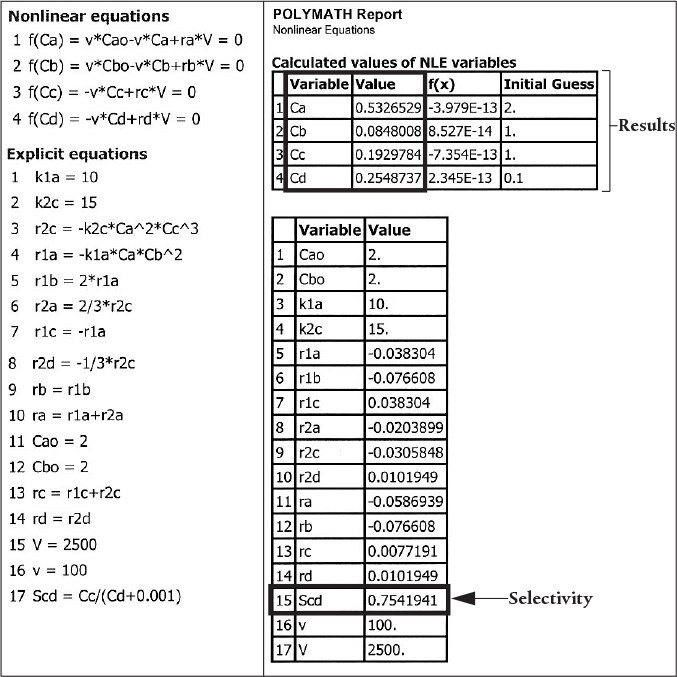
Example 8-6 Complex Reactions in a Liquid-Phase CSTR
The complex reactions discussed in Example 8-5 now take place in the liquid phase in a 2,500 dm3 CSTR. The feed is equimolar in A and B with FA0 = 200 mol/min and the volumetric flow rate is 100 dm3/min. The rate constants are
Find the concentrations of A, B, C, and D exiting the reactor, along with the exiting selectivity, .
A+2B→C 2A+3C→D
Solution
Liquid-Phase CSTR: υ = υ0 (Polymath Formulation)
Mole Balances
The Rate Laws, Relative Rates, and Net Rates are the same as in Example 8-5. In addition, Steps (5) through (14) (i.e., Equations (E8-5.5) through (E8-5.14) from Example 8-5) remain unchanged for this example. This fact is going to save us a lot of time in solving this example problem.
Selectivity
Note: We add a very small number (0.001 mol/min) to the term in the denominator to prevent Sc/D going to infinity when FD = 0.
Parameters
(16) u0=100dm3/min
(17) k1A=10(dm3/mol)2/min
(18) k2C=15(dm3/mol)4/min
(19) V= 2,500dm3
(20) CA0 = 2.0mol/dm3
(21) CB0 = 2.0mol/dm3
These equations are now used to solve for the exit concentrations using Polymath’s nonlinear equation solver.
Liquid-Phase CSTR
The exit concentrations are CA = 0.53 M, CB = 0.085 M, Cc = 0.19 M, and CD = 0.25 M with SC/D = 0.75. The corresponding conversion of A is
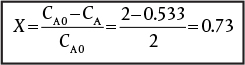
Analysis: The CRE algorithm for a complex reaction carried out in a CSTR was solved using the nonlinear equation solver. The CSTR exit concentrations shown in the results table correspond to a selectivity SC/D = 0.75, as shown in the Polymath report. While the CSTR conversion is reasonable, the selectivity is rather low. The PFR is a better choice for these reactions to maximize selectivity.
8.5.3 Complex Liquid-Phase Reactions in a Semibatch Reactor
Next up is the application of Tables 8-1 and 8-2 to a complex reaction carried out in a semibatch reactor.
Example 8-7 Complex Reactions in a Semibatch Reactor
The complex liquid-phase reactions discussed in Example 8-6 now take place in a semibatch reactor where A is fed to B with FA0 = 3 mol/min. The volumetric flow rate is 10 dm3/min and the initial reactor volume is 1,000 dm3. The rate constants are
A+2B→C 2A+3C→D
The maximum volume is 2,000 dm3. The inlet concentration of A is CA0 = 0.3 mol/dm3 and the initial concentration of B is CBi = 0.2 mol/dm3.
(a) Plot and analyze NA, NB, Nc, ND, and Sc/D as a function of time.
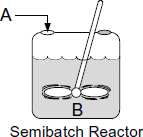
Solution
Mole Balances
Net Rates, Rate Laws, and Relative Rates are the same as liquid-phase CSTR.
Same as Step 2 in Example 8-5, i.e., Steps (5) through (14), Equations (E8-5.5) through (E8-5.14).
Stoichiometry
Tricks of the Trade. Because ND is 0 at time t = 0, the selective goes to infinity so we again use an “if” statement.
(21)
Parameters
New Parameters
(22) υ0 =10dm3/min
(23) V0 =1,000 dm3
(24) FA0 =3 mol/min
Putting this information into Polymath’s ODE solver, we obtain the following results.
Liquid-Phase Multiple Reactions in a Semibatch Reactor
Differential equations
1 d(Nb)/d(t) = rb*V
2 d(Na)/d(t) = ra*V +Fao
3 d(Nd)/d(t) = rd*V
4 d(Nc)/d(t) = rc*V
Explicit equations
1 k1a = 10
2 k2c = 15
3 Vo = 1000
4 vo = 10
5 V = Vo+vo*t
6 Ca = Na/V
7 Cb = Nb/V
8 r1a = -k1a*Ca*Cb^2
9 Cc = Nc/V
10 r1b = 2*r1a
11 rb = r1b
12 r2c = -k2c*Ca^2*Cc^3
13 Fao = 3
14 r2a = 2/3*r2c
15 r2d = -1/3*r2c
16 rlc = -r1a
17 rd = r2d
18 ra = r1a+r2a
19 Cd = Nd/V
20 rc = rlc+r2c
21 Scd = if(t>0.0001)then(Nc/Nd)else(0)
22 Nbo = 200
23 X = 1-Nb/Nbo
Calculated values of DEQ variables
Variable |
Initial value |
Final value |
|
1 |
Ca |
0 |
0.1034461 |
2 |
Cb |
0.2 |
0.0075985 |
3 |
Cc |
0 |
0.0456711 |
4 |
Cd |
0 |
0.0001766 |
5 |
Fao |
3. |
3. |
6 |
k1a |
10. |
10. |
7 |
k2c |
15. |
15. |
8 |
Na |
0 |
206.8923 |
9 |
Nb |
200. |
15.197 |
10 |
Nbo |
200. |
200. |
11 |
Nc |
0 |
91.34215 |
12 |
Nd |
0 |
0.3531159 |
13 |
r1a |
0 |
-5.973E-05 |
14 |
r1b |
0 |
-0.0001195 |
15 |
rlc |
0 |
5.973E-05 |
16 |
r2a |
0 |
-1.019E-05 |
17 |
r2c |
0 |
-1.529E-05 |
18 |
r2d |
0 |
5.097E-06 |
19 |
ra |
0 |
-6.992E-05 |
20 |
rb |
0 |
-0.0001195 |
21 |
re |
0 |
4.444E-05 |
22 |
rd |
0 |
5.097E-06 |
23 |
Scd |
0 |
258.6747 |
24 |
t |
0 |
100. |
25 |
V |
1000. |
2000. |
26 |
Vo |
1000. |
1000. |
27 |
vo |
10. |
10. |
28 |
X |
0 |
0.924015 |
Analysis: The CRE algorithm for a complex reaction was applied to a semibatch reactor and solved using the ODE solver Polymath. The maximum in the selectivity occurs after only 6.5 minutes (as shown in Figure E8-7.2); however, very little of the desired product, C, has been formed at this time. If ED > Eö, first try changing the temperature to see if that will improve the selectivity and the amount of product formed. If that does not work, an economical decision needs to be made. Are selectivity and the cost of separating C and D more important than making more C to sell?
