Chapter 1. Introduction to Separation Process Engineering
1.0 Summary—Objectives
In this chapter we explore some of the reasons for studying separations and some of the methods we will use. When finished studying this chapter, you should be able to satisfy the following objectives:
1. Explain how separations are used in a typical chemical plant
2. Define the concepts of equilibrium stages and unit operations
3. Explain what is meant by phase equilibrium
4. Explain the basic concepts of mass transfer
5. List the steps in the structured problem-solving approach and start to use this approach
6. Have some familiarity with the prerequisites
The first use of the Summary—Objectives section is to help you see where you are going. Then, when you’ve finished the chapter, the Summary—Objectives section can help you decide if you got there.
1.1 Importance of Separations
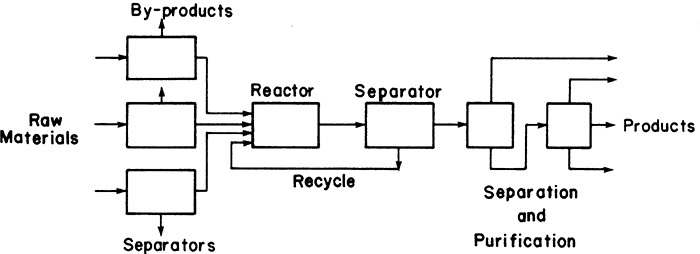
Chemical engineering requires the study of separation techniques because separations are crucial in chemical engineering. A typical chemical plant is a chemical reactor surrounded by separators, as diagramed in the schematic flowsheet of Figure 1-1. Raw materials are pre-purified in separation devices and fed to the chemical reactor; unreacted feed is separated from the reaction products and recycled back to the reactor. Products must be further separated and purified before they can be sold. This type of arrangement is very common. Examples for a variety of traditional processes are illustrated by Biegler et al. (1997), Chenier (2002), Couper et al. (2005), Matar and Hatch (2001), Shreve and Austin (1984), Speight (2002), and Turton et al. (2012), whereas recent processes often are shown in Chemical Engineering magazine. Chemical plants commonly have from 40% to 70% of both capital and operating costs in separations (Humphrey and Keller, 1997).
Since separations are ubiquitous in chemical plants and petroleum refineries, chemical engineers must be familiar with a variety of separation methods. We first focus on some of the most common chemical engineering separation methods: flash distillation, continuous column distillation, batch distillation, absorption, stripping, and extraction. These separations all contact two phases and can be designed and analyzed as equilibrium stage processes. Several other separation methods that can also be considered equilibrium stage processes are briefly discussed. Crystallization also contacts two phases to achieve separation but is discussed separately in Chapter 17 because, in addition to chemical purity, the distribution of crystal sizes and the exact crystal structure are critically important. Chapters 18 and 19 explore two important separations—membrane separators and adsorption processes—that do not operate as equilibrium stage systems.
The equilibrium stage concept is applicable when the process can be constructed as a series of discrete stages in which the two phases are contacted and then separated. The two separated phases are assumed to be in equilibrium with each other. For example, in distillation, a vapor and a liquid are commonly contacted on a metal plate with holes in it. Because of the intimate contact between the two phases, solute can transfer from one phase to another. Above the plate the vapor disengages from the liquid. Both liquid and vapor can be sent to additional stages for further separation. Assuming that the stages are equilibrium stages, the engineer can calculate concentrations and temperatures without detailed knowledge of flow patterns and heat and mass transfer rates. Although this example shows the applicability of the equilibrium stage method for equipment built with a series of discrete stages, we will see that the staged design method can also be used for packed columns where there are no discrete stages. The equilibrium stage concept greatly simplifies the design and analysis of chemical engineering separations that is used in Chapters 2 to 14 and 17.
A second useful concept is that of a unit operation. The idea here is that although the specific design may vary depending on what chemicals are being separated, the basic design principles for a given separation method are always the same. For example, the basic principles of distillation are always the same whether we are separating ethanol from water, separating several hydrocarbons, or separating liquid metals. Consequently, distillation is often called a unit operation, as are absorption, extraction, and so on.
