Lattice Defects and Diffusion in Nanomaterials
Abstract
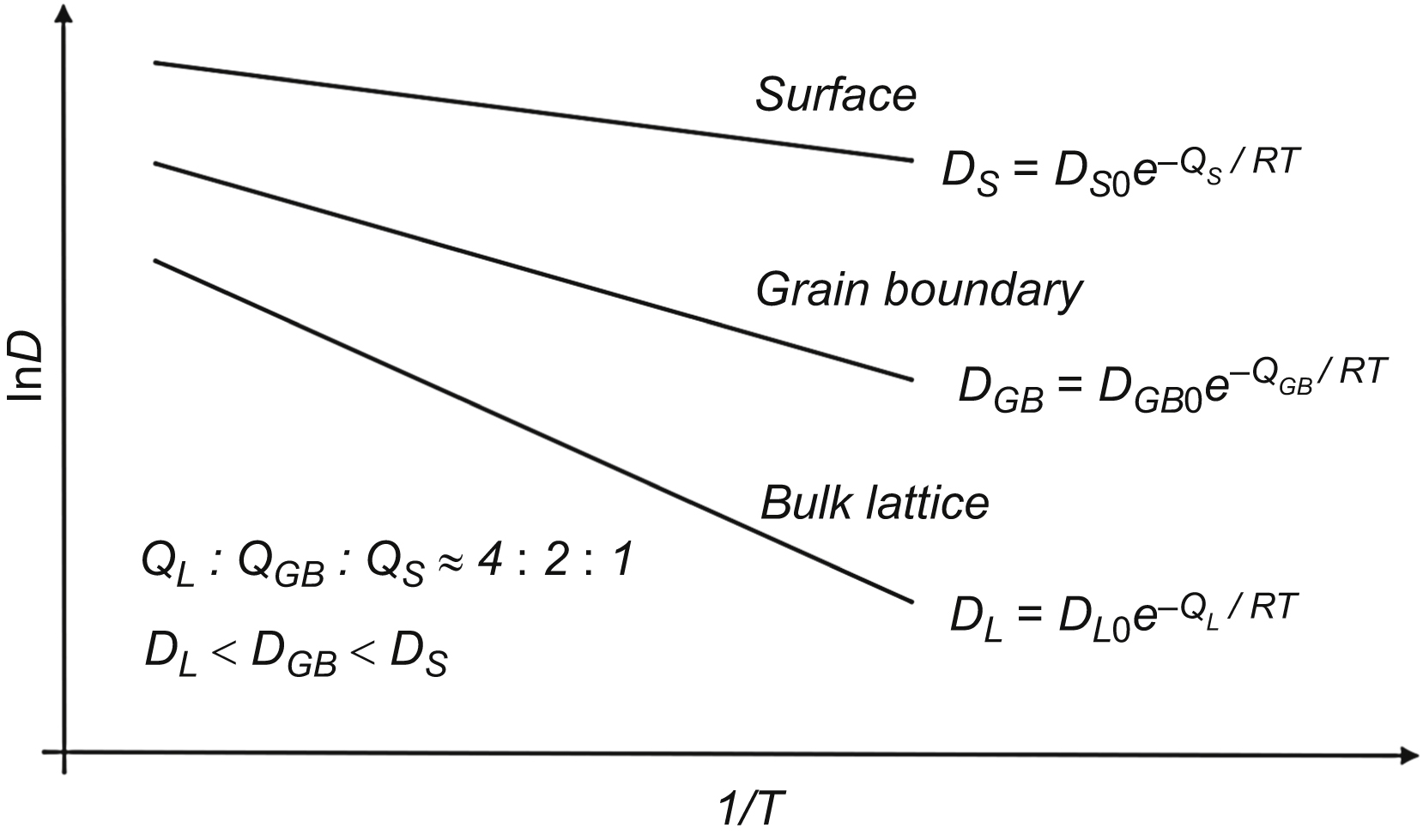
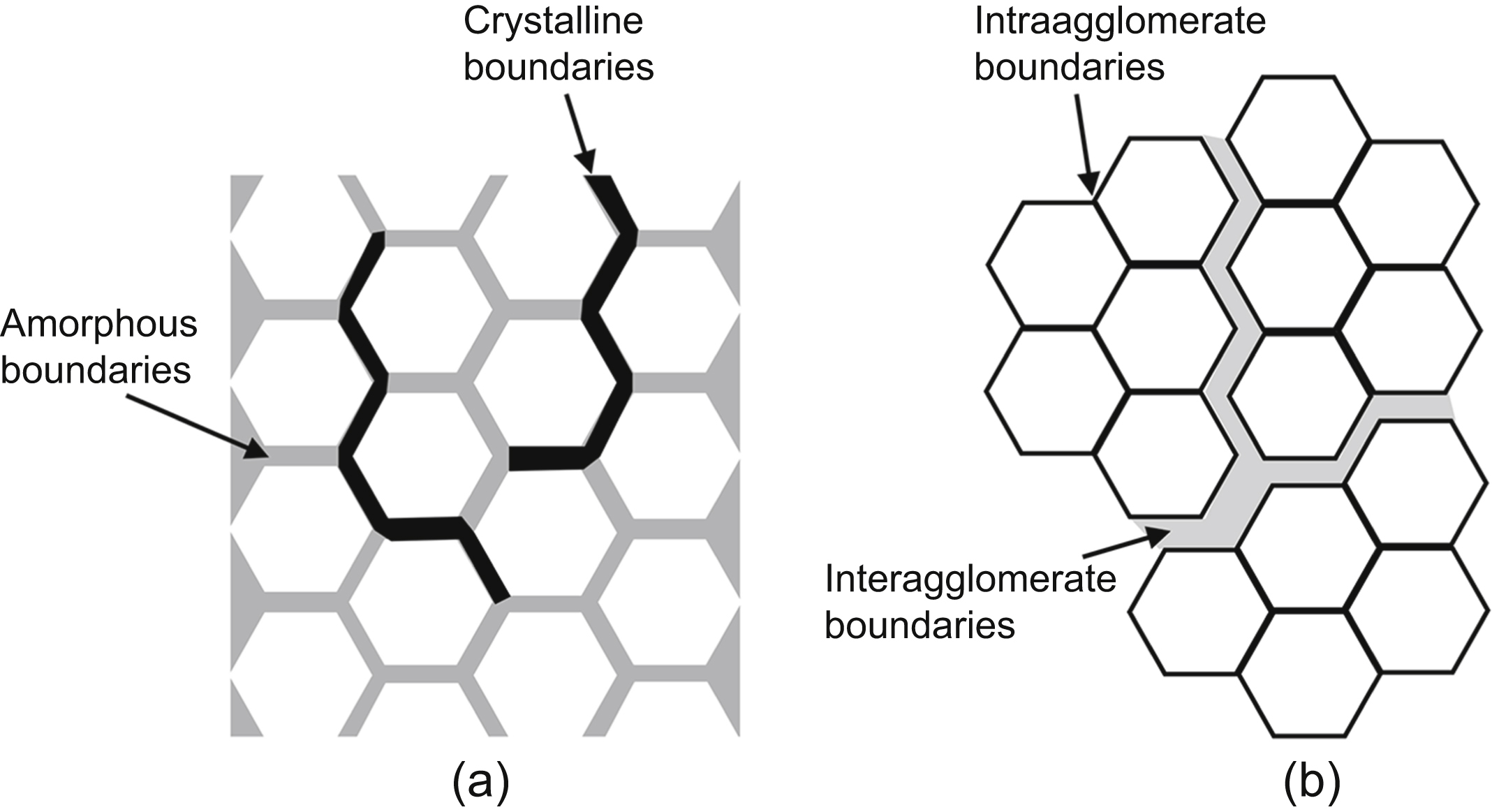
The diffusion rate in nanomaterials is influenced strongly by the type and densities of lattice defects. Generally, dislocations and grain boundaries are paths for fast diffusion; however, the actual atomic arrangement around these defects also influences the diffusion along them. In this chapter, the most important models, formulas, and methods for the evaluation of diffusion along grain boundaries and dislocations are overviewed. The effect of solute segregation and misorientation on grain boundary self-diffusion is revealed. It was found that the larger the free volume and the energy of grain boundaries, the faster the grain boundary diffusion. In ultrafine-grained metallic materials processed by severe plastic deformation (SPD), a hierarchical microstructure develops with nonequilibrium and relaxed grain boundaries, which are paths for fast and slow grain boundary diffusion, respectively. As the fraction of boundaries exhibiting fast diffusion is very small (under 0.5%), the diffusion rate in nanomaterials is determined by the relaxed boundaries. The diffusivity for these boundaries is very close to that observed for grain boundaries in coarse-grained materials. Therefore, the faster diffusion in nanomaterials compared to coarse-grained counterparts is caused basically by the larger amount of grain boundaries and not by their different quality. In nanomaterials processed by SPD, the fastest diffusion path is provided by the percolating pores at grain boundaries formed by agglomeration of excess vacancies. However, the contribution of these defects to the total diffusivity is very small due to their marginal volume fraction. In nanomaterials processed by powder metallurgy, the diffusivity for interagglomerate interfaces between particles and pores is several orders of magnitude larger than that for intraagglomerate boundaries. Similar bimodal diffusivity is observed for nanomaterials obtained by crystallization of amorphous materials. Here, the slow and fast diffusion pathways are the amorphous and conventional crystalline interfaces. The diffusion along amorphous boundaries is slower than that for crystalline interfaces.
