12. Steady-State Nonisothermal Reactor Design: Flow Reactors with Heat Exchange
Research is to see what everybody else sees, and to think what nobody else has thought.
—Albert Szent-Gyorgyi
12.1 Steady-State Tubular Reactor with Heat Exchange
In this section, we consider a tubular reactor in which heat is either added or removed through the cylindrical walls of the reactor (Figure 12-1). In modeling plug-flow reactors, we shall assume that “there are no radial gradients” in the reactor and that the heat flux through the wall per unit volume of reactor is as shown in Figure 12-1.1
1 Radial gradients are discussed in Section 12.7 and in Chapters 17 and 18.

Figure 12-1 Tubular reactor with heat gain or loss.
12.1.1 Deriving the Energy Balance for a PFR
We will carry out an energy balance on the volume ΔV. There is no work done, that is, , so Equation (11-10) becomes
The heat flow to the reactor, , is given in terms of the overall heat-transfer coefficient, U, the heat exchange area, ΔA, and the difference between the ambient temperature, Ta, and the reactor temperature T
where a is the heat exchange area per unit volume of reactor. For a tubular reactor
where D is the reactor diameter. Substituting for in Equation (12-1), dividing by ΔV, and then taking the limit as ΔV → 0, we get
Expanding
From a mole balance on species i, we have
Differentiating the enthalpy Equation (11-19) with respect to V
Substituting Equations (12-3) and (12-4) into Equation (12-2), we obtain
Rearranging, we arrive at
which is Equation (T11-1.G) in Table 11-1 on pages 547–549.
This form of the energy balance will also be applied to multiple reactions.
where
To help remember that Qr is the “heat” removed from the reacting mixture, we note the driving force is from “T” to “Ta”, that is, Ua (T – Ta).
For exothermic reactions, Qg will be a positive number. We note that when the heat “generated,” Qg, is greater than the heat “removed,” Qr (i.e., Qg > Qr), the temperature will increase down the reactor. When Qr > Qg, the temperature will decrease down the reactor.
For endothermic reactions ΔHRx will be a positive number and therefore the heat generated, Qg in Equation (12-5a), will be a negative number. The heat removed, Qr in Equation (12-5b), will also be a negative number because heat is added rather than removed Ta > T. See Chapter 12 Additional Material on the Web site for a sample calculation on endothermic reactions (http://www.umich.edu/~elements/6e/12chap/obj.html#/additional-materials/). This point is also illustrated in Example 12-2 for the case of constant ambient temperature.
We continue development of our algorithm by noting Equation (12-5) is coupled with the mole balances on each species, Equation (12-3). Next, we express rA as a function of either the concentrations for liquid systems or molar flow rates for gas systems, as described in Chapter 6. We will use the molar flow rate form of the energy balance for membrane reactors and also extend this form to multiple reactions.
We could also write Equation (12-5) in terms of conversion by recalling Fi = FA0(Θi + viX) and substituting this expression into the denominator of Equation (12-5).
PFR energy balance
For a packed-bed reactor dW = ρb dV where ρb is the bulk density
PFR energy balance
Equations (12-6) and (12-7) are also given in Table 11-1 as Equations (T11-1.D) and (T11-1.F). As noted earlier, having gone through the derivation to these equations, it will be easier to apply them accurately to CRE problems with heat effects.
12.1.2 Applying the Algorithm to Flow Reactors with Heat Exchange
We continue to use the algorithm described in the previous chapters and simply add a fifth building block, the energy balance.
Gas Phase
If the reaction is in gas phase and pressure drop is included, there are four differential equations that must be solved simultaneously. The differential equation describing the change in temperature with volume (i.e., distance) as we move down the reactor
Energy balance
must be coupled with the mole balance
Mole balance
and with the pressure-drop equation
Pressure drop
and solved simultaneously. If the temperature of the heat-exchange fluid, Ta, varies down the reactor, we must add the energy balance on the heat-exchange fluid. In the next section, we will derive the following equation for co-current heat transfer
Heat exchanger
Numerical integration of the coupled differential equations (A) to (D) is required.
along with the equation for countercurrent heat transfer. A variety of software packages (e.g., Polymath) can be used to solve these four coupled differential equations: (A), (B), (C), and (D).
Liquid Phase
For liquid-phase reactions, the rate is not a function of total pressure, so our mole balance is
Consequently, we need to only solve three equations (A), (D), and (E) simultaneously.
12.2 Balance on the Heat-Transfer Fluid
12.2.1 Co-Current Flow
The heat-transfer fluid will be a coolant for exothermic reactions and a heating medium for endothermic reactions. If the flow rate of the heat-transfer fluid is sufficiently high with respect to the heat released (or absorbed) by the reacting mixture, then the heat-transfer fluid temperature will be virtually constant along the length of the reactor. In the material that follows, we develop the basic equations for a coolant to remove heat from exothermic reactions; however, these same equations apply to endothermic reactions where a heating medium is used to supply heat.
We now carry out an energy balance on the coolant in the annulus between R1 and R2, and axially between V and V + ΔV, as shown in Figure 12-2. The mass flow rate of the heat-exchange fluid (e.g., coolant) is . We will consider the case when the reactor is cooled and the outer radius of the coolant channel R2 is insulated. Recall that by convention is the heat added to the system.
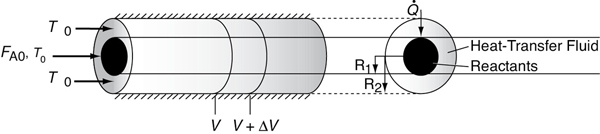
Figure 12-2 Co-current, double-pipe heat exchanger.
For co-current flow, the reactant and the coolant flow in the same direction.

The energy balance on the coolant in the volume between V and (V + ΔV) is
Coolant energy balance
where Ta is the temperature of the heat transfer fluid, that is, coolant, and T is the temperature of the reacting mixture in the inner tube.
Dividing by ΔV and taking limit as ΔV → 0
Analogous to Equation (12-4), the change in enthalpy of the coolant can be written as
The variation of coolant temperature Ta down the length of reactor is
The equation is valid whether the heat-transfer fluid is a coolant or a heating medium.
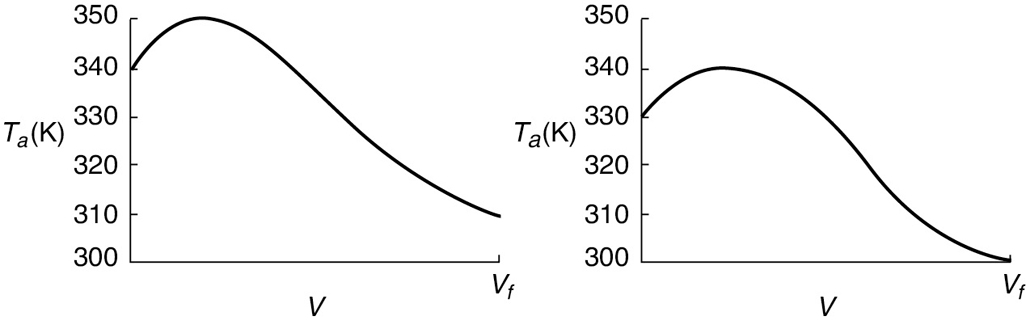
Typical heat-transfer fluid temperature profiles for both exothermic and endothermic reactions when the heat-transfer fluid enters at Ta0 are shown in Figure 12-3, parts (a) and (b) respectively.
Figure 12-3 Heat-transfer fluid temperature profiles for co-current heat exchanger. (a) Coolant. (b) Heating medium.
12.2.2 Countercurrent Flow
In countercurrent heat exchange, the reacting mixture and the heat-transfer fluid (e.g., coolant) flow in opposite directions. At the reactor entrance, V = 0, the reactants enter at temperature T0, and the coolant exits at temperature Ta2. At the end of the reactor, the reactants and products exit at temperature T, while the coolant enters at Ta0.
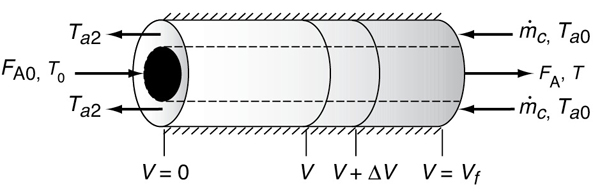
Figure 12-4 Countercurrent, double-pipe heat exchanger.
Again, we write an energy balance on the coolant over a differential reactor volume to arrive at
At the entrance, V = 0 X = 0 and Ta = Ta2.
At the exit, V = Vf Ta = Ta0.
We note that the only difference between Equations (12-10) and (12-11) is a minus sign, that is, (T – Ta) versus (Ta – T).
The solution to a countercurrent flow problem to find the exit conversion and temperature requires a trial-and-error procedure, as shown in Table 12-1.
Trial-and-error procedure required
TABLE 12-1 PROCEDURE TO SOLVE FOR THE EXIT CONDITIONS FOR PFRS WITH COUNTERCURRENT HEAT EXCHANGE
|
12.3 Examples of the Algorithm for PFR/PBR Design with Heat Effects
We now have all the tools to solve reaction engineering problems involving heat effects in PFRs and PBRs for the cases of both constant and variable coolant temperatures.
Table 12-2 gives the algorithm for the design of PFRs and PBRs with heat exchange: In Case A Conversion is the reaction variable and in Case B Molar-Flow-Rates are the reaction variables. The procedure in Case B must be used to analyze multiple reactions with heat effects.
TABLE 12-2 ALGORITHM FOR PFR/PBR DESIGN FOR GAS-PHASE REACTIONS WITH HEAT EFFECTS
The elementary gas-phase reaction
is to be carried out in a PBR with a co-current heat exchanger.
A. Conversion as the Reaction Variable
1. Mole Balance:
2. Rate Law:
Again we note that because δ ≡ 0, KC ≡ Ke
3. Stoichiometry (gas phase):
δ = (2 - 1 - 1) = 0, ∴ ε = 0

Mole Balance
Rate Law
Stoichiometry
Energy Balance
Parameters
Solution
Analysis
Combine Rate Law and Stoichiometry to find Xe
at equilibrium and and X = Xe
Solving for Xe
Because we are solving for the temperature as a function of catalyst weight down the reactor, we can use Equation (T12-2.4) to find the equilibrium constant, KC, profile and then use Equation (T12-2.11) to find the equilibrium conversion profile, Xe.
4. Energy Balances:
B. Molar Flow Rates as the Reaction Variable

1. Mole Balance:
2. Rate Law (elementary reaction):
3. Stoichiometry (gas phase):
4. Energy Balances:
Reactor:
Heat Exchangers: Same as for A. Conversion as the Reaction Variable
Case A: Conversion as the Independent Variable Example Calculation
5. Parameter Evaluation:
Now we enter all the explicit equations with the appropriate parameter values.
k1, E, R, CT0, Ta, T0, T1, T2, KC2, ΘB, ΘI, , CPA, CPB, CCA, Ua, ρb
Base Case for Parameter Values
with initial values T0 = 330 K, Ta0 = 320 K, p = 1, and X = 0 at W = 0 and final values: Wf = 5000 kg.
6. Solution:
Equations (T12-2.1)–(T12-2) are entered into the Polymath program along with the corresponding parameter values.
Differential equations
1 d(Ta)/d(W) = Uarho*(T-Ta)/(mc*Cpcool)
2 d(p)/d(W) = -alpha/2*(T/To)/p
3 d(T)/d(W) = (Qg-Qr)/(Fao*sumcp)
4 d(X)/d(W) = -ra/Fao

Explicit equations
1 Hr = -20000
2 thetaB = 1
3 Uarho = 0.5
4 alpha = .0002
5 To = 330
6 Qr = Uarho*(T-Ta)
7 mc = 1000
8 Cpcool = 18
9 Kc = 1000*(exp(Hr/1.987*(1/303-1/T)))
10 Fao = 5
11 thetaI = 1
12 CpI = 40
13 CpA = 20
14 yao = 1/(1+thetaB+thetaI)
15 CpB = 20
16 Cto = 0.3
17 Ea = 25000
18 Xe = ((thetaB+1)*Kc-(((thetaB+1)*Kc)^2-4*(Kc-4)*(Kc*thetaB))^0.5)/(2*(Kc-4))
19 k = .004*exp(Ea/1.987*(1/310-1/T))
20 Cao = yao*Cto
21 Cc = Cao*2*X*p*To/T
22 sumcp = (thetaI*CpI+CpA+thetaB*CpB)
23 Ca = Cao*(1-X)*p*To/T
24 Cb = Cao*(thetaB-X)*p*To/T
25 ra = -k*(Ca*Cb-Cc^2/Kc)
26 Qg = (-ra)*(-Hr)
http://www.umich.edu/~elements/6e/live/chapter12/T12-3/LEP-T12-2.pol
Calculated values of DEQ variables
| Variable | Initial value | Final value | |
|---|---|---|---|
| 1 | alpha | 0.0002 | 0.0002 |
| 2 | Ca | 0.1 | 0.0111092 |
| 3 | Cao | 0.1 | 0.1 |
| 4 | Cb | 0.1 | 0.0111092 |
| 5 | Cc | 0 | 0.0255273 |
| 6 | CpA | 20. | 20. |
| 7 | CpB | 20. | 20. |
| 8 | Cpcool | 18. | 18. |
| 9 | CpI | 40. | 40. |
| 10 | Cto | 0.3 | 0.3 |
| 11 | Ea | 2.5E+04 | 2.5E+04 |
| 12 | Fao | 5. | 5. |
| 13 | Hr | -2.0E+04 | -2.0E+04 |
| 14 | k | 0.046809 | 0.0303238 |
| 15 | Kc | 66.01082 | 93.4225 |
| 16 | mc | 1000. | 1000. |
| 17 | p | 1. | 0.2360408 |
| 18 | Qg | 9.361802 | 0.0706176 |
| 19 | Qr | 5. | 1.615899 |
| 20 | ra | -0.0004681 | -3.531E-06 |
| 21 | sumcp | 80. | 80. |
| 22 | T | 330. | 326.2846 |
| 23 | Ta | 320. | 323.0528 |
| 24 | thetaB | 1. | 1. |
| 25 | thetaI | 1. | 1. |
| 26 | To | 330. | 330. |
| 27 | Uarho | 0.5 | 0.5 |
| 28 | W | 0 | 4500. |
| 29 | X | 0 | 0.5346512 |
| 30 | Xe | 0.8024634 | 0.8285547 |
| 31 | yao | 0.3333333 | 0.3333333 |
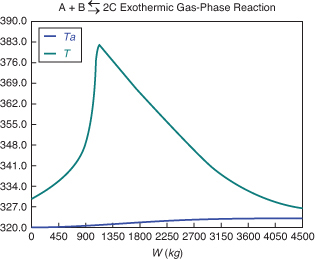
Analysis:
The temperature profiles are shown in Figure T12-2.1. One notes that due to the exothermic heat of reaction, the temperature of the gas in the reactor increases sharply near the entrance then decreases as the reactants are consumed and the fluid is cooled as it moves down the reactor. Figure T12-2.2 shows the conversion X increases and approaches its equilibrium value Xe at approximately W = 1125 kg of catalyst where the reaction rate approaches zero. At this point the conversion cannot increase further unless Xe increases. The increase in Xe occurs because of the cooling of the reactor’s contents by the heat exchanger thus shifting the equilibrium to the right. By the end of the reactor, the equilibrium conversion has been increased to 82.9% and the conversion in the reactor to 53.4%. One notes from Figure T12.2-2, that owing to the cooling and consumption of reactants the conversion does not increase significantly above a catalyst weight of 2000 kg, even though X is much below Xe (see Problem P12-1A).
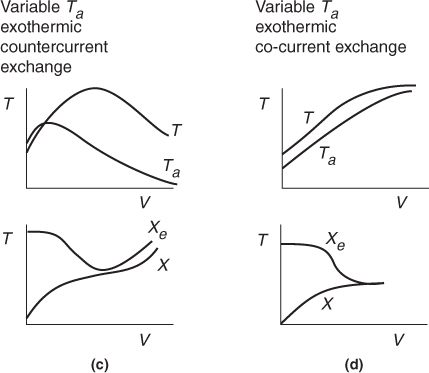
The following figures show “representative” profiles that would result from solving the above equations for different parameter values. The reader is encouraged to download the Living Example Problem for Table 12-2 and use Wolfram to vary a number of parameters, as discussed in Problem P12-1A. Be sure you can explain why these curves look the way they do.
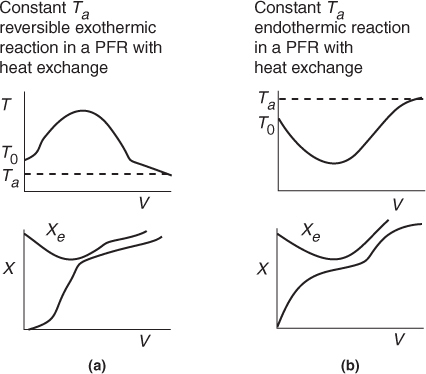
Be sure you can explain why these curves look the way they do.