A more general idea is that design methods for related unit operations are similar. Since distillation and absorption are both liquid-vapor contacting systems, the design is much the same for both. This similarity is useful because it allows us to apply a very few design tools to a variety of separation methods. We will use stage-by-stage methods where calculation is completed for one stage, and then the results are used for calculation of the next stage to develop basic understanding. Matrix solution of the mass and energy balances will be used for detailed computer simulations.
1.2 Concept of Equilibrium
The separation processes we are studying in Chapters 1 to 14 are based on the equilibrium stage concept, which states that streams leaving a stage are in equilibrium. What do we mean by equilibrium?
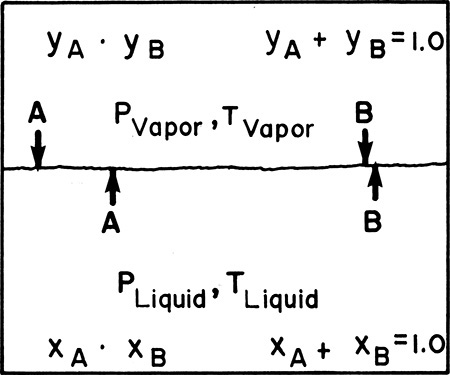
Consider a vapor and a liquid that are in contact with each other, as shown in Figure 1-2. Liquid molecules are continually vaporizing, while vapor molecules are continually condensing. If two chemical species are present, they will, in general, condense and vaporize at different rates. When not at equilibrium, the liquid and the vapor can be at different pressures and temperatures and be present in different mole fractions. At equilibrium the temperatures, pressures, and fractions of the two phases cease to change. Although molecules continue to evaporate and condense, the rate at which each species condenses is equal to the rate at which it evaporates. Although on a molecular scale nothing has stopped, on the macroscopic scale, where we usually observe processes, there are no further changes in temperature, pressure, or composition.
Equilibrium conditions can be conveniently subdivided into thermal, mechanical, and chemical potential equilibrium. In thermal equilibrium, heat transfer stops and the temperatures of the two phases are equal.
In mechanical equilibrium, the forces between vapor and liquid balance. In the staged separation processes we will study, this usually implies that the pressures are equal. Thus for the cases in this book,
If the interface between liquid and vapor is curved, equal forces do not imply equal pressures. In this case the Laplace equation can be derived (e.g., see Levich, 1962).
In phase equilibrium, the rate at which each species is vaporizing is just equal to the rate at which it is condensing. Thus there is no change in composition (mole fraction in Figure 1-2). However, in general, the compositions of liquid and vapor are not equal. If the compositions were equal, no separation could be achieved in any equilibrium process. If temperature and pressure are constant, equal rates of vaporization and condensation require a minimum in the free energy of the system. The resulting condition for phase equilibrium is
The development of Eq. (1-3), including the necessary definitions and concepts, is the subject of a large portion of many books on thermodynamics (e.g., Denbigh, 1981; Elliott and Lira, 2012; Franses, 2014; O’Connell and Haile, 2005; Sandler, 2006; Smith et al., 2005) but is beyond the scope of this book. However, Eq. (1-3) does require that there be some relationship between liquid and vapor compositions. In real systems this relationship may be very complex, and experimental data may be required. We will assume that the equilibrium data or appropriate correlations are known (see Chapter 2), and we will confine our discussion to the use of the equilibrium data in the design of separation equipment.
1.3 Mass Transfer Concepts
In the vapor-liquid contacting system shown in Figure 1-2, the vapor and liquid will not be initially at equilibrium. By transferring mass from one phase to the other we can approach equilibrium. The basic mass transfer equation in words is
In this equation the mass transfer rate will typically have units such as kmol/h or lbmol/h. The area is the area across which mass transfer occurs in m2 or ft2. The driving force is the concentration difference that drives the mass transfer. This driving force can be represented as a difference in mole fractions, a difference in partial pressures, a difference in concentrations in kmol/L, and so forth. The value and units of the mass transfer coefficient depend upon which driving forces are selected. The details are discussed in Chapter 15.
