Effect of Lattice Imperfections on Electrical Resistivity of Nanomaterials
Abstract
In this chapter the influence of lattice defects (vacancies, vacancy clusters, dislocations, planar faults, and grain boundaries) on the electrical resistivity of ultrafine-grained (UFG) and nanocrystalline materials is overviewed. First, the residual resistivities caused by different defects are compared with the intrinsic resistivity at different temperatures. It turned out that the resistivities of vacancies, dislocations, and twin faults are much smaller than that for high-angle grain boundaries (HAGBs), solute atoms, and the intrinsic resistivity at room temperature. For pure metals and equilibrium solid solutions, nanocrystallization by severe plastic deformation (SPD) methods yields only a few percentage increase in resistivity while the strength is improved considerably, thereby improving the strength-to-resistivity ratio. The addition of solute atoms to metallic matrices leads to an increment in both strength and resistivity in SPD-processed UFG materials; however, the strength-to-resistivity ratio is only slightly changed. The best combination of high strength and good conductivity in alloys is obtained if the strengthening is achieved by grain boundaries and nanosized secondary phase particles while the grain interiors are purified from solute elements. This microstructure can be processed by a sequential application of SPD and annealing. In pure metallic materials an improvement in strength-to-resistivity ratio can be achieved if the majority of HAGBs are substituted by coherent twin faults as the latter interfaces have very low specific electrical resistivity. In electrodeposited and sputtered films, nanoscale growth twin lamellae in UFG grains give a strong increase in strength while the conductivity is only slightly smaller than that for a coarse-grained defect-free material.
Keywords
9.1. Contribution of Lattice Defects to Electrical Resistivity

![]() (9.1)
(9.1)
![]() (9.2)
(9.2)
![]() (9.3)
(9.3)
![]() (9.4)
(9.4)
![]() (9.5)
(9.5)
![]() (9.6)
(9.6)
Table 9.1