12.3.1 Applying the Algorithm to an Exothermic Reaction
Example 12–1 Butane Isomerization Continued—OOPS!
When plant engineer Maxwell Anthony looked up the vapor pressure at the exit to the adiabatic reactor in Example 11-3, where the temperature is 360 K, he learned the vapor pressure for isobutene was about 1.5 MPa, which is greater than the rupture pressure of the glass vessel the company had hoped to use. Fortunately, when Max looked in the storage shed, he found there was a bank of 10 tubular reactors, each of which was 5 m3. The bank reactors were double-pipe heat exchangers with the reactants flowing in the inner pipe and with Ua = 5,000 kJ/m3·h·K. Max also bought some thermodynamic data from one of the companies he found on the Internet that used Colorimeter experiments to find ΔHRx for various reactions. One of the companies had the value of ΔHRx for his reaction on sale this week for the low, low price of $25,000.00. For this value of ΔHRx the company said it is best to use an initial concentration of A of 1.86 mol/dm3. The entering temperature of the reactants is 305 K and the entering coolant temperature is 315 K. The mass flow rate of the coolant, , is 500 kg/h and the heat capacity of the coolant, CPC, is 28 kJ/kg·K. The temperature in any one of the reactors cannot rise above 325 K. Carry out the following analyses with the newly purchased values from the Internet:
Co-current heat exchange: Plot X, Xe, T, Ta, and –rA, down the length of the reactor.
Countercurrent heat exchange: Plot X, Xe, T, Ta, and –rA down the length of the reactor.
Constant ambient temperature, Ta: Plot X, Xe, T, and –rA down the length of the reactor.
Adiabatic operation: Plot X, Xe, T, Ta, and –rA, down the length of the reactor.
Compare parts (a) through (d) above and write a paragraph describing what you find.
Additional information:
Recall from Example 11-3 that FT0 = 163 kmol/h and FA0 = 0.9 FT0, CPA = 141 kJ/kmol · K, CP0 = ΣΘiCPi = 159 kJ/kmol · K, and data from the company Maxwell got off the Internet are ΔHRx = −34500 kJ/kmol with ΔCP = 0 and CA0 = 1.86 kmol/m3 and E/R = 7906 K with k = 31.1 h-1 @ 360 K.
Solution
We shall first solve part (a), the co-current heat exchange case and then make small changes in the Polymath program for parts (b) through (d).
The molar flow rate of A to each of the 10 reactors in parallel
The mole balance, rate law, and stoichiometry are the same as in the adiabatic case previously discussed in Example 11-3; that is,
The Algorithm
Mole Balance:
Rate Law and Stoichiometry:
with
Same as Example 11-3
The equilibrium conversion is
Note: We could substitute for KC using Equation (E11-3.12) to write the reaction rate as
Energy Balance:
The energy balance on the reactor is
Qg = rAΔHRx
Qr = Ua(T - Ta)
Part (a) Co-Current Heat Exchange
We are now going to solve the coupled, ordinary differential and explicit equations (E11-3.1), (E11-3.7), (E11-3.10), (E11-3.11), (E11-3.12), and (E12-1.2), and the appropriate heat-exchange balance using Polymath. After entering these equations we will enter the parameter values. By using co-current heat exchange as our Polymath base case, we only need to change one line in the program for each of the other three cases and solve for the profiles of X, Xe, T, Ta, and –rA, and not have to reenter the program.
For co-current flow, the balance on the heat transfer fluid is
with T0 = 305 K and Ta = 315 K at V = 0. The Polymath program and solution are shown in Table E12-1.1.
Table E12-1.1 Part (a) CO-CURRENT HEAT EXCHANGE
Differential equations
1 d(Ta)/d(V) = Ua*(T-Ta)/m/Cpc
2 d(X)/d(V) = -ra/Fa0
3 d(T)/d(V) = (Qg-Qr)/(Cpo*Fa0)
Explicit equations
1 Cpc = 28
2 m = 500
3 Ua = 5000
4 deltaH = -34500
5 Qr = Ua*(T-Ta)
6 Ca0 = 1.86
7 Fa0 = 0.9*163*.1
8 Kc = 3.03*exp((deltaH/8.314)*((T-333)/(T*333)))
9 k = 31.1*exp((7906)*(T-360)/(T*360))
10 ra = -k*Ca0*(1-(1+1/Kc)*X)
11 Xe = Kc/(1+Kc)
12 Qg = ra*deltaH
13 Cpo = 159
14 rate = -ra
Calculated values of DEQ variables
| Variable | Initial value | Final value | |
|---|---|---|---|
| 1 | Ca0 | 1.86 | 1.86 |
| 2 | Cpc | 28. | 28. |
| 3 | Cpo | 159. | 159. |
| 4 | deltaH | -3.45E+04 | -3.45E+04 |
| 5 | Fa0 | 14.67 | 14.67 |
| 6 | k | 0.5927441 | 6.80861 |
| 7 | Kc | 9.512006 | 2.641246 |
| 8 | m | 500. | 500. |
| 9 | Qg | 3.804E+04 | 4077.238 |
| 10 | Qr | -5.0E+04 | 5076.445 |
| 11 | ra | -1.102504 | -0.1181808 |
| 12 | rate | 1.102504 | 0.1181808 |
| 13 | T | 305. | 336.7102 |
| 14 | Ta | 315. | 335.6949 |
| 15 | Ua | 5000. | 5000. |
| 16 | V | 0 | 5. |
| 17 | X | 0 | 0.7185996 |
| 18 | Xe | 0.9048707 | 0.7253687 |
Figure E12-1.1 shows the profiles for the reactor temperature, T, the coolant temperature, Ta, the conversion, X, the equilibrium conversion, Xe, and the reaction rate, –rA. Note that at the entrance to the reactor the reactor temperature T is below the coolant temperature, but as reacting mixture moves into and through the reactor the mixture heats up and T becomes greater than Ta. When you load the Poly-math, Python, or Wolfram Living Example Problem (LEP) code on your computer, one of the things you will want to explore is the value of the ambient temperature, Ta, or the entering temperature, T0, below which the reaction might never take off or “ignite.”
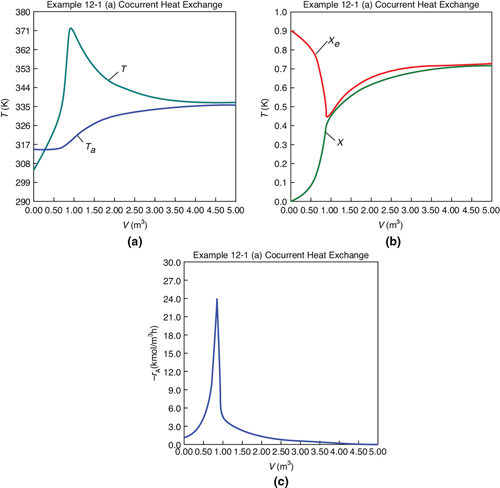
Figure E12-1.1 Profiles down the reactor for co-current heat exchange (a) temperature, (b) conversion, (c) reaction rate.
Analysis: Part (a) Co-Current Exchange: We note that reactor temperature goes through a maximum. Near the reactor entrance, the reactant concentrations are high and therefore the reaction rate is high (cf. Figure E12-1.1(a)) and Qg > Qr. Consequently, the temperature and conversion increase with increasing reactor volume while Xe decreases because of the increasing temperature. Eventually, X and Xe come close together (V = 0.95 m3) and the rate becomes very small as the reaction approaches equilibrium. At this point, the reactant conversion X cannot increase unless Xe increases. We also note that when the ambient heat-exchanger temperature, Ta, and the reactor temperature, T, are essentially equal, there is no longer a temperature driving force to cool the reactor. Consequently, the temperature does not change farther down the reactor, nor does the equilibrium conversion, which is only a function of temperature.
Part (b) Countercurrent Heat Exchange
For countercurrent flow, we only need to make two changes in the program. First, multiply the right-hand side of Equation (E12-1.6) by minus one to obtain
Next, we guess Ta at V = 0 and see whether it matches Ta0 at V = 5 m3. If it doesn’t, we guess again. In this example, we will guess Ta (V = 0) = 340.3 K and see whether Ta = Ta0 = 315 K at V = 5 m3.
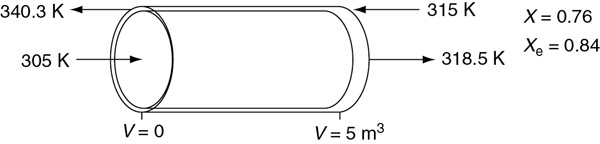
TABLE E12-1.2 PART (b) COUNTERCURRENT HEAT EXCHANGE
Differential equations
1 d(Ta)/d(V) = -Ua*(T-Ta)/m/Cpc
2 d(X)/d(V) = -ra/Fa0
3 d(T)/d(V) = (Qg-Qr)/Cpo*Fa0
The explicit equations (1)–(14) are the same as in Table E12-1.1 on page 605.

Good guess!

At V = 0, we guessed an entering coolant temperature of 340.3 K and found that at V = Vf, the calculation showed that it matched the emerging coolant temperature Ta0 = 315 K!! (Was that a lucky guess or what?!) The variable profiles are shown in Figure E12-1.2.

Figure E12-1.2 Profiles down the reactor for countercurrent heat exchange (a) temperature, (b) conversion, (c) reaction rate.
We observe that at V = 0.5 m3, X = 0.36 and the rate, –rA, drops to a low value as X approaches Xe. Because X can never be greater than Xe the reaction will not proceed further unless Xe is increased. In this case, the reactor is cooled, causing the temperature to decrease and resulting in an increase in the equilibrium conversion beyond a reactor volume of V = 0.5 m3.
Analysis: Part (b) Countercurrent Exchange: We note that near the entrance to the reactor, the coolant temperature is above the reactant entrance temperature. However, as we move down the reactor, the reaction generates “heat” and the reactor temperature rises above the coolant temperature. We note that Xe reaches a minimum (corresponding to the reactor temperature maximum) near the entrance to the reactor. At this point (V = 0.5 m3), X cannot increase above Xe. As we move down the reactor, the reactants are cooled and the reactor temperature decreases allowing X and Xe to increase. A higher exit conversion, X, and equilibrium conversion, Xe, are achieved in the countercurrent heat-exchange system than for the co-current system.
Part (c) Constant Ta
For constant Ta, use the Polymath program in part (a), but multiply the right side of Equation (E12-1.6) by zero in the program, that is,
TABLE E12-1.3 PART (c) CONSTANT Ta

The initial and final values are shown in the Polymath report and the variable profiles are shown in Figure E12-1.3.
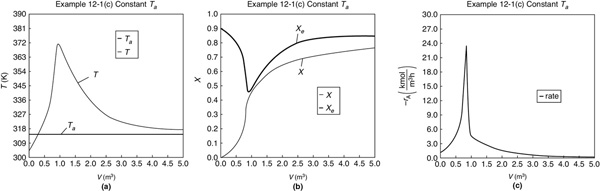
Figure E12-1.3 Profiles down the reactor for constant heat-exchange fluid temperature Ta; (a) temperature, (b) conversion, (c) reaction rate.
We note that for the parameter values chosen, the profiles for T, X, Xe, and –rA are similar to those for countercurrent flow. For example, as X and Xe become close to each other at V = 1.0 m3, but beyond that point Xe increases because the reactor temperature decreases.
Analysis: Part (c) Constant Ta: When the coolant flow rate is sufficiently large, the coolant temperature, Ta, will be essentially constant. If the reactor volume is sufficiently large, the reactor temperature will eventually reach the coolant temperature, as is the case here. At this exit temperature, which is the lowest achieved in this example (i.e., Part (c)), the equilibrium conversion, Xe, is the largest of the four cases studied in this example.
Part (d) Adiabatic Operation
In Example 11-3, we solved for the temperature as a function of conversion and then used that relationship to calculate k and KC. An easier way is to solve the general or base case of a heat exchanger for co-current flow and write the corresponding Poly-math program. Next, use Polymath (part (a)), but multiply the parameter Ua by zero, that is,
Ua = 5,000*0
and run the simulation again.
Adiabatic
TABLE E12-1.4 PART (d) ADIABATIC OPERATION
Differential equations
1 d(Ta)/d(V) = Ua*(T-Ta)/(m*Cpc)
2 d(X)/d(V) = -ra/Fa0
3 d(T)/d(V) = (Qg-Qr)/(Cpo*Fa0)
Explicit equations
1 Cpc = 28
2 m = 500
3 Ua = 5000*0
4 deltaH = -34500
5 Qr = Ua*(T-Ta)
6 Ca0 = 1.86
7 Fa0 = 0.9*163*.1
8 Kc = 3.03*exp((deltaH/8.314)*((T-333)/(T*333)))
9 k = 31.1*exp((7906)*(T-360)/(T*360))
10 ra = -k*Ca0*(1-(1+1/Kc)*X)
11 Xe = Kc/(1+Kc)
12 Qg = ra*deltaH
13 Cpo = 159
14 rate = -ra
Calculated values of DEQ variables
| Variable | Initial value | Final value | |
|---|---|---|---|
| 1 | Ca0 | 1.86 | 1.86 |
| 2 | Cpc | 28. | 28. |
| 3 | Cpo | 159. | 159. |
| 4 | deltaH | -3.45E+04 | -3.45E+04 |
| 5 | Fa0 | 14.67 | 14.67 |
| 6 | k | 0.5927441 | 124.2488 |
| 7 | Kc | 9.512006 | 0.5752071 |
| 8 | m | 500. | 500. |
| 9 | Qg | 3.804E+04 | -0.4109228 |
| 10 | Qr | 0 | 0 |
| 11 | ra | -1.102504 | 1.191E-05 |
| 12 | rate | 1.102504 | -1.191E-05 |
| 13 | T | 305. | 384.2335 |
| 14 | Ta | 315. | 315. |
| 15 | Ua | 0 | 0 |
| 16 | V | 0 | 5. |
| 17 | X | 0 | 0.3651629 |
| 18 | Xe | 0.9048707 | 0.3651628 |
The initial and exit conditions are shown in the Polymath report, while the profiles of T, X, Xe, and –rA are shown in Figure E12-1.4.

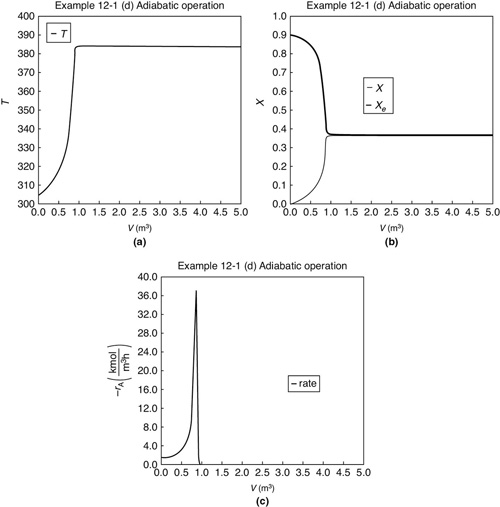
Figure E12-1.4 Profiles down the reactor for adiabatic reactor; (a) temperature, (b) conversion, (c) reaction rate.
Analysis: Part (d) Adiabatic Operation: Because there is no cooling, the temperature of this exothermic reaction will continue to increase down the reactor until equilibrium is reached, X = Xe = 0.365 at T = 384 K, which is the adiabatic equilibrium temperature. The profiles for X and Xe are shown in Figure E12-1.4(b) where one observes that the adiabatic equilibrium conversion Xe decreases down the reactor because of the increasing temperature until it becomes equal to the reactor conversion (i.e., X ≡ Xe), which occurs at circa 0.9 m3. There is no change in temperature, X or Xe, after this point because the reaction rate is virtually zero and thus the remaining reactor volume serves no purpose.
Finally, Figure E12-1.4(c) shows –rA increases as we move down the reactor as the temperature increases, reaching a maximum and then decreasing until X and Xe approach each other and the rate becomes virtually zero.
Overall Analysis: This is an extremely important example, as we applied our CRE PFR algorithm with heat exchange to a reversible exothermic reaction. We analyzed four types of heat-exchanger operations. We see that the countercurrent exchanger and the constant Ta cases give the highest conversion and adiabatic operation gives the lowest conversion.
12.3.2 Applying the Algorithm to an Endothermic Reaction
In Example 12-1 we studied the four different types of heat exchanger on an exothermic reaction. In this section we carry out the same study on an endothermic reaction.
Example 12–2 Production of Acetic Anhydride
Jeffreys, in a treatment of the design of an acetic anhydride manufacturing facility, states that one of the key steps is the endothermic vapor-phase cracking of acetone to ketene and methane is2
2 G. V. Jeffreys, A Problem in Chemical Engineering Design: The Manufacture of Acetic Anhydride, 2nd ed. London: Institution of Chemical Engineers.
CH3COCH3 → CH2CO + CH4
The article further states that this reaction is first-order with respect to acetone and that the specific reaction rate is given by
where k is in reciprocal seconds and T is in Kelvin. It is desired to feed 7850 kg of acetone per hour to a tubular reactor. The reactor consists of a bank of 1000 one-inch, schedule-40 tubes. We shall consider four cases of heat-exchanger operation. The inlet temperature and pressure are the same for all cases at 1035 K and 162 kPa (1.6 atm) and the entering heating-fluid temperature available is 1250 K. The heat-exchange fluid has a flow rate, , of 0.111 mol/s, with a heat capacity of 34.5 J/mol·K.
A bank of 1000 one-inch, schedule-40 tubes, 1.79 m in length corresponds to 1.0 m3 (0.001 m3/tube = 1.0 dm3/tube) and gives 20% conversion. Ketene is unstable and tends to explode, which is a good reason to keep the conversion low. However, the pipe material and schedule size should be checked to learn whether they are suitable for these temperatures and pressures. In addition, the final design and operating conditions need to be cleared by the safety committee before operation can begin. Also check the Web site, Process Safety Across the Chemical Engineering Curriculum (http://umich.edu/~safeche/index.html).
Case 1 The reactor is operated adiabatically.
Case 2 Constant heat-exchange fluid temperature Ta = 1250 K
Case 3 Co-current heat exchange with Ta0 = 1250 K
Case 4 Countercurrent heat exchange with Ta0 = 1250 K
Additional information:
CH3COCH3 (A):H°A(TR) = –216.67 kJ/mol, CPA = 163 J/mol · K
CH2CO (B):H°B(TR) = –61.09 kJ/mol, CPB = 83 J/mol · K
CH4 (C):H°C(TR) = –74.81 kJ/mol, CPC = 71 J/mol · K
N2 (I):CPI = —28.1 J/mol, · K
U = 110 J/S · m2 · K
The other parameters are given in Table E12-2.1.
Solution
Let A = CH3COCH3, B = CH2CO, and C = CH4. Rewriting the reaction symbolically gives us
A → B + C
Gas-phase endothermic reaction examples:
Adiabatic
Heat exchange Ta is constant
Co-current heat exchange with variable Ta
Countercurrent exchange with variable Ta
Algorithm for a PFR with Heat Effects
1. Mole Balance:
2. Rate Law:
Rearranging (E12-2.1)
3. Stoichiometry (gas-phase reaction with no pressure drop):
4. Combining yields
Before combining Equations (E12-2.2) and (E12-2.6), it is first necessary to use the energy balance to determine T as a function of X.
5. Energy Balance:
Reactor balance
Heat Exchanger. We will use the heat-exchange fluid balance for co-current flow as our base case. We will then show how we can very easily modify our ODE solver program (e.g., Polymath) to solve for the other cases by simply multiplying the appropriate line in the code by either zero or minus one.
For co-current flow:
6. Calculation of Mole Balance Parameters on a Per Tube Basis:
7. Calculation of Energy Balance Parameters:
Thermodynamics:
, using the standard heats of formation
ΔCp : Using the mean heat capacities
ΔCp = CPB + CPC – CPA = (83 + 71 – 163) J/mol · K
ΔCp = – 9 J/mol · K
Use Wolfram or Python to vary ΘI in the LEP.
Heat Exchange:
Energy balance. The heat-transfer area per unit volume of pipe is
Combining the overall heat transfer coefficient with the area yields
Ua = 16500 J/m3 · s · K
TABLE E12-2.1 SUMMARY OF PARAMETER VALUES
Parameter Values |
||
| T0 = 1035 K | ||
| FA0 = 0.0376 mol/s | ΔCP = =9 J/mol·K | TR = 298 K |
| CPA = 163 J/mol A/K | CA0 = 18.8 mol/m3 | |
| CPCool ≡ CPc = 34.5 J/mol/K | Ua = 16500 J/m3·s·K | Vf = 0.001 m3 |
We will solve for all four cases of heat-exchanger operation for this endothermic reaction example in the same way we did for the exothermic reaction in Example 12-1. That is, we will write the Polymath equations for the case of co-current heat exchange and use that as the base case. As noted in the LEP for this example one should use Wolfram or Python to explore the simulation. We will then manipulate the different terms in the heat-transfer fluid balance (Equations 12-10 and 12-11) to solve for the other cases. In the four cases that follow we will take ΘI = 0, but on the Wolfram and Python LEPs we allow the reader to vary ΘI. We will start with the adiabatic case where we multiply the heat transfer coefficient in the base case by zero.
Case 1 Adiabatic
We are going to start with the adiabatic case first to show the dramatic effects of how the reaction dies out as the temperature drops. In fact, we are now going to extend the length of each tube to make the total reactor volume 5 dm3 in order to observe this effect of a reaction dying out as well as showing the necessity of adding a heat exchanger. For the adiabatic case, we simply multiply the value of Ua in our Polymath program by zero. No other changes are necessary. For the adiabatic case, the answer will be the same whether we use a bank of 1000 reactors, each a 1-dm3 reactor, or one of 1 m3. To illustrate how an endothermic reaction can virtually die out completely, let’s extend the single-pipe volume from 1 to 5 dm3.
Ua = 16500*0
The Polymath program is shown in Table E12-2.2. Figure E12-2.1 shows the graphical output.
Adiabatic endothermic reaction in a PFR
TABLE E12-2.2 POLYMATH PROGRAM AND OUTPUT FOR ADIABATIC OPERATION
Differential equations
1 d(X)/d(V) = -ra/Fao
2 d(T)/d(V) = (Qg-Qr)/(Fao*(Cpa+X*delCp))
3 d(Ta)/d(V) = Ua*(T-Ta)/(mc*Cpc)
Explicit equations
1 To = 1035
2 Ua = 16500*0
3 Fao = .0376
4 Cpa = 163
5 delCp = -9
6 Cao = 18.8
7 ra = -Cao*3.58*exp(34222*(1/To-1/T))*(1-X)*(To/T)/(1+X)
8 deltaH = 80770+delCp*(T-298)
9 Qg = ra*deltaH
10 Qr = Ua*(T-Ta)
11 mc = .111
12 Cpc = 34.5
13 rate = -ra
Calculated values of DEQ variables
| Variable | Initial value | Final value | |
|---|---|---|---|
| 1 | Cao | 1.88 | 1.88 |
| 2 | Cpa | 163. | 163. |
| 3 | Cpc | 34.5 | 34.4 |
| 4 | delCp | -9. | -9. |
| 5 | deltaH | 7.414E+04 | 7.414E+04 |
| 6 | Fao | 0.0376 | 0.0376 |
| 7 | mc | 0.111 | 0.111 |
| 8 | Qg | -4.99E+06 | -2.79E+04 |
| 9 | Qr | 0 | 0 |
| 10 | ra | -67.304 | -0.3704982 |
| 11 | rate | 67.304 | 0.3704982 |
| 12 | T | 1035. | 904.8156 |
| 13 | Ta | 1250. | 1250. |
| 14 | To | 1035. | 1035. |
| 15 | Ua | 0 | 0 |
| 16 | V | 0 | 0.005 |
| 17 | X | 0 | 0.2817744 |


Figure E12-2.1 Adiabatic conversion and temperature (a), and reaction rate (b) profiles.
Analysis: Case 1 Adiabatic Operation: As temperature drops, so does k and hence the rate, –rA, drops to an insignificant value. Note that for this adiabatic endothermic reaction, the reaction virtually dies out after 3.5 dm3, owing to the large drop in temperature, and we observe very little conversion is achieved beyond this point. One way to increase the conversion would be to add a diluent such as nitrogen, which could supply the sensible heat for this endothermic reaction. The Wolfram LEP allows the reader to vary the nitrogen in the feed. However, if too much diluent is added, the concentration, and hence the rate, will be quite low. On the other hand, if too little diluent is added, the temperature will drop and virtually extinguish the reaction. How much diluent to add is left as an exercise. Figures E12-2.1(a) and (b) give the reactor volume as 5 dm3 in order to show the reaction “dying out.” However, because the reaction is nearly complete near the entrance to the reactor, that is, –rA ≅ 0, we are going to study and compare the heat-exchange systems in a 1-dm3 reactor (0.001 m3) in each of the next three cases.