Takeaway lesson: Reaction time is far too short for a batch or semibatch reactor
One notes in Figure E8-7.1 that after 70 minutes, very little change in the number of moles of species A, C, and D takes place; however, the number of moles of B continue to increase because it is continually fed and there isn’t sufficient A to react with B. The number of moles of D produced is so small that it is indistinguishable from the x-axis in Figure E8-7.1. Finally, we note that these times, 6.5 and 10 minutes, are far too short to use a semibatch reactor and, consequently, one should consider another reactor scheme such as Figure 8-2(g), where A is fed along the length of the reactor, or analogous to that shown in Figure 8-2(h), where A is fed to each of the CSTRs.
8.6 Membrane Reactors to Improve Selectivity in Multiple Reactions
In addition to using membrane reactors (MRs) to remove a reaction product in order to shift the equilibrium toward completion, we can use membrane reactors to increase selectivity in multiple reactions. This increase can be achieved by injecting one of the reactants along the length of the reactor. It is particularly effective in partial oxidation of hydrocarbons, as well as chlorination, ethoxy-lation, hydrogénation, nitration, and sulfonation reactions, to name a few.2

2 W. J. Asher, D. C. Bomberger, and D. L. Huestis, Evaluation of SRI’s Novel Reactor Process Permix™ (New York: AIChE, 2000).
In the top two reactions, the desired product is the intermediate (e.g., C2H40). However, because there is oxygen present, the reactants and intermediates can be completely oxidized to form undesired products, C02 and water. The desired product in the bottom reaction is xylene. We can enhance selectivity by keeping one of the reactants at a low concentration, which can be achieved by feeding it through the sides of a membrane reactor.
In the solved example problem in the Chapter 8 Expanded Material on the CRE Web site, we have used a membrane reactor (MR) for the hydrodealkylation of mesitylene reaction. In some ways, this CRE Web site example parallels the use of MRs for partial oxidation reactions. We will now do an example for a different reaction to illustrate the advantages of an MR for certain types of reactions.

Solved Problems
Example 8-8 Membrane Reactor (MR) to Improve Selectivity in Multiple Reactions
The reactions
take place in the gas phase. The overall selectivities, , are to be compared for a membrane reactor (MR) and a conventional PFR.† First, we use the instantaneous selectivity to determine which species should be fed through the membrane
We see that to maximize SD/U, we need to keep the concentration of A high and the concentration of B low; therefore, we feed B through the membrane. The molar flow rate of A entering the reactor is 4 mol/s and that of B entering through the membrane is 4 mol/s, as shown in Figure E8-8.1. For the PFR, B enters along with A.
† Prof. Dr. Sven Köttlov in the chemical engineering department at Jofostan University in Rica, Jofostan, is one of the best membrane analysts in that area of the world.
The reactor volume is 50 dm3 and the entering total concentration is 0.8 mol/dm3.
Plot and analyze the molar flow rates and the overall selectivity, , as a function of reactor volume for both the MR and PFR.
Solution
1. Mole Balances for Both the PFR and the MR
2. Net Rates and Rate Laws (same for PFR and MR)
3. Transport Law (MR)
We now discuss the rate of transport of B into the reactor, RB. From fluid mechanics we know that the volumetric flow rate through the membrane is given by Darcy’s Law.3
where K is the membrane permeability (m/s · kPa), Ps (kPa) and Pt (kPa) are the shell-side and tube-side pressures, and At is the membrane surface area (m2). The flow rate through the membrane can be controlled by adjusting the pressure drop across the membrane (Ps- Pt). Recall from Equation (6-5) that "a" is the membrane surface area per unit volume of reactor
The total molar flow rate of B through the sides of the reactor is
The molar flow rate of B into the side of the reactor per unit volume of reactor is
3J. O. Wilkes, Fluid Mechanics for Chemical Engineers with Microfluidics and CFD, 2nd Ed. (Upper Saddle River, NJ: Prentice Hall, 2006).
4. Stoichiometry (same for PFR and MR)
Isothermal (T = T0) and neglect pressure drop down the length of the reactor (P = P0,p= 1.0).
For no pressure drop down the length of the reactor and isothermal operation, the concentrations for both the PFR and MR are
Here, T = T0 and ΔP = 0.
5. Combine
The Polymath program will combine the mole balance, net rates, and stoichiometric equations to solve for the molar flow rate and selectivity profiles for both the conventional PFR and the MR, and also the selectivity profile.
A note of caution on calculating the overall selectivity
We again have to fool Polymath because at the entrance of the reactor Fö = 0, Polymath will look at Equation (E8-8.18) and will not run because it will say you are dividing by zero. Therefore, we need to add a very small number to the denominator, say 0.0001 mol/s (or smaller); that is
Fool Polymath!
TABLE E8-8.1 shows the Polymath program and report sheet.
Sketch the trends or results you expect before working out the details of the problem.

Living Example Problem
We can easily modify the program, shown in Table E8-8.1, for the PFR simply by setting RB equal to zero (RB = 0) and the initial condition for B to be 4.0.
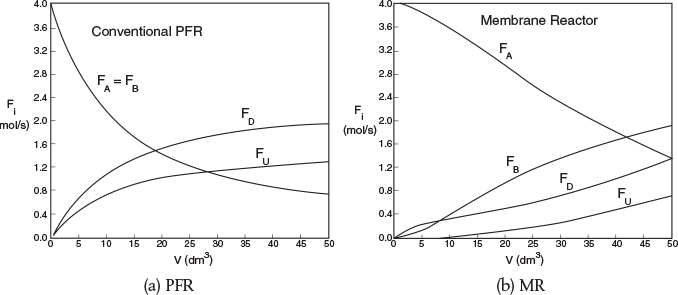
Figures E8-8.2(a) and E8-8.2(b) show the molar flow rate profiles for the conventional PFR and MR, respectively.

Figures E8-8.3(a) and E8-8.3(b) show the selectivity for the PFR and MR. One notices the enormous enhancement in selectivity the MR has over the PFR.