Specific electrical resistivities for vacancies, dislocations, stacking faults, twin faults, and general high-angle grain boundaries (HAGBs) in Cu
| Metal | Vacancy, ηv (Ωm) | Dislocation, ηd (Ωm3) | Stacking fault, ηsf (Ωm2) | Twin fault, ηTB (Ωm2) | Grain boundary, ηHAGB (Ωm2) |
| Cu | 1.9 × 10−6 | 1.3 × 10−25 | 3.4 × 10−17 | 1.7 × 10−17 | 3.4 × 10−16 |
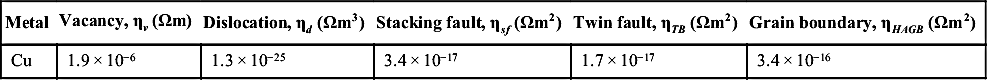
![]() (9.7)
(9.7)
![]() (9.8)
(9.8)
![]() (9.9)
(9.9)
![]() (9.10)
(9.10)
 (9.11)
(9.11)
![]() (9.12)
(9.12)
9.2. Change of Resistivity in Nanomaterials Processed by Severe Plastic Deformation
Table 9.2
Grain size, mechanical strength, conductivity in IACS, resistivity, and strength-to-resistivity ratio measured at RT for different UFG and nanocrystalline materials
| Material and processing method | Grain size (nm) | Strength (MPa) | Conductivity in IACS (%) | Resistivity (10−8 Ωm) | Strength-to-resistivity ratio (1010 MPa/Ωm) | References |
| 99.96% purity Cu, 16 ECAP at RT | 500 | 350 | 95 | 1.8 | 1.9 | [19] |
| 99.999% purity Cu film, magnetron sputtering, twin spacing: 7 nm | 70 | 930∗ | 74 | 2.3 | 4.0 | [10,26] |
| 99.999% purity Cu film, magnetron sputtering, twin spacing: 16 nm | 146 | 700∗ | 100 | 1.7 | 4.1 | [10,26] |
| 99.998% purity Cu, pulsed electrodeposition, twin spacing: 15 nm | 400 | 900 | 97 | 1.75 | 5.1 | [27] |
| Cu, SPS + CD | 220 | 590 | 78 | 2.2 | 2.7 | [28] |
| Cu–0.18 wt.% Zr with Cu5Zr precipitates, 15 HPT at RT | 200 | 530∗ | 59 | 2.9 | 1.8 | [22] |
| Cu–0.18 wt.% Zr solid solution, 15 HPT at RT | 200 | 570∗ | 51 | 3.4 | 1.7 | [22] |
| Cu–0.18 wt.% Zr with Cu5Zr precipitates, 15 HPT at RT + annealing for 1 h at 723 K | – | 570∗ | 75 | 2.3 | 2.5 | [22] |
| Cu–0.18 wt.% Zr solid solution, 15 HPT at RT + annealing for 1 h at 723 K | – | 600∗ | 57 | 3.0 | 2.0 | [22] |
| Cu–3 wt.% Ag, 8 ECAP at 343 K | 350 | 617 | 88 | 1.9 | 3.2 | [20] |
| Cu–1.1 wt.% Al2O3, cold spray | 37 | 700∗ | 31 | 5.5 | 1.3 | [14] |
| Cu–1.1 wt.% Al2O3, cold spray + annealing at 1223 K | 55 | 670∗ | 47 | 3.7 | 1.8 | [14] |
| Cu–1vol.% Al2O3, ball milling + HIP | 170 | 460 | 50 | 3.4 | 1.4 | [24] |