Case 2 Constant heat-exchange fluid temperature, Ta
We make the following changes in our program on line 3 of the base case (a)
The Polymath Program is shown in Table E12-2.3 and the profiles for T, X, and –rA are shown in Figure E12-2.2, parts (a), (b), and (c) respectively.
TABLE E12-2.3 POLYMATH PROGRAM AND OUTPUT FOR CONSTANT Ta
Differential equations
1 d(X)/d(V) = -ra/Fao
2 d(T)/d(V) = (Qg-Qr)/(Fao*(Cpa+X*delCp))
3 d(Ta)/d(V) = Ua*(T-Ta)/(mc*Cpc)*0
Explicit equations
1 To = 1035
2 Ua = 16500
3 Fao = .0376
4 Cpa = 163
5 delCp = -9
6 Cao = 18.8
7 ra = -Cao*3.58*exp(34222*(1/To-1/T))*(1-X)*(To/T)/(1+X)
8 deltaH = 80770+delCp*(T-298)
9 Qg = ra*deltaH
10 Qr = Ua*(T-Ta)
11 mc = .111
12 Cpc = 34.5
13 rate = -ra
| Variable | Initial value | Final value | |
|---|---|---|---|
| 1 | Cao | 1.88 | 1.88 |
| 2 | Caa | 163. | 163. |
| 3 | Cpc | 34.5 | 34.5 |
| 4 | delCp | -9. | -9. |
| 5 | deltaH | 7.414E+04 | 7.343E+04 |
| 6 | Fao | 0.0376 | 0.0376 |
| 7 | mc | 0.111 | 0.111 |
| 8 | Qg | -4.99E+06 | -1.211E+06 |
| 9 | Qr | -3.548E+06 | -2.242E+06 |
| 10 | ra | -67.304 | -16.48924 |
| 11 | rate | 67.304 | 16.48924 |
| 12 | T | 1035. | 1114.093 |
| 13 | Ta | 1250. | 1250. |
| 14 | To | 1035. | 1035. |
| 15 | Ua | 1.65E+04 | 1.65E+04 |
| 16 | V | 0 | 0.001 |
| 17 | X | 0 | 0.9508067 |
Endothermic reaction profiles
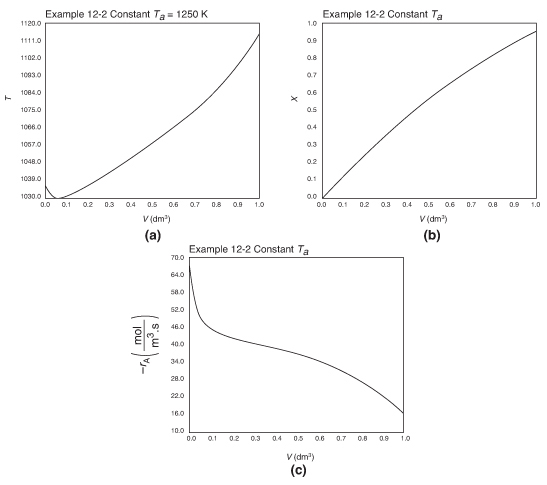
Figure E12-2.2 Profiles for constant heat exchanger fluid temperature, Ta; (a) temperature, (b) conversion, (c) reaction rate.
Analysis: Case 2 Constant Ta: Just after the reactor entrance, the reaction temperature drops as the sensible heat from the reacting fluid supplies the energy for the endothermic reaction. This temperature decrease in the reactor also causes the rate of reaction to decrease. As we move farther down the reactor, the reaction rate drops further as the reactants are consumed. Beyond V = 0.08 dm3, the heat supplied by the constant Ta heat exchanger becomes greater than that “consumed” by the endothermic reaction and the reactor temperature rises. In the range between V = 0.2 dm3 and V = 0.6 dm3, the rate decreases slowly owing to the depletion of reactants, which is counteracted, to some extent, by the increase in temperature and hence the rate constant k. Consequently, we are eventually able to achieve an exit conversion of 95%.
Case 3 Co-Current Heat Exchange
The energy balance on a co-current exchanger is
with Ta0 = 1250 K at V = 0.
The Polymath Program is shown in Table E12-2.4 and the variable profiles for T, Ta, X, and –rA are shown in Figure E12-2.3, parts (a), (b), and (c) respectively. Because the reaction is endothermic, Ta needs to start off at a high temperature at V = 0.
TABLE E12-2.4 POLYMATH PROGRAM AND OUTPUT FOR CO-CURRENT EXCHANGE
Differential equations
1 d(X)/d(V) = -ra/Fao
2 d(T)/d(V) = (Qg-Qr)/(Fao*(Cpa+X*delCp))
3 d(Ta)/d(V) = Ua*(T-Ta)/(mc*Cpc)
Explicit equations
1 To = 1035
2 Ua = 16500
3 Fao = .0376
4 Cpa = 163
5 delCp = -9
6 Cao = 18.8
7 ra = -Cao*3.58*exp(34222*(1/To-1/T))*(1-X)*(To/T)/(1+X)
8 deltaH = 80770+delCp*(T-298)
9 Qg = ra*deltaH
10 Qr = Ua*(T-Ta)
11 mc = .111
12 Cpc = 34.5
13 rate = -ra
Calculated values of DEQ variables
| Variable | Initial value | Final value | |
|---|---|---|---|
| 1 | Cao | 1.88 | 1.88 |
| 2 | Cpa | 163. | 163. |
| 3 | Cpc | 34.5 | 34.5 |
| 4 | delCp | -9. | -9. |
| 5 | deltaH | 7.414E+04 | 7.459E+04 |
| 6 | Fao | 0.0376 | 0.0376 |
| 7 | mc | 0.111 | 0.111 |
| 8 | Qg | -4.99E+06 | -3.654E+05 |
| 9 | Qr | -3.548E+06 | -1.881E+05 |
| 10 | ra | -67.304 | -4.899078 |
| 11 | rate | 67.304 | 4.899078 |
| 12 | T | 1035. | 984.8171 |
| 13 | Ta | 1250. | 996.215 |
| 14 | To | 1035. | 1035. |
| 15 | Ua | 1.65E+04 | 1.65E+04 |
| 16 | V | 0 | 0.001 |
| 17 | X | 0 | 0.456201 |
Analysis: Case 3 Co-Current Exchange: In co-current heat exchange, we see that the heat-exchanger fluid temperature, Ta, decreases rapidly initially and then continues to decrease along the length of the reactor as it supplies the energy to the heat drawn by the endothermic reaction. Eventually Ta decreases to the point where it approaches T and the rate of heat exchange is small; as a result, the temperature of the reactor, T, continues to decrease, as does the rate, resulting in a small conversion. Because the reactor temperature for co-current exchange is lower than that for Case 2 constant Ta, the reaction rate will be lower. As a result, significantly less conversion will be achieved than in the case of constant heat-exchange temperature Ta.
Endothermic reaction profiles
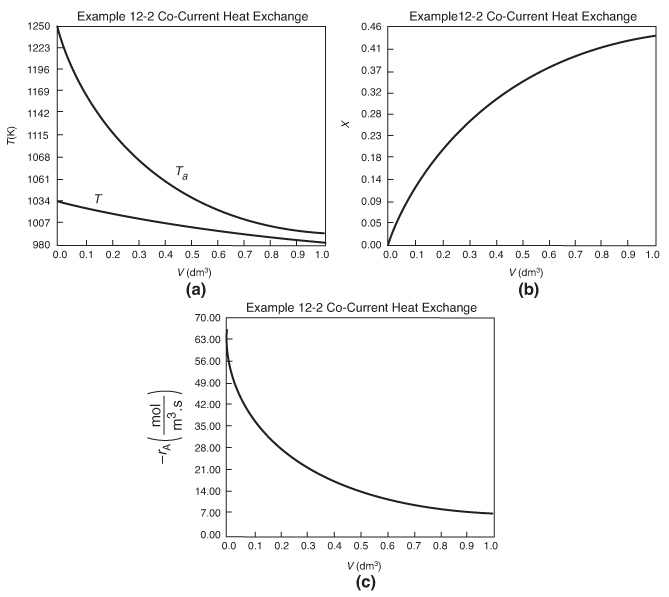
Figure E12-2.3 Profiles down the reactor for an endothermic reaction with co-current heat exchange; (a) temperature, (b) conversion, and (c) reaction rate.
Case 4 Countercurrent Heat Exchange
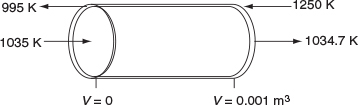
For countercurrent exchange, we first multiply the rhs of the co-current heat-exchanger energy balance by –1, leaving the rest of the Polymath program in Table 12-2.5 the same.
Next, guess Ta (V = 0) = 995.15 K to obtain Ta0 = 1250 K at V = 0.001m3. (Don’t you believe for a moment 995.15 K was my first guess.) Once this match is obtained as shown in Table E12-2.5, we can report the profiles shown in Figure E12-2.4.
Good guess!
TABLE E12-2.5 POLYMATH PROGRAM AND OUTPUT FOR COUNTERCURRENT EXCHANGE

Differential equations
1. d(X)/d(V) = -ra/Fao
2. d(T)/d(V) = (Qg-Qr)/(Fao*(Cpa+X*delCp))
3. d(Ta)/d(V) = -Ua*(T-Ta)/(mc*Cpc)
Explicit equations are the same as Case 3 Co-Current Heat Exchange.
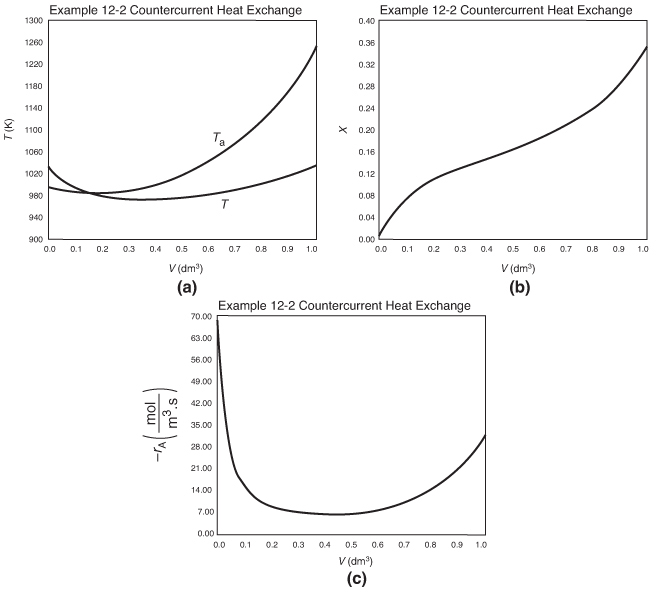
Figure E12-2.4 Profiles down the reactor for countercurrent heat exchange; (a) temperature, (b) conversion, (c) reaction rate.
Endothermic reaction profiles
Analysis: Case 4 Countercurrent Exchange: At the front of the reactor where the reactants enter, that is, V = 0, the reaction takes place very rapidly, drawing energy from the sensible heat of the gas and causing the gas temperature to drop because the heat exchanger cannot supply energy at an equal or greater rate to that being drawn by the endothermic reaction. Additional “heat” is lost at the entrance in the case of countercurrent exchange because the temperature of the exchange fluid, Ta, is below the entering reactor temperature, T. One notes there is a minimum in the reaction rate, –rA, profile that is rather flat. In this flat region, the rate is “virtually” constant between V = 0.2 dm3 and V = 0.8 dm3, because the increase in k caused by the increase in T is balanced by the decrease in rate brought about by the consumption of reactants. Just past the middle of the reactor, the rate begins to increase slowly as the reactants become depleted and the heat exchanger now supplies energy at a rate greater than the reaction draws energy and, as a result, the temperature eventually increases. This lower temperature coupled with the consumption of reactants causes the rate of reaction to be low in the plateau, resulting in a lower conversion than either the co-current or constant Ta heat exchange cases.
Analysis Summary of 4 Cases. The exit temperatures and conversion are shown in the Summary Table E12-2.6.
TABLE E12-2.6 SUMMARY TABLE
| Heat Exclusion | X | T(K) | Ta(K) |
|---|---|---|---|
| Adiabatic | 0.28 | 905 | --- |
| Constant Ta | 0.95 | 1114 | 1250 |
| Co-Current | 0.456 | 985 | 996 |
| Countercurrent | 0.35 | 1034 | 995 |
For this endothermic reaction, the highest conversion is obtained for constant Ta and the lowest conversion is for an adiabatic reaction. These two extremes correspond to the greatest and least amount of heat transfer to the reacting fluid.
AspenTech: Example 12-2 has also been formulated in AspenTech and can be downloaded on your computer directly from the CRE Web site.
12.4 CSTR with Heat Effects
In this section we apply the general energy balance (Equation (11-22)) to a CSTR at steady state. We then present example problems showing how the mole and energy balances are combined to design reactors operating adiabatically and non-adiabatically.
In Chapter 11 the steady-state energy balance was derived as
Recall that Ẇs is the shaft work, that is, the work done by a stirrer or mixer in the CSTR on the reacting fluid inside the CSTR. Consequently, because the convention that done by the system on the surroundings is positive, the CSTR stirrer work will be a negative number, for example, . (See Problem P12-6B, a California Professional Engineers’ Exam Problem.)
Note: In many calculations the CSTR mole balance derived in Chapter 2
(FA0X = –rAV)
These are the forms of the steady-state balance we will use.
will be used to replace the term following the brackets in Equation (11-28), that is, (FA0X) will be replaced by (–rAV) to arrive at Equation (12-12).
Rearranging yields the steady-state energy balance
Although the CSTR is well mixed and the temperature is uniform throughout the reaction vessel, these conditions do not mean that the reaction is carried out isothermally. Isothermal operation occurs when the feed temperature is identical to the temperature of the fluid inside the CSTR.
The Term in the CSTR
12.4.1 Heat Added to the Reactor,
Figure 12-6 shows the schematics of a CSTR with a heat exchanger. The heat-transfer fluid enters the exchanger at a mass flow rate (e.g., kg/s) at a temperature Ta1 and leaves at a temperature Ta2. The rate of heat transfer from the exchanger to the reactor fluid at temperature T is3
3 Information on the overall heat transfer coefficient may be found in J. R. Welty, G. L. Rorrer, and D. G. Foster, Fundamentals of Momentum Heat and Mass Transfer, 6th ed. New Jersey: Wiley, 2015, p. 370.
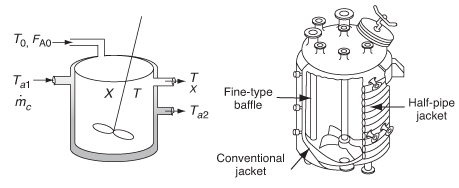
Figure 12-6 CSTR tank reactor with heat exchanger.
The following derivations, based on a coolant (exothermic reaction), apply also to heating mediums (endothermic reaction). As a first approximation, we assume a quasi-steady state operation for the coolant flow and neglect the accumulation term (i.e., dTa/dt = 0). An energy balance on the heat-exchanger fluid entering and leaving the exchanger is
For exothermic reactions (T>Ta2>Ta1)
For endothermic reactions (T1a>T2a>T)
Energy balance on heat-exchanger fluid
where CPc is the heat capacity of the heat exchanger fluid and TR is the reference temperature. Simplifying gives us
Solving Equation (12-16) for the exit temperature of the heat-exchanger fluid yields
From Equation (12-16)
Substituting for Ta2 in Equation (12-18), we obtain
Heat transfer to a CSTR
For large values of the heat-exchanger fluid flow rate, , the exponent will be small and can be expanded in a Taylor series (e–x = 1 – x + . . .) where second-order terms are neglected in order to give
Then
Valid only for large heat-transfer fluid flow rates!!
where Ta1 ≌ Ta2 = Ta.
With the exception of processes involving highly viscous materials such as in Problem P12-6B, a California Professional Engineers’ Exam Problem, the work done by the stirrer can usually be neglected. Setting equal to zero in Equation (11-27), neglecting ΔCP, in ΔHRx substituting for , and rearranging, we have the following relationship between conversion and temperature in a CSTR:
Solving for X
Equation (12-22) is coupled with the mole balance equation
to design CSTRs.
To help us more easily see the effect of the operating parameters of T, T0, and Ta, we collect terms and define three parameters: CP0, κ, and Tc.
We now will further rearrange Equation (12-21) after letting
then
Let κ and TC be non-adiabatic parameters defined by
Non-adiabatic CSTR heat exchange parameters: κ and Tc
Then
The parameters κ and Tc are used to simplify the equations for non-adiabatic operation. Solving Equation (12-24) for conversion X
Solving Equation (12-24) for the reactor temperature
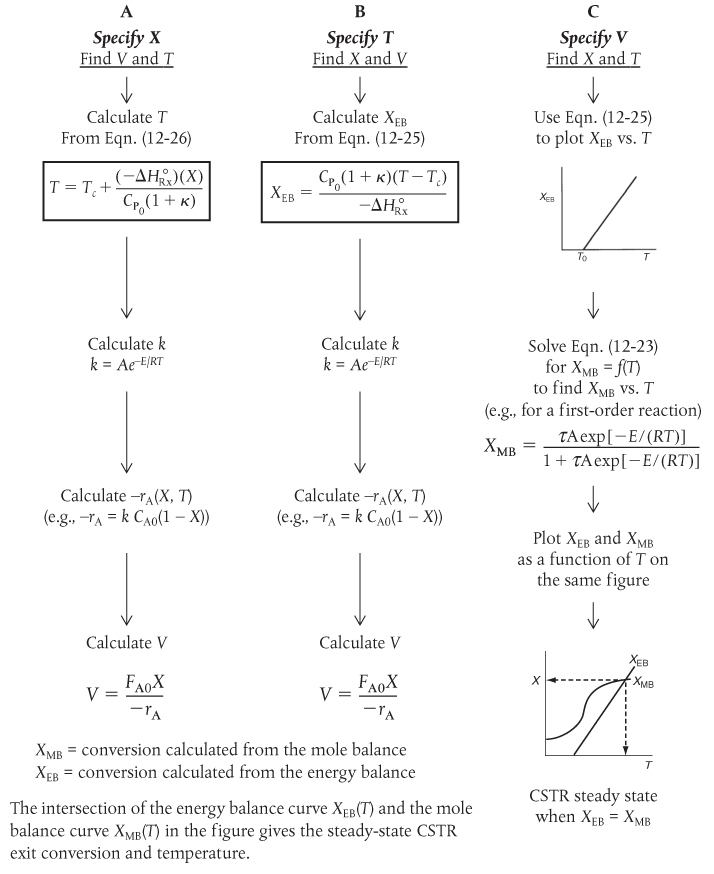
Table 12-3 shows three ways to specify the design of a CSTR. This procedure for nonisothermal CSTR design will be illustrated by considering a first-order irreversible liquid-phase reaction. To solve CSTR problems of this type, we have three variables X, T, and V and we specify one and then solve for the other two values. The algorithm for working through either cases A (X specified), B (T specified), or C (V specified) is shown in Table 12-3. Its application is illustrated in Example 12-3.
TABLE 12-3 WAYS TO SPECIFY THE SIZING OF A CSTR
Forms of the energy balance for a CSTR with heat exchange
Production, uses, and economics
Example 12–3 Production of Propylene Glycol in an Adiabatic CSTR
Propylene glycol is produced by the hydrolysis of propylene oxide:
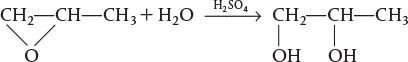
Over 900 million pounds of propylene glycol were produced in 2010 and the selling price was approximately $0.80 per pound. Propylene glycol makes up about 25% of the major derivatives of propylene oxide. The reaction takes place readily at room temperature when catalyzed by sulfuric acid.
You are the engineer in charge of an adiabatic CSTR producing propylene glycol by this method. Unfortunately, the reactor is beginning to leak, and you must replace it. (You told your boss several times that sulfuric acid was corrosive and that mild steel was a poor material for construction. He wouldn’t listen.) There is a nice-looking, bright, shiny overflow CSTR of 300-gal capacity standing idle in Professor Köttlov’s storage shed at his mountain vacation home. It is glass-lined, and you would like to use it.
We are going to work this problem in lbm, s, ft3, and lb-moles rather than g, mol, and m3 in order to give the reader more practice in working in both the English and metric systems. Why?? Many plants still use the English system of units.
You are feeding 2500 lbm/h (43.04 lb-mol/h) of propylene oxide (P.O.) to the reactor. The feed stream consists of (1) an equivolumetric mixture of propylene oxide (46.62 ft3/h) and methanol (46.62 ft3/h), and (2) water containing 0.1 wt % H2SO4. The volumetric flow rate of water is 233.1 ft3/h, which is 2.5 times the methanol–P.O. volumetric flow rate. The corresponding molar feed rates of methanol and water are 71.87 and 802.8 lb-mol/h, respectively. The water–propylene oxide–methanol mixture undergoes a slight decrease in volume upon mixing (approximately 3%), but you neglect this decrease in your calculations. The temperature of both feed streams is 58°F prior to mixing, but there is an immediate 17°F temperature rise upon mixing of the two feed streams caused by the heat of mixing. The entering temperature of all feed streams is thus taken to be 7°F (Figure E12-3.1).

