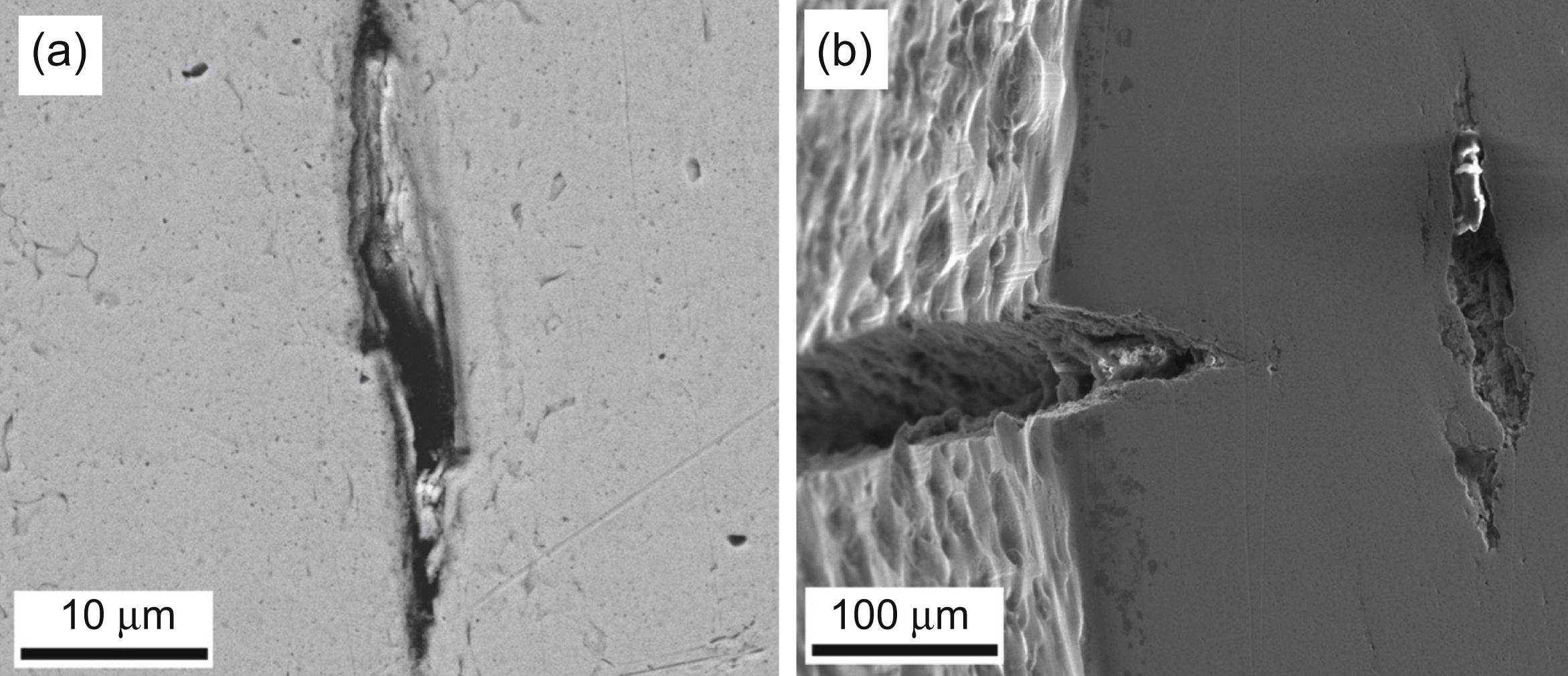
[1] Jiang H, Zhu Y.T, Butt D.P, Alexandrov I.V, Lowe T.C. Microstructural evolution, microhardness and thermal stability of HPT-processed Cu. Materials Science and Engineering, A. 2000;290:128–138.
[2] Takata N, Yamada K, Ikeda K, Yoshida F, Nakashima H, Tsuji N. Annealing behavior and recrystallized texture in ARB processed copper. Materials Science Forum. 2006;503–504:919–924.
[3] Gubicza J, Nam N.H, Balogh L, Hellmig R.J, Stolyarov V.V, Estrin Y, Ungár T. Microstructure of severely deformed metals determined by X-ray peak profile analysis. Journal of Alloys and Compounds. 2004;378:248–252.
[4] Gubicza J, Balogh L, Hellmig R.J, Estrin Y, Ungár T. Dislocation structure and crystallite size in severely deformed copper by X-ray peak profile analysis. Materials Science and Engineering, A. 2005;400–401:334–338.