For equilibrium staged separations, we would ideally calculate the mass transfer rate based on the transfer within each phase (vapor and liquid in Figure 1-2) using a driving force that is the concentration difference between the bulk fluid and the concentration at the interface. Since this is difficult, we often make a number of simplifying assumptions (see Section 15.4 for details) and use a driving force that is the difference between the actual concentration and the concentration we would have if equilibrium were achieved. For example, for the system shown in Figure 1-2 with concentrations measured in mole fractions, we could use the following rate expressions:
In these equations Ky and Kx are overall gas and liquid mass transfer coefficients, yA* is the mole fraction in the gas in equilibrium with the actual bulk liquid of mole fraction xA, xA* is the mole fraction in the liquid in equilibrium with the actual bulk gas of mole fraction yA, and the term “a” is the interfacial area per unit volume (m2/m3 or ft2/ft3).
By definition, at equilibrium we have yA* = yA and xA* = xA. Note that as yA→yA* and xA→xA*, the driving forces in Eqs. (1-5) approach zero and mass transfer rates decrease. In order to be reasonably close to equilibrium, the simplified model represented by Eq. (1-5) shows that we need high values of Ky and Kx and/or “a.” Generally speaking, the mass transfer coefficients will be higher if diffusivities are higher (details are in Chapter 15), which occurs with fluids of low viscosity. Since increases in temperature decrease viscosity, increasing temperature is favorable as long as it does not significantly decrease the differences in equilibrium concentrations and the materials are thermally stable. Mass transfer rates will also be increased if there is more interfacial area/volume between the gas and liquid (higher “a”). This can be achieved by having significant interfacial turbulence or by using a packing material with a large surface area (see Chapter 10).
Although some knowledge of what affects mass transfer is useful, we don’t need to know the details as long as we are willing to assume we have equilibrium stages. Thus, we will delay discussing the details until we need them (Chapters 15 through 19).
1.4 Problem-Solving Methods
To help develop your problem-solving abilities, an explicit strategy, which is a modification of the strategy developed at McMaster University (Woods et al., 1975), is used throughout this book. The seven stages of this strategy are
0. I want to, and I can
1. Define the problem
2. Explore or think about it
3. Plan
4. Do it
5. Check
6. Generalize
Step 0 is a motivation and confidence step. It is a reminder that you got this far in chemical engineering because you can solve problems. The more different problems you solve, the better a problem solver you will become. Remind yourself that you want to learn how to solve chemical engineering problems, and you can do it.
In step 1 you define the problem. Make sure that you clearly understand all the words. Draw the system and label its parts. List all the known variables and constraints. Describe what you are asked to do. If you cannot define the problem clearly, you will probably be unable to solve it.
In step 2 you explore and think about the problem. What are you really being asked to do? What basic principles should be applied? Can you find a simple limiting solution that gives you bounds to the actual solution? Is the problem over- or underspecified? Let your mind play with the problem and chew on it, and then go back to step 1 to make sure that you are still looking at the problem in the same way. If not, revise the problem statement and continue. Experienced problem solvers always include an explore step even if they don’t explicitly state it.
In step 3 the problem solver plans how to subdivide the problem and decides what parts to attack first. The appropriate theory and principles must be selected and mathematical methods chosen. The problem solver assembles required resources such as data, paper, and calculator. While doing this, new subproblems may arise; you may find there are not enough data to solve the problem. Recycle through the problem-solving sequence to solve these subproblems.
Step 4, do it, is often the first step that inexperienced problem solvers try. In this step the mathematical manipulations are done, the numbers are plugged in, and an answer is generated. If your plan was incomplete, you may be unable to carry out this step. In that case, return to step 2 (explore) or step 3 (plan) and recycle through the process.
In step 5, check your answer. Is it the right order of magnitude? For instance, commercial distillation columns are neither 12 centimeters nor 12 kilometers high. Does the answer seem reasonable? Have you avoided blunders such as plugging in the wrong number or incorrectly punching the calculator? Is there an alternative solution method that can serve as an independent check on the answer? If you find errors or inconsistencies, recycle to the appropriate step and solve the problem again.
The last step, generalize, is important but is usually neglected. In this step you try to learn as much as possible from the problem. What have you learned about the physical situation? Did including a particular phenomenon have an important effect, or could you have ignored it? Generalizing allows you to learn and become a better problem solver.