Figure E12-3.1 Propylene glycol manufacture in a CSTR.
The Furusawa Engineering Team in Japan state that under conditions similar to those at which you are operating, the reaction is apparent first-order in propylene oxide concentration and apparent zero-order in excess of water with the specific reaction rate4
4 T. Furusawa, H. Nishimura, and T. Miyauchi, J. Chem. Eng. Jpn., 2, 95.
k = Ae–E/RT = 16.96 × 1012 (e –32400/RT) h–1
The units of E are Btu/lb-mol and T is in °R.
There is an important constraint on your operation. Propylene oxide is a rather low-boiling-point substance. With the mixture you are using, the company’s safety team feels that you cannot exceed an operating temperature of 125°F, or you will lose too much propylene oxide by vaporization through the vent system.
Can you use the idle CSTR as a replacement for the leaking one if it will be operated adiabatically?
If so, what will be the expected conversion of propylene oxide to glycol?
Solution
(All data used in this problem were obtained from the CRC Handbook of Chemistry and Physics unless otherwise noted.) Let the reaction be represented by
A + B → C
where
A is propylene oxide (CPA = 35 Btu/lb-mol · °F)5
5 CPA and CPC are estimated from the observation that the great majority of low-molecular-weight oxygen-containing organic liquids have a mass heat capacity of 0.6 cal/g · °C ± 15%.
B is water (CPB = 18 Btu/lb-mol· °F)
C is propylene glycol (CPC = 46 Btu/lb-mol · °F)
M is methanol (CPM = 19.5 Btu/lb-mol· °F)
In this problem, neither the exit conversion nor the temperature of the adiabatic reactor is given. By application of the mole and energy balances, we can solve two equations with two unknowns (X and T), as shown on the right-hand pathway in Table 12-3. Solving these coupled equations, we determine the exit conversion and temperature for the glass-lined reactor to see whether it can be used to replace the present reactor.
Mole Balance and Design Equation:
FA0 – FA + rAV = 0
The design equation in terms of X is
Rate Law:
k = 16.96 1012 exp[–32400/R/T] h–1

Stoichiometry (liquid phase, (υ = υ0):
Combining yields
Solving for X as a function of T and recalling that τ = V/υ0 gives
This equation relates temperature and conversion through the mole balance.
Two equations, two unknowns
The energy balance for this adiabatic reaction in which there is negligible energy input provided by the stirrer is
This equation relates X and T through the energy balance. We see that two equations, Equations (E12-3.5) and (E12-3.6), and two unknowns, X and T, must be solved to find the conversion where XEB = XMB = X.
Calculations:
Rather than putting all those numbers in the mole and heat balance equations yourself, you can outsource this task, for a small fee, to Sven Köttlov Consulting Company, located on the third floor of the downtown Market Center Building (called the “MCB”) in Riça, Jofostan. The results of our outsourcing are given below.
Evaluate the mole balance terms (CA0, Θi, τ): The total liquid volumetric flow rate entering the reactor is
I know these are tedious calculations, but someone’s gotta know how to do them.

For methanol:
For water:
The conversion calculated from the mole balance, XMB, is found from Equation (E12-3.5)
Plot XMB as a function of temperature.
Evaluating the energy balance terms
(1) Heat of reaction at temperature T
(2) Heat capacity term
The conversion calculated from the energy balance, XEB, for an adiabatic reaction is given by Equation (11-29)
Substituting all the known quantities into the energy balance gives us
Adiabatic CSTR
Solving: There are a number of different ways to solve these two simultaneous algebraic equations (E12-3.10) and (E12-3.14). The easiest way is to use the Polymath nonlinear equation solver. However, to give insight into the functional relationship between X and T for the mole and energy balances, we shall obtain a graphical solution. Here, X is plotted as a function of T for both the mole (XMB) and energy (XEB) balances, and the intersection of the two curves gives the solution where both the mole and energy balance solutions are satisfied, that is, XEB = XMB. In addition, by plotting these two curves we can learn whether there is more than one intersection (i.e., multiple steady states) for which both the energy balance and mole balance are satisfied. If numerical root-finding techniques were used to solve for X and T, it would be quite possible that you would find only one root when there is actually more than one. If Polymath were used, you could learn whether multiple roots exist by changing your initial guesses in the nonlinear equation solver. We shall discuss multiple steady states further in Section 12-5. We now choose T and then calculate XMB and XEB (Table E12-3.1). The calculations for XMB and XEB are plotted in Figure E12-3.2. The virtually straight line corresponds to the energy balance, Equation (E12-3.14), and the curved line corresponds to the mole balance, Equation (E12-3.10). We observe from this plot that the only intersection
TABLE E12-3.1 CALCULATIONS OF XEB AND XMB AS A FUNCTION OF T
| T (°R) | XMB (Eq. (E12-3.10)) | XEB (Eq. (E12-3.14)) |
|---|---|---|
| 535 | 0.108 | 0.000 |
| 550 | 0.217 | 0.166 |
| 565 | 0.379 | 0.330 |
| 575 | 0.500 | 0.440 |
| 585 | 0.620 | 0.550 |
| 595 | 0.723 | 0.656 |
| 605 | 0.800 | 0.764 |
| 615 | 0.860 | 0.872 |
| 625 | 0.900 | 0.980 |
The reactor cannot be used because it will exceed the specified maximum temperature of 585°R.
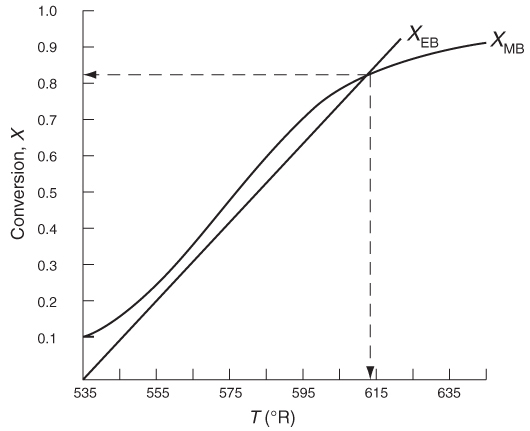
Figure E12-3.2 The conversions XEB and XMB as a function of temperature.
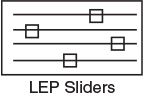
point is at 83% conversion and 613=R. At this point, both the energy balance and mole balance are satisfied. Because the temperature must remain below 125°F (585°R), we cannot use the 300-gal reactor as it is now.
Analysis: After using Equations (E12-3.10) and (E12-3.14) to make a plot of conversion as a function of temperature, we see that there is only one intersection of XEB(T) and XMB(T), and consequently only one steady state. The exit conversion is 83% and the exit temperature (i.e., the reactor temperature) is 613°R (153°F), which is above the acceptable limit of 585°R (125°F) and we thus cannot use the CSTR operating at these conditions. Be sure to go to the LEP 12-3 and use Wolfram to see the XMB and XEB versus T lines change along with their intersection change as you change the parameters in the slider box.
Ouch! Looks like our plant will not be able to be completed and our multimillion-dollar profit has flown the coop. But wait, don’t give up, let’s ask reaction engineer Maxwell Anthony to fly to our company’s plant in the country of Jofostan to look for a cooling coil heat exchanger that we could place in the reactor. See what Max found in Example 12-4.
Example 12–4 CSTR with a Cooling Coil
Fantastic! Max has located a cooling coil in an equipment storage shed in the small, mountainous village of Ölofasis, Jofostan, for use in the hydrolysis of propylene oxide discussed in Example 12-3. The cooling coil has 40 ft2 of cooling surface and the cooling-water flow rate inside the coil is sufficiently large that a constant coolant temperature of 85°F can be maintained. A typical overall heat-transfer coefficient for such a coil is 100 Btu/h·ft2·°F. Will the reactor satisfy the previous constraint of 125°F maximum temperature if the cooling coil is used?
Solution
If we assume that the cooling coil takes up negligible reactor volume, the conversion calculated as a function of temperature from the mole balance is the same as that in Example 12-3, Equation (E12-3.10).
Combining the mole balance, stoichiometry, and rate law, we have, from Example 12-3
T is in °R.
Energy balance: Neglecting the work by the stirrer, we combine Equations (11-27) and (12-20) to write
Solving the energy balance for XEB yields
The cooling-coil term in Equation (E12-4.2) is
Recall that the cooling temperature is
Ta = 85°F = 545°R
The numerical values of all other terms of Equation (E12-4.2) are identical to those given in Equation (E12-3.13), but with the addition of the heat exchange term, XEB becomes
We now have two equations, Equations (E12-3.10) and (E12-4.4), and two unknowns, X and T, which we can solve with Polymath. Recall Examples 4-5 and 8-6 to review how to solve nonlinear, simultaneous equations of this type with Polymath. (See Problem P12-1A(f) on pages 661–662 to plot X versus T on Figure E12-3.2.) We could generate Figure E12-3.2 by “fooling” Polymath to plot X and T, as explained in the tutorial on the Web site (http://www.umich.edu/~elements/6e/software/Polymath_fooling_tutorial.pdf ).

TABLE E12-4.1 POLYMATH: CSTR WITH HEAT EXCHANGE
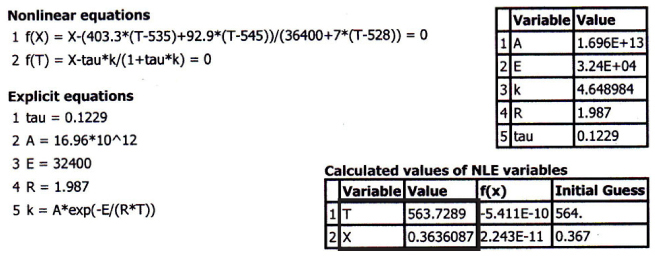
The Polymath program and solution to these two Equations (E12-3.10) for XMB, and (E12-4.4) for XEB, are given in Table E12-4.1. The exiting temperature and conversion are 103.7°F (563.7°R) and 36.4%, respectively, that is,
Be sure to go to the LEP 12-3 and use Wolfram or Python to see how XMB and XEB vary as you change the parameters in the slider box.
Analysis: We are grateful to the people of the village of Ölofasis in Jofostan for their help in finding this shiny new heat exchanger. By adding heat exchange to the CSTR, the XMB(T) curve is unchanged but the slope of the XEB(T) line in Figure E12-3.2 increases and intersects the XMB curve at X = 0.36 and T = 564°R. This conversion is low! We could try to reduce the cooling by increasing Ta or T0 to raise the reactor temperature closer to 585°R, but not above this temperature. The higher the temperature in this irreversible reaction, the greater the conversion.
We will see in the next section that there may be multiple exit values of conversion and temperature (multiple steady states, MSS) that satisfy the parameter values and entrance conditions.
12.5 Multiple Steady States (MSS)
In this section, we consider the steady-state operation of a CSTR in which a first-order reaction is taking place. An excellent experimental investigation that demonstrates the multiplicity of steady states was carried out by Vejtasa and Schmitz.6 They studied the reaction between sodium thiosulfate and hydrogen peroxide
6 S. A. Vejtasa and R. A. Schmitz, AIChE J., 16 (3), 415.
2Na2S2O3 + 4H2O2 → Na2S3O6 + Na2SO4 + 4H2O
in a CSTR operated adiabatically. The multiple steady-state temperatures were examined by varying the flow rate over a range of space times, τ.
To illustrate the concept of MSS, reconsider the XMB(T) curve, Equation (E12-3.10), shown in Figure E12-3.2, which has been redrawn and shown as dashed lines in Figure E12-3.2A. Now consider what would happen if the volu-metric flow rate ν0 is increased (τ decreased) just a little. The energy balance line, XEB(T), remains unchanged, but the mole balance line, XMB, moves to the right, as shown by the curved, solid line in Figure E12-3.2A. This shift of XMB(T) to the right results in the XEB(T) and XMB(T) intersecting three times, XMB = XEB, indicating three possible steady-state conditions at which the reactor could operate.
When more than one intersection occurs, there is more than one set of conditions that satisfy both the energy balance and mole balance (i.e., XEB = XMB); consequently, there will be multiple steady states at which the reactor may operate. These three steady states are easily determined from a graphical solution, but only one could show up in the Polymath equation solver solution. Thus, when using the Polymath nonlinear equation solver, we need to either choose different initial guesses to find whether there are other solutions that exist for which XMB = XEB. Or We could also fool Polymath to obtain the
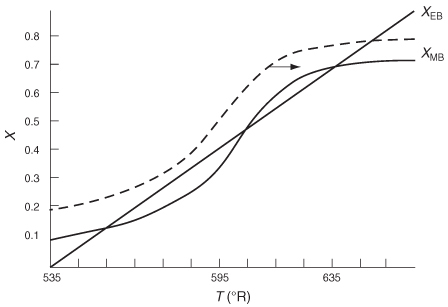
Figure E12-3.2A Plots of XEB(T) and XMB(T) for different spaces times τ.
plot in Figure E12-3.2A by using the ODE solver and setting and then plot XMB and XEB versus T, as in Example 12-3. Tutorials on “How to Fool Polymath” and generate G(T) and R(T) plots can be found at http://www.umich.edu/~elements/6e/software/Polymath_fooling_tutorial.pdf.
We begin by recalling Equation (12-24), which applies when one neglects shaft work and ΔCP (i.e., ΔCP = 0 and therefore )
where
“Fooling” Polymath
and
Using the CSTR mole balance , Equation (12-24) may be rewritten as
The left-hand side is referred to as the heat-generated term
G(T) = Heat-generated term
The right-hand side of Equation (12-28) is referred to as the heat-removed term (by flow and heat exchange) R(T)
R(T) = Heat-removed term
To study the multiplicity of steady states, we shall plot both R(T) and G(T) as a function of temperature on the same graph and analyze the circumstances under which we will obtain multiple intersections of R(T) and G(T).
12.5.1 Heat-Removed Term, R(T )
Vary Entering Temperature. From Equation (12-30), we see that R(T) increases linearly with temperature, with slope and intercept Tc. As the entering temperature T0 is increased, the line retains the same slope but shifts to the right as the intercept Tc increases, as shown in Figure 12-7.
Heat-removed curve R(T)
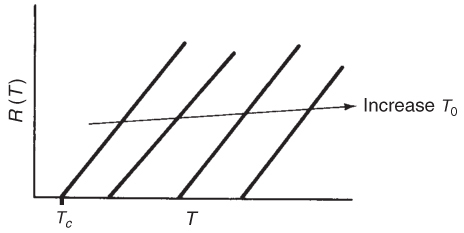
Figure 12-7 Variation of heat-removed line with inlet temperature.
Vary Non-adiabatic Parameter κ. If one increases κ by either decreasing the molar flow rate, FA0, or increasing the heat-exchange area, A, the slope increases and for the case of Ta < T0 the ordinate intercept moves to the left, as shown in Figure 12-8.

Figure 12-8 Variation of heat-removed line with κ (κ = UA/CP0FA0).
On the other hand, if Ta < T0, the intercept will move to the right as κ increases.
12.5.2 Heat-Generated Term, G(T )
The heat-generated term, Equation (12-29), can be written in terms of conversion. (Recall that X = –rAV/FA0.)
To obtain a plot of heat generated, G(T), as a function of temperature, we must solve for X as a function of T using the CSTR mole balance, the rate law, and stoichiometry. For example, for a first-order liquid-phase reaction, the CSTR mole balance becomes
Solving for X yields
First-order reaction
Substituting for X in Equation (12-31), we obtain
Finally, substituting for k in terms of the Arrhenius equation, we obtain
Note that equations analogous to Equation (12-33) for G(T) can be derived for other reaction orders and for reversible reactions simply by solving the CSTR mole balance for X. For example, for the second-order liquid-phase reaction
Second-order reaction
the corresponding heat-generated term is
Let’s now return to our first-order reaction, Equation (12-33), and examine the behavior of the G(T) curve. At very low temperatures, the second term in the denominator of Equation (12-33) for the first-order reaction can be neglected, so that G(T) varies as
Low T
(Recall that means that the standard heat of reaction is evaluated at TR.)
At very high temperatures, the second term on the right side in the denominator is large and dominates in Equation (12-33) so that the τk in the numerator and denominator cancel, and G(T) is reduced to
High T
G(T) is shown as a function of T for two different activation energies, E, in Figure 12-9. If the flow rate is decreased or the reactor volume increased so as to increase τ, the heat-generated term, G(T), changes, as shown in Figure 12-10.
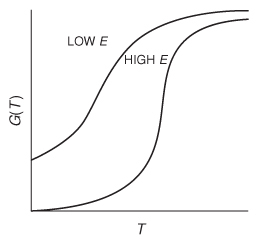
Figure 12-9 Variation of G(T) curve with activation energy.
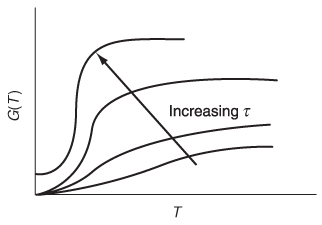
Figure 12-10 Variation of G(T) curve with space time.
Heat-generated curves, G(T)
Can you combine Figures 12-10 and 12-8 to explain why a Bunsen burner flame goes out when you turn up the gas flow to a very high rate? As an exercise, plot R(T) verses G(T) for different values of T0 and κ.
12.5.3 Ignition–Extinction Curve
The points of intersection of R(T) and G(T) give us the temperature at which the reactor can operate at steady state. This intersection is the point at which both the energy balance, R(T), and the mole balance, G(T), are satisfied. Suppose that we begin to operate our reactor at some relatively low temperature, T01. If we construct our G(T) and R(T) curves, illustrated by curve y = G(T) and line a = R(T), respectively, in Figure 12-11, we see that there will be only one point of intersection, point 1. From this point of intersection, one can find the steady-state temperature in the reactor, Ts1, by following a vertical line down to the T-axis and reading off the temperature, Ts1, as shown in Figure 12-11.
Ignition: The point of no return
If one were now to increase the entering temperature to T02, the G(T) curve, y, would remain unchanged, but the R(T) curve would move to the right, as shown by line b in Figure 12-11, and will now intersect the G(T) at point 2 and also be tangent at point 3. Consequently, we see from Figure 12-11 that there are two steady-state temperatures, Ts2 and Ts3, that can be realized in the CSTR for an entering temperature T02. If the entering temperature is further increased to T03, the R(T) curve, line c (Figure 12-12), intersects the G(T) curve three times and there are three steady-state temperatures, Ts4, Ts5, and Ts6. As we continue to increase T0, we finally reach line e, in which there are only two steady-state temperatures, a point of tangency at Ts10 and an intersection at Ts11. If the entering temperature is slightly increased beyond T05, say to T06, then the point of tangency disappears and there is a jump in reactor temperature, from Ts10 up to Ts12. This jump is called the ignition point for the reaction. By further increasing T0, we reach line f, corresponding to T06, in which we have only one reactor temperature that will satisfy both the mole and energy balances, Ts12. For the six entering temperatures, we can form Table 12-4, relating the entering temperature to the possible reactor operating temperatures.
By plotting steady-state reactor temperature Ts as a function of entering temperature T0, we obtain the well-known ignition–extinction curve shown in
Both the mole and energy balances are satisfied at the points of intersection or tangency.

