Environmental health and safety in buildings
Abstract:
A surprising range of environmental hazards are present in commercial and residential interiors. These range from issues of fire safety and the problems of combustible materials through to the hazards created by indoor air pollution. Decreased ventilation and the widening variety of indoor materials have enhanced the number of compounds to which we are exposed to indoors. Sick building syndrome and respiratory illnesses have been attributed to these exposures, so the management of occupied spaces has been an increased focus of regulation.
6.1 Introduction
The question of dangers in occupied spaces can seem somewhat surprising, especially when they refer to our homes which are familiar environments. Typically these are spaces we retire to to escape the noise and bustle of the outside world. They are environments where we usually feel in control, yet these have a range of problems in the modern world, that has made them an increasing area of study and even regulatory concern. The very notion of regulation raises issues of personal freedom and the problem is often blurred as it can be unclear which government agency is appropriate for indoor spaces. Indoor air, for example, is often not treated by the same government agency as outdoor air, thus departments of the environment are frequently replaced by departments of health, housing, safety or industry. The heterogeneous nature of the indoor environment has added to the difficulties of monitoring and regulating these volumes. Some issues have been rather mechanical and easy to approach, such as the flammability of indoor materials, where regulation is widespread, while others such as sick building syndrome, where the sociological context becomes an important factor, remain problematic.
Hazards in the residential environment have frequently been linked with adverse health outcomes, especially among children (Klitzman et al. 2005). These include: the problems of lead-based paint hazards that can be associated with elevated blood lead levels, illnesses that arise from dampness and fungi and the microbial volatile organic compounds (MVOCs), dust mite allergens with asthma and allergy and a range of risks associated with electrical hazards, fire hazards and emissions of materials that are flammable. Infants spend much time inside so there are issues here (Franklin 2007), and elderly or infirm people are also exposed to similar hazards.
There have been increasing pressures from buildings regulations to set standards for the design and construction of buildings to ensure the safety and health of people in or about those buildings. In the UK the Building Regulations are made under powers provided in the Building Act 1984, and apply in England and Wales (http://www.planningportal.gov.uk/england/genpub/en/1115313929034.html). These reflect concern over sustainability and climate change, but the Regulations also ensure that fuel and power are conserved. Schedule I to the Building Regulations contains a number of sections relevant to the concerns of this chapter, most notably Part D Toxic substances and Part F Ventilation, although the Schedule acknowledges that some buildings will require greater sensitivity and more flexibility. These include historic buildings, so English Heritage have produced, for example, an Interim Guidance Note on how to balance the needs for energy conservation with those of building conservation.
International regulation is also in evidence with the World Health Organisation (WHO) starting to show an interest in the indoor environment that parallels its work on outdoor air quality. The WHO list of pollutants recommended for guideline development (group 1) and those with insufficient scientific evidence and corresponding systematic reviews (group 2) are as follows:
• Group 1 pollutants: 1.1 formaldehyde, 1.2 benzene, 1.3 naphthalene, 1.4 nitrogen dioxide (NO2), 1.5 carbon monoxide (CO), 1.6 radon, 1.7 particulate matter (PM2.5 and PM10), 1.8 halogenated compounds (tetrachloroethylene, tricholoethylene, etc.), 1.9 polycyclic aromatic hydrocarbons (PAH), especially benzo-a-pyrene (BaP).
• Group 2 pollutants – current evidence uncertain or insufficient for guidelines: 2.1 toluene, 2.2 styrene, 2.3 xylenes, 2.4 acetaldehyde, 2.5 hexane, 2.6 nitric oxide (NO), 2.7 ozone (O3), 2.8 phthalates, 2.9 biocides, pesticides, 2.10 flame retardants, 2.11 glycol ethers, 2.12 asbestos, 2.13 carbon dioxide (CO2), 2.14 limonene, pinene, 2.15 total volatile organic compounds (TVOC).
In this context we can see a focus on a widening range of organic compounds driven especially through concerns over ‘tight buildings’ or ‘sick building syndrome’. Many of the larger organic molecules are not familiar from an engineering perspective, although useful textbooks in this area include Indoor Air Pollution edited by Peter Pluschke (2004), which treats the organic chemicals within the context of indoor air and for reference the classic data source on environmental organic compounds is Karel Verschueren’s Handbook of Environmental Data on Organic Chemicals (1996). A good listing of the compounds found in residential and commercial buildings is found in the 2003 Lawrence Berkeley National Laboratory report: Volatile Organic Compounds in Indoor Air: A Review of Concentrations Measured in North America Since 1990 (http://eetd.lbl.gov/ied/pdf/LBNL-51715.pdf) by Hodgson and Levin. The health effects of these compounds are not easy to interpret at the low concentrations found, but typically some such as aldehydes are irritants, others have odour and some are potential carcinogens. The difficulties are that the health outcomes at low concentration may arise from reactions or synergistic effects.
Our increasing worries about risk in pubic spaces received attention in the Commission for Architecture and the Built Environment report Living with risk – Promoting better public space design (CABE 2007), which recognised the need to carefully balance the risks in their design. The authors of the report envisaged that public spaces are in danger of becoming bland and standardised because of over-sensitivity to risk, arising from misplaced fears of a rampant compensation culture and unquestioning interpretations of health and safety aspects of indoor building regulations.
A range of issues have emerged in recent years in terms of public spaces. The public has been worried about the issue of carpets and allergens, and exposure to indoor dust and fungi from damp. This has broadened to strive to minimize the negative effects to individuals from exposure to perfumes, scents and other odours within our facilities. Perhaps of widest impact has been the increasing number of countries that have developed smoke-free legislation for public places to limit exposure to the dangers of second-hand tobacco smoke.
6.2 Safety issues in occupied spaces
There are a wide range of safety issues indoors that include escape, security and falls, but this chapter will focus on fire and what might be regarded as threats to the indoor environment. There has been a rising interest in indoor air pollution in recent years, with countries such as South Korea promulgating legislation and the WHO addressing the potential in its report from October 2006 Development of WHO Guidelines for Indoor Air Quality (WHO 2006) for regulation of pollutants that affect health. There has also been increased litigation over issues such as exposure to indoor mould and much concern from the occupants of new buildings about sick building syndrome and multiple chemical sensitivity, although both these have been resistant to clear understanding or ready solutions.
There is currently a view that we spend a great deal of time indoors and that this is increasing. From 1992 through 1994, the US Environmental Protection Agency used telephone interviews of participants who kept diaries to record their activities and locations. This probability-based National Human Activity Pattern Survey (NHAPS) was conducted with over 9000 respondents across the country (Brasche and Bischof 2005). The national results were generally consistent with an earlier Californian study, with the mean percentage of time spent indoors being 87%. This was split into 69% of time spent in a residence and 18% of the time spent in other indoor locations. Studies in Germany (Brasche and Bischof 2005) give very similar results: the overall mean time spent at home was 15.7 (65%) hours per day, consistent with the earlier German Environmental Survey (1990/92) and a small German study in 1987. Thus there is evidence of the large amount of time we spend indoors, but less convincing is that this is increasing dramatically; indoor activities may have changed along with the range of consumer products.
Additionally problems could have increased indoors because of pressures to decrease ventilation as part of energy conservation. This arises because of the desire to provide adequate ventilation at minimum energy cost (Sherman 1999). Typical ventilation rates from an engineering perspective are given in Table 6.1. There has also been an increased spread of heating and ventilation systems which have the potential to negatively affect indoor air quality, such as the outbreaks of Legionella (Diederen 2008) that have been particularly widely reported.
