List of Figures
Figure 1.1 Classification of processing routes of bulk nanomaterials. 1
Figure 1.2 (a) The schematic depiction of equal-channel angular pressing (ECAP) processing and (b) the four fundamental processing routes in ECAP. 2
Figure 1.3 A schematic of ECAP-Conform process [1]. 4
Figure 1.4 The principle of high-pressure torsion. 4
Figure 1.5 Schematic illustration of the steps of multidirectional forging procedure. The axes of the reference system attached to the sample are denoted as x, y, and z. The loading directions are indicated by black arrows. The letters a, b, and c denote the sample dimensions. 5
Figure 1.6 The principle of twist extrusion processing. 6
Figure 1.7 The principle of accumulative roll bonding method. 7
Figure 1.8 The principle of repetitive corrugation and straightening method. 7
Figure 1.9 Schematic picture of an inert gas condensation facility. 9
Figure 1.10 Schematic depiction of the apparatus of cryogenic melting. 9
Figure 1.11 Schematic diagram of the setup for electro-explosion of wire: (1) thin metal wire electrode, (2) metal plate electrode, (3) batteries, and (4) glass vessel. 10
Figure 1.12 The flowchart of the sonochemical method, resulting in Fe2O3 nanoparticles [20]. 11
Figure 1.13 Schematic of the main steps of hydrothermal synthesis of titanate nanotubes from anatase TiO2 powder. 12
Figure 1.14 Jar or drum mill. 13
Figure 1.15 Szegvari attritor. 13
Figure 1.16 Motion of balls and jars in a planetary mill. 14
Figure 1.17 Illustration of a vibratory ball mill. 15
Figure 1.18 Motion of balls in a magnetic mill. 15
Figure 1.19 Schematic depiction of shock wave consolidation process showing the experimental setup. 16
Figure 1.20 Illustration of the fusion of particles during pressureless sintering. 17
Figure 1.21 Schematic depiction of hot pressing. 17
Figure 1.22 Schematic depiction of the spark plasma sintering apparatus. 18
Figure 1.23 Schematic illustration of Ni electrodeposition. 19
Figure 1.24 Illustration of melt spinning. 20
Figure 1.25 Schematic depiction of copper mold casting. 21
Figure 2.1 Ranges of application for different experimental methods in the determination of vacancy concentration, dislocation density, and stacking or twin fault probability. 30
Figure 2.2 The division of reflecting crystallites into scattering columns according to Bertaut theorem. 31
Figure 2.3 Characterization of lattice distortions parallel to the diffraction vector g [or normal to lattice planes (hkl)]. 33
Figure 2.4 Schematic of some arrangements of dislocations yielding weak or strong screening of the strain fields that correspond to a larger or smaller value of the dislocation arrangement parameter M, respectively. 34
Figure 2.5 Full width at half maximum of diffraction peaks as a function of magnitude of diffraction vector (g) for the cases where the broadening is caused only by (a) crystallite size in Cu, (b) both size and dislocations in Cu, and (c) both size and twin faults in SiC. The datum points corresponding to harmonic reflection pairs are connected by dotted lines. 36
Figure 2.6 The convolutional multiple whole profile evaluation of the X-ray diffraction pattern for ultrafine-grained Au processed by severe plastic deformation. The open circles and the solid line represent the measured and the fitted X-ray diffraction patterns, respectively. A magnified part of the pattern is presented in the inset. The difference between the measured and the fitted patterns is also shown at the bottom of the figure. 37
Figure 2.7 Log-normal crystallite size distribution density function, f(x), and the arithmetic (〈x〉arit), the area- (〈x〉area), and the volume-weighted (〈x〉vol) mean crystallite sizes obtained from m and σ. 39
Figure 2.9 Grain boundary misorientation distribution for an ultrafine-grained Al-1%Mg alloy processed by high pressure torsion at room temperature under the pressure of 6 GPa at a rate of 1 rpm for 10 turns [24]. The columns and the solid curve represent the experimental result and the statistical prediction for a set of random orientations, respectively. 41
Figure 2.10 Schematic of the coordinate system attached to the sample and the pixels arranged in a square grid on electron backscatter diffraction image. The misorientations between the studied gray pixel and the two neighboring white pixels in x1 and x2 directions are characterized by quaternions Δq1 and Δq2, respectively. 42
Figure 2.11 Electron backscatter diffraction image (a) and the corresponding dislocation density map (b) obtained with the step size of 35 nm. In (b) the grain boundaries with misorientation angles larger than 5 degrees are indicated by thin blue lines, and the higher the dislocation density, the darker the gray contrast. 44
Figure 2.12 The dislocation density determined by electron backscatter diffraction as a function of the scan step size. The dislocation density obtained by X-ray line profile analysis is also indicated in the figure. 45
Figure 2.13 Schematic showing the difference between the grain and crystallite sizes determined by transmission electron microscopy and X-ray line profile analysis, respectively, in nanocrystalline and ultrafine-grained metallic materials processed by severe plastic deformation. 48
Figure 2.14 The correlation between the mean twin fault spacing values determined by transmission electron microscopy and X-ray line profile analysis for Ag and SiC samples. 49
Figure 2.15 The intrinsic resistivity in logarithmic scale as a function of temperature for 99.999% purity Cu. The resistivity for the vacancy concentration of cv =10−4 and dislocation density of ρ = 1015 m−2 are also indicated in the figure. 51
Figure 2.17 The poslston lifetime as a function of number of vacancies in clusters for Al. 54
Figure 3.1 The dislocation density and the crystallite size as a function of the number of equal channel angular pressing (ECAP) passes for 99.98% purity Cu. 59