[5] Schafler E, Steiner G, Korznikova E, Kerber M, Zehetbauer M.J. Lattice defect investigation of ECAP-Cu by means of X-ray line profile analysis, calorimetry and electrical resistometry. Materials Science and Engineering, A. 2005;410–411:169–173.
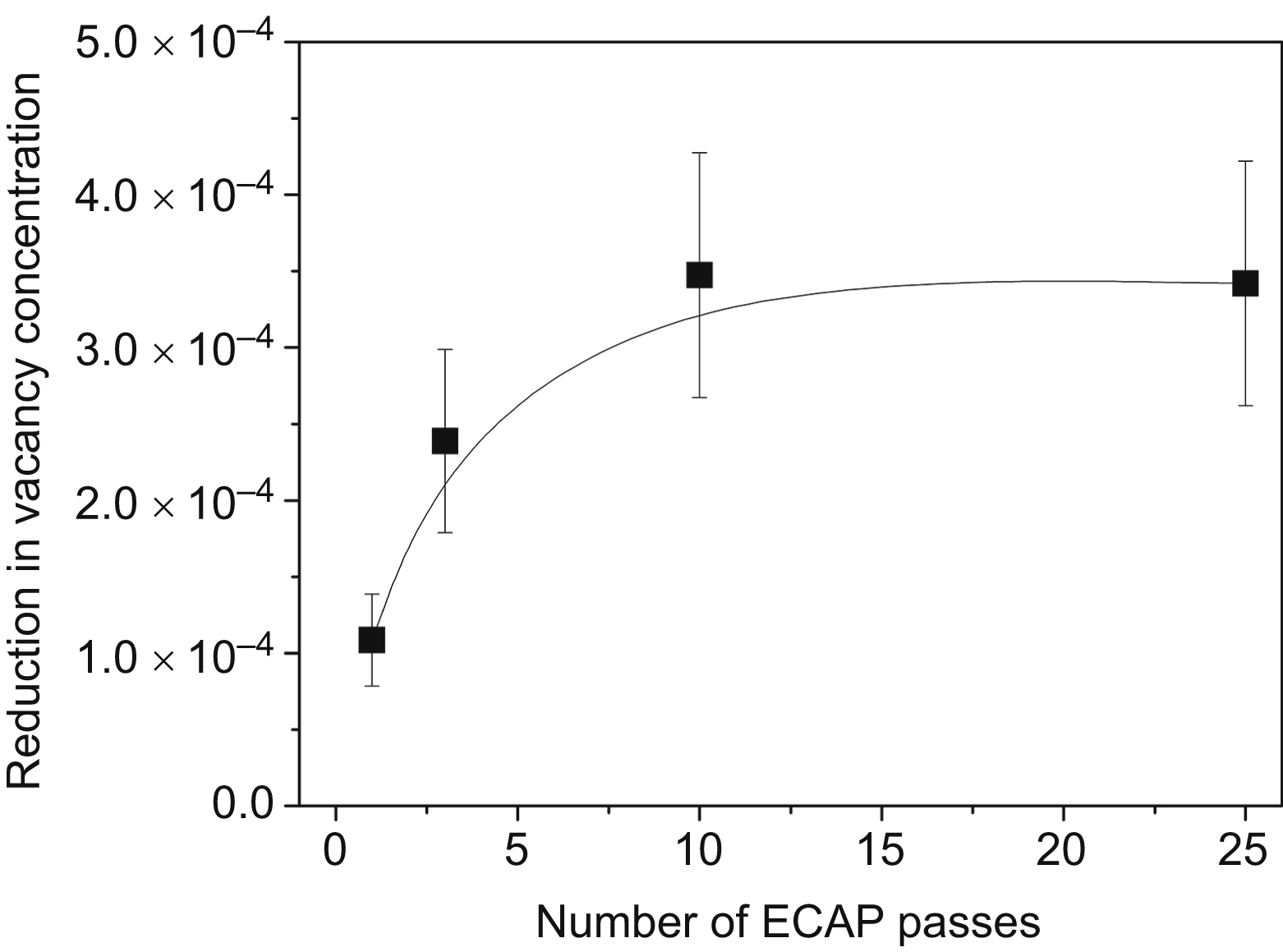
[6] Setman D, Schafler E, Korznikova E, Zehetbauer M.J. The presence and nature of vacancy type defects in nanometals detained by severe plastic deformation. Materials Science and Engineering, A. 2008;493:116–122.
[7] Gao N, Starink M.J, Langdon T.G. Using differential scanning calorimetry as an analytical tool for ultrafine grained metals processed by severe plastic deformation. Materials Science and Technology. 2009;25:687–698. .
[8] Dalla Torre F, Lapovok R, Sandlin J, Thomson P.F, Davies C.H.J, Pereloma E.V. Microstructures and properties of copper processed by equal channel angular extrusion for 1–16 passes. Acta Materialia. 2004;52:4819–4832.