Figure 12-11 Finding multiple steady states with T0 varied.
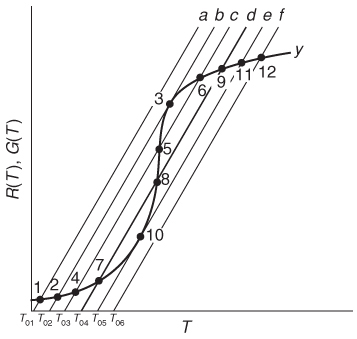
Figure 12-12 Finding multiple steady states with T0 varied.
TABLE 12-4 MULTIPLE STEADY-STATE TEMPERATURES
| Entering Temperature | Reactor Temperatures | ||||
|---|---|---|---|---|---|
T01 |
Ts1 |
||||
T02 |
Ts2 |
Ts3 |
|||
T03 |
Ts4 |
Ts5 |
Ts6 |
||
T04 |
Ts7 |
Ts8 |
Ts9 |
||
T05 |
Ts10 |
Ts11 |
|||
T06 |
Ts12 |
||||
Figure 12-13. From this figure, we see that as the entering temperature T0 is increased, the steady-state temperature Ts increases along the bottom line until T05 is reached. Any fraction-of-a-degree increase in temperature beyond T05 the point of tangency disappears and the steady-state reactor temperature Ts will jump up from Ts10 to Ts11, as shown in Figure 12-13. The temperature at which this jump occurs is called the ignition temperature. That is, we must exceed a certain feed temperature, T05, to operate at the upper steady state where the temperature and conversion are higher. The ignition point temperature is sometimes also called the onset temperature for a runaway reaction or the point of no return, especially when the upper steady state is at a very high temperature.
Runaway reaction?
If a reactor were operating at Ts12 and we began to cool the entering temperature down from T06, the steady-state reactor temperature, Ts3, would eventually be reached, corresponding to an entering temperature, T02. Any slight decrease below T02 the point of tangency on line b in Figure 12-12 would disappear and the temperature would drop the steady-state reactor temperature to immediately the lower steady-state value Ts2. Consequently, T02 is called the extinction temperature.
The middle points 5 and 8 in Figures 12-12 and 12-13 represent unstable steady-state temperatures. Consider the heat-removed line d in Figure 12-12, along with the heat-generated curve y, which are replotted in Figure 12-14. If we were operating at the middle steady-state temperature Ts8, for example, and a
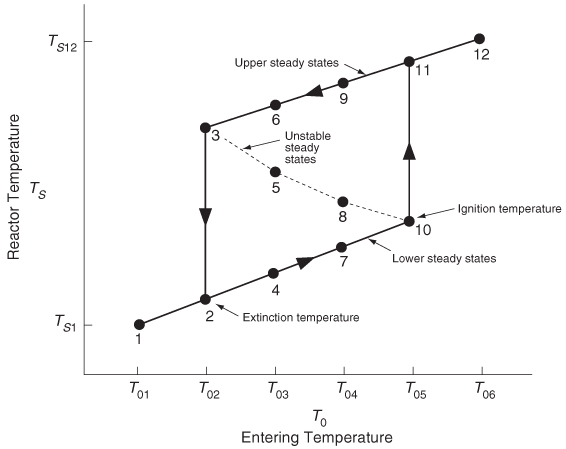
Figure 12-13 Temperature ignition–extinction curve.
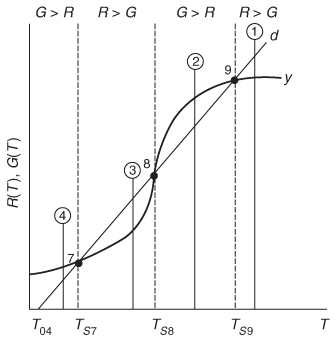
Figure 12-14 Stability of multiple steady-state temperatures.
Point of No Return

Beyond the onset point/temperature, if no action is taken the reaction will proceed to its maximum temperature.
pulse increase in reactor temperature occurred, we would find ourselves at the temperature shown by vertical line ![]() , between points 8 and 9. We see that along this vertical line
, between points 8 and 9. We see that along this vertical line ![]() , the heat-generated curve, y ≡ G(T), is greater than the heat-removed line d ≡ R(T), that is, (G > R). Consequently, the temperature in the reactor would continue to increase until point 9 is reached at the upper steady state. On the other hand, if we had a pulse decrease in temperature from point 8, we would find ourselves on a vertical line
, the heat-generated curve, y ≡ G(T), is greater than the heat-removed line d ≡ R(T), that is, (G > R). Consequently, the temperature in the reactor would continue to increase until point 9 is reached at the upper steady state. On the other hand, if we had a pulse decrease in temperature from point 8, we would find ourselves on a vertical line ![]() between points 7 and 8. Here, we see that the heat-removed curve d is greater than the heat-generated curve y (R > G), so the temperature will continue to decrease until the lower steady state is reached. That is, a small change in temperature either above or below the middle steady-state temperature, Ts8, will cause the reactor temperature to move away from this middle steady state. Steady states that behave in this manner are said to be unstable.
between points 7 and 8. Here, we see that the heat-removed curve d is greater than the heat-generated curve y (R > G), so the temperature will continue to decrease until the lower steady state is reached. That is, a small change in temperature either above or below the middle steady-state temperature, Ts8, will cause the reactor temperature to move away from this middle steady state. Steady states that behave in this manner are said to be unstable.
In contrast to these unstable operating points, there are stable operating points. Consider what would happen if a reactor operating at Ts9 were subjected to a pulse increase in reactor temperature indicated by line ![]() in Figure 12-14. We see that the heat-removed line d is greater than the heat-generated curve y (R > G), so that the reactor temperature will decrease and return to Ts9. On the other hand, if there is a sudden drop in temperature below Ts9, as indicated by line
in Figure 12-14. We see that the heat-removed line d is greater than the heat-generated curve y (R > G), so that the reactor temperature will decrease and return to Ts9. On the other hand, if there is a sudden drop in temperature below Ts9, as indicated by line ![]() , we see the heat-generated curve y is greater than the heat-removed line d (G > R), and the reactor temperature will increase and return to the upper steady state at Ts9. Consequently, Ts9 is a stable steady state.
, we see the heat-generated curve y is greater than the heat-removed line d (G > R), and the reactor temperature will increase and return to the upper steady state at Ts9. Consequently, Ts9 is a stable steady state.
Next, let’s look at what happens when the lower steady-state temperature at Ts7 is subjected to pulse increase to the temperature shown as line ![]() in Figure 12-14. Here, we again see that the heat removed, R, is greater than the heat generated, G, so that the reactor temperature will drop and return to Ts7. If there is a sudden decrease in temperature below Ts7 to the temperature indicated by line
in Figure 12-14. Here, we again see that the heat removed, R, is greater than the heat generated, G, so that the reactor temperature will drop and return to Ts7. If there is a sudden decrease in temperature below Ts7 to the temperature indicated by line ![]() , we see that the heat generated is greater than the heat removed (G > R), and that the reactor temperature will increase until it returns to Ts7. Consequently, Ts7 is a stable steady state. A similar analysis could be carried out for temperatures Ts1, Ts2, Ts4, Ts6, Ts11, and Ts12, and one would find that reactor temperatures would always return to locally stable steady-state values when subjected to both positive and negative fluctuations.
, we see that the heat generated is greater than the heat removed (G > R), and that the reactor temperature will increase until it returns to Ts7. Consequently, Ts7 is a stable steady state. A similar analysis could be carried out for temperatures Ts1, Ts2, Ts4, Ts6, Ts11, and Ts12, and one would find that reactor temperatures would always return to locally stable steady-state values when subjected to both positive and negative fluctuations.
While these points are locally stable, they are not necessarily globally stable. That is, a large perturbation in temperature or concentration may be sufficient to cause the reactor to fall from the upper steady state (corresponding to high conversion and temperature, such as point 9 in Figure 12-14), to the lower steady state (corresponding to low temperature and conversion, point 7).
12.6 Nonisothermal Multiple Chemical Reactions
Most reacting systems involve more than one reaction and do not operate isothermally. This section is one of the most important, if not the most important, sections of the book. It ties together all the previous chapters to analyze multiple reactions that do not take place isothermally.
12.6.1 Energy Balance for Multiple Reactions in Plug-Flow Reactors
In this section we give the energy balance for multiple reactions. We begin by recalling the energy balance for a single reaction taking place in a PFR, which is given by Equation (12-5)
When we have multiple reactions occurring, we have to account for, and sum up, all the heats of reaction in the reactor for each and every reaction. For q multiple reactions taking place in a PFR where there are m species, it is easily shown that Equation (12-5) can be generalized to (cf. Problem P12-1(j))
Energy balance for multiple reactions
i = Reaction number
j = Species
Note that we now have two subscripts on the heat of reaction, the reaction number “i” and species “j”. The heat of reaction for reaction i must be referenced to the same species in the rate, rij, by which ΔHRxij is multiplied, which is
where again we note the subscript j refers to the species, the subscript i refers to the particular reaction, q is the number of independent reactions, and m is the number of species. We are going to let
and
Then Equation (12-35) becomes
Equation (12-37) represents a nice compact form of the energy balance for multiple reactions.
12.6.1A Series Reactions in a PFR
Consider the following series reaction sequence carried out in a PFR:
The PFR energy balance becomes
One of the major goals of this text is that the reader will be able to solve multiple reactions with heat effects, and this section shows how!
Substituting for Qg and –Qr in Equation (12-37) we obtain
where ΔHRx1A = [J/mol of A reacted in reaction 1] and
ΔHRx2B = [J/mol of B reacted in reaction 2].
12.6.1B Parallel Reactions in a PFR
We will now give three examples of multiple reactions with heat effects: Example 12-5 discusses parallel reactions, Example 12-6 discusses series reactions, and Example 12-7 discusses complex reactions.
Example 12-5 Parallel Reactions in a PFR with Heat Effects
The following gas-phase reactions occur in a PFR:

Pure A is fed at a rate of 100 mol/s, at a temperature of 150=C and at a concentration of 0.1 mol/dm3. Neglect pressure drop and determine the temperature and molar flow rate profiles down the reactor.
Additional information:
ΔHRx1A = – 20000 J/(mol = of A reacted in reaction 1)
ΔHRx2A = – 60000 J/(mol = of A reacted in reaction 2)
Solution
0. Number Each Reaction: This step was given in the problem statement.
Mole Balances:

Rates:
Rate laws
Relative rates
Net rates
Stoichiometry (gas-phase but ΔP = 0, i.e., p = 1):
(T in K)
Selectivity:
Major goal of CRE: Analyze Multiple Reactions with Heat Effects
Energy Balance:
The PFR energy balance (cf. Equation (12-35))
becomes
Evaluation:
The Polymath program and its graphical outputs are shown in Table E12-5.1 and Figures E12-5.1 and E12-5.2.

TABLE E12-5.1 POLYMATH PROGRAM
Differential equations
1 d(Fa)/d(V) = r1a+r2a
2 d(Fb)/d(V) = -r1a
3 d(Fc)/d(V) = -r2a/2
4 d(T)/d(V) = (Qg-Qr)/(90*Fa+90*Fb+180*Fc)
Explicit equations
1 Qr = 4000*(T-373)
2 To = 423
3 k1a = 10*exp(4000*(1/300-1/T))
4 k2a = 0.09*exp(9000*(1/300-1/T))
5 Cto = 0.1
6 deltaH1 = -20000
7 deltaH2 = -60000
8 Ft = Fa+Fb+Fc
9 Ca = Cto*(Fa/Ft)*(To/T)
10 r2a = -k2a*Ca^2
11 Cb = Cto*(Fb/Ft)*(To/T)
12 Cc = Cto*(Fc/Ft)*(To/T
13 r1a = -k1a*Ca
14 Qg = (-r1a)*(-deltaH1)+(-r2a)*(-deltaH2)
Calculated values of DEQ variables
| Variable | Initial value | Final value | |
|---|---|---|---|
| 1 | Ca | 0.1 | 2.069E-09 |
| 2 | Cb | 0 | 0.0415941 |
| 3 | Cc | 0 | 0.016986 |
| 4 | Cto | 0.1 | 0.1 |
| 5 | Fa | 100. | 2.738E-06 |
| 6 | Fb | 0 | 55.04326 |
| 7 | Fc | 0 | 22.47837 |
| 8 | Ft | 100. | 77.52163 |
| 9 | k1a | 482.8247 | 2.426E+04 |
| 10 | k2a | 553.0557 | 3.716E+06 |
| 11 | Qg | 1.297E+06 | 1.003708 |
| 12 | Qr | 2.0E+05 | 1.396E+06 |
| 13 | r1a | -48.28247 | -5.019E-05 |
| 14 | r2a | -5.530557 | -1.591E-11 |
| 15 | T | 423. | 722.0882 |
| 16 | To | 423. | 423. |
| 17 | V | 0 | 1. |
Why does the temperature go through a maximum value?
Figure E12-5.1 Temperature profile.
Figure E12-5.2 Profile of molar flow rates FA, FB and FC
Analysis: From Figure E12-5.1 we see that the temperature increases slowly up to a reactor volume of 0.4 dm3 then suddenly jumps up (ignites) to a temperature of 850 K. Correspondingly, from Figure E12-5.2 the reactant molar flow rate decreases sharply at this point. The reactant is virtually consumed by the time it reaches a reactor volume V = 0.45 dm3; beyond this point,Qr > Qg, and the reactor temperature begins to drop. In addition, the selectivity remains constant after this point. If a high selectivity is required, then the reactor should be shortened to V = 0.3 dm3, at which point the selectivity is .
12.6.2 Energy Balance for Multiple Reactions in a CSTR
Recall that for the steady-state mole balance in a CSTR with a single reaction [–FA0X = rAV], and that , so that for T0 = Ti0 Equation (11-27) may be rewritten as
Again, we must account for the “heat generated” by all the reactions in the reactor. For q multiple reactions and m species, the CSTR energy balance becomes
Energy balance for multiple reactions in a CSTR
Substituting Equation (12-20) for , neglecting the work term, and assuming constant heat capacities and large coolant flow rates , Equation (12-39) becomes
For the two parallel reactions described in Example 12-5, the CSTR energy balance is
Major goal of CRE
As stated previously, one of the major goals of this text is to have the reader solve problems involving multiple reactions with heat effects (cf. Problems P12-23B, P12-24B, P12-25C, and P12-26C). That’s exactly what we are doing in the next two examples!
12.6.3 Series Reactions in a CSTR
Example 12-6 Multiple Reactions in a CSTR
The elementary liquid-phase reactions
take place in a 10-dm3 CSTR. What are the effluent concentrations for a volumetric feed rate of 1000 dm3/min at a concentration of A of 0.3 mol/dm3? The inlet temperature is 283 K.
Additional information:
Solution
The Algorithm:
0. Number Each Reaction:
1. Mole Balance on Every Species:
Species A: Combined mole balance and rate law for A
Solving for CA gives us
Species B: Combined mole balance and rate law for B

2. Rates:
The reactions follow elementary rate laws
3. Combine:
Substituting for r1B and r2B in Equation (E12-6.3) gives
Solving for CB yields
4. Energy Balances:
Applying Equation (12-41) to this system gives
Substituting for FA0 = υ0CA0, r1A, and r2B and rearranging, we have
5. Parameter Evaluation
We are going to generate G(T) and R(T) by "fooling" Polymath to first generate T as a function of a dummy variable, t. We then use our plotting options to convert T(t) to G(T) and R(T). The Polymath program to plot R(T) and G(T) vs. T is shown in Table E12-6.1, and the resulting graph is shown in Figure E12-6.1 (http://www.umich.edu/~elements/5e/tutorials/Polymath_fooling_tutorial_Example-12-6.pdf).
Fooling Polymath
Incrementing temperature in this manner is an easy way to generate R(T) and G(T) plots.

TABLE E12-6.1 POLYMATH: PROGRAM AND OUTPUT
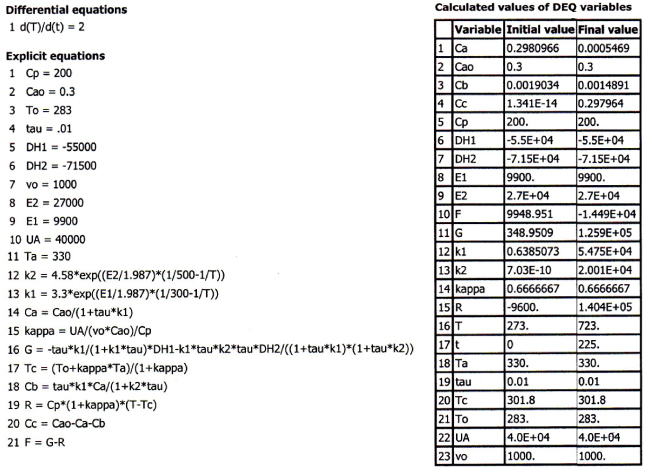
When F = 0, then G(T) = R(T) and the steady states can be found.
Wow!
Five (5) multiple steady states!
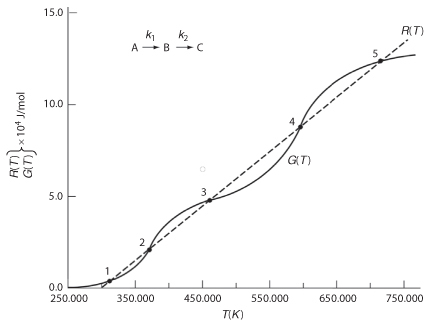
Figure E12-6.1 Heat-removed and heat-generated curves.
TABLE E12-6.2 EFFLUENT CONCENTRATIONS AND TEMPERATURES
SS |
T(K) |
CA (mol/dm3) |
CB (mol/dm3) |
CC (mol/dm3) |
|---|---|---|---|---|
| 1 | 310 | 0.284 | 0.016 | 0 |
| 2 | 363 | 0.189 | 0.111 | 0 |
| 3 | 449 | 0.033 | 0.267 | 0.00056 |
| 4 | 558 | 0.0041 | 0.167 | 0.129 |
| 5 | 677 | 0.00087 | 0.0053 | 0.294 |
Analysis: Wow! We see that five steady states (SS) exist!! The exit concentrations and temperatures listed in Table E12-6.2 were determined from the tabular output of the Polymath program. Steady states 1, 3, and 5 are stable steady states, while 2 and 4 are unstable. The selectivity at steady state 3 is , while at steady state 5 the selectivity is and is far too small. Consequently, to operate at a large selectivity we either have to operate at steady state 3 or find a different set of operating conditions. What do you think of the value of tau, that is, τ = 0.01 min? Is it a realistic number? (See Problem P12-1A (h).)
12.6.4 Complex Reactions in a PFR
Example 12-7 Complex Reactions with Heat Effects in a PFR
We will use the reactions that we discussed in Chapter 8 that were coded with dummy names, A, B, C, and D for reasons of national security.
The following complex gas-phase reactions follow elementary rate laws
and take place in a PFR. The feed is stoichiometric for reaction (1) in A and B with FA0 = 5 mol/min. The reactor volume is 10 dm3 and the total entering concentration is CT0 = 0.2 mol/dm3. The entering pressure is 100 atm and the entering temperature is 300 K. The coolant flow rate is 50 mol/min and the entering coolant fluid has a heat capacity of CPC0 = 10 cal/mol · K and enters at a temperature of 325 K.
Parameters
Plot FA, FB, FC, FD, p, T, and Ta as a function of V for
(a) Co-current heat exchange
(b) Countercurrent heat exchange
(c) Constant Ta
(d) Adiabatic operation
Solution
Gas Phase PFR No Pressure Drop (p = 1)
1. Mole Balances:

2. Rates:
2a. Rate Laws
2b. Relative Rates
2c. Net Rates of reaction for species A, B, C, and D are
3. Selectivity:
At V = 0, FD = 0 causing SC/D to go to infinity. Therefore, we set SC/D = 0 between V = 0 and a very small number, say, V = 0.0001 dm3 to prevent the ODE solver from crashing.
Major goal of CRE
4. Stoichiometry:
Neglect pressure drop
5. Parameters:
(24) CA0 = 0.2 mol/dm3
(25) R= 1.987 cal/mol/K
(26) E1 = 8,000 cal/mol
(27) E2 = 12,000 cal/mol
Other parameters are given in the problem statement, that is, Equations (28) to (34).
(28) CpA, (29) CpB, (30) Cpc, (31) , (32) ,
(33) , (34) CpC0
6. Energy Balance:
Recalling Equation (12-37)
The denominator of Equation (E12-7.35) is
The “heat removed” term is
The “heat generated” is
(a) Co-current heat exchange
The heat exchange balance for co-current exchange is
Part (a) Co-current flow: Plot and analyze the molar flow rates, and the reactor and coolant temperatures as a function of reactor volume. The Polymath code and output for co-current flow are shown in Table E12-7.1.
Co-current heat exchange