Figure 3.2 (a) A transmission electron microscopy micrograph of a subgrain in 99.99% purity Cu processed by repetitive corrugation and straightening. The inset in (a) is a high-resolution transmission electron microscopy (HRTEM) image showing that the subgrain boundaries are almost parallel to two sets of {111} planes. (b) A Fourier-filtered HRTEM image from the boundary as pointed out by a black arrowhead in (a). The white arrow in (b) points out the grain boundary (GB) orientation. The black and white circles mark interstitial and vacancy loops, respectively. 60
Figure 3.3 (a) A boundary in a 99.99% purity Cu sample processed by 14 cycles of repetitive corrugation and straightening. The grain boundary plane is curved and changes from the (5 5 12) plane to the (002) plane. (b) The corresponding electron diffraction pattern. (c) and (e) high-resolution transmission electron microscopy images from the upper-left and lower-right part of the boundary in (a) (see the framed areas). (d) A structural model of the boundary segment in (c). The two types of dislocations in the boundary are marked by black and white symbols. 62
Figure 3.4 Transmission electron microscopy images of the microstructure of Cu processed by equal channel angular pressing for the 5th (a) and 25th (b) passes. 64
Figure 3.5 The variation of (a) dislocation density, (b) fractions of 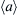 -type basal and nonbasal edge dislocations, and (c) screw dislocations in the center of an AZ31 disk as a function of high-pressure torsion (HPT) revolutions [35]. 65
-type basal and nonbasal edge dislocations, and (c) screw dislocations in the center of an AZ31 disk as a function of high-pressure torsion (HPT) revolutions [35]. 65
Figure 3.6 Schematic depiction of the grain refinement in hexagonal close-packed metals along the preexisting grain boundaries when the initial grain size is larger than a critical value. 66
Figure 3.7 Transmission electron microscopy images showing the microstructure of Cu specimens immediately after 20 cycles of multidirectional forging (a), 15 passes of twist extrusion (b), 25 passes of equal channel angular pressing (c) and 25 revolutions of high-pressure torsion (d). 68
Figure 3.8 Comparison of dislocation densities (a) and grain sizes (b) in different materials processed by equal channel angular pressing (ECAP) or high-pressure torsion (HPT) [55]. 70
Figure 3.9 The saturation grain and crystallite size values determined by transmission electron microscopy and X-ray line profile analysis, respectively, for severe plastic deformation-processed ultrafine-grained materials as a function of the saturation dislocation density. 73
Figure 3.10 The saturation crystallite size and dislocation density as a function of melting point for different pure face-centered cubic metals processed by equal channel angular pressing at room temperature. 73
Figure 3.11 The minimum grain size and the maximum dislocation density as a function of Mg content in Al solid solutions. 76
Figure 3.12 Transmission electron microscopy images taken on pure Al (a) and Al–3% Mg (b) processed by eight equal channel angular pressing passes at room temperature. 76
Figure 3.13 Schematic drawings of two edge dislocations climbing in opposite directions by increasing or decreasing the extension of the extra half plane that leads to a production or annihilation of vacancies, respectively. The direction of atomic migration is indicated by arrows. The produced and the annihilated vacancies are denoted by solid and dashed squares, respectively. 77
Figure 3.14 The vacancy cluster concentration in high-pressure torsion (HPT)-processed Cu as a function of the number of revolutions. The individual datum points given in Ref. [87] are not shown here, but all of them follow this line irrespective of the location in the HPT disk and the applied pressure (2 or 4 GPa). The scatter of datum points is illustrated by the error bar. 79
Figure 3.15 The concentration of vacancies in the center and at the periphery of Cu disks processed by different revolutions of high-pressure torsion (HPT). The individual datum points given in Ref. [88] are not shown here, but all of them follow this line irrespective of the applied pressure (2 or 4 GPa). The scatter of datum points is illustrated by the error bar. 79
Figure 3.16 The equilibrium Ag solute concentration in the Cu matrix as a function of the size of Ag nanoparticles at 623 K for two interface energies 0.38 and 1 J/m2. The horizontal dotted line represents the equilibrium Ag concentration in Cu matrix containing Ag particles with very large radius (c∞). 84
Figure 3.17 Reflection (220) of the Cu–3 at.% Ag sample in cryorolled state and after annealing at 623 K for 20 min. The symbols and the solid lines represent the measured data and the fitted curves, respectively. The diffraction peak in the annealed condition is a sum of two reflections related to Regions 1 and 2 having different average lattice parameters (for details see the text). In this figure the integrated intensity (the area under the peak after background subtraction) is normalized to unity for both cryorolled and annealed states. 85
Figure 3.18 Schematic of the development of heterogeneous microstructure in cryorolled ultrafine-grained Cu–3 at.% Ag alloy during annealing at 623 K up to 120 min. The darker the gray in the matrix, the higher the solute Ag content. 86
Figure 3.19 The variation of (a) the solute Ag concentration, (b) the X-ray intensity fraction, and (c) the dislocation density for Regions 1 and 2 in the Cu matrix as a function of annealing time. The solid curves serve only as guide to eyes. 87
Figure 4.1 High-resolution transmission electron microscopy image showing a dissociated screw dislocation bounded by partials in Ag processed by eight equal-channel angular pressing passes (at right) and a picture illustrating the corresponding crystallographic directions and lattice planes (at left). 95
Figure 4.2 The forces f1 and f2 acting on partials in dissociated screw dislocations due to the shear stress, τ, in the glide plane. The glide plane is lying in the sheet, and the stacking fault between partials is marked by light gray color. The Burgers vectors of partials are denoted by b1 and b2. The components of these vectors lying parallel and perpendicular to the dislocation line vector, l, are also presented. (a) and (b) show that if the stress is parallel to the Burgers vector of the undissociated dislocation, the forces acting on partials have the same directions; therefore they do not change the plitting distance between partials. If the stress is perpendicular to the Burgers vector of the undissociated dislocation, it may yield a decrease (c) or an increase (d) in the splitting distance between partials. 97