At first these steps will not “feel” right. You will want to get on with it and start calculating instead of carefully defining the problem and working your way through the procedure. Stick with a systematic approach. It works much better on difficult problems than a “start calculating, maybe something will work” method. The more you use this or any other strategy, the more familiar and less artificial it will become.
In this book, most example problems are solved using this strategy. To avoid repeating myself, I will not list step 0, but it is always there. The other six steps will usually be explicitly listed and developed. On the simpler examples some of the steps may be very short, but they are always present.
I strongly encourage you to use this strategy and write down each step as you do homework problems. In the long run this method will improve your problem-solving ability.
A problem-solving strategy is useful, but what do you do when you get stuck? In this case heuristics or rules of thumb are useful. A heuristic is a method that is often helpful but is not guaranteed to help. A large number of problem-solving heuristics have been developed. I have listed ten (Wankat and Oreovicz, 2015) that are often helpful to students.
Problem-Solving Heuristics
1. Try solving simplified, limiting cases.
2. Relate the problem to one you know how to solve. This heuristic encapsulates one of the major reasons for doing homework.
3. Generalize the problem.
4. Try putting in specific numbers. Heuristics 3 and 4 are the opposite of each other. Sometimes it is easier to see a solution path without all the details, and sometimes the details help.
5. Solve for ratios. Often problems can be solved for ratios, but there is not enough information to solve for individual values.
6. Do the solvable parts of the problem. This approach may provide information that allows you to solve previously unsolvable parts.
7. Look for information that you haven’t used.
8. Try guess and check. If you have a strong hunch, this may lead to an answer, but you must check your guess.
9. Take a break. Don’t quit, but do something else for a while. Coming back to the problem may help you see a solution path.
10. Ask someone for a little help. Then complete the problem on your own.
Ten heuristics is probably too many to use on a regular basis. Select four or five that fit you, and make them a regular part of your problem-solving method. If you want to read more about problem solving and heuristics, I recommend How to Model It: Problem Solving for the Computer Age (Starfield et al., 1994) and Strategies for Creative Problem Solving (Fogler and LeBlanc, 2013).
1.5 Units
In 1998 and 1999 the United States National Aeronautics and Space Administration (NASA) inadvertently did a large-scale demonstration of the importance of units during its Mars Climate Orbiter program (Civan, 2013). The thruster performance program was developed in the United States using English units. The output from this file was then read by a trajectory program that calculated the angular momentum and calculated the propulsion maneuvers necessary to arrive at the desired entry location into Martian atmosphere. The trajectory modelers in England used metric units. Their specifications for the interface of the two programs specified that the input data was in metric units. Both modeling groups thought the other group would make sure that any required unit conversions were done.
The $125 million spacecraft, the Mars Climate Orbiter, entered the Martian atmosphere on December 11, 1998, and NASA lost radio contact. Contact was never reestablished with the Orbiter, which presumably disintegrated. Later analysis showed that the Orbiter entered the planet’s atmosphere 49 seconds earlier and 170 km lower than it was supposed to. The error was due entirely to the inconsistent use of units.
Chemical engineers are unlikely to have anything as dramatic as loss of the Mars Orbiter occur, but the loss of several million dollars or a fatal accident because of incorrect units is quite possible and will not be helpful to your career. Best practice with respect to units is to convert all terms to a common set of units when you start the problem and carry units in the equations. These procedures are illustrated in the examples. Although problem solutions and Appendix C show unit conversion factors, it is assumed that you are very familiar with unit conversions, including conversion from weight to mole fractions, and vice versa.
Some students will protest that doing unit conversions and carrying units in the equations is a lot of unnecessary work. Why doesn’t the author put everything in a consistent set of units so that students do not have to worry about units? The NASA experience illustrated that different units occur in the “real” world and unit conversions are necessary. In this book data is reported in the units used by the original source. Use of data in school and in industry will often require conversion of the units.
1.6 Computers and Computer Simulations
Since the modern practice of chemical engineering is computer based, you need to have facility with common computer software, particularly with spreadsheets. Spreadsheets are the most common general-purpose calculation tool used in industry. Although Microsoft Excel is used for the example spreadsheets, any commercial spreadsheet program will work for the spreadsheets that do not use macros.