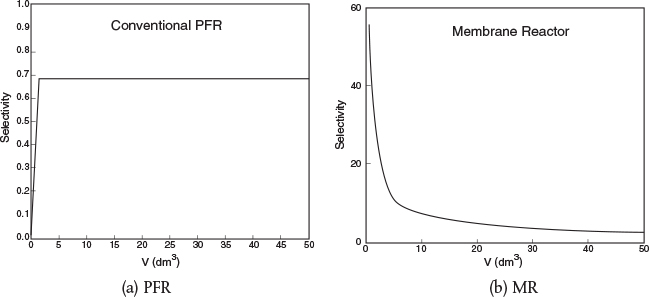
Figure E8-8.3 Selectivity.
LEP: http://www.umich.edu/~elementsße/08chap/live.html
Selectivities at V=5dm3
MR: SD/U= 14
PFR: SD/U=0.65
Be sure to download this Living Example Problem (LEP) from the CRE Web site and “play” with the reactions and reactors. With minor modifications, you can explore reactions analogous to partial oxidations
where oxygen (B) is fed through the side of the membrane (see Problem P8-15c).
Analysis: One notes that A is consumed more rapidly in the PFR than in the side-fed MR and that more of the desired product is also formed in the PFR. However, the selectivity is much higher in the MR than the PFR. One also notes, when comparing the molar flow rates, that the rates in the side-fed MR continue to change significantly after 30 dm3 down the reactor, while those in the PFR do not change significantly after 30 dm3.
8.7 Sorting It All Out
In Example 8-5 we were given the rate laws and asked to calculate the product distribution profiles. The inverse of the problem described in Example 8-5 must frequently be solved. Specifically the rate laws often must be determined from the variation in the product distribution profiles generated by changing the feed concentrations. In some instances, this determination may not be possible without carrying out independent experiments on some of the reactions in the sequence. The best strategy to use to sort out all of the rate-law parameters will vary from reaction sequence to reaction sequence. Consequently, the strategy developed for one system may not be the best approach for other multiple-reaction systems. One general rule is to start an analysis by looking for species produced in only one reaction; next, study the species involved in only two reactions, then three, and so on.
When some of the intermediate products are free radicals, it may not be possible to perform independent experiments to determine the rate-law parameters. Consequently, we must deduce the rate law parameters from changes in the distribution of reaction products with feed conditions. Under these circumstances, the analysis turns into an optimization problem to estimate the best values of the parameters that will minimize the sums of the squares between the calculated variables and measured variables. This process is basically the same as that described in Section 7.5, but more complex, owing to the larger number of parameters to be determined. We begin by estimating the parameter values using some of the methods just discussed. Next, we use these estimates in nonlinear regression techniques to determine the best estimates of our parameter values from the data for all of the experiments.4 Software packages are available for an analysis such as this one.
Nonlinear least squares
8.8 The Fun Part

I’m not talking about the fun you can have at an amusement park, but CRE fun. Now that we have an understanding on how to solve for the exit concentrations of multiple reactions in a CSTR and how to plot the species concentrations down the length of a PFR or PBR, we can address one of the most important and fun areas of chemical reaction engineering. This area, discussed in Section 8.3, is learning how to maximize the desired product and minimize the undesired product. It is this area that can make or break a chemical process financially. It is also an area that requires creativity in designing the reactor schemes and feed conditions that will maximize profits. Here, you can mix and match reactors, feed streams, and side streams, as well as vary the ratios of feed concentration in order to maximize or minimize the selectivity of a particular species. Problems of this type are what I call digital-age problems because almost always one needs to use ODE solvers, along with critical and creative thinking skills, to find the best answer.5·6 A number of problems at the end of this chapter will allow you to practice these critical and creative thinking skills. These problems offer the opportunity to explore many different solution alternatives to enhance selectivity and have fun doing it. The chemical reaction engineering students at Jofostan University in Rica, Jofostan, have often commented on how much they enjoy the LEPs and varying the parameters as they explore the problems and maximize the selective.
Multiple reactions with heat effects is unique to this book
However, to carry CRE to the next level and to have a lot more fun solving multiple-reaction problems, we will have to be patient a little longer. The reason is that in this chapter we consider only isothermal multiple reactions, and it is nonisothermal multiple reactions where things really get interesting. Consequently, we will have to wait to carry out schemes to maximize the desired product in nonisothermal multiple reactions until we study heat effects in Chapters 11, 12, and 13. After studying these chapters, we will add a new dimension to multiple reactions, as we now have another variable, temperature, that we may or may not be able to use to affect selectivity and yield. In one particularly interesting problem (P12-26c), we will study the production of styrene from ethylbenzene in which two side reactions, one endothermic and one exothermic, must be taken into account. Here, we may vary a whole slew of variables, such as entering temperature, diluent rate, and observed optima, in the production of styrene. However, we will have to delay gratification of the styrene study until we have mastered Chapters 11 and 12.
Summary
1. For the competing reactions
the instantaneous selectivity parameter is defined as
a. If ED > ΕU, the selectivity parameter SD/U will increase with increasing temperature.
b. If α1 > α2 and β2 > β1, the reaction should be carried out at high concentrations of A and low concentrations of B to maintain the selectivity parameter SD/U at a high value. Use a semibatch reactor with pure A initially or a tubular reactor in which B is fed at different locations down the reactor. Other cases discussed in the text are (α2 > α1, βl > β2), (α2 > α1, β2 > β1), and (α1 > α2, βl > β2).
The overall selectivity, based on molar flow rates leaving the reactor, for the reactions given by Equations (S8-1) and (S8-2) is
2. The overall yield is the ratio of the number of moles of a product at the end of a reaction to the number of moles of the key reactant that have been consumed
3. The algorithm for multiple reactions is shown in Table 8S-1. As noted earlier in this chapter, equations for the Rates Step are the major change in our CRE algorithm.
TABLE 8S-1 ALGORITHM FOR MULTIPLE REACTIONS
Number all reactions (1), (2), etc. |
||
Mole balances: |
||
Mole balance on each and every species |
||
PFR |
||
CSTR |
||
Batch |
||
Membrane ("i” diffuses in) |
||
Liquid-semibatch |
Rates:
Laws |
|
Relative rates |
|
Net rates |
Gas phase |
|
|
|
|
|
Liquid phase |
υ = υ0 |
|
CA, CB,... |
Combine:
Polymath will combine all the equations for you. Thank you, Polymath!!