Figure 4.3 A schematic illustration of a model for dissociated dislocation with wide stacking fault (SF) ribbon in a nanograin. Two Shockley partial dislocations, XEFY and XCDY, are emitted consecutively from the grain boundary XY, with their ends pinned at X and Y. 98
Figure 4.4 The effective equilibrium splitting distance between partials, dp,eff, in nanocrystalline Al as a function of grain size (solid line). The dashed line corresponds to the equilibrium splitting distance characteristic for coarse-grained counterparts. 100
Figure 4.5 Cross-slip of a dissociated screw dislocation.  and σs are the shear stresses pushing the partials toward each other on the initial glide plane (S1) and pulling the partials in opposite directions on the cross-slip plane (S2), respectively. 101
and σs are the shear stresses pushing the partials toward each other on the initial glide plane (S1) and pulling the partials in opposite directions on the cross-slip plane (S2), respectively. 101
Figure 4.6 Transmission electron microscopy images taken after (a) 1, (b) 4, (c) 8, and (d) 16 passes of equal-channel angular pressing. Examples of twin boundaries are indicated by white arrows. 104
Figure 4.7 The dislocation density and the twin boundary frequency as a function of number of equal-channel angular pressing (ECAP) passes for 4N5 purity Ag. 105
Figure 4.8 The saturation twin boundary frequency achieved by equal-channel angular pressing at room temperature versus the twin-fault energy, γT, for pure face-centered cubic metals. 105
Figure 4.9 The formation of twins at Lomer–Cottrell locks (a) and grain boundaries (b) by dissociation of lattice dislocations into twinning partials and their slip on successive {111} planes. The Schockley partials are denoted by “L.” 106
Figure 4.10 The untwinning process. The twin boundaries are indicated by “TB.” The partial dislocations are denoted as it is usual in a standard Thompson tetrahedron (see Fig. 4.11). When a dissociated dislocation meets a twin lamella, the leading partial (αB) splits into a sessile stair-rod dislocation (αδ) and a glissile Shockley partial (δB) that slips on a layer of the twin lamella resulting in untwinning on that layer (a). If dislocations on successive slip planes meet twin lamella, a complete disappearance of twin segment can occur (b). 107
Figure 4.11 (a) Thompson tetrahedron ABCD and (b) its two dimensional representation illustrating the possible slip planes and the Burgers vectors of dislocations in a face-centered cubic crystal. The four faces of the tetrahedron corresponding to the slip planes are denoted by a, b, c, and d while the centers of the faces are indicated as α, β, γ, and δ. 108
Figure 4.12 The dislocation density and the twin boundary frequency as a function of number of equal-channel angular pressing (ECAP) passes for 4N purity Ag. 109
Figure 4.13 Transmission electron microscopy image of the microstructure in 4N Ag processed by 20 high pressure torsion revolutions [34]. 112
Figure 4.14 The twin boundary frequency as a function of stacking fault energy (SFE). The grain size is also given at some data points. 114
Figure 4.15 Schematic illustration of the grain-refinement mechanism for the Cu–30 wt.% Zn alloy processed by high pressure torsion. 116
Figure 4.16 A high-resolution transmission electron microscopy image of a bent twin boundary (TB) in high pressure torsion-processed Cu–30% Zn alloy showing a high density of dislocations, which are indicated using white and black “T,” accumulated at the TB. Two white solid lines were drawn parallel to {111} at each side of the TB, respectively, and one dash white line was also drawn parallel to (111)M to indicate the misorientation between the {111} planes across the TB. 117
Figure 5.1 The mean crystallite size, the dislocation density, and the Mg concentration as a function of the milling period for the powder blend with the nominal composition of Al–6 wt.% Mg. 122
Figure 5.2 Schematic illustration of the particle/grain structure in milled metallic powders. 123
Figure 5.3 The parameters q and M describing the edge/screw character and the screening of dislocations, respectively, as a function of milling time for a powder blend with the nominal composition of Al–6 wt.% Mg. 123
Figure 5.4 The mean crystallite size, the dislocation density, and the Mg concentration in Al as a function of the nominal Mg content in Al–Mg powder blends milled for 3 h. 124
Figure 5.5 The parameters q and M describing the edge/screw character and the screening of dislocations, respectively, as a function of the nominal Mg content in Al–Mg powder blends milled for 3 h. 125
Figure 5.6 The smallest crystallite size in pure metal powders achieved by milling at room temperature as a function of the melting point. 132
Figure 5.7 (a) Bright-field transmission electron microscopy (TEM) image of Ni powder processed by electro-explosion of Ni wire. The particle size distribution obtained from TEM images is shown in (b) [47]. 133
Figure 5.8 The Volterra constructions of (a) wedge and (b) twist disclinations. 135
Figure 5.9 Decahedral (a) and icosahedral (b) nanoparticles. The twin faults in the decahedron are indicated by gray color. The disclinations are illustrated by the arrows, and the symbol ω in both decahedron and icosahedron. (c) shows that when five tetrahedra assembled with a common edge an angle gap of 7.35 degrees remains and after joining the tetrahedra a disclination is formed in the resulted decahedron. 136
Figure 5.10 The schematic of stepwise growth of twinned decahedral nanoparticles from tetrahedral units. 137
Figure 5.11 Energy-filtered transmission electron microscopy images on Ni nanopowder showing the element maps for (a) nickel and (b) oxygen, respectively. The lighter the color in (a) and (b), the higher the local nickel and oxygen contents, respectively. The corresponding X-ray diffraction pattern is presented in (c) [47]. 138