Keywords
10.1. Effect of Lattice Defects on Diffusion
![]() (10.1)
(10.1)
![]() (10.2)
(10.2)
![]() (10.3)
(10.3)
![]() (10.4)
(10.4)
![]() (10.5)
(10.5)

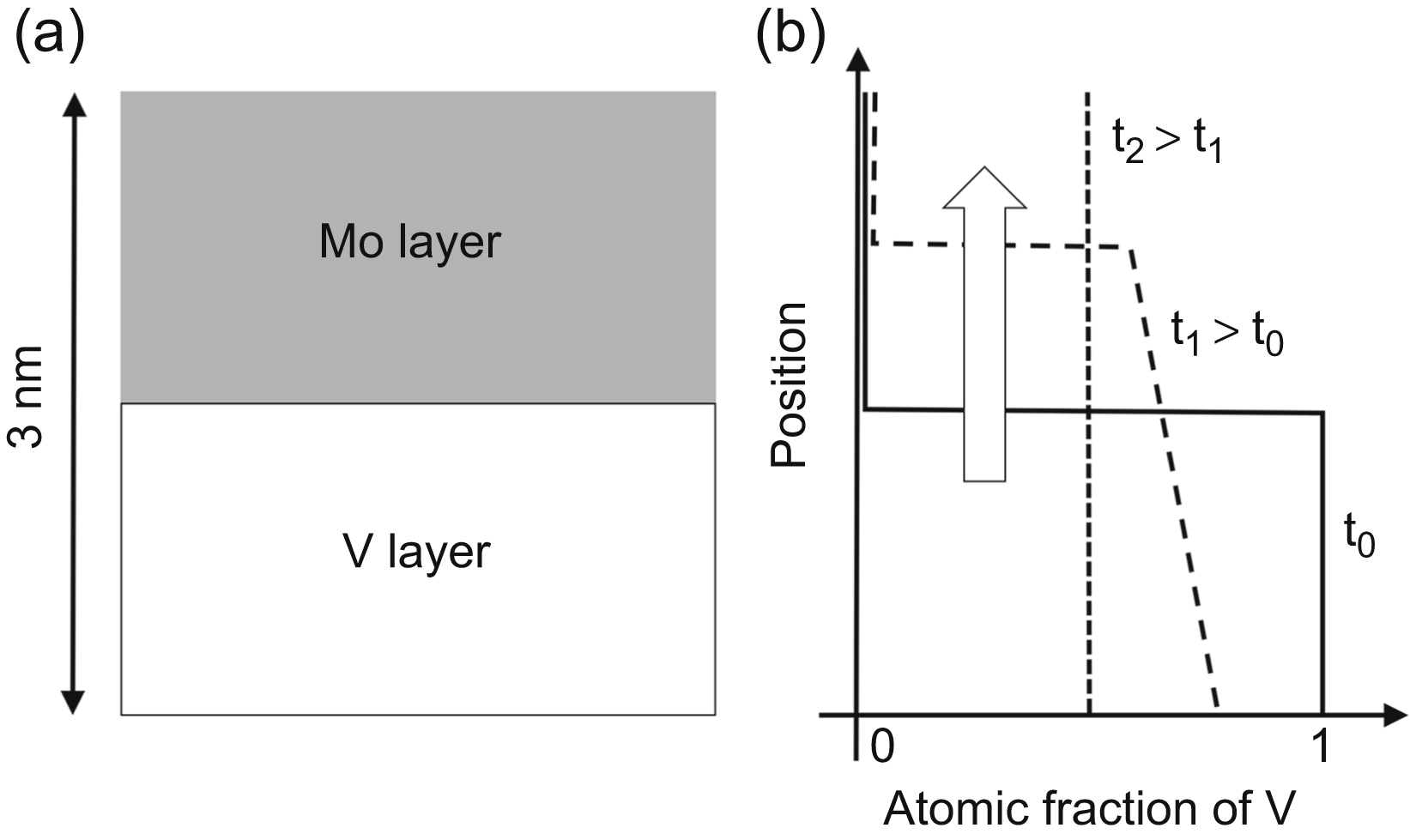
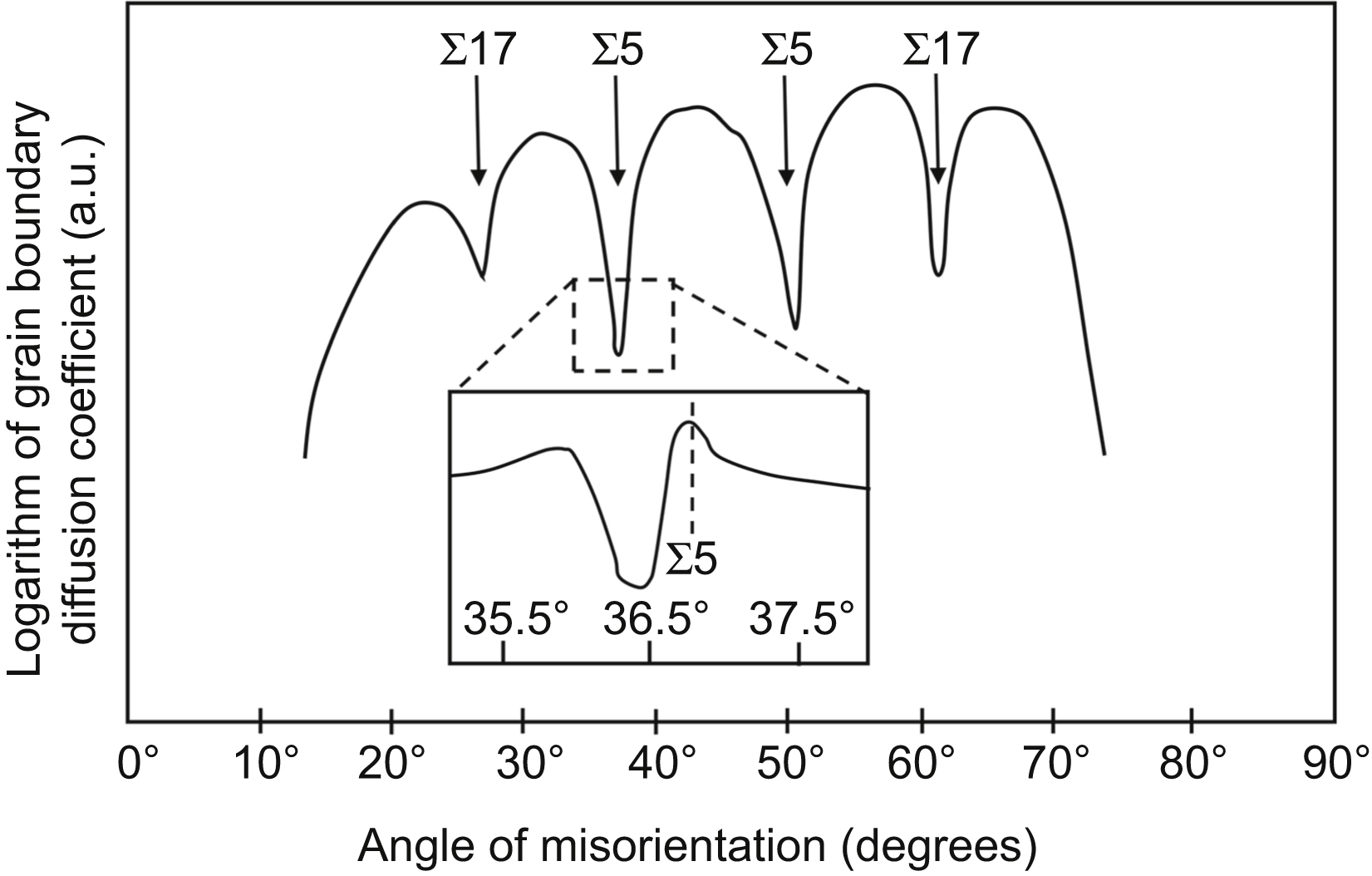
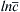 versus x6/5 gives a straight tail part for large x values, which is characteristic for grain boundary diffusion, as shown in (b). This straight line can be used for the determination of grain boundary diffusion coefficient (see the text for details).
