[1] Varin R.A, Czujko T, Wronski Z.S. Nanomaterials for Solid State Hydrogen Storage. New York: Springer Science + Business Media; 2009.
[2] Dornheim M, Doppiu S, Barkhordarian G, Boesenberg U, Klassen T, Gutfleisch O, Bormann R. Hydrogen storage in magnesium-based hydride composites. Scripta Materialia. 2007;56:841–846.
[3] David E. An overview of advanced materials for hydrogen storage. Journal of Materials Processing Technology. 2005;162–163:169–177. .
[4] Barkhordarian G, Klassen T, Bormann R. Kinetic investigation of the effect of milling time on the hydrogen sorption of magnesium catalyzed with different Nb2O5 contents. Journal of Alloys and Compounds. 2006;407:249–255.
[5] Bobet J.L, Even C, Nakamura Y, Akiba E, Darriet B. Synthesis of magnesium and titanium hydride via reactive mechanical alloying. Influence of 3d-metal addition on MgH2 synthesize. Journal of Alloys and Compounds. 2000;298:279–284.
[6] Barkhordarian G, Klassen T, Bormann R. Effect of Nb2O5 content on hydrogen reaction kinetics of Mg. Journal of Alloys and Compounds. 2004;364:242–246.
[7] Fátay D, Révész Á, Spassov T. Particle size and catalytic effect on the dehydriding of MgH2. Journal of Alloys and Compounds. 2005;399:237–241.
[8] Topler J, Buchner H, Saufferer H, Knorr K, Prandl W. Measurement of the diffusion of hydrogen atoms in magnesium and Mg2Ni by neutron scattering. Journal of the Less-Common Metals. 1982;88:397–404.
[9] Révész Á, Fátay D, Spassov T. Hydriding kinetics of ball-milled nanocrystalline MgH2 powders. Journal of Materials Research. 2007;22:3144–3151.
[10] Skripnyuk V.M, Rabkin E, Estrin Y, Lapovok R. The effect of ball milling and equal channel angular pressing on the hydrogen absorption/desorption properties of Mg-4.95 wt.% Zn-0.71 wt.% Zr (ZK60) alloy. Acta Materialia. 2004;52:405–414.
[11] Skripnyuk V, Buchman E, Rabkin E, Estrin Y, Popov M, Jorgensen S. The effect of equal channel angular pressing on hydrogen storage properties of eutectic Mg–Ni alloy. Journal of Alloys and Compounds. 2007;436:99–106.
[12] Kusadome Y, Ikeda K, Nakamori Y, Orimob S, Horita Z. Hydrogen storage capability of MgNi2 processed by high pressure torsion. Scripta Materialia. 2007;57:751–753.
[13] Skripnyuk V.M, Rabkin E, Estrin Y, Lapovok R. Improving hydrogen storage properties of magnesium based alloys by equal channel angular pressing. International Journal of Hydrogen Energy. 2009;34:6320–6324.
[14] Zehetbauer M, Grossinger R, Krenn H, Krystian M, Pippan R, Rogl P, Waitz T, Wurschum R. Bulk nanostructured functional materials by severe plastic deformation. Advanced Engineering Materials. 2010;12:692–700.
[15] Révész Á, Kánya Zs, Verebélyi T, Szabó P.J, Zhilyaev A.P, Spassov T. The effect of high-pressure torsion on the microstructure and hydrogen absorption kinetics of ball-milled Mg70Ni30. Journal of Alloys and Compounds. 2010;504:83–88.
[16] Edalati K, Yamamoto A, Horita Z, Ishihara T. High-pressure torsion of pure magnesium: evolution of mechanical properties, microstructures and hydrogen storage capacity with equivalent strain. Scripta Materialia. 2011;64:880–883.
[17] Danaie M, Mauer C, Mitlin D, Huot J. Hydrogen storage in bulk Mg-Ti and Mg-stainless steel multilayer composites synthesized via accumulative roll-bonding (ARB). International Journal of Hydrogen Energy. 2011;36:3022–3036.