Figure 5.12 Transmission electron microscope images of the sample processed by (a) Hot-isostatic pressing (HIP) and (b) Spark-plasma sintering (SPS). Grain size distributions for the (c) HIP- and (d) SPS-processed bulk samples determined from transmission electron microscopy images. The distributions of twinned grains are also shown by streaked columns [47]. 140
Figure 5.13 Scanning electron microscopy picture showing clusters in coarse-grained powder with an individual particle size of about 5 μm. 142
Figure 5.14 Scanning electron microscopy images of the microstructure for samples CG (a), A (b), and B (c). 143
Figure 5.15 Dislocation density and the crystallite size in nanodiamond samples as a function of consolidation pressure. 146
Figure 5.16 (a) Transmission electron microscopy images of specimens sintered at 1800 °C and 2 GPa, (b) 5.5 GPa, and (c) 8 GPa. Please note the different magnification for (b) [67]. 148
Figure 5.17 (a) The crystallite size, (b) the dislocation density, and (c) the twin density for 10 sintered specimens as a function of the sintering pressure and temperature. The wire grids in the figures are to guide the eye [67]. 149
Figure 6.1 Contour maps showing the influence of organic additive concentration and current density on crystallite size, dislocation density, and twin fault probability in textured Ni film processed by electrodeposition. 160
Figure 6.2 Schematic cross-sectional view of twin fault structures in two epitaxially grown sputtered Ag films where the foil surface is parallel to (a) plane (111) or (b) plane (110) [12]. These planes are perpendicular to the sheet. 161
Figure 6.3 Schematic of the structure for a pair of Cu and Nb layers in the Cu-Nb multilayer processed by direct current magnetron sputtering at room temperature. The arrows on the top surface indicate crystallographic directions 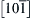 and
and 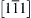 in Cu and Nb grains, respectively [13]. 162
in Cu and Nb grains, respectively [13]. 162
Figure 6.4 Schematic of the dislocation structure in Cu and Nb layers of the Cu-Nb multilayer processed by direct current magnetron sputtering at room temperature. 163
Figure 6.5 (a) Schematic of the formation of dislocations with Burgers vector [001] with the interaction of gliding dislocations with Burgers vectors 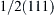 and
and 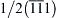 in Nb layers during rolling of a Cu-Nb multilayer. Figure (b) shows a Bravais cell of Nb with the crystallographic planes and directions which play important role in the dislocation reaction. 166
in Nb layers during rolling of a Cu-Nb multilayer. Figure (b) shows a Bravais cell of Nb with the crystallographic planes and directions which play important role in the dislocation reaction. 166
Figure 6.6 Schematic illustration of the confined glide of hairpin dislocation loops on two glide planes in a Nb layer of a nanoscale Cu-Nb multilayer, depositing misfit dislocations at the interfaces [21]. 167
Figure 6.7 Extension and retraction (detwinning) of coherent twin boundaries by moving of groups of three twinning partials at incoherent twin boundaries. 168
Figure 6.8 Schematic of the formation of interstitial dislocation loops in the vicinity of a pressurized He bubble (loop punching). 169
Figure 7.1 A schematic illustration of the model of a perfect screw dislocation emitted from grain boundary XY and dissociated into two partials in the slip plane (111). The stacking fault (SF) ribbon between the partials is indicated by color. 176
Figure 7.2 A schematic illustration of the dislocation model for the nucleation of deformation twins. The stacking sequence of the (111) planes is indicated by the letters A, B, and C. 176
Figure 7.3 A schematic illustration of the emission of a trailing partial from grain boundary XY that partially removes the stacking fault formed previously by passing a leading partial. 177
Figure 7.4 The shear stresses τP, τL, τtwin, τtrail, and τshrink as a function of the grain size, d, for nanocrystalline Al. The angle between the stress direction and the line of slipping dislocations is β = 30 degrees. 179
Figure 7.5 Schematic illustration of the Ashby–Verrall model for grain boundary sliding. The arrows in the grains indicate the sliding directions along the grain boundaries. At the bottom of the figure, the arrows show the directions of diffusion in the vicinity of grain boundaries that yields the change of the grain shape as a complementary process in addition to grain boundary sliding. 180
Figure 7.6 Two grains A and B with the size d in a polycrystalline material loaded by a tensile stress σ. The dashed lines represent the slip planes. In grain A, the plastic deformation has already been initiated by the external stress and a Frank–Read (FR) source emits dislocations that are gliding due to the shear stress τ. The stress field of the dislocations accumulated at the boundary in grain A assists the activation of FR sources in the unfavorable oriented grain B. 182
Figure 7.8 The stress (τ) required for the operation of a Frank–Read source versus the length (L) of the edge dislocation segment between the pinning points of the source in Cu. 184
Figure 7.10 Hardness of coarse-grained and ultrafine-grained (UFG) Pb, Sn, and In versus the homologous temperature, T/Tm (Tm is the melting point). The arrows indicate softening at RT due to grain refinement achieved by high-pressure torsion (HPT) processing. 187
Figure 7.11 The yield strength at room temperature (RT) reduced by the friction stress (σY − σ0) versus the product of Gbρ1/2 for different fcc metals and alloys processed by SPD. The errors on the individual datum points are indicated by solid horizontal and vertical lines at the symbol diamond. 189
Figure 7.12 The value of α in the Taylor equation as a function of the equilibrium splitting distance between partials in Burgers vector unit (dp/b) for pure fcc metals processed by equal-channel angular pressing at room temperature till saturation of the dislocation density. 190