Table 6.1
Air change rates recommended for some typical rooms and buildings (http://www.engineeringtoolbox.com/air-change-rate-room-d_867.html)
| Building/room | Air change rates (AC/h) |
| All spaces in general | min 4 |
| Attic spaces for cooling | 12 – 15 |
| Auditoriums | 8 – 15 |
| Banks | 4 – 10 |
| Barber shops | 6 – 10 |
| Bars | 20 – 30 |
| Beauty shops | 6 – 10 |
| Boiler rooms | 15 – 20 |
| Cafeterias | 12 – 15 |
| Churches | 8 – 15 |
| Computer rooms | 15 – 20 |
| Court houses | 4 – 10 |
| Department stores | 6 – 10 |
| Dress shops | 6 – 10 |
| Engine rooms | 4 – 6 |
| Factory buildings, ordinary | 2 – 4 |
| Foundries | 15 – 20 |
| Garages repair | 20 – 30 |
| Garages storage | 4 – 6 |
| Homes, night cooling | 10 – 18 |
| Kitchens | 15 – 60 |
| Laundries | 10 – 15 |
| Libraries, public | 4 |
| Medical centres | 8 – 12 |
| Municipal buildings | 4 – 10 |
| Museums | 12 – 15 |
| Offices, public | 3 |
| Offices, private | 4 |
| Post offices | 4 – 10 |
| Restaurants | 8 – 12 |
| Shopping centres | 6 – 10 |
| Supermarkets | 4 – 10 |
| Town halls | 4 – 10 |
| Theatres | 8 – 15 |
| Warehouses | 2 |
| Waiting rooms, public | 4 |
Air quality in schools has become a special problem that can often be increased through parental worry over the health of their children. Air pollution in schools has been studied frequently in recent years (e.g. Janssen et al. 2001), given the potential vulnerability of younger children to the effects of indoor air pollutants and disease. Indoor air pollution might also reduce the productivity of teachers and degrade the student learning experience (e.g. Yang et al. 2009).
Modern industries, such as electronics and nanotechnology, have emphasised the importance of clean production facilities where it has become important to keep particles and pollutants from sensitive nano-fabricated materials (Clark et al. 1992, Muller et al. 1994, Lebens et al. 1996, Bhushan and Chandra 1999). Telephone switching centres have proved vulnerable to air pollutants (e.g. Shields and Weschler 1992) and photographic and optical manufacturing (Spyak and Wolfe 1992) must maintain high standards.
Museums, historic properties, art galleries and major libraries focused on the traditional agents of deterioration: relative humidity, temperature and light in the past. These buildings have experienced air pollution (Brimblecombe 1990, 2008) and, more recently, dust (Lloyd et al. 2007) has been shown to be an important consideration in the management of their interiors.
6.3 Combustion, fire and combustible materials
Combustion indoors can be intentional, as in terms of heating or cooling or during fires, where very special risks are imposed on occupants and the fire services from exposure to combustion products, during fire-fighting.
There is a long history of exposure to indoor smoke. Seneca, Emperor Nero’s tutor, complained bitterly about exposure to the smoke of green wood from indoors fireplaces. In medieval England charcoal was often used for heating as few of the houses had chimneys. Exposure to wood smoke indoors remains a considerable problem in much of the world and has been the impetus for many studies that have health perspectives (Smith et al. 2000), along with pressures for simple, inexpensive but low emission cook-stoves. There are frequently poverty and gender issues here. It is women in the developing world that seem to be exposed at the highest concentrations and poor people who have less choice over the fuels they use so are disproportionately exposed to high indoor air pollution (Adonis and Gil 2001, Kavi Kumar and Viswanathan 2007). Space heating, especially the use of kerosene heaters, which was common in the past, can lead to high concentrations of air pollutants indoors (Leaderer 1982).
Carbon monoxide is a particularly critical indoor pollutant, and in many countries it accounts for more than half of the accidental poisonings (Raub et al. 2008) This can be especially severe as ventilation may be deliberately limited in winter to conserve heat, so carbon monoxide from poorly vented heaters can reach high concentrations, or gas lines may leak unnoticed into rooms. These problems can disproportionately affect the poor or the elderly, even in more salubrious neighbourhoods (Brimblecombe et al. 1996). Carbon monoxide poisoning can reach epidemic proportions in severe winter storms where electrical failures can cause residents to use unconventional methods of heating or attempt to generate their own electricity. Again there are significant social issues with high frequency of problems among ethnic minority groups with poor reading ability in the language used for warning labels (Houck and Hampson 1997).
Kerosene is used for cooking in some countries. In Indian households there were noticeable increases not only in indoor particulate materials, but also VOCs and PAH with naphthtalene, benzo(a)pyrene and phenanthrene all having I/O greater than 7 (Pandit et al. 2001). There have also been a series of investigations suggesting that gas cooking had the potential to increase indoor exposure to NO2. In kitchens these were associated, classically by Melia and his co-workers, with an increased risk of respiratory illnesses, especially among children (Melia et al. 1985). The problem can be improved by increasing the ventilation in kitchens, particularly the installation of hoods over stoves.
Fire statistics from the United Kingdom suggest that the majority of deaths in fires result from inhalation of toxic gases that are produced in the fires. Carbon monoxide once more is the most severe hazard, although the products of thermal decomposition or combustion of combustible material produces toxic gases. These vary according to the material burnt but cover a wide range, and toxic products are also dependent on the fire conditions. The gases can be asphyxiants which cause hypoxia. Others are respiratory irritants that stimulate nerve receptors in the eyes, nose, throat and upper respiratory tract. At sufficiently high concentrations, most irritants can also penetrate deeper into the lungs, causing pulmonary irritation effects which are normally related both to concentration and to the duration of exposure. After exposure respiratory distress and even death can occur from a few hours up to several days after exposure due to asphyxiation from pulmonary oedema (Hartzell 2001).
Multiple gas exposures can be considered in terms of a fractional effective dose (FeD), which can be calculated by summing the concentrations of the fire effluents and dividing by the lethal concentrations:
where the lethal concentration for 50% of the population (LC50) over a 30 min exposure time, Ac is acrolein. The first term, the effect of the CO, is enhanced by the increase in respiration rate caused by high concentrations of CO2 (expressed as a step function with one set of values of constants m and b for CO2 concentrations below 5% and another for those above 5% (Hull et al. 2008)). Table 6.2 presents potential exposure limits to toxic gases released during fires.
Table 6.2
Potential limits to exposure from toxic gases released during fires
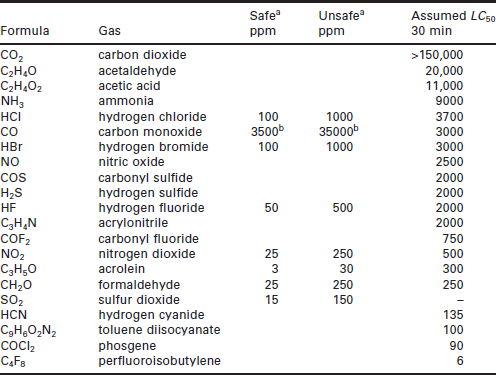
aConcept of limits on exposure of occupants as being safe or unsafe for most occupants.
bUnits: ppm min.