Solution
TABLE E12-7.1 POLYMATH PROGRAM AND OUTPUT FOR CO-CURRENT EXCHANGE
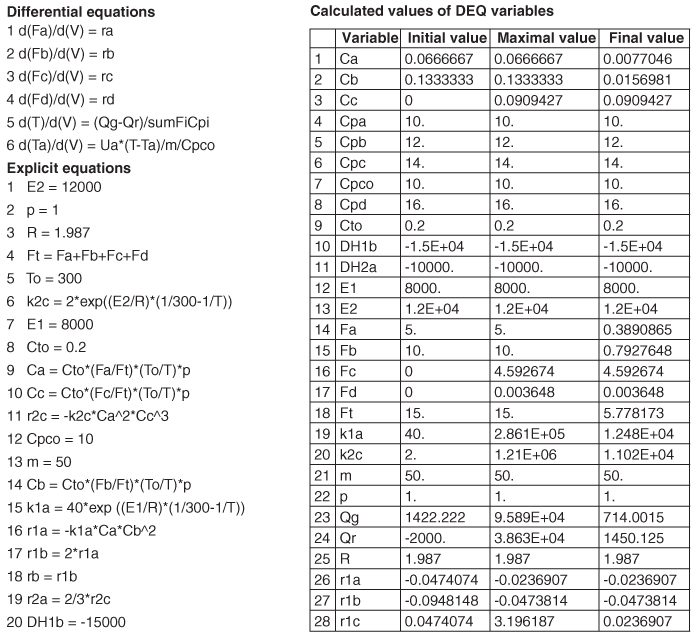


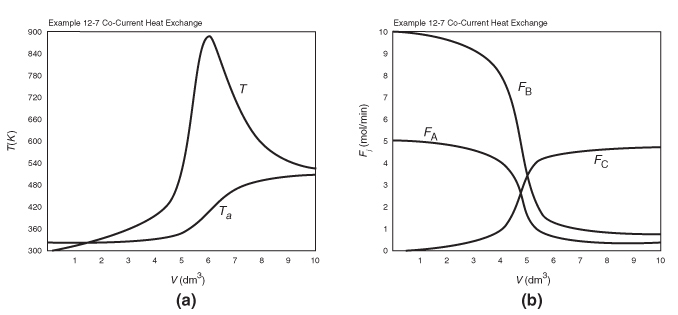
Figure E12-7.1 Profiles for co-current heat exchange; (a) temperature (b) molar flow rates. Note: The molar flow rate FD is very small and is essentially the same as the bottom axis.
The temperature and molar flow rate profiles are shown in Figure E12-7.1.
Analysis: Part (a): We also note that the reactor temperature, T, increases when Qg > Qr and reaches a maximum, T = 886 K, at approximately V = 6 dm3. After that, Qr > Qg the reactor temperature decreases and approaches Ta at the end of the reactor. For co-current heat exchange, the selectivity is really quite good.
Part (b) Countercurrent heat exchange: Solution: We will use the same program as part (a), but will change the sign of the heat-exchange balance and guess Ta at V = 0 to be 507 K and see whether the value gives us a Ta0 of 325 K at V = Vf.
We find our guess of 507 K matches Ta0 = 325 K. Are we lucky or what?!
The Polymath Program is shown in Table E12-7.2.
Countercurrent heat exchange
TABLE E12-7.2 POLYMATH PROGRAM AND OUTPUT FOR COUNTERCURRENT EXCHANGE
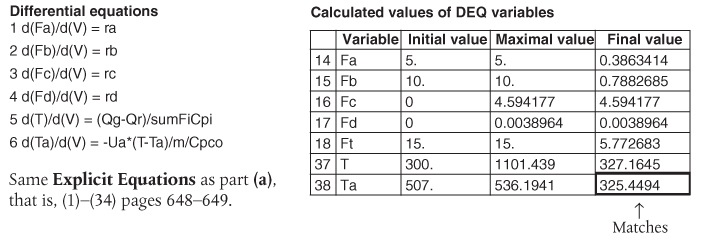

Figure E12-7.2 Profiles for countercurrent heat exchange; (a) temperature (b) molar flow rates.
Analysis: Part (b): For countercurrent exchange, the coolant temperature reaches a maximum at V = 1.3 dm3 while the reactor temperature reaches a maximum at V = 2.1 dm3. The reactor with a countercurrent exchanger reaches a maximum reactor temperature of 1100 K, which is greater than that for the co-current exchanger, (i.e., 930 K). Consequently, if there is a concern about additional side reactions occurring at this maximum temperature of 1100 K, one should use a co-current exchanger or if possible use a large coolant flow rate to maintain constant Ta in the exchanger. In Figure 12-7.2(a) we see that the reactor temperature approaches the coolant entrance temperature at the end of the reactor. The selectivity for the countercurrent systems, , is slightly lower than that for the co-current exchange.
Part (c) Constant Ta: Solution: To solve the case of constant heating-fluid temperature, we simply multiply the right-hand side of the heat-exchanger balance by zero, that is,
and use Equations (E12-7.1)–(E12-7.39). The Polymath Progam is shown in Table E12-7.3 and the temperature and molar flow rate profiles are shown in Figures E12-7.3(a) and E12-7.3(b), respectively.
Constant Ta
TABLE E12-7.3 POLYMATH PROGRAM AND OUTPUT FOR CONSTANT Ta

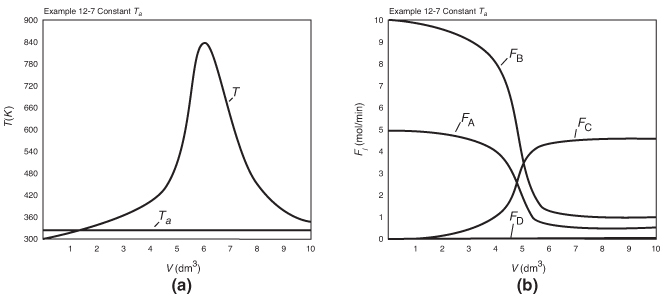
Figure E12-7.3 Profiles for constant Ta; (a) temperature (b) molar flow rates.
Analysis: Part (c): For constant Ta, the maximum reactor temperature, 837 K, is less than either co-current or countercurrent exchange while the selectivity, is greater than either co-current or countercurrent exchange. Consequently, one should investigate how to achieve sufficiently high mass flow of the coolant in order to maintain constant Ta.
Part (d) Adiabatic: To solve for the adiabatic case, we simply multiply the overall heat transfer coefficient by zero.
Ua = 80*0
The Polymath Program is shown in Table E12-7.4 while the temperature and molar flow rate profiles are shown in Figures E12-7.4(a) and E12-7.4(b), respectively.
Adiabatic operation
TABLE E12-7.4 POLYMATH PROGRAM AND OUTPUT FOR ADIABATIC OPERATION

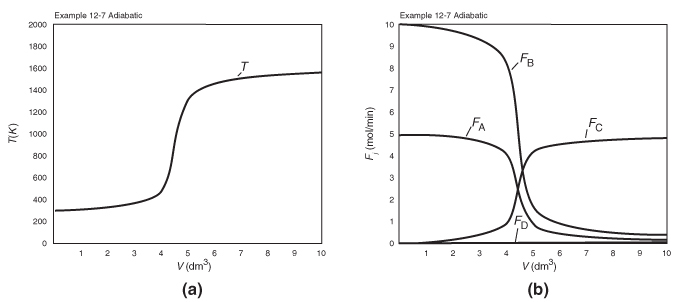
Figure E12-7.4 Profiles for adiabatic operation.
Analysis: Part (d): For the adiabatic case, the maximum temperature, which is the exit temperature, is higher than the other three exchange systems, and the selectivity is the lowest with . At this high temperature, the occurrence of unwanted side reactions certainly is a concern.
Overall Analysis Parts (a) to (d): Suppose the maximum temperature in each of these cases is outside the safety limit of 750 K for this system. Problem P12-1A (i) asks how you can keep the maximum temperature below 750 K.
12.7 Radial and Axial Temperature Variations in a Tubular Reactor
In the previous sections, we have assumed that there were no radial variations in velocity, concentration, temperature, or reaction rate in the tubular and packed-bed reactors so that the axial profiles could be determined using an ordinary differential equation (ODE) solver. However, we consider both radial and axial variations in our mole and energy balance, we obtain partial differential equations (PDEs), which cannot be solved with Polymath and Wolfram. Consequently, it was decided to move this discussion on radial variation to Chapter 18 where we use the PDE solver COMSOL to explore two-dimensional problems of this type.
12.8 And Now... A Word from Our Sponsor—Safety 12 (AWFOS–S12 Safety Statistics)†
† Also see http://umich.edu/~safeche.
12.8.1 The Process Safety Across the Chemical Engineering Curriculum Web site
The Process Safety Across the Chemical Engineering Curriculum Web site (http://umich.edu/~safeche/index.html) provides a safety module for every core course in chemical engineering. A module consists of viewing a Chemical Safety Board (CSB) video, carrying out a safety analysis of the incident and doing a calculation related to a specific course. The Web site includes tutorials on a number of safety algorithms such as the BowTie Diagram (Section 6.7), the NFPA Diamond (Section 2.7), and the process Safety Pyramid (Section 9.5). This Web site was developed by the author of this text and the tutorials are also given at the end of the various chapters in a section titled “A Word From Our Sponsor—Safety.” The CRE safety modules include the explosions at Monsanto, Synthron, and T2 Laboratories. The CRE and safety Web sites are open to all as they have no passwords or other to be accessed.
12.8.2 Safety Statistics
Scaling up exothermic chemical reactions can be very tricky. Tables 12-5 and 12-6 give reactions that have resulted in accidents and their causes, respectively.7 The reader should review the case histories of these reactions to learn how to avoid similar accidents.
7 Courtesy of J. Singh, Chemical Engineering, 92 (1997) and B. Venugopal, Chemical Engineering, 54 (2002).
TABLE 12-5 INCIDENCE OF BATCH PROCESS ACCIDENTS
| Process Type | Number of Incidents in United Kingdom, 1962–1987 |
|---|---|
Polymerization |
64 |
Nitration |
15 |
Sulfurization |
13 |
Hydrolysis |
10 |
Salt formation |
8 |
Halogenation |
8 |
Alkylation (Friedel-Crafts) |
5 |
Amination |
4 |
Diazolization |
4 |
Oxidation |
2 |
Esterification |
1 |
Total: |
134 |
Source: Courtesy of J. Singh, Chemical Engineering, 92 (1997).
TABLE 12-6 CAUSES OF BATCH REACTOR ACCIDENTS IN TABLE 12-5
| Cause | Contribution, % |
|---|---|
Lack of knowledge of reaction chemistry |
20 |
Problems with material quality |
9 |
Temperature-control problems |
19 |
Agitation problems |
10 |
Mis-charging of reactants or catalyst |
21 |
Poor maintenance |
15 |
Operator error |
5 |
Source: B. Venugopal, Chemical Engineering, 54 (2002).
Runaway reactions are the most dangerous in reactor operation, and a thorough understanding of how and when they could occur is part of the chemical reaction engineer’s responsibility. The reaction in Example 12-7 could be thought of as running away. Recall that as we moved down the length of the reactor, none of the cooling arrangements could keep the reactor from reaching an extremely high temperature (e.g., 800 K). In Chapter 13, we study case histories of two runaway reactions. One is the nitroaniline explosion discussed in Example E13-2 and the other is Example E13-6, concerning the explosion at T2 Laboratories. See Example 13-7 and videos on T2 Laboratories’ explosion and what caused it to happen (https://www.csb.gov/t2-laboratories-inc-reactive-chemical-explosion/ and https://www.youtube.com/watch?v=C561PCq5E1g).
There are many resources available for additional information on reactor safety and the management of chemical reactivity hazards. Guidelines for managing chemical reactivity hazards and other fire, explosion, and toxic release hazards are developed and published by the Center for Chemical Process Safety (CCPS) of the American Institute of Chemical Engineers. CCPS books and other resources are available at www.aiche.org/ccps. For example, the book Essential Practices for Managing Chemical Reactivity Hazards, written by a team of industry experts, is also provided free of charge by CCPS on the site at https://app.knovel.com/web/toc.v/cid:kpEPMCRH02/viewerType:toc/. A concise and easy-to-use software program that can be used to determine the reactivity of substances or mixtures of substances, the Chemical Reactivity Worksheet, is provided by the National Oceanic and Atmospheric Administration (NOAA) for free on its Web site, www.noaa.gov.
12.8.3 Additional Resources CCPS and SAChE
The Safety and Chemical Engineering Education (SAChE) program was formed in 1992 as a cooperative effort between the AIChE, CCPS, and engineering schools to provide teaching materials and programs that bring elements of process safety into the education of undergraduate and graduate students studying chemical and biochemical products and processes. The SAChE Web site (www.sache.org) has a great discussion of reactor safety with examples as well as information on reactive materials. These materials are also suitable for training purposes in an industrial setting.
The following instruction modules are available on the SAChE Web site (www.sache.org).
Chemical Reactivity Hazards: This Web-based instructional module contains about 100 Web pages with extensive links, graphics, videos, and supplemental slides. It can be used either for classroom presentation or as a self-paced tutorial. The module is designed to supplement a junior or senior chemical engineering course by showing how uncontrolled chemical reactions in industry can lead to serious harm, and by introducing key concepts for avoiding unintended reactions and controlling intended reactions.
Runaway Reactions: Experimental Characterization and Vent Sizing: This instruction module describes the ARSST and its operation, and illustrates how this instrument can easily be used to experimentally determine the transient characteristics of runaway reactions, and how the resulting data can be analyzed and used to size the relief vent for such systems. The theory along with an example behind the ARSST is given on the CRE Web site under Additional Material for Chapter 13 and the LEPs for Chapter 13, that is, PRS Example R13-1.
Rupture of a Nitroaniline Reactor: This case study demonstrates the concept of runaway reactions and how they are characterized and controlled to prevent major losses.
Seveso Accidental Release Case History: This presentation describes a widely discussed case history that illustrates how minor engineering errors can cause significant problems; problems that should not be repeated. The accident was in Seveso, Italy, in 1976. It was a small release of a dioxin that caused many serious injuries.
Note: Access is restricted to SAChE members and universities.
Membership in SAChE is required to view these materials. Virtually all U.S. universities and many non-U.S. universities are members of SAChE—contact your university SAChE representative, listed on the SAChE Web site, or your instructor or department chair to learn your university’s username and password. Companies can also become members—see the SAChE Web site for details.
Certificate Program
SAChE also offers several certificate programs that are available to all chemical engineering students. Students can study the material, take an online test, and receive a certificate of completion. The following two certificate programs are of value for reaction engineering:
Runaway Reactions: This certificate focuses on managing chemical reaction hazards, particularly runaway reactions.
Chemical Reactivity Hazards: This is a Web-based certificate that provides an overview of the basic understanding of chemical reactivity hazards.
Many students are taking the certificate test online and put the fact that they successfully obtained the certificate on their résumés.
More information on safety is given in the Summary Notes and Professional Reference Shelf on the Web. Particularly study the use of the ARSST to detect potential problems. These will be discussed in Chapter 13 Professional Reference Shelf R13.1 on the CRE Web site.
Summary
1. For single reactions, the energy balance on a PFR/PBR in terms of molar flow rate is
In terms of conversion
2. The temperature dependence of the specific reaction rate is given in the form
3. The temperature dependence of the equilibrium constant is given by van’t Hoff’s equation for ΔCp = 0
4. Neglecting changes in potential energy, kinetic energy, and viscous dissipation, and for the case of no work done on or by the system, large coolant flow rates (mc), and all species entering at the same temperature, the steady-state CSTR energy balance for single reactions is
5. Multiple steady states
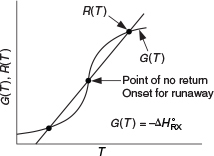
where
6. When q multiple reactions are taking place and there are m species
CRE Web Site Materials (http://www.umich.edu/~elements/6e/12chap/obj.html#/)
Interactive Computer Games that Talk to Each Other
Heat Effects I (http://www.umich.edu/~elements/6e/icm/heatfx1.html)
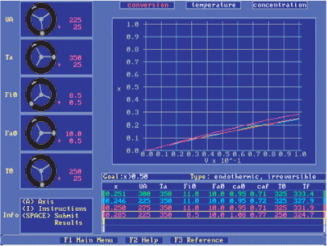
Heat Effects II (http://www.umich.edu/~elements/6e/icm/heatfx2.html)
The derivation of the user-friendly energy balances are color coded and derived in the tutorials in Heat Effects I and Heat Effects II. Here you see animation of the various terms as they move around the screen and talk to each other to arrive at the final form of the balance equation.

A step-by-step AspenTech tutorial is given on the CRE Web site.
See Example 12-2 (http://www.umich.edu/~elements/6e/software/aspen-example12-2.html). Formulated in AspenTech: Download AspenTech directly from the CRE Web site.
Questions, Simulations, and Problems
The subscript to each of the problem numbers indicates the level of difficulty: A, least difficult; D, most difficult.
In each of the questions and problems, rather than just drawing a box around your answer, write a sentence or two describing how you solved the problem, the assumptions you made, the reasonableness of your answer, what you learned, and any other facts that you want to include. See Preface Section G.2 for additional generic parts (x), (y), and (z) to the home problems.
Before solving the problems, state or sketch qualitatively the expected results or trends.
Questions
Q12-1A QBR (Question Before Reading). Before reading this chapter, identify the differences in applying the energy balance for co-current heat exchange and counter current heat exchange.
Q12-2A i>clicker. Go to the Web site (http://www.umich.edu/~elements/6e/12chap/iclicker_ch12_q1.html) and view five i>clicker questions. Choose one that could be used as is, or a variation thereof, to be included on the next exam. You also could consider the opposite case: explaining why the question should not be on the next exam. In either case, explain your reasoning.
Q12-3A Review Figure 12-13. Use this figure to write a few sentences (or at least draw an analogy) explaining why, when you strike the head of a safety match slowly on its pumice with little pressure, it may heat up a little, but does not ignite, yet when you put pressure on it and strike it rapidly, it does ignite. Thanks to Oscar Piedrahita, Medellín, Colombia.

Q12-4A Read over the problems at the end of this chapter. Make up an original problem that uses the concepts presented in this chapter. To obtain a solution:
(a) Make up your data and reaction.
(b) Use a real reaction and real data. See Problem P5-1A for guidelines.
(c) Prepare a list of safety considerations for designing and operating chemical reactors. (See www.sache.org.) The August 1985 issue of Chemical Engineering Progress may be useful for part (c).
Q12-5A Go to the LearnChemE screencast link for Chapter 12 (http://www.umich.edu/~elements/6e/12chap/learn-cheme-videos.html). View one or more of the screencast 5- to 6-minute videos and write a two-sentence evaluation. What would you suggest changing in the video to increase the comment from Recommended to Highly Recommended?
Q12-6A What did the section And Now… A Word From Our Sponsor point out or emphasize that was not done in the other AWFOS sections? What were the top two takeaway points in Chapter 12’s AWFOS?
Computer Simulations and Experiments
We will use the Living Example on the CRE Web site extensively to carry out simulations. Why carry out simulations to vary the parameters in the Living Example Problems? We do it in order to
Get a more intuitive feel reactor system.
Gain insight about the most sensitive parameters (e.g., E, KC) and how they affect outlet conditions.
Learn how reactors are affected by different operating conditions.
Simulate dangerous situations such as potential runaway reactions.
Compare the model and parameters with experimental data.
Optimize the reaction system.
P12-1B (a) LEP Table 12-2: Exothermic Reaction with Heat Exchange
Download the Polymath, MATLAB, Python, or Wolfram codes for the algorithm and data given in Table T12-2 for the exothermic gas phase reversible reaction
A + B ⇄ C
given on the Web in the Living Example Problems (LEPs).
Vary the following parameters in the ranges shown in parts (i) through (xi). Write a paragraph describing the trends you find for each parameter variation and why they look the way they do. Use the base case for parameters not varied. The feedback from students on this problem is that one should use Wolfram on the Web in the LEP T12-2 as much as possible to carry out the parameter variations. For each part, write two or more sentences describing the trends.
Wolfram and Python
(i) This is a Stop and Smell the Roses Simulation. View the base case X, Xe, and T profiles and explain why the conversion (X and Xe) and temperature profiles look the way they do.