Figure 7.13 The value of α in the Taylor equation as a function of the number of equal-channel angular pressing (ECAP) passes for pure Al and Cu. 191
Figure 7.14 Schematic illustration of decreasing ductility (εmax) with decreasing grain size according to Considere criterion. ultrafine-grained material. 193
Figure 7.15 Grain size dependence of uniform and total elongations at room temperature for interstitial free steel with various mean grain sizes. 194
Figure 7.16 The strain rate sensitivity, m, measured at room temperature as a function of grain size for (a) face-centered cubic Ni [64–66] and (b) body-centered cubic Fe processed by powder metallurgy methods. 195
Figure 7.17 Room temperature (RT) tensile engineering stress–strain curves for Cu with different microstructures. Ultrafine-grained (UFG): processed by eight passes of equal-channel angular pressing at RT and then rolled at liquid nlstogen temperature for a reduction of the cross-sectional area of 93%. Bimodal: sample UFG annealed at 200 °C for 3 min. Coarse-grained: conventional sample. The strain rate for all the tests is 10−4 s−1. 197
Figure 7.18 The dislocation density and the grain size (a) and the ultimate tensile strength and the elongation to failure (b) as a function of annealing temperature for Al–1% Mg processed by 10 turns of high-pressure torsion at room temperature [73]. 198
Figure 7.19 Engineering stress–strain curves for Cu–3 at.% Ag alloy rolled at liquid nlstogen temperature (LNT) and room temperature (RT), and annealed for a short time at 375 and 400 °C, respectively [74].199
Figure 7.20 Compression stress–strain curves for Ni samples consolidated from powders with the grain size of 50 or 100 nm, in Ar or air and by hot isostatic pressing (HIP) or spark plasma sintering (SPS). 204
Figure 7.21 Hall–Petch relation between the yield strength (σY) and the grain size (d) for Ni samples processed by electrodeposition and powder metallurgy. The solid line represents a linear fit on the data obtained for electrodeposited Ni specimens.204
Figure 7.22 The strength contribution of NiO dispersoids in sintered Ni samples as a function of the intensity ratio (INiO/INi) of the X-ray diffraction peaks for NiO and Ni at 2Θ = 37.4 and 44.6 degrees, respectively.205
Figure 7.23 (a) The dislocation density and (b) the twin boundary frequency for the hot isostatic pressing (HIP)- and spark plasma sintering (SPS)-processed samples before and after compression test as determined by X-ray line profile analysis. The distribution of the angle of misorientation between the grains in the (c) HIP-processed sample and after compression up to 10% strain and (d) in the SPS-processed sample and after compression up to 10% strain as determined by electron backscatter diffraction.206
Figure 7.24 (a) Transmission electron microscopy image showing twins in a large grain after compression test of Ni sample processed from a powder with particle size of 100 nm by spark plasma sintering in air. The arrows show steps on twin boundaries as a result of untwinning process. The untwinning mechanism is illustrated in (b) showing the Burgers vectors of dislocations interacting with a twin boundary (TB).207
Figure 7.25 Scanning electron microscopy images showing the surface of the sample processed by spark plasma sintering (SPS) in air deformed by compression to the strain values of (a) 7%, (b) 18%, and (c) 22% and (d) the surface of the hot isostatic pressing (HIP)-processed specimen at a strain of 25%. The loading axis is vertical in the figures. Some cracks are indicated by black arrows.209
Figure 7.26 (a) True stress–logarithmic plastic strain curves obtained by compression test at room temperature for samples CG, A, B, and ultrafine-grained (UFG) consolidated from blends of nano- and coarse-grained (CG) Ni powders with different volume fractions. The notations of the samples are explained in the text. The volume fraction of the CG component is also indicated. (b) The yield strength values obtained from the stress–strain curves in (a).210
Figure 7.27 The nanohardness distributions for (a) coarse-grained (CG) specimen, (b) sample A, (c) sample B, and (d) ultrafine-grained (UFG) specimen.211
Figure 7.28 The nanohardness of the coarse-grained (CG) and ultrafine-grained (UFG) fractions and their volume-weighted average as a function of the volume fraction of the UFG component for the as-processed specimen CG, sample A, sample B, and specimen UFG. The dashed line represents the linear interpolation between the values characteristic for the fully CG and UFG samples. 212
Figure 7.29 The yield strength, ultimate tensile strength, and strain to failure as a function of coarse-grained powder fraction for bimodal Al–7.5% Mg and 5083 Al alloys consolidated from blends of cryomilled NPs and unmilled coarse particles. 214
Figure 7.30 The flow stress at the strain of ε = 0.05 versus the logarithm of the strain rate for both CG-Zn and UFG-Zn. 215
Figure 7.31 Evolution of crystallite size and dislocation density in ultrafine-grained (UFG)-Zn sintered from nanopowder due to compression as a function of strain rate. The dashed lines indicate the crystallite size and the dislocation density in the as-sintered material. 216
Figure 8.1 Schematic of plasma spray forming of a blend of Al–Si powder and CNTs. 226
Figure 8.2 SEM image of fracture surface of plasma spray formed Al-CNT nanocomposite showing intergranular fracture and cluster of CNTs. 227
Figure 8.3 Schematic figure depicting the attachment of Cu ions to the functional groups on the surface of a CNT for the “molecular level mixing” process of Cu-CNT composite powders. 228
Figure 8.4 Schematic depiction of the Cu-CNT composite powder processed by “molecular level mixing”. 228
Figure 8.5 Schematic of aligned Cu-CNT composite samples obtained by (a) die-stretching and (b) electroplating. CNT, carbon nanotube. 229
Figure 8.6 High-resolution transmission electron microscopy image taken at the half-radius of the Cu–CNT-RT disk. The arrows indicate fragments of CNTs. 230