(data adapted from Hartzell 2001 and http://www.doctorfire.com/toxicity.html)
Plastic is often a significant hazard in fire; for example, as much as 0.3 g Hcl can be produced from 1 g of polyvinylchloride (PVc) during poorly ventilated fires (Hull et al. 2008). Polyurethanes are widely used in paints and yield a wide range of combustion products, including alkenyl isocyanates, propylene through to octylene derivatives during combustion (Boutin et al. 2004). in a recent eU project, ToXFire, that aimed to set guidelines for management of fires in chemical warehouses, test fires (Andersson et al. 2005) included three compounds with S, N, c and polymers: (1) tetramethylthiuram monosulphide (TmTm) used in vulcanising processes, (2) 4-chloro-3-nitro-benzoic acid (cNBA) used in dye production, (3) chlorobenzene and polymers, (4) nylon 6,6 (nylon, also containing N) and (5) polypropylene. Hydrogen chloride and sulphur dioxide yields are high compared with the maximum theoretical yields, which is in agreement with the assumption that these compounds are end products in the case of well-ventilated fires. Hydrogen cyanide (HcN) was formed in the experiments with nylon, TmTm and cNBA. But the yields are rather low, with a maximum yield of around 2%.
6.4 Infiltration of outdoor pollutants
Air pollutants found indoors can accompany incoming air or arise from processes within buildings. The same pollutant can have both indoor and outdoor sources. Advection of pollutants through windows or doors or through active ventilation via air conditioning systems is a most obvious source of indoor pollutants. Sensitive industrial facilities or archives carefully control the quality of incoming air, filtering and sometimes scrubbing the air free of pollutant gases.
Once indoors many pollutants react with the indoor surfaces and this reduces their concentration. This is part of the explanation for the advice given to sensitive individuals that they should remain indoors during air pollution episodes. The reduction in pollutant concentration can be represented as an indoor–outdoor ratio of pollutant gases. This has become an important concept in the understanding and management of indoor spaces, and can be described in terms of the simple equation:
where: Ci = indoor pollutant concentration, Co = outdoor pollutant concentration, E = air exchange rate of the room (hr− 1), A = surface area of interior (m2), V = volume (m3) of interior, ratio, and ![]() = average deposition velocity (m hr− 1 or converted from cm s− 1).
= average deposition velocity (m hr− 1 or converted from cm s− 1).
The air exchange rate has already been discussed in terms of the ventilation of typical spaces (see Table 6.1). The key notions are deposition velocity and air exchange rate in determining indoor–outdoor ratios. The deposition velocity represents the way in which a gas deposits and then sticks irreversibly to a surface. It can be thought of in terms of a number of separate resistances such that:
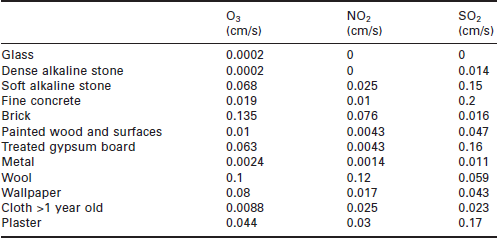
where ra, rb and rs are the aerodynamic resistance, the boundary layer resistance and the surface transfer resistance, respectively. The resistance at the surface can be thought of in terms of surface reactivity. Deposition velocity has been represented in terms of the uptake coefficient or sticking coefficient by Cano-Ruiz et al. (1992), where this is the ratio, in a molecular sense, of the fraction of all pollutant molecules colliding with the surfaces that result in irreversible removal. The magnitude of the deposition velocity can increase very dramatically with humidity or even the hygroscopicity of the surface. Typical values are given in Table 6.3 and more extensive tabulations onto indoor materials appear in Grontoft and Raychaudhuri (2004).
Table 6.3
Deposition velocities of gases to typical indoor materials at 70% RH (for full details see Grontoft and Raychaudhuri 2004)
The ventilation system itself can be a source of indoor pollutants. These are derived from compounds found deposited on the filters and within the ducting, but odour can also arise from heating coils and heat exchangers. These odours arise from volatile components within the dust. They are emitted more effectively when temperature and relative humidity increase. It is the longer chain aldehydes which contribute most to the odour, with nonanal and decanal especially important. Longer chain carboxylic acids have low odour thresholds, but they are bound to the dust more effectively. However, unsaturated nonenal, which has an unusually low odour threshold, might also be an important contributor to odour. Nitrogen containing compounds, such as indoles (e.g., 1H-isoindole-1,3(2H)-dione), pyrroles, quinolines, and pyridines (methyl and dimethylpyridine), could also be released through thermo-desorption (Hyttinen et al. 2007). Additionally n-aldehydes can also be produced via reaction with ozone with surfaces (see Wang and Morrison 2006).
6.5 Indoor emissions and outgassing
Construction materials and furnishings outgas volatile compounds over time as they age or degrade. This adds to outdoor pollutants or those produced from combustion processes indoors during cooking and heating. This section will take a brief look at some of the principal components of occupied spaces that are likely to release gases to the indoor environment, but more comprehensive lists of sources (Wang et al. 2007) and greater details of the chemistry are available elsewhere (Uhde and Salthammer 2007).
6.5.1 Wood
As wood ages it releases a range of materials, most notably acids and also aldehydes which derive from heat-treated woods. Formic and acetic acid are best known, but some longer chain carboxylic acids are also products from wood. Substantial emissions of acetic acid and/or furfural can also be observed for heat-treated wood (thermowood) or kiln-dried samples of various wood species (e.g. beech, rubber wood, etc.) (Uhde and Salthammer 2007). Terpenes such as α-pinene and 3-carene are commonly released from air-dried wood (Manninen et al. 2002) and can be an important source of these larger semi-volatile organic compounds indoors (Hodgson et al. 2002). Chloromethane can be found as fungi (Hymenochaetaceae) rot wood and additionally pectin reacts with chloride ion to form chloromethane (Watling and Harper 1998).
6.5.2 Floors
Floors may be wooden, but also incorporate glues and varnishes, etc. Parquet floors of birch, coated with acrylic lacquer and adhesive emit n-butylacrylate and n-butanol from the hydrolysis of n-butylacrylate ester along with pentanal and hexanal from the degradation of fatty acids (Uhde and Salthammer 2007). Horn (1998) has reported high emissions of furfural and acetic acid from composite cork products and Udhe and Salthammer (2007) list carbonyl disulfide, acetic acid, furfural styrene, benzaldehyde, cyclohexanone, phenol methylbenzoate, 4-phenylcyclohexene and benzophenone from the degradation of hemicellulose, adhesives binders and photo-initiators from a new cork parquet floor. Linoleum (Jensen et al. 1995a, Jensen et al. 1995b) can be a source of volatile organic compounds as the oxidation of linolenic acid leads to unsaturated compounds like 2,4-heptadienal (Belitz and Grosch 1992).
6.5.3 Paint, solvents and preservatives
Paints and varnishes are an important source of aromatic compounds (Edwards et al. 2001). Traditional paints, based on boiled linseed oil and other natural materials have become popular in recent years because the paint is said to last significantly longer than ordinary gloss paints primarily as the vegetable resins are impervious. Additionally there is a well advertised belief that these paints have no chemical emissions. However, hexanal, nonanal and propanal are released, while Toftum et al. (2008) have shown that beech boards painted with an ‘eco’ paint emitted large amounts of the terpene limonene and lesser amounts of carvone. Furniture coatings containing alkyd or natural resins (Afshari et al. 2003, Salthammer 1995a, Salthammer 1995b) on oxidation form many types of volatile aldehydes. Typical degradation products of oleic acid are saturated aldehydes from heptanal to decanal, while linoleic acid gives mainly hexanal. Organic solvents were used in paints more extensively in the past so compounds such as toluene, isopropanol, acetone and butyl acetate were emitted, but increasingly water-based paints have been adopted.