(ii) The slider parameters Ua/rho, ΘI, FA0, and ΔHRx all can be moved to make the X and Xe profiles to come together and to separate. What is the reason these sliders affect the profiles similarly?
(iii) Starting with the base case, determine which parameters when changed only a small amount most dramatically affects the conversion and temperature profiles.
(iv) What parameters separate X and Xe the most?
(v) Finally, write at least three conclusions about what you found in your experiments (i) through (iv).
Polymath
(vi) Vary T0: 310 K ≤ T0 ≤ 350 K and write a conclusion.
(vii) Vary Ta: 300 K ≤ Ta ≤ 340 K and write a conclusion.
Repeat (i) for countercurrent coolant flow.
Hint: In analyzing the trends it might be helpful to plot X, Xe, and p as a function of W on the same graph and T and Ta as a function of W on the same graph.
(viii) Repeat this problem for the case of constant Ta and adiabatic operation, and describe the most dramatic affect you find.
(b) Example 12-1: Butane Isomerization
Wolfram and Python Co-Current
This is another Stop and Smell the Roses Simulation.
(i) Describe the differences of the X, Xe, and T profiles between the four cases of heat exchange, co-current, counter current, constant Ta, and adiabatic.

(ii) Explain why the temperature (T and Ta) and conversion (X and Xe) look the way they do, and explain what happens to the X, Xe, T, and Ta profiles as you move slider yA0.
(iii) What parameter value brings T and Ta profiles close together?
(iv) What parameter when varied separates X and Xe the most?
(v) What parameter keeps the X and Xe profiles the closest together?
(vi) Write at least three conclusions about what you found in your experiments (i) through (v).
Polymath Co-Current
(vii) What is the entering value of the temperature Ta0 of the heat exchanger fluid below which the reaction will never “ignite”?
(viii) Vary some of the other parameters and see whether you can find unsafe operating conditions.
(ix) Plot Qr and Ta as a function of V necessary to maintain isothermal operation.
Wolfram and Python Countercurrent
(x) Explain why T, Ta, X, and Xe profiles look the way they do for the base case.
(xi) Vary Ua and Ta0 and describe what you find.
(xii) Vary yA0 and then one of the other parameters and describe what you find.
(xiii) Describe how Qr and Qg and their intersection change when you vary the molar flow rates of coolant, inert and yA0.
(xiv) Write at least three conclusions about what you found in your experiments (x) through (xiii).
Polymath Countercurrent
(xv) Describe and explain what happens to the X and Xe profiles as you vary ΔHRx.
(xvi) Describe what happens to the temperature profile T and Ta as you vary FA0.
(xvii) Compare the variations in the profiles for X, Xe, T, and Ta when you change the parameter values for all four cases: adiabatic, countercurrent exchange, co-current exchange, and constant Ta. What parameters when changed only a small amount dramatically change the profiles?
(xviii) The heat exchanger is designed for a maximum temperature of 370 K. Which parameter will you vary so that at least 75% conversion is still achieved while maintaining temperature under a safe limit?
Wolfram and Python Constant Ta
(xix) Vary yA0 and one other parameter of your choice, and describe how the X, Xe, and T profiles change.
(xx) Vary Ua and Ta and describe what you find.
(xxi) Vary and then one of the other parameters (e.g., yA0) and describe what you find.
(xxii) Write at least three conclusions about what you found in your experiments (xix)–(xxi).
Wolfram and Python Adiabatic Operation
(xxiii) Why do the shape of the profiles change in the way they do as you change yA0?
(xxiv) Write a set of three conclusions, one of which compares adiabatic operation with the other three modes of heat transfer (e.g., co-current).
(c) Example 12-2: Production of Acetic Anhydride-Endothermic Reaction Wolfram and Python
Co-Current

(i) Explain why the temperatures (T and Ta) and conversions (X and Xe) look the way they do for the base case slider values.
(ii) What parameter value when increased or decreased causes the reaction to die out the most quickly near the reactor entrance?
(iii) Which parameter, when varied most, drastically changes the profiles?
Adiabatic
(iv) How does the conversion change as heat capacity of A is increased?
Constant Ta
(v) You find that conversion has decreased after 6 months of operation. You checked the flow rate and material properties and found that these values have not changed. Which parameter would have changed?
Countercurrent
(vi) Explain why the temperatures (T and Ta) and conversions (X and Xe) look the way they do for the base case slider values.
(vii) Discuss the most profound differences between the four heat exchange modes (e.g., co-current, adiabatic) for this endothermic reaction.
(viii) Write two conclusions of what you learned from your experiments (i) through (vii).
Polymath
(ix) Let Qg = rAΔHRx and Qr = Ua (T – Ta), and then plot Qg and Qr on the same figure as a function of V.
(x) Repeat (vi) for V = 5 m3.
(xi) Plot Qg, Qr, and –rA versus V for all four cases on the same figure and describe what you find.
(xii) For each of the four heat exchanger cases, investigate the addition of an inert I with a heat capacity of 50 J/mol · K, keeping FA0 constant and letting the other inlet conditions adjust accordingly (e.g., ε).
(xiii) Vary the inert molar flow rate (i.e., ΘI, 0.0 < ΘI < 3.0 mol/s). Plot X and analyze versus ΘI.
(xiv) Finally, vary the heat-exchange fluid temperature Ta0 (1000 K < Ta0 < 1350 K). Write a paragraph describing what you find, noting interesting profiles or results.
(d) Example 12-2: AspenTech Formulation. Go to the Aspen LEP and repeat Example 12-2 using AspenTech.
(e) Example 12-3: Production of Propylene Glycol in a CSTR
Wolfram and Python
(i) Vary activation energy (E) and find the values of E for which there are at least two solutions.
(ii) Vary the flow rate of FB0 to find the temperature at which the conversion is 0.8.
(iii) What is the operating range of inlet temperatures such that at least one steady state solution exists while maintaining the reactor temperature below 640°R? Describe how your answers would change if the molar flow rate of methanol were increased by a factor of 4.
(iv) Vary V and find the points of (1) tangency and (2) intersection of XEB and XMB.
(v) Write a set of conclusions based on your experiments (i) through (iv).
(f) Example 12-4: CSTR with a Cooling Coil
Wolfram and Python
(i) Explore how variations in the activation energy (E) and in the heat of reaction, at reference temperature (), affect the conversions XEB and XMB.
(ii) What variables most affect the intersection of and the points of tangency of XEB and XMB?
(iii) Find a value of E and of that increase conversion to more than 80% while maintaining the constraint of 125°F maximum temperature.
(iv) Write two conclusions about what you found in your experiments (i) through (iii).
Polymath
(v) The space time was calculated to be 0.01 minutes. Is this realistic? Decrease ν0 and both reaction rate constants by a factor of 100 and describe what you find.
(vi) Use Figure E12-3.2 and Equation (E12-4.4) to plot X versus T to find the new exit conversion and temperature.
(vii) Other data on the Jofostan chemical engineering Web site show Btu/lb-mol and ΔCP = 29 Btu/lb-mol/°F. How would these values change your results?
(viii) Make a plot of conversion as a function of heat exchanger area. [0 < A < 200 ft2] and write a conclusion.
(g) Example 12-5: Parallel Reactions in a PFR with Heat Effects
Wolfram and Python
(i) Vary the sliders for the rate constants k1A0 and k2A0 and describe what you find.
(ii) What should be the initial concentration of A so that the company can produce equal flow rates of B and C?
(iii) Describe how the profiles for T, FA, FB, and FC change as you move the sliders. Comment specifically on the drastic changes that occur when you change the overall heat transfer coefficient.
(iv) What set of conditions give you the greatest selectivity, at the exit and what is the corresponding conversion.
(v) Write at least three conclusions about what you found in your experiments (i) through (iv).
Polymath
(vi) Why is there a maximum in temperature? How would your results change if there is a -pressure drop with α = 1.05 dm–3?
(vii) What if the reaction is reversible with KC= 10 at 450 K?
(viii) How would the selectivity change if Ua is increased? Decreased?
(h) Example 12-6: Multiple Reactions in a CSTR (Use the LEP)
Wolfram and Python
(i) What is the minimum value of Ta that causes the reactor temperature to jump to the single, upper steady-state value?
(ii) What is the operating range for the entering temperature T0 for which there are only three steady states?
(iii) Vary UA between 10,000 and 80,000, and describe how the number of steady-state solutions changes.
(iv) Write a set of conclusions from carrying out experiments (i) through (iii).
(i) Example 12-7: Complex Reactions with Heat Effects in a PFR—Safety
Wolfram and Python
(i) Co-current: Explore the effect of the coolant flow rate on the reactant temperature and coolant temperature profiles, and describe what you find.
(ii) Co-current: Explore the effect of Ua on the selectivity and describe what you find.
(iii) Constant Ta: How does the overall heat transfer coefficient affect the product flow rate distribution? Should it be kept minimum or maximum? Explain.
(iv) Constant Ta: Which are the variables that have virtually no effect on the profiles? Explain the reason.
(v) Adiabatic: You want to save capital cost by using a smaller volume reactor, that is, 5 dm3 instead of 10 dm3. Which parameters will you vary to achieve exit reactant and product flow rates same as base case?
(vi) Describe how the selectivity, changes as you change the parameters and operating conditions. What two variable slider(s) affect the most?
(vii) Write a set of conclusions of what you learned in experiments (i) through (vi).
Polymath
(viii) Plot Qg and Qr as a function of V. How can you keep the maximum temperature below 700 K? Would adding inerts help, and if so what should the flow rate be if CPI = 10 cal/mol/K?
(ix) Look at the figures. What happened to species D? What conditions would you suggest to make more species D?
(x) Make a table of the temperature (e.g., maximum T, Ta) and molar flow rates at two or three volumes, comparing the different heat-exchanger operations.
(xi) Why do you think the molar flow rate of C does not go through a maximum? Vary some of the parameters to learn whether there are conditions where it goes through a maximum. Start by increasing FA0 by a factor of 5.
(xii) Include pressure in this problem. Vary the pressure-drop parameter (0 < αρb < 0.0999 dm–3) and describe what you find and write a conclusion.
(j) Review the steps and procedure by which we derived Equation (12-5) and then, by analogy, derive Equation (12-35).
(k) CRE Web site SO2 Example PRS-R12.4-1. Download the SO2 oxidation LEP R12-1. How would your results change if (1) the catalyst particle diameter were cut in half? (2) The pressure were doubled? At what particle size does pressure drop become important for the same catalyst weight, assuming the porosity doesn’t change? (3) You vary the initial temperature and the coolant temperature? Write a paragraph with at least two conclusions describing what you find.

(l) SAChE. Go to the SAChE Web site, www.sache.org. Your instructor or department chair should have the username and password to enter the SAChE Web site in order to obtain the modules with the problems. On the left-hand menu, select “SAChE Products.” Select the “All” tab and go to the module titled, “Safety, Health and the Environment” (S, H & E). The problems are for KINETICS (i.e., CRE). There are some example problems marked “K” and explanations in each of the above S, H & E selections. Solutions to the problems are in a different section of the site. Specifically look at: Loss of Cooling Water (K-1), Runaway Reactions (HT-1), Design of Relief Values (D-2), Temperature Control and Runaway (K-4) and (K-5), and Runaway and the Critical Temperature Region (K-7). Go through the K problems and write a paragraph on what you have learned.
P12-2B (a) Example 12-2 Production of Acetic Anhydride Endothermic Reaction
Case 1 Adiabatic
(i) Vary inlet flow rate of inert and observe the conversion and temperature profiles. Describe what you find.
(ii) Find inlet temperature, T0, for which the reaction rate at V = 0.001 m3 is 0.1 times the rate at inlet. Note the conversion at the reactor exit for this temperature.
(iii) After varying two other parameters, write a set of conclusions from your experiments in (i) and (ii).
Case 2 Constant Ta
(iv) To reduce operating cost, the inlet temperature of heating fluid can be reduced. Can you find a minimum temperature of heating fluid at which exit conversion is virtually 100% for constant Ta.
(v) Find maximum value of the inlet concentration for which the conversion at exit is virtually 100% constant Ta.
(vi) Write a set of conclusions from your experiments in (iv) and (v).
P12-3B OEQ (Old Exam Question). Computer Experiments on a PFR for Reaction in Table 12-2.2. In order to develop a greater understanding of the temperature effects in PBRs, download the Living Example Problem Table 12-2, LEP T12-2, from the Web site to learn how changing the different parameters changes the conversion and temperature profiles.
This is a Sherlock Holmes Problem. Starting with the base case, one, and only one, parameter was varied between its maximum and minimum values to give the following figures. You need to decide what operating variable (e.g., T0) property value (e.g., CPA), or engineering variable (e.g., ) was varied. Choose from the following:
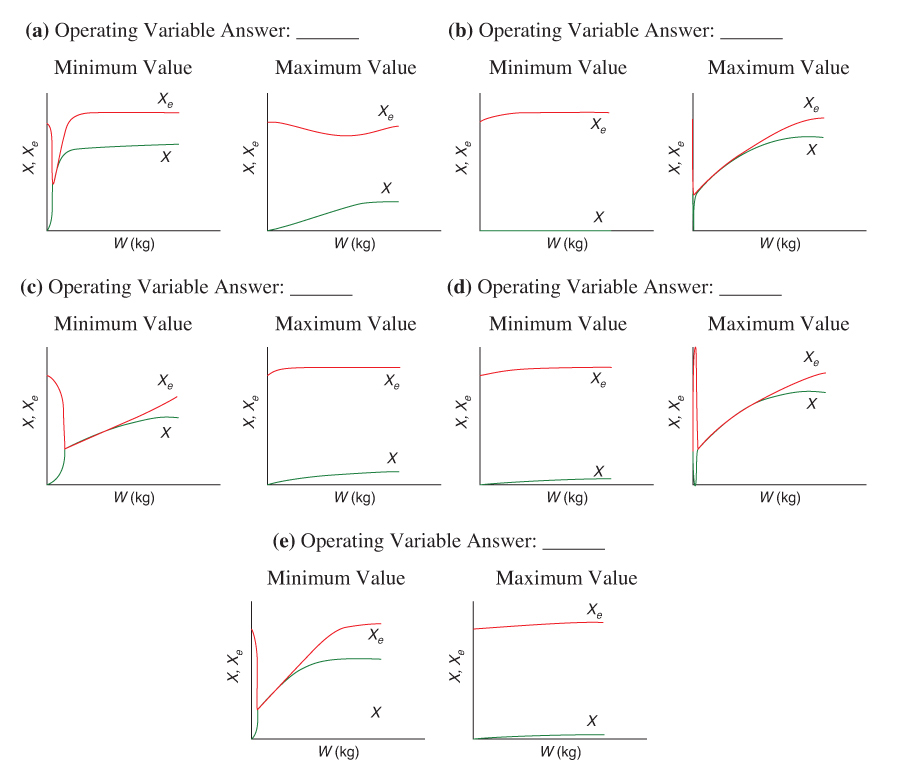
Interactive Computer Games
P12-4A Download the Interactive Computer Games (ICG) from the CRE Web site. Play the game, and then record your performance number for the module, which indicates your mastery of the material. Note: For simulation (b), only do the first three reactors, as reactors 4 and above do not work because of the technician tinkering with them.

(a) ICG Heat Effects Basketball 1 Performance # ________________.
(b) ICG Heat Effects Simulation 2 Performance # ________________.
Before attempting to play the ICG/Interactive Computer Games simulations, be sure to first go through the reviews to see the equations talking to each other.

Problems
P12-5C OEQ (Old Exam Question). Safety Problem. The following is an excerpt from The Morning News, Wilmington, Delaware (August 3, 1977): “Investigators sift through the debris from blast in quest for the cause [that destroyed the new nitrous oxide plant]. A company spokesman said it appears more likely that the [fatal] blast was caused by another gas—ammonium nitrate—used to produce nitrous oxide.” An 83% (wt) ammonium nitrate and 17% water solution is fed at 200°F to the CSTR operated at a temperature of about 510°F. Molten ammonium nitrate decomposes directly to produce gaseous nitrous oxide and steam. It is believed that pressure fluctuations were observed in the system and, as a result, the molten ammonium nitrate feed to the reactor may have been shut off approximately 4 min prior to the explosion.
Assume that at the time the feed to the CSTR stopped, there was 500 lbm of ammonium nitrate in the reactor. The conversion in the reactor is believed to be virtually complete at about 99.99%.
Additional information (approximate but close to the real case):
where M is the mass of ammonium nitrate in the CSTR (lbm) and k is given by the relationship below.
T (°F) |
510 |
560 |
k (h-1) |
0.307 |
2.912 |
The enthalpies of water and steam are
(a) Can you explain the cause of the blast? Hint: See Problem P13-3B.
(b) If the feed rate to the reactor just before shutoff was 310 lbm of solution per hour, what was the exact temperature in the reactor just prior to shutdown? Hint: Plot Qr and Qg as a function of temperature on the same plot.
(c) How would you start up or shut down and control such a reaction? Hint: See Problem P13-2B.
(d) Explore this problem and describe what you find. For example, add a heat exchanger UA (T – Ta), choose values of UA and Ta, and then plot R(T) versus G(T)?
(e) Discuss what you believe to be the point of the problem. The idea for this problem originated from an article by Ben Horowitz.
P12-6B OEQ (Old Exam Question)—Taken from California Professional Engineer’s Exam. The endothermic liquid-phase elementary reaction
A + B → 2C
proceeds, substantially, to completion in a single steam-jacketed, continuous-stirred reactor (Table P12-6B). From the following data, calculate the steady-state reactor temperature:
Reactor volume: 125 gal
Steam jacket area: 10 ft2
Jacket steam: 150 psig (365.9°F saturation temperature)
Overall heat-transfer coefficient of jacket, U: 150 Btu/h · ft2 · °F
Agitator shaft horsepower: 25 hp
Heat of reaction, Btu/lb-mol of A (independent of temperature)
TABLE P12-6B FEED CONDITIONS AND PROPERTIES
Component |
|||
A |
B |
C |
|
Feed (lb-mol/hr) |
10.0 |
10.0 |
0 |
Feed temperature (°F) |
80 |
80 |
— |
Specific heat (Btu/lb-mol·°F)* |
51.0 |
44.0 |
47.5 |
Molecular weight |
128 |
94 |
111 |
Density (lbm/ft3) |
63.0 |
67.2 |
65.0 |
* Independent of temperature. (Ans: T = 199°F)
(Courtesy of the California Board of Registration for Professional & land surveyors.)
P12-7B OEQ (Old Exam Question). Use the data in Problem P11-4A for the following reaction. The elementary, irreversible, organic liquid-phase reaction
A + B → C
is carried out in a flow reactor. An equal molar feed in A and B enters at 27°C, and the volumetric flow rate is 2 dm3/s and CA0 = 0.1 kmol/m3.
Additional information:
(a) Calculate the conversion when the reaction is carried out adiabatically in one 500-dm3 CSTR and then compare the results with the two adiabatic 250-dm3 CSTRs in series.
The reversible reaction (part (d) of Problem P11-4A) is now carried out in a PFR with a heat exchanger. Plot and then analyze X, Xe, T, Ta, Qr, Qg, and the rate, –rA, for the following cases:
(b) Constant heat-exchanger temperature Ta
(c) Co-current heat exchanger Ta (Ans: At V = 10 m3 then X = 0.36 and T = 442 K)
(d) Countercurrent heat exchanger Ta (Ans: At V = 10 m3 then X = 0.364 and T = 450 K)
(e) Adiabatic operation
(f) Make a table comparing all your results (e.g., X, Xe, T, Ta). Write a paragraph describing what you find.
(g) Plot Qr and Ta as a function of V necessary to maintain isothermal operation.
P12-8A The gas-phase reversible reaction as discussed in Problem P11-7B
A ⇄ B
is now carried out under high pressure in a packed-bed reactor with pressure drop. The feed consists of both inerts I and species A with the ratio of inerts to the species A being 2 to 1. The entering molar flow rate of A is 5 mol/min at a temperature of 300 K and a concentration of 2 mol/dm3. Work this problem in terms of volume. Hint:
Additional information:
Plot and then analyze X, Xe, T, Ta, and the rate (–rA) profiles in a PFR for the following cases. In each case, explain why the curves look the way they do.
(a) Co-current heat exchange
(b) Countercurrent heat exchange (Ans: When V = 20 dm3 then X = 0.86 and Xe = 0.94)
(c) Constant heat-exchanger temperature Ta
(d) Compare and contrast each of the above results and the results for adiabatic operation (e.g., make a plot or a table of X and Xe obtained in each case).
(e) Vary some of the parameters, for example, (0 < ΘI < 10) and describe what you find.
(f) Plot Qr and Ta as a function of V necessary to maintain isothermal operation.
P12-9A Algorithm for reaction in a PBR with heat effects and pressure drop
The elementary gas-phase reaction
A + B ⇄ 2C
in Problem P11-8B is now continued and carried out in packed-bed reactor. The entering molar flow rates are FA0 = 5 mol/s, FB0 = 2FA0, and FI = 2FA0 with CA0 = 0.2 mol/dm3. The entering temperature is 325 K and a coolant fluid is available at 300 K.
Additional information:
Plot X, Xe, T, Ta, and –rA down the length of the PFR for the following cases:
(a) Co-current heat exchange
(b) Countercurrent heat exchange
(c) Constant heat-exchanger temperature Ta
(d) Compare and contrast your results for (a), (b), and (c) along with those for adiabatic operation and write a paragraph describing what you find.
P12-10B Use the data and reaction in Problems P11-4A and P12-7B for the following reaction:
A+B → C+D
(a) Plot and then analyze the conversion, Qr, Qg, and temperature profiles up to a PFR reactor volume of 10 dm3 for the case when the reaction is reversible with KC = 10 m3/kmol at 450 K. Plot and then analyze the equilibrium conversion profile.
(b) Repeat (a) when a heat exchanger is added, Ua = 20 cal/m3/s/K, and the coolant temperature is constant at Ta = 450 K.
(c) Repeat (b) for both a co-current and a countercurrent heat exchanger. The coolant flow rate is 50 g/s, CPc = 1 cal/g · K, and the inlet coolant temperature is Ta0 = 450 K. Vary the coolant rate .
(d) Plot Qr and Ta as a function of V necessary to maintain isothermal operation.
(e) Compare your answers to (a) through (d) and describe what you find. What generalizations can you make?
(f) Repeat (c) and (d) when the reaction is irreversible but endothermic with . Choose Ta0 = 450 K.
P12-11B OEQ (Old Exam Question). Use the reaction data in Problems P11-4A and P12-7B for the case when heat is removed by a heat exchanger jacketing the reactor. The flow rate of coolant through the jacket is sufficiently high that the ambient exchanger temperature is constant at Ta = 50°C.
A+B → C
(a) (1) Plot and then analyze the temperature conversion, Qr, and Qg profiles for a PBR with where
where
ρb = bulk density of the catalyst (kg/m3)
a = heat-exchange area per unit volume of reactor (m2/m3)
U = overall heat transfer coefficient (J/s · m2 · K)
(2) How would the profiles change if Ua/=b were increased by a factor of 3000?
(3) If there is a pressure drop with α = 0.019 kg–1?
(b) Repeat part (a) for co-current and countercurrent flow and adiabatic operation with , CPc = 5000 J/kg K and an entering coolant temperature of 50°C.
(c) Find X and T for a “fluidized” CSTR with 80 kg of catalyst.
(d) Repeat parts (a) and (b) for W = 80.0 kg, assuming a reversible reaction with a reverse specific reaction rate of
Vary the entering temperature, T0, and describe what you find.
(e) Use or modify the data in this problem to suggest another question or calculation. Explain why your question requires either critical thinking or creative thinking. See Preface Section G and http://www.umich.edu/~scps.
P12-12C Derive the energy balance for a packed-bed membrane reactor. Apply the balance to the reaction in Problem P11-5A
A ⇄ B + C
for the case when it is reversible with KC = 1.0 mol/dm3 at 300 K. Species C diffuses out of the membrane with kC = 1.5 s–1.
(a) Plot and then analyze the concentration profiles for different values of KC when the reaction is carried out adiabatically.
(b) Repeat part (a) when the heat transfer coefficient is Ua = 30 J/s·kg-cat·K with Ta = 50°C.
P12-13B OEQ (Old Exam Question). Circle the correct answer.
(a) The elementary reversible isomerization of A to B was carried out in a packed-bed reactor. The following profiles were obtained:
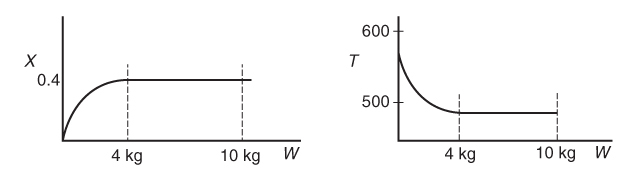
If the total entering volumetric flow rate remains constant, the addition of inerts to the feed stream will most likely
A) Increase conversion.
B) Decrease conversion.
C) Have no effect.
D) Insufficient information to tell
(b)
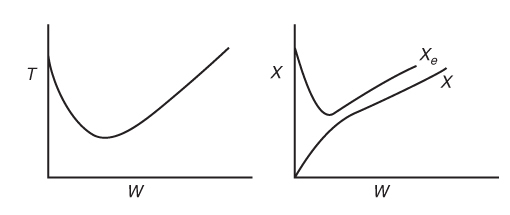
Which of the following statements are true?
A) The above reaction could be adiabatic.
B) The above reaction could be exothermic with constant cooling temperature.
C) The above reaction could be endothermic with constant heating temperature.
D) The above reaction could be second order.
(c) The conversion is shown below as a function of catalyst weight down a PBR.
Which of the following statements are false?
A) The reaction could be first-order endothermic and carried out adiabatically.
B) The reaction could be first-order endothermic and reactor is heated along the length with Ta being constant.
C) The reaction could be second-order exothermic and cooled along the length of the reactor with Ta being constant.
D) The reaction could be second-order exothermic and carried out adiabatically.
E) The reaction could be irreversible.
To view more conceptual problems similar to (a)–(c) above go to http://www.umich.edu/~elements/6e/12chap/iclicker_ch12_q1.html.
P12-14A OEQ (Old Exam Question). The irreversible reaction
A+B → C+D
is carried out adiabatically in a CSTR. The “heat generated” G(T) and the “heat removed” R(T) curves are shown in Figure P12-14A.