Many students naively believe that they are experts with Excel. To be an expert, you have to know how to use macros, including Visual Basic for Applications (VBA). Although some of the spreadsheets shown in the chapter appendices use VBA, these examples show only a small part of the power of VBA. Students who want to develop a true mastery of spreadsheets are advised to take an advanced course on spreadsheets or spend time with a good book on spreadsheets for engineers, such as Larson (2013). A portfolio showing your mastery of Excel will certainly provide you with numerous talking points during interviews.
Because most separation systems in industry are designed and simulated with the aid of process simulators, teaching you how to use process simulators is one of the major goals of this book. The computer labs in the appendices of Chapters 2, 4, 6, 8, 10, 12, 13, and 16 will step you through the process of learning how to use a process simulator. Although the process simulator used for these labs is Aspen Plus, with appropriate modifications of the instructions, the labs can be done with many process simulators.
1.7 Prerequisite Material
No engineering book exists in a vacuum, and some preparatory material is always required. The first prerequisite, which is often overlooked, is that you must be able to read well. If you don’t read well, get help immediately.
A second set of prerequisites involves certain mathematical abilities. You need to be comfortable with algebra and the solution of equations, as these skills are used throughout the text. Another required mathematical skill is graphical analysis, since many of the design methods are graphical methods. You need to be competent and to feel comfortable plotting curves and straight lines and solving simultaneous algebraic equations graphically. Familiarity with exponential and logarithmic manipulations is required for Chapter 7. The only chapters requiring calculus and the solution of ordinary differential equations are Section 8.5.2, Chapter 9, and Chapters 15 through 19. The solutions of partial differential equations are needed only in Sections 15.2.3 and 15.5.5 and at the very end of the book in Sections 19.6 and 19.7, and in all cases the solutions are presented.
The third area of prerequisites is use of mass balances, energy balances, and phase equilibria. Although the basics of mass and energy balances can be learned in a very short time, facility with their use requires practice. Thus, this book will normally be preceded by a course on mass and energy balances. Knowledge of the basic ideas of phase equilibrium, including the concept of equilibrium, Gibbs’ phase rule, distribution coefficients, familiarity with graphical representations of equilibrium data, and a working knowledge of vapor-liquid equilibrium (VLE) correlations will be helpful.
The fourth and fifth areas of prerequisites are problem-solving skills and facility with units. Because the chemical engineer must be a good problem solver, it is important to develop skills in this area. The ability to solve problems using modern computer tools is an expectation of all chemical engineering graduates. Units are critical because wrong answers due to incorrect units are just as wrong (and possibly deadly) as wrong answers due to misapplication of theory or incorrect experimental data.
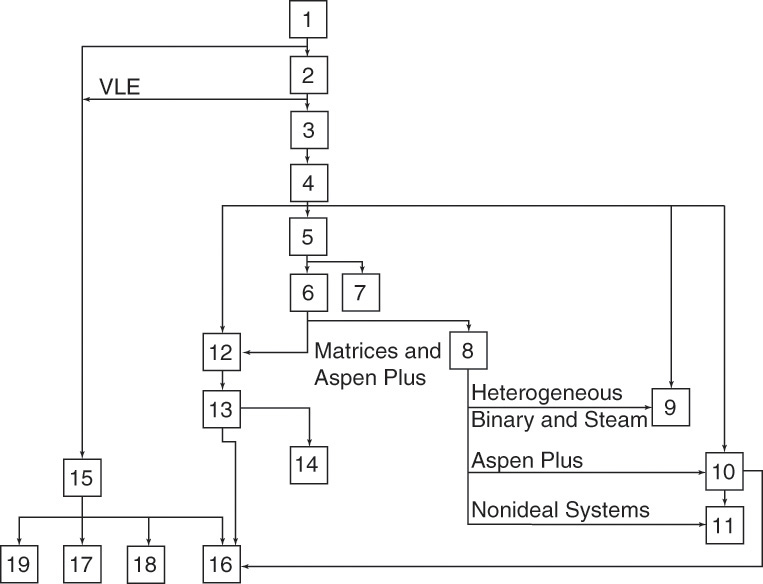
In general, later chapters depend on the earlier chapters, as shown schematically in Figure 1-3. Chapters 11, 14, 16, 17, and 18 are not required for the understanding of later chapters and can be skipped if time is short. Figure 1-3 should be useful in planning the order in which to cover topics and for adapting this book for special purposes.
