturated Al-Zn-Mg alloys. Materials Science and Engineering A. 2007;460–461:77–85.
[68] Alhamidi A, Edalati K, Horita Z, Hirosawa S, Matsuda K, Terada D. Softening by severe plastic deformation and hardening by annealing of aluminum–zinc alloy: significance of elemental and spinodal decompositions. Materials Science and Engineering A. 2014;610:17–27.
[69] Chinh N.Q, Jenei P, Gubicza J, Bobruk E.V, Valiev R.Z, Langdon T.G. Influence of Zn content on the microstructure and mechanical performance of ultrafine-grained Al-Zn alloys processed by high-pressure torsion. Materials Letters. 2016 submitted for publication.
[70] Krajnák T, Máthis K, Stráská J, Janecek M, Gubicza J. Microstructure and mechanical properties of severely deformed AX41 magnesium alloy. Acta Physica Polonica A. 2015;128:768–771.
[71] Krajnák T, Máthis K, Janecek M, Gubicza J. Comparison of the microstructure and the mechanical properties of AX41 magnesium alloy processed by EX-ECAP via three different routes A, Bc and C. IOP Conference Series: Materials Science and Engineering. 2014;63:012058.
[72] Dopita M, Janecek M, Kuzel R, Seifert H.J, Dobatkin S. Microstructure evolution of CuZr polycrystals processed by high-pressure torsion. Journal of Materials Science. 2010;45:4631–4644. .