Cre Web Site Materials
• Expanded Materials (http://umich.edu/~elements/5e/08chap/expanded.html)
1. Hydrodealkylation of Mesitylene in a PFR
(http://umich.edu/~elements/5e/08chap/expanded_08chap_l.pdf)
2. Hydrodealkylation of Mesitylene in a CSTR
(http://umich.edu/~elements/5e/08chap/expanded_08chap_l.pdf)
3. Calculating Concentration as a Function of Position for NH2 Oxidation in a PFR
(http://umich.edu/~elements/5e/08chap/prof-ammonia.html)
4. Puzzle Problem “What’s Wrong with this Solution?"
(http://umich.edu/~elements/5e/08chap/expanded_ch08_puzzle.pdfi
• Learning Resources (http://umich.edu/~elements/5e/08chap/learn.html)
1. Summary Notes
(http://umich.edu/~elements/5e/08chap/summary.html)
2. Self-Tests
(http://umich.edu/~elements/5e/08chap/add.html)
A. Exercises
(http://umich.edu/~elements/5e/08chap/add.html)
B. i>clicker Questions
(http://umich.edu/~elements/5e/08chap/iclicker_ch8_ql.html)
3. Web Modules
A. Cobra Bites
(http://www.umich.edu/~elements/5e/web_mod/cobra/index.html)
B. AspenTech (http://umich.edu/~elements/5e/08chap/learn-aspen.html)
C. Oscillating Reactions (http://umich.edu/~elements/5e/web_mod/oscil/module.htm)
4. Interactive Computer Games (http://umich.edu/~elements/5e/icm/index.html) The Great Race (http://umich.edu/~elements/5e/icm/grace.html)
5. Reactor Lab (See Learning Resources at the end of Chapter 5 for a description of these interactive computer exercises.) (http://umich.edu/~elements/5e/sofiware/reactorlab.html) Linfcs
6. Solved Problems (http://umich.edu/~elements/5e/08chap/live.html)
A. Blood Coagulation; also Living Example Problems (LEP)
B. Hydrodealkylation of Mesitylene in a PFR, CSTR, and Membrane Reactor; also Living Example Problems (LEPs)
C. All You Wanted to Know About Making Malic Anhydride and More; also Living Example Problems
D. Oxidation of Ammonia in a PFR; also Living Example Problems (LEP)
7. Clarification: PFR with feed streams along the length of the reactor (http://www.umich.edu/~elements/5e/08chap/learn-06lr.html)
• Living Example Problem (LEPs) (http://umich.edu/~elements/5e/08chap/live.html)
The LEPs are given using Polymath, MATLAB, and Wolfram
1. Example 8-1 Trambouze Reaction: Taking the output from the CSTR and feeding it to a PFR to increase conversion (decrease selectivity).
2. Example 8-3 Series Reactions in a BR
3. Example 8-4 Series Reactions in a CSTR
4. Example 8-5 Complex Gas-Phase Reactions in a PFR
5. Example 8-6 Complex Liquid-Phase Reactions in a CSTR
6. Example 8-7 Complex Liquid-Phase Reactions in a Semibatch Reactor
7. Example 8-8 Membrane Reactor to Improve Selectivity in Multiple Reactions
8. Example Web/Web Modules: Calculating Concentrations as a Function of Position for NH3 Oxidation in a PFR (See Chapter 8 Solved Problems on the CRE Web site for problem statement.)
9. Example Web/Web Modules: Cobra Bite Problem
10. Example Web/Web Modules: Solved Problems: Blood Coagulation
11. Example Web/Web Modules: Oscillating Reactions
12. AspenTech Example: Pyrolysis of Benzene
• FAQs (Frequently Asked Questions)—In Updates/FAQ icon section
(http://umich.edu/~elements/5e/08chap/faq.html)
• Professional Reference Shelf (http://umich.edu/~elements/5e/08chap/prof.html)
R8.1 Attainable Region Analysis (ARA)
(http://umich.edu/~elements/5e/08chap/pdf/CD-Ch06AttainableRegion.pay)
The ARA allows one to find the optimum reaction system for certain types of rate laws. The example used is one of modified van de Vusse kinetics

to find the optimum with respect to B using a combination of PFRs and CSTRs.

R8.2 Oxidation of Ammonia
(http://www.umich.edu/~elements/5e/08chap/prof-ammonia.html)
The coupled reactions for the oxidation of ammonia are modeled using a PFR.
R8.3 Calculating Pressure Drop in a Tubular Reactor (Pipes)
Questions and Problems
The subscript to each of the problem numbers indicates the level of difficulty: A, least difficult; D, most difficult.
A= • B = ▪ C = ♦ D= ♦♦
In each of the following questions and problems, rather than just drawing a box around your answer, write a sentence or two describing how you solved the problem, the assumptions you made, the reasonableness of your answer, what you learned, and any other facts that you want to include.

Questions
Q8-1A i>clicker. Go to the Web site (http://www.umich.edu/~elements/5e/08chap/iclicker_ch8_ql.html) and view at least five i>clicker questions. Choose one that could be used as is, or a variation thereof, to be included on the next exam. You also could consider the opposite case: explaining why the question should not be on the next exam. In either case, explain your reasoning.
Q8-2A (a) Reactant A has been unsuccessful in courting/dating reactant B because of a completing reaction. The matchmaker advises that the only way A will succeed is to raise the temperature. Is this a surefire idea? Will that work?
(b) Make up and solve an original problem to illustrate the principles of this chapter. See Problem P5-1A for guidelines.
(c) Write a question based on the material in this chapter that requires critical thinking. Explain why your question requires critical thinking. (Hint: See Preface Section G.)
(d) Are the overall and instantaneous selectivities identical for a CSTR, i.e., SMD/U≡ SD/U? Also, are the instantaneous and overall yields for a CSTR equal, i.e., YD = YD for a CSTR?
Q8-3C Read the cobra bite Web Module (http://www.umich.edu/~elements/5e/web_mod/cobra/index.html).
(a) Determine how many cobra bites are necessary in order that no amount of antivenom will save the victim.
(b) Suppose the victim was bitten by a harmless snake and not bitten by a cobra and anti-venom was injected. How much antivenom would need to be injected to cause death?
(c) What is the amount and latest possible time that anti-venom can be injected after a bite, such that the victim would not die?
(d) Apply one or more of the six ideas in Preface Table P-4, page xxvii, to this problem. (Hint: The Living Example Polymath program is on the CRE Web site.)
Computer Simulations and Experiments
P8-1A (a) Example 8-1: Trambouze Reactions Wolfram
(i) Vary the parameters kh k2, k3, and υ0 to learn their effects on conversion and selectivity in both a CSTR and a PFR.
(ii) Write a conclusion about your experiments.
Polymath
(iii) What would have been the selectivity, SB/XY, and conversion, X, if the reaction had been carried out in a single PFR with the same volume as the CSTR?
(iv) Recalling C = P RT, how would your answers change if the pressure were increased by a factor of 100?
(b) Example 8-2: Reactor Choice. Make a table/list for each reactor shown in Figure 8-2, identifying all the types of reactions that would be best carried out in this reactor. For example, Figure 8-2(d) Semibatch: used for (1) highly exothermic reactions and (2) increased selectivity.
(c) Example 8-3: LEP Series Reactions in Batch Reactor Wolfram
(i) Describe how the trajectories CA, CB, Cc, SB/C, and YB/C change by setting Ex and E2 equal to zero and then varying kx and k2 from their maximum to minimum values.