[9] Gubicza J, Chinh N.Q, Lábár J.L, Dobatkin S, Hegedűs Z, Langdon T.G. Correlation between microstructure and mechanical properties of severely deformed metals. Journal of Alloys and Compounds. 2009;483:271–274.
[10] Wang Y.M, Ma E. Three strategies to achieve uniform tensile deformation in a nanostructured metal. Acta Materialia. 2004;52:1699–1709.
[11] Valiev R.Z. Nanomaterial advantage. Nature. 2002;419:887–889.
[12] Gubicza J, Dobatkin S.V, Khosravi E, Kuznetsov A.A, Lábár J.L. Microstructural stability of Cu processed by different routes of severe plastic deformation. Materials Science and Engineering, A. 2011;528:1828–1832.
[13] Setman D, Kerber M.B, Schafler E, Zehetbauer M.J. Activation enthalpies of deformation-induced lattice defects in severe plastic deformation nanometals measured by differential scanning calorimetry. Metallurgical and Materials Transactions, A. 2010;41:810–815.
[14] Zhilyaev A.P, Nurislamova G.V, Surinach S, Baró M.D, Langdon T.G. Calorimetric measurements of grain growth in ultrafine-grained nickel. Material Physics and Mechanics. 2002;5:23–30.
[15] Zhilyaev A.P, Gubicza J, Nurislamova G, Révész Á, Suriñach S, Baró M.D, Ungár T. Microstructural characterization of ultrafine-grained nickel. Physica Status Solidi, (a). 2003;198:263–271.
[16] Kissinger H.E. Reaction kinetics in differential thermal analysis. Analytical Chemistry. 1957;29:1702–1706.
[17] Lugo N, Llorca N, Sunol J.J, Cabrera J.M. Thermal stability of ultrafine grains size of pure copper obtained by equal-channel angular pressing. Journal of Materials Science. 2010;45:2264–2273.
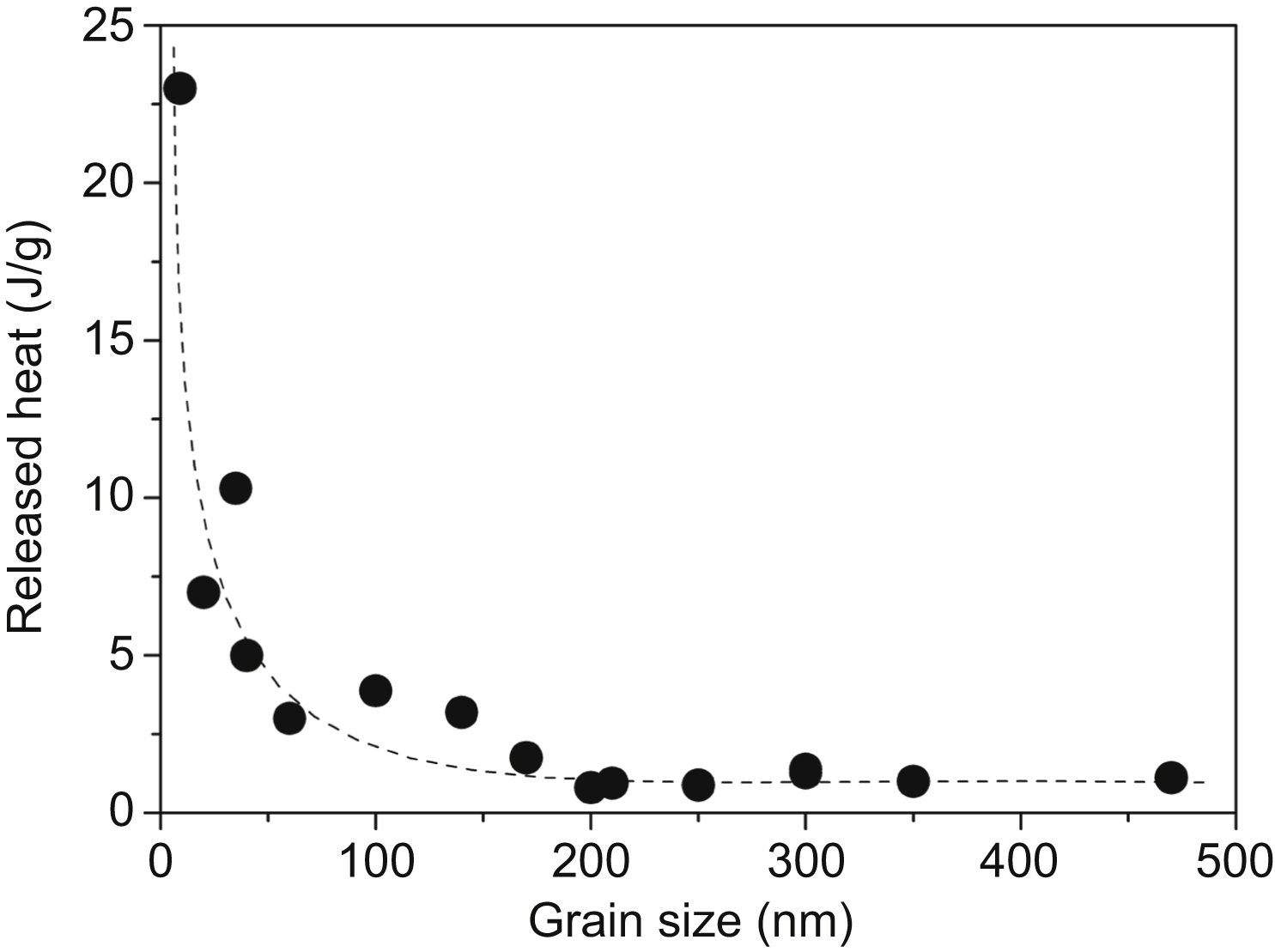
[18] Cao W.Q, Gu C.F, Pereloma E.V, Davies C.H.J. Stored energy, vacancies and thermal stability of ultrafine grained copper. Materials Science and Engineering, A. 2008;492:74–79.
[19] Cizek J, Prochazka I, Cieslar M, Kuzel R, Kuriplach J, Chmelik F, Stulikova I, Becvar F, Melikhova O. Thermal stability of ultrafine grained copper. Physical Review, B. 2002;65:094106.
[20] Jenei P, Gubicza J, Yoon E.Y, Kim H.S, Lábár J.L. High temperature thermal stability of pure copper and copper – carbon nanotube composites consolidated by high pressure torsion. Composites: A. 2013;51:71–79.