The desire to reduce the volatile compounds emitted from paints and lacquers has encouraged manufacturers to use more UV-curable formulations. These require a photo-initiator. Uhde and Salthammer (2007) investigated the importance of the photo-initiator (a blend 1-hydroxy-cyclohexyl-phenone (HCPK)/benzophenone) and its degradation products as a pollutant source to indoor air. Cyclohexanone was formed from the hydroxyl–cyclohexyl radical upon cleavage of HCPK. The photo-initiator benzophenone was found along with its degradation products benzaldehyde and methyl benzoate.
Some indoor materials are protected with pesticides. Naphthalene concentrations can be high indoors, particularly in warmer climates where it is frequently used as an insecticide. Pesticides can be deliberately sprayed into air by householders or evaporate from treated products. Given our sensitivity to pesticides, risks from these need to be reduced and often require more thought in our approach to cleaning (see Section 6.6.2). Some pesticides degrade within materials over time. An important example of this is the production of indoor pollutants from pentachlorophenol, commonly used to protect wood from fungi, as the compound degrades over time and gives rise to emissions of chloroanisoles (Gunschera et al. 2004).
6.5.4 Fabrics and coverings
Indoors large areas of fabrics cover the floors and furnishings in addition to acting as curtains. The tendency in many countries for increased areas of floor to be covered by carpets raised concern in the late 20th century because of an increasing frequency of asthma and its potential link to dust mites that inhabit carpet. Homeowners were sometimes advised to remove carpet from their rooms to limit the risk to allergy and asthma patients. There has been much disagreement about this because clean, dry, well-maintained carpets can also improve air quality as they can absorb both inorganic pollutants such as ozone and SO2 and organic compounds (Won et al. 2001). Additionally, emissions from other types of flooring could impose other types of risk.
Volatile organic substances released from carpets are highly variable and depend on their type. Often these are not related to the carpet surface itself, but to the foam backing. Quite exotic substances can be formed during the reaction, which is a radical-induced polymerisation that creates the styrene–butadiene copolymers (SBR) in foam. Residual monomers styrene and butadiene are removed by distillation, but odour-intensive compounds 4-phenylcyclohexene (4-PCH) can remain (Uhde and Salthammer 2007). PVC-backed carpet emitted formaldehyde, vinyl acetate, isooctane, 1,2-propanediol and 2-ethyl-1-hexanol with vinyl acetate and propanediol at the highest emission rates, while a polyurethane-backed carpet emitted mostly butylated hydroxytoluene (Hodgson et al. 1993). Some materials with a high sulphur content such as wool can give off large amounts of carbonyl and hydrogen sulphide (Brimblecombe et al. 1992).
Other more exotic coverings can be important in some environments. Chang et al. (1995) studied volatile organic compounds (VOCs) released from athletic running tracks which included: 2-methyl furan, butanal, methyl ethyl ketone, benzene, heptane, methyl isobutyl ketone, toluene, octane, hexanal, nonane + ethylbenzene, xylenes + styrene, propyl benzene, decane, 1,3,5-trimethyl benzene, 1,2,4-trimethyl benzene, 1,2,3-trimethyl benzene and undecane. Hexanal was the most common and the principal compound from the synthetic rubber and polyurethane tracks studied.
6.5.5 Flame retardants
Flame retardants are used to make materials, especially plastics, wood and wood-based textiles flame-proof. Worldwide in 2000, usage of flame retardants was in excess of 1.08 million tons of which nearly a quarter were organophosphate flame retardants. These also include a range of phthalates (possible endocrine disruptors) and pose potential risk as they outgas and become additionally incorporated into indoor dust.
Added to this, retardants can release volatile products derived from rather non-volatile precursors that yield 1-chloro-2-propanol, 2-chloro-1-propanol and 1,3-dichloro-2-propanol. These can be found in indoor air samples following hydrolysis of flame retardants such as tri(chloropropyl) phosphate and tris(dichloropropyl)phosphate, respectively (Salthammer et al. 2003). The latter requires increased attention since 1,3-dichloro-2-propanol has been acknowledged as a carcinogenic substance.
A study by Ni et al. (2007) of wallpapers showed a significant positive correlation between emissions of tris(2-chloroisopropyl)phosphate (TCPP) and its content in wallpaper samples. Additionally a linear relationship (Arrhenius like) was found between reciprocal temperature and the logarithm rate of the TCPP emission rate at different temperatures. The observed maximum emissions of TCPP from 5 w/w% content wallpaper materials was 645 μg m− 2 h− 1, at 25 °C.
Nevertheless, the inhalation of phthalates and particulate organic chlorine at levels reaching the allowable daily intake seems unlikely under normal living conditions. However, the evaluation of the exposure to household dust is far more difficult if we include ingestion (Wensing et al. 2003).
6.5.6 Plastics, foams and glues
Glue is an especially important source of indoor air pollutants, notably formaldehyde. It has a wide range of sources in air and is a frequent oxidation product outdoors. Formaldehyde is characteristic of hospitals where releases to air will occur when it is used for sterilisation. More general indoor sources are from formaldehyde-based insulation and furniture foams, cigarette smoke and carpets, resins, wood, paints and glue, which mean indoor concentrations can be very high (see Table 6.4). It has long been known that the hydrolysis of ureids (glue based on urea-formaldehyde), due to the unavoidable presence of water, leads to hydrolysis of the N–O bond and, as a consequence, to the release of formaldehyde (Uhde and Salthammer 2007). Fibreboards can be especially problematic because of the urea-formaldehyde resins used to bond the constituent parts together. Other aldehydes and organic acids arise from woods, insulating foams, textiles and glues. Sensory irritation of some shorter aldehydes (e.g., formaldehyde and acrolein) might be higher than that of the longer ones (in passing, Hyttinen et al. 2007).
Table 6.4
Some reported occupational and environmental concentrations of formaldehyde (http://www.mflohc.mb.ca/fact_sheets_folder/formaldehyde.html)

Because of the importance of furniture in enhancing the exposure to formaldehyde in the home, there has been increasing regulation of formaldehyde in furnishings. The UK Department for Environment, Food and Rural Affairs (DEFRA) introduced Building Regulations to prohibit the use of urea-formaldehyde foam (UFF) in buildings and to control its installation elsewhere. Broadly the regulations only permit UFF insulation between cavity walls with masonry inner and outer leaves. Here the inner leaf should afford good resistance to vapour penetration.
Plastics often contain plasticisers to improve the plasticity of the product. Those present in phthalates and adipate containing plastics tend to hydrolyse over time. A typical reaction product found in indoor air samples is 2-ethyl-1-hexanol from the hydrolysis of diethylhexylphthalate or butanol from the hydrolysis of butylphthalates or n-butylacrylate. Both substances have a strong odour and can lower the perceived air quality.
6.5.7 Secondary production
There are also contributions to indoor air from the reaction of pollutant gases with surfaces. Such secondary emissions arise when ozone reacts with paints to give formaldehyde (Reiss et al. 1995) and on carpets (Weschler et al. 1992) or counter tops and releases a range of aldehydes, especially nonanal (Wang and Morrison 2006).