| Cu–5vol.% Al2O3, ball milling + HIP | 170 | 620 | 44 | 3.9 | 1.6 | [24] |
| Cu–4 wt.% Al2O3, ball milling + compaction by hot-pressing | – | 600∗ | 47 | 3.7 | 1.6 | [25] |
| Cu–0.5 wt.% Al2O3, 4 ECAP at RT | 370 | 525 | 86 | 2.0 | 2.6 | [23] |
| Table Continued | ||||||

| Material and processing method | Grain size (nm) | Strength (MPa) | Conductivity in IACS (%) | Resistivity (10−8 Ωm) | Strength-to-resistivity ratio (1010 MPa/Ωm) | References |
| Cu–1.1 wt.% Al2O3, 4 ECAP at RT | 350 | 560 | 82 | 2.1 | 2.7 | [23] |
| Cu–0.3 wt.% Mg–0.05 wt.% Ce, 8 ECAP at RT | 600 | 520 | 72 | 2.4 | 2.2 | [29] |
| Cu–0.3 wt.% Mg–0.05 wt.% Ce, 8 ECAP at RT + annealing for 2 h at 573 K | – | 510 | 75 | 2.3 | 2.2 | [29] |
| Cu–0.2 wt.% Mg, 4 ECAP + CR + CD at RT | 100–200 | 567 | 82 | 2.1 | 2.7 | [30] |
| Cu–0.2 wt.% Mg, 4 ECAP + CR + CD at RT + annealing for 2 h at 573 K | – | 426 | 87 | 2.0 | 2.1 | [30] |
| Cu–0.4 wt.% Mg, 4 ECAP + CR + CD at RT | 100–200 | 597 | 72 | 2.4 | 2.5 | [30] |
| Cu–0.4 wt.% Mg, 4 ECAP + CR + CD at RT + annealing for 2 h at 573 K | – | 488 | 80 | 2.1 | 2.3 | [30] |
| Cu–0.75 wt.% Cr, Q + 5 HPT at RT | 209 | 580∗ | 34 | 5.0 | 1.2 | [31] |
| Cu–0.75 wt.% Cr, Q + 5 HPT at RT + annealing for 1 h at 523 K | 245 | 610∗ | 35 | 4.9 | 1.2 | [31] |
| Cu–0.75 wt.% Cr, SC + 5 HPT at RT | – | 537∗ | 61 | 2.8 | 1.9 | [31] |
| Cu–0.75 wt.% Cr, SC + 5 HPT at RT + annealing for 1 h at 523 K | – | 478∗ | 72 | 2.4 | 2.0 | [31] |
| Cu–9.85 wt.% Cr, Q + 5 HPT at RT | 143 | 713∗ | 29 | 5.9 | 1.2 | [31] |
| Cu–9.85 wt.% Cr, Q + 5 HPT at RT + annealing for 1 h at 773 K | 229 | 584∗ | 67 | 2.6 | 2.2 | [31] |
| Cu–9.85 wt.% Cr, SC + 5 HPT at RT | – | 702∗ | 54 | 3.2 | 2.2 | [31] |
| Cu–9.85 wt.% Cr, SC + 5 HPT at RT + annealing for 1 h at 773 K | – | 631∗ | 76 | 2.3 | 2.7 | [31] |
| Cu–27 wt.% Cr, as-cast + 5 HPT at RT | 40 | 900∗ | 20 | 8.6 | 1.1 | [31] |
| Cu–27wt.% Cr, as-cast + 5 HPT at RT + annealing for 1 h at 773 K | 96 | 879∗ | 42 | 4.1 | 2.1 | [31] |
| Table Continued | ||||||

| Material and processing method | Grain size (nm) | Strength (MPa) | Conductivity in IACS (%) | Resistivity (10−8 Ωm) | Strength-to-resistivity ratio (1010 MPa/Ωm) | References |