Figure 8.7 The area-weighted mean crystallite size, 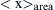 (a), the dislocation density, ρ (b), and the twin boundary frequency, β (c) at the center, half-radius, and periphery of the HPT-processed Cu, Cu–CNT-RT, and Cu–CNT-373 disks. 232
(a), the dislocation density, ρ (b), and the twin boundary frequency, β (c) at the center, half-radius, and periphery of the HPT-processed Cu, Cu–CNT-RT, and Cu–CNT-373 disks. 232
Figure 8.8 Dark-field TEM images for samples (a) Cu, (b) Cu–CNT-RT, and (c) Cu–CNT-373. Some twin boundaries in sample Cu–CNT-RT are indicated by white arrows in (d). 233
Figure 8.9 The Young's modulus and the yield strength as a function of the volume fraction of MWNTs in Al-CNT composites. 235
Figure 8.10 The ratio of the Young's moduli, the yield and tensile strengths and the elongations to failure in tension obtained for CNT composites and their pure matrices. 240
Figure 8.11 The microhardness (HV) as a function of the distance from the center of the HPT-processed Cu, Cu–CNT-RT, and Cu–CNT-373 disks (a). The hardness values determined at the half-radius of the disks at RT and 350 K (b). 241
Figure 8.12 The calculated yield strength versus the measured values obtained as one-third of the hardness at the center, half-radius, and periphery of the HPT-processed Cu, Cu–CNT-RT, and Cu–CNT-373 disks. The yield strength was calculated from the grain size obtained by TEM using the Hall–Petch formula and also from the dislocation density measured by X-ray line profile analysis using the Taylor equation. 242
Figure 8.13 The ratio of the conductivities and the strength-to-resistivity values obtained for laminar Cu-CNT composites and the pure Cu matrix processed by electroplating [24]. 244
Figure 9.1 The electrical resistivity of coarse-grained pure Cu as a function of temperature between 1 and 1356 K (the melting point of Cu) [1]. 247
Figure 9.2 Typical electrical resistivity ranges for different lattice defects and Ni solute atoms in nanostructured Cu. The intrinsic resistivity is also indicated for different temperatures. 250
Figure 9.3 Variation of the porosity factor of electrical resistivity as a function of volume fraction of pores according to Eqs. (9.11) and (9.12). 251
Figure 9.4 Strength versus resistivity for ultrafine-grained pure copper and its alloys. The different processing routes are indicated by various symbols. The strength-to-resistivity ratio values are reflected by the slopes of the lines connecting the data points and the origin of the coordinate system. The materials with the lowest and the highest strength-to-resistivity ratios are indicated by dashed lines. 259
Figure 9.5 The variation of strength and conductivity due to high-pressure torsion (HPT) and subsequent annealing in Al–5.4 wt.% Ce–3.1 wt.% La alloy and pure Al. 261
Figure 9.6 Schematic of nanotwinned ultrafine-grained microstructure in electrodeposited polycrystalline Cu possessing high strength and good conductivity. 262
Figure 9.7 Three-dimensional schematic view of highly twinned, epitaxially grown Cu film processed by magnetron sputtering. The domains are separated by Σ3{112} twin interfaces, and they contain many Σ3{111} twin faults. These films have high strength and low resistivity. 264
Figure 9.8 Strength, resistivity, and strength-to-resistivity ratio as a function of layer thickness for Cu/Cr multilayers processed by sputtering. 267
Figure 10.1 Schematic of diffusion coefficient (D) versus reciprocal of temperature (T) for bulk lattice, general grain boundaries (GBs), and free surface. The diffusion coefficient is plotted in logarithmic scale. DS, DGB, and DL stand for the diffusion coefficients for surface, grain boundary and lattice diffusion. 272
Figure 10.2 (a) Schematic showing the basic diffusion processes and the tracer atom diffusion profile (indicated by light gray color) developed in the vicinity of a grain boundary due to simultaneous lattice and grain boundary (GB) diffusion. δ is the thickness of the grain boundary. Plot of 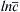 versus x6/5 gives a straight tail part for large x values, which is characteristic for grain boundary diffusion, as shown in (b). This straight line can be used for the determination of grain boundary diffusion coefficient (see the text for details). 273
versus x6/5 gives a straight tail part for large x values, which is characteristic for grain boundary diffusion, as shown in (b). This straight line can be used for the determination of grain boundary diffusion coefficient (see the text for details). 273
Figure 10.3 Schematic of the three basic tracer spatial distribution types: A, B, and C. The tracer atoms diffuse from the surface layer at the right side. The volumes containing larger amount of tracer atoms than a given concentration are indicated by gray color (tracer profile) [6]. 275
Figure 10.4 (a) Schematic of the tracer penetration profiles before and after a boundary moving parallel to the surface layer. (b) The improved Fisher's model with secondary short-circuit diffusion paths (dislocations, subgrain, and interface boundaries). This diffusion regime is also referred to as type D kinetics. 277
Figure 10.5 Schematic showing the variation of the logarithm of grain boundary diffusion coefficient in Cu as a function of misorientation angle for [001] symmetric tilt boundaries. The plot was constructed according to the data presented in [11] and [13]. The dashed line in the inset shows the ideal misorientation angle for Σ5 coincidence site lattice boundary. 279
Figure 10.6 Dependence of self-diffusion activation energy on high-angle grain boundary energy for symmetric tilt grain boundaries in Cu [15]. The error bar at the straight line reflects the difference between the data measured parallel and perpendicular to the tilt axis. 280
Figure 10.7 Schematic of diffusion profile obtained in ultrafine-grained materials in which both slow and fast grain boundary diffusion occur. 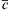 is the average tracer concentration determined by serial sectioning method while x is the tracer penetration depth. 282
is the average tracer concentration determined by serial sectioning method while x is the tracer penetration depth. 282