[73] Kuzel R, Janecek M, Matej Z, Cizek J, Dopita M, Srba O. Microstructure of Equal-channel angular pressed Cu and Cu-Zr samples studied by different methods. Metallurgical and Materials Transactions A. 2010;41:1174–1190.
[74] Dobatkin S.V, Gubicza J, Shangina D.V, Bochvar N.R, Tabachkov N.U. High strength and good electrical conductivity in Cu-Cr alloys processed by severe plastic deformation. Materials Letters. 2015;153:5–9.
[75] Shangina D.V, Gubicza J, Dodony E, Bochvar N.R, Straumal P.B, Tabachkova N.Yu, Dobatkin S.V. Improvement of strength and conductivity in Cu-alloys with the application of High Pressure Torsion and subsequent heat-treatments. Journal of Materials Science. 2014;49:6674–6681.
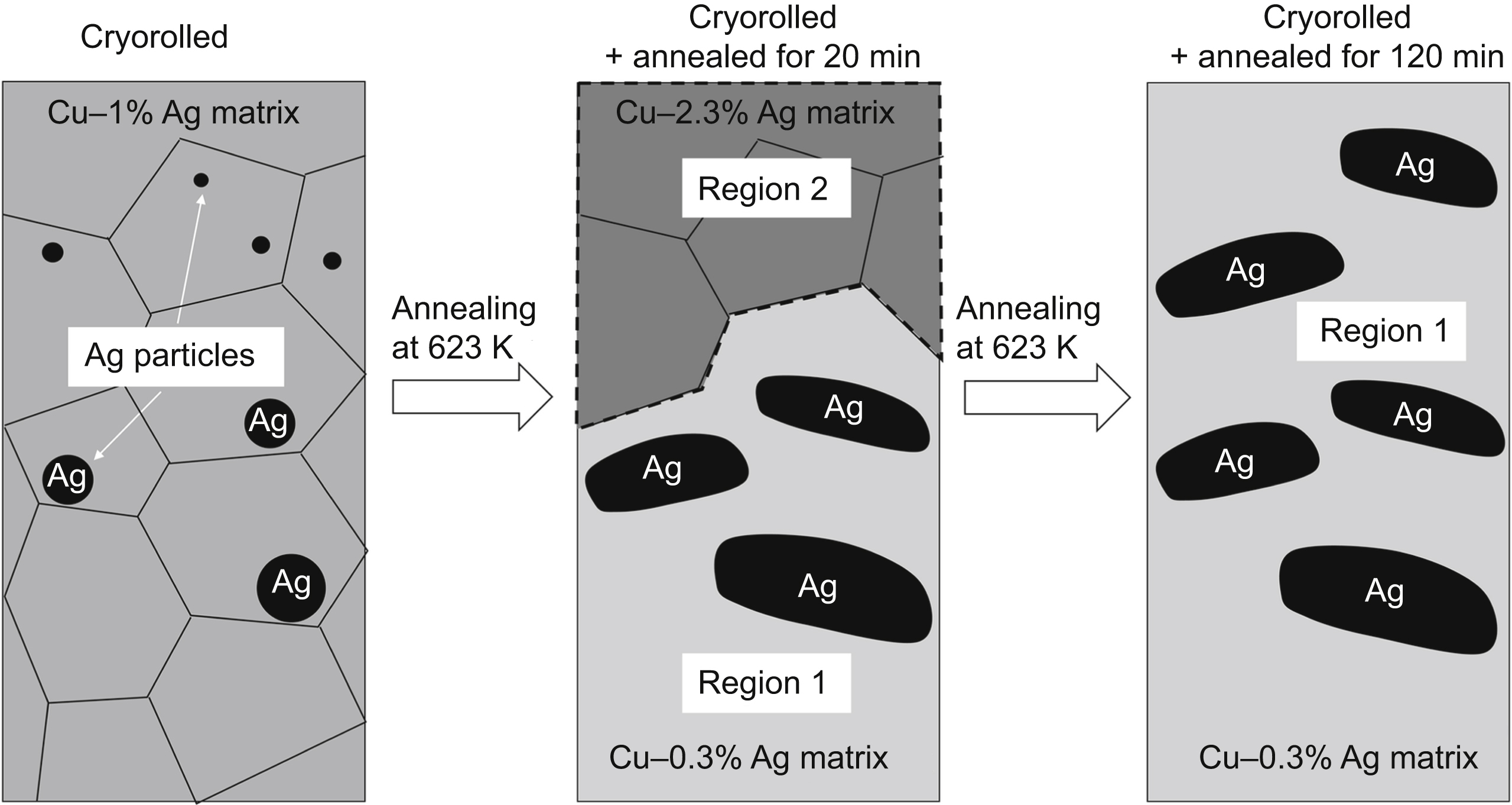
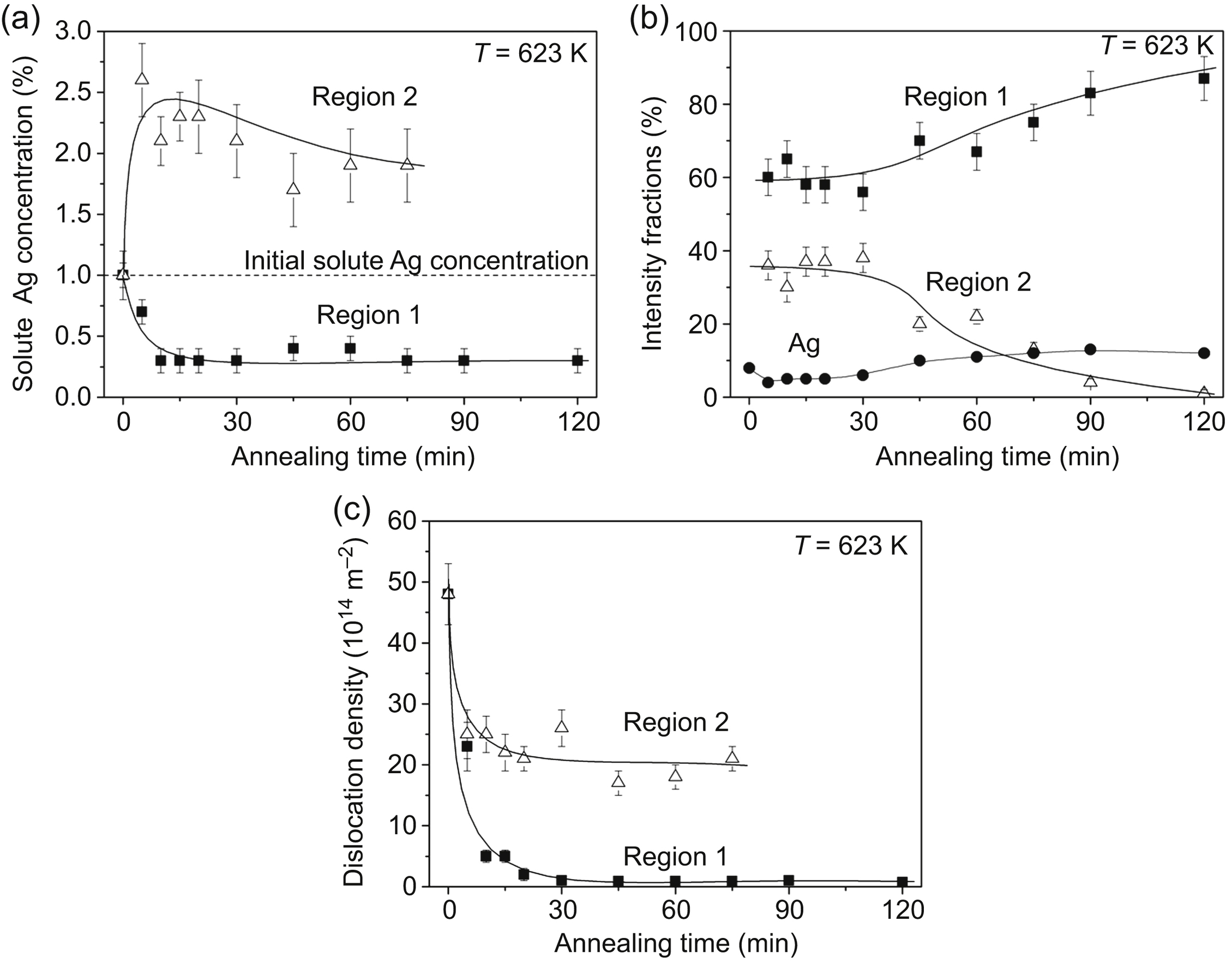
[76] Gubicza J, Hegedűs Z, Lábár J.L, Kauffmann A, Freudenberger J, Subramanya Sarma V. Solute redistribution during annealing of a cold rolled Cu–Ag alloy. Journal of Alloys and Compounds. 2015;623:96–103.
[77] Frost H.J, Ashby M.F. Deformation-Mechanism Maps: The Plasticity and Creep of Metals and Ceramics. Oxford: Pergamon; 1982.
[78] Schumacher S, Birringer R, Straus R, Gleiter H. Diffusion of silver in nanocrystalline copper between 303 and 373K. Acta Metallurgica. 1989;37:2485–2488.