(ii) Reset (i.e., reload) to the initial conditions in the example and vary temperature between 273 K and 400 K and describe what you find.
(iii) Next, vary Ex and E2 and describe what you find.
(iv) How would topt change if fei = fe2 = 0.2 5/h at 300 K?
(d) Example 8-4: LEP Series Reactions in a CSTR Wolfram
(i) Use Wolfram to describe how the trajectories CA, CB, Cc, SB/C, and YB/C change by setting Ex and E2 equal to zero and then varying kx and fe2 from their maximum to minimum values.
(ii) Reset (i.e., reload) to the initial conditions in the example and vary temperature between 273 K and 400 K and describe what you find.
Polymath
(iii) What CSTR operating temperature (with t = 0.5s) would you recommend to maximize B for CA0 = 5 mol/dm3, kx = 0.4 s-1 and k2 = 0.01s-1, with Ex = 10 kcal/mol and E2 = 20 kcal/mol?
(Hint: Plot CB versus T. Use either Wolfram or Polymath.)
(e) Example 8-5: LEP Multiple Gas-Phase Reactions in a PBR Wolfram
(i) Vary CT0 and then describe what happens when you go from its minimum value to its maximum value. Describe how varying k and CT0 affect the selectivity, SqD, and the yield, Yc and YD.
(ii) After reviewing Generating Ideas and Solutions on the Web site (http://www.umich.edu/~elements/5e/toc/SCPS,3rdEdBook(Ch07).pdf), choose one of the brainstorming techniques (e.g., lateral thinking) to suggest two questions that should be included in this problem.
Polymath
(iii) Make slight modifications in the Polymath program to explore the case when the first reaction is reversible
with K = 0.002 (dm3/mol)2. Compare with the original problem and describe the difference you observe. Vary the ratio of entering flow rates of A to B to learn the effect on selectivity and then do the same by varying the same for volumetric flow rate.
(f) Example 8-6: LEP Complex Reactions in a Liquid-Phase CSTR Wolfram
(i) Explore the problem and describe what you find—i.e., which parameter has the greatest effect on selectivity. (One hint: Repeat (e)—e.g., vary CB.)
(g) Example 8-7: LEP Complex Reactions in a Semibatch Reactor Wolfram
(i) Vary FA0 and υ0 between the minimum and maximum values and then describe what happens to the profiles when compared to the base case. Which parameter has the greatest effect on selectivity?
(h) Example 8-8: LEP Membrane Reactor to Improve Selectivity Wolfram
(i) Vary CT0, fe1A, and k2A between their minimum and maximum values to describe what happens to the profiles of the molar flow rates and to the selectivity.
Polymath
(ii) Describe how your answers would change for the base case if FA0 = 2FA0. What if the reaction were A + 2B —> D with the rate law remaining the same? Vary the parameters and describe what you find.
(i) AspenTech Benzene Pyrolysis Example. (1) Change the activation energies to Ex = 28 kcal/mol and E2 = 32 kcal/mol, run the AspenTech program, and describe what you find. Compare with original data. (2) Repeat (1) by changing Ex = 32 kcal/mol and E2 = 28 kcal/mol, and describe what you find. (3) Double the reactor volume and compare the molar flow rate profiles. Describe what you find.
(j) Web Example. PFR Mesitylene Reaction. Download the Living Example Problem (LEP) from the CRE Web site. (1) How would your answers change if the feed were equal molar in hydrogen and mesitylene? (2) What is the effect of ΘΗ on τopt? On SX/T?
(k) Web Example. CSTR Mesitylene Reaction. Same question as P8-l(j).
(l) Web Example. Oxidation of Ammonia. Consider the following set of reactions:
Rate Laws Determined from Totusimetry Data (11/2/2019)
Use Wolfram to investigate the set of reactions in a PFR. Describe what you find.
(m) Read Solved Blood-Coagulation Problem. Download the Living Example Problem. (1) If blood was flowing out of a small wound at a rate of 0.05 dm3/minute, what is the value of k1 below which would cause you to bleed to death? (2) Plot out some of the other concentrations, such as TF-VIIa and TF-VIIaX. (3) Why do the curves look the way they do? What reaction in the cascade is most likely to be inhibited causing one to bleed to death? (4) What reactions, if eliminated, could cause one to die of a blood clot? (Hint: Look at ATIIII and/or TFPI.)
(n) Web Module Living Example: Oscillating Reactions. Use the Living Example Polymath Program for oscillating reactions on the CRE Web site. For the (IO–) and (I) reactions set k1 = 0.0001/min–1 and for reaction (1) CP0 = 0.01 mol/dm3. (1) What did you find? Look at the linearized stability analysis on the CRE Web site. (2) What factors affect the frequency and onset of the oscillations? (3) Explore and write a paragraph describing what you find. (4) Download the Living Example Polymath Program for the BZ reaction. Vary the parameters and write a paragraph describing what you find.
Interactive Computer Games
P8-2A Download the Interactive Computer Game (ICG) The Great Race from the CRE Web site (http://www.umkh .edu/~elements/5e/icm/index.html). Play the game and then record your performance number for the module, which indicates your mastery of the material. Your professor has the key to decode your performance number.
Performance #____________________
take place in a batch reactor.
Additional information:
k1 = 1.0 min–1, K1A = 10
k2 = 100 min–1, K2A = 1.5
CA0 = 1 mol/dm3
(Adapted from a problem by Prof. John Falconer, University of Colorado.)
(a) Plot and analyze conversion and the concentrations of A, D, and U as a function of time. When would you stop the reaction to maximize the concentration of D? Describe what you find.
(b) When does the maximum concentration of U occur? (Ans.: t = 0.31 min)
(c) What are the equilibrium concentrations of A, D, and U?
(d) What would be the exit concentrations from a CSTR with a space time of 1.0 min? Of 10.0 min? Of 100 min?
P8-4A Consider the following system of gas-phase reactions:
B is the desired product, and X and Y are foul pollutants that are expensive to get rid of. The specific reaction rates are at 27°C. The reaction system is to be operated at 27°C and 4 atm. Pure A enters the system at a volumetric flow rate of 10 dm3/min.
(a) Sketch the instantaneous selectivities (SB/X, SB/Y, and SB/XY = rB/(rx + rY)) as a function of the concentration of CA.
(b) Consider a series of reactors. What should be the volume of the first reactor?
(c) What are the effluent concentrations of A, B, X, and Y from the first reactor?
(d) What is the conversion of A in the first reactor?
(e) If 99% conversion of A is desired, what reaction scheme and reactor sizes should you use to maximize SB/XY?
(f) Suppose that E1 = 20,000 cal/mol, E2 = 10,000 cal/mol, and E3 = 30,000 cal/mol. What temperature would you recommend for a single CSTR with a space time of 10 min and an entering concentration of A of 0.1 mol/dm3?