We are all aware of the smell of tobacco smoke that persists long after smoking has ceased. This can be due to the sorption of organic compounds such as nicotine and their slow desorption which may take years (Van Loy et al. 2001). Some of these compounds are organic bases and subtle shifts in the acidity of wall coverings could affect the rate of release.
6.6 Occupant activity
6.6.1 Cooking
Obviously kitchens expose occupants to combustion products, and in many countries this exposure may be largest for women and children. Beyond combustion products, the preparation of food can involve the release of ethanol or acetic acid (vinegar).
The cooking process produces organic materials such as n-alkanes (C25–C36), saturated and unsaturated monocarboxylic acids (C16–C24), saturated and unsaturated dicarboxylic acids (C6–C14), nonanal, lactones, levoglucosan, along with aromatic compounds, particularly toluene and the xylenes (Edwards et al. 2001; Zhao et al. 2007) that can be found as films on window glass (Liu et al. 2003). Oil droplets are also forced into the air along with particles of ingredients. We are all aware of the irritancy of capsaicin in kitchen air when chilli peppers are being fried. Frying itself with modern Teflon-coated pans releases a range of compounds such as polyfluoro- and/or polychlorofluoro- (C3–C14) carboxylic acids, a pyrolysis product of polytetrafluoroethylene (Teflon). Although there are few cases of effects on humans, their impact on birds is well known (Boucher et al. 2000).
6.6.2 Cleaning and fragrances
Cleaning products and fragrances have an impact on the composition of indoor air. In addition to abrasion, cleaning releases a range of volatile compounds and other particles to indoor air (Wolkoff et al. 1998). These include surfactants (from detergents), complexing agents as water softeners (e.g. EDTA), disinfectants and waxes. Typical volatile compounds found in cleaning agents include alkanes and alkenes and various halogenated derivatives, alcohols, ethers and carboxyl compounds. There have been relatively few studies that assess the risk balance involved in cleaning within buildings.
In domestic interiors, fragrances in cleaning products represent an additional source of the terpenes, α-pinene and limonene (Edwards et al. 2001) along with synthetic aromatic nitro-musks and polycyclic musks, scenting agents in cosmetics, perfumes and cleaning products, that are widely dispersed in the environment. These have largely replaced natural musks, but increasing concern about their environmental impact may have led to lower production (Weschler 2009). Terpentine oils are produced in large amounts (~ 0.3 Mt/a) and limonene is increasingly seen as a safe fragrance, cleaner and solvent and is globally produced in very large amounts (~ 70kt/a). Little is known of the risks through inhalation exposure to d-limonene, although it is an irritant. However enhanced risks may arise from its oxidation products and indoor air chemistry.
Increasing numbers of people express a sensitivity towards perfumes and scents. There is also a growing concern about exposure to the volatile materials from household products such as air fresheners. There was more than a 30% increase in babies that suffered diarrhoea in homes where air fresheners were used every day, compared with homes where they were used once a week, while their mothers had more headaches and depression (Farrow et al. 2003).
6.6.3 Ozone generation
Indoor activities are contributors to the generation of ozone gas. It is sometimes argued that ozone can arise from electrical equipment and photocopying machines, but the amounts are quite small. Ozone is produced in larger amounts from ozone generators designed to remove odours and to destroy organic compounds, though there is increased awareness that these should not be used for occupied spaces. Elevated concentrations of ozone may be used for deodorising hotel rooms or homes affected by fires and to disinfect mould-afflicted buildings. Here ozonation can lead to the formation and emission of carbonyls (Poppendieck et al. 2007).
6.7 Transformations within the interior
Indoor air is chemically active. As with secondary pollutants produced by reactions in outdoor air that are characteristic of the Los Angeles smog, indoor air has its own transformations (Weschler and Shields 1997). The transformations of compounds in indoor air can be a function of light. In particular, this occurs as the result of the decrease in the amount of light as air moves indoors. This occurs in large rooms with weakly absorbing walls (metal, polymer or glass). NO2 can be generated as the ‘equilibrium’ shifts to the right (Brimblecombe et al. 1999):
In buildings with a high surface to volume ratio (i.e. small rooms), surface absorption can result in the ozone and to a lesser extent nitrogen oxides being absorbed by walls. However, surface chemistry can transform NO2, so the situation may be different:
There is also an active organic chemistry within interior spaces. Oxidation reactions in indoor air have been studied because of the potential that the oxidation products of volatile organic matter may be more harmful to health than the precursors. Oxidation products include free radicals, secondary ozonides, epoxides, aldehydes, ketones, acids, diacids and dicarbonyls (Weschler 2009).
Ozone reactions with the terpenes typically found in rooms produce particles. Such chemical transformations of limonene and α-pinene reactions may be responsible for much of the formaldehyde, almost all of the p-tolualdehyde and a substantial fraction of the particle mass generated indoors (Weschler and Shields 1999, Fan et al. 2003). This can be an important source as they are components of air fresheners and ozone is advected through open widows or more critically arises where ozone generators are used. The particles produced in these reactions are in the submicron range and thus a potential health risk as they reach deep into the lung.
6.8 Particles in buildings that impact on environmental health and safety
A wide variety of particles are found indoors and these range from the large particles that trouble households as dust, often containing fibres as much as a millimetre in length through to much smaller particles. Soot arises from incomplete combustion and is common indoors, normally being associated with wax candles, incense, mosquito coils, gas stoves or smoking, and many of the particles are in the submicron range (Afshari et al. 2005).
6.8.1 Non-viable particles
Concrete is known to yield small alkaline particles (Toishi and Kenjo 1967, 1975) and plaster indoors is likely to give fine gypsum as it degrades (Camuffo et al. 1999). Mechanical abrasion and cleaning is a source, especially vacuum cleaners which produce fine particles. The degradation of materials containing asbestos has been a special problem. Asbestos was widely used in the building industry because of their resistance in fires. Asbestos minerals are highly fibrous and exposure can lead to tumours of the respiratory system and lung cancer, so asbestos is well regulated and within the UK. the three previous sets of regulations covering the prohibition of asbestos, the control of asbestos at work and asbestos licensing now fall within the Control of Asbestos Regulations 2006. It is important to distinguish between ‘friable’ and ‘non-friable’ asbestos, because it is the friable material that produces dangerous fibres. Friable materials are those that contain more than 1% asbestos and can be reduced to powder by the human hand.
6.8.2 Spores and bacteria
In addition to non-viable particles there are also numerous fungal spores, bacteria and fragments of biological material. The production of these has become particularly associated with dampness in buildings which encourages the growth of fungi. Although the future climate of Britain may be drier, warmer conditions could enhance fungal growth. Fungal infections are often associated with microbial volatile organic compounds (MVOCs) which include alcohols, ketones, aldehydes, aromatic hydrocarbons, amines and terpenes (Kuske et al. 2005). The best known indoor bacterial infection is from Legionella, which is associated with the growth of the bacteria in water particularly associated with cooling systems or spas (Diederen 2008). It can even be a problem with domestic electric showers, because although domestic hot water systems are often 60 °C and kill legionella, electric showers are cold water fed.