| Cu–0.9 wt.% Hf, Q + 5 HPT at RT | 155 | 660∗ | 41 | 4.2 | 1.6 | [32] |
| Cu–0.9 wt.% Hf, Q + 5 HPT at RT + annealing for 1 h at 723 K | 189 | 800∗ | 71 | 2.4 | 3.3 | [32] |
| Cu–0.9 wt.% Hf, SC + 5 HPT at RT | – | 660∗ | 50 | 3.4 | 1.9 | [32] |
| Cu–0.9 wt.% Hf, SC + 5 HPT at RT + annealing for 1 h at 723 K | – | 600∗ | 80 | 2.1 | 2.9 | [32] |
| Cu–0.7 wt.% Cr–0.9 wt.% Hf, Q + 5 HPT at RT | 108 | 800∗ | 23 | 7.4 | 1.1 | [32] |
| Cu–0.7 wt.% Cr–0.9 wt.% Hf, Q + 5 HPT at RT + annealing for 1 h at 773 K | 131 | 933∗ | 61 | 2.8 | 3.3 | [32] |
| Cu–0.7 wt.% Cr–0.9 wt.% Hf, SC + 5 HPT at RT | – | 673∗ | 46 | 3.7 | 1.8 | [32] |
| Cu–0.7 wt.% Cr–0.9 wt.% Hf, SC + 5 HPT at RT + annealing for 1 h at 773 K | – | 770∗ | 67 | 2.6 | 3.0 | [32] |
| 99.5 wt.% Al, 10 HPT at RT | 810 | 200∗ | 57 | 3.0 | 0.7 | [33] |
| Al–0.3 wt.% Si–0.4 wt.% Fe (AA 1050), 4 HE at RT | 449 | 167∗ | 60 | 2.8 | 0.6 | [34] |
| Al–0.3 wt.% Si–0.4 wt.% Fe (AA 1050), 4 ECAP at RT | 539 | 163∗ | 60 | 2.8 | 0.6 | [34] |
| Al–0.3 wt.% Si–0.4 wt.% Fe (AA 1050), 2 ECAP + 4 HE at RT | 454 | 167∗ | 59 | 2.9 | 0.6 | [34] |
| Al–4.6 wt.% Mg–0.9 wt.% Mn–0.1 wt.% Si–0.1 wt.% Fe (AA 5483), 4 HE at RT | 199 | 510∗ | 26 | 6.6 | 0.8 | [34] |
| Al–4.6 wt.% Mg–0.9 wt.% Mn–0.1 wt.% Si–0.1 wt.% Fe (AA 5483), 2 ECAP at RT | 215 | 437∗ | 26 | 6.6 | 0.7 | [34] |
| Al–4.6 wt.% Mg–0.9 wt.% Mn–0.1 wt.% Si–0.1 wt.% Fe (AA 5483), 2 ECAP + 4 HE at RT | 185 | 493∗ | 26 | 6.6 | 0.8 | [34] |
| Al–0.8 wt.% Mg–0.8 wt.% Si–0.1 wt.% Fe alloy (Al 6201), 20 HPT at RT | 130 | 448 | 48 | 3.6 | 1.2 | [35] |
| Table Continued | ||||||

| Material and processing method | Grain size (nm) | Strength (MPa) | Conductivity in IACS (%) | Resistivity (10−8 Ωm) | Strength-to-resistivity ratio (1010 MPa/Ωm) | References |
| Al–0.8 wt.% Mg–0.8 wt.% Si–0.1 wt.% Fe alloy (Al 6201), 20 HPT at 403 K | 280 | 380 | 56 | 3.1 | 1.2 | [35] |
| Al–0.8 wt.% Mg–0.8 wt.% Si–0.1 wt.% Fe alloy (Al 6201), 20 HPT at 453 K | 440 | 326 | 58 | 2.9 | 1.1 | [35] |