(g) If you could vary the pressure between 1 and 100 atm, what pressure would you choose?
P8-5B Pharmacokinetics concerns the ingestion, distribution, reaction, and elimination reaction of drugs in the body. Consider the application of pharmacokinetics to one of the major problems we have in the United States, drinking and driving. Here, we shall model how long one must wait to drive after having a tall martini. In most states, the legal intoxication limit is 0.8 g of ethanol per liter of body fluid. (In Sweden it is 0.5 g/L, and in Eastern Europe and Russia it is any value above 0.0 g/L.)

The ingestion of ethanol into the bloodstream and its subsequent elimination can be modeled as a series reaction. The rate of absorption from the gastrointestinal tract into the bloodstream and body is a first-order reaction with a specific reaction-rate constant of 10 h_1. The rate at which ethanol is broken down in the bloodstream is limited by regeneration of a coenzyme. Consequently, the process may be modeled as a zero-order reaction with a specific reaction rate of 0.192 g/h-L of body fluid.
Suppose you immediately drank two tall martinis after arriving at a party. How long would you have to wait before your blood-alcohol concentration is below the legal limit in order to drive (a) in the United States, (b) in Sweden, and (c) in Russia? How would your answer change if (d) the drinks were taken ½ hour apart? (e) and if the two drinks were consumed at a uniform rate during the first hour? (Ans.: (b) t = 7.8 h) (f) Suppose that one went to a party, had two tall martinis right away, and then received a phone call saying an emergency had come up and the person needed to drive home immediately. How many minutes would the individual have to reach home before he/she became legally intoxicated, assuming that the person had nothing further to drink? (g) How would a plot of blood alcohol concentration-time curve change in part (e) if you continuously drank Bud Light at a rate of one, 1-oz. swallow per minute for two hours? (h) How would your answers be different for a thin person? A heavy person? (Hint: Base all ethanol concentrations on the volume of body fluid. Plot the concentration of ethanol in the blood as a function of time.) What generalizations can you make? (i) What is the major unspoken point of this problem?

Additional information:
Ethanol in a tall martini: 40 g
Volume of b ody fluid: 40 L (SADD -MADD problem)
(See Web site Chapter 9, PRS R9-7 for a more in-depth look at alcohol metabolism; http://www.umich.edu/~elements/5e/09chap/prof-Tpharmacokinetics.html.)
P8-6B Pharmacokinetics. Tarzlon is a liquid antibiotic that is taken orally to treat infections of the spleen. It is effective only if it can maintain a concentration in the bloodstream (based on volume of body fluid) above 0.4 mg per dm3 of body fluid. Ideally, a concentration of 1.0 mg/dm3 in the blood should be realized. However, if the concentration in the blood exceeds 1.5 mg/dm3, harmful side effects can occur. Once the Tarzlon reaches the stomach, it can proceed in two pathways, both of which are first order: (1) It can be absorbed into the bloodstream through the stomach walls; (2) it can pass out through the gastrointestinal tract and not be absorbed into the blood. Both these processes are first order in Tarzlon’s concentration in the stomach. Once in the bloodstream, Tarzlon attacks bacterial cells and is subsequently degraded by a zero-order process. Tarzlon can also be removed from the blood and excreted in urine through a first-order process within the kidneys. In the stomach:
Absorption into blood |
k1 = 0.15 h−1 |
Elimination through gastrointestinal |
k2 = 0.6 h−1 |
In the bloodstream:
Degradation of Tarzlon |
k3 = 0.1 mg/dm3.h |
Elimination through gastrointestinal |
k4 = 0.2 h−1 |

One dose of Tarzlon is 250 mg in liquid form: Volume of body fluid = 40 dm3.
(a) Plot and analyze the concentration of Tarzlon in the blood as a function of time when 1 dose (i.e., one liquid capsule) of Tarzlon is taken.
(b) How should the Tarzlon be administered (dosage and frequency) over a 48-h period to be most effective? (Hint: Recall what it says on many antibiotic prescriptions regarding the first dose.)
(c) Comment on the dose concentrations and potential hazards.
(d) How would your answers change if the drug were taken on a full or empty stomach?
P8-7C Reactor selection and operating conditions. For each of the following sets of reactions, describe your reactor system and conditions to maximize the selectivity to D. Make sketches where necessary to support your choices. The rates are in (mol/dm3 · s), and concentrations are in (mol/dm3).
(a) |
(1) |
|
(2) |
||
(1) |
||
(2) |
||
(c) |
(1) |
|
(2) |
||
(d) |
(1) |
|
(2) |
||
(3) |
||
(e) |
(1) |
|
(2) |
||
(3) |
||
(f) |
(1) |
|
(2) |
||
(3) |
P8-8B Consider the reaction
Pure A is fed to a 1.0-dm3 CSTR where it reacts to form a desired product (D), which can then react further to produce an undesired product (U); both reactions are elementary and irreversible, and everything is liquid phase. The entering concentration of A is 1 mole/dm3 at a molar flow rate of 1 mol/min.
(a) Sketch the conversion of A, X, the instantaneous selectivity of D to U, SDiu, and the instantaneous yield of D, YD, as a function of space time (make sure to label them on the plot). You may want to write a sentence or two of reasoning for partial credit purposes.
(b) If at τ = 1.0 minutes the instantaneous selectivity, SOjV, is (1/2) and the conversion of A is (0.5), what are the specific reactions rates kx and k2?
P8-9B Exam Problem. The elementary liquid-phase series reaction
is carried out in a 500-dm3 batch reactor. The initial concentration of A is 1.6 mol/dm3. The desired product is B, and separation of the undesired product C is very difficult and costly. Because the reaction is carried out at a relatively high temperature, the reaction is easily quenched.
(a) Plot and analyze the concentrations of A, B, and C as a function of time. Assume that each reaction is irreversible, with kx = 0.4 Ir1 and fe2 = 0.01 h_1.
(b) Plot and analyze the concentrations of A, B, and C as a function of time when the first reaction is reversible, with k_x = 0.3 h_1.
(c) Plot and analyze the concentrations of A, B, and C as a function of time for the case where both reactions are reversible, with k_2 = 0.005 h_1.
(d) Compare (a), (b), and (c) and describe what you find.
(e) Vary kx, k2, fe-χ, and fe_2. Explain the consequence of kx > 100 and fe2 < 0.1 with k_x = fe_2 = 0 and with fe_2 = 1, fe_! = 0, and fe_2 = 0.25.
(f) Apply one or more of the six ideas in Preface Table P-4, page xxvii, to this problem.
P8-10B Terephthalic acid (TPA) finds extensive use in the manufacture of synthetic fibers (e.g., Dacron) and as an intermediate for polyester films (e.g., Mylar). The formation of potassium terephthalate from potassium benzoate was studied using a tubular reactor [Ind. Eng. Chem. Res., 26, 1691].