Biologically derived particles can also incorporate allergens. Of particular concern are those from the dust mite which are associated with asthma and allergy. Allergens are also associated with pets and although pet ownership has increased over time, a Mintel report in June 2005 shows recent declines as people have less time to care for pets and keep them company, particularly in a period where children have many other activities. Dog ownership in Britain fell by 26% between 1985 and 2004, but the outcome in terms of susceptibility to allergens is not easy to predict.
6.9 Materials and toxicity
Modern materials and lifestyles have imposed new risks within occupied spaces and the choice of construction and furnishing materials has become especially difficult. We have to balance the risks imposed by flame retardants, cleaning agents or pesticides against the problems that arise if they have not been used. There is much advice on making such choices, but the reasoning behind such advice is not always obvious. There is a general tendency to regard natural materials as preferable to artificial, on the basis that they have been in use for a long time. Use over a long time is often a good guide as we learn correct approaches to the materials, but it does not seem to make them inherently safe.
6.9.1 Regulation
There is a widening regulatory framework that controls the quality of indoor air within occupational, public and domestic spaces. Best known in the UK are the the Building Regulations where Part C concerns resistance to contaminants, Part D to toxic substances and Part F ventilation. Formaldehyde from a number of sources has been of concern and the UK Department for Environment, Food and Rural Affairs pressed for Building Regulations Part D to prohibit the use of UFF in unsuitable buildings and to control its installation and use elsewhere. In parallel there is regulatory concern over formaldehyde from medium density fibreboard, such that in the US emissions must limit yield from MDF to 0.3 ppm and in Germany the exposure limit is 0.l ppm, while Britain’s Health and Safety Executive has issued a number of hazard assessments (e.g. Medium Density Fibreboard – Hazard Assessment Document, EH75/1). Despite the regulatory progress, high levels of formaldehyde are still found when buildings are refurbished, as illustrated by the renovation or construction of schools in Korea where the indoor concentrations of HCHO were higher in schools less than a year old (Yang et al. 2009).
Materials such as lead in paint or asbestos have been revealed as problematic, but the range of problem materials is more extensive than this. Asbestos is now well covered by the Control of Asbestos Regulations 2006. The use of lead pigments in primer paints on some prefabricated domestic wooden windows continued through to the 1980s, but the UK implemented the provisions of an EU Directive (89/677/EEC) and restricts the use of lead paint. The Regulations allow the manufacture and use of lead, but in controlled circumstances for the redecoration of Grade I and II* listed historic buildings, though the general sale of lead paint in the UK is prohibited. Nevertheless, during the redecoration of older houses, paintwork containing lead pigment can be mobilised, so must be treated as a potential health risk. Furthermore, if it is flaking, likely to be chewed by children or animals, additional risks are imposed.
6.10 Advanced material requirements
Those who wish to create buildings with low environmental risk suggest avoiding materials that are likely to produce persistent organic pollutants. These have the potential to accumulate in fatty tissues concentrating as they move up the food chain and to act as endocrine disruptors. Chlorinated building materials, notably the plastics are likely to be associated with chlorinated dibenzodioxins and furans during their manufacture or when they burn. They also advise against brominated flame retardants, particularly polybrominated diphenyl ether and various perfluorocompounds such as Teflon and Scotchguard. The latter has been used to protect furnishings and fabrics. The key ingredient was perfluorooctane sulfonate, although this has now been reformulated with the less persistent perfluorobutane sulfonate. It is suggested that heavy metals are to be avoided, mercury in switches, lead flashing and solder and copper.
The choices advocated for interior materials typically focus on the use of natural substances. This approach to the use of flooring materials can potentially make a big change to indoor safety. Natural linoleum is made from linseed, cork, tree resin, limestone and jute. It is biodegradable and anti-microbial. Natural cork flooring is produced from the bark of the cork oak tree and often layered with UV cured acrylic. It is highly durable, comfortable, sound and thermally insulating. In the case of carpeting and furnishing fabrics, wool is often seen as a non-toxic alternative to synthetic fibres which can outgas, but wool releases sulfides (Brimblecombe et al. 1992). Bamboo is seen as a durable, anti-microbial and water resistant material that can be used for floors and walls. As a plywood, it can be used for kitchen cutting boards, plates, bowls, utensils, countertops and walls and furniture.
Paints and finishes are preferred where they contain low or no VOCs, no fungicides or biocides and natural pigments or they are from naturallyderived materials such as citrus peel extracts, essential oils, seed oils, tree resins, inert mineral fillers, tree and bee waxes.
The careful choice of materials in improving the indoor environment makes sense, but it must be recognised that even the materials in the recommendations above are not without problems. In particular, a focus on natural resins are a problem as they can produce significant concentrations of highly reactive terpenes as indoor VOCs and the UV curing process can release VOCs (Uhde and Salthammer 2007).
6.11 Future trends
As emphasised in the recent article by Weschler (2009), there have been enormous changes in the pollutants we encounter in air over the last fifty years. These will no doubt continue. It is also hard to appreciate the magnitude of potential social change given what we have witnessed in the last fifty years. Social changes have an impact that is difficult to predict. If we look at the European heat-wave of 2003, it is possible to see that changing family structures affected the stress/death among elderly people in that very hot summer, possibly from indoor ozone. Our lifestyles have also changed with increasing use of computers and entertainment within the home rather than outside. It is hard to predict how our indoor lives will change in the future (time inside, electronics, food, etc.). Furthermore, climate change will impose novel requirements on heating and ventilation systems.
There is no doubt that we will see an increasing regulation of consumer products and building materials. It is likely that regulations will develop to establish a wider range of criteria for indoor air quality. Since 2004 South Korea has guidelines for formaldehyde (210 μg m− 3), benzene (30 μg m− 3), toluene (1000 μg m− 3), ethylbenzene (360 μg m− 3), xylene (700 μg m− 3) and styrene (300 μg m− 3) in public buildings as part of the Indoor Air Quality Management Act (Kim et al. 2006). In other countries, including China, Singapore, Canada, United Kingdom and Germany, air quality guidelines have been developed also for the indoor environment, but they are highly variable in terms of the compounds and factors considered.
Standards relating to the emissions from materials are increasingly common but in some countries, such as China, rapid development has led to a deterioration of indoor air quality because of the use of new materials, e.g. the extensive use of chipboard with high emissions of formaldehyde. In the future there may be entirely new materials such as nano-dusts from emerging nanotechnology.
In parallel we can expect an increase in monitoring of indoor pollutants. Carbon monoxide is a particularly critical indoor pollutant, as it accounts for so many deaths. This has meant a great interest in monitors, which are increasingly common in rooms to detect leaks.
Changes in the way we ventilate spaces, the range of materials and modern activities pose new dangers within our buildings. The changes are not all for the worse, but we have to recognise the types of risks our choices impose. Flame retardants are dangerous and cleaning agents are risky, yet these substances can save lives or reduce the chance of infection. Modern approaches should favour the adoption of materials and processes that lead to the broadest reduction of risk and are compatible with notions of sustainability.
6.12 References
Adonis, M., Gil, L. Indoor air pollution in a zone of extreme poverty of metropolitan Santiago, Chile. Indoor and Built Environment. 2001; 10:138–146.
Afshari, A., Lundgren, B., Ekberg, L.E. ‘Comparison of three small chamber test methods for the measurement of VOC emission rates from paint. Indoor Air. 2003; 13:156–165.
Afshari, A., Matson, U., Ekberg, L.E. Characterization of indoor sources of fine and ultrafine particles, a study conducted in a full-scale chamber. Indoor Air. 2005; 15:141–150.