[79] Li Y.S, Zhang Y, Tao N.R, Lu K. Effect of the Zener-Hollomon parameter on the microstructures and mechanical properties of Cu subjected to plastic deformation. Acta Materialia. 2009;57:761–772.
[80] Mishra A, Kad B.K, Gregori F, Meyers M.A. Microstructural evolution in copper subjected to severe plastic deformation: experiments and analysis. Acta Materialia. 2007;55:13–28.
[81] Dubravina A, Zehetbauer M.J, Schafler E, Alexandrov I.V. Correlation between domain size obtained by X-ray Bragg profile analysis and macroscopic flow stress in severely plastically deformed copper. Materials Science and Engineering A. 2004;387–389:817–821.
[82] Kuzel R, Matej Z, Cherkaska V, Pesicka J, Cizek J, Prochazka I, Islamgaliev R.K. Microstructure of severely deformed metals and its thermal stability. Journal of Alloys and Compounds. 2004;378:242–247.
[83] Schafler E, Dubravina A, Mingler B, Karnthaler H.P, Zehetbauer M. On the microstructure of HPT processed Cu under variation of deformation parameters. Materials Science Forum. 2006;503–504:51–56.
[84] Gubicza J, Chinh N.Q, Lábár J.L, Hegedűs Z, Langdon T.G. Twinning and dislocation activity in silver processed by severe plastic deformation. Journal of Materials Science. 2009;44:1656–1660.
[85] Kawasaki M, Horita Z, Langdon T.G. Microstructural evolution in high purity aluminum processed by ECAP. Materials Science and Engineering A. 2009;524:143–150.