[18] Delmas C, Tessier C. Stacking faults in the structure of nickel hydroxide: a rationale of its high electrochemical activity. Journal of Materials Chemistry. 1997;7(8):1439–1443.
[19] Carpenter G.J.C, Wronski Z.S. Nanocrystalline NiO and NiO-Ni(OH)2 composite powders prepared by thermal and mechanical dehydroxylation of nickel hydroxide. Nanostructured Materials. 1999;11(1):67–80.
[20] Bernard F, Gaffet E, Champion Y, Boudarina N, Ustinov A. Correlation between ball milling conditions and planar effects on Cu-nanostructured powders. Journal of Physics IV. 2002;12:455–460.
[21] Huhn P.A, Dornheim M, Klassen T, Bormann R. Thermal stability of nanocrystalline magnesium for hydrogen storage. Journal of Alloys and Compounds. 2005;404–406:499–502.
[22] Fátay D, Spassov T, Delchev P, Ribárik G, Révész Á. Microstructural development in nanocrystalline MgH2 during H-absorption/desorption cycling. International Journal of Hydrogen Energy. 2007;32:2914–2919.
[23] Friedrichs O, Aguey-Zinsou F, Ares Fernandez J.R, Sanchez-Lopez J.C, Justo A, Klassen T, Bormann R, Fernandez A. MgH2 with Nb2O5 as additive, for hydrogen storage: chemical, structural and kinetic behaviour with heating. Acta Materialia. 2006;54:105–110. .
[24] Révész Á, Fátay D. Microstructural evolution of ball-milled MgH2 during a complete dehydration-hydrogenation cycle. Journal of Power Sources. 2010;195:6997–7002.
[25] Inui H, Yamamoto T, Hirota M, Yamaguchi M. Lattice defects introduced during hydrogen absorption-desorption cycles and their effects on P–C characteristics in some intermetallic compounds. Journal of Alloys and Compounds. 2002;330–332:117–124.
[26] Decamps B, Joubert J.-M, Cerny R, Percheron-Guegan A. TEM study of the dislocations generated by hydrogen absorption/desorption in LaNi5 and derivatives. Journal of Alloys and Compounds. 2005;404–406:570–575.
[27] Matsuda J, Nakamura Y, Akiba E. Lattice defects introduced into LaNi5-based alloys during hydrogen absorption/desorption cycling. Journal of Alloys and Compounds. 2011;509:7498–7503.
[28] Matsuda J, Nakamura Y, Akiba E. Microstructure of Ti–V–Mn BCC alloys before and after hydrogen absorption-desorption. Journal of Alloys and Compounds. 2011;509:4352–4356.
[29] Janot R, Cuevas F, Latroche M, Percheron-Guegan A. Influence of crystallinity on the structural and hydrogenation properties of Mg2X phases (X = Ni, Si, Ge, Sn). Intermetallics. 2006;14:163–169.
[30] Liu D, Zhu Y, Li L. Crystal defect analysis and surface characteristics of Mg2NiH4 produced by hybriding combustion synthesis. International Journal of Hydrogen Energy. 2007;32:2417–2421.
[31] Lamari Darkrim F, Malbrunot P, Tartaglia G.P. Review of hydrogen storage by absorption in carbon nanotubes. International Journal of Hydrogen Energy. 2002;27:193–202.
[32] Bhalerao G.M, Sinha A.K, Sathe V. Defect-dependent annealing behaviour of multi-walled carbon nanotubes. Physics E: Low-dimensional Systems and Nanostructures. 2008;41:54–59.
[33] Liu F, Zhang X, Cheng J, Tu J, Kong F, Huang W, Chen C. Preparation of short carbon nanotubes by mechanical ball milling and their hydrogen absorption behaviour. Carbon. 2003;41:2527–2532.
[34] Gao L, Yoo E, Nakamura J, Zhang W, Chua H.T. Hydrogen storage in Pd-Ni doped defective carbon nanotubes through the formation of CHx (x = 1,2). Carbon. 2010;48:3250–3255.