Andersson, B., Markert, F., Holmstedt, G. Combustion products generated by hetero-organic fuels on four different fire test scales. Fire Safety Journal. 2005; 40:439–465.
Belitz, H.-D., Grosch, W.Lehrbuch der Lebensmittelchemie. Berlin: Springer, 1992.
Bhushan, B., Chandra, S. Detection and sizing of particulate contamination in rigid disk drives: instruments and sampling techniques. IEEE Transactions on Magnetics. 1999; 35:956–962.
Boucher, M., Ehmler, T.J., Bermudez, A.J. Polytetrafluoroethylene gas intoxication in broiler chickens. Avian Diseases. 2000; 44:449–453.
Boutin, M., Lesage, J., Ostiguy, C., Pauluhn, J., Bertrand, M.J. Identification of the isocyanates generated during the thermal degradation of a polyurethane car paint. Journal of Analytical and Applied Pyrolysis. 2004; 71:791–802.
Brasche, S., Bischof, W. Daily time spent indoors in German homes: baseline data for the assessment of indoor exposure of German occupants. International Journal of Hygiene and Environmental Health. 2005; 208:247–253.
Brimblecombe, P. Composition of museum atmospheres. Atmospheric Environment. 1990; 24B:1–8.
Brimblecombe, P. Understanding the composition of museum air. Zeitschrift für Kunsttechnologie und Konservierung. 2008; 22:235–239.
Brimblecombe, P., Shooter, D., Kaur, A. Wool and reduced sulphur gases in museum air. Studies in Conservation. 1992; 37:53–60.
Brimblecombe, N., Dorling, D., Shaw, M. Where the poor die in a rich city: the case of Oxford. Health and Place. 1996; 5:287–300.
Brimblecombe, P., Blades, N., Camuffo, D., Sturaro, G., Valentino, A., Gysels, K., Van Grieken, R., Busse, H.-J., Kim, O., Ulrych, U., Wieser, M. The indoor environment of a modern museum building, the Sainsbury Centre for Visual Arts, Norwich, UK. Indoor Air. 1999; 9:146–164.
CABELiving with risk – Promoting better public space design. London: WHO, 2007.
Camuffo, D., Brimblecombe, P., Van Grieken, R., Busse, H., Sturaro, G., Valentino, A., Bernardi, A., Blades, N., Shooter, D., De Bock, L., Gysels, K., Wieser, M., Kim, O. Indoor air quality at the Correr Museum, Venice, Italy. Science of the Total Environment. 1999; 236:135–152.
Cano-Ruiz, J.A., Kong, D., Balas, R.B., Nazaroff, W.W. Removal of reactive gases at indoor surfaces: combining mass transport and surface kinetics. Atmospheric Environment. 1992; 27:2039–2050.
Chang, F.H., Lin, T.C., Huang, C.I., Chao, H.R., Chang, T.Y., Lu, C.S. Emission characteristics of VOCs from athletic tracks. Journal of Hazardous Materials. 1995; 23:1–20.
Clark, L.A., Hastie, T., Psotakelty, L.A., Sinclair, J.D., Rauchut, J. Sources of particle contamination in an IC manufacturing environment. Aerosol Science and Technology. 1992; 16:43–50.
Diederen, B.M.W. Legionella spp. and Legionnaires’ disease. Journal of Infection. 2008; 56:1–12.
Edwards, R.D., Jurvelin, J., Koistinen, K., Saarela, K., Jantunen, M. OC source identification from personal and residential indoor, outdoor and workplace microenvironment samples in EXPOLIS – Helsinki, Finland. Atmospheric Environment. 2001; 35:4829–4841.
Fan, Z.H., Lioy, P., Weschler, C., Fiedler, N., Kipen, H., Zhang, J.F. Ozone-initiated reactions with mixtures of volatile organic compounds under simulated indoor conditions. Environmental Science and Technology. 2003; 37:1811–1821.
Farrow, A., Taylor, H., Northstone, K., Golding, J. Symptoms of mothers and infants related to total volatile organic compounds in household products. Archives of Environmental Health. 2003; 58:633–641.
Franklin, P. Indoor air quality and respiratory health of children. Paediatric Respiratory Reviews. 2007; 8:281–286.
Grontoft, T., Raychaudhuri, M.R. Compilation of tables of surface deposition velocities for O-3, NO2 and SO2 to a range of indoor surfaces. Atmospheric Environment. 2004; 38:533–544.
Gunschera, J., Fuhrmann, F., Salthammer, T., Schulze, A., Uhde, E. Formation and emission of chloroanisoles as indoor pollutants. Environmental Science and Pollution Research. 2004; 11:147–151.
Hartzell, G.E. Engineering analysis of hazards to life safety in fires: the fire effluent toxicity component. Safety Science. 2001; 38:147–155.
Hodgson, A.T., Levin, H.Volatile Organic Compounds in Indoor Air: a Review of Concentrations Measured in North America Since 1990. Lawrence Berkeley National Laboratory Report, 2003. [LBNL–51715].
Hodgson, A.T., Wooley, J.D., Daisey, J.M. Emissions of volatile organic compounds from new carpets measured in a large-scale environmental chamber. Journal of the Air and Waste Management Association. 1993; 43:316–324.
Hodgson, A.T., Beal, D., McIlvaine, J.E.R. Sources of formaldehyde, other aldehydes and terpenes in a new manufactured house. Indoor Air. 2002; 12:235–242.
Horn, W. VOC emissions from cork products for indoor use. Indoor Air. 1998; 8:39–46.
Houck, P.M., Hampson, N.B. Epidemic carbon monoxide poisoning following a winter storm. Journal of Emergency Medicine. 1997; 15:469–473.
Hull, T.R., Lebek, K., Pezzani, M., Messa, S. Comparison of toxic product yields of burning cables in bench and large-scale experiments. Fire Safety Journal. 2008; 43:140–150.
Hyttinen, M., Pasanen, P., Bjorkroth, M., Kalllokoski, P. Odors and volatile organic compounds released from ventilation filters. Atmospheric Environment. 2007; 41:4029–4039.
Janssen, N.A.H., van Vliet, P.H.N., Aarts, F., Harssema, H., Brunekreef, B. Assessment of exposure to traffic related air pollution of children attending schools near motorways. Atmospheric Environment. 2001; 35:3875–3884.
Jensen, B., Wolkoff, P., Wilkins, C.K., Clausen, P.A. Characterization of linoleum. Part 1: measurement of volatile organic compounds by use of the field and laboratory emission cell, FLEC. Indoor Air. 1995; 5:38–43.
Jensen, B., Wolkoff, P., Wilkins, C.K. Characterization of linoleum. Part 2: preliminary odor evaluation. Indoor Air. 1995; 5:44–49.
Kavi Kumar, K.S., Viswanathan, B. Changing structure of income indoor air pollution relationship in India. Energy Policy. 2007; 35:5496–5504.
Kim, S.S., Kang, D.H., Choi, D.H., Yeo, M.S., Kim, K.W. Comparison of strategies to improve indoor air quality at the pre-occupancy stage in new apartment buildings. Building and Environment. 2006; 43:320–328.
Klitzman, S., Caravanos, J., Belanoff, C., Rothenberg, L. A multihazard, multistrategy approach to home remediation: results of a pilot study. Environmental Research. 2005; 99:294–306.