It was found that the intermediates (primarily K-phthalates) formed from the dissociation of K-benzoate over a CdCl2 catalyst reacted with K-terephthalate in an autocatalytic reaction step
where A = K-benzoate, R = lumped intermediates (K-phthalates, K-isophthalates, and K-benzenecarboxy-lates), and S = K-terephthalate. Pure A is charged to the reactor at a pressure of 110 kPa. The specific reaction rates at 410 °C are kx = 1.08 X 10~3 s"1 with Ex = 42.6 kcal/mol, fe2 = 1.19 X 10~3 s"1 with E2 = 48.6 kcal/mol, and fe3 = 1.59 X 10~3 dm3/mol · s with E3 = 32 kcal/mol.
(a) Plot and analyze the concentrations of A, R and S as a function of time in a batch reactor at 410°C, noting when the maximum in R occurs.
(b) Repeat (a) for temperatures of 430° C and 390 °C.
(c) What would be the exit concentrations from a CSTR operated at 410° C and a space time of 1200 s?
Sketch the trends or results you expect before working out the details of the problem.
P8-11A The following liquid-phase reactions were carried out in a CSTR at 325 K:
The concentrations measured inside the reactor were CA = 0.10, CB = 0.93, Cc = 0.51, and CD = 0.049 all in mol/dm3.
(a) What are the values of r1A, r2A, and r3A? (r1A = —0.7 mol/dm3-min)
(b) What are the values of r1B, r2ß, and r3ß?
(c) What are the values of rlC, r2C, and r3C? (rlc = 0.23 mol/dm3-min)
(d) What are the values of r1D, r2D and r3D
(e) What are the values of r1E, r2E, and r3E?
(f) What are the values of the net rates of formation of A, B, C, D, and E?
(g) The entering volumetric flow rate is 100 dm3/min and the entering concentration of A is 3 M. What is the CSTR reactor volume? (Ans.: 400 dm3.)
(h) What are the exit molar flow rates from the 400 dm3 CSTR?
Note: The following pans require an ODE solver and are of"B level” difficulty.
(i) PFR. Now assume the reactions take place in the gas phase. Use the preceding data to plot the molar flow rate’s selectivity and p as a function of PFR volume up to 400 dm3. The pressure-drop parameter is 0.001 dirr3, the total concentration entering the reactor is 0.2 mol/dm3, and υ0 = 100 dm3/min. What are the values of and ?
(j) Membrane Reactor. Repeat (i) when species C diffuses out of a membrane reactor and the transport coefficient, fec, is 10 min-1. Compare your results with part (i).
P8-12B In this problem, the complex reactions described below will first be carried out in the liquid phase (parts (a) through (d)) and then in the gas phase (parts (e) through (g)). One need not solve the liquid phase to solve the gas-phase problems.
The following reactions are carried out isothermally.
k1D = 0.25 dm6/mol2 · min |
υ0 = 10dm3/min |
k2E = 0.1 dm3/mol·min |
CA0 = 1.5 mol/dm3 |
k3F = 5.0dm9/mol2·min |
CB0 = 2.0 mol/dm3 |
(a) Consider the reactions to be liquid phase and plot the species concentrations and the conversion of A as a function of the distance (i.e., volume) down a 50-dm3 PFR. Note any maxima.
(b) Consider the reactions to be liquid phase and determine the effluent concentrations and conversion from a 50-dm3 CSTR. (Ans.: CA = 0.61, CB = 0.79, CF = 0.25, and CD = 0.45 mol/dm3.)
(c) Plot and analyze the species concentrations and the conversion of A as a function of time when the reaction is carried out in a semibatch reactor initially containing 40 dm3 of liquid. Consider two cases: (1) A is fed to B, and (2) B is fed to A. What differences do you observe for these two cases? Describe what you find.
(d) Vary the ratio of B to A (1 < ΘΒ < 10) in the feed to the PFR and describe what you find. What generalizations can you make from this problem?
(e) Rework (a) for the case when the reaction is a gas-phase reaction. We will keep the constants the same so you won’t have to make too many changes in your Polymath program, but we will make υ0 = 100 dm3/min, CT0 = 0.4 mol/dm3, V= 500 dm3, and an equalmolar feed of A and B. Plot the molar flow rates and Sc/D and SE/F down a PFR. Describe what you find.
(f) Repeat (e) when D diffuses out through the sides of a membrane reactor where the mass-transfer coefficient, feCD, can be varied between 0.1 min-1 and 10 min-1. What trends do you find?
(g) Repeat (e) when B is fed through the sides of a membrane reactor. Describe what you find.
P8-13B The gas-phase reactions take place isothermally in a membrane reactor packed with catalyst. Pure A enters the reactor at 24.6 atm and 500 K, and a flow rate of A of 10 mol/min.
Only species B diffuses out of the reactor through the membrane.
Additional Information:
Overall mass-transfer coefficient fec = 1.0 dm3 / kg-cat · min
k1C = 2 dm3 / kg-cat · min |
k3E = 5.0 dm3 / mol2 · kg-cat · min |
K1C = 0.2 mol / dm3 |
Wf = 100 kg |
k2D = 0.4 dm3 / kg-cat · min |
α = 0.008 kg-1 |
(a) Plot and analyze the concentrations down the length of the reactor.
(b) Explain why your curves look the way they do.
(c) Describe the major differences you observe when C diffuses out instead of B, with the same mass-transfer coefficient.
(d) Vary some of the parameters (e.g., feB, felc, Klc) and write a paragraph describing what you find.
P8-14B The complex reactions involved in the oxidation of formaldehyde to formic acid over a Vanadium titanium oxide catalyst [Ind. Eng. Chem. Res. 28, 387] are shown below. Each reaction follows an elementary rate law.
Let A = HCHO, B = 02, C = HCOOH, D = HCOOCH3, E = CO, W = H20, and G = CH3OH.
The entering flow rates are FA0 = 10 mol/s and FB0 = 5 mol/s, and υ0 = 100 dm3/s. At a total entering concentration CT0 = 0.147 mol/dm3, the suggested reactor volume is 1,000 dm3.
Additional information:
At 300 K
(a) Plot the molar flow rates of each species along the volume (length) of the reactor on the same figure and then analyze why the profiles look the way they do.
(b) Plot and analyze , , , and along the length of the reactor. Note and explain any maximums and the volume at which they occur.
(c) Plot and analyze the overall HCOOH yield and overall selectivity of HCOH to CO, of HCOOCH3 to CH3OH, and of HCOOH to HCOOCH3 as a function of the ΘO2 . Suggest some conditions to best produce formic acid. Write a paragraph describing what you find.
(d) Compare your plot in part (a) with a similar plot when pressure drop is taken into account with α = 0.002 dm−3. Note any unusual differences between parts (a) and (d).
(e) Suppose that E1 = 10,000 cal/mol, E2 = 30,000 cal/mol, E3 = 20,000 cal/mol, and E4 = 10,000 cal/mol, what temperature would you recommend for a 1000-dm3 PFR?