[21] Huang Y.K, Menovsky A.A, de Boer F.R. Calorimetric analysis of the grain growth in nanocrystalline copper samples. Nanostructured Materials. 1993;2:587–595.
[22] Kumpmann A, Günther B, Kunze H.-D. Thermal stability of ultrafine-grained metals and alloys. Materials Science and Engineering, A. 1993;168:165–169.
[23] Abib K, Hadj Larbi F, Rabahi L, Alili B, Bradai D. DSC analysis of commercial Cu–Cr–Zr alloy processed by equal channel angular pressing. Transactions of Nonferrous Metals Society of China. 2015;25:838–843.
[24] Zhilyaev A.P, Kim B.-K, Szpunar J.A, Baro M.D, Langdon T.G. The microstructural characteristics of ultrafine-grained nickel. Materials Science and Engineering, A. 2005;391:377–389.
[25] Zhilyaev A.P, Nurislamova G.V, Valiev R.Z, Baro M.D, Langdon T.G. Thermal stability and microstructural evolution in ultrafine-grained nickel after equal-channel angular pressing (ECAP). Metallurgical and Materials Transactions, A. 2002;33:1865–1868.
[26] Wang N, Wang Z, Aust K.T, Erb U. Isokinetic analysis of nanocrystalline nickel electrodeposits upon annealing. Acta Materialia. 1997;45:1655–1669.
[27] Hegedűs Z, Gubicza J, Kawasaki M, Chinh N.Q, Süvegh K, Fogarassy Z, Langdon T.G. High temperature thermal stability of ultrafine-grained silver processed by equal-channel angular pressing. Journal of Materials Science. 2013;48:1675–1684.
[28] Hegedűs Z, Gubicza J, Szommer P, Chinh N.Q, Huang Y, Langdon T.G. Inhomogeneous softening during annealing of ultrafine-grained silver processed by HPT. Journal of Materials Science. 2013;48:7384–7391.
[29] Zhou F, Liao X.Z, Zhu Y.T, Dallek S, Lavernia E.J. Microstructural evolution during recovery and recrystallization of a nanocrystalline Al–Mg alloy prepared by cryogenic ball milling. Acta Materialia. 2003;51:2777–2791. .
[30] Adamczyk-Cieslak B, Mizera J, Kurzydłowski K.J. Thermal stability of model Al–Li alloys after severe plastic deformation – effect of the solute Li atoms. Materials Science and Engineering, A. 2010;527:4716–4722.
[31] Scholz F, Woldt E. The release of stored energy during recovery and recrystallization of cold rolled ultra high purity iron. Journal of Thermal Analysis and Calorimetry. 2001;64:895–903.