versus x6/5 gives a straight tail part for large x values, which is characteristic for grain boundary diffusion, as shown in (b). This straight line can be used for the determination of grain boundary diffusion coefficient (see the text for details). (10.6)
(10.6)
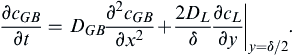 (10.7)
(10.7)
 (10.8)
(10.8)

Table 10.1
The grain boundary diffusion kinetics types, the related experimental conditions, and the evaluated function of concentration ![]() versus penetration depth (x)
versus penetration depth (x)
| Diffusion type | Condition | Evaluated function | Obtained parameter |
| A | fDGB + (1−f)DL | ||
| B | sδDGB | ||
| C | DGB |



10.2. Diffusion in Ultrafine-Grained and Nanocrystalline Materials Processed by Severe Plastic Deformation

 is the average tracer concentration determined by serial sectioning method while x is the tracer penetration depth. HAGB, high-angle grain boundary.
is the average tracer concentration determined by serial sectioning method while x is the tracer penetration depth. HAGB, high-angle grain boundary.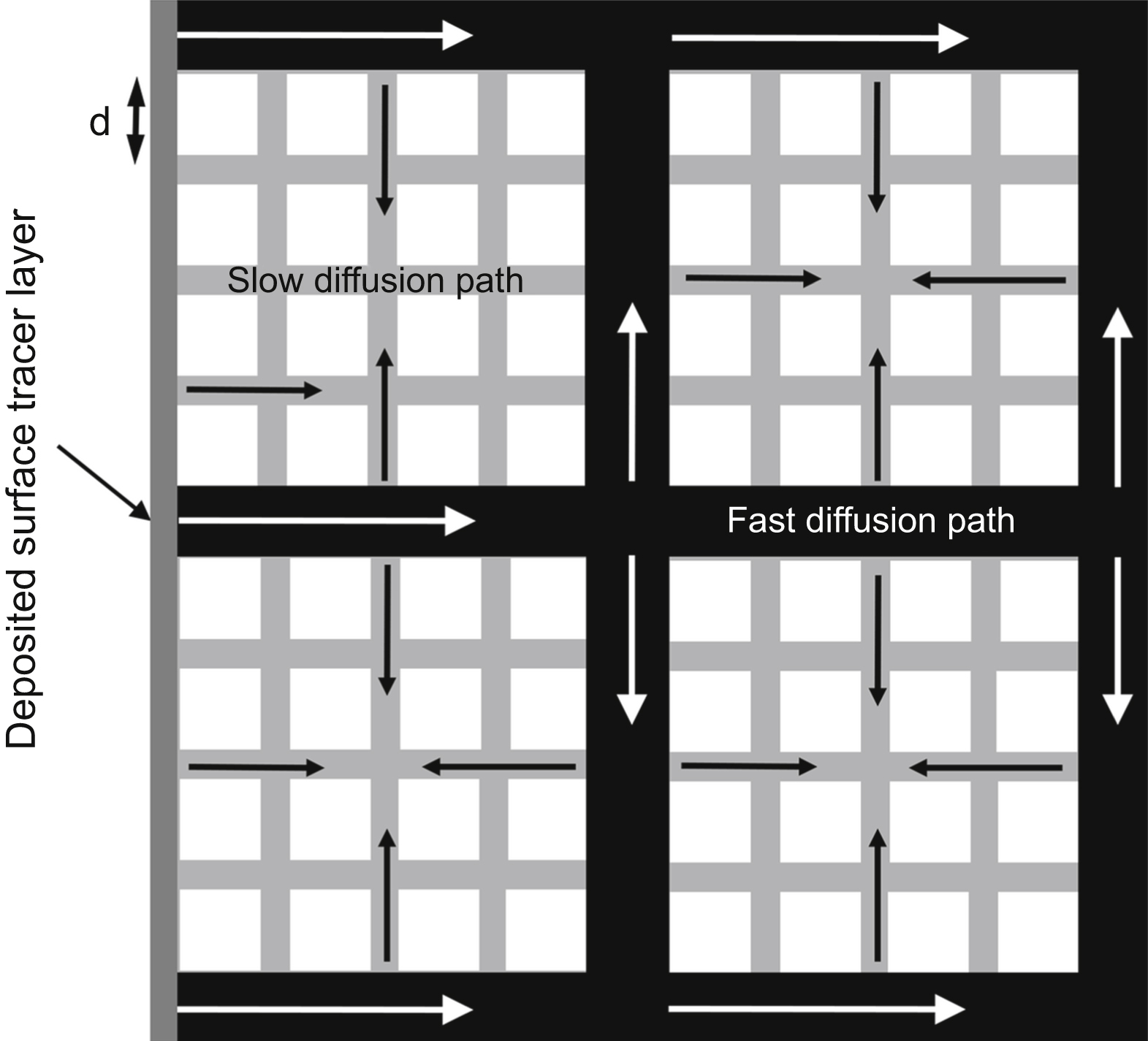
 (10.9)
(10.9)
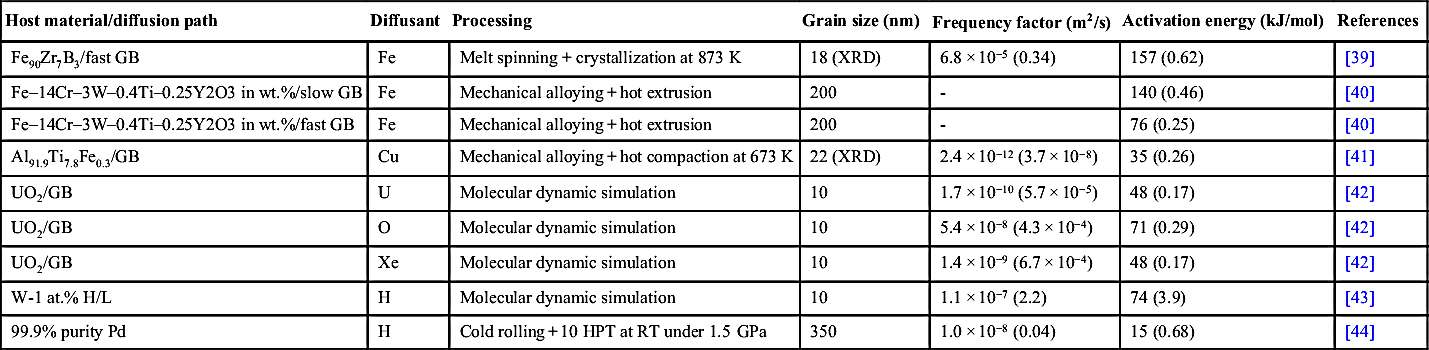
Table 10.2
The frequency factor (D0), the activation energy (Q) of diffusion for different UFG and nanocrystalline materials
| Host material/diffusion path | Diffusant | Processing | Grain size (nm) | Frequency factor (m2/s) | Activation energy (kJ/mol) | References |