[86] Xu C, Horita Z, Langdon T.G. Microstructural evolution in an aluminum solid solution alloy processed by ECAP. Materials Science and Engineering A. 2011;528:6059–6065.
[87] Liao X.Z, Kilmametov A.R, Valiev R.Z, Gao H, Li X, Mukherjee A.K, Bingert J.F, Zhu Y.T. High-pressure torsion-induced grain growth in electrodeposited nanocrystalline Ni. Applied Physics Letters. 2006;88:021909.
[88] Cizek J, Janecek M, Srba O, Kuzel R, Barnovska Z, Prochazka I, Dobatkin S. Evolution of defects in copper deformed by high-pressure torsion. Acta Materialia. 2011;59:2322–2329.
[89] Watts B.R. Conduction electrons scattering in dislocated metals. In: Nabarro F.R.N, ed. Dislocation in Solids. vol. 8. Amsterdam: Elsevier; 1989:175–419.
[90] Wollenberger H.J. Point defects. In: Cahn R.W, Haasen P, eds. Physical Metallurgy. vol. 9. Amsterdam: Elsevier; 1983:1189–1221.
[91] Varotsos P, Eftaxias K. High-temperature vacancy concentration in Cu. Physical Review B. 1989;40:9963–9964.
[92] Ungár T, Schafler E, Hanák P, Bernstorff S, Zehetbauer M. Vacancy production during plastic deformation in copper determined by in situ X-ray diffraction. Materials Science and Engineering A. 2007;462:398–401.
[93] Sitarama Raju K, Subramanya Sarma V, Kauffmann A, Hegedűs Z, Gubicza J, Peterlechner M, Freudenberger J, Wilde G. High strength and ductile ultrafine grained Cu-Ag alloy through bimodal grain size, dislocation density and solute distribution. Acta Materialia. 2013;61:228–238. .
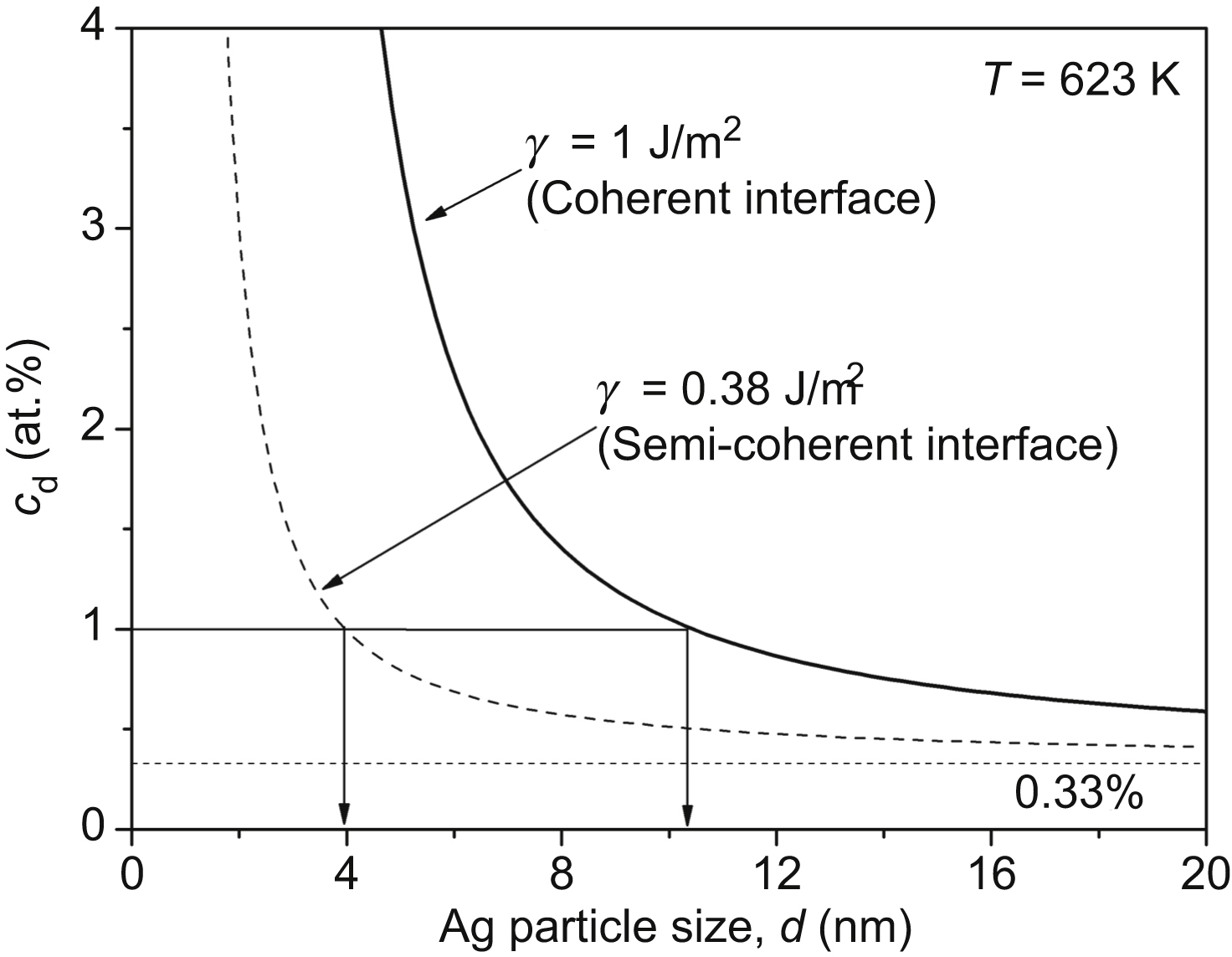
[94] Perez M. Gibbs-Thomson effect in phase transformations. Scripta Materialia. 2005;52:709–712.