[32] Malow T.R, Koch C.C. Grain growth in nanocrystalline iron prepared by mechanical attrition original. Acta materialia. 1997;45:2177–2186.
[33] Hazra S.S, Gazder A.A, Pereloma E.V. Stored energy of a severely deformed interstitial free steel. Materials Science and Engineering, A. 2009;524:158–167.
[34] Hibbard G, Aust K.T, Palumbo G, Erb U. Thermal stability of electrodeposited nanocrystalline cobalt. Scripta Materialia. 2001;44:513–518.
[35] Hoseini M, Pourian M.H, Bridier F, Vali H, Szpunar J.A, Bocher P. Thermal stability and annealing behaviour of ultrafine grained commercially pure titanium. Materials Science and Engineering, A. 2012;532:58–63.
[36] Zhang X, Wang H, Koch C.C. Mechanical behavior of bulk ultrafine-grained and nanocrystalline Zn. Reviews in Advanced Materials Science. 2004;6:53–93.
[37] Lian J, Valiev R.Z, Baudelet B. On the enhanced grain growth in ultrafine grained metals. Acta Metallurgica et Materialia. 1995;43:4165–4170.
[38] Tjong S.C, Chen H. Nanocrystalline materials and coatings. Materials Science and Engineering, R. 2004;45:1–88.
[39] Kuo C.-M, Lin C.-S. Static recovery activation energy of pure copper at room temperature. Scripta Materialia. 2007;57:667–670.
[40] Divinski S, Ribbe J, Schmitz G, Herzig C. Grain boundary diffusion and segregation of Ni in Cu. Acta Materialia. 2007;55:3337–3346.
[41] Murr L.E. Interfacial Phenomena in Metals and Alloys. Reading: Addison Wesley; 1975.
[42] Hirth J.P, Lothe J. Theory of Dislocations. New York: Wiley; 1982.
[43] Folegati P, Makkonen I, Ferragut R, Puska M.J. Analysis of electron-positron momentum spectra of metallic alloys as supported by first-principles calculations. Physical Review B. 2007;75:054201.
[44] Linderoth S, Hidalgo C. Direct evidence for positron annihilation from shallow traps. Physical Review B. 1987;36:4054.
[45] Hakkinen H, Makinen S, Manninen M. Edge dislocations in fcc metals: microscopic calculations of core structure and positron states in Al and Cu. Physical Review B. 1990;41:12441.
[46] Schaefer H.-E, Wurschum R, Birringer R, Gleiter H. Structure of nanometer-sized polycrystalline iron investigated by positron lifetime spectroscopy. Physical Review B. 1988;38:9545.
[47] Humphreys F.J, Hatherly M. Recrystallization and Related Annealing Phenomena. second ed. Oxford: Elsevier; 2004.
[48] Wolf D. Effect of interatomic potential on the calculated energy and structure of high-angle coincident site grain boundaries—II. (100) Twist boundaries in Cu, Ag and Au. Acta Metallurgica. 1984;32:735–748.
[49] Kawasaki M, Horita Z, Langdon T.G. Microstructural evolution in high purity aluminum processed by ECAP. Materials Science and Engineering, A. 2009;524:143–150.
[50] An X.H, Wu S.D, Zhang Z.F, Figueiredo R.B, Gao N, Langdon T.G. Evolution of microstructural homogeneity in copper processed by high-pressure torsion. Scripta Materialia. 2010;63:560–563.
[51] Tokunaga T, Kaneko K, Horita Z. Production of aluminium–matrix carbon nanotube composite using high pressure torsion. Materials Science and Engineering, A. 2008;490:300–304.
[52] Li H, Misra A, Zhu Y, Horita Z, Koch C.C, Tokunaga T.G. Processing and characterization of nanostructured Cu–carbon nanotube composites. Materials Science and Engineering, A. 2009;523:60–64.
[53] Zhou K, Li H, Pang J.B, Wang Z. Investigation of microstructure thermal evolution in nanocrystalline Cu. Physica B. 2011;406:760–765. .
[54] Hegedűs Z, Gubicza J, Kawasaki M, Chinh N.Q, Lábár J.L, Langdon T.G. Stability of the ultrafine-grained microstructure in silver processed by ECAP and HPT. Journal of Materials Science. 2013;48:4637–4645.
[55] Figueiredo R.B, Cetlin P.R, Langdon T.G. Using finite element modeling to examine the flow processes in quasi-constrained high-pressure torsion. Materials Science and Engineering, A. 2011;528:8198–8204.