P8-15c The ethylene epoxydation is to be carried out using a cesium-doped silver catalyst in a packed-bed reactor.
Along with the desired reaction, the complete combustion of ethylene also occurs
[M. Al-Juaied, D. Lafarga, and A. Varma, Chem. Eng. Sei. 56, 395 (2001)].
It is proposed to replace the conventional PBR with a membrane reactor in order to improve the selectivity. As a rule of thumb, a 1% increase in the selectivity to ethylene oxide translates to an increase in profit of about $2 million/yr. The feed consists of 12% (mole) oxygen, 6% ethylene, and the remainder nitrogen at a temperature of 250° C and a pressure of 2 atm. The total molar flow rate is 0.0093 mol/s to a reactor containing 2 kg of catalyst.
Additional information:
(a) What conversion and selectivity of ethylene epoxide to CO2 are expected in a conventional PBR?
(b) What would be the conversion and selectivity if the total molar flow rate were divided and the 12% oxygen stream (no ethylene) were uniformly fed through the sides of the membrane reactor, and 6% ethylene (no oxygen) were fed at the entrance?
(c) Repeat (b) for a case when ethylene is fed uniformly through the sides and oxygen is fed at the entrance. Compare with parts (a) and (b). Describe what you find.
P8-16B Solar energy capture has great potential to help meet the world’s growing energy demand, which is 12 terawatts in 2010 and is expected to rise to 36 terawatts in 2050 (cf. P3-15B). Professor Al Weiner and his students at the University of Colorado are engaged in developing methods of utilizing solar-thermal energy. In solar-thermal reactors, mirrors are used to focus and concentrate the sun’s energy on a flow-type cavity reactor where temperatures as high as 1200°C can be realized, as shown below.

Figure P8-16.1 Solar field design. Five 265-m-tall towers with three heliostat fields/tower, on 275 acres of land in Daggett, CA. Net concentration 3,868 suns and 295 MW delivered to each solar reactor. (Melinda M. Channel, Jonathan Scheffe, Allan Lewandowski, and Alan W. Weimer, November 11, 2009. Also see: Chemical Engineering, 116, p. 18, March 2009). (http://www.sciencedirect.com/science/article/pii/S0009250907005878 and http://www.sciencedirect.com/science/article/pii/S0038092X03004663)
The switch grass is fed to the 1200°C solar-thermal reactor. At these temperatures, biomass can be converted to CO and H2, i.e., syn gas, which then can be used for liquid fuels. Switch grass, which is approximately ⅔ cellulose (C6H10O5) and ⅓ lignin (C10H12O3) will be fed with steam to produce CO, H2 and a small amount of ash, which we will neglect. In order to simplify this process into a tractable home problem, we assume the switch grass is volatilized immediately upon entering the plug-flow reactor and that the reactions and postulated rate laws are
(1) Cellulose: C6H10O5(C) + H20(W) → 6H2 + 6CO
(2) Lignin: C10H12O3(L )+ 7H20(W) → 13H2 + 10CO
[AIChEJ. 55, p. 286 (2009)]. Also see Science p. 326,1472 (2009).
The rate laws and constants are hypothesized to be
Total gas concentration in the feed and reactor with the entering molar flow rates of cellulose, lignin, and water are Fco = 0.00411 mol/s and FL0 = 0.00185 mol/s, Fwo = 0.02 mol/s, respectively.
(a) Plot and analyze the molar flow rates as a function of PFR volume up to V = 0.417 dm3.
(b) Plot and analyze Yc, Yw, YL, and down the reactor. Describe what you find.
(c) Repeat (a) for different molar flow rates of water.
P8-17B Solar-thermal biochar gasification has also been studied at the University of Colorado (see P8-16B). [Chemical Engineering and Processing: Process Intensification 48, p. 1279 (2009) and AIChE). 55 p.286 (2009).] While this process follows a shrinking core model (see the Expanded Material for Chapter 14 on the CRE Web site), for the purposes of this example, we will use the following sequence:
(1) Lignin: C10H12O3(L)+ 3H2O(W) → 3H2 +3CO + Char (e.g., cresol)
(2) Char: Char(Ch)+ 4H2O → 10H2 + 7CO
The rate laws at 1200°C are hypothesized to be
The entering molar flow rates are FL0 = 0.0123 mol/s, Fw0 = 0.111 mol/s, the total entering concentration is CT0 = 0.2 mol/dm3, and the reactor volume is 0.417 dm3.
(a) Plot and analyze FCh, FL, FW, FC0, and down the length of a plug-flow reactor.
(b) Repeat (a) for the concentrations Cc, Cch, etc.
(c) Plot and analyze the selectivity and yields and YL down the PFR.
(d) At what point is the char molar flow rate a maximum? How does it change with changing feed conditions, such as the ratio of (Fwo/ FL0), CT0, etc? Describe what you found in parts (a) through (d).
P8-18A Go to Professor Herz’s Reactor Lab on the CRE Web site at www.reactoriab.net.
(a) Download Division 5, Lab 2 of the Reactor Lab from the CRE Web site for the selective oxidation of ethylene to ethylene oxide. Click the [i] info button to get information about the system. Perform experiments and develop rate equations for the reactions. Write a technical memo that reports your results and includes plots and statistical measurements of how well your kinetic model fits experimental data.
(b) Download Division 5, Labs 3 and 4 of the Reactor Lab for batch reactors in which parallel and series reactions, respectively, can be carried out. Investigate how dilution with solvent affects the selectivity for different reaction orders and write a memo describing your findings.
• Additional Homework Problems
A number of homework problems that can be used for exams or supplementary problems or examples are found on the CRE Web site, http://www.umich.edu/~elements/5e/index.html.
Supplementary Reading
1. Selectivity, reactor schemes, and staging for multiple reactions, together with evaluation of the corresponding design equations, may or may not be presented in
BURGESS, THORNTON W, The Adventures of Chatterer the Red Squirrel, New York: Dover Publications, Inc., 1915.
BUTT, JOHN B, Reaction Kinetics and Reactor Design, Second Edition, Revised and Expanded, New York: Marcel Dekker, Inc., 1999.
DENBIGH, K. G., and J. C. R. TURNER, Chemical Reader Theory, 2nd ed. Cambridge: Cambridge University Press, 1971, Chap. 6.
2. Many analytical solutions for parallel, series, and combination reactions are presented in
WALAS, S. M., Chemical Reaction Engineering Handbook of Solved Problems. Newark, NJ: Gordon and Breach, 1995.
4 See, for example, Y. Bard, Nonlinear Parameter Estimation (San Diego, CA: Academic Press, 1974).
5 H. Scott Fogler, Teaching Critical Thinking, Creative Thinking, and Problem Solving in the Digital Age, Phillips Lecture (Stillwater, OK: OSU Press, 1997).
6 H. S. Fogler and S. E. LeBlanc, with B. Rizzo, Strategies for Creative Problem Solving, 3rd Ed. (Upper Saddle River, N.J.: Prentice Hall, 2014).