| Al–0.8 wt.% Mg–0.8 wt.% Si–0.1 wt.% Fe alloy (Al 6201), 20 HPT at 503 K | 960 | 218 | 59 | 2.9 | 0.8 | [35] |
| Al–0.6 wt.% Mg–0.5 wt.% Si–0.1 wt.% Fe alloy (Al 6101), 20 HPT at RT | 180 | 570∗ | 47 | 3.7 | 1.5 | [36] |
| Al–0.6 wt.% Mg–0.5 wt.% Si–0.1 wt.% Fe alloy (Al 6101), 20 HPT at 373 K | 240 | 440∗ | 52 | 3.3 | 1.3 | [36] |
| Al–0.6 wt.% Mg–0.5 wt.% Si–0.1 wt.% Fe alloy (Al 6101), 20 HPT at 443 K | 430 | 240∗ | 59 | 2.9 | 0.8 | [36] |
| Al–5.4 wt.% Ce–3.1 wt.% La, 20 HPT at RT | 136 | 475 | 40 | 4.3 | 1.1 | [37] |
| Al–5.4 wt.% Ce–3.1 wt.% La, 20 HPT at RT + annealing for 1 h at 553 K | 203 | 495 | 45 | 3.9 | 1.3 | [37] |
| Al–5.4 wt.% Ce–3.1 wt.% La, 20 HPT at RT + annealing for 1 h at 673 K | 385 | 255 | 52 | 3.3 | 0.8 | [37] |
| Ti (50% α + 50% ω), 10 HPT at 100 K | 54 | 1300∗ | 2.1 | 80 | 0.16 | [38] |
| Ti (20% α + 80% ω), 10 HPT at RT | 118 | 1200∗ | 1.9 | 90 | 0.13 | [38] |
| Fe–0.2 wt.% Mn (low carbon steel), 4 CGP at RT | 231 | 400 | 1.7 | 100 | 0.04 | [21] |

9.3. Processing of Nanomaterials With High Hardness and Good Conductivity
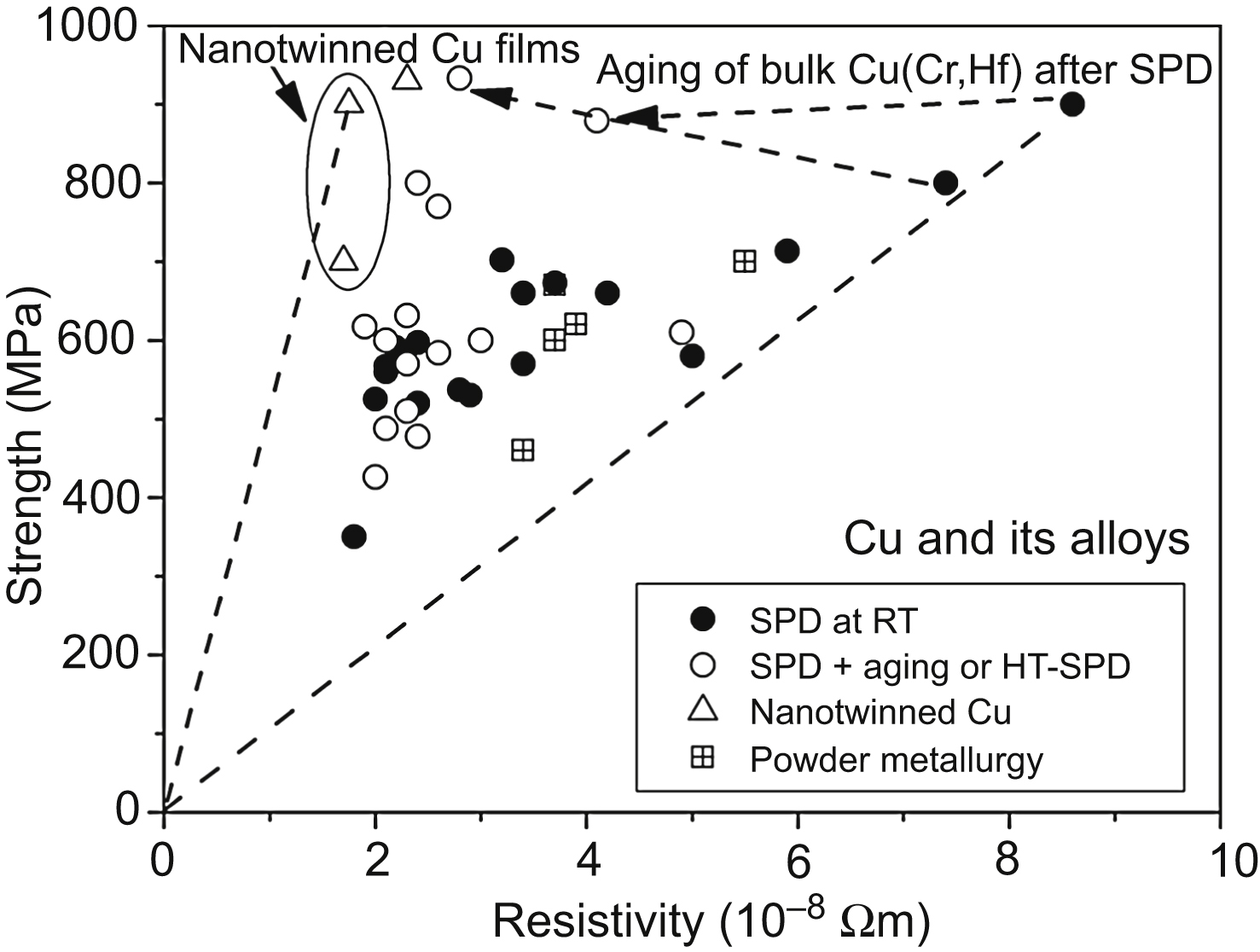
9.4. Electrical Resistivity of Nanostructured Films
![]() (9.14)
(9.14)
Table 9.3
Grain size, mechanical strength, conductivity in International Annealed Copper Standard (IACS), resistivity, and strength-to-resistivity ratio measured at room temperature (RT) for Cu/Cr multilayers with different layer thicknesses. The strength is calculated as one-third of the hardness [41,43]
| Material and processing method | Grain size (nm) | Strength (MPa) | Conductivity in IACS (%) | Resistivity (10−8 Ωm) | Strength-to-resistivity ratio (1010 MPa/Ωm) |
| Cu–Cr multilayer sputtered at RT, layer thickness: 50 nm | 32/10 (Cu/Cr) | 2000 | 24 | 7.0 | 2.9 |
| Cu–Cr multilayer, sputtered at RT, layer thickness: 25 nm | – | 2070 | 18 | 9.5 | 2.2 |
| Cu–Cr multilayer sputtered at RT, layer thickness: 10 nm | 9/7.5 (Cu/Cr) | 2330 | 11 | 15 | 1.6 |
| Cu–Cr multilayer sputtered at RT, layer thickness: 5 nm | 5/5 (Cu/Cr) | 2330 | 9 | 20 | 1.2 |
| Cu–Cr multilayer sputtered at RT, layer thickness: 2.5 nm | 2.5/2.5 (Cu/Cr) | 2400 | 6 | 27.5 | 0.9 |