1.8 Other Resources on Separation Process Engineering
Since students have different learning styles, you need to customize the way you use this book to adapt to your learning style. Of course, you will have to take charge of your learning and do this for yourself. If you are interested in exploring your learning style, a good place to start is the Index of Learning Styles, which was developed by Richard M. Felder and Linda K. Silverman. This index is available free on the Internet at www4.ncsu.edu/unity/lockers/users/f/felder/public/ILSpage.html. Alternatively, you may search on the term “Felder” using a search engine such as Google.
Since students (and professors) have different learning styles, no single approach to teaching or writing a book can be best for all students. Thus, there will undoubtedly be parts of this book that do not make sense to you. Many students use other students, then the teaching assistant, and finally the professor as resources. Fortunately, a number of good textbooks and Web pages exist that can be helpful because their presentations differ from those in this textbook. Table 1-1 presents a short annotated bibliography of some of the available handbook and textbook resources. A large number of useful websites are available but are not listed because URLs change rapidly. They can be accessed by searching on the terms “distillation,” “extraction,” “crystallization,” “reverse osmosis,” and so forth, using any popular search engine.
Belter, P. A., E. L. Cussler, and W.-S. Hu, Bioseparations. Downstream Processing for Biotechnology, Wiley-Interscience, New York, 1988. Separations textbook with emphasis on bioseparations.
Cussler, E. L., Diffusion: Mass Transfer in Fluid Systems, 3rd ed., Cambridge University Press, Cambridge, UK, 2009. Textbook on basics of diffusion and mass transfer with applications to a variety of separation processes in addition to other applications.
Doherty, M. F., and M. F. Malone, Conceptual Design of Distillation Systems, McGraw-Hill, New York, 2001. Advanced distillation textbook that uses residue curve maps to analyze complex distillation processes.
Geankoplis, C. J., Transport Processes and Separation Process Principles, 4th ed., Prentice Hall PTR, Upper Saddle River, NJ, 2003. Unit operations textbook that has expanded coverage of separation processes and transport phenomena.
Harrison, R. G., P. Todd, S. R. Rudge, and D. P. Petrides, Bioseparations Science and Engineering, 2nd ed., Oxford University Press, New York, 2015. Separations textbook with emphasis on bioseparations.
Hines, A. L., and R. M. Maddox, Mass Transfer: Fundamentals and Applications, Prentice-Hall PTR, Upper Saddle River, NJ, 1985. Textbook on basics of diffusion and mass transfer with applications to separation processes.
Humphrey, J. L., and G. E. Keller II, Separation Process Technology, McGraw-Hill, New York, 1997. Industrially oriented book that includes performance, selection, and scale-up information.
King, C. J., Separation Processes, 2nd ed., McGraw-Hill, New York, 1980. Textbook that seeks to integrate knowledge of separation processes and has extensive case studies.
McCabe, W. L., J. C. Smith, and P. Harriott, Unit Operations of Chemical Engineering, 7th ed., McGraw-Hill, New York, 2004. Unit operations textbook that includes extensive coverage of separations and transport phenomena.
Noble, R. D., and P. A. Terry, Principles of Chemical Separations with Environmental Applications, Cambridge University Press, Cambridge, UK, 2004. Basic separation principles with environmental examples and problems in a non-calculus-based format.
Perry, R. H., and D. W. Green (Eds.), Perry’s Chemical Engineers’ Handbook, 8th ed., McGraw-Hill, New York, 2008. General handbook that has extensive coverage on separations, but coverage often assumes reader has some prior knowledge of technique.
Rousseau, R.W. (Ed.), Handbook of Separation Process Technology, Wiley-Interscience, New York, 1987. Handbook containing detailed information on a number of different separation methods.