[56] Figueiredo R.B, Pereira P.H.R, Aguilar M.T.P, Cetlin P.R, Langdon T.G. Using finite element modeling to examine the temperature distribution in quasi-constrained high-pressure torsion. Acta Materialia. 2012;60:3190–3198.
[57] Jeong H.J, Yoon E.Y, Lee D.J, Kim N.J, Lee S, Kim H.S. Nanoindentation analysis for local properties of ultrafine grained copper processed by high pressure torsion. Journal of Materials Science. 2012;47:7828–7834.
[58] Song Y, Yoon E.Y, Lee D.J, Lee J.H, Kim H.S. Mechanical properties of copper after compression stage of high-pressure torsion. Materials Science and Engineering, A. 2011;528:4840–4844.
[59] Gubicza J, Dobatkin S.V, Khosravi E. Microstructural stability of Cu processed by different routes of severe plastic deformation. Materials Science and Engineering, A. 2010;527:6102–6104.
[60] Mishin O.V, Godfrey A. Microstructure of ECAE processed copper after long-term room-temperature storage. Metallurgical and Materials Transactions, A. 2008;39:2923–2930.
[61] Ungár T, Schafler E, Hanák P, Bernstorff S, Zehetbauer M. Vacancy production during plastic deformation in copper determined by in situ X-ray diffraction. Materials Science and Engineering, A. 2007;462:398–401.
[62] Ungár T, Gubicza J, Ribárik G, Borbély A. Crystallite size distribution and dislocation structure determined by diffraction profile analysis: principles and practical application to cubic and hexagonal crystals. Journal of Applied Crystallography. 2001;34:298–310.
[63] Varotsos P, Eftaxias K. High-temperature vacancy concentration in Cu. Physical Review, B. 1989;40:9963–9964.
[64] Cohen J.B, Weertman J. A dislocation model for twinning in fcc metals. Acta Metallurgica. 1963;11:996–998.
[65] Wang G, Wu S.D, Zuo L, Esling C, Wang Z.G, Li G.Y. Microstructure, texture, grain boundaries in recrystallization regions in pure Cu ECAE samples. Materials Science and Engineering, A. 2003;346:83–90.
[66] Estrin Y, Isaev N.V, Lubenets S.V, Malykhin S.V, Pugachov A.T, Pustovalov V.V, Reshetnyak E.N, Fomenko V.S, Fomenko L.S, Shumilin S.E, Janecek M, Hellmig R.J. Effect of microstructure on plastic deformation of Cu at low homologous temperatures. Acta Materialia. 2006;54:5581–5590.
[67] Gunther B, Kumpmann A, Kunze H.-D. Secondary recrystallization effects in nanostructured elemental metals. Scripta Metallurgica et Materialia. 1992;27:833–838.
[68] Gertsman V.Y, Birringer R. On the structure and strength of ultrafine-grained copper produced by severe plastic deformation. Scripta Metallurgica et Materialia. 1994;30:577–581.
[69] Yin K.B, Xia Y.D, Chan C.Y, Zhang W.Q, Wang Q.J, Zhao X.N, Li A.D, Liu Z.G, Bayes M.W, Yee K.W. The kinetics and mechanism of room-temperature microstructural evolution in electroplated copper foils. Scripta Materialia. 2008;58:65–68.
[70] Stangl M, Lipták M, Fletcher A, Acker J, Thomas J, Wendrock H, Oswald S, Wetzig K. Influence of initial microstructure and impurities on Cu room-temperature recrystallization (self-annealing). Microelectronic Engineering. 2008;85:534–541.
[71] Hansen K, Pantleon K. Microstructure stability of silver electrodeposits at room temperature. Scripta Materialia. 2008;58:96–98.
[72] Paik J.-M, Park Y.-J, Yoon M.-S, Lee J.-H, Joo Y.-C. Anisotropy of grain boundary energies as cause of abnormal grain growth in electroplated copper films. Scripta Materialia. 2003;48:683–688.
[73] Pantleon K, Somers M.A.J. X-ray diffraction investigation of self-annealing in nanocrystalline copper electrodeposits. Scripta Materialia. 2006;55:283–286. .
[74] Gubicza J, Chinh N.Q, Lábár J.L, Hegedűs Z, Langdon T.G. Principles of self-annealing in silver processed by equal-channel angular pressing: the significance of a very low stacking fault energy. Materials Science and Engineering, A. 2010;527:752–760.
[75] Argon A.S, Moffatt W.C. Climb of extended edge dislocations. Acta Metallurgica. 1981;29:293–299.
[76] Escaig P.B. Sur le glissement dévié des dislocations dans la structure cubique à faces centrées. Journal de Physique. 1968;29:225–239.