[95] Kaptay G. Nano-Calphad: extension of the Calphad method to systems with nano-phases and complexions. Journal of Materials Science. 2012;47:8320–8335.
[96] Bacher P, Wynblatt P, Foiles S.M. A Monte Carlo study of the structure and composition of (001) semicoherent interphase boundaries in Cu-Ag-Au alloys. Acta Metallurgica et Materialia. 1991;39:2681–2691.
[97] Delogu F.J. Free energy differences between Ag−Cu nanophases with different chemical order. Journal of Physical Chemistry C. 2010;114:19946–19951.
[98] Han K, Vasquez A.A, Xin Y, Kalu P.N. Microstructure and tensile properties of nanostructured Cu-25wt%Ag. Acta Materialia. 2003;51:767–780.
[99] Liu J.B, Zeng Y.W, Meng L. Interface structure and energy in Cu–71.8 wt.% Ag. Journal of Alloys and Compounds. 2008;464:168–173.
[100] Tian Y.Z, Zhang Z.F. Bulk eutectic Cu–Ag alloys with abundant twin boundaries. Scripta Materialia. 2012;66:65–68.
[101] Hong S.I, Hill M.A. Microstructural stability and mechanical response of Cu-Ag microcomposite wires. Acta Materialia. 1998;46:4111–4122.
[102] Liu J.B, Zhang L, Yao D.W, Meng L. Microstructure evolution of Cu/Ag interface in the Cu–6 wt.% Ag filamentary nanocomposite. Acta Materialia. 2011;59:1191–1197.
[103] Chinh N.Q, Valiev R.Z, Sauvage X, Varga G, Havancsák K, Kawasaki M, Straumal B.B, Langdon T.G. Grain boundary phenomena in an ultrafine-grained Al–Zn alloy with improved mechanical behavior for micro-devices. Advanced Engineering Materials. 2014;16:1000–1009.





 -type basal and nonbasal edge dislocations, and (c) screw dislocations in the center of an AZ31 disk as a function of high-pressure torsion (HPT) revolutions [35].