Schweitzer, P. A. (Ed.), Handbook of Separation Techniques for Chemical Engineers, 3rd ed., McGraw-Hill, New York, 1997. Handbook containing detailed information on many separations. Coverage often assumes reader has some prior knowledge of technique.
Seader, J. D., E. J. Henley, and D. J. Roper, Separation Process Principles, 3rd ed., Wiley, New York, 2011. Textbook covering an introduction to mass transfer and a large variety of separation processes.
Seidel, A. (Ed.), Kirk-Othmer Encyclopedia of Chemical Technology, 5th Ed., Wiley-Interscience, New York, 2004. Extensive encyclopedia with many entries by authorities on separation processes.
Treybal, R. E., Mass-Transfer Operations, 3rd ed., McGraw-Hill, New York, 1980. Textbook on basics of diffusion and mass transfer with detailed applications to separation processes.
Wankat, P. C., Mass Transfer Limited Separations, Springer, Berlin, 1990. Advanced textbook on crystallization, adsorption, chromatography, ion exchange, and membrane separations.
http://www.engineeringtoolbox.com/ and http://www.cheric.org/research/kdb/ are excellent sources for data needed for separation problems.
TABLE 1-1. Annotated bibliography of resources on separation process engineering
References
Biegler, L. T., I. E. Grossmann, and A. W. Westerberg, Systematic Methods of Chemical Process Design, Prentice Hall, Upper Saddle River, NJ, 1997.
Chenier, P. J., Survey of Industrial Chemistry, Springer-Verlag, Berlin, 2002.
Civan, F., “Avoid Problems with Units of Measurement,” Chemical Engineering Progress, 109 (2), 43, February 2013.
Couper, J. R., W. R. Penney, J. R. Fair, and S. M. Walas, Chemical Process Equipment: Selection and Design, 2nd ed., Elsevier, Amsterdam, 2005.
Denbigh, K., The Principles of Chemical Equilibrium, 4th ed., Cambridge University Press, Cambridge, UK, 1981.
Elliott, J. R., and C. T. Lira, Introductory Chemical Engineering Thermodynamics, 2nd ed., Prentice Hall, Upper Saddle River, NJ, 2012.
Fogler, H. S., and S. E. LeBlanc, Strategies for Creative Problem Solving, 3rd ed., Prentice Hall, Upper Saddle River, NJ, 2013.
Franses, E. I., Thermodynamics with Chemical Engineering Applications, Cambridge University Press, Cambridge, UK, 2014.
Humphrey, J. L., and G. E. Keller II, Separation Process Technology, McGraw-Hill, New York, 1997.
Larsen, R. W., Engineering with Excel, 4th ed., Pearson (published as Prentice Hall), Upper Saddle River, NJ, 2013.
Levich, V. G., Physiochemical Hydrodynamics, Prentice Hall, Upper Saddle River, NJ, 1962.
Matar, S., and L. F. Hatch, Chemistry of Petrochemical Processes, 2nd ed., Gulf Publishing Co., Houston, TX, 2001.
O’Connell, J. P. and J. M. Haile, Thermodynamics. Fundamentals and Applications, Cambridge University Press, New York, 2005.
Sandler, S. I., Chemical and Engineering Thermodynamics, 4th ed. Wiley, New York, 2006.
Shreve, R. N., and G. T. Hatch, Chemical Process Industries, 5th ed., McGraw-Hill, New York, 1984.
Smith, J. M., H. C. Van Ness, and M. M. Abbott, Introduction to Chemical Engineering Thermodynamics, 7th ed., McGraw-Hill, New York, 2005.
Speight, J. G., Chemical and Process Design Handbook, McGraw-Hill, New York, 2002.
Starfield, A. M., K. A. Smith, and A. L. Bleloch, How to Model It: Problem Solving for the Computer Age, 2nd ed., McGraw-Hill, New York, 1994.
Turton, R., R. C. Bailie, W. B. Whiting, J. A. Shaeiwitz, and D. Bhattacharya, Analysis, Synthesis and Design of Chemical Processes, 4th ed., Prentice Hall, Upper Saddle River, NJ, 2012.
Wankat, P. C., and F. S. Oreovicz, Teaching Engineering, 2nd ed., Purdue University Press, West Lafayette, Indiana, 2015.