[77] Paul H, Driver J.H, Maurice C, Piatkowski A. The role of shear banding on deformation texture in low stacking fault energy metals as characterized on model Ag crystals. Acta Materialia. 2007;55:575–588.
[78] Kumar M, Schwartz A.J, King W.E. Microstructural evolution during grain boundary engineering of low to medium stacking fault energy fcc materials. Acta Materialia. 2002;50:2599–2612.
[79] Mishin O.V, Bowen J.R. Through-thickness variations of deformed and annealed microstructures in ECAE-processed copper. Metallurgical and Materials Transactions, A. 2009;40:1684–1692.
[80] Terhune S.D, Swisher D.L, Oh-ishi K, Horita Z, Langdon T.G, McNelley T.R. An investigation of microstructure and grain-boundary evolution during ECA pressing of pure aluminum. Metallurgical and Materials Transactions, A. 2002;33:2173–2184.
[81] Matsunaga H, Horita Z. Softening and microstructural coarsening without twin formation in FCC metals with low stacking fault energy after processing by high-pressure torsion. Materials Transactions. 2009;50:1633–1637.
[82] Hegedűs Z, Gubicza J, Kawasaki M, Chinh N.Q, Fogarassy Z, Langdon T.G. The effect of impurity level on ultrafine-grained microstructures and their stability in low stacking fault energy silver. Materials Science and Engineering, A. 2011;528:8694–8699.
[83] Zhang N.X, Kawasaki M, Huang Y, Langdon T.G. The significance of self-annealing in two-phase alloys processed by high-pressure torsion. IOP Conference Series: Materials Science and Engineering. 2014;63:012126.
[84] Zhang N.X, Chinh N.Q, Kawasaki M, Huang Y, Langdon T.G. Self-annealing in a two-phase Pb–Sn alloy after processing by high-pressure torsion. Materials Science Engineering, A. 2016;666:350–359.
[85] Edalati K, Horita Z. Significance of homologous temperature in softening behavior and grain size of pure metals processed by high-pressure torsion. Materials Science and Engineering, A. 2011;528:7514–7523.
[86] Jin R, Cao Y.W, Mirkin C.A, Kelly K.L, Schatz G.C, Zheng J.G. Photoinduced conversion of silver nanospheres to nanoprisms. Science. 2001;294:1901–1903.
[87] Xiong Y, Siekkinen A.R, Wang J, Yin Y, Kim M.J, Xia Y. Synthesis of silver nanoplates at high yields by slowing down the polyol reduction of silver nitrate with polyacrylamide. Journal of Materials Chemistry. 2007;17:2600–2602.