Kuske, M., Romain, A.-C., Nicolas, J. Microbial volatile organic compounds as indicators of fungi. Can an electronic nose detect fungi in indoor environments? Building and Environment. 2005; 40:824–831.
Leaderer, B.P. Air pollutant emission from kerosene stove. Science. 1982; 218:1113–1115.
Lebens, J.A., McColgin, W.C., Russell, J.B., Mori, E.J., Shive, L.W. Unintentional doping of wafers due to organophosphates in the clean room ambient. Journal of the Electrochemical Society. 1996; 143:2906–2909.
Liu, Q.T., Chen, R., McCarry, B.E., Diamond, M.L., Bahavar, B. Characterization of polar organic compounds in the organic film on indoor and outdoor glass windows. Environmental Science and Technology. 2003; 37:2340–2349.
Lloyd, H., Brimblecombe, P., Lithgow, K. The economics of dust. Studies in Conservation. 2007; 52:135–146.
Manninen, A.M., Pasanen, P., Holopainen, J.K. Comparing the VOC emissions between air-dried and heat-treated Scots pine wood. Atmospheric Environment. 2002; 36:1763–1768.
Melia, R.J., Florey, C.D., Chinn, S., Morris, R.W., Goldstein, B.D., John, H.H., Clark, D. Investigations into the relations between respiratory illness in children, gas cooking and nitrogen dioxide in the UK. The Tokai Journal of Experimental and Clinical Medicine. 1985; 10:3775–3787.
Muller, A.J., Psotakelty, L.A., Krautter, H.W., Sinclair, J.D. Volatile cleanroom contaminants – sources and detection. Solid State Technology. 1994; 37:61.
Ni, Y., Kumagai, K., Yanagisawa, Y. Measuring emissions of organophosphate flame retardants using a passive flux sampler. Atmospheric Environment. 2007; 41:3235–3240.
Pandit, G.G., Srivastava, P.K., Mohan Rao, A.M. Monitoring of indoor volatile organic compounds and polycyclic aromatic hydrocarbons arising from kerosene cooking fuel. Science of the Total Environment. 2001; 279:159–165.
Pluschke, P. Indoor Air Pollution. Berlin: Springer, 2004.
Poppendieck, D.G., Hubbard, H.F., Weschler, C.J., Corsi, R.L. Formation and emissions of carbonyls during and following gas-phase ozonation of indoor materials. Atmospheric Environment. 2007; 41:7614–7626.
Raub, J.A., Mathieu-Nolf, M., Hampson, N.B., Thom, S.R. Carbon monoxide poisoning: a public health perspective. Toxicology. 145, 2008.
Reiss, R., Ryan, P.B., Koutrakis, P., Tibbetts, S.J. Ozone reactive chemistry on interior latex paint. Environmental Science and Technology. 1995; 29:1906–1912.
Salthammer, T. Emission of volatile organic compounds from furniture coatings. Indoor Air. 1995; 7:189–197.
Salthammer, T. Volatile organic ingredients of household and consumer, products. In: Salthammer T., ed. Organic Indoor Air Pollutants. Wiley VCH: Weinheim; 1995:219–232.
Salthammer, T., Fuhrmann, F., Uhde, E. Flame retardants in the indoor environment – Part II: Release of VOCs (triethylphosphate and halogenated degradation products) from polyurethane. Indoor Air. 2003; 13:49–52.
Sherman, M.H.Air Change Rate and Airtightness in Buildings. West Conshohocken, PA: ASTM International, 1999. [Vol STP 1067].
Shields, H.C., Weschler, C.J. Volatile organic compounds measured at a telephone switching center from 5/30/85–12/6/88 – a detailed case study. Journal of the Air and Waste Management Association. 1992; 42:792–804.
Smith, K.R., Samet, J.M., Romieu, I., Bruce, N. Indoor air pollution in developing countries and acute lower respiratory infections in children. Thorax. 2000; 55:518–532.
Spyak, P.R., Wolfe, W.L. Scatter from particulate-contaminated mirrors. 3. Theory and experiment for dust and lambda = 10.6 μm. Optical Engineering. 1992; 31:1764–1774.
Toftum, J., Freund, S., Salthammer, T., Weschler, C.J. Secondary organic aerosols from ozone-initiated reactions with emissions from wood-based materials and a green paint. Atmospheric Environment. 2008; 42:7632–7640.
Toishi, K., Kenjo, T. Some aspects of the conservation of art works in buildings of new concrete. Journal of Paint Technology. 1967; 39:52–55.
Toishi, K., Kenjo, T. Some aspects of the conservation of art works in buildings of new concrete. Studies in Conservation. 1975; 20:118–122.
Uhde, E., Salthammer, T. Impact of reaction products from building materials and furnishings on indoor air quality – a review of recent advances in indoor chemistry. Atmospheric Environment. 2007; 41:3111–3128.
Van Loy, M.D., Riley, W.J., Daisey, J.M., Nazaroff, W.W. Dynamic behavior of semivolatile organic compounds in indoor air. 2. Nicotine and phenanthrene with carpet and wallboard. Environmental Science and Technology. 2001; 35:560–567.
Verschueren, K.Handbook of Environmental Data on Organic Chemicals. New York: Wiley, 1996.
Wang, H., Morrison, G.C. Ozone-initiated secondary emission rates of aldehydes from indoor surfaces in four homes. Environmental Science and Technology. 2006; 40:5263–5268.
Wang, S., Ang, H.M., Tade, M.O. Volatile organic compounds in indoor environment and photocatalytic oxidation: state of the art. Environment International. 2007; 33:694–705.
Watling, R., Harper, D.B. Chloromethane production by wood-rotting fungi and an estimate of the global flux to the atmosphere. Mycological Research. 1998; 102:769–787.
Wensing, M., Uhde, E., Salthammer, T. Plastics additives in the indoor environment – flame retardants and plasticizers. Science of the Total Environment. 2003; 339:9–40.
Weschler, C.J. Changes in indoor pollutants since the 1950s. Atmospheric Environment. 2009; 43:153–169.
Weschler, C.J., Shields, H.C. Potential reactions among indoor pollutants. Atmospheric Environment. 1997; 31:3487–3495.
Weschler, C.J., Shields, H.C. Indoor ozone/terpene reactions as a source of indoor particles. Atmospheric Environment. 1999; 33:2301–2312.
Weschler, C.J., Hodgson, A.T., Wooley, J.D. Indoor chemistry – ozone, volatile organic compounds, and carpets. Environmental Science and Technology. 1992; 26:2371–2377.
WHODevelopment of WHO Guidelines for Indoor Air Quality. Copenhagen: World Health Organization Regional Office for Europe, 2006.
Wolkoff, P., Schneider, T., Kildesø, J., Degerth, R., Jaroszewski, M., Schunk, H. Risk in cleaning: chemical and physical exposure. Science of the Total Environment. 1998; 215:135–156.
Won, D., Sander, D.M., Shaw, C.Y., Corsi, R.L. Validation of the surface sink model for sorptive interactions between VOCs and indoor materials. Atmospheric Environment. 2001; 35:4479–4488.
Yang, W., Sohn, J., Kim, J., Son, B., Park, J. Indoor air quality investigation according to age of the school buildings in Korea. Journal of Environmental Management. 2009; 90:348–354.
Zhao, Y., Hu, M., Slanina, S., Zhang, Y. The molecular distribution of fine particulate organic matter emitted from Western-style fast food cooking. Atmospheric Environment. 2007; 41:8163–8171.