| 99.98 wt.% Cu/slow GB | Ni | 4 ECAP at RT | 300 | 1.6 × 10−7 (2.3 × 10−3) | 85 (0.38) | [21] |
| Cu–0.17 wt.% Zr/slow GB | Ni | 4 ECAP at RT | 300 | 1.4 × 10−7 (2.0 × 10−3) | 84 (0.37) | [23] |
| Cu–0.17 wt.% Zr/fast GB | Ni | 4 ECAP at RT | 300 | 1.6 × 10−3 (22.9) | 96 (0.43) | [23] |
| 99.6 wt.% purity Ni/fast GB | Ni | 4 ECAP at RT | 300 | 1.2 × 10−8 (1.3 × 10−4) | 67 (0.24) | [28] |
| Ni/GB | Cu | ECAP at RT + annealing at 398 K for 1 h | 300 | - | 43 (0.17) | [26] |
| Ni/GB | Ni | Compaction of powder at 773 K and 4.4 GPa | 70 | 2.2 × 10−12 (2.4 × 10−8) | 46 (0.17) | [35] |
| γ-Fe−40 wt.% Ni/slow GB | Fe | Compaction of powder at 1123 K and 1.25 GPa | 80–100 (XRD) | 4.2 × 10−3 (4.8) | 187 (0.62) | [36] |
| γ-Fe–40 wt.% Ni/fast GB | Fe | Compaction of powder at 1123 K and 1.25 GPa | 80–100 (XRD) | 3.4 × 10−3 (3.9) | 148 (0.49) | [36] |
| γ-Fe–40 wt.% Ni/slow GB | Ni | Compaction of powder at 1123 K and 1.25 GPa | 80–100 (XRD) | 9.3 × 10−4 (1.2) | 177 (0.58) | [37] |
| γ-Fe–40 wt.% Ni/fast GB | Ni | Compaction of powder at 1123 K and 1.25 GPa | 80–100 (XRD) | 1.8 × 10−3 (2.3) | 134 (0.44) | [37] |
| γ-Fe–40 wt.% Ni/slow GB | Ag | Compaction of powder at 1123 K and 1.25 GPa | 80–100 (XRD) | 4.7 × 10−4 (0.39) | 173 (0.62) | [38] |
| γ-Fe–40 wt.% Ni/fast GB | Ag | Compaction of powder at 1123 K and 1.25 GPa | 80–100 (XRD) | 8.1 × 10−5 (0.07) | 91 (0.33) | [38] |