[88] Xiong Y, Cai H, Wiley B.J, Wang J, Kim M.J, Xia Y. Synthesis and mechanistic study of palladium nanobars and nanorods. Journal of the American Chemical Society. 2007;129:3665–3675.
[89] Bajáki Á, Lábár J, Csanády Á, Geszti O, Hargitai H, Kármán F.H. Investigation of noble metal nanoparticles (Ag, Au, Pd, Pt) produced by chemical reduction. Materials Science Forum. 2010;659:115–120.
[90] Gubicza J, Lábár J.L, Quynh L.M, Nam N.H, Luong N.H. Evolution of size and shape of gold nanoparticles during long-time ageing. Materials Chemistry and Physics. 2013;138:449–453.
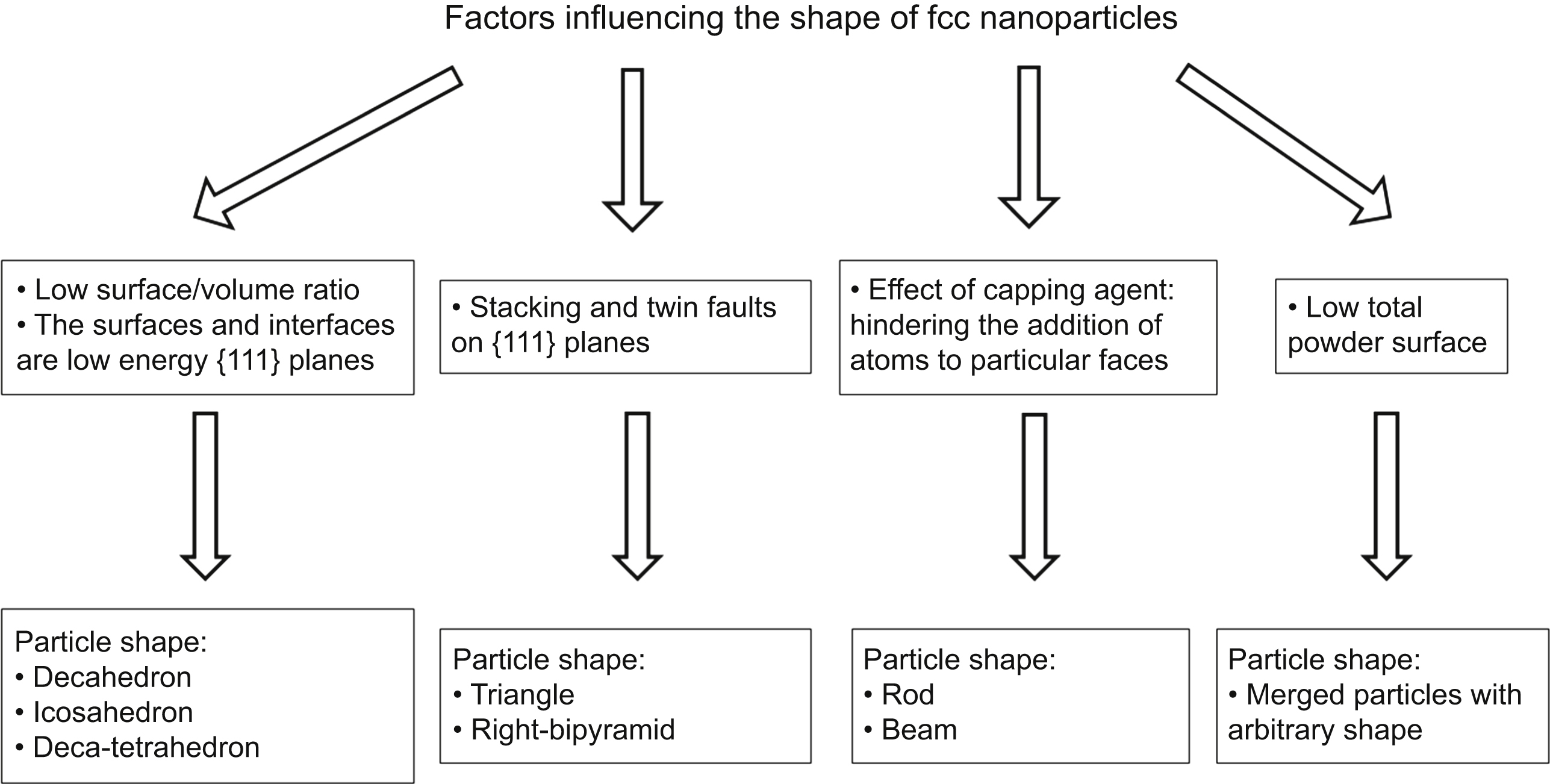
[91] Xia Y, Xiong Y, Lim B, Skrabalak S.E. Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angewandte Chemie International Edition. 2009;48:60–103.
[92] Gryaznov V.G. Pentagonal symmetry and disclinations in small particles. Crystal Research and Technology. 1999;34:1091–1119.
[93] Tsuji M, Ogino M, Matsuo R, Kumagae H, Hikino S, Kim T, Yoon S.-H. Stepwise growth of decahedral and icosahedral silver nanocrystals in DMF. Crystal Growth and Design. 2010;10:296–301.
[94] Tao A.R, Habas S, Yang P. Shape control of colloidal metal nanocrystals. Small. 2008;4:310–325. .
[95] Sun Y, Xia Y. Shape-controlled synthesis of gold and silver nanoparticles. Science. 2002;298:2176–2179.
[96] Xiong Y, Cai H, Yin Y, Xia Y. Synthesis and characterization of fivefold twinned nanorods and right bipyramids of palladium. Chemical Physical Letters. 2007;440:273–278.
[97] Murphy C.J, Sau T.K, Gole A.M, Orendorff C.J, Gao J, Gou L, Hunyadi S.E, Li T. Anisotropic metal nanoparticles: synthesis, assembly, and optical applications. Journal of Physical Chemistry, B. 2005;109:13857–13870.
[98] Wiley B.J. Synthesis and electrical characterization of silver nanobeams. Nano Letters. 2006;6:2273–2278.
[99] Jang E, Lim E.-K, Choi J, Park J, Huh Y.-J, Suh J.-S, Huh Y.-M, Haam S. Br-assisted ostwald ripening of Au nanoparticles under H2O2 redox. Crystal Growth and Design. 2012;12:37–39.






 -,
-,  -, and
-, and  -type dislocations as a function of annealing temperature.
-type dislocations as a function of annealing temperature.![]() (12.1)
(12.1)


![]() (12.2)
(12.2)


 (12.3)
(12.3)![]() (12.4)
(12.4)![]() (12.5)
(12.5)![]() (12.6)
(12.6) (12.7)
(12.7)