[35] Chen C.-H, Huang C.-C. Enhancement of hydrogen spillover onto carbon nanotubes with defect feature. Microporous and Mesoporous Materials. 2008;109:549–559.
[36] Mu S.-C, Tang H.-L, Qian S.-H, Pan M, Yuan R.-Z. Hydrogen storage in carbon nanotubes modified by microwave plasma etching and Pd decoration. Carbon. 2006;44:762–767.
[37] McDaniel F.D, Naab F.U, Holland O.W, Dhoubhadel M, Mitchell L.J, Duggan J.L. Low energy ion irradiation effects on hydrogen absorption and desorption in carbon nanotubes. Surface & Coatings Technology. 2007;201:8564–8567.
[38] Krasheninnikov A.V, Nordlund K. Irradiation effects in carbon nanotubes. Nuclear Instruments and Methods in Physics Research, Section B: Beam Interactions With Materials and Atoms. 2004;216:355–366.
[39] Chen C.-H, Huang C.-C. Hydrogen absorption in defective carbon nanotubes. Separation and Purification Technology. 2009;65:305–310.
[40] Zhao J.-X, Ding Yi-H, Wang X.-G, Cai Q.-H, Wang X.-Z. Theoretical studies of the CNx nanotube with four nitrogen divacancy (4ND) defects. Diamond and Related Materials. 2011;20:36–41.
![]() (11.1)
(11.1)![]() (11.2)
(11.2)![]() (11.3)
(11.3)![]() (11.4)
(11.4)
![]() (11.5)
(11.5)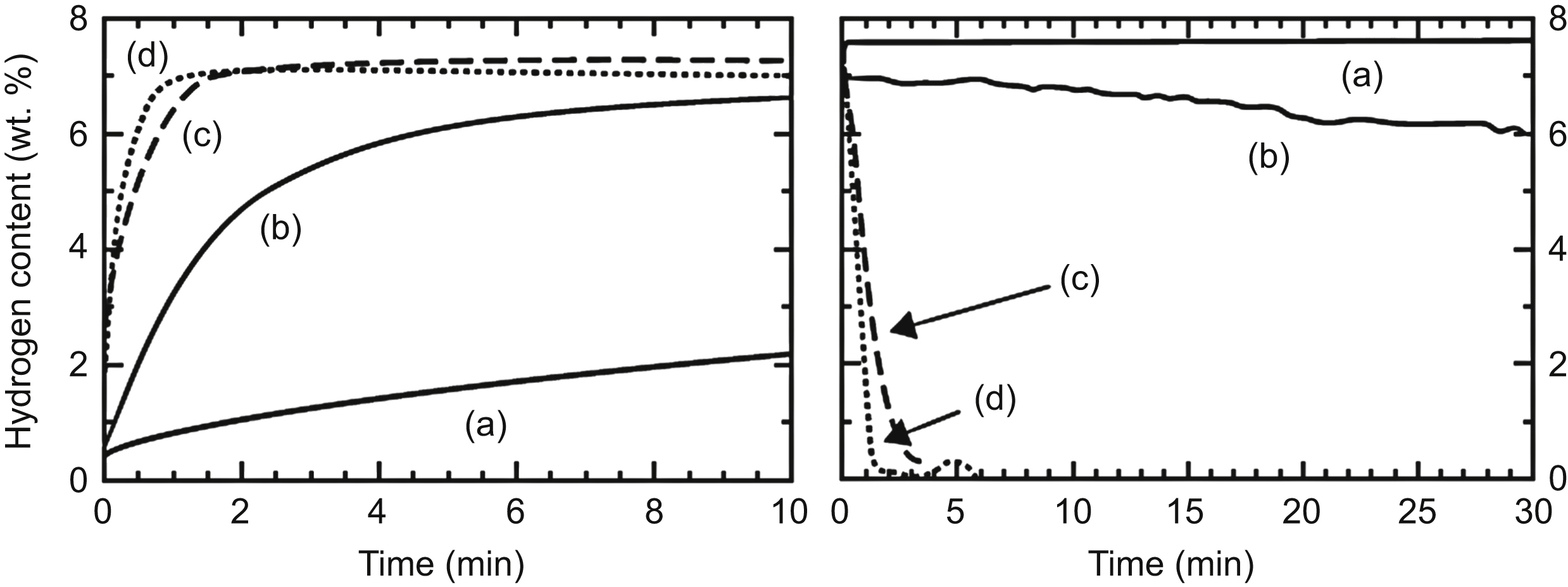
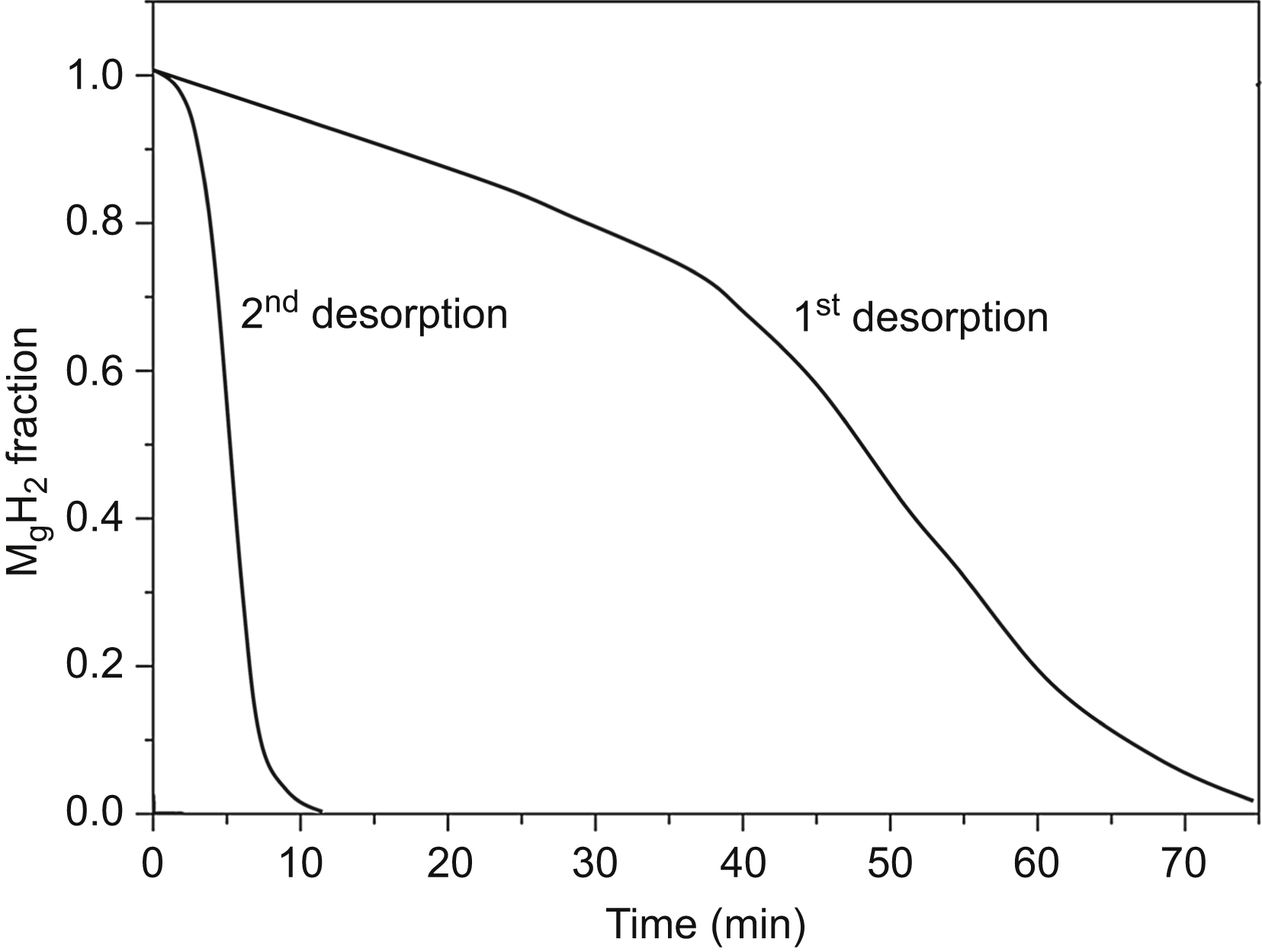


 (11.6)
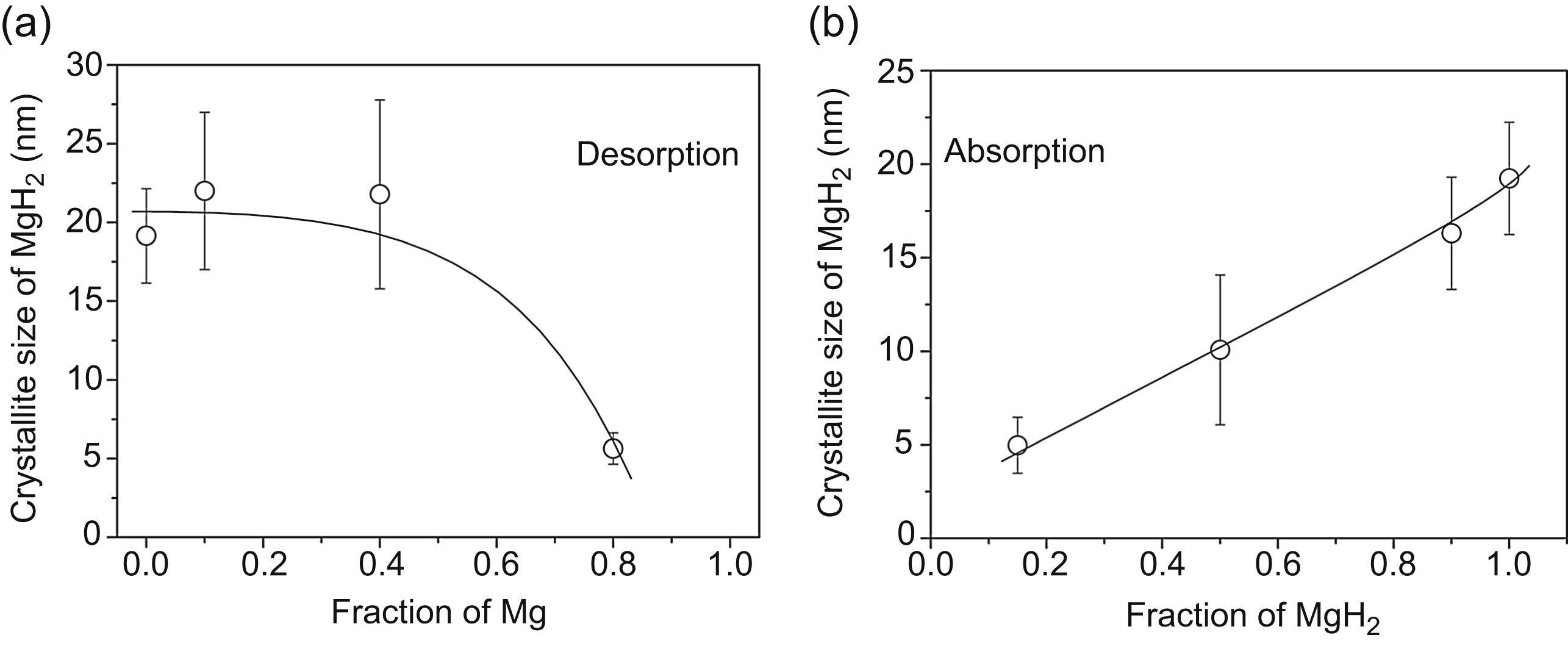
(11.6)![]() (11.7)
(11.7)