Woods, D. R., J. D. Wright, T. W. Hoffman, R. K. Swartman, and I. D. Doig, “Teaching Problem Solving Skills,” Engineering Education, 66 (3), 238 (Dec. 1975).
Homework
A. Discussion Problems
A1. Return to your successful solution of a fairly difficult problem in one of your previous technical courses (preferably chemical engineering). Look at this solution but from the point of view of the process used to solve the problem instead of the technical details. Did you follow a structured method? Most people don’t at first. Did you eventually do most of the steps listed? Usually, the define, explore, plan, and do it steps are done sometime during the solution. Rearrange your solution so that these steps are in order. Did you check your solution? If not, do that now. Finally, try generalizing your solution.
A2. Without returning to the book, answer the following:
a. Define a unit operation. Give a few examples.
b. What is the equilibrium stage concept?
c. What are the steps in the systematic problem solving approach? Explain each step in your own words.
A3. The equilibrium stage concept
a. is a hypothetical construct.
b. assumes that phases leaving the stage are in equilibrium.
c. is useful even when phases are not in equilibrium.
d. all of the above.
A4. If you have studied heat transfer, relate Eq. (1-4) to the similar basic definition of heat transfer by conduction and convection.
A5. Do you satisfy the prerequisites? If not, how can you remedy this situation?
A6. Develop a key relations chart (one page or less) for this chapter. A key relations chart is a summary of everything you need to solve problems or answer questions from the chapter. In general, it will include equations, sketches, and key words. Organize it in your own way. The purpose of developing a key relations chart is to force your brain to actively organize the material. This practice will greatly aid you in remembering the material.
A7. The results of Web searches can often give surprising results. Use a standard search engine and search for “separation processes,” and see what the results are.
B1. List as many products and how they are purified or separated as you can. Go to a large supermarket and look at some of the household products. How many of these could you separate? At the end of this book you will know how to purify most of the liquid products and many of the solid products.
B2. Some separation methods are common in homes in the United States. Most of these are concerned with water treatment. List the separations that you are familiar with, and briefly describe how you think they work.
B3. The body uses several membrane separation methods. List as many of these as you can, and describe how you think they work.
B4. Separation operations are very common in chemistry laboratories. List the separations that you employed in various chemistry labs.
C. Derivations
C1. Write the mass and energy balances (in general form) for the separator shown in Figure 1-1. If you have difficulty with this task, review a book on mass and energy balances.
D. Problems
D1. One of the prerequisites for study of separations is the ability to convert from weight to mole fractions, and vice versa. As a refresher in this conversion, solve the following problem: We have a flow rate of 1500.0 kmol/h of a feed that is 40.0 mol% ethanol and 60.0 mol% water. What is the weight fraction of ethanol, and what is the total flow rate in pounds per hour?
Data: The locations in this textbook of different data are given in Appendix D.
D2. We have 100.0 kg/h of a feed that is 40.0 wt% ethanol and 60.0 wt% water. What is the mole fraction of ethanol, and what is the total flow rate in moles per hour?
Data: The locations in this textbook of different data are given in Appendix D.
D3. If the pressure is 900.0 mm Hg, what is the pressure in kPa?
Note: Unit conversions are in Appendix C.
D4. If you have an energy requirement of 13.0 kJ/s, how many kW is this?
Note: Unit conversions are in Appendix C.
E. Complex Problems
There are no complex problems for this chapter.
F. Problems Using Other Resources
F1. Look through several recent issues of Chemical Engineering magazine or similar technical magazines and find an article that contains a process flow chart. Read the article and write a short (less than one page) critique. Explicitly comment on whether the flowsheet for the process fits (at least approximately) the general flowsheet shown in Figure 1-1.
F2. Arrange a tour of the unit operations laboratory in your institution to observe the different types of separation equipment. Note that although this equipment is often much larger than the separation equipment that you used in chemistry laboratory, it is much smaller than industrial-scale equipment.
G. Computer Simulation Problems
There are no computer simulation problems for this chapter.
H. Computer Spreadsheet Problems
There are no computer spreadsheet problems for this chapter.