Figure 10.8 Model of the hierarchical microstructure with nonequilibrium and relaxed grain boundaries, acting as fast and slow diffusion paths, respectively [23]. 283
Figure 10.9 Schematic illustrating the difference between the diffusivities of Ni for slow and fast grain boundaries in Cu–0.17 wt.% Zr alloy processed by equal channel angular pressing at room temperature. The diffusivity of grain boundaries in coarse-grained pure Cu is also shown [23]. 286
Figure 10.10 Heterogeneous grain boundary structures with slow and fast diffusion pathways in nanomaterials processed by (a) crystallization of amorphous materials or (b) sintering from nanopowders. 288
Figure 10.11 Comparison of grain boundary diffusion coefficients for nanomaterials processed by different techniques: severe plastic deformation–processing, sintering from powders, and crystallization of amorphous materials. The diffusivity levels for conventional lattices, grain boundaries, and surfaces are also indicated by dashed horizontal lines. 290
Figure 10.12 Variation of the logarithm of bulk lattice self-diffusion coefficient normalized by the frequency factor as a function of grain size for Cu at room temperature, if the influence of grain size on melting point is taken into account. 291
Figure 10.13 Illustration of dissolution of Mo in V for Mo/V multilayer while the interface between the two materials remains atomically sharp. (a) Schematic of a pair of Mo and V layers. (b) Change of V atom distribution perpendicular to the layers as a function of time of diffusion experiment. 292
Figure 11.1 Schematic diagram of pressure–composition isotherm. 298
Figure 11.2 Schematic depiction of phase transformation according to (a) Johnson–Mehl–Avrami–Kolmogorov and (b) contracting volume models. The dark areas represent the growing new phase. 300
Figure 11.3 Absorption (left side) and desorption curves (right side) of (a) conventional coarse-grained MgH2; (b) nanocrystalline MgH2 processed by ball milling for 20 h; (c) MgH2 ball-milled for 700 h, and (d) Nb2O5-catalyzed ball-milled MgH2. 302
Figure 11.4 The first and the second desorption curves measured in vacuum at 300 °C for MgH2 ball-milled for 10 h. The first desorption corresponds to the activation of the powder. 305
Figure 11.5 Variation of the average crystallite size of MgH2 as a function of the number of dehydrogenation–hydrogenation cycles. 305
Figure 11.6 Schematic depiction of the crystallite structure in MgH2 (a) immediately after milling, after (b) the first and (c) the second dehydrogenation–hydrogenation cycles. 306
Figure 11.7 Variation of the average crystallite size of MgH2 as a function of the fraction of Mg and MgH2 during (a) desorption and (b) absorption, respectively. 307
Figure 11.8 Variation of the normalized crystallite size of MgH2 as a function of the transformed fraction of Mg during desorption according to Eq. (11.7). 308
Figure 11.9 Front walls of one and the same single-walled nanotubes just after ion impact (a) and after annealing (b). During annealing the divacancy (D) transformed to an agglomeration of nonhexagonal rings. The numbers in the center of the rings indicate the numbers of atoms that constitute the rings. The single vacancy (S) and the nearby carbon adatom (A) in the upper right-hand corner in (A) transformed to a Stone–Wales 5–7 defect in (B). 311
Figure 11.10 TEM images of (a) defective multiwalled carbon nanotubes (MWCNTs) processed by oxidation, (b) defective MWCNTs doped with Pd–Ni catalyst nanoparticles (appeared as black dots). 311
Figure 11.11 Schematic representation of hydrogen spillover in (a) defect free and (b) defective multiwalled nanotubes decorated by catalyst particles. H, atomic hydrogen, H2, hydrogen molecule. 312
Figure 12.1 Differential scanning calorimetry thermograms taken at the heating rate of 10 K/min on 99.995% (4N5) purity Ag processed by 1, 4, 8, and 16 passes of equal-channel angular pressing (ECAP). 318
Figure 12.2 (a) The temperature of the maximum of the differential scanning calorimetry (DSC) peaks presented in Fig. 12.1 for 4N5 Ag processed by different number of passes of equal-channel angular pressing (ECAP). (b) The heat released in the DSC peaks and the activation energies determined by the Kissinger method as a function of number of ECAP passes.318
Figure 12.3 The mean crystallite size and the dislocation density as a function of annealing temperature for Ti processed by eight equal-channel angular pressing passes. The corresponding differential scanning calorimetry (DSC) thermogram is also presented. 321
Figure 12.4 The relative fractions of 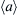 -,
-,  -, and
-, and  -type dislocations as a function of annealing temperature. 321
-type dislocations as a function of annealing temperature. 321
Figure 12.5 The released heat obtained in differential scanning calorimetry experiments as a function of grain size for pure face-centered cubic metals. 326
Figure 12.6 The microstructure of 4N5 purity Ag (a) immediately after equal-channel angular pressing processing and (b) after the differential scanning calorimetry peak as determined by transmission electron microscope and electron backscatter diffraction. 328
Figure 12.7 Transmission electron microscope image of the interior of a recrystallized grain in equal-channel angular pressing–processed Ag heat-treated up to the end of the exothermic differential scanning calorimetry peak. The black spots indicate small dislocation loops. 329
Figure 12.8 Differential scanning calorimetry thermograms for high-pressure torsion (HPT)–processed bulk-Cu and the counterpart consolidated by HPT from a microcrystalline Cu powder. 334
Figure 12.9 Evolution of crystallite size (a) and dislocation density (b) in high-pressure torsion (HPT)–processed bulk-Cu and the counterpart consolidated by HPT from a microcrystalline Cu powder, as determined by X-ray diffraction line profile analysis (XLPA). The notation “recryst.” indicates the occurrence of recrystallization, which made the XLPA evaluation impossible. 334
Figure 12.10 Differential scanning calorimetry thermograms measured at 40 K/min for consolidated-Cu and Cu–carbon nanotube (CNT) samples. 337