-type basal and nonbasal edge dislocations, and (c) screw dislocations in the center of an AZ31 disk as a function of high-pressure torsion (HPT) revolutions [35].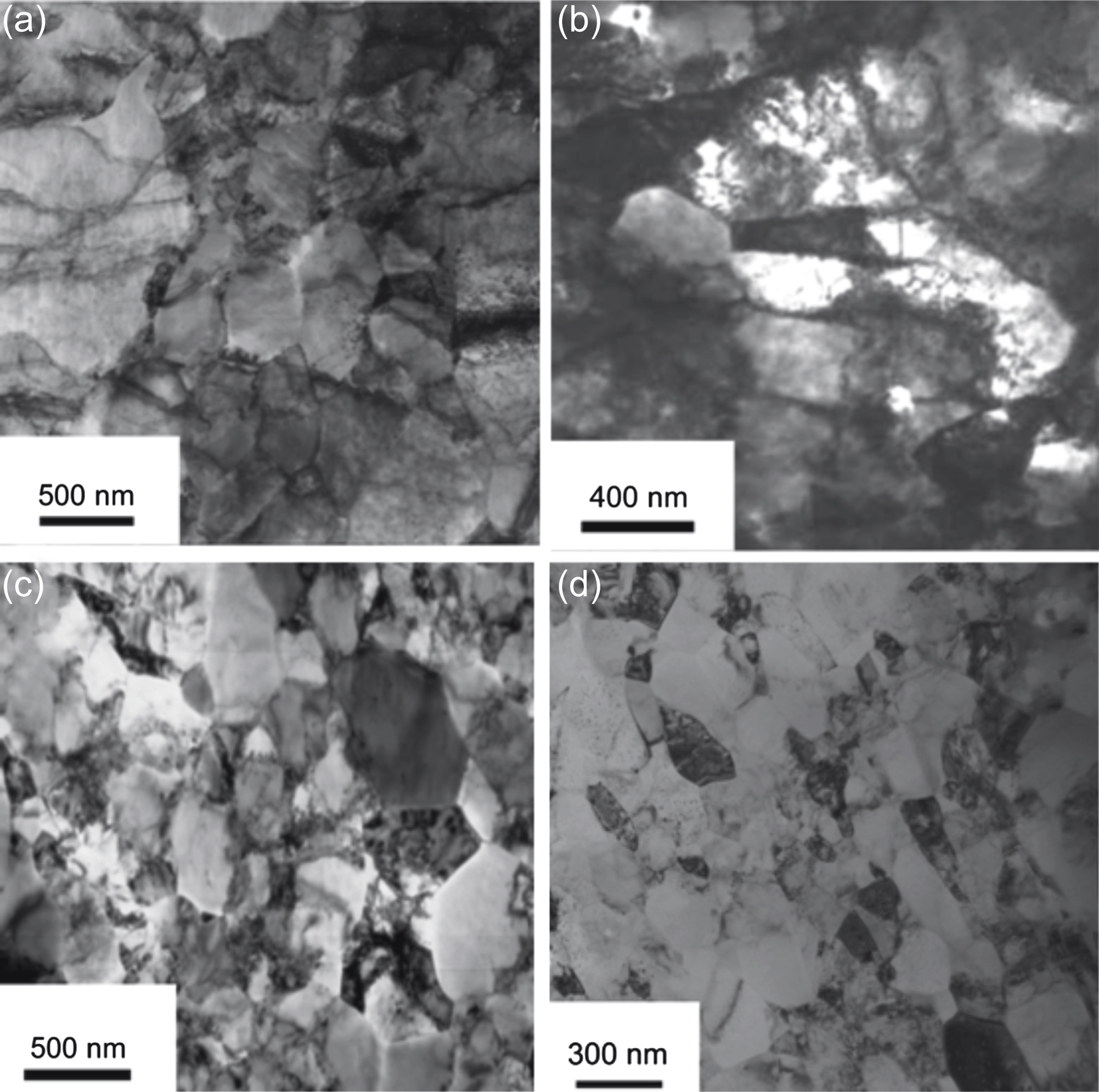


![]() (3.1)
(3.1)![]() (3.2)
(3.2)![]() (3.3)
(3.3)![]() (3.4)
(3.4)


![]() (3.5)
(3.5)


![]() (3.6)
(3.6)![]() (3.7)
(3.7)![]() (3.8)
(3.8)