| Fe90Zr7B3/slow GB | Fe | Melt spinning + crystallization at 873 K | 18 (XRD) | 2.8 × 10−7 (1.4 × 10−3) | 163 (0.65) | [39] |
| Table Continued | ||||||

| Host material/diffusion path | Diffusant | Processing | Grain size (nm) | Frequency factor (m2/s) | Activation energy (kJ/mol) | References |
| Fe90Zr7B3/fast GB | Fe | Melt spinning + crystallization at 873 K | 18 (XRD) | 6.8 × 10−5 (0.34) | 157 (0.62) | [39] |
| Fe–14Cr–3W–0.4Ti–0.25Y2O3 in wt.%/slow GB | Fe | Mechanical alloying + hot extrusion | 200 | - | 140 (0.46) | [40] |
| Fe–14Cr–3W–0.4Ti–0.25Y2O3 in wt.%/fast GB | Fe | Mechanical alloying + hot extrusion | 200 | - | 76 (0.25) | [40] |
| Al91.9Ti7.8Fe0.3/GB | Cu | Mechanical alloying + hot compaction at 673 K | 22 (XRD) | 2.4 × 10−12 (3.7 × 10−8) | 35 (0.26) | [41] |
| UO2/GB | U | Molecular dynamic simulation | 10 | 1.7 × 10−10 (5.7 × 10−5) | 48 (0.17) | [42] |
| UO2/GB | O | Molecular dynamic simulation | 10 | 5.4 × 10−8 (4.3 × 10−4) | 71 (0.29) | [42] |
| UO2/GB | Xe | Molecular dynamic simulation | 10 | 1.4 × 10−9 (6.7 × 10−4) | 48 (0.17) | [42] |
| W-1 at.% H/L | H | Molecular dynamic simulation | 10 | 1.1 × 10−7 (2.2) | 74 (3.9) | [43] |
| 99.9% purity Pd | H | Cold rolling + 10 HPT at RT under 1.5 GPa | 350 | 1.0 × 10−8 (0.04) | 15 (0.68) | [44] |

10.3. Diffusion in Nanomaterials Processed by Bottom-Up Methods

![]() (10.10)
(10.10)

![]() (10.11)
(10.11)