Figure 12.11 The average crystallite size (a), the dislocation density (b), and the twin-fault probability (c) in consolidated-Cu and Cu–carbon nanotube samples obtained by X-ray diffraction line profile analysis (XLPA) as a function of the temperature in differential scanning calorimetry annealing at a heating rate of 40 K/min. “Recryst.” indicates the occurrence of recrystallization, which yielded higher crystallite size and lower dislocation density than the detection limits of XLPA. 338
Figure 12.12 Dark field transmission electron microscopy micrographs taken on consolidated-Cu specimen (a) immediately after high-pressure torsion (HPT) and after subsequent heating up to (b) 750 K and (c) 1000 K, as well as on Cu–carbon nanotube composite (d) immediately after HPT and after subsequent heating up to (e) 750 K and (f) 1000 K. 339
Figure 12.13 Scanning electron microscopy images of the polished cross sections at the half-radius of the high-pressure torsion processed disks after heating up to 1000 K: (a) consolidated-Cu and (b) Cu–carbon nanotube composite. 340
Figure 12.14 The hardness as a function of temperature for consolidated-Cu and Cu–carbon nanotube (CNT) composite specimens in differential scanning calorimetry annealing at a heating rate of 40 K/min. 341
Figure 12.15 Differential scanning calorimetry thermogram obtained at a heating rate of 10 K/min for 4N purity Ag sample processed by 10 revolutions of high-pressure torsion. The temperatures of heat treatments are indicated by solid circles. 342
Figure 12.16 Nanoindentation layout on the cross section of the high-pressure torsion (HPT)-processed Ag disk. 343
Figure 12.17 Nanohardness distributions as a function of the distance from the bottom of the high-pressure torsion (HPT)–processed Ag disk measured on the cross section in the axial direction. The lines serve only as guides to the distributions. The typical error bar is illustrated on the left side of the figure. 344
Figure 12.18 Electron backscatter diffraction micrographs showing the ultrafine-grained (UFG) microstructure before the exothermic differential scanning calorimetry (DSC) peaks at 400 K (a) and after the second peak at 497 K (b). The microstructure after the first DSC peak at 440 K is shown in (c) where the transition layer between the recrystallized interior and the UFG surface layer is indicated by a dashed line. The inset in (c) shows a part of the recrystallized grain in a higher magnification, illustrating that the large grain contains smaller twinned subgrains. A part of the UFG microstructure in the surface region is shown in a higher magnification in (d). 345
Figure 12.19 Differential scanning calorimetry thermograms obtained immediately after equal-channel angular pressing (ECAP) and storage at room temperature for 4 years in the case of Cu samples processed by 1 and 10 passes. 347
Figure 12.20 The reduction in vacancy concentration determined from the decrease of stored energy in Cu as a function of number of equal-channel angular pressing (ECAP) passes. 348
Figure 12.21 Transmission electron microscopy images taken immediately after high-pressure torsion processing (a) and 4 years of storage (b). 350
Figure 12.22 The microhardness of samples processed by different numbers of equal-channel angular pressing (ECAP) passes as a function of the time of storage at room temperature. 352
Figure 12.23 Debye–Scherrer rings for the 220 reflections of X-rays (a) immediately after eight equal-channel angular pressing (ECAP) passes and (b) after eight ECAP passes and storage at room temperature for 4 months. 353
Figure 12.24 Bright field transmission electron microscopy images from a sample processed through eight equal-channel angular pressing passes (a) and after storage at room temperature for 4 months (b). 354
Figure 12.25 (a) The dislocation density and (b) the twin boundary frequency in the recovered volumes of samples stored at room temperature up to 4 months after processing by equal-channel angular pressing (ECAP) through 1, 4, 8, and 16 passes. 354
Figure 12.26 Values of the microhardness after processing by equal-channel angular pressing (ECAP) for 1, 4, 8, and 16 passes as a function of the storage time at room temperature for 4N purity Ag. 357
Figure 12.27 Bright field transmission electron microscopy images showing the microstructures of (a) the 4N5 purity sample processed through eight equal-channel angular pressing (ECAP) passes and stored at room temperature for 4 months and (b) the 4N purity specimen after eight ECAP passes and storage for 1 year. 358
Figure 12.28 Schematic graph of the hardness distribution along the diameter of the Zn–22% Al disk processed by one turn of high-pressure torsion (HPT). The hardness before HPT and the evolution of the hardness distribution due to storage at room temperature are also shown [83]. 359
Figure 12.29 Transmission electron microscopy images obtained on Au–cetyltrimethylammonium bromide nanoparticles. (a) Immediately after production the size of the spherical Au nanoparticles was 2–5 nm, and (b) after 1 year of storage the gold particles grew to about 25 nm and exhibited different shapes. 361
Figure 12.30 Transmission electron microscopy images showing the different types nanoparticles with regular morphology after 1 year of storage. (a) Decahedron (D) and triangular plate (TP). (b) Deca-tetrahedron (DT) and rod (R). The inset shows a schematic drawing of the three-dimensional morphology of a DT. (c) Bipyramid (BP). (d) The arrow indicates that the twin boundaries in a rod are lying parallel to its longitudinal axis. 362
Figure 12.31 Transmission electron microscopy image of fused Au nanoparticles after storing them for 1 year. The arrow indicates the joint surface of two fused nanoparticles. 364
Figure 12.32 The factors influencing the shape of face-centered cubic nanoparticles and the resulted crystal morphologies. 365
Figure 12.33 Williamson–Hall plot of the full width at half maximum (FWHM) of the X-ray diffraction peaks as a function of the length of the diffraction vector (g) for Au nanoparticles after 1 year of storage. 366
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.