Figure P12-14A Heat removed R(T) and heat “generated” G(T) curves.
(a) What is the ΔHRx of the reaction?
(b) What are the inlet ignition and extinction temperatures?
(c) What are all the temperatures in the reactor corresponding to the inlet ignition and extinction temperatures?
(d) What are the conversions at the ignition and extinction temperatures?
P12-15B The first-order, irreversible, exothermic liquid-phase reaction
A → B
is to be carried out in a jacketed CSTR. Species A and an inert I are fed to the reactor in equimolar amounts. The molar feed rate of A is 80 mol/min.
Additional information:
(a) What is the reactor temperature for a feed temperature of 450 K?
(b) Plot and then analyze the reactor temperature as a function of the feed temperature.
(c) To what inlet temperature must the fluid be preheated for the reactor to operate at a high conversion? What are the corresponding temperature and conversion of the fluid in the CSTR at this inlet temperature?
(d) Suppose that the fluid inlet temperature is now heated 5°C above the reactor temperature in part (c) and then cooled 20°C, where it remains. What will be the conversion?
(e) What is the inlet extinction temperature for this reaction system? (Ans: T0 = 87°C)
P12-16B The elementary reversible liquid-phase reaction

takes place in a CSTR with a heat exchanger. Pure A enters the reactor.
(a) Derive an expression (or set of expressions) to calculate G(T) as a function of the heat of reaction, equilibrium constant, temperature, and so on. Show a sample calculation for G(T) at T = 400 K.
(b) What are the steady-state temperatures? (Ans: 310, 377, 418 K)
(c) Which steady states are locally stable?
(d) What is the conversion corresponding to the upper steady state?
(e) Vary the ambient temperature Ta and make a plot of the reactor temperature as a function of Ta, identifying the ignition and extinction temperatures.
(f) If the heat exchanger in the reactor suddenly fails (i.e., UA = 0), what would be the conversion and the reactor temperature when the new upper steady state is reached? (Ans: 431 K)
(g) What heat exchanger product, UA, will give the maximum conversion?
(h) Write a question that requires critical thinking and then explain why your question requires critical thinking. Hint: See Preface Section G.
(i) What is the adiabatic blowout flow rate, υ0?
(j) Suppose that you want to operate at the lower steady state. What parameter values would you suggest to prevent runaway, for example, the upper SS?
Additional information:
P12-17B OEQ (Old Exam Question). The reversible liquid-phase reaction
A ⇄ B
is carried out in a 12-dm3 CSTR with heat exchange. Both the entering temperature, T0, and the heat exchange fluid, Ta, are at 330 K. An equal molar mixture of inerts and A enter the reactor.
(a) Choose a temperature, T, and carry out a calculation to find G(T) to show that your calculation agrees with the corresponding G(T) value on the curve shown in Figure P12-17B at the temperature you choose.
(b) Find the exit conversion and temperature from the CSTR. X = _____ T = _____ Answers
(c) What entering temperature T0 would give you the maximum conversion? T0 = _____ X = _____
(d) What would the exit conversion and temperature be if the heat-exchange system failed (i.e., U = 0)?
(e) Can you find the inlet ignition and extinction temperatures? If yes, what are they? If not, go on to the next problem.
(f) Use Preface Section G to ask another question.
Additional information:
The G(T) curve for this reaction is shown in Figure P12-17B.
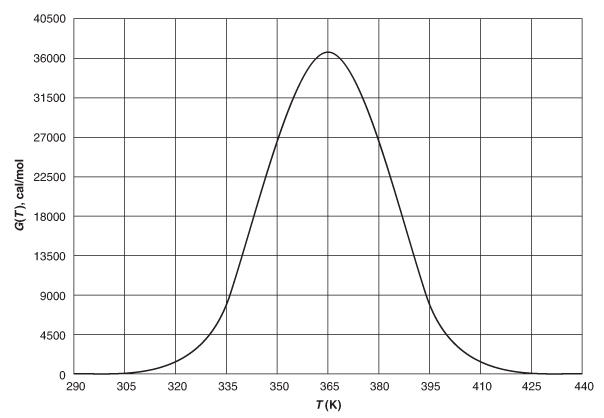
Figure P12-17B Heat removed curve, G(T), for a reversible reaction.
P12-18C The elementary gas-phase reaction
2A ⇄ C
is carried out in a packed-bed reactor. Pure A enters the reactor at a 450-K flow rate of 10 mol/s, and a concentration of 0.25 mol/dm3. The PBR contains 90 kg of catalyst and is surrounded by a heat exchanger for which cooling fluid is available at 500 K. Compare the conversion achieved for the four types of heat exchanger operation: adiabatic, constant Ta, co-current flow, and countercurrent flow.
Additional information:
P12-19C A reaction is to be carried out in the packed-bed reactor shown in Figure P12-19C.

Figure P12-19C PFR with heat exchange.
The reactants enter the annular space between an outer insulated tube and an inner tube containing the catalyst. No reaction takes place in the annular region. Heat transfer between the gas in this packed-bed reactor and the gas flowing countercurrently in the annular space occurs along the length of the reactor. The overall heat-transfer coefficient is 5 W/m2 · K. Plot the conversion and temperature as a function of reactor length for the data given in Problem P12-7B.
P12-20B OEQ (Old Exam Question). The reaction
is carried out in a packed-bed reactor. Match the following temperature and conversion profiles for the four different heat-exchange cases: adiabatic, constant Ta, co-current exchange, and countercurrent exchange.
(a) Figure 1 matches Figure ___
(b) Figure 2 matches Figure ___
(c) Figure 3 matches Figure ___
(d) Figure 4 matches Figure ___
P12-21B OEQ (Old Exam Question). Also Hall of Fame Problem. The irreversible liquid-phase reactions
are carried out in a PFR with heat exchange. The temperature profiles shown in Figure P12-21B were obtained for the reactor and the coolant stream:
Figure P12-21B Reactant temperature T and coolant temperature Ta profiles.
The concentrations of A, B, C, and D were measured at the point down the reactor where the liquid temperature, T, reached a maximum, and they were found to be CA = 0.1, CB = 0.2, CC = 0.5, and CD = 1.5, all in mol/dm3. The product of the overall heat transfer coefficient and the heat-exchanger area per unit volume, Ua, is 10 cal/s · dm3 · K. The entering molar flow rate of A is 10 mol/s.
Additional information:
(a) What is the activation energy for Reaction (1)?
P12-22B The following elementary reactions are to be carried out in a PFR with a heat exchange with constant Ta:
The reactants all enter at 400 K. Only A and B enter the reactor. The entering concentration of A and B are 3 molar and 1 molar at a volumetric flow rate of 10 dm3/s.
Additional information:
What constant coolant temperature, Ta, is necessary such that at the reactor entrance, that is, V = 0, ?
P12-23B The complex gas-phase reactions are elementary
and carried out in a PFR with a heat exchanger. Pure A enters at a rate of 5 mol/min, a concentration of 0.2 mol/dm3, and temperature 300 K. The entering temperature of an available coolant is 320 K.
Additional information:
k1A1 = 50 dm3/mol · min@305 K with E1 = 8000 J/mol
k2C2 = 4000 dm9/mol3 · min@310 K with E2 = 4000 J/mol
KCA = 10 dm3/mol@315 K
Note: This is the equilibrium constant with respect to A in reaction 1 when using van’t Hoff’s equation, that is, (S12-4),
The reactor volume is 10 dm3.
(a) Plot (FA, FB, FC) on one graph and (T and Ta) on another along the length of the reactor for adiabatic operation, heat exchange with constant Ta, and co-current and countercurrent heat exchange with variable Ta. Only turn in a copy of your code and output for co-current exchange.
Adiabatic operation
(b) What is the maximum temperature and at what reactor volume is it reached?
(c) At what reactor volume is the flow rate of B a maximum, and what is FBmax at this value?
Constant Ta
(d) What is the maximum temperature and at what reactor volume is it reached?
(e) At which reactor volume is the flow rate of B a maximum, and what is FBmax at this volume?
Co-current exchange
(f) At what reactor volume does Ta become greater than T? Why does it become greater?
Countercurrent exchange
(g) At what reactor volume does Ta become greater than T?
Hint: Guess Ta at entrance around 350 K.
P12-24B The elementary liquid-phase reactions
(1) A+ 2B → 2C
(2) A+C→ 2D
are carried out adiabatically in a 10 dm3 PFR. After streams A and B mix, species A enters the reactor at a concentration of CA0 = 2 mol/dm3 and species B at a concentration of 4 mol/dm3. The entering volumetric flow rate is 10 dm3/s.
Assuming you could vary the entering temperature between 300 K and 600 K, what entering temperture would you recommend to maximize the concentration of species C exiting the reactor? (±25°K). Assume all species have the same density.
Additional information:
P12-25C OTHEQ (Old Take Home Exam Question). Multiple reactions with heat effects. Xylene has three major isomers, m-xylene (A), o-xylene (B), and p-xylene (C). When m-xylene (A) is passed over a Cryotite catalyst, the following elementary reactions are observed. The reaction to form p-xylene is irreversible:
The feed to the reactor is pure m-xylene (A). For a total feed rate of 2 mol/min and the reaction conditions below, plot the temperature and the molar flow rates of each species as a function of catalyst weight up to a weight of 100 kg.
(a) Plot the concentrations of each of xylenes down the length (i.e., V) of a PBR.
(b) Find the lowest concentration of o-xylene achieved in the reactor.
(c) Find the maximum concentration of o-xylene in the reactor.
(d) Repeat part (a) for a pure feed of o-xylene (B). What is the maximum concentration of meta-xylene and where does it occur in the reactor?
(e) Vary some of the system parameters and describe what you learn.
(f) What do you believe to be the point of this problem?
Additional information8
8 Obtained from inviscid pericosity measurements.
All heat capacities are virtually the same at 100 J/mol · K.
P12-26C OTHEQ (Old Take Home Exam Question). Comprehensive problem on multiple reactions with heat effects. Styrene can be produced from ethylbenzene by the following reaction:
However, several irreversible side reactions also occur:
(J. Snyder and B. Subramaniam, Chem. Eng. Sci., 49, 5585 (1994)). Ethylbenzene is fed at a rate of 0.00344 kmol/s to a 10.0-m3 PFR (PBR), along with inert steam at a total pressure of 2.4 atm. The steam/ethylbenzene molar ratio is initially, that is, parts (a) to (c), 14.5:1 but can be varied.
Given the following data, find the exiting molar flow rates of styrene, benzene, and toluene along with for the following inlet temperatures when the reactor is operated adiabatically:

(a) T0 = 800 K
(b) T0 = 930 K
(c) T0 = 1100 K
(d) Find the ideal inlet temperature for the production of styrene for a steam/ethylbenzene ratio of 58:1. Hint: Plot the molar flow rate of styrene versus T0. Explain why your curve looks the way it does.
(e) Find the ideal steam/ethylbenzene ratio for the production of styrene at 900 K. Hint: See part (d).
(f) It is proposed to add a countercurrent heat exchanger with Ua = 100 kJ/m3/min/K, where Ta is virtually constant at 1000 K. For an entering stream to ethylbenzene ratio of 20, what would you suggest as an entering temperature? Plot the molar flow rates and .
(g) What do you believe to be the major points of this problem?
(h) Ask another question or suggest another calculation that can be made for this problem.
Note: Whenever I teach Chemical Reaction Engineering, I always assign this problem.
Additional information
Heat capacities |
|
Methane |
68 J/mol · K |
Ethylene |
90 J/mol·K |
Benzene |
201 J/mol · K |
Toluene |
249 J/mol · K |
Styrene |
273 J/mol · K |
Ethylbenzene |
299 J/mol·K |
Hydrogen |
30 J/mol · K |
Steam |
40 J/mol · K |
The kinetic rate laws for the formation of styrene (St), benzene (B), and toluene (T), respectively, are as follows (EB = ethylbenzene).
The temperature T is in Kelvin and Pi is in atm.
P12-27B OTHEQ (Old Take Home Exam Question). The liquid-phase, dimer-quadmer series addition reaction
4 A → 2A2 → A4
can be written as
and is carried out in a 10-dm3 PFR. The mass flow rate through the heat exchanger surrounding the reactor is sufficiently large so that the ambient temperature of the exchanger is constant at Ta = 315 K. The reactants enter at a temperature T0, of 300 K. Pure A is fed to the rector at a volumetric flow rate of 50 dm3/s and a concentration of 2 mol/dm3.
(a) Plot, compare, and analyze the profiles FA, FA2, and FA4 down the length of the reactor up to 10 dm3.
(b) The desired product is A2 and it has been suggested that the current reactor may be too large. What reactor volume would you recommend to maximize FA2?
(c) What operating variables (e.g., T0, Ta) would you change and how would you change them to make the reactor volume as small as possible and to still maximize FA2? Note any opposing factors in maximum production of A2. The ambient temperature and the inlet temperature must be kept between 0°C and 177°C.
Additional information:
Turn in your recommendation of reactor volume to maximize and the molar flow rate at this maximum.
Supplementary Reading
1. An excellent development of the energy balance is presented in
R. ARIS, Elementary Chemical Reactor Analysis. Upper Saddle River, NJ: Prentice Hall, 1969, Chaps. 3 and 6. JOSEPH FOGLER, A Reaction Engineer’s Handbook of Thermochemical Data. Riça, Jofostan: Jofostan Press, (2025).
2. Safety
CENTER FOR CHEMICAL PROCESS SAFETY (CCPS), Guidelines for Chemical Reactivity Evaluation and Application to Process Design. New York: American Institute of Chemical Engineers (AIChE), 1995.
DANIEL A. CROWL and JOSEPH F. LOUVAR, Chemical Process Safety: Fundamentals with Applications, 3rd ed. Upper Saddle River, NJ: Prentice Hall, 2011.
G. A. MELHEM and H. G. FISHER, International Symposium on Runaway Reactions and Pressure Relief Design. New York: Center for Chemical Process Safety (CCPS) of the American Institute of Chemical Engineers (AIChE) and The Institution of Chemical Engineers, 1995.
See the Center for Chemical Process Safety (CCPS) Web site, www.aiche.org/ccps.
3. A number of example problems dealing with nonisothermal reactors may or may not be found in
THORNTON W. BURGESS, The Adventures of Jerry Muskrat. New York: Dover Publications, Inc., 1914.
G. F. FROMENT and K. B. BISCHOFF, Chemical Reactor Analysis and Design, 3rd ed. New York: Wiley, 2010.
S. M. WALAS, Chemical Reaction Engineering Handbook of Solved Problems. Amsterdam: Gordon and Breach, 1995. See the following solved problems: Problem 4.10.1, page 444; Problem 4.10.08, page 450; Problem 4.10.09, page 451; Problem 4.10.13, page 454; Problem 4.11.02, page 456; Problem 4.11.09, page 462; Problem 4.11.03, page 459; Problem 4.10.11, page 463.
4. A review of the multiplicity of the steady state and reactor stability is discussed by
D. D. PERLMUTTER, Stability of Chemical Reactors. Upper Saddle River, NJ: Prentice Hall, 1972.
5. The heats of formation, (Hi T), Gibbs free energies, (Gi TR), and the heat capacities of various compounds can be found in
DON W. GREEN and ROBERT H. PERRY, Perry’s Chemical Engineers’ Handbook, 8th ed. (Chemical Engineers Handbook). New York: McGraw-Hill, 2008.
DAVID R. LIDE, CRC Handbook of Chemistry and Physics, 90th ed. Boca Raton, FL: CRC Press, 2009.